Abstract
While chemotherapy provides useful palliation, advanced lung cancer remains incurable since those tumors that are initially sensitive to therapy rapidly develop acquired resistance. Resistance may arise from impaired drug delivery, extracellular factors, decreased drug uptake into tumor cells, increased drug efflux, drug inactivation by detoxifying factors, decreased drug activation or binding to target, altered target, increased damage repair, tolerance of damage, decreased proapoptotic factors, increased antiapoptotic factors, or altered cell cycling or transcription factors. Factors for which there is now substantial clinical evidence of a link to small cell lung cancer (SCLC) resistance to chemotherapy include MRP (for platinum-based combination chemotherapy) and MDR1/P-gp (for non-platinum agents). SPECT MIBI and Tc-TF scanning appears to predict chemotherapy benefit in SCLC. In non-small cell lung cancer (NSCLC), the strongest clinical evidence is for taxane resistance with elevated expression or mutation of class III β-tubulin (and possibly α tubulin), platinum resistance and expression of ERCC1 or BCRP, gemcitabine resistance and RRM1 expression, and resistance to several agents and COX-2 expression (although COX-2 inhibitors have had minimal impact on drug efficacy clinically). Tumors expressing high BRCA1 may have increased resistance to platinums but increased sensitivity to taxanes. Limited early clinical data suggest that chemotherapy resistance in NSCLC may also be increased with decreased expression of cyclin B1 or of Eg5, or with increased expression of ICAM, matrilysin, osteopontin, DDH, survivin, PCDGF, caveolin-1, p21WAF1/CIP1, or 14-3-3sigma, and that IGF-1R inhibitors may increase efficacy of chemotherapy, particularly in squamous cell carcinomas. Equivocal data (with some positive studies but other negative studies) suggest that NSCLC tumors with some EGFR mutations may have increased sensitivity to chemotherapy, while K-ras mutations and expression of GST-pi, RB or p27kip1 may possibly confer resistance. While limited clinical data suggest that p53 mutations are associated with resistance to platinum-based therapies in NSCLC, data on p53 IHC positivity are equivocal. To date, resistance-modulating strategies have generally not proven clinically useful in lung cancer, although small randomized trials suggest a modest benefit of verapamil and related agents in NSCLC.
Keywords: lung cancer, chemotherapy, resistance
1.0 BACKGROUND
1.1 Lung cancer and resistance
Lung cancer is the leading cause of cancer death in the United States and has a 5-year relative survival rate of only 16%1. Chemotherapy yields response rates of 20–50% in advanced non-small cell lung cancer (NSCLC) and 60–80% in extensive small cell lung cancer (SCLC), but almost all tumors that are not intrinsically resistant rapidly develop acquired resistance, often with broad cross-resistance to other unrelated chemotherapy agents. Alternating chemotherapy agents with differing mechanisms of action does not overcome this resistance2.
1.2 Types of resistance
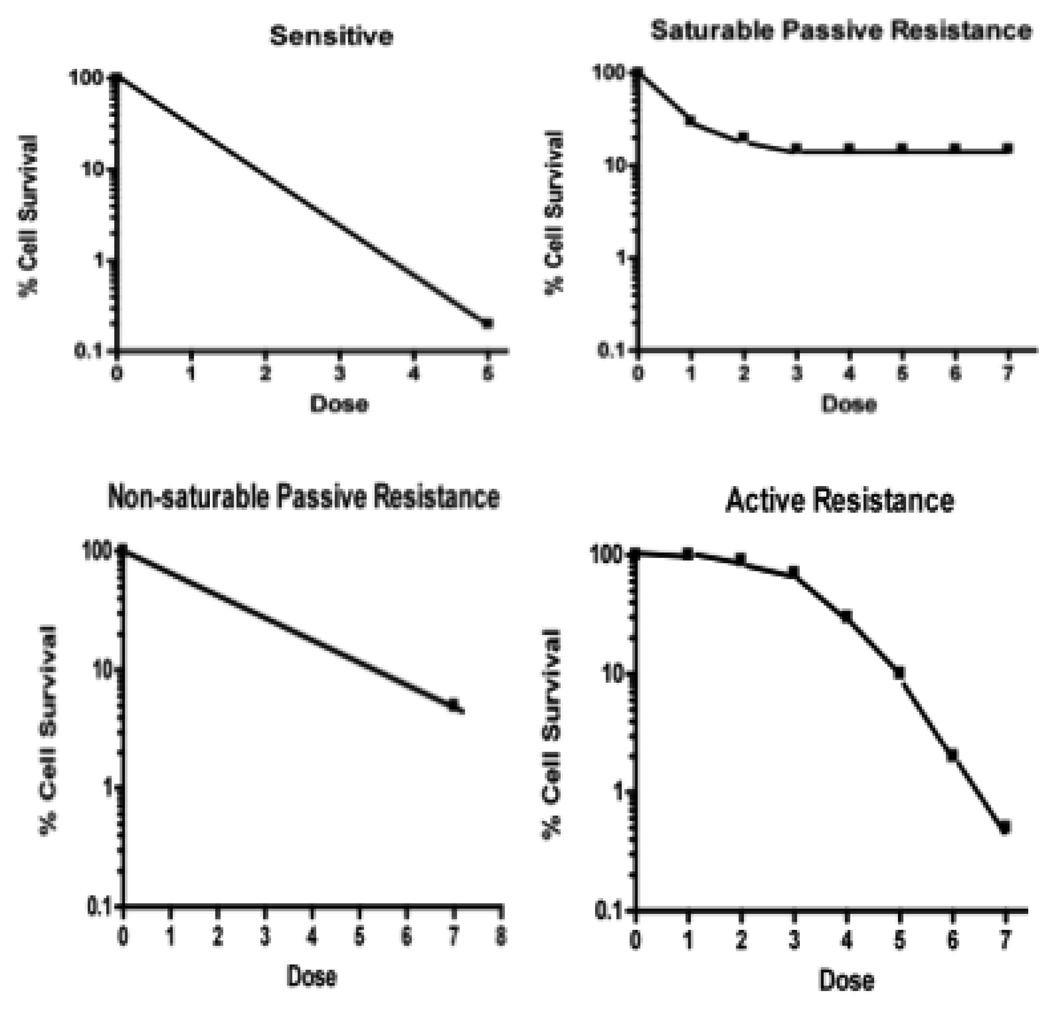
Resistance may be classified in several ways (Table 1). In addition to being intrinsic vs acquired, it may also be classified pharmacodynamically as “active” (due to excess of a resistance factor, analogous to competitive inhibition of drug effect) vs “non-saturable passive” (due to mutation or alteration of a factor, analogous to decreased affinity of a drug for its receptor) vs “saturable passive” (due to deficiency or saturation of a factor required for drug efficacy, analogous to non-competitive inhibition of drug effect)3. Just as dose-response curve shapes reflect whether drug inhibition is competitive vs non-competitive, they may also reflect the predominant type of resistance (Fig. 1)3. Dose response curve flattening at higher drug doses in NSCLC and the failure of bone marrow transplant approaches to impact other epithelial tumors suggests that the major reason for not being able to cure metastatic NSCLC and other epithelial tumors is due to saturable passive resistance (deficiency or saturation of factors required for drug efficacy)4.
Table 1.
Classification of resistance
| Classification Method | Types of resistance |
|---|---|
| Time of onset | Intrinsic (de novo) Acquired |
| Pharmacodynamic | Active (competitive inhibition) Non-saturable passive (decreased affinity) Saturable passive (non-competitive inhibition) |
| Kinetic | Quiescent Accelerated |
| Genetic | Mutation Epigenetic |
| Host vs tumor | Host factor Tumor factor |
| Cell involved | Tumor cell factor Microenvironment/stromal cell factor Abscopal effect of distant resistant tumor |
Fig 1.
Cell kill by first order kinetics gives a straight line in sensitive cells. Deficiency of a factor needed for tumor cell killing (“saturable passive resistance”) gives a terminal plateau on the dose-response curve, analogous to non-competitive inhibition of drug effect. Alteration or mutation of a factor (“non-saturable passive resistance”) results in a decreased dose-response curve slope (analogous to decreased affinity of a drug for its receptor). Excess of a mutation factor (“active resistance”) gives a shoulder on a dose-response curve, analogous to competitive inhibition of drug effect.
Resistance may also be classified as “quiescent” (due to lack of cell cycling through a sensitive cell cycle phase) vs “accelerated” (with treatment failure due to rapid repopulation after initial tumor cell killing). Resistance may be classified as genetic (due to selection of tumor cell subpopulations with mutations that render the cells resistant) or it may arise epigenetically (with rapid up-regulation or down-regulation of relevant genes). In genetic resistance, mutations could involve simple point mutations or could be associated with extensive chromosomal changes5 including aneuploidy6 or with amplification of resistance factors as a result of repeated chromosomal breakage-fusion-bridge cycles7. Epigenetic changes could arise due to alterations of DNA methylation8.
Resistance may also be classified as arising due to tumor cell characteristics or due to factors in the tumor environment. With few exceptions9, in vitro sensitivity testing in lung cancers has done well at predicting resistance with very few false negatives (ie, there have been few patients predicted to be resistant but who responded clinically), while false positives have been relatively common (predicted sensitive but failed clinically)10–15. Cases that are resistant clinically despite in vitro testing predicting sensitivity are potential examples of resistance arising from tumor environmental factors despite intrinsic sensitivity of the tumor cells.
More than one of these classifications may be relevant for a single resistance mechanism. For example, the broad down-regulation of membrane transporters reported previously in cisplatin-resistant hepatoma and cervical carcinoma cell lines16 would be an example of resistance that is both epigenetic and saturable passive. The development of reversible senescence17 and autophagy18 in response to chemotherapy exposure has characteristics of each of acquired, epigenetic, quiescent and saturable passive resistance. Clinically, quiescent tumor cells may survive the first cycle of chemotherapy, and surviving tumor cells may then undergo rapid epigenetic changes leading to enhanced resistance due to upregulation of active resistance mechanisms and to accelerated repopulation4. Alternatively, the epigenetic changes may lead to down-regulation of growth factors, transporters, etc, and saturable passive resistance associated with reversible quiescence4.
In preclinical models, a sensitive tumor may also be rendered resistant if a resistant tumor is implanted on the opposite flank19. While the mechanism of this effect is unknown, one might speculate that it is mediated through cytokines produced by the resistant tumor. Such cytokines could potentially directly impact characteristics of tumors located in other sites, or, alternatively they could have an indirect effect (eg, mediated by their stimulation of release of mesenchymal stem cells from the bone marrow20).
1.3 Resistance factors and normal tissues
Many putative cancer resistance factors are the same factors that normal cells use to protect themselves from oxygen radicals, ingested toxins, radiation, temperature extremes, and other components of a hostile environment. The dependence of normal tissues on these may explain in part our limited ability to successfully and safely counter them sufficiently with resistance modulators to augment sensitivity of tumors to therapy. Adding to the problem is the fact that multiple different resistance factors probably enter into play simultaneously21–23.
1.4 Cross-resistance
The problem is also magnified by the reality that factors that render a tumor resistant to one drug may also simultaneously increase resistance to several other agents. As a result, alternating up to 4 regimens2 or combining 6 chemotherapy agents24 with differing mechanisms of action in patients with NSCLC did not appear to improve outcome. In preclinical studies in lung cancer cell lines and xenografts, cross-resistance is seen frequently, but is not universal25–33. While there are no completely consistent patterns, there may be a higher probability of lack of lung cancer cross-resistance for platinums vs taxanes25, 27–29 or vs pemetrexed34, or for gemcitabine vs other agents26, 30, 33, 35, 36.
1.5 Implications of correlations of resistance with the host genotype
In addition to tumor cell characteristics being important, the observed relationship between chemotherapy-induced leukopenia and tumor response in SCLC37 and in NSCLC38 supports a role for host factors in treatment outcome. For example, patient genotype for various drug metabolism pathways could alter both drug efficacy and drug toxicity. We will not discuss these further in this paper, but several examples of these have been described in lung cancer patients39–41. However, we will discuss in later sections how host genotype for specific resistance factors impacts therapy outcome, and examples are presented in Table 2. Resistance to chemotherapy may correlate with a resistance factor’s gene copy number, gene mutation status, mRNA expression, or protein expression within a tumor, or with host genotype polymorphisms. Little work has been done to correlate host genotype polymorphisms with tumor expression of a gene, but genotype variations may lead to alteration of a factor’s enzymatic activity or protein stability or half-life. Hence, altered (increased or decreased) expression of a resistance or sensitivity factor in a tumor could result from increased or decreased production related to gene copy number or due to up-regulation or down-regulation in response to cellular factors, or it could be due to prolonged or shortened protein half-life due either to tumor gene mutations or due to the tumor inheriting from the host a specific gene factor polymorphism that prolongs or shortens the half-life of the gene product. Similarly, enzymatic activity of the protein could be increased or decreased due to tumor gene mutations or due to polymorphisms inherited from the host. Hence, for a specific factor that may be important in resistance, there could be lack of correlations of outcome with mRNA expression or protein expression because of mutations or polymorphisms that alter gene product half-life or activity. During tumorigenesis, oncogene mutations often drive tumor growth, and are selected for, but one might not expect to see selection for specific resistance factor mutations until after initiation of therapy; hence, host genotype polymorphisms might hypothetically be particularly likely to play a major role in intrinsic resistance, with a possibility of resistance factor mutation or gene amplification or deletion playing a bigger role in acquired resistance.
Table 2.
Resistance factors for which host gene polymorphisms correlate with resistance clinically
| Platinum regimens: |
| MRP2 |
| Glutathione-S-transferase-π |
| Other glutathione-related genes |
| ERCC1 |
| Xeroderma pigmentosum Ca |
| Xeroderma pigmentosum Da |
| Xeroderma pigmentosum G |
| XRCC1a |
| BRCA1 |
| NQO1 |
| P53 |
| Cyclin D1 |
| SHH |
| Gemcitabine: |
| Deoxycytidine deaminase |
| Irinotecan (with cisplatin): |
| MRP2 |
| Etoposide/vinorelbine (with cisplatin): |
| MDR1/P-glycoproteinb |
Data were negative or equivocal in some individual trials
No association with outcome in patients treated with cisplatin-docetaxel
1.6 Chemotherapy as “targeted” therapy
There is now substantial interest in “targeted therapies” for cancer, but chemotherapy agents may also be “targeted”. The fact that there is any selectivity of effect, with marked shrinkage of some tumors in response to chemotherapy, suggests the possibility that those tumors not only are deficient in resistance factors but that they also possess the target required for drug effect. We have traditionally thought of chemotherapy as simply targeting DNA, tubulin, topoisomerases, etc, but all normal cells also possess these, and the ability to selectively kill some (but not other) tumor cells suggests that the sensitive tumor cells may possess an additional target (or activating system, etc) required for sensitivity, and hence, resistance may arise from the absence or saturation of a required target, etc, rather than from the presence of a resistance factor. Dose-response curve flattening at higher drug doses would be in keeping with this. On the whole, we have not done a good job of searching for these hypothetical unique chemotherapy targets. In the past, the sensitivity of cells to chemotherapy has been attributed to rapid cell growth, but this does not explain things well. For example, cisplatin is active against many types of tumors and is toxic to cochlear hair cells, renal convoluted tubule cells, dorsal root ganglion neurons and gastrointestinal enterochromaffin cells but is not toxic to most rapidly dividing normal tissues.
While tumor type may be used to guide choice of agents (eg, pemetrexed was more effective in lung adenocarcinomas than was gemcitabine, while gemcitabine was more effective than pemetrexed against squamous cell carcinomas42), molecular characteristics are only infrequently used in choosing patients for clinical trials. We feel that it is important to raise the efficacy bar by putting substantially more effort into defining molecular determinants of sensitivity vs resistance43. Resistance modulators have generally had equivocal or negative effects in solid tumors. Modulators aimed at resistance factors such as efflux pumps might not be expected to make much of a difference if the tumor lacks a target, activating system, etc required for chemotherapy action.
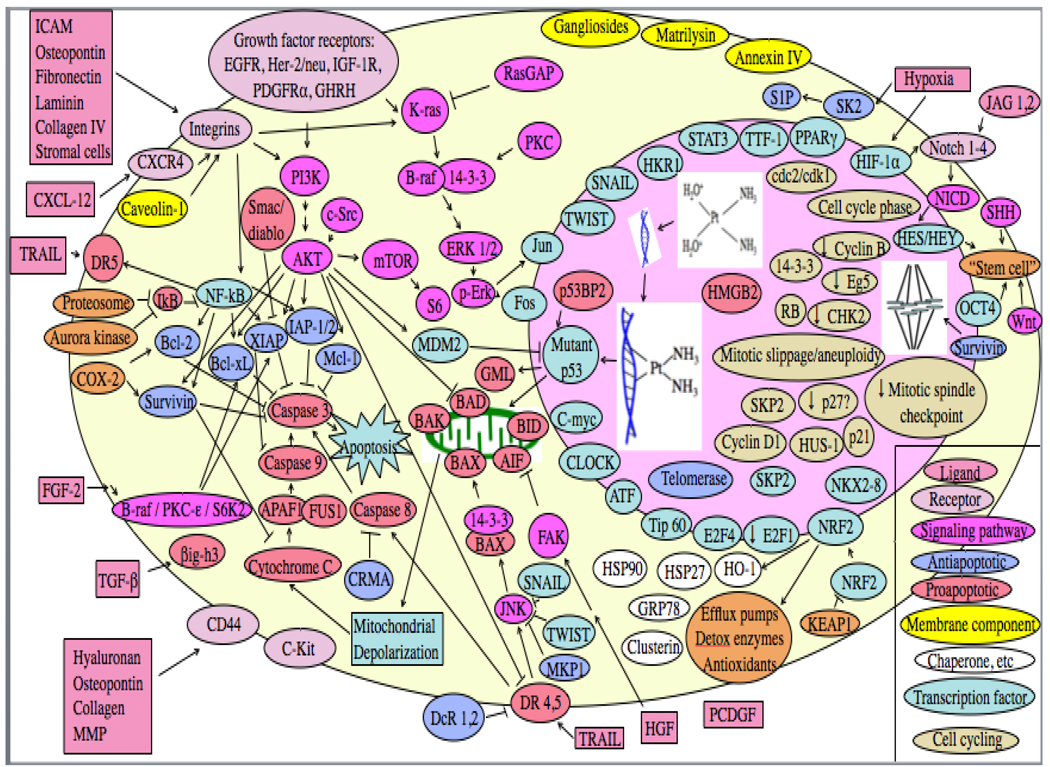
Below we will discuss resistance to a variety of chemotherapy agents in lung cancer. Resistance mechanisms, agents affected and presence or absence of supporting clinical evidence is summarized in Table 3.
Table 3.
Tumor factors contributing to lung cancer resistance to chemotherapy
| Factor | Agents affected preclinically | Clinical data support link to resistance | |
|---|---|---|---|
| Yes | No | ||
| Decreased tumor blood flow: | |||
| ↓ Drug delivery | All? | Cisplatin combinations | |
| ↓ Oxygen delivery | Etoposide, paclitaxel (not cisplatin, topotecan) | ||
| ↑ sphingosine kinase 2 / sphingosine- 1-phosphate |
Etoposide | ||
| ↑ HIF-1α | Cisplatin, doxorubicin, paclitaxel | Cisplatin-gemcitabine | |
| ↑ VEGF | Cisplatin-gemcitabine | ||
| Alterations of tumor extracellular pH: | |||
| ↓ pH | Weak bases (doxorubicin, vinca alkaloids) | ||
| ↑ pH | Weak acids (platinums, alkylating agents) | ||
| Decreased drug uptake: | |||
| ↑ Cell membrane rigidity/sphingomyelin/cholesterol |
Platinums, etoposide, paclitaxel | ||
| ↓ Long chain/unsaturated fatty acids | Platinums | ||
| ↓ CTR1 | Platinums | ||
| ↓ Multiple membrane transporters | Platinums | ||
| ↓ Na+, K+ ATPase/ ↑ thromboxane A2/ ↑ sorbitol |
Platinums | Platinums a | |
| ↑ Intracellular chloride or zinc | Platinums | ||
| ↓ Human equilibrative nucleoside transporter 1 |
Gemcitabine | Gemcitabinea | |
| ↓ Folate transporters | Pemetrexed | Pemetrexed | |
| Increased drug efflux: | |||
| ↑ MRP/GSH-conjugate pump | Platinumsa, anthracyclines, vincas, etoposide, taxanes, gemcitabinea |
Multiple platinum regimensa; vindesine + etoposide |
|
| ↑ MDR1/p-glycoproteinb | Anthracyclines, vincas, etoposide, taxanes | Multiple regimensa | |
| ↓ Connexin 32 | Vinorelbine | ||
| ↑ Breast cancer resistance protein | Platinum regimens | ||
| ↑ RLIP76/RALBP1 | Vinorelbine, doxorubicin | ||
| ↑ Lung resistance proteinc | Cisplatina, etoposidea | Platinum regimensa | Taxanes, CAV, some cisplatin regimens |
| ↑ P-type adenosine triphosphatase 7B | Cisplatin | ||
| Increased drug detoxification: | |||
| ↑ Glutathione (GSH) | Cisplatin, etoposidea, anthracyclinesa, vincasa, radiation, camptothecins, mitomycin, alkylating agents, methotrexate |
||
| ↑ Glutamate-cysteine ligase | Cisplatin | ||
| ↑ GSH peroxidase/GSH reductase | Cisplatin | ||
| ↑ Glutathione-S-transferase-π | Cisplatina | Platinum regimensa | Vinorelbine regimens |
| ↑ Metallothioneins | Cisplatin, etoposide | Cisplatin-etoposide/CAV | |
| ↑ Dihydrodiol dehydrogenase | Cisplatin, doxorubicin, taxanesa, vincas, melphalan | ||
| ↑ Thymidine & folate pools | Pemetrexed | ||
| ↑ Peroxiredoxin V | Doxorubicin, etoposide | ||
| ↑ Deoxycytidine deaminase | Gemcitabine | ||
| Decreased drug activation or binding: | |||
| ↓ Deoxycytidine kinase activity | Gemcitabine | ||
| ↓ drug binding/ ↑ intracellular pH | Cisplatin | ||
| Increased, decreased or altered target: | |||
| ↑ Ribonucleotide reductase M1 | Gemcitabine | Gemcitabine regimens | Platinum + etoposide |
| ↑ Ribonucleotide reductase M2 | Gemcitabine + docetaxel | ||
| ↑ Folate pathway enzymes | Pemetrexed | ||
| ↓ Stathmin (oncoprotein 18) | Vincas | Cisplatin-vinorelbineg | |
| ↑/mutated III β tubulin (+/− α tubulin) | Taxanes, vincasa, cisplatin, doxorubicin, etoposide | Taxanes, cisplatin- vinorelbinea |
|
| ↑ histone deacetylase 6 (↑ tubulin staility) |
Taxanes | ||
| ↓ /mutated Topoisomerase II-α | Etoposide, anthracyclines | Etoposide | |
| ↑ Fragile histidine triad gene | Etoposide, camptothecins | ||
| Increased DNA damage repair: | |||
| ↑ Topoisomerase II-α | Cisplatin, radiation, vincas | Cisplatin regimens | |
| ↑ ERCC1 | Platinumsa | Platinum regimensa | Gemcitabine/docetaxel, gemcitabine/epirubicin |
| ↑ Xeroderma Pigmentosum A | Platinums | ||
| ↑ Rad23A | Cisplatin | ||
| ↑ Ribonucleotide reductase M1 | Gemcitabine, cisplatin | Gemcitabine regimens | Platinum + etoposide |
| ↑ Rad51 (homologous DNA repair) | Platinums, etoposide | Cisplatin + gemcitabine | |
| ↑ DNA-dependent protein kinase | Etoposide | ||
| ↑ Hus1 | Cisplatin | ||
| ↑ BRCA1 | Platinumsd | Cisplatin + gemcitabinea, cisplatin regimens |
Gemcitabine + epirubicin or docetaxeld |
| ↑ FANCD2 | Platinum regimens | ||
| ↑ apurinic/apyridinimic endonuclease | Cisplatin | Cisplatin regimens | |
| ↑ Eme1 endonuclease | Cisplatin | ||
| ↑ PARP | Cisplatin, topotecan, temozolamide | ||
| ↓ High mobility group box 2 | Cisplatin | ||
| ↓ Fragile histidine triad gene | Cisplatin | ||
| ↑ Thymidylate synthase | Platinums | ||
| ↑ Dihdropyrimidine dehydrogenase | Platinums | ||
| Decreased apoptotic response: | |||
| ↓ DNA mismatch repair | Platinums | Platinum regimensa | |
| p53 mutation/ ↑ IHC expression | Cisplatin, etoposide, camptothecin, methotrexate, anthracyclines, radiation, taxanesa, others |
Platinum regimensa,c, CAV | Taxanes or vincas without platinums |
| ↓ p53-binding protein 2 | Cisplatin, radiation | ||
| ↓ GML protein | Cisplatin | Cisplatin | |
| ↓ Caspase-8 activity | Cisplatin, topotecan, radiation | ||
| ↓ Caspase-9 activity | Cisplatin | ||
| ↓ FUS1 | Cisplatin | ||
| ↓ SAPK/c-Jun N-terminal kinase | Platinums, gemcitabine | ||
| ↓ Bak, Bad, Bid | Cisplatin, etoposide, radiation, Fas ligand | Vinorelbine | |
| ↓ Baxa | Cisplatin, etoposide, taxanes, doxorubicin | Cisplatin regimens, Vinorelbine/docetaxel |
|
| ↓ Apoptosis signal transduction | Cisplatin, taxanes | ||
| ↓ ERK1/2 & MAPK/ERK kinase | Taxanesa, cisplatina | ||
| ↓ p-ERK | Gemcitabine | Platinum regimens, taxane regimens |
|
| ↓ βig-h3 | Etoposide | ||
| Increased Apoptosis Inhibitors: | |||
| ↑ Cyclooxygenase-2 | Cisplatin, anthracycline, etoposide, vinca, taxane, gemcitabine |
Carboplatinh, gemcitabineh, vinorelbinei, docetaxel |
|
| ↑ Bcl-2a,f | Cisplatin, camptothecin, doxorubicin, etoposide, vincas | Platinum regimens, vincas, taxanes, etoposide regimens |
|
| ↑ Bcl-xL | Cisplatin, gemcitabine, doxorubicin, vincas, taxanes, etoposide, others |
Vinorelbine | |
| ↑ Mcl-1 | Cisplatin, etoposide, taxanes, radiation | ||
| ↑ Survivin | Cisplatin, gemcitabine, taxanes | Cisplatin/etoposide | |
| ↑ Livin | Etoposide | ||
| ↑ XIAP | Cisplatin, etoposide | ||
| ↑ IAPs | Gemcitabine | ||
| ↑ Telomerase | Cisplatin, docetaxel, etoposide | ||
| ↑ TRAIL decoy receptors 1 & 2 | Doxorubicin, etoposide | ||
| ↑ Epidermal growth factor receptor (by IHC or gene copy number)j |
Cisplatina, doxorubicin, etoposide, vincas, taxanes, camptothecin, pemetrexed, gemcitabine, others |
Platinums, taxane, XRT, gemcitabine, vinorelbine |
|
| EGFR wild type vs exon 19 deletion | Platinum regimens | ||
| ↑ HER-2/neu (erbB-2, p185) | Cisplatin, etoposide, doxorubicin, taxanes, gemcitabinea,e |
Platinum regimensa | |
| ↑ IGF-1R | Platinums, etoposide, taxanes | Carboplatin/paclitaxel | |
| ↑ Attachment to ECM: integrins | Cisplatin, doxorubicin, taxanes, etoposide, others | ||
| ↑ Intercellular adhesion molecule | Cisplatin/paclitaxel | ||
| ↑ Osteopontin | Cisplatin | Carboplatin/paclitaxel | |
| ↑ Stromal-cell-derived factor- 1/CXCL12 |
Etoposide | ||
| ↑ Matrix metalloproteinase-7 (matrilysin) |
Platinum regimens | ||
| ↑ Hyaluronan/CD44 | Cisplatin | ||
| ↑ Growth hormone releasing hormone | Taxanes | ||
| ↓ c-Kit | Cisplatin + etoposide | ||
| ↑ PDGFRα | Cisplatin | ||
| ↑ Hepatocyte growth factor | Cisplatin | ||
| ↑ Fibroblast growth factor 2 | Etoposide | ||
| ↑ PC cell-derived growth factor | Platinum regimens | ||
| ↑ PTEN/PI3K/Akt/mTOR pathway activation |
Cisplatin, etoposide, taxanes, gemcitabine, others | Platinum regimens, taxanes |
|
| ↑ p70S6K & S6 phosphorylation | Cisplatin | ||
| K-ras mutation | Taxanes | Platinum regimensa,j | |
| ↑ ERK1/2 | Etoposide, cisplatina, taxanesa | ||
| ↑ MAPK phosphatase-1 | Cisplatin | ||
| ↑ PKC-α | Platinumsa, vincas, taxanesa, doxorubicin, others | Cisplatin + gemcitabine | |
| ↓ PKC-β | Cisplatin, etoposide | ||
| ↑ PKC-δ | Etoposide, cisplatin | ||
| ↑ PKC-ε | Etoposide, doxorubicin | ||
| ↑ PKC-η | Platinumsa, vincas, taxanesa, doxorubicin, others | ||
| ↑ Caveolin-1/Caveolae organelles | Etoposide, paclitaxel | Gemcitabine/cisplatin, gemcitabine/epirubicin |
|
| Altered membrane gangliosides | Cisplatin | ||
| ↑ Annexin IV | Taxanes | ||
| ↑ c-Src | Amrubicin | ||
| ↑ Heat shock protein 90 | Taxanes | ||
| ↑ Heat shock protein 27 | Vinorelbinei | ||
| ↑ Clusterin | Paclitaxel, gemcitabine | ||
| ↑ Nrf2/heme oxygenase-1 | Platinums, etoposide, doxorubicin | ||
| ↓ / mutated Keap1 | Platinums, etoposide, doxorubicin | ||
| ↑ Glucose-regulated protein 78 | Etoposide | ||
| Altered cell cycling: | |||
| Cell cycle phase | Varies with drug | ||
| Abnormal mitotic spindle checkpoint | Vinorelbine, taxanes | ||
| ↓ mitotic slippage/ ↓ aneuploidy | Taxanes | ||
| ↑ aneuploidy | Etoposide, topotecan, gemcitabine | ||
| ↓ CHK2 kinase | Cisplatin | ||
| ↑ HUS1 | Cisplatin | ||
| ↑ RB/ ↓ pRB | Cisplatin, etoposide, taxanes, 5-FU | Cisplatin regimensa | |
| ↑ 14-3-3ζ | Cisplatin | ||
| ↑ 14-3-3σ | Cisplatin + gemcitabine | ||
| ↑ p27Kip1 | Cisplatin regimensa | ||
| ↑ P21WAF1/CIP1 | Cisplatin, camptothecin, doxorubicin, etoposide | Platinum regimens | |
| ↑ SKP2 | Cisplatin, camptothecin, others | ||
| ↑ Cdc2/cdk1 | Platinum regimens | ||
| Cyclin D1 polymorphisms | Platinum regimens | ||
| ↓ Eg5 | Cisplatin + antimitotic agent |
||
| ↓ Cyclin B1 | Platinums + antimitotic agents |
||
| Increased transcription factors: | |||
| ↑ NF-κB | Cisplatin, doxorubicin a, etoposidea, gemcitabine, taxanes |
||
| ↑ Clock | Cisplatin, etoposide | ||
| ↑ Activating Transcription Factor 4 | Cisplatin, etoposide | ||
| ↑ HIV-1 Tat Interacting Protein 60 | Cisplatin | ||
| ↑ TWIST | Cisplatin | ||
| ↑ SNAIL | Cisplatin | ||
| ↑ Kruppel-related zinc finger protein-1 (HKR-1) |
Cisplatin | Cisplatin | |
| ↑ STAT3 | Cisplatin | ||
| ↑ Thyroid Transcription Factor-1 | Cisplatin | ||
| ↑ NKX2–8 | Cisplatin | ||
| ↑ PPARγ splice variant | Cisplatin | ||
| ↑ c-myc | Cisplatin | ||
| ↑ E2F4/ ↓ E2F1 | Cisplatin, etoposide | ||
| ↑ Oct4 | Cisplatin | ||
| Stem cell markers and pathways | |||
| ↑ CD133 | Cisplatin, etoposide, doxorubicin, paclitaxel | Platinum regimens | |
| SHH polymorphisms | Cisplatin, carboplatin | Platinum regimens | |
| “Global” factors | |||
| Gene expression arrays | Cisplatin, taxanes, irinotecan, gemcitabine, vinorelbine | Cisplatin regimens, pemetrexed |
|
| Chromosomal alterations | Platinums, taxanes, etoposide, topotecan | ||
| DNA methylation | |||
| In vitro sensitivity | Platinums, vincas, taxanes, topo I & II inhibitors, gemcitabine, cyclophosphamide |
||
Data were not consistent, and were equivocal or negative in some studies
No association preclinically with resistance to platinums; may sensitize to gemcitabine
No association preclinically with resistance to anthracyclines, vinca alkaloids, bleomycin, irinotecan/SN-38
BRCA1 expression sensitized cells to antimicrotubule agents, and improved clinical outcome with gemcitabine + docetaxel
Paradoxical increase in sensitivity to gemcitabine-cisplatin combination in one preclinical study
Paradoxical increase in sensitivity to taxanes in some preclinical studies
Effect clinically was opposite to preclinical effect, with worse outcome in patients with tumors with high stathmin
Paradoxical increase in efficacy for patients with tumors positive for p53 by IHC
Trend present
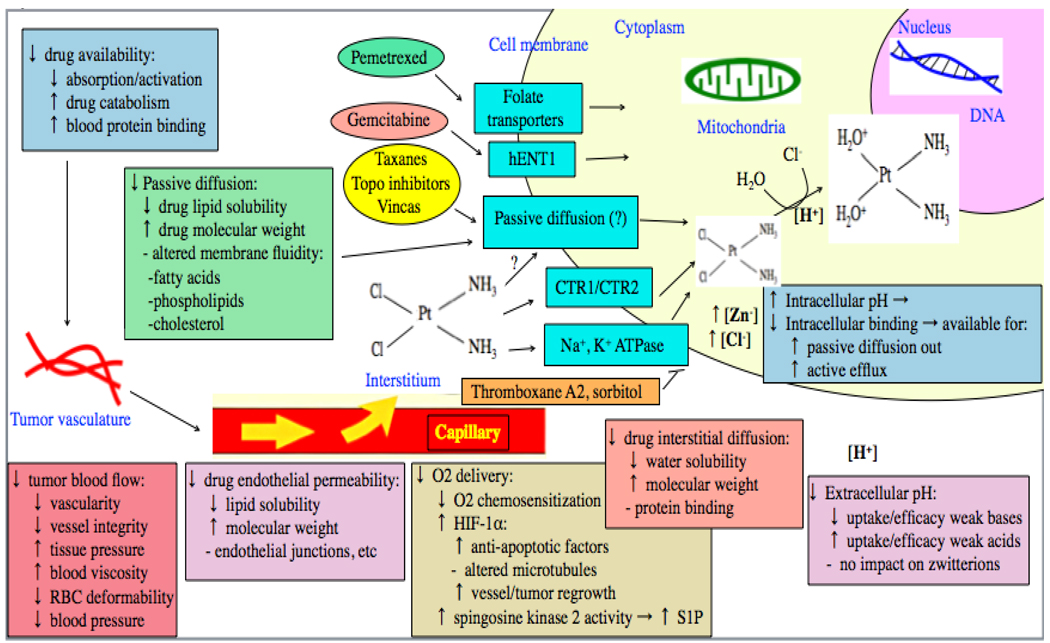
2.0 Drug and oxygen delivery (Fig. 2)
Fig. 2.
Drug effect may be decreased by a decrease in drug bioavailability, hepatic activation, penetration across vessel endothelium or interstitium, uptake across cell membranes, or intracellular activation, or by decrease in tumor blood flow or oxygenation.
Drug concentrations achieved in tumor may conform to either a “flow-limited model” (proportional to tumor blood flow)44 or to a “membrane-limited model” (not proportional to blood flow and presumably limited by ability of the drug to cross cell membranes)45, 46. In a SCLC model, uptake of gemcitabine appeared to be flow-limited47, but correlation with tumor blood flow has not been adequately assessed in lung cancer for most drugs. A variety of observations suggest that delivery of cisplatin to tissues may be primarily membrane-limited48. As recently reviewed48, several factors associated with tumors may reduce tumor blood flow, including high tissue pressure, high blood viscosity (eg, due to fibrinogen), and decreased red blood cell membrane deformability. Due to impaired vascular autoregulation, tumor blood flow is more susceptible to blood pressure fluctuations than is blood flow to normal tissues49. As reviewed48, delivery of drugs such as cisplatin could also be reduced by rapid, irreversible binding to blood and extracellular proteins.
Factors limiting blood flow to tumor also secondarily reduce delivery of oxygen, and hypoxia has a variety of effects on therapy efficacy50. Some agents that are important in the treatment of lung cancer (eg, etoposide51 and paclitaxel52) are less effective in hypoxic tumor cells, while others (eg, topotecan53) are more effective in hypoxic tissues, and still others (eg, cisplatin53, 54) are equally effective in aerobic and hypoxic conditions. While hypoxia itself may not alter efficacy of some agents, cell exposure to hypoxia may activate anti-apoptotic signaling pathways that augment drug resistance51. For example, hypoxia activates sphingosine kinase 2, which in turn promotes the synthesis and release of sphingosine-1-phosphate (S1P)55. S1P then binds to S1P receptors, thereby activating p42/44 mitogen-activated protein kinase and protecting lung cancer cells from etoposide cytotoxicity55.
Hypoxia also leads to increased expression of hypoxia inducible factor-1α (HIF-1α), which in turn augments resistance of NSCLC cell lines to cisplatin56, doxorubicin56, and paclitaxel52, alters conformation and dynamics of microtubules52, and fosters tumor regrowth through stimulating angiogenesis by up-regulation of expression of vascular endothelial growth factor (VEGF) and platelet derived growth factor (PDGF)57.
Clinically, in patients with advanced NSCLC treated with platinum-based regimens, there was no evidence that therapy efficacy was increased by the addition of any of pentoxifylline (which may improve blood flow by decreasing red blood cell membrane rigidity)58, tiripazamine (which sensitizes hypoxic tissues to therapy)59, or motexafin gadolinium (which increases the formation of superoxide and other reactive oxygen species in the presence of oxygen)60. Limited clinical data suggested possible benefit for NSCLC patients from addition of the hypoxic cell sensitizer metronidazole to doxorubicin, carmustine or mitomycin-C single agent chemotherapy, with responses in 4 of 8 patients61, although little efficacy was seen in NSCLC or other tumor types when metronidazole was added to epirubicin62, carboplatin63 or etoposide64 concurrently with multiple other potential resistance modulators including ketoconazole62–64, dipyridamole62–64, tamoxifen62–64, pentoxifylline63, novobiocin63 and cyclosporin62, 64. While administration of the vasodilator nitroglycerin prior to surgical removal of NSCLCs did reduce the rate of immunohistochemistry (IHC) positivity for HIF-1α and VEGF65, imaging studies using (18)F-Fluoromisonidazole (which concentrates in hypoxic tissues) suggested that the hypoxic cell fraction of primary NSCLC is low in humans, and there was no significant correlation between hypoxia and glucose metabolism as assessed by (18)F-Fluorodeoxyglucose (FDG) scanning66. Furthermore, in operable NSCLC patients treated with neoadjuvant cisplatin-gemcitabine, HIF-1α mRNA in post chemotherapy resected specimens did not correlate with patient survival67, serum or plasma levels of VEGF did not correlate with survival in patients with advanced NSCLC receiving platinum-based chemotherapy alone68 or combined with tirapazamine69 or bevacizumab70, and tumor IHC expression of VEGF did not correlate with response or survival in advanced NSCLC patients treated with a variety of cisplatin-based combinations71. Overall, it remains unclear whether tumor blood flow and hypoxia-related factors play a major role in resistance of human lung cancers to chemotherapy.
In addition to trying to improve delivery of drugs and/or oxygen to tissues by manipulations that could alter blood flow, other strategies have also been tested. Liposome encapsulation of drug overcame resistance to cisplatin72, daunorubicin73 and vinorelbine74 in lung cancer cell lines. Howver, administration of liposomeencapsulated cisplatin75–77, docetaxel plus gemcitabine combined with liposomal doxorubicin78 or docetaxel plus liposomal doxorubicin plus radiotherapy79 to patients with NSCLC did not appear to augment efficacy in phase II trials, and pegylated liposomal doxorubicin was inactive in recurrent SCLC80. Inhaled aerosolized liposomal cisplatin has also undergone phase I trials, but none of 16 patients experienced tumor regression81.
Drug administration via nanoparticles has also been tested. Preclinical studies suggested that cisplatin delivery via inhaled biotinylated-EGF-modified gelatin nanoparticles would improve delivery to EGFR-expressing tumor cells82, and a micellar nanoparticle formulation increased the ability of paclitaxel to radiosensitize lung cancer cell lines83. Nanoparticle albumin-bound paclitaxel (NAB-paclitaxel) reached higher tumor concentrations and was more active than cremophor-based paclitaxel in preclinical models that included lung cancer84. In a phase I/II trial of NAB-paclitaxel, the observed response rate of 30% was somewhat higher than what would be expected with standard taxane therapy85, and a phase II trial of NAB-paclitaxel with carboplatin and bevacizumab yielded a median survival time (16.8 months) which was longer than generally seen with advanced NSCLC86. Hence, while liposomal administration of drug did not appear to improve outcome, preliminary clinical data with nanoparticle administration are suggestive of possible enhancement of efficacy. Randomized phase III trials are ongoing to assess this further.
3.0 Extracellular pH
Extracellular pH (which may be manipulated by systemic administration of a glucose load, by medications such as amiloride or by bicarbonate87) may also be important (Fig. 2). Tumor extracellular pH is often acidic, while tumor intracellular pH is often neutral or alkaline. A low extracellular pH favors uptake into tumor cells and enhances cytotoxicity of weak acids such as cisplatin88 and alkylating agents89, while a higher extracellular pH favors uptake into tumor cells of weak bases such as doxorubicin89 and vinca alkaloids87, and has little net effect on zwitterions like paclitaxel89. Hence, both dietary factors and concurrent medications could potentially contribute to tumor resistance by altering tumor pH.
4.0 Drug uptake
Tumor uptake of some drugs is by passive diffusion, whereas other drugs enter cells by active transport or by a combination of active transport and passive diffusion (Fig.2).
4.1 Cisplatin
Reduced uptake of cisplatin has been noted in many resistant NSCLC32, 90–94 and SCLC32, 93, 95 cell lines, although it is not a universal finding. Cisplatin-resistant lung cancer cell lines with reduced platinum uptake have also been noted to have increased intracellular chloride96 or zinc content91, increased cell membrane rigidity97, increased sphingomyelin content97, and increased density of membrane lipid packing, with reduced long chain and unsaturated fatty acids98. These changes could potentially alter passive diffusion of cisplatin into cells or might reduce the activity of membrane transporters. Culturing cisplatin-resistant SCLC cells in long chain fatty acids (that were then incorporated into cell phospholipids) increased cisplatin cellular uptake and DNA binding, and reduced resistance99. Dipyridamole increased the concentration of both cisplatin and its active aquated species in cells through mechanisms that were uncertain100. However, when dipyridamole was added to high dose cisplatin in the treatment of chemonaive NSCLC, the response rate was only 14%, with a median survival time of 8 months, suggesting that clinical activity was not increased substantially101.
4.1.1 CTR1 and CTR2
Platinum-resistance may be associated with broad cross-resistance and with down-regulation of expression of a wide range of membrane transporters16. Platinums may be transported into cells by the copper transporter CTR1, and platinum resistance in lung cancer may be associated with decreased CTR1 expression102. A role for the related copper transporter CTR2 in platinum uptake has also been suggested in other tumor types103 but has not yet been assessed in lung cancer. Decreased CTR1 expression was noted in patients with a variety of malignancies who had been exposed to a wide range of chemotherapy and targeted agents within the previous 3 months104 suggesting that exposure to many types of drugs could lead to platinum resistance by down-regulating expression of CTR1104, 105.
4.1.2 Na+, K+ ATPase
Na+, K+ ATPase and agents that activate it are also associated with increased intracellular accumulation and/or efficacy of cisplatin, with the association being stronger in NSCLC than in SCLC cell lines106–108. Inhibition of factors (such as thromboxane A2108, 109) that antagonize Na+, K+ ATPase also increased cisplatin uptake and cytotoxicity. Inhibition of thromboxane A2 also resulted in upregulation of expression of interleukin-1β-converting enzyme, the expression of which is reduced in some platinum-resistant NSCLC cell lines109. Whether N+, K+ ATPase plays a direct role in cisplatin cellular uptake or whether it instead exerts an effect indirectly through alteration of intracellular pH110 or other mechanisms is unclear. In NSCLC cell lines, the glucose metabolite sorbitol decreased cisplatin cytotoxicity, Na+, K+-ATPase activity, and cisplatin uptake, suggesting a possible mechanism for cisplatin resistance in poorly controlled diabetes111.
Tumor retention of Thallium-201 (T201) on SPECT scanning may reflect Na+, K+ ATPase activity, and in one study in which SCLC patients were treated with cisplatin-based chemotherapy, response correlated with pretreatment T201 retention112, but this was not noted in another study in which SCLC patients were treated with a broader spectrum of chemotherapy agents113, and no correlation between T201 retention and outcome was seen in NSCLC patients treated with mitomycin-vindesine-cisplatin114. Hence, it is unclear if T201 scanning is of any value in this setting.
4.2 Taxanes, vinca alkaloids and etoposide
There is relatively little information available on mechanisms by which taxanes, vinca alkaloids or etoposide enter cancer cells, nor on the potential role of decreased drug influx in resistance, although etoposide uptake is significantly higher in sensitive SCLC cell lines than in more resistant NSCLC lines115. The nonionic detergent Tween-80 augmented uptake and cytotoxicity of etoposide in NSCLC cell lines116, and some etoposide-resistant cell lines have greatly increased content of cholesterol117, which would increase cell membrane rigidity. Transfection into NSCLC cells of the human HERG K+ channel gene significantly increased the cytotoxicity of vincristine, paclitaxel and hydroxycamptothecin (but not cisplatin)118, but it is unknown if it plays any role in drug transport.
4.3 Gemcitabine
For gemcitabine, human equilibrative nucleoside transporter 1 (hENT1) plays a role in cellular uptake, and hENT 1-deficient cells are resistant to gemcitabine119. A deficiency in hENT 1 may be more important in intrinsic than in acquired gemcitabine resistance120. Liposome encapsulation may augment gemcitabine uptake and efficacy121.
While pretreatment hENT-1 expression did not correlate with response or survival in one NSCLC study of gemcitabine-based chemotherapy in which only 16% expressed hENT 1 by IHC119, in another trial, no NSCLC patient in whom hENT 1 was absent by IHC responded to gemcitabine-based therapy122.
4.4 Pemetrexed
For the multitargeted antifolate pemetrexed, the proton-coupled folate receptor123, the reduced folate carrier123, 124 and the folate receptor-α125 all appear to play a role in cellular uptake, although they have not been extensively assessed in lung cancer. Pemetrexed is more active in lung adenocarcinomas than in squamous cell carcinomas clinically42, possibly related to the fact that adenocarcinomas have significantly higher expression of folate receptor-α and a strong trend towards increased expression of the reduced folate carrier compared to squamous cell carcinomas126.
Overall, low drug uptake may be an important cause of resistance, and uptake may vary with extracellular pH. With cisplatin, CTR1, Na+, K+ ATPase, and membrane fluidity may play a role in uptake and resistance, while hENT1 may play a role with gemcitabine and folate carriers may play a role with pemetrexed. Little is known regarding how other drugs enter cells.
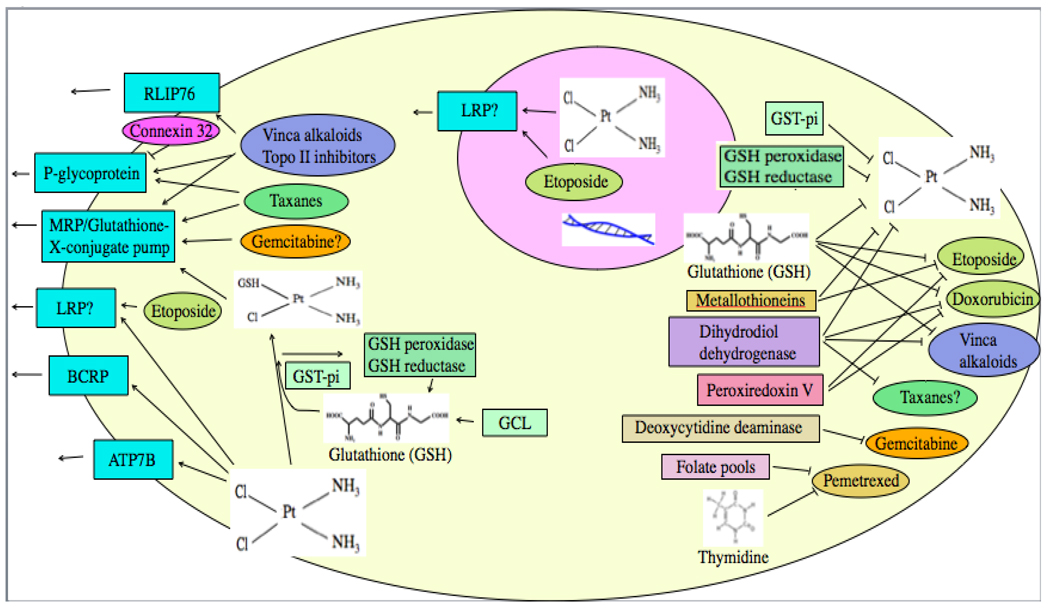
5.0 Drug efflux
More is known about the role of efflux (Fig. 3) than about the role of uptake in drug resistance. Various membrane efflux pumps have been assessed that may play a role in reducing drug intracellular concentrations.
Fig. 3.
Drug intracellular concentration may be decreased by efflux pumps, or drug may be detoxified by various factors.
5.1 Multidrug resistance protein (MRP)
A high proportion of SCLC127, 128 and NSCLC127, 129 cell lines express MRP mRNA, with greater MRP protein and/or mRNA expression in NSCLC than in SCLC cell lines130, 131. MRP expression is associated with decreased cellular drug accumulation of cisplatin95, paclitaxel132 and other agents133. In NSCLC heterotransplants in nude mice, there was a statistically non-significant trend toward a higher response rate to paclitaxel in MRP-negative tumors134. In both SCLC95, 127, 128, 130–132 and NSCLC29, 127, 129–133, 135 cell lines, MRP mRNA or protein expression correlated significantly with resistance to anthracyclines95, 127, 128, 130, 131, 133, 136 vinca alkaloids95, 129, 130, 133, 136, etoposide127, 130, 131, 136, docetaxel29, paclitaxel132, gemcitabine135 and cisplatin95, 130. However, an association between MRP expression and resistance was not seen in some cell lines137–139, and in some, MRP was not associated with resistance to cisplatin despite contributing to resistance to other agents127, 133. Paradoxically, in still other lines, resistance to gemcitabine was decreased instead of being increased by MRP1 expression139. Similarly, in another study, the anti-inflammatory agent indomethacin induced apoptosis preferentially in SCLC cells that were doxorubicin resistant and that overexpressed MRP1, and this apoptosis was decreased if MRP1 was inhibited by siRNA140
MRP1130, MRP3130, and MRP7129, 132 may be particularly important in resistance, with less of a role for MRP2129, 130 and MRP3129. The MRP7 inhibitor sulfin-pyrazone increased NSCLC cell line sensitivity to vinorelbine129. 5-Fluorouracil may sensitize cells to cisplatin by inhibiting MRP expression141, and verapamil may also modulate MRP effect131. Depleting cells of glutathione reversed resistance associated with MRP in one study, suggesting that MRP functions as a glutathione S-conjugate (“GS-X”) pump that exports molecules that are bound to glutathione136. Increased GS-X pump activity is associated with cisplatin resistance and decreased cellular cisplatin accumulation142.
MRP mRNA and/or protein by IHC was found in 32–100% of clinical NSCLC tumor specimens127, 143–145, but gene amplification has not been noted127. MRP expression was significantly higher in more differentiated tumors than in less differentiated tumors143, in squamous cell carcinomas than in other NSCLCs145, and in tumors with mutant p53144. MRP expression is also common in SCLC clinical tumor samples128.
There is not a consensus on whether tumor MRP expression correlates with clinical outcome, although tumor type and chemotherapy type may have a bearing on the relationship. In SCLC patients treated with platinum-based combinations, response rates were significantly lower for tumors that were IHC-positive vs −negative for MRP1146, 147 or MRP2148. After being taken up by cells, Tc-99m methoxyisobutyl isonitrile (MIBI) and technetium-99m tetrofosmin (Tc-TF) may be exported from cells by either MRP or by MDR1/p-glycoprotein, and retention of these compounds on SPECT scanning has been used clinically as an indicator of efflux pump activity. Higher tumor uptake of MIBI149 and Tc-TF150 on SPECT scanning was associated with better response to chemotherapy, and, in keeping with preclinical predictions, uptake of MIBI and Tc-TF correlated negatively with tumor MRP and MDR1 expression149–151). Furthermore, MRP1 expression in SCLC tumors was markedly increased at relapse after cisplatin-etoposide compared to chemonaive values146 or there was a strong trend towards an increase in MRP IHC positivity after treatment with etoposide or teniposide152, suggesting that chemotherapy exposure either upregulates expression of MRP or else selects for resistant, MRP-positive cells. Similarly, in autopsy NSCLC tumor tissues, mRNA expression levels of MRP3153 and MRP5154 (but not MRP4153) were significantly higher in patients who had been exposed to platinum drugs ante mortem than in patients who had not received platinum agents.
In advanced NSCLC, response rates to cisplatin plus irinotecan were higher and survival was longer in patients with some MRP2 host genotypes than with other genotypes155, tumor MRP2 (but not MRP1 or MRP3) IHC expression correlated with survival (but not with response) in patients treated with platinum-based combination chemotherapy156, and tumor MRP expression was associated with poor survival in patients treated with vindesine plus etoposide144, 145. However, in another NSCLC study, MRP mRNA expression only correlated inversely with response in lung adenocarcinoma, and not in squamous cell carcinoma157, there was no correlation between MRP IHC expression and response to platinum-based combinations in other studies156, 158, MIBI scanning did not correlate with response to chemotherapy in a study involving 14 NSCLC and 9 SCLC patients151, and tumor MRP1 or MRP2 IHC expression did not correlate with survival in patients with resected NSCLC receiving adjuvant cisplatin plus a vinca alkaloid or etoposide159.
Overall, preclinical data strongly support a role for MRP in resistance to several types of chemotherapy. Clinical observations suggest that MRP is probably associated with resistance to chemotherapy in SCLC. The clinical data remain less convincing in NSCLC, but are suggestive of a possible role for MRP in chemoresistance, and the increased MRP expression seen in treated NSCLC and SCLC tumors suggest the possibility that it may play a greater role in acquired than in intrinsic resistance.
5.2 MDR1/p-glycoprotein (P-gp)
Like MRP, P-gp may also render tumors resistant to chemotherapy by transporting drugs out of cells. In NSCLC cell lines, increased MDR1 mRNA and/or protein expression levels were associated with resistance to anthracyclines25, 160, 161, vinca alkaloids25, 129, 160–162, etoposide160, 161, and taxanes25, 160, 161, 163, 164. With occasional exceptions165, MDR1/P-gp expression did not correlate significantly with sensitivity to cisplatin25, 92, 137 or carboplatin160, nor with intracellular92, 137 or intranuclear137 cisplatin accumulation, and cisplatin and carboplatin may actually increase cellular concentrations of some other agents by inhibiting P-gp166. Increased expression of P-gp is also frequently seen in cell lines that are sensitive to cisplatin despite resistance to paclitaxel167. In addition, some NSCLC and SCLC cell lines transfected with the MDR1 gene had augmented sensitivity to gemcitabine, and this augmented sensitivity was reversed by the P-gp inhibitor verapamil139. MDR1 gene amplification was detected in some resistant lines25.
MDR1 gene overexpression was also seen in SCLC cell lines selected for resistance by exposure to paclitaxel132, anthracyclines33 or etoposide168, 169, although MDR1 mRNA expression was relatively uncommon in SCLC cell lines not selected for resistance128.
Some resistant lines that overexpress P-gp also overexpressed caveolin 1, and P-gp localized to caveolin-rich membrane domains in these cells117. P-gp expression correlated with HIF-1α expression in NSCLC cell lines170 and in resected NSCLC tumors65. P-gp expression was increased when lung adenocarcinoma cells were cultured in hypoxia170 and was reduced in tumors of patients who had nitroglycerin patches applied to improve tumor blood flow and oxygenation prior to surgical resection65.
Expression of Connexin 32 in a NSCLC cell line significantly potentiated vinorelbine-induced cytotoxicity and induction of apoptosis by down-regulating expression of MDR1162. Connexins are important in gap junctions and normally play a role in electrical signaling between cells.
Clinically, in chemonaive NSCLC patients, MDR1 mRNA and/or P-gp were expressed in 11–32% of surgical specimens152, 165, 171–173, but were expressed in 61% of tumors that had been treated with chemotherapy172. MDR1 expression in NSCLC did not correlate with histopathology or with clinical details171. In SCLC, MDR1 expression was seen in 13–60% of tumor biopsy samples128, 152, 173, and in 50% of SCLCs xenografted from treated and untreated patients into nude mice174.
With MDR1/P-gp, as with MRP, there is stronger evidence of an association of expression with outcome in SCLC than in NSCLC, so we will discuss SCLC first. In SCLC, there was a negative correlation between expression of P-gp and response146, 147, 149, 173, 175, 176 and/or survival176, 177 in patients treated with cisplatin-etoposide146, 147, 149, 173, 175, 176, cyclophosphamide-doxorubicin-vincristine (CAV)173, 176, or other doxorubicin or etoposide regimens177, and P-gp expression was significantly increased in tumors previously exposed to therapy compared to pretreatment expression146, 152. Response of SCLC patients to cisplatin plus etoposide also correlated with MDR1 host polymorphisms, with a significantly better chemotherapy response in patients with the 3435 CC genotype (exon 26) compared with the combined 3435 CT and TT genotypes, and patients harboring the 2677G-3435C haplotype also responded significantly better than did other patients 178. Similarly there was a strong correlation between uptake of MIBI113, 149, 179 or Tc-TF150, 175, 180 on SPECT scanning and response of SCLC to cisplatin plus etoposide149, 150, 175, 179, 180 (EP), to EP plus doxorubicin181, and to CAV plus mitomycin-C and vindesine113, or there was a trend towards a correlation of response with MIBI182, 183. Patients with SCLC demonstrated significantly greater MIBI uptake than those with NSCLC184.
In NSCLC, there was a significant correlation of tumor P-gp IHC expression with response in 2 studies using paclitaxel and a platinum185, 186 and in one study using cisplatin, ifosfamide, vinblastine and radiation187, and P-gp expression also correlated inversely with survival in this latter study187. The MDR1 3435 CC host genotype was associated with a significantly better response to cisplatin-vinorelbine compared with the combined 3435 CT and TT genotypes, and patients with the 2677G-3435C haplotype also had significantly better responses to chemotherapy compared to patients with other haplotypes combined 188. In another NSCLC study, tumor MDR1 mRNA expression did correlate with shortened survival even though it did not correlate with response to chemotherapy157. In NSCLC, while tumor P-gp status did not correlate with MIBI uptake on SPECT scanning in some studies189, 190, it did correlate in other studies185, 191. Furthermore, MIBI114, 185, 190, 191 and Tc-TF192, 193 uptake on SPECT scanning correlated significantly with response to chemotherapy with cisplatin, mitomycin-C plus vindesine114, 190, with paclitaxel185, with paclitaxel-based regimens191, or with radiation combined with cisplatin plus etoposide193, and also correlated with survival190.
However, in a number of other studies involving patients with advanced156, 173, 194 or locally advanced195 NSCLC, P-gp expression by IHC did not correlate with response to cisplatin-based regimens156, 173, 194 or carboplatin-based regimens195 that also included vinca alkaloids156, 173, 195, irinotecan156, taxanes156, gemcitabine156 or radiation195, and host MDR1 C3435T polymorphisms did not correlate with outcome in patients with advanced NSCLC treated with docetaxel-cisplatin196 despite their association with efficacy of cisplatin-vinorelbine in another study, as noted above188. Similarly, preoperative MIBI uptake did not correlate with in vitro sensitivity (for etoposide, doxorubicin, vindesine, cyclophosphamide and cisplatin) of resected specimens189, nor with clinical response to chemotherapy151 in some studies.
P-gp antagonists have been assessed in both NSCLC and in SCLC. The P-gp antagonist verapamil augmented paclitaxel accumulation in human lung cancer cells overexpressing P-gp138, increased vinorelbine efficacy in NSCLC cell lines overexpressing MDR1129, and improved the efficacy of a cyclophosphamide, cisplatin, doxorubicin, and etoposide regimen against SCLC xenografts197. The epidermal growth factor receptor (EGFR) inhibitor gefitinib also reversed P-gp mediated taxane resistance in NSCLC cell lines164, 198, but did not improve efficacy of chemotherapy in NSCLC in randomized clinical trials199, 200.
In a phase II clinical trial in which the P-gp antagonist cyclosporine A was added to paclitaxel in NSCLC patients (18 of whom had received one prior chemotherapy regimen and 8 of whom were chemonaive), the response rate was 26%, median time to progression was 3.5 months and median overall survival was 6 months, suggesting a possible modest beneficial impact of the cyclosporine on paclitaxel efficacy201. However, when cyclosporine was added to etoposide plus cisplatin as front line therapy in advanced NSCLC, there was no evidence of benefit, with a response rate of 22.7% and a 2-year survival rate of 8%, and better outcome in patients receiving a lower vs higher dose of cyclosporine202.
In a small randomized phase II trial with 54 NSCLC patients, addition of the P-gp antagonist dexverapamil to vindesine plus etoposide was associated with a response rate of 31.3 % compared to only 11.1 % for the chemotherapy alone203, and addition of verapamil to vindesine plus ifosfamide/mesna in a randomized study involving 72 NSCLC patients was associated with an increase in response rate from 18% to 41% (p=0.057) and the overall survival time was increased significantly (p=0.02). However the calcium channel blocker nifedipine (which reverses resistance to chemotherapy both through P-gp antagonism plus through other mechanisms, as previously reviewed204) plus the methylxanthine pentoxifylline did not reverse resistance to combination chemotherapy in previously-treated patients with NSCLC and other malignancies58. Similarly, while hydroxyurea is thought to reverse MDR1-associated resistance by breaking down double minute chromosomes carrying amplified MDR1205, the addition to paclitaxel of hydroxyurea in previously-treated patients with advanced NSCLC did not appear to improve outcome, with a response rate of only 3% and a median survival time of 4.6 months206.
In randomized clinical trials in SCLC, the P-gp modulator megestrol acetate did not improve outcome with chemotherapy207 and verapamil did not improve response or survival rates in patients receiving cyclophosphamide, doxorubicin, vincristine plus etoposide208.
Based on the above, we can conclude that there is strong preclinical evidence of an association between MDR1/P-gp expression and resistance to several agents in lung cancer cell lines, but probably no increased resistance to platinum agents, and possible collateral sensitivity to gemcitabine. Clinically, there is relatively strong evidence of an association between MDR1/P-gp expression in SCLC and resistance to combination chemotherapy. The clinical evidence is inconclusive for an association with outcome in NSCLC. With respect to P-gp modulation, verapamil or dexverapamil may possibly increase chemotherapy efficacy in NSCLC, but other strategies have been ineffective, and neither verapamil nor megestrol helped in SCLC.
5.3 Lung Resistance Protein/Major Vault Protein (LRP)
Vaults are ribonucleoparticles involved in nuclear-cytoplasmic transport, cell signaling and immune responses, and LRP is a major component of vaults209. In some NSCLC cell lines, LRP expression correlated significantly with resistance to cisplatin210, 211 or etoposide212. However, in other NSCLC cell lines, resistance to cisplatin92, 137 and cisplatin intracellular concentration92, 137 did not correlate with LRP mRNA92, 137 and protein137 expression. LRP expression also did not correlate with resistance of NSCLC cell lines to anthracyclines210, 211, etoposide210, 211, 213, vinca alkaloids210, 211, bleomycin210, 211 or the irinotecan metabolite SN-38211.
In some clinical studies, NSCLC tumor LRP expression was associated with reduced response to platinum-based chemotherapy157, 172, 214. However, this association was only noted in squamous cell carcinomas in one study214, and in still other studies, there was no correlation with response to platinum-based chemotherapy (including platinum combinations with taxanes and epipodophyllotoxins158, 215) or to taxane-based chemotherapy216. Autopsy tumor LRP gene expression was not increased by prior platinum exposure217. Tumor LRP expression correlated with MRP expression in one NSCLC study157, but it did not correlate with MIBI191 or Tc-TF150, 192 uptake on SPECT scanning.
In SCLC, there was a statistically insignificant trend towards decreased response to CAV in patients whose tumors overexpressed LRP146, while in other studies, there was no apparent association between tumor LRP expression and response to CAV215 or with response to cisplatin plus etoposide147. Overall, there is no conclusive evidence that LRP plays a major role in resistance of lung cancer to chemotherapy.
5.4 P-type adenosine triphosphatase (ATP 7B)
As recently reviewed48, the copper transporter ATP 7B is thought to play a role in cisplatin efflux. While ATP 7B mRNA and IHC expression significantly correlated with cisplatin resistance in NSCLC xenografts218, it did not correlate with resistance in some lung cancer cell lines102. There is evidence of a link between ATP 7B expression and resistance to cisplatin-based chemotherapy in some other tumor types219, but data from clinical tumor specimens are not yet available for lung cancer. It remains uncertain whether ATP 7B is important in lung cancer resistance to chemotherapy.
5.5 Breast Cancer Resistance Protein (BCRP)
In advanced NSCLC patients undergoing platinum-based chemotherapy, tumor IHC expression of BCRP (a transporter and member of the ATP binding cassette family) was significantly associated with shortened survival in two studies156, 220, and blood BCRP concentrations were significantly higher in chemoresistant patients than in chemosensitive patients221. Tumor BCRP IHC expression correlated with lack of response in one study222, but not in another study220. Overall, the data suggest a possible role for BCRP in lung cancer chemotherapy resistance.
5.6 Ral-interacting protein (RLIP76) (RALBP1)
In NSCLC and SCLC cell lines, overexpression of the non-ABC glutathione-conjugate transporter RLIP76 (which is involved in receptor-ligand endocytosis and in multispecific drug transport) was associated with augmented efflux, decreased intracellular concentrations and resistance to vinorelbine223 and doxorubicin224–226, and its inhibition enhanced cisplatin-vinorelbine efficacy in lung cancer xenografts227. RLIP76 transport activity appeared to be regulated by protein-kinase-C-α (PKC-α)-mediated phosphorylation226, 228. The greater resistance to doxorubicin in NSCLC cells compared to SCLC cells may be mediated through differential phosphorylation of RLIP76 in NSCLC vs SCLC226. Lung cancer clinical data are unavailable.
6.0 Drug detoxification
Drugs may also be neutralized or detoxified by various cellular mechanisms (Fig. 3).
6.1 Glutathione (GSH)
GSH may bind cisplatin, thereby decreasing formation of platinum-DNA adducts. It may also play a role in increased repair of platinum-DNA adducts 229 and it plays a role in drug efflux via pumps that expel GSH-conjugates from cells (“GS-X pumps”). In lung cancer cell lines that had been rendered resistant to anthracyclines, vinca alkaloids and etoposide by transfection with the MRP gene, GSH depletion by DL-buthionine-S,R-sulfoximine (BSO) reversed resistance, suggesting that MRP functions as a GS-X pump136. While efficacy of cisplatin against NSCLC cell lines90, NSCLC xenografts230, or SCLC cell lines231, 232 did not correlate with GSH content in some studies, in several other NSCLC cell lines92, 93, 233, 234 and SCLC cell lines 32, 93, 142, 233, 235–237, increase in GSH content was accompanied by increase in cisplatin resistance32, 92, 93, 142, 233–237, with decreased platinum-DNA binding93, 236, 237, and with decreased intracellular platinum accumulation32. Factors that reduced cellular GSH augmented sensitivity to cisplatin93, 141 or other agents238.
Etoposide-resistant and doxorubicin-resistant NSCLC cell lines also may have increased GSH content 238, and in some cases, cisplatin resistance and increased GSH content was accompanied by cross-resistance to vinca alkaloids236, anthracyclines32, etoposide32, camptothecins32, mitomycin-C32, alkylating agents32, methotrexate32, and radiation234, while in other instances, resistance to cisplatin with augmented GSH was accompanied by lack of cross-resistance to anthracyclines or epipodophyllotoxins236, or by collateral sensitivity to vinca alkaloids and 5-fluorouracil32.
Expression of genes involved in GSH synthesis (glutamate-cysteine ligase [GCL], also known as gammaglutamylcysteine synthetase [GGCS]) in NSCLC xenografts also enhanced resistance to cisplatin239, 240, and inhibition of GCL decreased cisplatin resistance241. The expression level of the GCL light chain subunit gene was significantly higher in NSCLC than in SCLC239. Survival of NSCLC patients treated with cisplatin-based regimens was significantly associated with host genotype for some GSH metabolic genes242.
In vitro sensitivity testing of lung cancers that had been resected demonstrated increased resistance to cisplatin for tumors that were IHC positive for GSH peroxidase and GSH reductase, and IHC expression also varied significantly with lung cancer histologic types243.
Overall, the preponderance of preclinical evidence supports a role for GSH in resistance to cisplatin and perhaps other agents in both NSCLC and SCLC, although the relationship is not consistent across all studies, and clinical data are limited.
6.2 Glutathione-S-transferase-pi (GST-pi)
GST catalyzes the binding of GSH to various compounds. There has been particular interest in the GST-pi variant in chemotherapy resistance, and SCLC cell lines had lower GST-pi expression than did NSCLC cell lines244, 245. Cisplatin resistance correlated with GST-pi expression244–246, GST activity 231, or absence of GST inhibitor232, 247 in cells harvested from resected NSCLC tumors246, in some NSCLC cell lines244, and in some SCLC cell lines231, 232, 244, 245, 247, but in other NSCLC cell lines90, 92, 93, 160, 248 and SCLC cell lines93, 95, 236, resistance to cisplatin90, 92, 93, 95, 236, 248, anthracyclines95, 160, vinca alkaloids95, 160, 236, etoposide160, 248, irinotecan248, taxanes248, 5-fluorouracil160 and radiation95 did not correlate with GST-pi expression.
Clinically, NSCLC tumors were positive for GST-pi or had high GST-pi IHC expression in 56–69% of patients246, 249–251. Some clinical studies showed a correlation between lack of response187, 249–251 or increased risk of relapse or shortened survival187 and high tumor187, 249–251 or serum252 GST-pi expression in NSCLC patients treated with platinum-vindesine249, platinum-etoposide250, cisplatin, ifosfamide, vinblastine and radiation187 or various platinum-based regimens251, 252 for advanced disease249–252, for locally advanced disease187, or with adjuvant cisplatin-based chemotherapy for resected disease246. NSCLC patients treated with platinum-based regimens who had the GST-pi exon 6 wild type host genotype (Ala/Ala) had significantly worse survival than did patients with the variant genotype (Ala/Val or Val/Val), while GSTpi exon 5 genotype was not associated with survival253.
On the other hand, some other NSCLC studies showed no correlation between tumor GST-pi IHC expression and response194, 195 or survival195 in patients with locally advanced disease undergoing radiotherapy combined with vinorelbine +/− carboplatin195 or in patients with advanced disease receiving platinum-based chemotherapy194, 254. Furthermore, a second pharmacogenetic study failed to demonstrate an association between GST-pi exon 5 and exon 6 polymorphisms and response to chemotherapy or survival, although patients possessing the variant alleles GSTP1 105Val or GSTP1*B demonstrated trends toward inferior response and survival255.
Overall, as with GSH, the association of GST-pi with resistance of lung cancer to platinums and other agents is suggestive but is not consistent across studies, and there is little experience with attempts to clinically modulate GST-pi in lung cancer patients receiving chemotherapy.
6.3 Metallothioneins
Metallothioneins are a family of cysteine-rich low molecular weight proteins that are involved in zinc homeostasis and may also be involved in chemotherapy binding and detoxification. Increased metallothionein expression was noted in some cisplatin-resistant NSCLC92 and SCLC232 cell lines, and metallothionein expression also correlated with resistance to etoposide256. Exposure of lung cancer cell lines to cadmium or zinc increased metallothionein synthesis and increased resistance to etoposide256.
In patients with SCLC treated with alternating cisplatin-etoposide/CAV, IHC for metallothionein (45% positive) correlated significantly with short survival257. Overall, IHC expression of metallothionein in human tumors was less common in SCLC than in NSCLC, and it increased post exposure to chemotherapy258.
6.4 Dihydrodiol dehydrogenase (DDH)
NSCLC cells overexpressing or transfected with DDH (a cytoplasmic aldo-keto oxidoreductase enzyme that plays an important role in the detoxification process259 and in the metabolism of steroid hormones, prostaglandins and xenobiotics260) had increased resistance to cisplatin259–261, doxorubicin259, 261, vincristine261, melphalan261 and radiation259. While some assessments suggested an association between DDH expression and paclitaxel resistance261, a systematic review of cell lines with an inverse resistance relationship between cisplatin and paclitaxel (resistant to cisplatin but sensitive to paclitaxel) suggested that increased expression of DDH was a common characteristic of such cells167. Proinflammatory mediators such as interleukin-6 induced DDH expression in NSCLC cells, but this induction could be prevented by protein kinase C inhibitors260. DDH expression inversely correlated with the DNA repair factor Nijmegen breakage syndrome 1 (NBS1) and with apoptosis-inducing factor (AIF)260. DDH is often overexpressed in NSCLC cells, and patients with DDH overexpression are at increased risk of recurrence259, and have a poor prognosis and chemoresistance260. Hence, preclinical data and limited clinical data suggest a role for DDH in lung cancer resistance.
6.5 Deoxycytidine deaminase
Gemcitabine is activated by conversion to the metabolite gemcitabine triphosphate (see below), and gemcitabine triphosphate may then be inactivated by the enzyme deoxycytidine deaminase. Deoxycytidine deaminase activity was lower in SCLC33, 139 and NSCLC139 variants that were sensitive to gemcitabine compared to resistant variants. In pharmacogenetic analyses in patients with advanced NSCLC treated with cisplatin-gemcitabine, a cytidine deaminase host polymorphism associated with significantly reduced enzymatic activity correlated with better clinical benefit, toxicity and longer time to progression and overall survival262.
6.6 Other potential detoxifying mechanisms
Down-regulation of expression of the redox factor peroxiredoxin V in lung cancer cell lines augmented efficacy of etoposide and doxorubicin, suggesting a role for redox reactions in resistance to these agents263. In NSCLC and other cell lines, thymidine administration inhibited cytotoxicity of pemetrexed, and dipyridamole prevented this rescue by blocking thymidine transport264. High extracellular folate pools also markedly decreased cell killing by pemetrexed265. The clinical importance of these observations is uncertain.
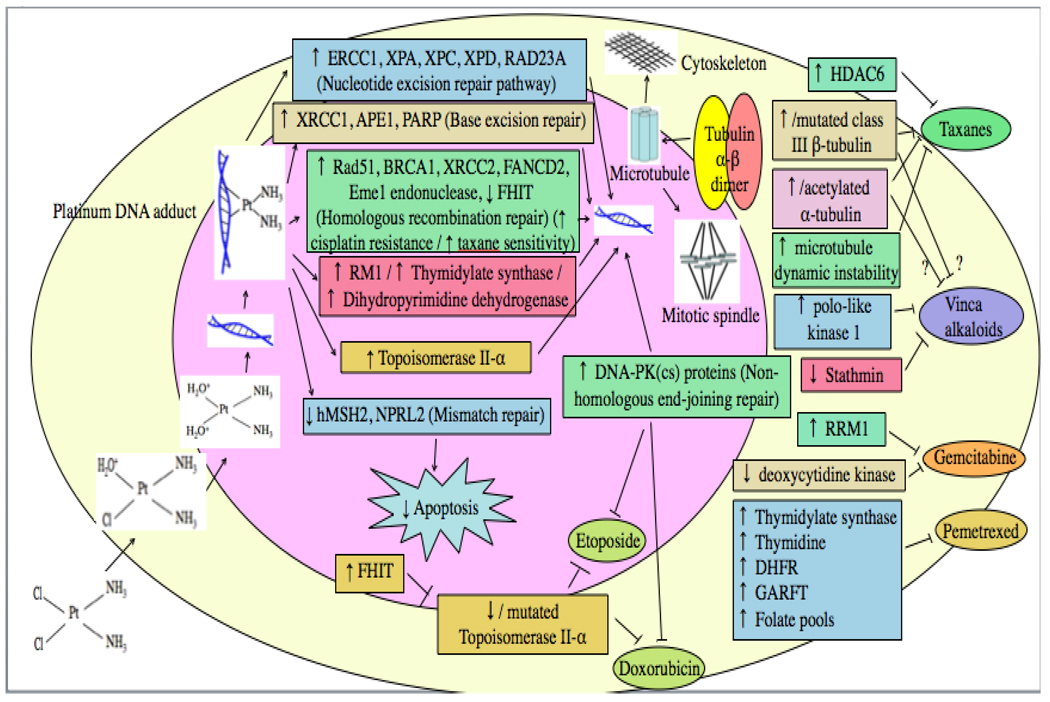
7.0 Drug activation and binding
Resistance may also occur if there is defective drug activation (Fig. 2 and 4).
Fig. 4.
Drug-induced DNA damage may be repaired by various DNA repair pathways, or apoptosis may be triggered as a result of attempted repair of platinum-induced damage by the Mismatch Repair pathway. Drug effect can also be reduced due to increased, decreased or mutated target.
7.1 Deoxycytidine kinase
Some NSCLC21, 120, 135, 139, 266, 267 and SCLC33, 139 cell lines resistant to gemcitabine had decreased activity of the enzyme deoxycytidine kinase which is responsible for conversion of gemcitabine to its active metabolite, gemcitabine triphosphate. Observations in some lines suggested that deoxycytidine kinase deficiency may play more of a role in acquired gemcitabine resistance than in intrinsic resistance120.
MRP1 expression was associated with increased deoxycytidine kinase expression and with augmented sensitivity to gemcitabine, and antagonism of MRP1 by verapamil reduced sensitivity to gemcitabine, possibly be decreasing expression of deoxycytidine kinase139, 268. Corticosteroids alone decreased deoxycytidine kinase activity and also decreased gemcitabine efficacy in NSCLC cell lines268. Synergism between gemcitabine and pemetrexed in NSCLC cell lines appears to be mediated in part through the up-regulation of expression of deoxycytidine kinase expression by pemetrexed135, 267, although upregulation of expression of the gemcitabine transporter (hENT1) and decreased phosphorylation of Akt may also play a role267. Clinical data on the importance of deoxycytidine kinase remain limited.
7.2 DNA binding of platinum agents and intracellular pH
DNA binding of cisplatin (ie, platinum-DNA adduct formation) was increased in some cisplatin sensitive NSCLC93, 94, 269 and SCLC93, 99, 232, 237, 270 cell lines compared to resistant variants, although this was not seen in all cases93, 233. Through mechanisms that are unclear, the antifungal agent amphotericin B increased sensitivity to cisplatin while increasing cisplatin uptake and DNA binding in both NSCLC and SCLC cell lines271. As outlined in an earlier review48, when cisplatin or carboplatin enter tumor cells, chloride adducts are lost in the low-chloride intracellular milieu and are replaced by water molecules to form highly reactive, positively charged aquated species or relatively non-reactive, neutral hydroxy moieties. A more acidic intracellular pH favors formation of the positively charged species, and it is the positively charged species that is the active form of cisplatin that binds to DNA. An acidic intracellular pH was also associated with a reduction in cisplatin efflux in NSCLC cell lines, possibly due to both more rapid intracellular binding as well as decreased ability to passively diffuse back out across cell membranes269. Resistant lung cancer variants with decreased DNA binding had a more alkaline intracellular pH compared to sensitive variants269, 272. However, the proton pump inhibitor AG-2000 did not alter sensitivity of a cisplatin-resistant NSCLC cell line273. In more resistant lines with higher intracellular pH, platinums tended to concentrate more in acidic organelles due to their tropism for an acidic environment272.
In NSCLC patients treated with daily low dose cisplatin and XRT, cisplatin-DNA-adduct staining in buccal cells was an independent prognostic factor, with shorter survival times for patients with low adduct staining274. Overall, preclinical data support a role for reduced DNA-adduct formation and for increased intracellular pH in lung cancer resistance to platinums, but clinical data remain limited.
8.0 Increased, decreased or altered target
Depending on the drug and its target, resistance may also occur if drug target is increased, decreased or mutated (Fig. 4).
8.1 Tubulin
NSCLC cell lines rendered resistant to taxanes had significantly increased expression of class III β-tubulin275–279 encoded by the Hβ4 gene279, and may also have had increased α-tubulin278. Findings were less consistent in NSCLC lines rendered resistant to vinca alkaloids, in that some had significantly increased β-tubulin280 while others instead had significantly decreased expression of class III β-tubulin275. Increased expression of β-tubulin after taxane exposure was inhibited in the presence of wild type p53276.
In two NSCLC cell lines, siRNA vs βIII-tubulin inhibited its expression, and increased sensitivity not only to the tubulin binding agents paclitaxel, vincristine, and vinorelbine, but also to cisplatin, doxorubicin, and etoposide281. In some NSCLC cell lines that were more resistant to paclitaxel under hypoxic conditions than under normoxic conditions, the expression level of β-tubulin was comparable under normoxic and hypoxic conditions, but the distribution of β-tubulin and cell morphology were changed according to HIF-1α expression levels, suggesting that HIF-1 influences the conformation and dynamics of microtubules52.
Polo-like kinases (PLKs) play a role in mitotic entry, spindle pole function and cytokinesis282. Expression of PLK 1 was elevated in NSCLC, and inhibiting it abolished microtubule polymerization, led to abnormalities in spindle formation and to abnormalities in staining for α-tubulin, arrested cells in G2/M, induced apoptosis, and substantially potentiated the efficacy of vinorelbine283.
In NSCLC284 and SCLC285 cell lines resistant to taxanes, there was increased microtubule dynamic instability284 or increased acetylation of α-tubulin285 and partial dependence on taxane for growth284, 285. In addition, histone deacetylase 6 (HDAC6) decreased microtubule stability and antagonized the effect of paclitaxel on NSCLC cells, while the farnesyl transferase inhibitor lonafarnib blocked the effect of HDAC6 on tubulin and was synergistic with paclitaxel286.
In IHC assessments of advanced NSCLC, all tumors stained with pan-β tubulin antibody and class I tubulin isotype287. A majority of the tumor samples expressed class II and class III tubulins, although the percentage of positive cells varied significantly between tumors287. Expression of delta2 α tubulin was uncommon287. In another study involving European patients, β-tubulin mutations in exons 1 or 4 were noted in 33% of NSCLC patients, and β-tubulin mutations were also detected in serum DNA of 42% of 131 NSCLC patients (vs none of a control group)288, although they were uncommon in tumors of Japanese patients with NSCLC or SCLC289. In NSCLC patients treated with surgery alone, high class III β-tubulin expression was associated with poorer relapse-free and overall survival290.
In locally advanced or metastatic NSCLC treated with taxane-containing regimens, patients with tumor β- tubulin mutations had substantially lower response rates288 or a trend towards lower response rates287 than did patients with wild type β-tubulin. Response rates were also lower in patients with high tumor expression of β-tubulin III compared to those with low expression291, 292. There was also a longer time to progression287, 291, 292 and longer survival292 with low β-tubulin III mRNA expression in patients treated with taxane-containing regimens, but not in patients receiving non-tubulin-binding regimens292.
Advanced NSCLC patients treated with vinorelbine/cisplatin who had low β-tubulin III mRNA expression also had a significantly longer time to progression291, 293, lower likelihood of tumor progression while on therapy293 and longer overall survival293 than patients with high expression. Low Delta2 α-tubulin expression was also associated with a significantly longer overall survival in patients treated with cisplatin/vinorelbine293, while tubulin II levels did not correlate with patient outcome293. Conversely, when cisplatin/vinorelbine was administered as adjuvant therapy to patients who had undergone surgical resection of their NSCLC tumors, greater benefit of the therapy was seen in patients with high vs low class III β-tubulin, and the adjuvant therapy appeared to overcome the negative prognostic effect of high β-tubulin290. It is unclear why the effects of tubulin on vinorelbine efficacy appear to be different in the adjuvant setting than in advanced disease.
Overall, the available data suggest that elevated expression or mutation of class III β-tubulin (and possibly α-tubulin) increases resistance to taxanes, with somewhat more equivocal data for its effect on resistance to vinca alkaloids.
8.2 Topoisomerase II-α (topo II-α)
Ordinarily, topo II temporarily breaks DNA, permitting it to unwind, then puts it back together again. Epipodophyllotoxins such as etoposide and teniposide, and anthracyclines such as doxorubicin and epirubicin bind to topo II and permit it to break DNA, but block its ability to rejoin the cleaved strands, leading to potentially lethal DNA breaks. A variety of topo II-α abnormalities have been found in resistant lines.
On average, SCLC cell lines are more sensitive to topo II inhibitors than are NSCLC cell lines115, 294. SCLC cell lines had only a modest increase in topo II-α levels and topo II catalytic activity compared to NSCLC cell lines in one study, and no clear association across all unrelated cell lines between sensitivity and topo II-α levels or topo II activity294. However, in another cell line comparison, nuclear topo II activity was twofold higher in SCLC cell lines than in NSCLC cell lines115, and when resistant variants were compared to their sensitive parent line in matched comparisons, expression of topo II-α mRNA and protein levels were lower in the resistant variants295.
One etoposide-resistant SCLC cell line had no alteration of topo II activity, but did have increased topoisomerase I (topo I) activity (in addition to increased GSH and GST activity)32, with cross-resistance to platinums, mitomycin-C, camptothecins, alkylating agents, methotrexate and anthracyclines, but collateral sensitivity to vinca alkaloids and 5-fluorouracil32. Another etoposide-resistant SCLC cell line had decreased nuclear localization of topo II-α, with a shift of the enzyme from nucleus to cytoplasm, but no change in overall cellular catalytic activity296. Other etoposide-resistant SCLC cell lines derived from a patient who developed drug-resistant relapse after initial response to an etoposide regimen had cross-resistance to doxorubicin, reduced topo II unknotting activity, and reduced topo II α expression297.
Still other etoposide-resistant SCLC169, 236, 298–301 or NSCLC299, 302 cell lines with cross-resistance to doxorubicin299, 301 or teniposide236, 299 (but not to cisplatin298 or vinca alkaloids236, 298) had mutated topo II-α236, 298, 299, 302 (in some cases with relocalization of topo II-α to cytoplasm from the nucleus302) and reduced levels of topo II-α protein and/or mRNA236, 298, 299, 301, or reduced topo II activity169, 300, compared to their sensitive parent lines. Some also had decreased cleaved complex formation in the presence of etoposide298, 300, 301 or decreased expression of topo II- β301.
Drug-resistant variants selected by exposure of a NSCLC cell line to doxorubicin frequently had reduced topo II-α mRNA and protein levels, whereas clones selected with vincristine showed normal levels of topo II-α303. No alterations of topo II β levels were detected303. On the other hand, some SCLC cell lines resistant to cisplatin95, 236 with cross-resistance to radiation95 and vinca alkaloids95, 236 had increased expression of topo II-α95, 236 (but continued sensitivity to etoposide236) in keeping with a possible role for topo II-α in repair of DNA damage caused by cisplatin or radiation.
In clinical tumor specimens, there was a significantly greater topo II-α expression by IHC in SCLC than in NSCLC152, 304, and topo II-α IHC expression decreased significantly in SCLC tumors after therapy with etoposide or teniposide152. A topo II-α mutation (transversions of G to A at codon 486) associated with resistance in cell lines was detected in tumor samples obtained from a patient with SCLC previously treated with etoposide152, 305.
In patients with advanced NSCLC158, or with limited306, 307 or extensive disease SCLC307 treated with a platinum158, 306, 307 combined with either paclitaxel158, teniposide158, etoposide158, 306, 307, ifosfamide158 or other agents307, patients with high topo II-α expressing tumors had a significantly worse survival158, 307 or a significantly lower response rate306 than did patients with tumors with low topo II-α expression, while high topo II-β expression was associated with lower response rates in one study307.
Overall, preclinical data suggest that reduced expression or mutation of topo II-α may increase resistance to epipodophyllotoxins and anthracyclines (putatively since these drugs rely on an intact topo II system to break DNA strands) while high topo II expression may increase resistance to platinums, radiation and some other agents (potentially through the role topo II may play in DNA repair). Clinical data are inconclusive. On the one hand, higher topo II-α expression in SCLC than in NSCLC might help explain the greater sensitivity of SCLC to topo II inhibitors. Conversely, the majority of clinical studies assessing impact of topo II expression within a tumor type support, if anything, a role of topo II in increasing resistance to platinum-based combination therapy, whether or not the platinum is combined with a topo II inhibitor.
8.3 Ribonucleotide reductase M1 (RRM1)
RRM1 (a major target for gemcitabine) encodes the regulatory subunit for ribonucleotide reductase308. Because of the role of ribonucleotide reductase in synthesis of deoxyribonucleotides, this enzyme is important in cell growth and DNA repair. RRM1 encodes the regulatory subunit of ribonucleotide reductase and is a molecular target of gemcitabine308. While an association has not been found in all lung cancer cell line studies309, RRM1 gene polymorphisms310, gene amplification311, and mRNA over-expression308, 312 did correlate with NSCLC cell line resistance to gemcitabine in other studies. RRM1 over-expression was also associated with a modest degree of platinum resistance in NSCLC cell lines308. Bexarotene (which prevents or reverses RRM1 gene amplification311) and RRM1 siRNA135 both reduced resistance to gemcitabine. In a panel of 15 NSCLC and 5 SCLC cell lines, significantly higher RRM1 mRNA expression was found in SCLC vs NSCLC309.
In tumors from patients with advanced NSCLC313–316 and SCLC306, there was a strong correlation between expression levels of RRM1 and of the nucleotide excision repair pathway factor ERCC1306, 313–316, and in some studies a correlation was also noted with the DNA repair factor XPD315. While no significant correlation between RRM1 mRNA and survival was seen in patients treated with gemcitabine plus vinorelbine and ifosfamide in one study313, a number of other studies have suggested a link between tumor RRM1 expression and gemcitabine efficacy. For example, in NSCLC patients treated with gemcitabine alone314 or combined with a platinum291, 308, 313–315, with docetaxel317, or with pemetrexed318, RRM1 mRNA expression levels correlated inversely with survival291, 313–315, 317, time to progression291, 317, or response308, 315, 318, and patients with low levels of both RRM1 and ERCC1 mRNA expression had significantly longer survival than did patients in whom both were high313, 314. The related factor RRM2 also correlated inversely with response in one study317, and patients whose tumors had high expression of both RRM1 and RRM2 had significantly lower response rates and shorter time to progression and overall survival than did patients in whom both were low317.
In NSCLC pharmacogenetic studies, while RRM1 host gene polymorphisms did not correlate with outcome in patients treated with cisplatin plus docetaxel196, the RRM1 promoter host allelotype in patients treated with gemcitabine +/− a platinum did correlate significantly with response (but not with overall survival or progression-free survival)319.
In limited disease and extensive disease SCLC patients treated with a platinum plus etoposide, tumor RRM1 mRNA expression did not correlate with response or survival306.
Overall, available preclinical and clinical data indicate that tumor RRM1 expression probably is associated with resistance to gemcitabine-based therapy in NSCLC, and further studies are underway to explore RRM1 tumor expression as a tool to help in selecting therapy for patients with advanced NSCLC. Data are insufficient in SCLC to draw any conclusions.
8.4 Folate pathway
Pemetrexed cytotoxicity is mediated through the inhibition of the enzymes thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), and increased expression of these enzymes was associated with decreased efficacy of pemetrexed in NSCLC cell lines267.
8.5 Stathmin
Stathmin (oncoprotein 18) is a protein that plays an important regulatory role in tubulin dynamics. Transfection of the gene into lung cancer cells increased sensitivity to vinca alkaloids320, but in patients with advanced NSCLC treated with vinorelbine plus cisplatin, time to progression was shorter in patients with high stathmin than with low stathmin mRNA expression291. Hence, its role in resistance remains unclear.
9.0 DNA repair
While some resistant cell lines appear to survive exposure to cisplatin and other agents by developing tolerance to drug-induced DNA damage, other resistant lines show evidence of significant repair of DNA damage93 (Fig. 4). Clinically, several genes associated with DNA repair are overexpressed in NSCLCs321. DNA repair pathways that could potentially be relevant for selected chemotherapy agents include the nucleotide excision repair pathway, homologous recombination repair, non-homologous end-joining repair, base excision repair, and mismatch repair48.
9.1 Nucleotide Excision Repair Pathway
Components of the nucleotide excision repair pathway include XPA-XPG, ERCC1, Replication Protein A (RPA), Rad23A, DNA polymerases δ and ε, and DNA ligase.
9.1.1 Excision repair cross-complementation group 1 protein (ERCC1)
As outlined in a recent review48, ERCC1 probably plays a major role in repair of cisplatin-induced DNA damage. In chemosensitivity testing of resected NSCLC tumors322 and in a panel of NSCLC cell lines323, high NER activity323 and ERCC1 expression322 correlated with cisplatin resistance, but ERCC1 expression did not correlate with NSCLC in vitro sensitivity to paclitaxel, vinorelbine, etoposide, irinotecan, 5-fluorouracil or gemcitabine322, and in another panel of 15 NSCLC and 5 SCLC cell lines, there were no correlations between mRNA expression for ERCC1 or ERCC2 and chemosensitivity to cisplatin, carboplatin or gemcitabine309.
The ERCC1 protein was detected by IHC in approximately 40–55% of patients with early stage324 or advanced325–327 NSCLC. ERCC1 mRNA308, 314, 328–330 or IHC220, 325, 331, 332 expression was assessed in tumors from patients with advanced220, 308, 314, 325, 328–331 or resected324, 326, 332 NSCLC treated with therapeutic220, 308, 314, 325, 328–331, neoadjuvant326, 332 or postoperative adjuvant324 regimens that combined a platinum with a taxane326, 329, 330, 332, etoposide324, 326, 329, a vinca alkaloid324, 325, 329, 330, gemcitabine308, 314, 325, 327–329, 331, irinotecan332, mitomycin-C plus ifosfamide, radiation326, 332, or with a variety of agents220. Patients treated with a combination of epirubicin plus gemcitabine have also been assessed331. ERCC1 expression did not correlate with response220, 328–332, progression-free survival220 or survival331, 332 in some studies. However, there was a significantly higher response rate332 or a trend towards a higher response rate in ERCC1-negative tumors in other studies308, 325. Furthermore, in some studies, median progression-free survival329 and/or overall survival220, 314, 324, 326–329 was significantly longer in patients whose tumors had low (or negative) vs high (or positive) ERCC1 expression in tumors, or there was a trend towards longer progression-free survival326 or overall survival325, 330. Patients with resected ERCC1-negative tumors derived substantially more survival benefit from platinum-based adjuvant chemotherapy than did patients with ERCC1-positive tumors324. Assigning patients with advanced NSCLC who had high ERCC1 mRNA expression to chemotherapy with docetaxel plus gemcitabine instead of to docetaxel plus cisplatin resulted in a significant increase in response rate333.
In patients with advanced NSCLC treated with platinum-based regimens, ERCC1 C8092A334, 335 and ERCC1 C118T262, 336 host genetic polymorphisms did not correlate independently with treatment outcome in some analyses, but ERCC1 C118T genotype was significantly associated with response337 or survival196, 335, 338 in other studies, and in still other NSCLC studies, the ERCC1 C8092A genotype was significantly associated with survival336, 339.
In patients with limited disease SCLC treated with platinum combinations (mainly combined with etoposide306, etoposide plus ifosfamide340 or irinotecan340) high expression of ERCC1 was associated with poor overall survival. Little340 or no306 significant association with therapy efficacy was seen in extensive SCLC.
Overall, while not all studies agree, the data suggest a probable role for ERCC1 in resistance of NSCLC to platinum-based chemotherapy, while there are insufficient clinical data to draw conclusions in SCLC. Additional studies are underway assessing ERCC1 as a marker for allocation of patients to therapy with a platinum vs other agent.
9.1.2 Other components of the NER pathway
Antisense to xeroderma pigmentosum group A (XPA) gene reduced cellular NER capacity in NSCLC cells and sensitized cells to cisplatin341, and the cell cycle checkpoint abrogator UCN-01 inhibited DNA repair by attenuating the interaction of XPA and ERCC1342. Sensitivity to cisplatin was also increased by silencing of the NER component RAD23A343.
In clinical pharmacogenetic analyses of components of the NER pathway in advanced NSCLC patients receiving platinum-based chemotherapy, response rates varied significantly with XPC SS vs LL host genotype334, XPD Asp312Asn genotype344, XPD *A haplotype345, and with XPG 46His/His genotype346. However, in other studies, response did not vary with XPDAsp312Asn genotype262, with XPD Lys751Gln genotype334, with XPG 1104His/Asp genotype346, and survival did not correlate with XPDAsp312Asn genotype262, 338 or with XPD Lys751Gln genotype196, 338, 347. Even if individual polymorphisms did not correlate with outcome, there were significant interactions between XPC, XPD and ERCC1 genetic polymorphisms334 or between XPD and the base excision repair pathway component XRCC1348 in some studies, and survival worsened significantly as a patient’s number of different unfavorable genotypes within the NER pathway increased, with ERCC1 8092, XPC intron 9, XPD 751 and ERCC6 1097 each contributing to outcome339. This latter model was further strengthened when polymorphisms for XPG 1104, NQO1, p53 and GSTpi exon 5 were added339.
Overall, the pharmacogenetic data available suggest a role for XPC and XPD in resistance of NSCLC to platinum-based chemotherapy.
9.2 Base excision repair
Components of the base excision repair pathway include DNA glycosylases, AP endoculeases, DNA polymerase β, XRCC1, poly(ADP-ribose) polymerase (PARP) and DNA ligase III. As outlined below, data are limited, but suggest a possible role for base excision repair in NSCLC resistance to platinum agents.
9.2.1 XRCC1
In clinical pharmacogenetic analyses in advanced NSCLC patients receiving platinum-based chemotherapy, response rates varied significantly with XRCC1 Arg194Trp genotype346, 348. While response did not vary with XRCC1 399Arg/Gln genotype346, survival did vary significantly with this genotype in both NSCLC and SCLC patients treated with platinum-based regimens347. The pharmacogenetic data suggest a possible role for XRCC1 in resistance of NSCLC to platinum-based chemotherapy.
9.2.2 Apurinic/apyrimidinic endonuclease (APE1)
APE1 is involved in base excision repair and in redox signaling. Exposure of NSCLC cells to cisplatin in vitro resulted in increased expression of APE1, and APE1 inhibition by siRNA sensitized cells to cisplatin349. In NSCLC patients treated with cisplatin-based regimens, 84% of resistant tumors had high APE1 expression, compared to only 8% of responding tumors, and survival was better in patients with low APE1 expression (p<0.01)349.
9.2.3 PARP
PARP inhibitors potentiated the effect of temozolomide350, toptecan350 and cisplatin351 in lung cancer cell lines, suggesting that PARP played a role in resistance. Clinical trials of PARP inhibitors are in progress.
9.3 Homologous recombination repair
Components of the homologous recombination repair pathway include Rad51, DMC1, XRCC2, XRCC3, BRCA1, BRCA2, Eme 1 endonuclease, Fanconi Anemia proteins and DNA polymerase eta.
9.3.1 Rad51
In SCLC cell lines, resistance to etoposide correlated with protein levels of RAD51, and downregulation or overexpression of the RAD51 gene altered both etoposide efficacy and repair of etoposide-induced DNA double strand breaks352.
Rad51 was expressed in 41% of clinical NSCLC tumor samples (particularly in squamous cell and poorly differentiated tumors)322. In NSCLC cell lines, Rad51 expression was associated with reduced platinum efficacy, particularly if co-expressed with ERCC1, while its expression did not correlate with resistance to paclitaxel, etoposide, vinorelbine, gemcitabine, 5-FU, or irinotecan322. In NSCLC cell lines, cisplatin exposure increased ERK1/2 activation353 and Rad51 protein induction353. Increased ERK1/2 signaling augmented expression of Rad51 protein, and blockage of ERK1/2 signaling activation by administering the EGFR tyrosine kinase inhibitor gefitinib concurrently with cisplatin potentiated the efficacy of cisplatin by decreasing cisplatin-induced augmentation of Rad51 protein expression through reduction of Rad51 mRNA and destabilization of the Rad51 protein353. Si-RNA reduction of Rad51 also significantly increased cell kill by cisplatin353. However, in one clinical study involving patients with NSCLC treated with cisplatin plus gemcitabine, hRad51 expression by IHC did not correlate with response or survival331.
Overall, available preclinical data suggest that Rad51 might play a role in lung cancer resistance to platinums and etoposide, although this has not been confirmed clinically, and its role in resistance to other agents has not been explored in any detail.
9.3.2 Breast Cancer 1 (BRCA1)
BRCA1 interacts with Rad51 during repair of DNA double strand breaks. Its overexpression in resected early stage NSCLC was associated with poor survival354, and in cell lines, high expression correlated with resistance to platinums but sensitivity to antimicrotubule agents167, 355. In patients with advanced NSCLC treated with cisplatin-gemcitabine or epirubicin-gemcitabine, IHC for tumor BRCA1 expression did not correlate with response or survival331, and in patients treated with gemcitabine plus docetaxel, response rate increased and probability of tumor progression decreased with higher tumor BRCA1 mRNA expression356. On the other hand, in NSCLC patients who received neoadjuvant cisplatin plus gemcitabine, survival was worse with high than with low tumor BRCA1 mRNA levels357, and high tumor BRCA1 mRNA expression was associated with worse progression-free survival in advanced NSCLC patients receiving second line platinum-based therapy356. In an assessment of impact of BRCA1 host genotype, patients with stage III-IV NSCLC treated with platinum regimens also had significantly worse survival if they had 2 copies of the wild-type BRCA1 AACC haplotype than if they had none or one copy, particularly if they had squamous cell lung cancer358. Overall, available data suggest that BRCA1 may increase resistance to platinum regimens but may increase sensitivity to taxanes.
9.3.3 Fragile histidine triad gene (FHIT)
FHIT blocks DNA homologous recombination repair and promotes apoptosis in cells with damaged DNA359. FHIT increased sensitivity to cisplatin in NSCLC cells that possessed wild type p53 and partially restored sensitivity to cisplatin in resistant cells that overexpressed Bcl-2- and Bcl-x(L)360. FHIT also increased sensitivity of lung cancer cells to paclitaxel in some assessments361, but not in others360. However, transfection of FHIT-negative NSCLC cells with FHIT reduced sensitivity to etoposide, doxorubicin, and topotecan due to Fhit-induced downregulation of DNA topoisomerases I and II360.
9.3.4 Other homologous recombination repair pathway components
The Eme1 endonuclease was associated with resistance to cisplatin in lung cancer and other cell lines362. In vitro sensitivity of resected lung cancer samples to cisplatin and carboplatin varied significantly with XRCC2 C41657T host genotype, but not with the XRCC2 G4234C genotype, although sensitivity was significantly greater with the 41567T/4234G haplotype than with the 41567T/4234C haplotype363. Expression of FANCD2 protein (a marker for Fanconi Anemia pathway functioning) was detected by IHC in 32% of tumor specimens from NSCLC patients, but did not correlate with response to platinum-based chemotherapy or with patient survival364.
9.4 Non-homologous end-joining repair
Components of the non-homologous end-joining repair pathway include Ku70, Ku80, DNA ligase IV, XRCC4, XRCC5, XLF, DNA-dependent protein kinase (DNA-PK(cs)) protein, and DNA polymerases λ and μ. Etoposide efficacy and etoposide-induced double strand breaks in SCLC cell lines varied with expression of DNA-PK(cs) proteins, suggesting a role for non-homologous end-joining repair in etoposide resistance352. However, in vitro sensitivity of resected lung cancers to cisplatin and carboplatin did not vary with XRCC5 G74582A and C74468A host genotypes363.
9.5 Mismatch repair
Components of the mismatch repair pathway include MSH2, MSH3, MSH6, MLH1 and PMS1. As recently reviewed48, cells deficient in mismatch repair may be paradoxically resistant to platinum agents, possibly since attempts at mismatch repair trigger apoptosis. The tumor suppressor gene NPRL2 is thought to be involved in DNA mismatch repair365. The expression of NPRL2 in NSCLC cell lines correlated with cisplatin sensitivity, transfection with NPRL2 gene augmented cisplatin sensitivity in NSCLC cell lines, and systemic administration of NPRL2 nanoparticles to mice bearing an orthotopic lung cancer model significantly enhanced the therapeutic efficacy of cisplatin and overcame cisplatin-induced resistance365.
In patients with stage III NSCLC receiving vinorelbine +/− cisplatin with radiotherapy, response to induction chemotherapy and survival were worse in patients with low vs high IHC expression of hMSH2, while outcome did not correlate with hMLH1 expression195. In NSCLC patients receiving gemcitabine with cisplatin, hMLH1 and hMSH2 expression by IHC did not significantly impact response or survival366. Hence, preclinical data and some clinical data support a role for mismatch repair deficiency in lung cancer resistance, but further clinical data are needed.
9.6 Deoxyribonucleotides needed for DNA repair and cell division: Thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD)
In resected NSCLC tumor samples, in vitro sensitivity to cisplatin and carboplatin correlated significantly with mRNA expression of TS and DPD, 2 enzymes that are critical in nucleic acid metabolism367, and that could thereby play a role in DNA repair. The correlation between TS expression and platinum sensitivity was particularly important for tumors from patients with some specific TS polymorphisms367.
9.7 High mobility group box 2 (HMG2)
HMG2 are chromatin-associated proteins that bend DNA and may be involved in DNA repair. However, NSCLC cells transfected with HMG2 gene actually have increased sensitivity to cisplatin compared to non transfected cells, with increased cellular platinum levels and decreased repair of DNA interstrand cross-links368.
10.0 Damage tolerance
In addition to resistance being due to a decrease in ability of chemotherapy to cause damage or an increase in ability to repair damage, resistance may also arise from increased damage tolerance, such that cellular damage occurs and is not repaired, but fails to cause cell death369. This could occur due to defective proapoptotic factors or to increased antiapoptotic factors (Fig. 5).
Fig. 5.
Drug resistance can also occur due to deficient or defective proapoptotic factors or due to antiapoptotic effects of growth factors, growth factor receptors, signaling pathways, various membrane components, chaperones, transcription factors and cell cycle factors. Tumor cells are most sensitive to platinums during G1 and to taxanes during G2.
11.0 Reduced apoptotic response (Fig. 5)
11.1 P53
Mutations in p53 are very common in lung cancer cell lines370 and mutations were also found in 40–90% of resected NSCLC tumors371–373. A significant increase in radiosensitivity was found for mutations in p53 exon 7 compared with mutations in other p53 exons370. While no correlation was found between type of p53 mutation and sensitivity to chemotherapy agents in some studies370, 372, ability of p53 mutations to induce chemotherapy resistance did vary with the mutation type in another study374. NSCLC cell lines371, 374–376 or heterotransplants134 with mutant (MT) p53 were resistant to cisplatin371, 374–376 (particularly low dose cisplatin374), cyclophosphamide371, 5-fluorouracil375, etoposide374, 375, methotrexate375, anthracyclines375, and bleomycin375, but were not resistant to paclitaxel134, 375. Introduction of MTp53 into NSCLC cell lines bearing wild type (WT) p53 rendered the cells more resistant to cisplatin377, 378, etoposide378, and camptothecin378 in some instances, while transfection of WTp53 into cells bearing MTp53 augmented sensitivity375, 378, 379. Presence of MTp53 in NSCLC cell lines correlated significantly with overexpression of MRP144.
Transfection of WTp53 into p53 null NSCLC cell lines only modestly increased sensitivity to carboplatin in one trial380, while transfection with WTp53 into p53 null379, 381–386 or WTp53381, 384 cells did augment sensitivity to cisplatin379, 381–386, 5-fluorouracil379, 384, doxorubicin384, taxanes379, 384, irinotecan379, 384, and etoposide384 in other NSCLC cell lines. Induced expression of p53 in p53 null NSCLC cells led to cellular senescence385, 387, enhanced the cytotoxic effect of cisplatin385 but protected against etoposide385 and paclitaxel387 cytotoxicity, and decreasing p53 expression by siRNA increased resistance to paclitaxel in NSCLC cells in one study163. Induction of p21waf1 overexpression conferred increased resistance to both etoposide and cisplatin385. Similarly, transfection of p73 (a homologue of p53) into p53 null and WTp53 NSCLC cells enhanced cell sensitivity to cisplatin and increased apoptosis, independently of p53388. The presence of hypoxia may elicit a downstream transcriptional response to p53 activation that favors cell cycle arrest over apoptosis389.
Exposure of WTp53 NSCLC cell lines to docetaxel strongly induced expression of p53 mRNA, and docetaxel induced increased β-tubulin gene transcription in p53 null cells, but not in WTp53 cells unless p53 was silenced by siRNA276. This suggests that p53 plays a role in preventing a compensatory increase in β-tubulin production in NSCLC cells exposed to taxanes.
Overall, the NSCLC cell line data suggest that MTp53 may confer resistance to several agents. Loss of p53 function may possibly be associated with increased sensitivity to taxanes (instead of resistance) but the data are inconclusive.
In lung cancer cell lines, higher mRNA expression of the proapoptotic p53-binding protein 2 (53BP2) was also associated with increased sensitivity to cisplatin and radiation390.
Approximately 31%–63% of NSCLC tumors expressed p53 by IHC71, 173, 194, 327, 391, 392. Positive IHC staining for p53 tended to be more frequent in tumors bearing p53 mutations, with a 70% concordance between IHC overexpression of p53 protein and mutation in p53371, although this concordance was not seen in all studies393. Because of the frequent concordance between IHC positivity and p53 mutation status, IHC positivity is often used as a surrogate indicator of p53 mutation. However, correlations of drug resistance with tumor p53 IHC positivity do not reflect well the correlations seen between p53 mutation and resistance in cell lines. With in vitro sensitivity testing of resected NSCLC tumors, positive IHC expression of p53 correlated with a high degree of resistance to etoposide and doxorubicin but with reduced resistance to platinums, taxanes and gemcitabine in one study6. In another study, tumors that were p53 IHC positive had significantly greater in vitro resistance to 5FU and slightly higher resistance to doxorubicin than p53 negative tumors, while p53 IHC expression did not correlate with in vitro sensitivity to cisplatin, etoposide, vindesine or mitomycin-C394. In other in vitro sensitivity testing, cells from resected NSCLC tumors that had p53 mutations determined by gene sequencing were significantly more resistant to combined cyclophosphamide, etoposide plus epirubicin than were tumors with WTp53, while sensitivity to carboplatin plus paclitaxel or etoposide did not correlate with p53 mutation status395.
Several NSCLC clinical studies have also assessed the relationship between tumor p53 mutations or IHC expression and outcome, and findings have been inconsistent across studies. In many of these studies, platinum agents were combined with one or more other agents, and in studies using more than one combination, there was generally no breakdown with respect to interaction (if any) of p53 abnormality and individual agent added to the platinum. Hence, it remains possible that some of the inconsistency is due to differences between different agents with respect to impact of the p53 abnormality. In patients receiving preoperative neoadjuvant cisplatin plus etoposide and radiation396 or in patients receiving cisplatin-based combinations for advanced disease393, presence of MTp53 in tumor (defined by DNA sequencing) was associated with significantly lower response rates and shorter overall survival compared to patients with WTp53, while there was no significant association between response and tumor p53 mutation status in patients with locally advanced NSCLC treated with radiotherapy and a taxane (without a platinum)373.
NSCLC studies assessing p53 IHC positivity have included patients receiving neoadjuvant therapy397, 398 and therapy for locally advanced195, 274, 399, 400 or metastatic71, 173, 194, 214, 327, 329, 391, 401–406 disease. P53 IHC positivity was associated with a lower probability of response71, 173, 194, 195, 400, 401, 406, with a trend towards a lower response rate397, or with significantly shorter survival71, 194, 195, 329, 401, 402 for some studies combining a platinum with a vinca alkaloid173, 329, 401, a taxane329, etoposide71, 401, gemcitabine71, 329, ifosfamide401, mitomycin-C/ifosfamide71, mitomycin-C/vindesine400, 5FU/folinic acid401, a vinca alkaloid plus radiotherapy (with or without a platinum)195, or unspecified platinum combinations194, 406. In one study in which an association was noted between p53 IHC expression and shorter overall survival, no significant association was seen with progression-free survival329.
On the other hand, there was no significant correlation between tumor p53 IHC expression and response391, 393, 398, 399, 403 or survival327, 391, 393, 398, 399, 404 in other studies in which a vinca alkaloid alone403 or combined with gemcitabine391 was used, or in which a platinum was combined with etoposide404, gemcitabine327, 404, irinotecan391, radiotherapy274, a vinca alkaloid398, 399, a vinca alkaloid plus radiotherapy399, mitomycin-C/ifosfamide404 or unspecified concurrent agents393. In 2 studies, response214, 405 or survival405 were paradoxically increased in patients whose tumors expressed p53 by IHC and who received a platinum combined with a vinca alkaloid214, a taxane214, etoposide214, irinotecan214, mitomycin-C214, or unspecified platinum combinations405. In a metaanalysis of 16 published studies involving 1070 NSCLC patients treated with cisplatin-based regimens the association of p53 expression with response did not achieve significance, with a combined odds hazard ratio of 1.37 (0.84–2.24)392.
SCLC tumors were positive for p53 by IHC in 48%–63% of patients173, 257. Positivity for p53 correlated with positivity for metallothioneins257. Tumor p53 IHC positivity has been assessed in SCLC patients treated with a platinum combined with other agents including etoposide173, 257, 340, 402, 407, irinotecan340, ifosfamide340, cyclophosphamide257, doxorubicin257, and vincristine257. It has also been assessed in SCLC patients treated with CAV without a platinum173, 402, and with unspecified regimens148. In SCLC, high p53 IHC expression did not correlate with patient outcome in some trials148, 173, 340, 402, although there was a trend towards shorter survival in patients whose tumors were p53 IHC positive in another trial257, and significantly shorter survival in another407.
The possible importance of host p53 genotype in patient outcome was supported by the observation that in patients with advanced NSCLC treated with cisplatin combinations, p53 mRNA expression was significantly higher in the lymphocytes of nonresponders than in the lymphocytes of responders, and p21waf1 mRNA expression in lymphocytes was also significantly higher in non-responders408. In patients with advanced NSCLC treated with platinum-based chemotherapy, a higher response rate was seen in patients with the p53 codon 72 Pro allele and in those with p73 exon 2 G4C14/A4T14 or A4T14/A4T14 genotypes compared to the G4C14/G4C14 genotype409. The response rate in those carrying the WT genotypes for both p53 and p73 was only 7.7%, whereas the response rates in patients carrying 1, 2, or more than 2 variant alleles of p53 and p73 were 34.8%, 42.2% and 40.7%, respectively409. However, in another study, patients with p53 codon 72 Pro/Pro genotype had a significantly lower response rate to cisplatin plus irinotecan and shorter survival than did patients with the Arg/Pro or Arg/Arg genotypes391. The p53 genotype did not correlate with response to gemcitabine plus vinorelbine391. For MDM2 (a protein that binds to p53), SNP309 genotype did not correlate significantly with response to cisplatin-irinotecan or gemcitabine-vinorelbine, but the TT genotype was associated with significantly better survival than were the TG or GG genotypes391.
Injection of adenovirus/WTp53 into tumors of NSCLC patients receiving carboplatin plus paclitaxel or cisplatin plus vinorelbine did not increase response rate, although the injected lesions did have a greater decrease in tumor size than did non injected lesions, particularly for patients receiving cisplatin plus vinorelbine410.
Overall, there is fairly strong preclinical evidence for a role of MTp53 in lung cancer resistance to chemotherapy. While there have been few studies of actual p53 mutational status, the data from the available studies do support an association between p53 mutation and resistance to platinum-based therapy in NSCLC. Limited host genotyping studies have given conflicting results. IHC positivity for p53 (which generally correlates with p53 mutational status, but which is not necessarily synonymous with p53 mutation) correlated with benefit of platinum based therapy in some NSCLC and SCLC trials but not in others, and a metaanalysis in NSCLC was associated with a statistically insignificant trend towards an unfavorable outcome with p53 IHC positivity. Together the data indicate that clinical decisions re therapy choices should not be made based on p53 IHC positivity but that more studies should be done assessing actual p53 mutational status.
11.2 Caspases
In NSCLC cell lines, chemotherapy resistance was associated with defects in expression of caspase-3, -8, and -9411. NSCLC xenograft transfection with caspase-8 and -9 strongly induced apoptosis and also sensitized the tumors to cisplatin-induced cell death411. In other studies, NSCLC and SCLC cell lines exhibited normal caspase-9 activation in response to cisplatin, and while some NSCLC lines expressed the caspase-9 inhibitor TUCAN/CARDINAL/CARD8, siRNA down-regulation of TUCAN did not affect cisplatin-induced caspase-9 activation or cisplatin sensitivity412. In NSCLC cells, inhibition of caspase 9 by XIAP did not block apoptosis induction by cisplatin, topotecan, and gemcitabine, but stable expression of caspase-8 inhibitors (such as cytokine response modifier A) almost completely abrogated apoptosis and enhanced clonogenic survival413.
Dexamethasone increased resistance of tumor cells from resected lung cancer specimens to both cisplatin and gemcitabine414. Glucocorticoids induced chemotherapy resistance in lung carcinoma cells by down-regulating proapoptotic elements of the death receptor and mitochondrial apoptosis pathways, resulting in a decreased activity of caspase-8, caspase-9, and caspase-3415. Transfection of glucocorticoid-treated cells with caspase-8 and -9 resensitized tumor cells and xenografts to cisplatin induced cell death415. Clinical data on role of caspase expression in lung cancer resistance are very limited, but clinical assessments are warranted based on the preclinical data.
11.3 SAPK/c-Jun N-terminal kinase (JNK) and c-Jun
Activation of JNK plays a role in TRAIL-induced apoptosis416. JNK phosphorylates 14-3-3, a cytoplasmic anchor of the proapoptotic protein Bax, thereby promoting apoptosis by releasing Bax for translocation to mitochondria417. In NSCLC cells, gemcitabine induced activation of JNK (for which the transcription factor target is c-Jun) in sensitive parental cells, but not in gemcitabine-resistant variants418. JNK inhibitors abrogated gemcitabine-induced apoptosis in sensitive cells, while transfection with JNK partially restored gemcitabine sensitivity in the resistant variant418. Cisplatin also induced activation of JNK, and this in turn mediated induction of apoptosis419.
In SCLC cells, cisplatin420, 421, tumor suppressive anti-GD2-monoclonal antibodies421, and other anticancer agents421 stimulated activity of JNK420, 421, suppressed tumor cell growth421 and induced apoptosis421. The anti-GD2-antibodies were synergistic with cisplatin in phosphorylating JNK and inducing apoptosis421. The JNK inhibitor curcumin decreased the effect of cisplatin and anti-GD2 antibodies421. On the other hand, transfection with mutant JNK or overexpression of the JNK target, c-Jun, significantly protected SCLC cells from platinum compounds, but did not alter expression of genes involved in DNA repair, glutathione synthesis, or drug accumulation420.
11.4 βig-h3
Expression of βig-h3 (a secreted protein induced by transforming growth factor-β that may modulate cell adhesion and tumor formation, and that upregulates IGFBP3) was absent or reduced by more than two-fold in 35% of primary lung carcinomas relative to normal lung tissues422. Lung cancer cells with low βig-h3 expression were resistant to etoposide, and restoration of βig-h3 expression resulted in a significant increase in sensitivity to etoposide, and also significantly suppressed tumor cell growth and tumorigenicity422.
11.5 FUS1
In NSCLC cells deficient in FUS1, a tumor suppressor gene located in the human chromosome 3p21.3 region, restoration of function inhibited tumor cell growth, sensitized cells to cisplatin in vitro and in an orthotopic xenograft mouse model, down-regulated MDM2, and led to accumulation of p53 and activation of the Apaf-1-dependent apoptosis pathway423. Clinical trials are underway in NSCLC and SCLC to restore FUS1 by intravenous infusion of the FUS1 gene entrapped in nanoparticles.
11.6 Glycosylphosphatidylinositol-anchored molecule-like (GML) protein gene
The GML gene, which is induced by WT p53, may participate in apoptosis. GML gene mRNA was detected in 30% of resected NSCLC tumor samples, and tumors that were p53 IHC-negative or WT were significantly more likely to express GML than were p53 positive or mutated tumors 406. Cisplatin sensitivity in vitro and response to cisplatin-based chemotherapy clinically correlated with tumor expression of GML and with lack of IHC staining for p53406.
12.0 Apoptosis inhibitors
In some NSCLC cell lines, cisplatin424, etoposide425, radiation425, or Fas ligand425 induced normal activation of caspase-8424, -9424, 425 and -3424, 425, and normal release of cytochrome c424, 425, but did not demonstrate cleavage of nuclear substrates of caspase-3, such as PARP425, and did not demonstrate relocalization of caspase-3 from cytosol to nucleus425 or other aspects of apoptosis inducibility424. This suggests that the inhibition of apoptosis in NSCLC occurs downstream of mitochondrial changes and caspase activation, and upstream of nuclear events425. Several factors in the cell could augment resistance to anticancer therapies by specifically or non-specifically opposing apoptosis:
12.1 Antiapoptotic factors (Fig. 5)
12.1.1 Cyclooxygenase-2 (COX-2)
NSCLC cells have constitutively high expression of COX-2, cytosolic phospholipase A2 (cPLA2), and prostaglandin (PGE2), and many also express 12-lipoxygenase and 12(S)-hydroxyeicosatetraenoic acid426. Constitutive expression of COX-2 resulted in increased expression of the apoptosis inhibitor survivin427. Exposure to cisplatin induced COX-2 expression in p53 wild type NSCLC cells, but not in p53 mutant cell, while paclitaxel exposure induced COX-2 expression in both p53 wild type and mutant cells428. Many non-steroidal anti-inflammatory drugs (NSAIDs) inhibit COX-2, and NSAIDs may also have effects on lung cancer cell lines that are mediated by targets other than COX-2429.
In one study using human NSCLC cell lines, indomethacin, Sulindac, tolmetin and other NSAIDs significantly increased the cytotoxicity of anthracyclines, epipodophyllotoxins, and vincristine (independently of their COX inhibitory ability, and possibly through impact on MRP), but did not enhance efficacy of the other vinca alkaloids, various antimetabolites, alkylating agents, platinums, paclitaxel and camptothecin429. However, in other studies, the COX-2 inhibitor celecoxib significantly potentiated the effect of docetaxel against NSCLC cell lines430, 431 and xenografts431. Sulindac sulfide (a COX-1 and COX-2 inhibitor), sulindac sulfone (exisulind, a proapoptotic agent that does not inhibit COX), and nordihydroguaiaretic acid (a lipoxygenase inhibitor) each inhibited growth of NSCLC and SCLC cell lines, and were synergistic with paclitaxel, cisplatin, and 13-cis-retinoic acid426. The COX-2 inhibitor nimesulide inhibited proliferation and induced apoptosis in NSCLC cell lines (particularly in cell lines with high COX-2 expression), and potentiated the effect of docetaxel, cisplatin or etoposide432. The NSAID indomethacin substantially augmented the efficacy of vincristine in resistant NSCLC cells, and its ability to modulate efficacy varied with the chemotherapy agent used433. The COX-2 inhibitor NS-398 enhanced the efficacy of gemcitabine against a NSCLC cell line, and this potentiation was associated with up-regulation of the cyclin dependent kinase inhibitors p21WAF1 and p27KIP1 protein434. Growth hormone releasing hormone (GHRH) antagonists that inhibited growth of NSCLC xenografts also significantly decreased protein expression of COX-2, as well as decreasing expression of various other factors435.
From a prognostic perspective, COX-2 expression in NSCLC tumors has only a minor negative impact on survival, with the greatest negative impact seen in stage I patients in a meta-analysis436. However, it may increase resistance to chemotherapy in patients with advanced disease. NSCLC IHC expression of COX-2 correlated strongly with expression of the efflux pumps P-gp, MRP1 and BCRP437. Patients with advanced NSCLC receiving platinum-based chemotherapy had significantly worse survival if they had high baseline serum levels of the inflammatory marker C-reactive protein, and patients who had a reduction in serum C-reactive protein over the course of therapy had substantially better survival than did patients who did not have a decrease in serum C-reactive protein438. Similarly, in a phase II trial adding celecoxib to carboplatin plus paclitaxel in advanced NSCLC, responding patients had a greater drop in urinary excretion of PGE-M (a major metabolite of prostaglandin E(2) within one week of starting celecoxib compared to nonresponders, and very high initial urinary levels of PGE-M were associated with poor response to the chemotherapy439. In another trial in which celecoxib was combined with docetaxel in previously treated NSCLC patients, patients with the greatest reduction in urinary PGE-M had the longest survival440. In a randomized study combining the COX-2 inhibitor celecoxib with chemotherapy, COX-2 expression was not a negative prognostic factor for patients receiving celecoxib with chemotherapy, but it was a negative prognostic factor for those receiving chemotherapy alone441. In pharmacogenetic analyses, there was a trend towards better outcome in patients with NSCLC treated with vinorelbine if they possessed the COX-2 1195G genotype188. Overall, the data suggest that biomarkers related to COX-2 are associated with decreased chemotherapy efficacy in advanced NSCLC.
In clinical trials in which COX-2 inhibitors were added to chemotherapy, a few have suggested a beneficial impact of the COX-2 inhibitor, but most have not. In nonrandomized trials, patients with stage IB-IIIA NSCLC treated with preoperative carboplatin, paclitaxel plus celecoxib, had a response rate of 65%, which was somewhat better than expected with chemotherapy alone442, and in patients with platinum-refractory NSCLC treated with docetaxel plus celecoxib, the response rate of 24%, median time to progression of 5 months and median survival of 11 months were superior to outcomes typically seen with docetaxel alone443. However, in other nonrandomized trials, there was no indication of benefit from addition of celecoxib to carboplatin/paclitaxel439 or to docetaxel in chemonaive patients with advanced NSCLC444, from addition of celecoxib to docetaxel in previously treated NSCLC patients440, 445, 446, nor from addition of celecoxib to cisplatin-etoposide in patients with SCLC447.
In randomized trials, addition of celecoxib to carboplatin plus gemcitabine in advanced NSCLC improved overall survival and progression-free survival for patients whose tumors expressed COX-2, while having no significant impact on those whose tumors did not express COX-2441. Addition of the COX-2 inhibitor rofecoxib to gemcitabine did not significantly prolong survival but it did significantly improve response rate and quality-of-life448. Addition of celecoxib to irinotecan plus docetaxel vs gemcitabine in previously treated NSCLC patients led to a median survival of only 6.3 months in patients receiving celecoxib vs 9 months in patients receiving chemotherapy alone449.
Overall, both preclinical and clinical data suggest that COX-2 and related factors impact efficacy of some chemotherapy agents in NSCLC. However, COX-2 inhibitors have had little impact on chemotherapy efficacy. The data available suggest that any further trials of COX-2 inhibitors should be limited to patients whose tumors overexpress COX-2.
12.1.2 Bcl-2 and related proteins
12.1.2.1 Bcl-2
In sensitive NSCLC cells, cisplatin exposure resulted in activation of the mitochondrial death pathway, with activation of caspase-9 and downregulation of the antiapoptotic protein Bcl-2 through dephosphorylation, ubiquination and enhanced proteosomal degradation450. The downregulation of Bcl-2 in sensitive cells appeared to be mediated by cisplatin-induced generation of peroxide and other reactive oxygen species, and this Bcl-2 downregulation was prevented by the antioxidant enzymes catalase and glutathione peroxidase450. Bcl-2 downregulation was also prevented by nitric oxide, which induced Bcl-2 S-nitrosylation, thereby inhibiting its ubiquination and degradation451.
In some NSCLC451–454 and SCLC455, 456 cell lines, high expression of the antiapoptotic protein Bcl-2 was associated with increased resistance to cisplatin451–456, camptothecin453, doxorubicin453, 455, 456 and etoposide455, 456, and Bcl-2 was constitutively phosphorylated in a cisplatin-sensitive parental SCLC cell line, but not in the cisplatin-resistant variant457. In NSCLC cell lines, nicotine exposure resulted in increased phosphorylation of Bcl-2 and resistance to cisplatin plus etoposide458. However, some other NSCLC160 and SCLC457 cell lines demonstrated no relationship between Bcl-2 expression and resistance to doxorubicin160, vincristine160, etoposide160, 457, paclitaxel160, 457, 5-fluorouracil160, or carboplatin160.
In NSCLC cell lines453, 459–462 and orthotopic xenografts463 and in SCLC cell lines456, downregulation of Bcl-2 expression by siRNA459, 462, by an antisense oligonucleotide453, 456, 463, or by an aurora kinase inhibitor461 increased spontaneous apoptosis459 and reduced resistance to cisplatin453, 456, 459, 460, etoposide456, 460, 461, anthracyclines453, 456, 460, 461, camptothecin453, and vinorelbine463, but was paradoxically antagonistic with cisplatin, doxorubicin and etoposide in one SCLC cell line with low baseline bcl-2 expression456.
Taxanes may interact with Bcl-2 in a different manner than do other agents. Some NSCLC cell lines demonstrated no correlation between Bcl-2 expression and sensitivity to paclitaxel160, 457, and cells derived from NSCLC tumor samples demonstrated increased sensitivity to docetaxel in the presence of high Bcl-2 expression, rather than increased resistance165. In other NSCLC studies involving tumors heterotransplanted from patients into nude mice, all tumors that responded to paclitaxel were Bcl-2 positive at baseline, while only 43% of non-responding tumors were Bcl-2 positive (p = 0.02)134. Furthermore, unlike the situation with most other chemotherapy agents, Bcl-2 downregulation did not increase efficacy of docetaxel462. Docetaxel inactivated NSCLC cellular Bcl-2 by phosphorylation, while cisplatin exposure led to Bcl-2 dephosphorylation462.
Expression of Bcl-2 was seen in 5–37% of NSCLC tumors71, 165, 327, 464. Despite the frequent association between tumor cell Bcl-2 expression and resistance in lung cancer cell lines, Bcl-2 expression was associated with a significant improvement (rather than worsening) in NSCLC prognosis across different stages and histologies in a meta-analysis465 and in individual large studies466. In locally advanced195, 274 or advanced71, 214, 327, 403, 464 NSCLC and in limited and extensive SCLC340, tumor Bcl-2 expression by IHC did not correlate with response195, 214, 403, 464 or survival195, 274, 327, 340, 464, or was unexpectedly associated with significantly improved survival71 in patients treated with radiation plus vinorelbine +/− carboplatin195, radiation plus daily low dose cisplatin274, docetaxel plus vinorelbine464, vinorelbine alone403, etoposide or irinotecan +/− ifosfamide340, or a platinum combined with a vinca alkaloid214, taxane214, etoposide71, 214, irinotecan214, gemcitabine71, 327, mitomycin-C214 or mitomycin-C/ifosfamide71. However, in SCLC patients receiving cyclophosphamide, epirubicin, and etoposide or cyclophosphamide, epirubicin and vincristine alternating with carboplatin and etoposide, shorter survival was seen with high tumor Bcl-2 IHC expression in multivariate analysis307.
In patients receiving neoadjuvant etoposide-cisplatin, tumor Bcl-2 IHC expression decreased following treatment467, while serum Bcl-2 levels did not change with therapy in patients with advanced NSCLC receiving platinum-based chemotherapy, and bcl-2 serum levels did not correlate with survival68.
In a randomized trial in extensive SCLC of carboplatin plus etoposide +/− the bcl-2 antisense oligonucleotide oblimersen, the oblimersen did not alter response rates and was associated with worsening of failure-free survival and overall survival468, due in part to increased Myelosuppression and toxic deaths in the oblimersen arm.
Overall, despite substantial preclinical data suggesting that Bcl-2 is associated with resistance to chemotherapy, there is little indication that Bcl-2 expression is associated with worsening of outcome clinically. Across all stages high tumor Bcl-2 expression was associated with favorable survival, and most clinical studies have not found it to be a predictive factor negatively associated with response to chemotherapy.
12.1.2.2 Bcl-xL
Overexpression of the antiapoptotic protein Bcl-xL was found in NSCLC cell lines160, 469 and xenografts469 resistant to doxorubicin160, vincristine160, etoposide160, paclitaxel160, 5-fluorouracil160 and gemcitabine469, and Bcl-xL expression was up-regulated following exposure to gemcitabine in a gemcitabine-resistant cell line470. However, a cisplatin-resistant SCLC cell line with reduced apoptosis in response to cisplatin also had reduced Bcl-xL levels compared to the sensitive parent, rather than having increased levels457.
In resistant NSCLC cell lines, downregulation of Bcl-xL expression by siRNA470, 471, by an antisense oligonucleotide472, by an aurora kinase inhibitor461, or by a histone deacetylase inhibitor473, or Bcl-xL inhibition by a small molecule inhibitor474, augmented sensitivity to gemcitabine470, cisplatin471, 474, paclitaxel472, 474, etoposide461, 473, 474, doxorubicin461, 474, and vincristine472. In keeping with this, augmentation of expression of Bcl-xL in response to FGF-2 augmented chemoresistance in SCLC cells475.
Clinically, response of NSCLC to vinorelbine did not correlate with tumor Bcl-xL expression403. Overall, preclinical data suggest a possible role for Bcl-XL in lung cancer chemoresistance, but clinical data are limited.
12.1.2.3 Myeloid cell leukemia-1 (Mcl-1) protein
NSCLC cells express abundant amounts of the antiapoptotic Bcl-2 family member Mcl-1, and resected NSCLC tumors expressed elevated levels of Mcl-1 protein compared with normal adjacent lung tissue476. Lung cancer cells overexpressing Mcl-1 were less sensitive to apoptosis induced by cisplatin, etoposide, paclitaxel and gefitinib, and depletion of Mcl-1 levels by antisense Mcl-1 oligonucleotides induced apoptosis in NSCLC cells and sensitized cells to apoptosis induced by chemotherapy agents and radiation476. Epidermal growth factor (EGF) enhanced Mcl-1 protein levels in an ERK-dependent manner476.
The pan-Bcl-2 inhibitor GX15-070 is a small molecule agent that binds to anti-apoptotic Bcl-2 proteins and interferes with their ability to interact with pro-apoptotic proteins477. GX15-070 disrupted Mcl-1:Bak interactions, induced apoptosis, and was synergistic with cisplatin in NSCLC cells477.
12.1.2.4 Other Bcl-2 family members
In still other NSCLC cell lines, apoptosis was increased on exposure to etoposide, paclitaxel, doxorubicin or cisplatin if a transfected gene for the pro-apoptotic Bcl-2 family member BAK was turned on vs off478, and in heterotransplants of resected NSCLC tumors from patients into nude mice, there was a trend toward a higher response rate in tumors positive for Bax (another pro-apoptotic Bcl-2 family member)134. A cisplatin-resistant SCLC cell line also had a slight reduction in Bax levels457. However, overexpression (rather than under expression) of Bax has also been noted in resistant lines160. Bax expression was reported in 63–68% of NSCLC tumor samples464. In patients with advanced NSCLC treated with vinorelbine, response rate did not vary with tumor expression of Bad403, Bak403, Bid403 or Bax464. Furthermore, in patients with advanced NSCLC treated with cisplatin-gemcitabine, survival did not correlate with Bax expression327, and outcome in SCLC patients treated with a platinum combined with etoposide or irinotecan +/− ifosfamide did not correlate with tumor bax expression by IHC340,
12.1.3 Survivin
Survivin expression in SCLC cells was increased by exposure to cisplatin or by constitutive activation of Akt479. In resistant NSCLC cells480–482, SCLC cells479 and NSCLC xenografts483, transfection with an siRNA479, 481 or antisense oligonucleotide480, 482 to survivin caused apoptosis480, enhanced caspase-3 activity480, and enhanced efficacy of cisplatin479–481, 483, etoposide482 and paclitaxel481. Nicotine protected NSCLC cells from apoptosis induced by gemcitabine, cisplatin, and paclitaxel by mediating the recruitment of E2F1, which led to dissociation of the retinoblastoma tumor suppressor protein (Rb) from the survivin promoter, thereby leading to induction of XIAP and survivin484. In patients with NSCLC treated with cisplatin plus etoposide, survivin expression by in situ hybridization correlated significantly with lack of response and with shorter survival485.
12.1.4 Livin
The anti-apoptotic protein Livin (also called ML-IAP or KIAP) is expressed in a significant proportion of NSCLC tissue samples and cell lines, and Livin siRNA sensitized Livin-expressing NSCLC cells to various pro-apoptotic stimuli, such as UV-irradiation and etoposide486.
12.1.5 X-linked Inhibitor of Apoptosis Protein (XIAP) and Smac/DIABLO
XIAP suppresses apoptosis by binding to caspases-3, -7, and -9. Exposure of NSCLC cells to nicotine led to Akt-mediated upregulation of expression of XIAP and increased resistance to cisplatin plus etoposide, and this antiapoptotic effect of nicotine was blocked by inhibitors of mitochondrial anion channels, Akt or MAPK458. The apoptogenic protein Smac/DIABLO487, 488 (or small molecule Smac mimics487 or an IAP-binding peptide488) overcame the effect of XIAP, enhancing cisplatin-induced487 or etoposide-induced488 apoptosis, with an increase in caspase-3 activity487. However, Smac deficiency does not appear to play a major role in resistance488. FGF-2 increased expression of XIAP and Bcl-xL and triggered resistance to etoposide in SCLC cells in a process mediated through the formation of a complex consisting of B-Raf, PKCepsilon and S6K2475.
12.1.6 Inhibitor of Apoptosis Proteins (IAPs)
In NSCLC cells (unlike SCLC cells), the apoptotic process was blocked downstream of caspase activation489. NSCLC cell lines had a stronger cIAP-2 expression than SCLC cell lines, while the SCLC cell lines had a higher level of XIAP protein489. However, following exposure to etoposide or radiation, expression of cIAP-1, cIAP-2, and XIAP did not appear to be sufficient to explain differences in sensitivity between NSCLC and SCLC cell lines489.
In NSCLC cell lines, exposure to gemcitabine decreased expression of IκB-α protein, thereby leading to increased activity of NF-κB, which in turn led to increased expression of the caspase inhibitor IAP-1 490. Suppression of NF-κB activation blocked the increase of IAP-1 protein and potentiated the action of gemcitabine, while overexpression of IAP-1 restored resistance to gemcitabine490.
12.1.7 Telomeres and Telomerase
Many cancers have increased telomerase expression. This permits them to maintain telomeres, and this in turn permits ongoing propagation without apoptosis being triggered in response to telomere shortening. In NSCLC cells, inhibition of telomerase led to increased baseline apoptosis and enhanced induction of apoptosis by cisplatin, docetaxel and etoposide491, and a telomerase-specific oncolytic adenovirus OBP-301 enhanced the effect of gemcitabine492 and docetaxel493 in NSCLC xenografts. A histone deacetylase inhibitor that inhibited expression of mRNA for human telomerase reverse transcriptase (hTERT) also inhibited growth of SCLC cells that were resistant to etoposide, irinotecan and cisplatin494. Four of four NSCLC clinical samples expressed flap endonuclease I (FEN1), a protein involved in DNA repair and in preservation of telomere stability495. In multivariate analyses, serum hTERT levels were an independent predictor of short time to progression and overall survival in NSCLC patients treated with cisplatin plus docetaxel496, while high tumor expression of RAP1 (which negatively regulates telomere length) was associated with improved survival in patients undergoing NSCLC resection497, and tumor hTERT gene amplification498 and high tumor telomerase activity499 were associated with worse recurrence-free survival. However, results have not been consistent across all studies: in other studies of early stage NSCLC patients treated with surgery alone, high tumor hTERT mRNA expression500 was associated with improved 5-year survival, and telomere shortening was associated with worse outcome501. The telomerase antisense compound GRN163L is currently undergoing early clinical trials in combination with chemotherapy in advanced NSCLC502.
12.1.8 Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Decoy receptor 1 (DcR1) and 2 (DcR2)
The TRAIL receptors DcR1503 and DcR2504 suppress TRAIL-induced apoptosis and their overexpression conferred resistance to doxorubicin and etoposide in lung cancer cell lines503, 504, while their silencing enhanced chemotherapy-induced apoptosis504.
12.2.0 Growth Factor Receptors
12.2.1 Epidermal growth factor receptor (EGFR)
In some NSCLC cell lines505, 506 or resected tumors tested in vitro for chemosensitivity243, higher EGFR expression correlated with increased resistance to cisplatin243, 505, doxorubicin505, etoposide505, vinorelbine506, paclitaxel506, camptothecin506, and 5-fluorouracil506. However, in other NSCLC cell lines323 or resected NSCLC tumor samples6, EGFR expression did not correlate with cisplatin resistance323, or EGFR positive tumors were actually less likely to have extreme resistance to platinums than were EGFR negative tumors6. In NSCLC heterotransplants into nude mice, there was a statistically insignificant trend toward a lower response rate to paclitaxel in EGFR-positive tumors134.
EGFR down-regulation by siRNA507 or inhibition by tyrosine kinase inhibitors (TKIs)198, 505, 508–513 or by the anti-EGFR monoclonal antibody cetuximab514, 515 increased sensitivity of NSCLC cell lines198, 505, 507–511, 513 or xenografts512, 514, 515 to platinums505, 507, 511, 512, 514, 515, doxorubicin505, 507, etoposide505, taxanes198, 507, 508, 511–515, or pemetrexed509, 510 and did not alter efficacy of gemcitabine in one study512, while it did increase gemcitabine efficacy in another514. The addition of the EGFR TKI erlotinib to pemetrexed inhibited the increase in EGFR phosphorylation509 and the induction of an EGFR-mediated activation of the PI3-knase/AKT pathway510 seen when cells were treated with pemetrexed alone509. Erlotinib also significantly reduced the expression and activity of the pemetrexed target thymidylate synthase509. The presence of P-gp overexpression did not prevent the EGFR TKI gefitinib from reversing resistance to paclitaxel198.
EGFR TKI augmentation of chemotherapy efficacy was schedule-dependent. For docetaxel, the optimum schedule was docetaxel followed by the EGFR TKI gefitinib508 or erlotinib513. Similarly, with pemetrexed, synergism was seen in NSCLC cell lines when erlotinib was administered concurrently with and/or following pemetrexed509, 510, while antagonism (associated with erlotinib-induced G1 blockade) was seen if erlotinib preceded pemetrexed510 or docetaxel513. In one series of NSCLC cell lines, gefitinib antagonized the effect of cisplatin in 13 of 15 lines tested, and was also antagonistic to cisplatin combined with paclitaxel516. The antagonism appeared to possibly be related to a gefitinib-induced reduction in cisplatin uptake at higher cisplatin doses, and was not seen if the gefitinib was delayed until 24 hours after the cisplatin516.
The synergism (or antagonism516) between an EGFR TKI and pemetrexed509, 510, cisplatin511, 516 or paclitaxel511 did not vary with EGFR mutation status509–511 or with sensitivity to erlotinib alone510, whereas cetuximab improved efficacy of cisplatin or paclitaxel against NSCLC xenografts only in models that were sensitive to cetuximab alone515. In some studies, the sensitization to chemotherapy by EGFR inhibitors was seen only in cell lines with high EGFR expression, and not in lines with low EGFR expression505. In other studies, the cell lines that were most likely to display synergism between the EGFR TKI gefitinib and cisplatin or paclitaxel were those that exhibited an increase in phospho-EGFR (pEGFR) following exposure to chemotherapy alone, while cell lines that showed no change or a decrease in pEGFR following chemotherapy exposure were more likely to exhibit an antagonistic or additive interaction between gefitinib and the chemotherapy511.
Approximately 49–54% of NSCLC tumor samples express EGFR517. In a NSCLC meta-analysis, EGFR expression was significantly associated with poor survival when EGFR expression was assessed by IHC517. However, when EGFR expression was assessed by Northern blot, PCR or ligand competition methods, there was no correlation with survival517.
In patients with locally advanced195 or advanced518–520 NSCLC undergoing treatment with chemoradiotherapy195 or with front-line chemotherapy518–520, response rate195, 518–520, progression-free survival519 and overall survival195, 519 did not correlate with EGFR IHC expression195, 518, 519 or with EGFR gene copy number by FISH519, 520. While presence of EGFR mutations did not correlate with response to chemotherapy or time to progression in one NSCLC study521, patients with advanced NSCLC treated with chemotherapy in another study did survive significantly longer if their tumor had an EGFR mutation rather than being wild type522, and in still another study, among patients with EGFR gene mutations, response to chemotherapy was observed only in those with exon 19 deletion518.
In advanced NSCLC, addition of gefitinib to gemcitabine plus cisplatin199 or to paclitaxel plus carboplatin200 did not improve survival, time to progression, or response rate, and erlotinib added to cisplatin and gemcitabine also failed to improve outcome523, while erlotinib added to carboplatin plus paclitaxel improved outcome only in patients who had never smoked524.
Overall, preclinical studies suggest that EGFR expression may increase resistance to chemotherapy and that antagonizing EGFR may reduce resistance to chemotherapy, with the interaction between chemotherapy and EGFR inhibitors being schedule-dependent. However, clinical studies suggest that EGFR inhibitors generally do not potentiate the effect of chemotherapy, and that EGFR expression has little impact on resistance to chemotherapy, but tumors with EGFR mutations (particularly exon 19 deletions) have increased sensitivity to chemotherapy.
12.2.2 HER-2/neu (erbB-2)
HER-2/neu expression is present more frequently in NSCLC cell lines than in SCLC (where it is present infrequently)525. In NSCLC cell lines, high expression of glycoprotein p185neu (the product of HER-2/neu gene expression, a membrane-bound receptor with tyrosine kinase activity)372, 526, 527 or high HER-2/neu mRNA expression528 correlated with resistance to etoposide372, 526–528, doxorubicin372, 526, 528, cisplatin372, 526–528, and other agents528. High p185neu expression also correlated with low S-phase fraction372, and long doubling time372. In NSCLC heterotransplants in nude mice, resistance to paclitaxel correlated strongly with HER-2/neu expression134. High HER-2/neu expression was not associated with resistance to gemcitabine in NSCLC cell lines, and the combination of gemcitabine plus cisplatin had greater cytotoxicity against the high- than the low-p185neu expressors527. High HER-2/neu expression (but not EGFR expression) correlated with nucleotide excision repair pathway activity and with cisplatin resistance323, and HER-2/neu depletion down-regulated DNA repair mechanism529.
Her-2/neu depletion529 or inhibition by a TKI526, 530 or by the monoclonal antibody trastuzumab525 increased sensitivity of NSCLC cells to cisplatin525, 526, 529, 530, gemcitabine525, paclitaxel525, vinorelbine525, doxorubicin526, 530 and etoposide526, 530 in Her-2/neu positive NSCLC cell lines.
In a meta-analysis, overexpression of Her-2/neu appeared to be a potential marker of poor prognosis for survival in NSCLC531. In patients with advanced NSCLC, response rate to front-line chemotherapy did not correlate with HER2 gene copy number by FISH518. Similarly, Her-2/neu IHC expression did not correlate significantly with response to induction or neoadjuvant chemotherapy195, 397, 399, 532, 533 or with survival195, 399, 532, 533 in patients with stage II397 or III195, 397, 399, 532, 533 NSCLC treated with a vinca alkaloid +/− cisplatin plus radiation195, 399, neoadjuvant cisplatin-vinorelbine397 or neoadjuvant cisplatin plus etoposide (followed by postoperative radiotherapy plus either cisplatin, etoposide532 or carboplatin, vindesine533). However, there was a nonsignificant trend towards chemoresistance in tumors that were Her-2/neu positive in two studies397, 533, and in one of these two studies, chemoresistance was observed statistically significantly more often when one or both of Her-2/neu and p53 were positive397. There was also a trend to a higher proportion of tumors being positive after therapy vs pre therapy in one study, suggesting induction of Her-2/neu expression or else selection of positive tumor cells533.
Overall, preclinical data suggest an association between Her-2/neu expression and chemoresistance in NSCLC, while clinical data are at best equivocal.
12.2.3 Insulin-like growth factor-1 receptor (IGF-1R)
In a NSCLC cell line and in a NSCLC xenograft model, inhibition of IGF-1R significantly enhanced sensitivity to cisplatin and increased apoptosis534. Similarly, in SCLC cell lines, IGF-1R was a potent growth factor and inhibited etoposide- and carboplatin-induced apoptosis through activation of the PI3K/Akt pathway535. Clinically in a randomized phase II trial in advanced NSCLC, addition of the anti-IGF-1R antibody CP-751,871 to paclitaxel plus carboplatin was associated with an increase in response rate from 42% with chemotherapy alone to 54% with the addition of the IGF-1R antagonist, with the greatest apparent benefit being seen in squamous cell lung cancer536. Response of squamous cell carcinoma to single agent CP-751,871 was also seen in 2 patients536.
12.2.4 β(1)-integrins, extracellular matrix and cell adhesion
Interaction of cells with extracellular matrix (ECM) proteins such as fibronectin (FN) initiates clustering of integrin receptors in the cell membrane, leading to activation of signaling pathways regulating survival, proliferation, differentiation, adhesion, and migration537. Cisplatin, paclitaxel, mitomycin-C or radiation stimulated clustering of β(1) integrins in NSCLC cells only if the cells were attached to FN, and attachment to FN led to a significant reduction in sensitivity to these agents537.
Attachment of SCLC cells to ECM485, 538, 539, or stromal cells539 or the ECM components FN540, laminin540 or collagen IV540 conferred resistance to apoptosis induction by etoposide485, 538–541, cisplatin540, doxorubicin540, cyclophosphamide541 and radiation485, 541. SCLC cellular attachment was also associated with morphological changes, with down-regulation of neuroendocrine markers, and with up-regulation of several factors, including epithelial differentiation markers (cyclin D1 and endothelin), and cell adhesion molecules (CD 44 and integrin subunits α2, β3 and β4) 541. Transfection of SCLC cells with the gene for osteopontin (a hypoxia-associated cytokine that plays a role in cell adhesion and binds to integrin-α-V / integrin-β-3) reduced sensitivity to cisplatin-induced apoptosis542. Similarly, transfection with hyalouronan/CD44 (a receptor for hyaluronate, osteopontin, collagen and matrix metalloproteinases that plays a role in cell adhesion) increased cisplatin resistance of NSCLC cells cultured on hyaluronate-coated plates by inducing increased MRP2 expression543.
SCLC cells also express high levels of CXCR4 receptors for the chemokine stromal-cell-derived factor-1 (SDF-1/CXCL12)539. CXCL12-induced integrin activation resulted in an increased adhesion of SCLC cells to fibronectin and collagen, mediated by α2, α4, α5, and β1 integrins along with CXCR4 activation539. CXCR4 inhibitors decreased the protection from etoposide-induced apoptosis afforded by stromal cells539.
Cell attachment was also associated with augmented activation of phosphoinositide-3-OH kinase (PI3K)538, Akt541, MAP kinase541, other protein tyrosine kinases 540, Bad protein541 and nuclear factor-κB (NF-κB)541. These adhesion-related events led to suppression of caspase-3 activation and apoptosis538, and prevention of etoposide- and radiation-induced G2/M cell cycle arrest (by blocking up-regulation of p21Cip1/WAF1 and p27Kip1 and by down-regulation of cyclins E, A and B)538. The ability of adhesion to ECM to block therapy effects was abrogated by PI3K inhibition538. Cultivation of adherent SCLC sublines on uncoated surfaces reversed their adherent phenotype immediately, and Akt activity reverted to low levels541. Similarly, conditioned medium from primary tumor fibroblasts decreased efficacy of paclitaxel (but not of cisplatin) against NSCLC cells, and this resistance appeared to be mediated by activation of ERK 1/2 and Akt544.
In patients with advanced NSCLC treated with cisplatin/paclitaxel +/− bevacizumab, high vs low baseline plasma levels of the integrin ligand intercellular adhesion molecule (ICAM) were associated with significantly lower response rates and shorter overall survival time, and beneficial impact of bevacizumab on survival was greatest for patients with low ICAM levels70. Overexpression of matrix metalloproteinase-7 (MMP-7, matrilysin) (which helps maintain ECM homeostasis and stabilizes cell-matrix interactions) in patients with advanced NSCLC treated with platinum regimens was associated with a significant reduction in response rates and survival, particularly in patients with lung adenocarcinomas545. Matrilysin exposure also increased resistance of a NSCLC cell line to cisplatin546. For patients with advanced NSCLC on both arms of a randomized trial of carboplatin/paclitaxel +/− tiripazamine, progression-free and overall survival was significantly longer and response probability was higher in patients with low vs high plasma osteopontin levels69. Patient outcome and plasma osteopontin levels did not correlate with tumor osteopontin levels69.
Overall, available preclinical data suggest that adherence and integrins are important in resistance, and limited clinical data support this.
12.2.5 Other receptors
In NSCLC orthotopic xenografts, cells were positive for growth hormone releasing hormone (GHRH) receptors and peptide, and inhibition of GHRH decreased tumor growth, significantly decreased protein expression of K-Ras, COX-2 and pAkt, and was synergistic with docetaxel435. In SCLC patients treated with cisplatin plus etoposide, time to progression and survival was longer for patients positive for c-kit by IHC than for those negative547. Imatinib inhibited the growth of a cisplatin-resistant NSCLC cell line, and synergistically augmented tumor cell killing by cisplatin, probably through its ability to inhibit phosphorylation of platelet derived growth factor receptor (PDGFR)α548. By IHC of clinical samples, 89% of squamous lung carcinomas, 100% of lung adenocarcinomas, and 100% of small cell lung cancers expressed PDGFRα548.
12.3 Growth factors
Hepatocyte growth factor (HGF) (the ligand for the c-Met receptor) is frequently overexpressed in NSCLC, and addition of HGF to NSCLC cell lines increased cisplatin resistance549. This augmentation of cisplatin resistance appeared to be mediated by suppression of apoptosis-inducing factor (AIF) via the HGF receptor’s downstream effector, focal adhesion kinase (FAK)549. Fibroblast growth factors (FGF)-2 increased the expression of the antiapoptotic proteins, XIAP and Bcl-X(L), and rendered SCLC cells resistant to etoposide475. These effects were mediated through the formation of a specific multiprotein complex comprising B-Raf, PKCepsilon and S6K2475. In patients with advanced NSCLC treated with platinum-based regimens, expression in blood of PC cell-derived growth factor (PCDGF) (a growth factor originally derived from the PC teratoma cell line) was somewhat higher in chemoresistant patients than in chemosensitive patients, and tumors expressing PCDGF by IHC were also more resistant to platinum-based chemotherapy222.
12.4.0 Signaling pathways
12.4.1 Phosphatase and tensin homolog (PTEN)/Phosphoinositide 3-kinase (PI3K)/ Protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway
The PI3K/Akt pathway is important in many cellular functions, and activating mutations of PI3K are present in many malignancies. The tumor suppressor gene PTEN inhibits PI3K, but PTEN may be mutated, deleted or hypermethylated in cancers, or may be unable to silence PI3K if the PI3K gene has an activating mutation.
12.4.1.1 PTEN/PI3K
In NSCLC cell lines, SiRNA inactivation of PTEN increased resistance to paclitaxel163. Similarly, hypoxia activated the PI3K/Akt pathway and induced resistance to UV light and etoposide51. This hypoxia-induced resistance was reversed by blocking activation of PI3K/Akt51. In SCLC cell lines, β1 integrin (expressed following adhesion to ECM538) and components of important SCLC autocrine loops (stem cell factor and insulin-like growth factor550) potently activated PI3K538, 550, and activated PI3K prevented etoposideinduced caspase-3 activation and subsequent apoptosis538.
Inhibition of PI3K sensitized SCLC cell lines to etoposide550, and sensitized NSCLC cell lines51, 454, 551–553 or xenografts554 to apoptosis induced by paclitaxel551, 552, 554, docetaxel454, cisplatin552, etoposide51, 552, 553, gemcitabine552, trastuzumab552 and radiation552.
12.4.1.2 Akt
Akt is a key factor in the suppression of anoikis and in the modulation of chemotherapy-induced apoptosis555, and a high proportion of NSCLC cell lines have PI3K-dependent constitutive activation of Akt1552. Akt1 overexpression556, gene amplification556 or constitutive activation552, 557 rendered NSCLC cell lines resistant to cisplatin556, 557, doxorubicin557, mitoxantrone557, paclitaxel557, etoposide552, 5-fluorouracil557 and radiation552. Akt modulated the apoptotic threshold of several apoptotic pathways towards increasing the threshold of onset557. Nicotine exposure led to increased Akt-mediated resistance to cisplatin/etoposide-induced apoptosis in NSCLC cells458. Following exposure to cisplatin, increased expression of Bcl-x(l) and a delayed onset of the p53 pathway was noted in resistant Akt1-expressing cells557.
Akt activity551, 552 and phosphorylation552 were decreased by PI3K inhibition, and inhibition of Akt by PI3K inhibitors sensitized NSCLC cell lines to a variety of chemotherapy agents and radiation, as outlined above in the PI3K section. The augmentation by PI3K inhibition of chemotherapy-induced apoptosis was seen predominantly in cells with high Akt levels552. The effect of PI3K inhibitors on etoposide efficacy in SCLC cell lines was reversed by expression of constitutively activated Akt550. Inhibition of Akt by transfection with dominant negative Akt552, 556, by siRNA558 or by growth hormone releasing hormone antagonists435 also decreased NSCLC cell resistance to etoposide552, cisplatin552, 556, 558, docetaxel435 and paclitaxel552, and significantly decreased ERK activity558. The c-Src antagonist dasatinib decreased expression of Akt in SCLC cells and sensitized them to the anthracycline amrubicin559.
Adhesion of SCLC cells to ECM components increased Akt activation541, 560 and decreased sensitivity to a variety of chemotherapy agents541. Inhibitors of the PI3K/Akt/mTOR pathway abrogated ECM-mediated survival560, and cultivation of cells on uncoated surfaces resulted in reversion of Akt activity to low levels541.
In patients with advanced NSCLC, response rate to front-line chemotherapy did not correlate with p-Akt status by IHC518, 521.
12.4.1.3 mTOR
In NSCLC cell lines, Akt1-induced resistance to cisplatin appeared to be mediated through mTOR556, and the mTOR inhibitor CCI-779 enhanced the effect of cisplatin in cisplatin resistant cell lines561.
12.4.1.4 P70 S6 kinase
Activation of p70 S6 kinase by mTOR results in phosphorylation of the S6 ribosomal protein, and this in turn induces protein synthesis by ribosomes. In SCLC cells, levels of phosphorylated p70S6K and S6 (but not total p70S6K or S6) were elevated in a cisplatin-resistant variant compared to the sensitive parent562. Cell exposure to cisplatin activated p70S6K, and downregulation of p70S6K or its inhibition by rapamycin augmented cisplatin-induced apoptosis562.
Overall, there is substantial preclinical evidence that the PTEN/PI3K/Akt/mTOR pathway can render lung cancer cells resistant to several chemotherapy agents, but clinical data remain very limited.
12.4.2 K-ras
In a panel of 20 NSCLC cell lines, presence of K-ras mutation did not correlate significantly with resistance to doxorubicin, cisplatin, melphalan, or carmustine, and mutant lines were slightly more sensitive to etoposide and mitomycin-C than were K-ras wild type lines528. In lung cancer cell lines with both mutant and wild-type K-ras, the farnesyl transferase inhibitor lonafarnib was synergistic with paclitaxel when administered after the paclitaxel, but was antagonistic when administered before paclitaxel563. Lonafarnib prevents post-translational modifications of K-ras protein that are required for localization of K-ras in membrane, where it can participate in signaling. RasGTPase-activating protein (RasGAP) (the main regulator of Ras GTPase family members) was cleaved at low caspase activity into an N-terminal fragment that triggered potent anti-apoptotic signals via activation of the Ras/PI3K/Akt pathway564. When caspase activity was increased, RasGAP fragment N was further processed into two fragments that effectively potentiated apoptosis564. Hence, whether RasGAP was anti-apoptotic or pro-apoptotic depended on the degree of caspase activation. SCLC cell lines (which were sensitive to therapy) had lower RasGAP expression levels and higher spontaneous cleavage than did more resistant NSCLC cell lines564. Constitutive formation of RasGAP fragment N could potentially contribute to primary resistance of NSCLC and to acquired resistance in SCLC564.
K-ras mutations were noted in approximately 21% of patients with NSCLC522 and in 26% of lung adenocarcinomas565. In a meta-analysis, there was a significantly worse survival for patients with NSCLC whose tumors had K-ras mutations than with K-ras wild type566, and in a phase II trial of paclitaxel in NSCLC, K-ras mutations appeared to correlate with resistance567. However, in patients with advanced NSCLC treated with a mesna, ifosfamide, carboplatin, etoposide combination565 or with carboplatin plus paclitaxel522, K-ras mutations did not correlate with response or survival522, 565, although there was a trend towards a lower response rate in patients with K-ras mutations in one trial of advanced NSCLC patients treated with platinum-based chemotherapy568. In patients with resected NSCLC, adjuvant chemotherapy with cisplatin plus vinorelbine was associated with significant improvement in survival in patients whose tumors were K-ras wild type, with a hazard ratio of 0.69, while in patients with K-ras mutant tumors, chemotherapy did not significantly improve survival (hazard ratio = 0.95)569. However, in multivariate analysis in this study, there was no significant interaction between the impact of chemotherapy and the presence of a K-ras mutation (p=0.27), and K-ras mutation was not found to be a prognostic factor569. In patients treated with adjuvant cisplatin plus etoposide plus radiotherapy after NSCLC resection, there was also a trend towards worse outcome in patients with K-ras mutant tumors, while K-ras mutational status was not associated with outcome in patients treated with postoperative radiotherapy alone570. Survival was significantly shorter in advanced NSCLC patients with K-ras mutations (but not in K-ras wild type patients) if the EGFR TKI erlotinib was added to chemotherapy compared to chemotherapy alone522.
In NSCLC patients with taxane-refractory NSCLC who received the farnesyltransferase inhibitor lonafarnib (which inhibits mutant K-ras function) combined with paclitaxel, 10% of patients experienced a response and 38% had stable disease571, suggesting that paclitaxel efficacy may have been modestly augmented by the lonafarnib.
Overall, available data suggest a possible role for K-ras mutations in lung cancer resistance to chemotherapy, but the data are not conclusive. Available data do suggest a harmful effect of adding an EGFR TKI to chemotherapy in patients with mutant K-ras.
12.4.3 Extracellular signal-regulated kinases 1 and 2 (ERK1/2)
ERK1 and ERK2 are examples of mitogen-activated protein kinases (MAPKs), and are downstream from Ras, Raf and MEK in the Ras pathway. Some etoposide-resistant SCLC cell lines had markedly increased MAPK activity541. Hypoxia activated the ERK pathway and increased resistance of NSCLC lines to UV light or etoposide, and blocking the ERK pathway reversed this hypoxia-induced resistance51. Inhibition of ERK in NSCLC cell lines with constitutive ERK1/2 activity also potentiated paclitaxel-induced apoptosis (suggesting that constitutive ERK1/2 activity in NSCLC cells promotes cell survival and chemotherapy resistance), while MEK inhibitors had no effect on apoptosis572. In taxane-sensitive cells, taxanes inhibited MAPK activity and p34cdc2 kinase activity, and this kinase inhibition in turn played a role in altering microtubule dynamics by enhancing the interaction of microtubule-associated proteins with α and β tubulins573. This inhibition of MAPK was not seen in a taxaneresistant SCLC cell line, suggesting an alteration in the MAPK cascade in resistant cells573.
However, in other studies, inhibition of ERK reduced sensitivity of NSCLC cell lines to paclitaxel574, and exposure of NSCLC cells to cisplatin led to a marked increase in ERK activity, while pretreatment of these cells with a selective MEK inhibitor reduced the level of cisplatin-induced apoptosis, implying that cisplatin-induced MEK/ERK activation mediates apoptotic cell death558.
In clinical studies, response rates and time to progression for platinum-based and paclitaxel-based regimens in advanced NSCLC were not influenced by p-Erk IHC expression521. While all responders to gemcitabine exhibited p-Erk IHC expression, time to progression for gemcitabine was not affected by p-Erk expression521. Overall, the role (if any) of ERK/MAPK in chemotherapy resistance is unclear.
12.4.4 Mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP1)
MKP1 negatively regulates MAPK signaling575, ERK576, N-terminal-c-Jun kinase (JNK) 419, 576 and p38576, and may play a role in cell survival in response to stressful stimuli575. MKP1 was expressed in a high proportion of NSCLC tumor samples419, 576, and was expressed more commonly in NSCLC than in SCLC cell lines576. Cisplatin induced MKP1 in human lung cancer cell lines, and overexpression of MKP1 rendered human lung cancer cells resistant to cisplatin575. Cisplatin-induced cell death was inhibited by blocking the apoptosis induction mediator JNK (which is negatively regulated by MPK1), while MKP1 inhibition sensitized lung cancer cell lines to cisplatin419, 575. This sensitization was associated with a marked increase in cisplatin-induced activation of JNK and p38419. Similarly, inhibition of MKP1 in NSCLC xenografts decreased the rate of tumor growth and increased sensitivity to cisplatin419.
12.4.5 Protein kinase C (PKC)
Impact of PKC on chemotherapy resistance may depend on the PKC isoform present and on the agents used. In SCLC cell lines, paclitaxel resistance was not associated with alteration of expression of any of the PKC isozymes, while cisplatin-resistant and etoposide-resistant SCLC cells had significant reduction (rather than increase) in PKC activity577. A cisplatin-resistant SCLC variant had a decrease in PKC-α and -β expression and no change in PKC-zeta or-iota577, while an etoposide-resistant SCLC variant had decreased PKC β expression577. On the other hand, PKC-delta expression was increased in cisplatin- and etoposide-resistant SCLC variants577, and PKC-epsilon expression was associated with resistance to etoposide and doxorubicin, with blockage of apoptosis578. PKC-epsilon formed a multiprotein complex with B-raf and S6K2 that increased expression of the antiapoptotic proteins XIAP and Bcl-xL and increased resistance to etoposide in SCLC cells475.
Expression of PKC-epsilon was also associated with chemotherapy resistance in NSCLC cell lines578, and down-regulation of PKC-eta reduced resistance to vincristine and paclitaxel and augmented vincristine- and paclitaxel-induced caspase-3 activity472, while expression of other PKC isoforms did not correlate with resistance in NSCLC cell lines578. Although PKC-α antisense augmented sensitivity to carboplatin, vincristine, doxorubicin and other agents in NSCLC cell lines579, addition of PKC-α inhibitors to cisplatin plus gemcitabine in phase II trials in NSCLC yielded response rates and survival times similar to what one would expect with the chemotherapy alone580, 581.
Overall, preclinical data suggest that PKC-epsilon expression confers resistance in both SCLC and NSCLC cell lines, resistance may be associated with PKC-delta expression in SCLC and with PKC-eta in NSCLC, while PKC-α and -β expression may be associated with chemotherapy sensitivity in SCLC cell lines.
12.5.6 Caveolin-1
Caveolin is a principal component of caveolae membranes, and interaction of the caveolin scaffolding domain with signaling molecules can functionally inhibit the activity of these molecules582. Caveolin 1 expression is downregulated in various cancer cell lines, but its expression is substantially increased in several drug-resistant cell lines117. Significantly increased expression of caveolin-1117, 582, caveolae organelles582, and cholesterol117 (the major lipid component of caveolae) (but not of caveolin-2582) was reported in NSCLC cell lines that were resistant to paclitaxel582 and etoposide117, and paclitaxel exposure led to upregulation of caveolin-1 expression in paclitaxel-sensitive NSCLC cells582.
In patients with advanced NSCLC treated with gemcitabine plus cisplatin or epirubicin or with gemcitabine alone, caveolin-1 IHC expression was found in 16.4% of patients583. Patients with caveolin-1 expression had a significantly lower response rate and significantly shorter progression-free survival and overall survival compared to those without caveolin-1 expression583.
12.4.7 Other signaling pathway components
Gangliosides (cell membrane components composed of glycosphingolipid with one or more sialic acids linked on the sugar chain) modulate cell signal transduction. A cisplatin-resistant SCLC cell line showed a simplified ganglioside pattern, expressing only gangliosides GM3 and GM2, and had a marked increase in ganglioside GM3 compared to the parent line584. Annexins are proteins that undergo calcium-dependent binding to negatively charged phospholipids, and annexin IV plays a role in ion channel regulation, exocytosis and calcium-dependent signal transduction585. In a paclitaxel-resistant NSCLC cell line, annexin IV (but not annexin II) was overexpressed586. Short-term treatment of NSCLC cells with paclitaxel resulted in induction of annexin IV expression, and transfection of annexin IV cDNA into sensitive NSCLC cells augmented their resistance to paclitaxel586. Clinically, amplification and overexpression of c-myc was associated with poor prognosis in SCLC587 and amplification of N-myc in SCLC cell lines was associated with increased resistance to cisplatin588. Inhibition of c-myc increased sensitivity of a cisplatin-resistant SCLC cell line to cisplatin, but not to doxorubicin or vincristine589.
12.5.0 Stress proteins and chaperones
12.5.1 Heat shock proteins (HSPs)
HSPs are chaperones that protect cellular proteins from degradation under hostile intracellular conditions. Hsp90 inhibitors, such as 17-allylamino-17-demethoxygeldanamycin (17-AAG), induced the degradation of Hsp90-interacting proteins (including mutant EGFR), and significantly potentiated the efficacy of paclitaxel against NSCLC xenografts590. In a panel of lung carcinoma cell lines, siRNA to HSP72 did not sensitize cells to cisplatin, etoposide or ionizing radiation591, but in NSCLC patients receiving front-line single agent vinorelbine, there was a trend towards Hsp27-positive tumors being more likely to show progression (p=0.068)403.
12.5.2 Clusterin
Clusterin is a stress-associated cytoprotective chaperone protein that is up-regulated by various apoptotic triggers in many cancers and confers treatment resistance when overexpressed592. Clusterin was expressed by IHC in more than 80% of human NSCLCs592. Clusterin antisense or siRNA significantly enhanced sensitivity of NSCLC cell lines to paclitaxel, and clusterin antisense enhanced the efficacy of paclitaxel and gemcitabine against NSCLC xenografts592.
12.5.3 Nrf2/heme oxygenase-1 (HO-1)
HO-1 is a molecular chaperone protein that plays an important role in tumor cell growth through anti-oxidative and anti-apoptotic effects, and HO-1 expression was mediated by transcriptional activation by the transcription factor Nrf2593. Inhibition of HO-1 by siRNA or by MAPK inhibitors increased apoptosis, degradation of procaspase-3, cisplatin cytotoxicity and cisplatin-induced production of reactive oxygen species in a resistant NSCLC cell line that expressed high levels of HO-1, suggesting that HO-1 may modulate the chemosensitivity of NSCLC cells to cisplatin through the MAPK-Nrf2 pathway593. In another study, treatment of lung cancer cells with cisplatin augmented HO-1 expression, inhibitors of EGFR or of Akt blocked this augmentation of HO-1 expression, and HO-1 inhibition augmented cisplatin cytotoxicity594.
Decrease in Keap1 activity (eg, due to mutation) resulted in constitutive activation of Nrf2 in lung cancer cell lines595, 596. Increased expression of Nrf2 led to resistance to cisplatin595, 597, carboplatin596, doxorubicin597 and etoposide596, 597 by causing up-regulation of expression of cytoprotective genes encoding multidrug resistance pumps, phase II detoxifying enzymes, and antioxidative stress enzymes/proteins595, 596.
12.5.4 Glucose-regulated protein78 (GRP78)
In NSCLC cell lines, induction of increased expression of GRP78 (a member of the endoplasmic reticulum chaperone family) increased resistance to etoposide598.
13.0 Cell cycling
Factors related to cell cycling may also contribute to resistance (Fig. 5).
13.1 Cell cycle phase
In NSCLC cells, maximal sensitivity to cisplatin occurred with exposure during the G1 phase of the cell cycle, and least effect was seen with exposure during G2599. Exposure to cisplatin led to a prolonged S-phase and accumulation of cells at the S/G2 transition599. While quiescent cells are resistant to some agents, platinum drugs retain the ability to evoke apoptosis in quiescent cells54.
Unlike cisplatin, etoposide and doxorubicin were least cytotoxic to a murine lung cancer cell line during G1, possibly due to the disappearance of topoisomerase II during G1 phase600. Similarly, pretreatment of NSCLC cells with tumor necrosis factor601 or erlotinib510 blocked cells in G1510, 601 or G0601 and proved antagonistic to pemetrexed510 and doxorubicin601, while not altering efficacy of cisplatin601.
One NSCLC variant with resistance to vinorelbine, cisplatin, fluorouracil, paclitaxel, etoposide, decarbonizes, ifosfamide, and epirubicin (but not to gemcitabine or irinotecan) had a significantly higher proportion of cells in G0/G1 and significantly fewer in S phase than did the sensitive parent26. Treatment of NSCLC cells with a monoclonal antibody that significantly increased the percentage of cells in the G2 phase of the cell cycle led to increased sensitivity to taxanes602. On the other hand, transfection of WT p53 gene into p53-null NSCLC cells induced senescence, cell cycle arrest in G1 and decreased sensitivity to paclitaxel387.
13.2 Mitotic spindle checkpoint and mitotic slippage/aneuploidy
Ordinarily, cells with abnormal mitoses undergo G2-M arrest. The mitotic spindle checkpoint, which blocks segregation of abnormal chromosomes, is often defective in human lung cancer cell lines, and this is thought to contribute to chromosomal instability603. Anti-microtubule agents such as vinca alkaloids and taxanes activate the mitotic spindle checkpoint603. In NSCLC cell lines, impairment of the mitotic spindle checkpoint was associated with marked reduction in the ability of vinorelbine and docetaxel to induce apoptosis, compared to cell lines with an intact mitotic spindle checkpoint, while the status of the mitotic spindle checkpoint had no impact on the ability of cisplatin to induce apoptosis603.
Cells may also escape from the G2-M arrest without undergoing cell division (“mitotic slippage), resulting in the formation of aneuploidy G1 cells, and this process of mitotic slippage may also trigger apoptosis604. Microtubule-stabilizing agents such as taxanes and related agents produced aneuploidy populations in NSCLC cell lines that had defective mitotic blocks, and aneuploidy cells were diminished in resistant variants604. In contrast, microtubule-destabilizing agents such as the vinca alkaloids were unable to initiate aneuploidy605. Hence, factors permitting aberrant mitoses and aneuploidy may reduce resistance to taxanes, while factors that promote a mitotic block (thereby reducing aberrant mitoses and aneuploidy) may augment resistance605.
13.3 CHK2
CHK2 kinase is a tumor suppressor that plays an important role in DNA damage signaling, cell cycle regulation and DNA-damage-induced apoptosis. DNA damage leads to its phosphorylation, and the activated protein then blocks entry of cells into mitosis. Cisplatin-resistant NSCLC cell lines had decreased expression of CHK2, related to hypermethylation of the CHK2 promoter606. Hypermethylation of the CHK2 promoter was also found in all of 10 NSCLC tumor samples assessed, and decreased IHC staining for CHK2 was found in 83% of 87 NSCLC samples assessed606.
13.4 Hus1
Hus1, a component of the radiation sensitive (Red) machinery that plays a role in DNA repair and cell cycle G2/M checkpoint control pathways, forms a discrete complex with the Red family members, Rad1 and Rad9. Antisense-mediated downregulation of expression of Hus1 sensitized p53 deficient NSCLC cells to cisplatin607, suggesting that Hus1 may play a role in cisplatin resistance.
13.5.0 Cell cycle regulators
13.5.1 Retinoblastoma protein (RB)
The retinoblastoma tumor suppressor (RB) is a key regulator of cell cycle progression and is functionally inactivated in the majority of NSCLC tumors608. Hypophosphorylated RB blocks cell cycle progression, and phosphorylation of RB (to form pRB) inactivates RB, permitting progression through the cell cycle. Transfection of RB into RB-deficient NSCLC cells enhanced the G1 checkpoint response to chemotherapy608. Transfection of RB into RB-deficient NSCLC608 or SCLC609 cells also significantly increased resistance608 or resulted in a trend towards increased resistance609 to cisplatin608, 609, etoposide608, 609, doxorubicin609 and 5-fluorouracil608, while dexamethasone-induced dephosphorylation of pRB in NSCLC cells was associated with antagonism of paclitaxel-induced cytotoxicity574. On the other hand, one study found that expression of RB protein in cell lines derived from biopsies of patients with NSCLC or SCLC did not correlate with in vitro chemotherapy resistance, nor with response to chemotherapy or survival of the patients from whom the cells were derived 609.
By IHC, 38% of pre-chemotherapy advanced NSCLC tumors71 and 10% of limited and extensive SCLC tumors257 expressed RB. Response rates to chemotherapy (cisplatin combined with gemcitabine, etoposide or with mitomycin-c plus ifosfamide) were significantly lower in RB positive vs negative NSCLC patients71, while survival did not correlate with RB expression in either these NSCLC patients71 nor in SCLC patients treated with alternating cisplatin-etoposide/CAV257. Hence, RB expression is associated with chemotherapy resistance preclinically and with reduced lung cancer clinical response to chemotherapy, but did not correlate with patient survival.
13.5.2 14-3-3
14-3-3 is a family of highly conserved regulatory proteins that bind to numerous signaling proteins and appear to play a role in cell signaling, cell cycle control, and apoptotic death610. Down-regulation of 14-3-3zeta in lung cancer cells significantly increased sensitivity to cisplatin610.
In NSCLC patients, increased 14-3-3zeta expression was positively correlated with a more advanced pathologic stage and grade610. Methylation of the mitotic checkpoint gene 14-3-3sigma in serum-derived circulating tumor DNA was associated with significant prolongation of overall survival and time to progression, and with a trend to increased probability of response (p=0.06) in NSCLC patients treated with cisplatin plus gemcitabine611.
Overall, the limited data available suggest that 14-3-3 might contribute to resistance.
13.5.3 The cyclin-dependent kinase inhibitors P27kp1 and P21WAF1/CIP1
In patients with advanced NSCLC treated with platinum-based chemotherapy regimens, higher tumor IHC expression of the cyclin-dependent kinase inhibitor p27Kip1 correlated with improved response612 or survival612, 613. In keeping with this, overexpression in NSCLC cells of S-phase kinase-associated protein 2 (SKP2) (a member of the F-box family of ubiquitin-protein ligase complexes that controls the stability of several cell cycle-related proteins614) was associated with reduced expression of each of p27kip1, p21WAF/cip1 and cyclin E1, with increased S-phase cells and with chemoresistance against camptothecin, cisplatin, and other agents614.
On the other hand, in patients with resected NSCLC, adjuvant chemotherapy (cisplatin plus vinca alkaloid or etoposide) resulted in significantly longer overall survival compared with controls among patients whose tumors were IHC negative for p27Kip1, while overall survival was not different between patients treated with cisplatin-based chemotherapy and controls in patients with p27Kip1-positive tumors615. By IHC, other cell cycle regulators including p16INK4A, cyclin D1, cyclin D3, cyclin E, and Ki-67 did not predict benefit of adjuvant cisplatin-based chemotherapy, and none of these biomarkers was significantly associated with overall survival of the patients in the total study population615.
Furthermore, a NSCLC cell line resistant to camptothecin, doxorubicin and cisplatin had markedly attenuated caspase-3-like protease activity and elevated expression of p21Waf1/Cip1453. Transfection of p21Waf1/Cip1 cDNA into the parental sensitive cell line resulted in resistance to apoptosis, while p21Waf1/Cip1 antisense oligonucleotides restored sensitivity, particularly if combined with bcl-2 antisense oligonucleotides453. Induction of p21Waf1/Cip1 overexpression in a p53 null NSCLC cell line also conferred increased resistance to killing by etoposide or cisplatin385 and high pre-chemotherapy expression of p21WAF1/CIP1 in tumors of stage IIIA-B NSCLC patients undergoing neoadjuvant platinum-based chemotherapy was associated with shorter time to failure616.
Hence, some data suggest that cyclin-dependent kinase inhibitors decrease chemotherapy resistance while other data suggest that they increase resistance.
13.5.4 Other cell cycle regulators
Cells in human lung cancers that survived induction chemotherapy (and hence were presumably at least partially resistant) overexpressed Cdc2/Cdk1617. Host polymorphisms for the cyclin D1 gene correlated significantly with tumor response or stabilization618, and patients with advanced NSCLC treated with an antimitotic agent plus a platinum had significantly higher response rates if their tumors were IHC positive vs negative for cyclin B1 or for the microtubule motor protein Eg5 (which functions in bipolar spindle assembly)619.
14.0 Transcription factors
Several transcription factors have also been linked to chemotherapy resistance (Fig. 5).
14.1 Nuclear factor-κB (NF-κB)
NF-κB augments the expression of a variety of anti-apoptotic proteins, including IAPs490, 551, Bcl-XL461, 551 and Bcl-2461, and NF-κB activity in NSCLC cells was significantly increased by exposure to cisplatin620, taxanes620, doxorubicin503, etoposide503, and gemcitabine490, 621, 622. This suggests that inhibition of NF-κB activity could augment chemotherapy efficacy by blocking an NF-κB-mediated upregulation of expression of antiapoptotic proteins. In keeping with this, introduction of the NF-κ B antagonist IκBα into resistant NSCLC cells623, 624, upregulation of IκB via inhibition of PI3K551, presence of IκB in a sensitive variant622, inhibition of the IκB antagonist Aurora-A kinase461, and exposure to BAY11-0782551, to genistein620, or to a proteosome inhibitor621, 625 blocked NF-κB activation461, 551, 620, 623, 624, reduced expression of NF-κB-regulated antiapoptotic proteins (including IAPs490, 551, Bcl-XL461, 551 and Bcl-2461), and augmented apoptosis induced by cisplatin620, 626, doxorubicin461, 620, 626, etoposide461, gemcitabine490, 621, 622 and taxanes551, 620, 623–625. Overexpression of IAP-1 protein in NSCLC cells expressing IκBα restored resistance to gemcitabine, suggesting that IAP-1 may be responsible for the chemoresistance induced by NF-κB490. Transfection with IκBα624 or exposure to a proteosome inhibitor621 also blocked paclitaxel-624 or gemcitabine621-induced upregulation of expression of p65 (which heterodimerizes with NF-κB to form the NF-κB complex)624, NF-κB binding to DNA621 and transcription of several NF-κB-regulated genes621. Proteosomal inhibition also potentiated the effect of gemcitabine against NSCLC xenografts621. However, exposure of NSCLC cells to doxorubicin and etoposide induced the NF-κB-dependent expression of the pro-apoptotic proteins TRAIL and DR5 (in addition to increasing the NF-κB-dependent expression of anti-apoptotic proteins such as IAPs), and inhibition of NF-κB led to loss of cell surface expression of TRAIL and DR5, and to chemoresistance of tumor xenografts in nude mice503. Hence, because of the range of factors regulated by NF-κB, inhibition of it could result in either increased or decreased chemotherapy efficacy.
Hence, while clinical data are limited, most (but not all) preclinical data suggest that NF-κB could play a role in resistance of NSCLC to several types of agents.
14.2 Clock-related transcription factors
The circadian transcription factor Clock was overexpressed in cisplatin-resistant cells627 and Clock’s target627 Activating Transcription Factor 4 (ATF4) was cisplatin-inducible at the transcriptional level628. In human lung cancer cell lines, cisplatin resistance correlated with expression of each of Clock627, ATF4628 and of another Clock target, HIV-1 Tat interacting protein629 (Tip60) (a histone acetyltransferase [HAT] gene that plays a role in chromatin remodeling, transcription, DNA repair, and signal transduction). Hyperacetylation of H3K14 and H4K16 was found in cisplatin-resistant cells629. Expression of two other HAT genes, HAT1 and MYST1 did not correlate with cisplatin resistance629. Downregulation of Clock and ATF4 augmented sensitivity of NSCLC cells to cisplatin and etoposide627, and knockdown of Tip60 expression downregulated expression of several DNA repair genes and rendered cells sensitive to cisplatin (but not to oxaliplatin, vincristine, and etoposide)629. Conversely, transfection with ATF4 decreased sensitivity to cisplatin, but not to vincristine628. ATF4 expression also correlated with expression of genes for glutathione metabolism627.
14.3 Zinc finger transcription factors
In NSCLC cells, siRNA inhibition of the zinc finger transcription factors TWIST630 and SNAIL631 (which may play a role in epithelial-mesenchymal transition354, 631 and in regulation of N-cadherin expression354) significantly augmented efficacy of cisplatin by inducing activation of the JNK/mitochondrial pathway630, 631, while mRNA expression of the Kruppel-related zinc finger protein-1(HKR1) transcription factor was induced by exposure to cisplatin632. High expression levels of HKR1 in autopsy lung cancer tissues were associated with antemortem platinum drug administration632.
14.4 Other transcription factors
The transcription factors HIF-1 and p53 have been discussed previously. Other transcription factors that have been associated with cisplatin resistance in NSCLC cell lines include signal transducer and activator of transcription (STAT)-3633, Thyroid Transcription Factor-1 (TTF-1) (when coactivated with the transcription factor NKX2-8)634, and a splice variant of the nuclear receptor protein/transcription factor peroxisome proliferator-activated receptor gamma (PPARγ)1 635. Exposure of a NSCLC cell line to cisplatin and etoposide led to decreased levels of E2F4 (which suppresses proliferation-associated genes) and to induction of E2F1 (which mediates cell proliferation)636. Cells in which the E2F4 gene was lacking or inhibited were more sensitive to apoptosis induced by cisplatin and etoposide, while E2F1-deficient cells were less sensitive636.
15.0 Cancer stem cells and resistance
A small proportion of cells present within tumors have stem cell properties, including self-renewal capability. Cancer stem cells are highly resistant to chemotherapy637–644, and exposure to therapy selects for tumor cells with stem-cell properties639, 640, 643. While stem cells may have high expression of a variety of resistance factors637, 638, 642, part of the resistance of stem cells has been attributed to some stem cell populations being quiescent, but still having the capacity to proliferate637, 638, 644. Such quiescent populations could explain the flattening of the chemotherapy dose-response curve seen clinically in NSCLC4.
Lung cancer cell populations with stem cell properties have been reported640–643, 645–648, and more than one distinct stem cell population may be present in a single cancer cell line648. A population of CD133-positive (CD133+) cells expands during repair of lung damage, and CD133+ cells have been found in human lung cancers638. CD133 may normally play a role in the organization of cell membrane topology649. Lung cancer CD133+ cells generated long-term chemotherapy-resistant spheroids in vitro, indicating potential stem cell function638. CD133+ lung cancer cell lines were resistant to cisplatin642, 643, eotposide642, doxorubicin642, paclitaxel642 and radiotherapy642, and CD133 expression correlated with clinical resistance to platinum regimens in patients with advanced NSCLC643. Resistance to cisplatin of CD133+ NSCLC cell lines and xenografts was reversed by siRNA against the stem cell transcription factor Oct4642.
Lung cancer cells with increased aldehyde dehydrogenase (ALDH) activity also have stem cell properties646, 647, and maintain longer telomeres than do cells with low ALDH levels647. In lung cancer cell lines, ALDH1 expression correlated with CD133 expression641. ALDH is thought to suppress stem cell differentiation by processing retinoic aldehydes. Clinically, lung cancer expression of ALDH1 correlated with tumor stage and grade, and was associated with poor prognosis641.
In breast cancers, cells that are CD44-positive but also CD24-negative are highly invasive650, have stem cell properties651 and are chemotherapy-resistant651. CD44 is a transmembrane glycoprotein that plays a role in cell adhesion and in angiogenesis652, while CD24 is a mucin-like adhesion molecule653 that may recruit beta1integrins into membrane lipid raft domains654. In resected NSCLC tumors, 36.4% had cells with a CD44+/ESA+/CD24- phenotype655.
Each of the Notch, Sonic Hedgehog and Wnt pathways may help maintain stem cell populations. For example, mammalian cells have 4 Notch receptors (Notch 1–4) and 5 membrane-bound Notch ligands (Delta-like1, 3 and 4, Jagged1 and 2)656, 657. Binding of ligand to receptor on an adjacent cell results in a 2-step cleavage of Notch. The second cleavage step is catalyzed by γ-secretase657, and releases Notch intracellular domain (NICD). NICD then translocates to the nucleus where it interacts with the proteins CSL and Mastermind to promote transcription of HES and HEY family members, which then repress various tissue-specific transcription factors657, 658. Notch cleavage may inhibit cellular differentiation, thereby promoting asymmetric cell division and maintenance of a stem cell phenotype in daughter cells that are in contact with cells bearing Notch ligands657. Notch function in inhibiting differentiation and in promoting stem cell renewal is enhanced by hypoxia through an effect of HIF-1α and HIF-2α659. The Notch pathway also may play an important role in epithelial-mesenchymal transition (EMT)660.
A high proportion of NSCLC cell lines expressed Notch receptors, particularly Notch 2 and 3661. Notch 3 expression661, 662 and expression of the Notch ligand Jag1662 was also common clinically in resected lung cancer specimens, and expression of the activated NICD form of Notch 3 was noted in 41% of lung cancer cell lines661. Notch-1 expression was substantially increased in lung cancer cell lines in the presence of hypoxia663. Expression of the Notch ligand Jagged1 and the Notch transcriptional target genes HES1 and HEY1 was also very common in lung cancer cell lines661, the Notch targets HES-1 and HES-5 were elevated in lung tumors663, and ALDH-positive lung cancer stem cells had increased expression of Notch664, Notch ligands646 and Notch effectors646.
Since there is evidence that stem cells may play a major role in chemotherapy and radiotherapy resistance637–640, 643, 644, and since Notch may be important in maintaining stem cell populations, there is interest in targeting Notch665. Inhibition of γ-secretase inhibits Notch activity by preventing the cleavage of Notch to NICD. γ-Secretase inhibitors were active in lung cancer cell lines and xenografts661, 666, including in putative lung cancer stem cells that overexpressed both ALDH and Notch receptors664, in a NSCLC cell line that overexpressed Notch 3667, and in lung cancer cell lines grown in hypoxic conditions that expressed Notch 1663. Because of their potential to target cancer stem cells, there is interest in assessing ability of γ-secretase inhibitors to modulate lung cancer resistance to chemotherapy, and early clinical trials are in progress.
With respect to other stem cell pathways, Wnt appeared to be active in only a small proportion of lung cancer cell lines in one study668, but Wnt activation was found in 50% of NSCLC tumors in another study669. Wnt1 was expressed in 40–50% of NSCLC clinical specimens669, 670, and its expression correlated with Ki-67 expression and with poor survival670. Similarly, Sonic Hedgehog (SHH) was detected in 84% of clinical NSCLC and SCLC tissue specimens671. However, SHH pathway activation (as manifested by expression of its targets Gli1 and PTCH1) was only detected in 10.5% of clinical tumor specimens in one study671, and Gli1 expression was uncommon in SCLC cell lines672, but Gli1 was detected in 85% of clinical SCLC tumor samples in another study672. Host genetic polymorphisms for the SHH pathway correlated with survival in advanced NSCLC treated with platinum-based chemotherapy regimens673.
Overall, available preclinical data support a role for cancer stem cells in resistance to chemotherapy, and limited clinical data are also supportive.
16.0 “Global” indicators of chemotherapy sensitivity and resistance
As noted above, there are several different factors that have been associated with resistance to chemotherapy in lung cancer, and it is difficult to predict clinically how expression of any one of these factors or any group of factors would translate into success or failure of a therapy. Hence, various “global” indicators have also been assessed that would take into consideration several factors simultaneously. We will describe in more detail below some examples to which we alluded briefly above:
16.1 Gene expression arrays
In resected NSCLC, significant associations were found between expression levels of dozens of genes and in vitro chemosensitivity for docetaxel, paclitaxel, irinotecan, cisplatin, gemcitabine, and vinorelbine22. When gene expression profiles were compared to chemosensitivity in a range of NSCLC cell lines, gemcitabine belonged to an isolated cluster, while taxanes, 5-FU, SN-38, and platinums were gathered together into one large cluster36. In a gemcitabine-resistant line, approximately 18.8% of the total DNA elements had substantially altered level of expression compared to the sensitive parent by cDNA microarray, with differences seen in expression of oncogenes, tumor suppressor genes, cell cycle regulators, heat shock proteins, apoptotic and antiapoptotic factors, DNA transcription factors, DNA repair and recombination factors, signal transduction genes, protein translation genes, and many metabolic genes21. When expression of 1291 sensitivity-related genes were assessed using cDNA macroarrays, substantial differences were found between SCLC and NSCLC cell lines674.
Gene signatures were defined for response of NSCLC patients to cisplatin-based therapy, with a positive predictive value of 78% and a negative predictive value of 100%34. There was an inverse correlation between the likelihood of response to cisplatin vs pemetrexed34. In another study involving SCLC and NSCLC patients receiving platinum-based chemotherapy, there was a significant increase in the expression of nine genes in non-responders compared with responders23. Allogeneic inflammatory factor, HLA-DR antigen associated invariant subunit and MHC class II HLA-DR-β precursor were significantly associated with chemotherapy resistance23. One or more resistance genes were overexpressed in 28% of SCLC tumors and in 66% of NSCLC tumors (p=0.012)23. In patients with NSCLC treated with paclitaxel plus irinotecan, cDNA microarray analysis of peripheral blood samples for expression levels of 1176 genes revealed that the genes encoding protein phosphatase, IL-1α and IgA were independent predictive factors for chemosensitivity, while the thyrotropin-releasing hormone receptor and alkylation repair genes were independent prognostic factors675.
Overall, gene expression arrays hold promise as tools for guiding therapy choice in patients, but substantial work remains to be done.
16.2 Chromosomal alterations
In a SCLC cell line, the process of rendering cells resistant by exposure to cisplatin and oxaliplatin caused similar chromosomal changes to emerge5. The resistant cell lines lost their resistant phenotype after 3 months of drug-free culture, and many (but not all) of the acquired chromosomal abnormalities were also lost at the same time, suggesting a link between the chromosomal changes and emergence of resistance5. Emergence of resistance to taxanes has been linked in a NSCLC cell line to amplification of resistance factors as a result of repeated chromosomal breakage-fusion-bridge cycles with telomere addition7.
With in vitro sensitivity testing of resected NSCLC tumor samples, aneuploid tumors tended to have increased resistance to etoposide and topotecan, while aneuploidy had less of an impact on resistance to gemcitabine, and no major impact with platinums6.
16.3 DNA hypermethylation
In a NSCLC cell line and in a xenograft model, pulse exposures to etoposide, doxorubicin, vinca alkaloids, cisplatin, hydroxyurea, 1-β-D-arabinofuranosylcytosine, 5-fluorouracil, 5-fluorodeoxyuridine, and methotrexate caused DNA hypomethylation at drug concentrations which produced mild DNA synthesis inhibition and which killed less than 50% of exposed cells, while marked drug-induced DNA hypermethylation was noted when the degree of DNA synthesis inhibition caused by the drug exceeded 90% and when drug exposure was sufficient to kill 90–100% of exposed cells8. Drug-induced DNA hypermethylation could be blocked by preexposure to hypomethylating agents administered at low concentrations8. Drug-induced DNA hypermethylation may be capable of creating drug-resistant phenotypes by inactivating genes the products of which are required for drug cytotoxicity, and may also contribute to resistance by potentiating the process of gene amplification8.
16.4 In vitro sensitivity testing
Several studies have assessed the ability of in vitro sensitivity testing to predict response of lung cancer to chemotherapy. In some studies of in vitro sensitivity testing involving patients with lung cancer10–13 and other malignancies10 using cisplatin10–13, carboplatin13, vindesine13, vinorelbine13, docetaxel12, 13, paclitaxel12, 13, etoposide12, 13, irinotecan12, 13, gemcitabine12, 13, mitomycin-C10–12, 5-fluorouracil10, 12, doxorubicin10–12, mitoxantrone11, and other agents11, there were relatively frequent false positives (predicted sensitive but failed clinically, with true positive rates of up to 73%) but no false negatives (predicted resistant but succeeded clinically). In still other studies involving patients with chemonaive unresectable NSCLC assessed for in vitro sensitivity to platinums, the positive/negative predictive values were 61.1% and 78.6% with a predictive accuracy of 68.8%14. The group predicted to be sensitive had better clinical response (P=.036), longer progression-free survival (P=.060), and longer overall survival (P=.025) than those predicted to be resistant14. Occasional studies did not find that in vitro sensitivity testing did any better than empiric therapy in NSCLC9.
With limited SCLC patients treated with 4 cycles of etoposide plus platinum plus radiotherapy followed by either 4 cycles of cyclophosphamide plus doxorubicin plus vincristine vs 4 cycles of therapy based on in vitro sensitivity testing, median survival was 19 months with standard therapy vs 38.5 months with therapy based on sensitivity testing, but other factors besides in vitro sensitivity testing may have contributed15. In other studies in SCLC patients treated with therapy selected by in vitro sensitivity testing either after 12 weeks of etoposide-platinum or at relapse, there was a trend towards a higher response rate than in patients with therapy selected empirically9.
Overall, the results of in vitro sensitivity testing suggest that it may be fairly good at predicting resistance, but somewhat less good at predicting sensitivity. Factors that operate against it being used routinely include the frequency of false postives, as well as the amount of fresh tissue required and the amount of time required to perform the tests.
17.0 Summary
There are numerous factors that directly or indirectly may contribute to lung cancer resistance to chemotherapy. Of the many factors that have been linked to resistance in preclinical systems, several have also been linked to resistance in the clinic, although conflicting clinical data are common. Issues that muddy the water clinically include the fact that in some instances it is difficult to determine whether a factor is primarily a prognostic factor (correlating with survival whether or not therapy is given) vs a predictive factor (correlating with benefit of the therapy being assessed). The use of chemotherapy agents in combination may also blunt the impact of a resistance factor, and the presence of co-morbid conditions and the administration of subsequent therapy may make it more difficult to discern the effect of a resistance factor on patient survival.
Factors for which there is now substantial clinical evidence of a link to SCLC resistance to chemotherapy include MRP (for platinum-based combination chemotherapy) and MDR1/P-gp (for non-platinum agents). SPECT MIBI and Tc-TF scanning appears to predict chemotherapy benefit in SCLC. In NSCLC, the strongest clinical evidence is for taxane resistance with elevated expression or mutation of class III β-tubulin (and possibly α tubulin), platinum resistance and expression of ERCC1, platinum resistance and BCRP expression, gemcitabine resistance and RRM1 expression, and resistance to several agents and COX-2 expression (although COX-2 inhibitors have had minimal impact on drug efficacy clinically). Tumors expressing high BRCA1 may have increased resistance to platinums but increased sensitivity to taxanes. Limited early clinical data suggest that chemotherapy resistance in NSCLC may also be increased with decreased expression of cyclin B1 or of the microtubule motor protein Eg5, or with increased expression of stem cell markers, ICAM, matrilysin, osteopontin, DDH, survivin, PCDGF, caveolin-1, p21WAF1/CIP1, or 14-3-3sigma, and that IGF-1R inhibitors may increase efficacy of chemotherapy, particularly in squamous cell carcinomas. Equivocal data (with some positive studies but other negative studies) suggest that NSCLC tumors with EGFR mutations (particularly exon 19 deletions) may have increased sensitivity to chemotherapy, while K-ras mutations and expression of GST-pi or RB may possibly confer resistance. While limited clinical data suggest that p53 mutations are associated with resistance to platinum-based therapies in NSCLC, data on p53 IHC positivity are equivocal, with some studies showing a link to resistance, some showing no correlation, and some instead unexpectedly showing increased sensitivity to therapy in p53 positive tumors. Some limited clinical studies suggested increased resistance with p27kip1 while others suggested increased sensitivity.
18.0 Future directions
Future studies might benefit from attempting to identify targets that must be present within tumor cells if a therapy is going to be of value, as well as identifying tumor cell, microenvironment and host factors that will render a tumor resistant despite the presence of the required target. Concurrent combined correlations of tumor expression of putative targets and resistance markers, in vitro tumor cell sensitivity to therapy, and clinical tumor shrinkage on therapy might help in this regard. Future studies of resistance modulating strategies also need to take into consideration the fact that such trials could potentially fail because a required therapy target is lacking (and hence, response could not occur even if resistance factors were neutralized), because host/microenvironment factors protect the tumor despite neutralization of the resistance factor, because several resistance factors are at play in addition to the one being targeted, or because the tumor does not express the resistance factor being targeted. We also need to do a better job of assessing not just which resistance factors are at play at initiation of therapy (leading to intrinsic resistance) but also which ones emerge after exposure to therapy, leading to acquired resistance. Since many factors may lead to broad cross-resistance to several agents, and since some of these cross-resistance factors either limit uptake of drug into tumor cells or else increase drug efflux, progress might also be helped by concentrating on new therapies that work at the outer cell membrane level and do not require entry into tumor cells as a prerequisite for efficacy.
Future studies also need to do a better job of differentiating whether a factor that is associated with outcome is just a prognostic factor (correlating with tumor cell growth rate or with relapse probability) or whether instead it is a predictive factor (correlating with ability of a chemotherapy agent to kill tumor cells). Factors that correlated with tumor shrinkage on therapy might be expected to be predictive, while factors correlating with tumor stability or survival outcomes could be either predictive or prognostic. Future studies might also benefit from assessing host genotype polymorphisms for targets and resistance factors at the same time that tumor mRNA and protein expression are assessed. The host genotype polymorphisms could impact half life or enzymatic activity of the target/resistance factor, and could help explain discrepancies between target/resistance factor mRNA expression, protein expression and therapy efficacy.
Overall, epithelial tumors in general and lung cancer in particular are likely to remain a major challenge for some time to come.
Acknowledgements
I would like to thank John V. Heymach, MD, PhD for reviewing the manuscript and offering very helpful comments and suggestions. Supported in part by Cancer Center Support Grant number 5-P30 CA16672-32 and by Department of Defense grant number W81XWH-07-1-0306
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009 doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Stewart DJ, Tomiak E, Shamji FM, Maziak DE, MacLeod P, Phase II. study of alternating chemotherapy regimens for advanced non-small cell lung cancer. Lung Cancer. 2004;44:241–249. doi: 10.1016/j.lungcan.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Stewart DJ, Raaphorst GP, Yau J, Beaubien AR. Active vs. passive resistance, dose-response relationships, high dose chemotherapy, and resistance modulation: a hypothesis. Invest New Drugs. 1996;14:115–130. doi: 10.1007/BF00210782. [DOI] [PubMed] [Google Scholar]
- 4.Stewart DJ, Chiritescu G, Dahrouge S, Banerjee S, Tomiak EM. Chemotherapy dose--response relationships in non-small cell lung cancer and implied resistance mechanisms. Cancer Treat Rev. 2007;33:101–137. doi: 10.1016/j.ctrv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Stordal B, Peters G, Davey R. Similar chromosomal changes in cisplatin and oxaliplatin-resistant sublines of the H69 SCLC cell line are not associated with platinum resistance. Genes Chromosomes Cancer. 2006;45:1094–1105. doi: 10.1002/gcc.20373. [DOI] [PubMed] [Google Scholar]
- 6.d'Amato TA, Landreneau RJ, Ricketts W, et al. Chemotherapy resistance and oncogene expression in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133:352–363. doi: 10.1016/j.jtcvs.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Kitada K, Yamasaki T. The complicated copy number alterations in chromosome 7 of a lung cancer cell line is explained by a model based on repeated breakage-fusion-bridge cycles. Cancer Genet Cytogenet. 2008;185:11–19. doi: 10.1016/j.cancergencyto.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Nyce J. Drug-induced DNA hypermethylation and drug resistance in human tumors. Cancer Res. 1989;49:5829–5836. [PubMed] [Google Scholar]
- 9.Shaw GL, Gazdar AF, Phelps R, et al. Correlation of in vitro drug sensitivity testing results with response to chemotherapy and survival: comparison of non-small cell lung cancer and small cell lung cancer. J Cell Biochem Suppl. 1996;24:173–185. doi: 10.1002/jcb.240630513. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y, Kubota T, Asanuma F, et al. The predictability of clinical antitumor effects using two distinctive in vitro chemosensitivity tests: an analysis of true positive cases. Surg Today. 1993;23:193–199. doi: 10.1007/BF00309227. [DOI] [PubMed] [Google Scholar]
- 11.Ohnoshi T, Hiraki S. In vitro drug-sensitivity test using human tumor clonogenic assay in lung cancer patients. Gan To Kagaku Ryoho. 1985;12:1582–1587. [PubMed] [Google Scholar]
- 12.Yoshimasu T, Oura S, Hirai I, et al. Data acquisition for the histoculture drug response assay in lung cancer. J Thorac Cardiovasc Surg. 2007;133:303–308. doi: 10.1016/j.jtcvs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura M, Gika M, Abiko T, et al. Clinical evaluation of chemosensitivity testing for patients with unresectable non-small cell lung cancer (NSCLC) using collagen gel droplet embedded culture drug sensitivity test (CD-DST) Cancer Chemother Pharmacol. 2007;59:507–513. doi: 10.1007/s00280-006-0292-8. [DOI] [PubMed] [Google Scholar]
- 14.Moon YW, Choi SH, Kim YT, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007;109:1829–1835. doi: 10.1002/cncr.22601. [DOI] [PubMed] [Google Scholar]
- 15.Cortazar P, Gazdar AF, Woods E, et al. Survival of patients with limited-stage small cell lung cancer treated with individualized chemotherapy selected by in vitro drug sensitivity testing. Clin Cancer Res. 1997;3:741–747. [PubMed] [Google Scholar]
- 16.Shen DW, Su A, Liang XJ, Pai-Panandiker A, Gottesman MM. Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer. 2004;91:270–276. doi: 10.1038/sj.bjc.6601956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puig PE, Guilly MN, Bouchot A, et al. Tumor cells can escape DNA-damaging cisplatin through DNA endoreduplication and reversible polyploidy. Cell Biol Int. 2008;32:1031–1043. doi: 10.1016/j.cellbi.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007;3:464–467. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- 19.Teicher BA, Chatterjee D, Liu JT, Holden SA, Ara G. Protection of bone-marrow granulocyte-macrophage colony-forming units in mice bearing in vivo alkylating-agent-resistant EMT-6 tumors. Cancer Chemother Pharmacol. 1993;32:315–319. doi: 10.1007/BF00686178. [DOI] [PubMed] [Google Scholar]
- 20.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 21.Dong M, Feng FY, Lin C, et al. Mechanisms of the drug resistance of a 2', 2-difluorodeoxycytide (gemcitabine)-resistant variant of the human lung adenocarcinoma cell line. Zhonghua Yi Xue Za Zhi. 2004;84:323–328. [PubMed] [Google Scholar]
- 22.Kikuchi T, Daigo Y, Katagiri T, et al. Expression profiles of non-small cell lung cancers on cDNA microarrays: identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene. 2003;22:2192–2205. doi: 10.1038/sj.onc.1206288. [DOI] [PubMed] [Google Scholar]
- 23.Oshita F, Ikehara M, Sekiyama A, et al. Genomic-wide cDNA microarray screening to correlate gene expression profile with chemoresistance in patients with advanced lung cancer. J Exp Ther Oncol. 2004;4:155–160. [PubMed] [Google Scholar]
- 24.Stewart DJ, Goel R, Gertler SZ, et al. Concurrent use of multiple low dose chemotherapy agents with differing mechanisms of action as a strategy vs passive resistance: a pilot study. Int J Oncol. 1999;15:693–699. doi: 10.3892/ijo.15.4.693. [DOI] [PubMed] [Google Scholar]
- 25.Yabuki N, Sakata K, Yamasaki T, et al. Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet Cytogenet. 2007;173:1–9. doi: 10.1016/j.cancergencyto.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Chen GY, Yang ZY, Hong X, Wang M, Lu L, Zhang CH. Establishment of a multi drug-resistant human lung adenocarcinoma cell line and biological characteristics there of. Zhonghua Yi Xue Za Zhi. 2007;87:924–926. [PubMed] [Google Scholar]
- 27.Stordal B, Davey M. Understanding cisplatin resistance using cellular models. IUBMB Life. 2007;59:696–699. doi: 10.1080/15216540701636287. [DOI] [PubMed] [Google Scholar]
- 28.Jensen PB, Holm B, Sorensen M, Christensen IJ, Sehested M. In vitro cross-resistance and collateral sensitivity in seven resistant small-cell lung cancer cell lines: preclinical identification of suitable drug partners to taxotere, taxol, topotecan and gemcitabin. Br J Cancer. 1997;75:869–877. doi: 10.1038/bjc.1997.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang Y, O'Driscoll L, McDonnell S, et al. Enhanced in vitro invasiveness and drug resistance with altered gene expression patterns in a human lung carcinoma cell line after pulse selection with anticancer drugs. Int J Cancer. 2004;111:484–493. doi: 10.1002/ijc.20230. [DOI] [PubMed] [Google Scholar]
- 30.Fujita F, Fujita M, Fujita M, Sakamoto Y. Acquired resistance and cross-resistance of gemcitabine to cisplatin or vindesine in human lung cancer xenografted in nude mice. Gan To Kagaku Ryoho. 1994;21:2749–2755. [PubMed] [Google Scholar]
- 31.Chikamori M, Takigawa N, Kiura K, et al. Establishment of a 7-ethyl-10-hydroxy-camptothecin-resistant small cell lung cancer cell line. Anticancer Res. 2004;24:3911–3916. [PubMed] [Google Scholar]
- 32.Moritaka T, Kiura K, Ueoka H, et al. Cisplatin-resistant human small cell lung cancer cell line shows collateral sensitivity to vinca alkaloids. Anticancer Res. 1998;18:927–933. [PubMed] [Google Scholar]
- 33.Bergman AM, Munch-Petersen B, Jensen PB, et al. Collateral sensitivity to gemcitabine (2',2'-difluorodeoxycytidine) and cytosine arabinoside of daunorubicin- and VM-26-resistant variants of human small cell lung cancer cell lines. Biochem Pharmacol. 2001;61:1401–1408. doi: 10.1016/s0006-2952(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu DS, Balakumaran BS, Acharya CR, et al. Pharmacogenomic strategies provide a rational approach to the treatment of cisplatin-resistant patients with advanced cancer. J Clin Oncol. 2007;25:4350–4357. doi: 10.1200/JCO.2007.11.0593. [DOI] [PubMed] [Google Scholar]
- 35.Dong M, Lin C, Feng FY, et al. Development and characterization of a gemcitabine-resistant variant of human lung adenocarcinoma cell line A549. Ai Zheng. 2004;23:667–671. [PubMed] [Google Scholar]
- 36.Gemma A, Li C, Sugiyama Y, et al. Anticancer drug clustering in lung cancer based on gene expression profiles and sensitivity database. BMC Cancer. 2006;6:174. doi: 10.1186/1471-2407-6-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jereczek-Fossa B, Jassem J, Karnicka-Mlodkowska H, et al. Does chemotherapy-induced leukopenia predict a response in small-cell lung cancer? J Cancer Res Clin Oncol. 1998;124:106–112. doi: 10.1007/s004320050141. [DOI] [PubMed] [Google Scholar]
- 38.Pallis AG, Agelaki S, Kakolyris S, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with advanced non-small cell lung cancer treated with front-line docetaxel-gemcitabine chemotherapy. Lung Cancer. 2008;62:356–363. doi: 10.1016/j.lungcan.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Pan JH, Han JX, Wu JM, Sheng LJ, Huang HN. CYP450 polymorphisms predict clinic outcomes to vinorelbine-based chemotherapy in patients with non-small-cell lung cancer. Acta Oncol. 2007;46:361–366. doi: 10.1080/02841860600902197. [DOI] [PubMed] [Google Scholar]
- 40.Han JY, Lim HS, Shin ES, et al. Influence of the organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on irinotecan-pharmacokinetics and clinical outcome of patients with advanced non-small cell lung cancer. Lung Cancer. 2008;59:69–75. doi: 10.1016/j.lungcan.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Han JY, Lim HS, Shin ES, et al. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol. 2006;24:2237–2244. doi: 10.1200/JCO.2005.03.0239. [DOI] [PubMed] [Google Scholar]
- 42.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 43.Stewart DJ, Kurzrock R. Cancer: the road to Amiens. J Clin Oncol. 2009;27:328–333. doi: 10.1200/JCO.2008.18.9621. [DOI] [PubMed] [Google Scholar]
- 44.Mapleson WW. An electric analogue for uptake and exchange of inert gases and other agents. J Appl Physiol. 1963;18:197–204. doi: 10.1152/jappl.1963.18.1.197. [DOI] [PubMed] [Google Scholar]
- 45.Lutz RJ, Galbraith WM, Dedrick RL, Shrager R, Mellett LB. A model for the kinetics of distribution of actinomycin-D in the beagle dog. J Pharmacol Exp Ther. 1977;200:469–478. [PubMed] [Google Scholar]
- 46.Bischoff KB. Some fundamental considerations of the applications of pharmacokinetics to cancer chemotherapy. Cancer Chemother Rep. 1975;59:777–793. [PubMed] [Google Scholar]
- 47.Kristjansen PE, Brown TJ, Shipley LA, Jain RK. Intratumor pharmacokinetics, flow resistance, and metabolism during gemcitabine infusion in ex vivo perfused human small cell lung cancer. Clin Cancer Res. 1996;2:359–367. [PubMed] [Google Scholar]
- 48.Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Hori K, Abe I, Saito S, Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981;67:663–669. [PubMed] [Google Scholar]
- 50.Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem. 2008;8:790–797. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- 51.Lee SM, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Hypoxia confers protection against apoptosis via PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett. 2006;242:231–238. doi: 10.1016/j.canlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Zeng L, Kizaka-Kondoh S, Itasaka S, et al. Hypoxia inducible factor-1 influences sensitivity to paclitaxel of human lung cancer cell lines under normoxic conditions. Cancer Sci. 2007;98:1394–1401. doi: 10.1111/j.1349-7006.2007.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lund EL, Hansen LT, Kristjansen PE. Augmenting tumor sensitivity to topotecan by transient hypoxia. Cancer Chemother Pharmacol. 2005;56:473–480. doi: 10.1007/s00280-005-1008-1. [DOI] [PubMed] [Google Scholar]
- 54.Knight LA, Conroy M, Fernando A, Polak M, Kurbacher CM, Cree IA. Pilot studies of the effect of zoledronic acid (Zometa) on tumor-derived cells ex vivo in the ATP-based tumor chemosensitivity assay. Anticancer Drugs. 2005;16:969–976. doi: 10.1097/01.cad.0000176500.56057.66. [DOI] [PubMed] [Google Scholar]
- 55.Schnitzer SE, Weigert A, Zhou J, Brune B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res. 2009;7:393–401. doi: 10.1158/1541-7786.MCR-08-0156. [DOI] [PubMed] [Google Scholar]
- 56.Song X, Liu X, Chi W, et al. Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol. 2006;58:776–784. doi: 10.1007/s00280-006-0224-7. [DOI] [PubMed] [Google Scholar]
- 57.Weinberg RA. The Biology of Cancer. New York, NY: Garland Science; 2007. [Google Scholar]
- 58.Stewart DJ, Evans WK, Logan D. Addition of pentoxifylline plus nifedipine to chemotherapy in patients with cisplatin-resistant cancers of the lung and other sites. Am J Clin Oncol. 1994;17:313–316. doi: 10.1097/00000421-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Williamson SK, Crowley JJ, Lara PN, Jr, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005;23:9097–9104. doi: 10.1200/JCO.2005.01.3771. [DOI] [PubMed] [Google Scholar]
- 60.William WN, Jr, Zinner RG, Karp DD, et al. Phase I trial of motexafin gadolinium in combination with docetaxel and cisplatin for the treatment of non-small cell lung cancer. J Thorac Oncol. 2007;2:745–750. doi: 10.1097/JTO.0b013e31811f4719. [DOI] [PubMed] [Google Scholar]
- 61.Stewart DJ, Maroun JA, Young V, et al. Feasibility study of combining metronidazole with chemotherapy. J Clin Oncol. 1983;1:17–23. doi: 10.1200/JCO.1983.1.1.17. [DOI] [PubMed] [Google Scholar]
- 62.Stewart DJ, Cripps MC, Goel R, et al. Pilot study of multiple chemotherapy resistance modulators plus epirubicin in the treatment of resistant malignancies. Cancer Chemother Pharmacol. 1997;41:1–8. doi: 10.1007/s002800050700. [DOI] [PubMed] [Google Scholar]
- 63.Stewart DJ, Goel R, Cripps MC, Huan S, Yau J, Verma S. Multiple resistance modulators combined with carboplatin for resistant malignancies: a pilot study. Invest New Drugs. 1997;15:267–277. doi: 10.1023/a:1005993705237. [DOI] [PubMed] [Google Scholar]
- 64.Stewart DJ, Goel R, Cripps MC, et al. Concurrent use of multiple chemotherapy resistance modulators with etoposide in patients with resistant malignancies. Int J Oncol. 1997;11:709–716. doi: 10.3892/ijo.11.4.709. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda H, Nakayama K, Watanabe M, et al. Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin Cancer Res. 2006;12:6748–6757. doi: 10.1158/1078-0432.CCR-06-1124. [DOI] [PubMed] [Google Scholar]
- 66.Cherk MH, Foo SS, Poon AM, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non-small cell lung cancer assessed by 18F-Fluoromisonidazole and 18F-FDG PET. J Nucl Med. 2006;47:1921–1926. [PubMed] [Google Scholar]
- 67.Felip E, Taron M, Rosell R, et al. Clinical significance of hypoxia-inducible factor-1a messenger RNA expression in locally advanced non-small-cell lung cancer after platinum agent and gemcitabine chemotherapy followed by surgery. Clin Lung Cancer. 2005;6:299–303. doi: 10.3816/clc.2005.n.009. [DOI] [PubMed] [Google Scholar]
- 68.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Serum vascular endothelial growth factor (VEGF) and bcl-2 levels in advanced stage non-small cell lung cancer. Cancer Invest. 2006;24:576–580. doi: 10.1080/07357900600894781. [DOI] [PubMed] [Google Scholar]
- 69.Mack PC, Redman MW, Chansky K, et al. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol. 2008;26:4771–4776. doi: 10.1200/JCO.2008.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab--an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 71.Ludovini V, Gregorc V, Pistola L, et al. Vascular endothelial growth factor, p53, Rb, Bcl-2 expression and response to chemotherapy in advanced non-small cell lung cancer. Lung Cancer. 2004;46:77–85. doi: 10.1016/j.lungcan.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 72.Carvalho Junior AD, Vieira FP, Melo VJ, et al. Preparation and cytotoxicity of cisplatin-containing liposomes. Braz J Med Biol Res. 2007;40:1149–1157. doi: 10.1590/s0100-879x2006005000125. [DOI] [PubMed] [Google Scholar]
- 73.Sadava D, Coleman A, Kane SE. Liposomal daunorubicin overcomes drug resistance in human breast, ovarian and lung carcinoma cells. J Liposome Res. 2002;12:301–309. doi: 10.1081/lpr-120016196. [DOI] [PubMed] [Google Scholar]
- 74.Webb MS, Johnstone S, Morris TJ, et al. In vitro and in vivo characterization of a combination chemotherapy formulation consisting of vinorelbine and phosphatidylserine. Eur J Pharm Biopharm. 2007;65:289–299. doi: 10.1016/j.ejpb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Ravaioli A, Papi M, Pasquini E, et al. Lipoplatin monotherapy: A phase II trial of second-line treatment of metastatic non-small-cell lung cancer. J Chemother. 2009;21:86–90. doi: 10.1179/joc.2009.21.1.86. [DOI] [PubMed] [Google Scholar]
- 76.White SC, Lorigan P, Margison GP, et al. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br J Cancer. 2006;95:822–828. doi: 10.1038/sj.bjc.6603345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim ES, Lu C, Khuri FR, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer. 2001;34:427–432. doi: 10.1016/s0169-5002(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 78.Patlakas G, Bouros D, Tsantekidou-Pozova S, Koukourakis MI. Triplet chemotherapy with docetaxel, gemcitabine and liposomal doxorubicin, supported with subcutaneous amifostine and hemopoietic growth factors, in advanced non-small cell lung cancer. Anticancer Res. 2005;25:1427–1431. [PubMed] [Google Scholar]
- 79.Koukourakis MI, Romanidis K, Froudarakis M, et al. Concurrent administration of Docetaxel and Stealth liposomal doxorubicin with radiotherapy in non-small cell lung cancer : excellent tolerance using subcutaneous amifostine for cytoprotection. Br J Cancer. 2002;87:385–392. doi: 10.1038/sj.bjc.6600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samantas E, Kalofonos H, Linardou H, et al. A Hellenic Cooperative Oncology Group Study. Phase II study of pegylated liposomal doxorubicin: inactive in recurrent small-cell lung cancer. Ann Oncol. 2000;11:1395–1397. doi: 10.1023/a:1026523316736. [DOI] [PubMed] [Google Scholar]
- 81.Wittgen BP, Kunst PW, van der Born K, et al. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–2421. doi: 10.1158/1078-0432.CCR-06-1480. [DOI] [PubMed] [Google Scholar]
- 82.Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30:3476–3485. doi: 10.1016/j.biomaterials.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Negishi T, Koizumi F, Uchino H, et al. NK105, a paclitaxel-incorporating micellar nanoparticle, is a more potent radiosensitising agent compared to free paclitaxel. Br J Cancer. 2006;95:601–606. doi: 10.1038/sj.bjc.6603311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 85.Rizvi NA, Riely GJ, Azzoli CG, et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non-small-cell lung cancer. J Clin Oncol. 2008;26:639–643. doi: 10.1200/JCO.2007.10.8605. [DOI] [PubMed] [Google Scholar]
- 86.Reynolds C, Barrera D, Jotte R, et al. Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel, Carboplatin, and Bevacizumab in First-line Patients with Advanced Nonsquamous Non-small Cell Lung Cancer. J Thorac Oncol. 2009 doi: 10.1097/JTO.0b013e3181c0a2f4. [DOI] [PubMed] [Google Scholar]
- 87.Raghunand N, Martinez-Zaguilan R, Wright SH, Gillies RJ. pH and drug resistance. II. Turnover of acidic vesicles and resistance to weakly basic chemotherapeutic drugs. Biochem Pharmacol. 1999;57:1047–1058. doi: 10.1016/s0006-2952(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 88.Laurencot CM, Kennedy KA. Influence of pH on the cytotoxicity of cisplatin in EMT6 mouse mammary tumor cells. Oncol Res. 1995;7:371–379. [PubMed] [Google Scholar]
- 89.Mahoney BP, Raghunand N, Baggett B, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics. I. Acid pH affects the distribution of chemotherapeutic agents in vitro. Biochem Pharmacol. 2003;66:1207–1218. doi: 10.1016/s0006-2952(03)00467-2. [DOI] [PubMed] [Google Scholar]
- 90.Matsumura T, Takigawa N, Kiura K, et al. Determinants of cisplatin and irinotecan activities in human lung adenocarcinoma cells: evidence of cisplatin accumulation and topoisomerase I activity. In Vivo. 2005;19:717–721. [PubMed] [Google Scholar]
- 91.Shimura M, Saito A, Matsuyama S, et al. Element array by scanning X-ray fluorescence microscopy after cis-diamminedichloro-platinum(II) treatment. Cancer Res. 2005;65:4998–5002. doi: 10.1158/0008-5472.CAN-05-0373. [DOI] [PubMed] [Google Scholar]
- 92.Kawai H, Kiura K, Tabata M, et al. Characterization of non-small-cell lung cancer cell lines established before and after chemotherapy. Lung Cancer. 2002;35:305–314. doi: 10.1016/s0169-5002(01)00430-5. [DOI] [PubMed] [Google Scholar]
- 93.Shellard SA, Fichtinger-Schepman AM, Lazo JS, Hill BT. Evidence of differential cisplatin-DNA adduct formation, removal and tolerance of DNA damage in three human lung carcinoma cell lines. Anticancer Drugs. 1993;4:491–500. doi: 10.1097/00001813-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 94.Bungo M, Fujiwara Y, Kasahara K, et al. Decreased accumulation as a mechanism of resistance to cis-diamminedichloroplatinum( II) in human non-small cell lung cancer cell lines: relation to DNA damage and repair. Cancer Res. 1990;50:2549–2553. [PubMed] [Google Scholar]
- 95.Henness S, Davey MW, Harvie RM, Davey RA. Fractionated irradiation of H69 small-cell lung cancer cells causes stable radiation and drug resistance with increased MRP1, MRP2, and topoisomerase IIalpha expression. Int J Radiat Oncol Biol Phys. 2002;54:895–902. doi: 10.1016/s0360-3016(02)03037-7. [DOI] [PubMed] [Google Scholar]
- 96.Salerno M, Yahia D, Dzamitika S, de Vries E, Pereira-Maia E, Garnier-Suillerot A. Impact of intracellular chloride concentration on cisplatin accumulation in sensitive and resistant GLC4 cells. J Biol Inorg Chem. 2009;14:123–132. doi: 10.1007/s00775-008-0430-3. [DOI] [PubMed] [Google Scholar]
- 97.Popovic P, Wong PTT, Kates M, et al. Membrane fluidity and lipids in cisplatin resistant cells with low cisplatin uptake. Proc AACR. 1994;35:440. [Google Scholar]
- 98.Liang XJ, Shen DW, Gottesman MM. A pleiotropic defect reducing drug accumulation in cisplatin-resistant cells. J Inorg Biochem. 2004;98:1599–1606. doi: 10.1016/j.jinorgbio.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 99.Timmer-Bosscha H, Hospers GA, Meijer C, et al. Influence of docosahexaenoic acid on cisplatin resistance in a human small cell lung carcinoma cell line. J Natl Cancer Inst. 1989;81:1069–1075. doi: 10.1093/jnci/81.14.1069. [DOI] [PubMed] [Google Scholar]
- 100.Howell SB, Vick J, Andrews PA. Biochemical modulation of cisplatin by dipyridamole. Proc AACR. 1987;28:313. [Google Scholar]
- 101.Vallejo CT, Rabinovich MG, Perez JE, et al. High-dose cisplatin with dipyridamole in advanced non-small cell lung cancer. A Grupo Oncologico Cooperativo del Sur study. Am J Clin Oncol. 1995;18:185–188. doi: 10.1097/00000421-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Song IS, Savaraj N, Siddik ZH, et al. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3:1543–1549. [PubMed] [Google Scholar]
- 103.Blair BG, Larson CA, Safaei R, Howell SB. Copper Transporter 2 Regulates the Cellular Accumulation and Cytotoxicity of Cisplatin and Carboplatin. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-09-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stewart DJ, Issa JP, Kurzrock R, et al. Decitabine effect on tumor global DNA methylation and other parameters in a phase I trial in refractory solid tumors and lymphomas. Clin Cancer Res. 2009;15:3881–3888. doi: 10.1158/1078-0432.CCR-08-2196. [DOI] [PubMed] [Google Scholar]
- 105.Stewart DJ. Gefitinib maintenance in stage III non-small-cell lung cancer. J Clin Oncol. 2008;26:4849–4850. doi: 10.1200/JCO.2008.18.7625. author reply 50–51. [DOI] [PubMed] [Google Scholar]
- 106.Bando T, Fujimura M, Kasahara K, Ueno T, Matsuda T. Selective beta2-adrenoceptor agonist enhances sensitivity to cisplatin in non-small cell lung cancer cell line. Oncol Rep. 2000;7:49–52. doi: 10.3892/or.7.1.49. [DOI] [PubMed] [Google Scholar]
- 107.Bando T, Fujimura M, Kasahara K, Matsuda T. Significance of Na+, K(+)-ATPase on intracellular accumulation of cis-diamminedichloroplatinum(II) in human non-small-cell but not in small-cell lung cancer cell lines. Anticancer Res. 1998;18:1085–1089. [PubMed] [Google Scholar]
- 108.Bando T, Fujimura M, Kasahara K, Matsuda T. Role of thromboxane receptor on the intracellular accumulation of cis-diamminedichloroplatinum(II) in non-small-cell but not in small-cell lung cancer cell lines. Anticancer Res. 1998;18:1079–1084. [PubMed] [Google Scholar]
- 109.Fujimura M, Kasahara K, Shirasaki H, et al. Up-regulation of ICH-1L protein by thromboxane A2 antagonists enhances cisplatin-induced apoptosis in non-small-cell lung-cancer cell lines. J Cancer Res Clin Oncol. 1999;125:389–394. doi: 10.1007/s004320050291. [DOI] [PubMed] [Google Scholar]
- 110.Muto S, Nemoto J, Okada K, et al. Intracellular Na+ directly modulates Na+,K+-ATPase gene expression in normal rat kidney epithelial cells. Kidney Int. 2000;57:1617–1635. doi: 10.1046/j.1523-1755.2000.00006.x. [DOI] [PubMed] [Google Scholar]
- 111.Bando T, Fujimura M, Kasahara K, et al. Exposure to sorbitol induces resistance to cisplatin in human non-small-cell lung cancer cell lines. Anticancer Res. 1997;17:3345–3348. [PubMed] [Google Scholar]
- 112.Tokuchi Y, Isobe H, Takekawa H, et al. Predicting chemotherapeutic response to small-cell lung cancer of platinum compounds by thallium-201 single-photon emission computerized tomography. Br J Cancer. 1998;77:1363–1368. doi: 10.1038/bjc.1998.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamamoto Y, Nishiyama Y, Satoh K, et al. Comparative study of technetium-99m-sestamibi and thallium-201 SPECT in predicting chemotherapeutic response in small cell lung cancer. J Nucl Med. 1998;39:1626–1629. [PubMed] [Google Scholar]
- 114.Nishiyama Y, Yamamoto Y, Satoh K, et al. Comparative study of Tc-99m MIBI and TI-201 SPECT in predicting chemotherapeutic response in non-small-cell lung cancer. Clin Nucl Med. 2000;25:364–369. doi: 10.1097/00003072-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 115.Kasahara K, Fujiwara Y, Sugimoto Y, et al. Determinants of response to the DNA topoisomerase II inhibitors doxorubicin and etoposide in human lung cancer cell lines. J Natl Cancer Inst. 1992;84:113–118. doi: 10.1093/jnci/84.2.113. [DOI] [PubMed] [Google Scholar]
- 116.Tsujino I, Yamazaki T, Masutani M, Sawada U, Horie T. Effect of Tween-80 on cell killing by etoposide in human lung adenocarcinoma cells. Cancer Chemother Pharmacol. 1999;43:29–34. doi: 10.1007/s002800050859. [DOI] [PubMed] [Google Scholar]
- 117.Belanger MM, Gaudreau M, Roussel E, Couet J. Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther. 2004;3:954–959. doi: 10.4161/cbt.3.10.1112. [DOI] [PubMed] [Google Scholar]
- 118.Chen SZ, Jiang M, Zhen YS. HERG K+ channel expression-related chemosensitivity in cancer cells and its modulation by erythromycin. Cancer Chemother Pharmacol. 2005;56:212–220. doi: 10.1007/s00280-004-0960-5. [DOI] [PubMed] [Google Scholar]
- 119.Seve P, Mackey JR, Isaac S, et al. cN-II expression predicts survival in patients receiving gemcitabine for advanced non-small cell lung cancer. Lung Cancer. 2005;49:363–370. doi: 10.1016/j.lungcan.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 120.Achiwa H, Oguri T, Sato S, Maeda H, Niimi T, Ueda R. Determinants of sensitivity and resistance to gemcitabine: the roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci. 2004;95:753–757. doi: 10.1111/j.1349-7006.2004.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim MJ, Lee HJ, Lee IA, et al. Preparation of pH-sensitive, long-circulating and EGFR-targeted immunoliposomes. Arch Pharm Res. 2008;31:539–546. doi: 10.1007/s12272-001-1190-9. [DOI] [PubMed] [Google Scholar]
- 122.Oguri T, Achiwa H, Muramatsu H, et al. The absence of human equilibrative nucleoside transporter 1 expression predicts nonresponse to gemcitabine-containing chemotherapy in non-small cell lung cancer. Cancer Lett. 2007;256:112–119. doi: 10.1016/j.canlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 123.Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Mol Pharmacol. 2008;74:854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chattopadhyay S, Wang Y, Zhao R, Goldman ID. Lack of impact of the loss of constitutive folate receptor alpha expression, achieved by RNA Interference, on the activity of the new generation antifolate pemetrexed in HeLa cells. Clin Cancer Res. 2004;10:7986–7993. doi: 10.1158/1078-0432.CCR-04-1225. [DOI] [PubMed] [Google Scholar]
- 125.Theti DS, Jackman AL. The role of alpha-folate receptor-mediated transport in the antitumor activity of antifolate drugs. Clin Cancer Res. 2004;10:1080–1089. doi: 10.1158/1078-0432.ccr-03-0157. [DOI] [PubMed] [Google Scholar]
- 126.Nunez MI, Behrens C, Woods DM, et al. Immunohistochemical expression of membrane transporters correlates with histology type of non-small cell lung carcinoma. Proc of the 100th Annual Meeting of the American Association of Cancer Research. 2009 Abstract #1594. [Google Scholar]
- 127.Giaccone G, van Ark-Otte J, Rubio GJ, et al. MRP is frequently expressed in human lung-cancer cell lines, in non-small-cell lung cancer and in normal lungs. Int J Cancer. 1996;66:760–767. doi: 10.1002/(SICI)1097-0215(19960611)66:6<760::AID-IJC9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 128.Campling BG, Young LC, Baer KA, et al. Expression of the MRP and MDR1 multidrug resistance genes in small cell lung cancer. Clin Cancer Res. 1997;3:115–122. [PubMed] [Google Scholar]
- 129.Bessho Y, Oguri T, Ozasa H, et al. ABCC10/MRP7 is associated with vinorelbine resistance in nonsmall cell lung cancer. Oncol Rep. 2009;21:263–268. [PubMed] [Google Scholar]
- 130.Young LC, Campling BG, Cole SP, Deeley RG, Gerlach JH. Multidrug resistance proteins MRP3, MRP1, and MRP2 in lung cancer: correlation of protein levels with drug response and messenger RNA levels. Clin Cancer Res. 2001;7:1798–1804. [PubMed] [Google Scholar]
- 131.Narasaki F, Oka M, Fukuda M, et al. Multidrug resistance-associated protein gene expression and drug sensitivity in human lung cancer cells. Anticancer Res. 1997;17:3493–3497. [PubMed] [Google Scholar]
- 132.Oguri T, Ozasa H, Uemura T, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther. 2008;7:1150–1155. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- 133.Gonzalez Manzano R, Versanvoort C, Wright K, Twentyman PR. Rapid recovery of a functional MDR phenotype caused by MRP after a transient exposure to MDR drugs in a revertant human lung cancer cell line. Eur J Cancer. 1996;32A:2136–2141. doi: 10.1016/s0959-8049(96)00263-8. [DOI] [PubMed] [Google Scholar]
- 134.Perez-Soler R, Kemp B, Wu QP, et al. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice. Clin Cancer Res. 2000;6:4932–4938. [PubMed] [Google Scholar]
- 135.Oguri T, Achiwa H, Sato S, et al. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther. 2006;5:1800–1806. doi: 10.1158/1535-7163.MCT-06-0025. [DOI] [PubMed] [Google Scholar]
- 136.Zaman GJ, Lankelma J, van Tellingen O, et al. Role of glutathione in the export of compounds from cells by the multidrug-resistance-associated protein. Proc Natl Acad Sci U S A. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ikuta K, Takemura K, Sasaki K, et al. Expression of multidrug resistance proteins and accumulation of cisplatin in human non-small cell lung cancer cells. Biol Pharm Bull. 2005;28:707–712. doi: 10.1248/bpb.28.707. [DOI] [PubMed] [Google Scholar]
- 138.van Ark-Otte J, Samelis G, Rubio G, Lopez Saez JB, Pinedo HM, Giaccone G. Effects of tubulin-inhibiting agents in human lung and breast cancer cell lines with different multidrug resistance phenotypes. Oncol Rep. 1998;5:249–255. [PubMed] [Google Scholar]
- 139.Bergman AM, Pinedo HM, Talianidis I, et al. Increased sensitivity to gemcitabine of P-glycoprotein and multidrug resistance-associated protein-overexpressing human cancer cell lines. Br J Cancer. 2003;88:1963–1970. doi: 10.1038/sj.bjc.6601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Groot DJ, van der Deen M, Le TK, Regeling A, de Jong S, de Vries EG. Indomethacin induces apoptosis via a MRP1-dependent mechanism in doxorubicin-resistant small-cell lung cancer cells overexpressing MRP1. Br J Cancer. 2007;97:1077–1083. doi: 10.1038/sj.bjc.6604010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhan M, Liu X. Schedule-dependent reversion of cisplatin resistance by 5-fluorouracil in a cisplatin-resistant human lung adenocarcinoma cell line A549DDP. Chin Med J (Engl) 1999;112:336–339. [PubMed] [Google Scholar]
- 142.Kurokawa H, Ishida T, Nishio K, et al. Gamma-glutamylcysteine synthetase gene overexpression results in increased activity of the ATP-dependent glutathione S-conjugate export pump and cisplatin resistance. Biochem Biophys Res Commun. 1995;216:258–264. doi: 10.1006/bbrc.1995.2618. [DOI] [PubMed] [Google Scholar]
- 143.Xu M, Li J, Xia Q. Expression of multidrug resistance-associated protein gene in non-small cell lung cancer. Zhonghua Jie He He Hu Xi Za Zhi. 1999;22:268–270. [PubMed] [Google Scholar]
- 144.Oshika Y, Nakamura M, Tokunaga T, et al. Multidrug resistance-associated protein and mutant p53 protein expression in non-small cell lung cancer. Mod Pathol. 1998;11:1059–1063. [PubMed] [Google Scholar]
- 145.Ota E, Abe Y, Oshika Y, et al. Expression of the multidrug resistance-associated protein (MRP) gene in non-small-cell lung cancer. Br J Cancer. 1995;72:550–554. doi: 10.1038/bjc.1995.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Triller N, Korosec P, Kern I, Kosnik M, Debeljak A. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54:235–240. doi: 10.1016/j.lungcan.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 147.Yeh JJ, Hsu NY, Hsu WH, Tsai CH, Lin CC, Liang JA. Comparison of chemotherapy response with P-glycoprotein, multidrug resistance-related protein-1, and lung resistance-related protein expression in untreated small cell lung cancer. Lung. 2005;183:177–183. doi: 10.1007/s00408-004-2532-1. [DOI] [PubMed] [Google Scholar]
- 148.Ushijima R, Takayama K, Izumi M, et al. Immunohistochemical expression of MRP2 and clinical resistance to platinum-based chemotherapy in small cell lung cancer. Anticancer Res. 2007;27:4351–4358. [PubMed] [Google Scholar]
- 149.Kao A, Shiun SC, Hsu NY, Sun SS, Lee CC, Lin CC. Technetium-99m methoxyisobutylisonitrile chest imaging for small-cell lung cancer. Relationship to chemotherapy response (six courses of combination of cisplatin and etoposide) and p-glycoprotein or multidrug resistance related protein expression. Ann Oncol. 2001;12:1561–1566. doi: 10.1023/a:1013133801173. [DOI] [PubMed] [Google Scholar]
- 150.Kuo TH, Liu FY, Chuang CY, Wu HS, Wang JJ, Kao A. To predict response chemotherapy using technetium-99m tetrofosmin chest images in patients with untreated small cell lung cancer and compare with p-glycoprotein, multidrug resistance related protein-1, and lung resistance-related protein expression. Nucl Med Biol. 2003;30:627–632. doi: 10.1016/s0969-8051(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 151.Dirlik A, Burak Z, Goksel T, et al. The role of Tc-99m sestamibi imaging in predicting clinical response to chemotherapy in lung cancer. Ann Nucl Med. 2002;16:103–108. doi: 10.1007/BF02993712. [DOI] [PubMed] [Google Scholar]
- 152.Kreisholt J, Sorensen M, Jensen PB, Nielsen BS, Andersen CB, Sehested M. Immunohistochemical detection of DNA topoisomerase IIalpha, P-glycoprotein and multidrug resistance protein (MRP) in small-cell and non-small-cell lung cancer. Br J Cancer. 1998;77:1469–1473. doi: 10.1038/bjc.1998.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oguri T, Isobe T, Fujitaka K, Ishikawa N, Kohno N. Association between expression of the MRP3 gene and exposure to platinum drugs in lung cancer. Int J Cancer. 2001;93:584–589. doi: 10.1002/ijc.1369. [DOI] [PubMed] [Google Scholar]
- 154.Oguri T, Isobe T, Suzuki T, et al. Increased expression of the MRP5 gene is associated with exposure to platinum drugs in lung cancer. Int J Cancer. 2000;86:95–100. doi: 10.1002/(sici)1097-0215(20000401)86:1<95::aid-ijc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 155.Han JY, Lim HS, Yoo YK, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110:138–147. doi: 10.1002/cncr.22760. [DOI] [PubMed] [Google Scholar]
- 156.Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–1697. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 157.Wang J, Liu X, Jiang W. Expression of LRP, MRP and MDR1 in non-small-cell lung cancer and its clinical significance. Zhonghua Zhong Liu Za Zhi. 2000;22:304–307. [PubMed] [Google Scholar]
- 158.Dingemans AC, van Ark-Otte J, Span S, et al. Topoisomerase IIalpha and other drug resistance markers in advanced non-small cell lung cancer. Lung Cancer. 2001;32:117–128. doi: 10.1016/s0169-5002(00)00224-5. [DOI] [PubMed] [Google Scholar]
- 159.Filipits M, Haddad V, Schmid K, et al. Multidrug resistance proteins do not predict benefit of adjuvant chemotherapy in patients with completely resected non-small cell lung cancer: International Adjuvant Lung Cancer Trial Biologic Program. Clin Cancer Res. 2007;13:3892–3898. doi: 10.1158/1078-0432.CCR-06-2446. [DOI] [PubMed] [Google Scholar]
- 160.NicAmhlaoibh R, Heenan M, Cleary I, et al. Altered expression of mRNAs for apoptosis-modulating proteins in a low level multidrug resistant variant of a human lung carcinoma cell line that also expresses mdr1 mRNA. Int J Cancer. 1999;82:368–376. doi: 10.1002/(sici)1097-0215(19990730)82:3<368::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 161.Pesic M, Markovic JZ, Jankovic D, et al. Induced resistance in the human non small cell lung carcinoma (NCI-H460) cell line in vitro by anticancer drugs. J Chemother. 2006;18:66–73. doi: 10.1179/joc.2006.18.1.66. [DOI] [PubMed] [Google Scholar]
- 162.Sato H, Fukumoto K, Hada S, et al. Enhancing effect of connexin 32 gene on vinorelbine-induced cytotoxicity in A549 lung adenocarcinoma cells. Cancer Chemother Pharmacol. 2007;60:449–457. doi: 10.1007/s00280-006-0406-3. [DOI] [PubMed] [Google Scholar]
- 163.Ji D, Deeds SL, Weinstein EJ. A screen of shRNAs targeting tumor suppressor genes to identify factors involved in A549 paclitaxel sensitivity. Oncol Rep. 2007;18:1499–1505. [PubMed] [Google Scholar]
- 164.Kitazaki T, Oka M, Nakamura Y, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer. 2005;49:337–343. doi: 10.1016/j.lungcan.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 165.Inoue Y, Gika M, Abiko T, et al. Bcl-2 overexpression enhances in vitro sensitivity against docetaxel in non-small cell lung cancer. Oncol Rep. 2005;13:259–264. [PubMed] [Google Scholar]
- 166.Bogush TA, Konukhova AV, Ravcheeva AB, et al. Inhibition of ABC-transporter(s)' function in nonsmall cell lung cancer cells by platinum drugs. Antibiot Khimioter. 2003;48:11–15. [PubMed] [Google Scholar]
- 167.Stordal B, Davey R. A systematic review of genes involved in the inverse resistance relationship between cisplatin and paclitaxel chemotherapy: role of BRCA1. Curr Cancer Drug Targets. 2009;9:354–365. doi: 10.2174/156800909788166592. [DOI] [PubMed] [Google Scholar]
- 168.Glisson BS, Alpeter MD. Multidrug resistance in a small cell lung cancer line: rapid selection with etoposide and differential chemosensitization with cyclosporin A. Anticancer Drugs. 1992;3:359–366. doi: 10.1097/00001813-199208000-00007. [DOI] [PubMed] [Google Scholar]
- 169.Takigawa N, Ohnoshi T, Ueoka H, Kiura K, Kimura I. Establishment and characterization of an etoposide-resistant human small cell lung cancer cell line. Acta Med Okayama. 1992;46:203–212. doi: 10.18926/AMO/32669. [DOI] [PubMed] [Google Scholar]
- 170.Xia S, Yu SY, Yuan XL, Xu SP. Effects of hypoxia on expression of P-glycoprotein and multidrug resistance protein in human lung adenocarcinoma A549 cell line. Zhonghua Yi Xue Za Zhi. 2004;84:663–666. [PubMed] [Google Scholar]
- 171.Oka M, Fukuda M, Sakamoto A, et al. The clinical role of MDR1 gene expression in human lung cancer. Anticancer Res. 1997;17:721–724. [PubMed] [Google Scholar]
- 172.Peng ZM, Luo J, Wang WB, Wang XH, Chen JH, Lan SM. Predictive value of drug resistance-related genes expression in neoadjuvant chemotherapy in patients with non-small cell lung cancer of stage III. Ai Zheng. 2004;23:963–967. [PubMed] [Google Scholar]
- 173.Kawasaki M, Nakanishi Y, Kuwano K, Takayama K, Kiyohara C, Hara N. Immunohistochemically detected p53 and P-glycoprotein predict the response to chemotherapy in lung cancer. Eur J Cancer. 1998;34:1352–1357. doi: 10.1016/s0959-8049(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 174.Canitrot Y, Bichat F, Cole SP, et al. Multidrug resistance genes (MRP) and MDR1 expression in small cell lung cancer xenografts: relationship with response to chemotherapy. Cancer Lett. 1998;130:133–141. doi: 10.1016/s0304-3835(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 175.Yeh JJ, Hsu WH, Huang WT, Wang JJ, Ho ST, Kao A. Technetium-99m tetrofosmin SPECT predicts chemotherapy response in small cell lung cancer. Tumour Biol. 2003;24:151–155. doi: 10.1159/000073845. [DOI] [PubMed] [Google Scholar]
- 176.Savaraj N, Wu CJ, Xu R, et al. Multidrug-resistant gene expression in small-cell lung cancer. Am J Clin Oncol. 1997;20:398–403. doi: 10.1097/00000421-199708000-00016. [DOI] [PubMed] [Google Scholar]
- 177.Tabata M, Ohnoshi T, Ueoka H, Kiura K, Kimura I. MDR1 gene expression and treatment outcome in small cell lung cancer: MDR1 gene expression as an independent prognostic factor. Acta Med Okayama. 1993;47:243–248. doi: 10.18926/AMO/31553. [DOI] [PubMed] [Google Scholar]
- 178.Sohn JW, Lee SY, Lee SJ, et al. MDR1 polymorphisms predict the response to etoposide-cisplatin combination chemotherapy in small cell lung cancer. Jpn J Clin Oncol. 2006;36:137–141. doi: 10.1093/jjco/hyi231. [DOI] [PubMed] [Google Scholar]
- 179.Bom HS, Lim SC, Kim YC, et al. Dipyridamole modulated Tc-99m sestamibi lung SPECT in small cell lung cancer. Clin Nucl Med. 1999;24:97–101. doi: 10.1097/00003072-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 180.Kao CH, Ho YJ, Shen YY, Lee JK. Evaluation of chemotherapy response in patients with small cell lung cancer using Technetium-99m-tetrofosmin. Anticancer Res. 1999;19:2311–2315. [PubMed] [Google Scholar]
- 181.Bom HS, Kim YC, Song HC, Min JJ, Kim JY, Park KO. Technetium-99m-MIBI uptake in small cell lung cancer. J Nucl Med. 1998;39:91–94. [PubMed] [Google Scholar]
- 182.Changlai SP, Tsai CS, Ding HJ, Huang WT, Kao A, Hsu WH. Using technetium-99m methoxyisobutylisonitrile lung single-photon-emission computed tomography to predict response to chemotherapy and compare with P-glycoprotein expression in patients with untreated small cell lung cancer. Med Oncol. 2003;20:247–253. doi: 10.1385/mo:20:3:247. [DOI] [PubMed] [Google Scholar]
- 183.Akgun A, Cok G, Karapolat I, Goksel T, Burak Z. Tc-99m MIBI SPECT in prediction of prognosis in patients with small cell lung cancer. Ann Nucl Med. 2006;20:269–275. doi: 10.1007/BF02984643. [DOI] [PubMed] [Google Scholar]
- 184.Ceriani L, Giovanella L, Bandera M, Beghe B, Ortelli M, Roncari G. Semi-quantitative assessment of 99Tcm-sestamibi uptake in lung cancer: relationship with clinical response to chemotherapy. Nucl Med Commun. 1997;18:1087–1097. doi: 10.1097/00006231-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 185.Hsu WH, Yen RF, Kao CH, et al. Predicting chemotherapy response to paclitaxel-based therapy in advanced non-small-cell lung cancer (stage IIIb or IV) with a higher T stage (> T2). Technetium-99m methoxyisobutylisonitrile chest single photon emission computed tomography and P-glycoprotein express ion. Oncology. 2002;63:173–179. doi: 10.1159/000063811. [DOI] [PubMed] [Google Scholar]
- 186.Yeh JJ, Hsu WH, Wang JJ, Ho ST, Kao A. Predicting chemotherapy response to paclitaxel-based therapy in advanced non-small-cell lung cancer with P-glycoprotein expression. Respiration. 2003;70:32–35. doi: 10.1159/000068411. [DOI] [PubMed] [Google Scholar]
- 187.Vlachogeorgos GS, Manali ED, Blana E, et al. Placental isoform glutathione S-transferase and P-glycoprotein expression in advanced nonsmall cell lung cancer: association with response to treatment and survival. Cancer. 2008;114:519–526. doi: 10.1002/cncr.23981. [DOI] [PubMed] [Google Scholar]
- 188.Pan JH, Han JX, Wu JM, Sheng LJ, Huang HN, Yu QZ. MDR1 single nucleotide polymorphisms predict response to vinorelbine-based chemotherapy in patients with non-small cell lung cancer. Respiration. 2008;75:380–385. doi: 10.1159/000108407. [DOI] [PubMed] [Google Scholar]
- 189.Sasaki M, Kuwabara Y, Ichiya Y, et al. Prediction of the chemosensitivity of lung cancer by 99mT-chexakis-2-methoxyisobutyl isonitrile SPECT. J Nucl Med. 1999;40:1778–1783. [PubMed] [Google Scholar]
- 190.Yuksel M, Cermik TF, Doganay L, et al. 99mTc-MIBI SPET in non-small cell lung cancer in relationship with Pgp and prognosis. Eur J Nucl Med Mol Imaging. 2002;29:876–881. doi: 10.1007/s00259-002-0804-7. [DOI] [PubMed] [Google Scholar]
- 191.Shih CM, Hsu WH, Huang WT, Wang JJ, Ho ST, Kao A. Usefulness of chest single photon emission computed tomography with technetium-99m methoxyisobutylisonitrile to predict taxol based chemotherapy response in advanced non-small cell lung cancer. Cancer Lett. 2003;199:99–105. doi: 10.1016/s0304-3835(03)00335-5. [DOI] [PubMed] [Google Scholar]
- 192.Shih CM, Shiau YC, Wang JJ, Ho ST, Kao A. Using technetium-99m tetrofosmin chest imaging to predict taxol-based chemotherapy response in non-small cell lung cancer but not related to lung resistance protein expression. Lung. 2003;181:103–111. doi: 10.1007/s00408-003-1011-4. [DOI] [PubMed] [Google Scholar]
- 193.Fukumoto M, Yoshida D, Hayase N, Kurohara A, Akagi N, Yoshida S. Scintigraphic prediction of resistance to radiation and chemotherapy in patients with lung carcinoma: technetium 99m-tetrofosmin and thallium-201 dual single photon emission computed tomography study. Cancer. 1999;86:1470–1479. [PubMed] [Google Scholar]
- 194.Miyatake K, Gemba K, Ueoka H, et al. Prognostic significance of mutant p53 protein, P-glycoprotein and glutathione S-transferase-pi in patients with unresectable non-small cell lung cancer. Anticancer Res. 2003;23:2829–2836. [PubMed] [Google Scholar]
- 195.Brooks KR, To K, Joshi MB, et al. Measurement of chemoresistance markers in patients with stage III non-small cell lung cancer: a novel approach for patient selection. Ann Thorac Surg. 2003;76:187–193. doi: 10.1016/s0003-4975(03)00131-0. discussion 93. [DOI] [PubMed] [Google Scholar]
- 196.Isla D, Sarries C, Rosell R, et al. Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann Oncol. 2004;15:1194–1203. doi: 10.1093/annonc/mdh319. [DOI] [PubMed] [Google Scholar]
- 197.Arvelo F, Poupon MF, Bichat F, et al. Adding a reverser (verapamil) to combined chemotherapy overrides resistance in small cell lung cancer xenografts. Eur J Cancer. 1995;31A:1862–1868. doi: 10.1016/0959-8049(95)00386-w. [DOI] [PubMed] [Google Scholar]
- 198.Yang CH, Huang CJ, Yang CS, et al. Gefitinib reverses chemotherapy resistance in gefitinib-insensitive multidrug resistant cancer cells expressing ATP-binding cassette family protein. Cancer Res. 2005;65:6943–6949. doi: 10.1158/0008-5472.CAN-05-0641. [DOI] [PubMed] [Google Scholar]
- 199.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 200.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 201.Kruijtzer CM, Schellens JH, Mezger J, et al. Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4508–4516. doi: 10.1200/JCO.2002.04.058. [DOI] [PubMed] [Google Scholar]
- 202.Ross HJ, Cho J, Osann K, et al. Phase I/II trial of low dose cyclosporin A with EP for advanced non-small cell lung cancer. Lung Cancer. 1997;18:189–198. doi: 10.1016/s0169-5002(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 203.Gatzemeier U, Schneider A, von Pawel J. Randomised trial of vindesine and etoposide +/− dexverapamil in advanced non-small cell lung cancer: first results. J Cancer Res Clin Oncol. 1995;121 Suppl 3:R17–R20. doi: 10.1007/BF02351066. [DOI] [PubMed] [Google Scholar]
- 204.Stewart DJ, Evans WK. Non-chemotherapeutic agents that potentiate chemotherapy efficacy. Cancer Treat Rev. 1989;16:1–40. doi: 10.1016/0305-7372(89)90002-9. [DOI] [PubMed] [Google Scholar]
- 205.Christen RD, Shalinsky DR, Howell SB. Enhancement of the loss of multiple drug resistance by hydroxyurea. Semin Oncol. 1992;19:94–100. [PubMed] [Google Scholar]
- 206.Stewart DJ, Tomiak EM, Goss G, et al. Paclitaxel plus hydroxyurea as second line therapy for non-small cell lung cancer. Lung Cancer. 1996;15:115–123. doi: 10.1016/0169-5002(96)00576-4. [DOI] [PubMed] [Google Scholar]
- 207.Wood L, Palmer M, Hewitt J, et al. Results of a phase III, double-blind, placebo-controlled trial of megestrol acetate modulation of P-glycoprotein-mediated drug resistance in the first-line management of small-cell lung carcinoma. Br J Cancer. 1998;77:627–631. doi: 10.1038/bjc.1998.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Milroy R West of Scotland Lung Cancer Research Group, and the Aberdeen Oncology Group. A randomised clinical study of verapamil in addition to combination chemotherapy in small cell lung cancer. Br J Cancer. 1993;68:813–818. doi: 10.1038/bjc.1993.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Berger W, Steiner E, Grusch M, Elbling L, Micksche M. Vaults and the major vault protein: novel roles in signal pathway regulation and immunity. Cell Mol Life Sci. 2009;66:43–61. doi: 10.1007/s00018-008-8364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Berger W, Elbling L, Micksche M. Expression of the major vault protein LRP in human non-small-cell lung cancer cells: activation by short-term exposure to antineoplastic drugs. Int J Cancer. 2000;88:293–300. [PubMed] [Google Scholar]
- 211.Ikeda K, Oka M, Narasaki F, et al. Lung resistance-related protein gene expression and drug sensitivity in human gastric and lung cancer cells. Anticancer Res. 1998;18:3077–3080. [PubMed] [Google Scholar]
- 212.Lee E, Lim SJ. The association of increased lung resistance protein expression with acquired etoposide resistance in human H460 lung cancer cell lines. Arch Pharm Res. 2006;29:1018–1023. doi: 10.1007/BF02969286. [DOI] [PubMed] [Google Scholar]
- 213.Trussardi A, Poitevin G, Gorisse MC, et al. Sequential overexpression of LRP and MRP but not P-gp 170 in VP16-selected A549 adenocarcinoma cells. Int J Oncol. 1998;13:543–548. doi: 10.3892/ijo.13.3.543. [DOI] [PubMed] [Google Scholar]
- 214.Harada T, Ogura S, Yamazaki K, et al. Predictive value of expression of P53, Bcl-2 and lung resistance-related protein for response to chemotherapy in non-small cell lung cancers. Cancer Sci. 2003;94:394–399. doi: 10.1111/j.1349-7006.2003.tb01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dingemans AM, van Ark-Otte J, van der Valk P, et al. Expression of the human major vault protein LRP in human lung cancer samples and normal lung tissues. Ann Oncol. 1996;7:625–630. doi: 10.1093/oxfordjournals.annonc.a010681. [DOI] [PubMed] [Google Scholar]
- 216.Chiou JF, Liang JA, Hsu WH, Wang JJ, Ho ST, Kao A. Comparing the relationship of Taxol-based chemotherapy response with P-glycoprotein and lung resistance-related protein expression in non-small cell lung cancer. Lung. 2003;181:267–273. doi: 10.1007/s00408-003-1029-7. [DOI] [PubMed] [Google Scholar]
- 217.Oguri T, Fujiwara Y, Ochiai M, et al. Expression of lung-resistance protein gene is not associated with platinum drug exposure in lung cancer. Anticancer Res. 1998;18:4159–4162. [PubMed] [Google Scholar]
- 218.Nakagawa T, Inoue Y, Kodama H, et al. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) correlates with cisplatin resistance in human non-small cell lung cancer xenografts. Oncol Rep. 2008;20:265–270. [PubMed] [Google Scholar]
- 219.Katoh R, Takebayashi Y, Takenoshita S. Expression of copper-transporting P-type adenosine triphosphatase (ATP7B) as a chemoresistance marker in human solid carcinomas. Ann Thorac Cardiovasc Surg. 2005;11:143–145. [PubMed] [Google Scholar]
- 220.Ota S, Ishii G, Goto K, et al. Immunohistochemical expression of BCRP and ERCC1 in biopsy specimen predicts survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Lung Cancer. 2009;64:98–104. doi: 10.1016/j.lungcan.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 221.Hu Y, Feng FY, Cheng SJ, Gao YN, Xiao T, Liu YN. Expression of serum breast drug-resistance protein in predicting chemosensitivity of NSCLC. Zhonghua Zhong Liu Za Zhi. 2006;28:750–752. [PubMed] [Google Scholar]
- 222.Hu Y, Lin DM, Cheng SJ, Liu YN, Feng FY. Influences of PC cell-derived growth factor and breast cancer resistance protein on the curative effects of platinum-based chemotherapeutic regimens for advanced non-small cell lung cancer. Zhonghua Yi Xue Za Zhi. 2006;86:2611–2614. [PubMed] [Google Scholar]
- 223.Stuckler D, Singhal J, Singhal SS, Yadav S, Awasthi YC, Awasthi S. RLIP76 transports vinorelbine and mediates drug resistance in non-small cell lung cancer. Cancer Res. 2005;65:991–998. [PubMed] [Google Scholar]
- 224.Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem Pharmacol. 2005;70:481–488. doi: 10.1016/j.bcp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 225.Awasthi S, Singhal SS, Singhal J, Yang Y, Zimniak P, Awasthi YC. Role of RLIP76 in lung cancer doxorubicin resistance: III. Anti-RLIP76 antibodies trigger apoptosis in lung cancer cells and synergistically increase doxorubicin cytotoxicity. Int J Oncol. 2003;22:721–732. [PubMed] [Google Scholar]
- 226.Singhal SS, Wickramarachchi D, Singhal J, Yadav S, Awasthi YC, Awasthi S. Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett. 2006;580:2258–2264. doi: 10.1016/j.febslet.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 227.Singhal SS, Singhal J, Yadav S, et al. Regression of lung and colon cancer xenografts by depleting or inhibiting RLIP76 (Ral-binding protein 1) Cancer Res. 2007;67:4382–4389. doi: 10.1158/0008-5472.CAN-06-4124. [DOI] [PubMed] [Google Scholar]
- 228.Sharma R, Singhal SS, Wickramarachchi D, Awasthi YC, Awasthi S. RLIP76 (RALBP1)-mediated transport of leukotriene C4 (LTC4) in cancer cells: implications in drug resistance. Int J Cancer. 2004;112:934–942. doi: 10.1002/ijc.20516. [DOI] [PubMed] [Google Scholar]
- 229.Meijer C, Mulder NH, Hospers GA, Uges DR, de Vries EG. The role of glutathione in resistance to cisplatin in a human small cell lung cancer cell line. Br J Cancer. 1990;62:72–77. doi: 10.1038/bjc.1990.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.D'Incalci M, Bonfanti M, Pifferi A, et al. EORTC SPG and PAMM Groups. The antitumour activity of alkylating agents is not correlated with the levels of glutathione, glutathione transferase and O6-alkylguanine-DNA-alkyltransferase of human tumour xenografts. Eur J Cancer. 1998;34:1749–1755. doi: 10.1016/s0959-8049(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 231.Sharma R, Singhal SS, Srivastava SK, Bajpai KK, Frenkel EP, Awasthi S. Glutathione and glutathione linked enzymes in human small cell lung cancer cell lines. Cancer Lett. 1993;75:111–119. doi: 10.1016/0304-3835(93)90195-f. [DOI] [PubMed] [Google Scholar]
- 232.Kasahara K, Fujiwara Y, Nishio K, et al. Metallothionein content correlates with the sensitivity of human small cell lung cancer cell lines to cisplatin. Cancer Res. 1991;51:3237–3242. [PubMed] [Google Scholar]
- 233.Fokkema E, Groen HJ, Helder MN, de Vries EG, Meijer C. JM216-, JM118-, and cisplatin-induced cytotoxicity in relation to platinum-DNA adduct formation, glutathione levels and p53 status in human tumour cell lines with different sensitivities to cisplatin. Biochem Pharmacol. 2002;63:1989–1996. doi: 10.1016/s0006-2952(02)00983-8. [DOI] [PubMed] [Google Scholar]
- 234.Oshita F, Fujiwara Y, Saijo N. Radiation sensitivities in various anticancer-drug-resistant human lung cancer cell lines and mechanism of radiation cross-resistance in a cisplatin-resistant cell line. J Cancer Res Clin Oncol. 1992;119:28–34. doi: 10.1007/BF01209484. [DOI] [PubMed] [Google Scholar]
- 235.Meijer C, Mulder NH, Timmer-Bosscha H, Sluiter WJ, Meersma GJ, de Vries EG. Relationship of cellular glutathione to the cytotoxicity and resistance of seven platinum compounds. Cancer Res. 1992;52:6885–6889. [PubMed] [Google Scholar]
- 236.Jain N, Lam YM, Pym J, Campling BG. Mechanisms of resistance of human small cell lung cancer lines selected in VP-16 and cisplatin. Cancer. 1996;77:1797–1808. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1797::AID-CNCR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 237.Hospers GA, Meijer C, de Leij L, Uges DR, Mulder NH, de Vries EG. A study of human small-cell lung carcinoma (hSCLC) cell lines with different sensitivities to detect relevant mechanisms of cisplatin (CDDP) resistance. Int J Cancer. 1990;46:138–144. doi: 10.1002/ijc.2910460125. [DOI] [PubMed] [Google Scholar]
- 238.Curtin NJ, Turner DP. Dipyridamole-mediated reversal of multidrug resistance in MRP over-expressing human lung carcinoma cells in vitro. Eur J Cancer. 1999;35:1020–1026. doi: 10.1016/s0959-8049(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 239.Nishi M, Abe Y, Fujimori S, et al. The modifier subunit of glutamate cysteine ligase relates to cisplatin resistance in human small cell lung cancer xenografts in vivo. Oncol Rep. 2005;14:421–424. [PubMed] [Google Scholar]
- 240.Fujimori S, Abe Y, Nishi M, et al. The subunits of glutamate cysteine ligase enhance cisplatin resistance in human non-small cell lung cancer xenografts in vivo. Int J Oncol. 2004;25:413–418. [PubMed] [Google Scholar]
- 241.Inoue Y, Tomisawa M, Yamazaki H, et al. The modifier subunit of glutamate cysteine ligase (GCLM) is a molecular target for amelioration of cisplatin resistance in lung cancer. Int J Oncol. 2003;23:1333–1339. [PubMed] [Google Scholar]
- 242.Yang P, Mandrekar SJ, Hillman SH, et al. Evaluation of glutathione metabolic genes on outcomes in advanced non-small cell lung cancer patients after initial treatment with platinum-based chemotherapy: an NCCTG-97-24-51 based study. J Thorac Oncol. 2009;4:479–485. doi: 10.1097/jto.0b013e31819c7a2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ogawa J, Iwazaki M, Inoue H, Koide S, Shohtsu A. Immunohistochemical study of glutathione-related enzymes and proliferative antigens in lung cancer. Relation to cisplatin sensitivity. Cancer. 1993;71:2204–2209. doi: 10.1002/1097-0142(19930401)71:7<2204::aid-cncr2820710707>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 244.Hida T, Ariyoshi Y, Kuwabara M, et al. Glutathione S-transferase pi levels in a panel of lung cancer cell lines and its relation to chemo-radiosensitivity. Jpn J Clin Oncol. 1993;23:14–19. [PubMed] [Google Scholar]
- 245.Nakagawa K, Yokota J, Wada M, et al. Levels of glutathione S transferase pi mRNA in human lung cancer cell lines correlate with the resistance to cisplatin and carboplatin. Jpn J Cancer Res. 1988;79:301–304. doi: 10.1111/j.1349-7006.1988.tb01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Inoue T, Ishida T, Sugio K, Maehara Y, Sugimachi K. Glutathione S transferase Pi is a powerful indicator in chemotherapy of human lung squamous-cell carcinoma. Respiration. 1995;62:223–227. doi: 10.1159/000196451. [DOI] [PubMed] [Google Scholar]
- 247.Awasthi S, Sharma R, Singhal SS, Herzog NK, Chaubey M, Awasthi YC. Modulation of cisplatin cytotoxicity by sulphasalazine. Br J Cancer. 1994;70:190–194. doi: 10.1038/bjc.1994.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Miyara H, Hida T, Nishida K, et al. Modification of chemo-radiosensitivity of a human lung cancer cell line by introduction of the glutathione S-transferase pi gene. Jpn J Clin Oncol. 1996;26:1–5. doi: 10.1093/oxfordjournals.jjco.a023171. [DOI] [PubMed] [Google Scholar]
- 249.Nakanishi Y, Kawasaki M, Bai F, et al. Expression of p53 and glutathione S-transferase-pi relates to clinical drug resistance in non-small cell lung cancer. Oncology. 1999;57:318–323. doi: 10.1159/000012068. [DOI] [PubMed] [Google Scholar]
- 250.Arai T, Yasuda Y, Takaya T, et al. Immunohistochemical expression of glutathione transferase-pi in untreated primary non-small-cell lung cancer. Cancer Detect Prev. 2000;24:252–257. [PubMed] [Google Scholar]
- 251.Bai F, Nakanishi Y, Kawasaki M, et al. Immunohistochemical expression of glutathione S-transferase-Pi can predict chemotherapy response in patients with nonsmall cell lung carcinoma. Cancer. 1996;78:416–421. doi: 10.1002/(SICI)1097-0142(19960801)78:3<416::AID-CNCR6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 252.Hida T, Kuwabara M, Ariyoshi Y, et al. Serum glutathione S-transferase-pi level as a tumor marker for non-small cell lung cancer. Potential predictive value in chemotherapeutic response. Cancer. 1994;73:1377–1382. doi: 10.1002/1097-0142(19940301)73:5<1377::aid-cncr2820730511>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 253.Lu C, Spitz MR, Zhao H, et al. Association between glutathione S-transferase pi polymorphisms and survival in patients with advanced nonsmall cell lung carcinoma. Cancer. 2006;106:441–447. doi: 10.1002/cncr.21619. [DOI] [PubMed] [Google Scholar]
- 254.Unsal M, Akpolat I, Kandemir B. Glutathione-S transferase-pi expression in non small cell lung cancer in the assessment of response to chemotherapy. Saudi Med J. 2003;24:493–498. [PubMed] [Google Scholar]
- 255.Booton R, Ward T, Heighway J, Ashcroft L, Morris J, Thatcher N. Glutathione-S-transferase P1 isoenzyme polymorphisms, platinum-based chemotherapy, and non-small cell lung cancer. J Thorac Oncol. 2006;1:679–683. [PubMed] [Google Scholar]
- 256.Shimoda R, Achanzar WE, Qu W, et al. Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci. 2003;73:294–300. doi: 10.1093/toxsci/kfg095. [DOI] [PubMed] [Google Scholar]
- 257.Joseph MG, Banerjee D, Kocha W, Feld R, Stitt LW, Cherian MG. Metallothionein expression in patients with small cell carcinoma of the lung: correlation with other molecular markers and clinical outcome. Cancer. 2001;92:836–842. [PubMed] [Google Scholar]
- 258.Matsumoto Y, Oka M, Sakamoto A, et al. Enhanced expression of metallothionein in human non-small-cell lung carcinomas following chemotherapy. Anticancer Res. 1997;17:3777–3780. [PubMed] [Google Scholar]
- 259.Hung JJ, Chow KC, Wang HW, Wang LS. Expression of dihydrodiol dehydrogenase and resistance to chemotherapy and radiotherapy in adenocarcinoma cells of lung. Anticancer Res. 2006;26:2949–2955. [PubMed] [Google Scholar]
- 260.Wang HW, Lin CP, Chiu JH, et al. Reversal of inflammation-associated dihydrodiol dehydrogenases (AKR1C1 and AKR1C2) overexpression and drug resistance in nonsmall cell lung cancer cells by wogonin and chrysin. Int J Cancer. 2007;120:2019–2027. doi: 10.1002/ijc.22402. [DOI] [PubMed] [Google Scholar]
- 261.Deng HB, Adikari M, Parekh HK, Simpkins H. Ubiquitous induction of resistance to platinum drugs in human ovarian, cervical, germ-cell and lung carcinoma tumor cells overexpressing isoforms 1 and 2 of dihydrodiol dehydrogenase. Cancer Chemother Pharmacol. 2004;54:301–307. doi: 10.1007/s00280-004-0815-0. [DOI] [PubMed] [Google Scholar]
- 262.Tibaldi C, Giovannetti E, Vasile E, et al. Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2008;14:1797–1803. doi: 10.1158/1078-0432.CCR-07-1364. [DOI] [PubMed] [Google Scholar]
- 263.Kropotov A, Gogvadze V, Shupliakov O, et al. Peroxiredoxin V is essential for protection against apoptosis in human lung carcinoma cells. Exp Cell Res. 2006;312:2806–2815. doi: 10.1016/j.yexcr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 264.Smith PG, Marshman E, Newell DR, Curtin NJ. Dipyridamole potentiates the in vitro activity of MTA ( LY231514) by inhibition of thymidine transport. Br J Cancer. 2000;82:924–930. doi: 10.1054/bjoc.1999.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chattopadhyay S, Tamari R, Min SH, Zhao R, Tsai E, Goldman ID. Commentary: a case for minimizing folate supplementation in clinical regimens with pemetrexed based on the marked sensitivity of the drug to folate availability. Oncologist. 2007;12:808–815. doi: 10.1634/theoncologist.12-7-808. [DOI] [PubMed] [Google Scholar]
- 266.van Bree C, Castro Kreder N, Loves WJ, Franken NA, Peters GJ, Haveman J. Sensitivity to ionizing radiation and chemotherapeutic agents in gemcitabine-resistant human tumor cell lines. Int J Radiat Oncol Biol Phys. 2002;54:237–244. doi: 10.1016/s0360-3016(02)02891-2. [DOI] [PubMed] [Google Scholar]
- 267.Giovannetti E, Mey V, Nannizzi S, et al. Cellular and pharmacogenetics foundation of synergistic interaction of pemetrexed and gemcitabine in human non-small-cell lung cancer cells. Mol Pharmacol. 2005;68:110–118. doi: 10.1124/mol.104.009373. [DOI] [PubMed] [Google Scholar]
- 268.Bergman AM, Pinedo HM, Peters GJ. Steroids affect collateral sensitivity to gemcitabine of multidrug-resistant human lung cancer cells. Eur J Pharmacol. 2001;416:19–24. doi: 10.1016/s0014-2999(01)00858-5. [DOI] [PubMed] [Google Scholar]
- 269.Chau Q, Stewart DJ. Cisplatin efflux, binding and intracellular pH in the HTB56 human lung adenocarcinoma cell line and the E-8/0.7 cisplatin-resistant variant. Cancer Chemother Pharmacol. 1999;44:193–202. doi: 10.1007/s002800050967. [DOI] [PubMed] [Google Scholar]
- 270.Meijera C, van Luyn MJ, Nienhuis EF, Blom N, Mulder NH, de Vries EG. Ultrastructural morphology and localisation of cisplatin-induced platinum-DNA adducts in a cisplatin-sensitive and -resistant human small cell lung cancer cell line using electron microscopy. Biochem Pharmacol. 2001;61:573–578. doi: 10.1016/s0006-2952(00)00584-0. [DOI] [PubMed] [Google Scholar]
- 271.Morikage T, Ohmori T, Nishio K, Fujiwara Y, Takeda Y, Saijo N. Modulation of cisplatin sensitivity and accumulation by amphotericin B in cisplatin-resistant human lung cancer cell lines. Cancer Res. 1993;53:3302–3307. [PubMed] [Google Scholar]
- 272.Huang Z, Huang Y. The change of intracellular pH is involved in the cisplatin-resistance of human lung adenocarcinoma A549/DDP cells. Cancer Invest. 2005;23:26–32. [PubMed] [Google Scholar]
- 273.Bando T, Kasahara K, Shibata K, Nakatsumi Y, Fujimura M, Matsuda T. Effect of proton pump inhibitor on cell growth and sensitivity to cis-diamminedichloroplatinum(II) in non-small cell lung cancer cell lines. Anticancer Res. 1994;14:2727–2730. [PubMed] [Google Scholar]
- 274.van de Vaart PJ, Belderbos J, de Jong D, et al. DNA-adduct levels as a predictor of outcome for NSCLC patients receiving daily cisplatin and radiotherapy. Int J Cancer. 2000;89:160–166. [PubMed] [Google Scholar]
- 275.Wehbe H, Kearney CM, Pinney KG. Combretastatin A-4 resistance in H460 human lung carcinoma demonstrates distinctive alterations in beta-tubulin isotype expression. Anticancer Res. 2005;25:3865–3870. [PubMed] [Google Scholar]
- 276.Chang JT, Chang GC, Ko JL, et al. Induction of tubulin by docetaxel is associated with p53 status in human non small cell lung cancer cell lines. Int J Cancer. 2006;118:317–325. doi: 10.1002/ijc.21372. [DOI] [PubMed] [Google Scholar]
- 277.Verdier-Pinard P, Wang F, Martello L, Burd B, Orr GA, Horwitz SB. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry. Biochemistry. 2003;42:5349–5357. doi: 10.1021/bi027293o. [DOI] [PubMed] [Google Scholar]
- 278.Han EK, Gehrke L, Tahir SK, et al. Modulation of drug resistance by alpha-tubulin in paclitaxel-resistant human lung cancer cell lines. Eur J Cancer. 2000;36:1565–1571. doi: 10.1016/s0959-8049(00)00145-3. [DOI] [PubMed] [Google Scholar]
- 279.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br J Cancer. 1999;80:1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chan MW, Chiang CD, Song EJ, Yang VC. Effects of cytoskeletal inhibitors on the accumulation of vincristine in a resistant human lung cancer cell line with high level of polymerized tubulin. Cancer Biochem Biophys. 1998;16:347–363. [PubMed] [Google Scholar]
- 281.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67:9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 282.Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 283.Zhou Q, Bai M, Su Y. Effect of antisense RNA targeting polo-like kinase 1 on cell cycle and proliferation in A549 cells. Chin Med J (Engl) 2004;117:1642–1649. [PubMed] [Google Scholar]
- 284.Goncalves A, Braguer D, Kamath K, et al. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc Natl Acad Sci U S A. 2001;98:11737–11742. doi: 10.1073/pnas.191388598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ohta S, Nishio K, Kubota N, et al. Characterization of a taxol-resistant human small-cell lung cancer cell line. Jpn J Cancer Res. 1994;85:290–297. doi: 10.1111/j.1349-7006.1994.tb02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Marcus AI, Zhou J, O'Brate A, et al. The synergistic combination of the farnesyl transferase inhibitor lonafarnib and paclitaxel enhances tubulin acetylation and requires a functional tubulin deacetylase. Cancer Res. 2005;65:3883–3893. doi: 10.1158/0008-5472.CAN-04-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Dumontet C, Isaac S, Souquet PJ, et al. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull Cancer. 2005;92:E25–E30. [PubMed] [Google Scholar]
- 288.Rosell R, Felip E. Predicting response to paclitaxel/carboplatin-based therapy in non-small cell lung cancer. Semin Oncol. 2001;28:37–44. doi: 10.1016/s0093-7754(01)90058-2. [DOI] [PubMed] [Google Scholar]
- 289.Tsurutani J, Komiya T, Uejima H, et al. Mutational analysis of the beta-tubulin gene in lung cancer. Lung Cancer. 2002;35:11–16. doi: 10.1016/s0169-5002(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 290.Seve P, Lai R, Ding K, et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR.10. Clin Cancer Res. 2007;13:994–999. doi: 10.1158/1078-0432.CCR-06-1503. [DOI] [PubMed] [Google Scholar]
- 291.Rosell R, Scagliotti G, Danenberg KD, et al. Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene. 2003;22:3548–3553. doi: 10.1038/sj.onc.1206419. [DOI] [PubMed] [Google Scholar]
- 292.Seve P, Mackey J, Isaac S, et al. Class III beta-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol Cancer Ther. 2005;4:2001–2007. doi: 10.1158/1535-7163.MCT-05-0244. [DOI] [PubMed] [Google Scholar]
- 293.Seve P, Isaac S, Tredan O, et al. Expression of class III {beta}-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin Cancer Res. 2005;11:5481–5486. doi: 10.1158/1078-0432.CCR-05-0285. [DOI] [PubMed] [Google Scholar]
- 294.Yamazaki K, Isobe H, Hanada T, et al. Topoisomerase II alpha content and topoisomerase II catalytic activity cannot explain drug sensitivities to topoisomerase II inhibitors in lung cancer cell lines. Cancer Chemother Pharmacol. 1997;39:192–198. doi: 10.1007/s002800050559. [DOI] [PubMed] [Google Scholar]
- 295.Houlbrook S, Harris AL, Carmichael J, Stratford IJ. Relationship between topoisomerase II levels and resistance to topoisomerase II inhibitors in lung cancer cell lines. Anticancer Res. 1996;16:1603–1610. [PubMed] [Google Scholar]
- 296.Wessel I, Jensen PB, Falck J, Mirski SE, Cole SP, Sehested M. Loss of amino acids 1490Lys-Ser-Lys1492 in the COOH-terminal region of topoisomerase IIalpha in human small cell lung cancer cells selected for resistance to etoposide results in an extranuclear enzyme localization. Cancer Res. 1997;57:4451–4454. [PubMed] [Google Scholar]
- 297.Andoh T, Nishizawa M, Hida T, Ariyoshi Y, Takahashi T, Ueda R. Reduced expression of DNA topoisomerase II confers resistance to etoposide (VP-16) in small cell lung cancer cell lines established from a refractory tumor of a patient and by in vitro selection. Oncol Res. 1996;8:229–238. [PubMed] [Google Scholar]
- 298.Mirski SE, Evans CD, Almquist KC, Slovak ML, Cole SP. Altered topoisomerase II alpha in a drug-resistant small cell lung cancer cell line selected in VP-16. Cancer Res. 1993;53:4866–4873. [PubMed] [Google Scholar]
- 299.Giaccone G, Gazdar AF, Beck H, Zunino F, Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992;52:1666–1674. [PubMed] [Google Scholar]
- 300.de Jong S, Zijlstra JG, de Vries EG, Mulder NH. Reduced DNA topoisomerase II activity and drug-induced DNA cleavage activity in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1990;50:304–309. [PubMed] [Google Scholar]
- 301.Evans CD, Mirski SE, Danks MK, Cole SP. Reduced levels of topoisomerase II alpha and II beta in a multidrug-resistant lung-cancer cell line. Cancer Chemother Pharmacol. 1994;34:242–248. doi: 10.1007/BF00685084. [DOI] [PubMed] [Google Scholar]
- 302.de Lucio B, Manuel V, Barrera-Rodriguez R. Characterization of human NSCLC cell line with innate etoposide-resistance mediated by cytoplasmic localization of topoisomerase II alpha. Cancer Sci. 2005;96:774–783. doi: 10.1111/j.1349-7006.2005.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Eijdems EW, de Haas M, Timmerman AJ, et al. Reduced topoisomerase II activity in multidrug-resistant human non-small cell lung cancer cell lines. Br J Cancer. 1995;71:40–47. doi: 10.1038/bjc.1995.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Guinee DG, Jr, Holden JA, Benfield JR, et al. Comparison of DNA topoisomerase II alpha expression in small cell and nonsmall cell carcinoma of the lung. In search of a mechanism of chemotherapeutic response. Cancer. 1996;78:729–735. doi: 10.1002/(SICI)1097-0142(19960815)78:4<729::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 305.Kubo A, Yoshikawa A, Hirashima T, et al. Point mutations of the topoisomerase IIalpha gene in patients with small cell lung cancer treated with etoposide. Cancer Res. 1996;56:1232–1236. [PubMed] [Google Scholar]
- 306.Ceppi P, Longo M, Volante M, et al. Excision repair cross complementing-1 and topoisomerase IIalpha gene expression in small-cell lung cancer patients treated with platinum and etoposide: a retrospective study. J Thorac Oncol. 2008;3:583–589. doi: 10.1097/JTO.0b013e3181734f24. [DOI] [PubMed] [Google Scholar]
- 307.Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999;5:2048–2058. [PubMed] [Google Scholar]
- 308.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 309.Shimizu J, Horio Y, Osada H, et al. mRNA expression of RRM1, ERCC1 and ERCC2 is not associated with chemosensitivity to cisplatin, carboplatin and gemcitabine in human lung cancer cell lines. Respirology. 2008;13:510–517. doi: 10.1111/j.1440-1843.2008.01302.x. [DOI] [PubMed] [Google Scholar]
- 310.Kwon WS, Rha SY, Choi YH, et al. Ribonucleotide reductase M1 (RRM1) 2464G>A polymorphism shows an association with gemcitabine chemosensitivity in cancer cell lines. Pharmacogenet Genomics. 2006;16:429–438. doi: 10.1097/01.fpc.0000204999.29924.da. [DOI] [PubMed] [Google Scholar]
- 311.Tooker P, Yen WC, Ng SC, Negro-Vilar A, Hermann TW. Bexarotene (LGD1069, Targretin), a selective retinoid X receptor agonist, prevents and reverses gemcitabine resistance in NSCLC cells by modulating gene amplification. Cancer Res. 2007;67:4425–4433. doi: 10.1158/0008-5472.CAN-06-4495. [DOI] [PubMed] [Google Scholar]
- 312.Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–3766. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 313.Rosell R, Danenberg KD, Alberola V, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2004;10:1318–1325. doi: 10.1158/1078-0432.ccr-03-0156. [DOI] [PubMed] [Google Scholar]
- 314.Ceppi P, Volante M, Novello S, et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol. 2006;17:1818–1825. doi: 10.1093/annonc/mdl300. [DOI] [PubMed] [Google Scholar]
- 315.Rosell R, Felip E, Taron M, et al. Gene expression as a predictive marker of outcome in stage IIB-IIIA-IIIB non-small cell lung cancer after induction gemcitabine-based chemotherapy followed by resectional surgery. Clin Cancer Res. 2004;10:4215s–4219s. doi: 10.1158/1078-0432.CCR-040006. [DOI] [PubMed] [Google Scholar]
- 316.Simon G, Sharma A, Li X, et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:2741–2746. doi: 10.1200/JCO.2006.08.2099. [DOI] [PubMed] [Google Scholar]
- 317.Souglakos J, Boukovinas I, Taron M, et al. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008;98:1710–1715. doi: 10.1038/sj.bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Bepler G, Sommers KE, Cantor A, et al. Clinical efficacy and predictive molecular markers of neoadjuvant gemcitabine and pemetrexed in resectable non-small cell lung cancer. J Thorac Oncol. 2008;3:1112–1118. doi: 10.1097/JTO.0b013e3181874936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Kim SO, Jeong JY, Kim MR, et al. Efficacy of gemcitabine in patients with non-small cell lung cancer according to promoter polymorphisms of the ribonucleotide reductase M1 gene. Clin Cancer Res. 2008;14:3083–3088. doi: 10.1158/1078-0432.CCR-07-4591. [DOI] [PubMed] [Google Scholar]
- 320.Nishio K, Nakamura T, Koh Y, Kanzawa F, Tamura T, Saijo N. Oncoprotein 18 overexpression increases the sensitivity to vindesine in the human lung carcinoma cells. Cancer. 2001;91:1494–1499. doi: 10.1002/1097-0142(20010415)91:8<1494::aid-cncr1157>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 321.Saviozzi S, Ceppi P, Novello S, et al. Non-small cell lung cancer exhibits transcript overexpression of genes associated with homologous recombination and DNA replication pathways. Cancer Res. 2009;69:3390–3396. doi: 10.1158/0008-5472.CAN-08-2981. [DOI] [PubMed] [Google Scholar]
- 322.Takenaka T, Yoshino I, Kouso H, et al. Combined evaluation of Rad51 and ERCC1 expressions for sensitivity to platinum agents in non-small cell lung cancer. Int J Cancer. 2007;121:895–900. doi: 10.1002/ijc.22738. [DOI] [PubMed] [Google Scholar]
- 323.Tsai CM, Chang KT, Li L, Perng RP, Yang LY. Interrelationships between cellular nucleotide excision repair, cisplatin cytotoxicity, HER-2/neu gene expression, and epidermal growth factor receptor level in non-small cell lung cancer cells. Jpn J Cancer Res. 2000;91:213–222. doi: 10.1111/j.1349-7006.2000.tb00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 325.Huang PY, Liang XM, Lin SX, Luo RZ, Hou JH, Zhang L. Correlation analysis among expression of ERCC-1, metallothionein, p53 and platinum resistance and prognosis in advanced non-small cell lung cancer. Ai Zheng. 2004;23:845–850. [PubMed] [Google Scholar]
- 326.Hwang IG, Ahn MJ, Park BB, et al. ERCC1 expression as a prognostic marker in N2(+) nonsmall-cell lung cancer patients treated with platinum-based neoadjuvant concurrent chemoradiotherapy. Cancer. 2008;113:1379–1386. doi: 10.1002/cncr.23693. [DOI] [PubMed] [Google Scholar]
- 327.Lee HW, Choi YW, Han JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in advanced non-small cell lung cancer patients treated with platinum-based doublet chemotherapy. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 328.Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res. 2002;8:2286–2291. [PubMed] [Google Scholar]
- 329.Azuma K, Komohara Y, Sasada T, et al. Excision repair cross-complementation group 1 predicts progression-free and overall survival in non-small cell lung cancer patients treated with platinum-based chemotherapy. Cancer Sci. 2007;98:1336–1343. doi: 10.1111/j.1349-7006.2007.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Booton R, Ward T, Ashcroft L, Morris J, Heighway J, Thatcher N. ERCC1 mRNA expression is not associated with response and survival after platinum-based chemotherapy regimens in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2:902–906. doi: 10.1097/JTO.0b013e318155a637. [DOI] [PubMed] [Google Scholar]
- 331.Wachters FM, Wong LS, Timens W, Kampinga HH, Groen HJ. ERCC1, hRad51, and BRCA1 protein expression in relation to tumour response and survival of stage III/IV NSCLC patients treated with chemotherapy. Lung Cancer. 2005;50:211–219. doi: 10.1016/j.lungcan.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 332.Fujii T, Toyooka S, Ichimura K, et al. ERCC1 protein expression predicts the response of cisplatin-based neoadjuvant chemotherapy in non-small-cell lung cancer. Lung Cancer. 2008;59:377–384. doi: 10.1016/j.lungcan.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 333.Cobo M, Isla D, Massuti B, et al. Customizing cisplatin based on quantitative excision repair cross-complementing 1 mRNA expression: a phase III trial in non-small-cell lung cancer. J Clin Oncol. 2007;25:2747–2754. doi: 10.1200/JCO.2006.09.7915. [DOI] [PubMed] [Google Scholar]
- 334.Yuan P, Miao XP, Zhang XM, et al. Correlation of genetic polymorphisms in nucleotide excision repair system to sensitivity of advanced non-small cell lung cancer patients to platinum-based chemotherapy. Ai Zheng. 2005;24:1510–1513. [PubMed] [Google Scholar]
- 335.Park SY, Hong YC, Kim JH, et al. Effect of ERCC1 polymorphisms and the modification by smoking on the survival of non-small cell lung cancer patients. Med Oncol. 2006;23:489–498. doi: 10.1385/MO:23:4:489. [DOI] [PubMed] [Google Scholar]
- 336.Zhou W, Gurubhagavatula S, Liu G, et al. Excision repair cross-complementation group 1 polymorphism predicts overall survival in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Clin Cancer Res. 2004;10:4939–4943. doi: 10.1158/1078-0432.CCR-04-0247. [DOI] [PubMed] [Google Scholar]
- 337.Su D, Ma S, Liu P, et al. Genetic polymorphisms and treatment response in advanced non-small cell lung cancer. Lung Cancer. 2007;56:281–288. doi: 10.1016/j.lungcan.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 338.Ryu JS, Hong YC, Han HS, et al. Association between polymorphisms of ERCC1 and XPD and survival in non-small-cell lung cancer patients treated with cisplatin combination chemotherapy. Lung Cancer. 2004;44:311–316. doi: 10.1016/j.lungcan.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 339.Wu X, Lu C, Ye Y, et al. Germline genetic variations in drug action pathways predict clinical outcomes in advanced lung cancer treated with platinum-based chemotherapy. Pharmacogenet Genomics. 2008;18:955–965. doi: 10.1097/FPC.0b013e32830efdd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lee HW, Han JH, Kim JH, et al. Expression of excision repair cross-complementation group 1 protein predicts poor outcome in patients with small cell lung cancer. Lung Cancer. 2008;59:95–104. doi: 10.1016/j.lungcan.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 341.Fan W, Zhang HL, Wu XM. Enhancement effect of nucleotide excision repair gene xeroderma pigmentosun group a antisense RNA on sensitivity of human lung adenocarcinoma cell line A549 to cisplatin. Ai Zheng. 2005;24:403–407. [PubMed] [Google Scholar]
- 342.Jiang H, Yang LY. Cell cycle checkpoint abrogator UCN-01 inhibits DNA repair: association with attenuation of the interaction of XPA and ERCC1 nucleotide excision repair proteins. Cancer Res. 1999;59:4529–4534. [PubMed] [Google Scholar]
- 343.Chiang YY, Chen SL, Hsiao YT, et al. Nuclear expression of dynamin-related protein 1 in lung adenocarcinomas. Mod Pathol. 2009 doi: 10.1038/modpathol.2009.83. [DOI] [PubMed] [Google Scholar]
- 344.Gurubhagavatula S, Liu G, Park S, et al. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J Clin Oncol. 2004;22:2594–2601. doi: 10.1200/JCO.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 345.Booton R, Ward T, Heighway J, et al. Xeroderma pigmentosum group D haplotype predicts for response, survival, and toxicity after platinum-based chemotherapy in advanced nonsmall cell lung cancer. Cancer. 2006;106:2421–2427. doi: 10.1002/cncr.21885. [DOI] [PubMed] [Google Scholar]
- 346.Sun X, Li F, Sun N, et al. Polymorphisms in XRCC1 and XPG and response to platinum-based chemotherapy in advanced non-small cell lung cancer patients. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 347.Giachino DF, Ghio P, Regazzoni S, et al. Prospective assessment of XPD Lys751Gln and XRCC1 Arg399Gln single nucleotide polymorphisms in lung cancer. Clin Cancer Res. 2007;13:2876–2881. doi: 10.1158/1078-0432.CCR-06-2543. [DOI] [PubMed] [Google Scholar]
- 348.Yuan P, Miao XP, Zhang XM, et al. XRCC1 and XPD genetic polymorphisms predict clinical responses to platinum-based chemotherapy in advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2006;28:196–199. [PubMed] [Google Scholar]
- 349.Wang D, Xiang DB, Yang XQ, et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 350.Delaney CA, Wang LZ, Kyle S, et al. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6:2860–2867. [PubMed] [Google Scholar]
- 351.Miknyoczki SJ, Jones-Bolin S, Pritchard S, et al. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol Cancer Ther. 2003;2:371–382. [PubMed] [Google Scholar]
- 352.Hansen LT, Lundin C, Spang-Thomsen M, Petersen LN, Helleday T. The role of RAD51 in etoposide (VP16) resistance in small cell lung cancer. Int J Cancer. 2003;105:472–479. doi: 10.1002/ijc.11106. [DOI] [PubMed] [Google Scholar]
- 353.Ko JC, Ciou SC, Cheng CM, et al. Involvement of Rad51 in cytotoxicity induced by epidermal growth factor receptor inhibitor (gefitinib, IressaR) and chemotherapeutic agents in human lung cancer cells. Carcinogenesis. 2008;29:1448–1458. doi: 10.1093/carcin/bgn130. [DOI] [PubMed] [Google Scholar]
- 354.Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS ONE. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rosell R, Cuello M, Cecere F, et al. Usefulness of predictive tests for cancer treatment. Bull Cancer. 2006;93:E101–E108. [PubMed] [Google Scholar]
- 356.Boukovinas I, Papadaki C, Mendez P, et al. Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS ONE. 2008;3:e3695. doi: 10.1371/journal.pone.0003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Taron M, Rosell R, Felip E, et al. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13:2443–2449. doi: 10.1093/hmg/ddh260. [DOI] [PubMed] [Google Scholar]
- 358.Kim HT, Lee JE, Shin ES, et al. Effect of BRCA1 haplotype on survival of non-small-cell lung cancer patients treated with platinum-based chemotherapy. J Clin Oncol. 2008;26:5972–5979. doi: 10.1200/JCO.2008.16.6496. [DOI] [PubMed] [Google Scholar]
- 359.Hu B, Wang H, Wang X, et al. Fhit and CHK1 have opposing effects on homologous recombination repair. Cancer Res. 2005;65:8613–8616. doi: 10.1158/0008-5472.CAN-05-1966. [DOI] [PubMed] [Google Scholar]
- 360.Andriani F, Perego P, Carenini N, Sozzi G, Roz L. Increased sensitivity to cisplatin in non-small cell lung cancer cell lines after FHIT gene transfer. Neoplasia. 2006;8:9–17. doi: 10.1593/neo.05517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Kim CH, Yoo JS, Lee CT, et al. FHIT protein enhances paclitaxel-induced apoptosis in lung cancer cells. Int J Cancer. 2006;118:1692–1698. doi: 10.1002/ijc.21573. [DOI] [PubMed] [Google Scholar]
- 362.Tomoda Y, Katsura M, Okajima M, Hosoya N, Kohno N, Miyagawa K. Functional evidence for Eme1 as a marker of cisplatin resistance. Int J Cancer. 2009 doi: 10.1002/ijc.24268. [DOI] [PubMed] [Google Scholar]
- 363.Wang R, Wang W, Zhang JW. Correlation between XRCC2 and XRCC5 single nucleotide polymorphisms and drug-sensitivity of human lung cancer cells. Zhonghua Yi Xue Za Zhi. 2008;88:3059–3062. [PubMed] [Google Scholar]
- 364.Ferrer M, Span SW, Vischioni B, et al. FANCD2 expression in advanced non-small-cell lung cancer and response to platinum-based chemotherapy. Clin Lung Cancer. 2005;6:250–254. doi: 10.3816/CLC.2005.n.005. [DOI] [PubMed] [Google Scholar]
- 365.Ueda K, Kawashima H, Ohtani S, et al. The 3p21.3 tumor suppressor NPRL2 plays an important role cisplatin-induced resistance in human non-small-cell lung cancer cells. Cancer Res. 2006;66:9682–9690. doi: 10.1158/0008-5472.CAN-06-1483. [DOI] [PubMed] [Google Scholar]
- 366.Scartozzi M, Franciosi V, Campanini N, et al. Mismatch repair system (MMR) status correlates with response and survival in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2006;53:103–109. doi: 10.1016/j.lungcan.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 367.Takizawa M, Kawakami K, Obata T, et al. In vitro sensitivity to platinum-derived drugs is associated with expression of thymidylate synthase and dihydropyrimidine dehydrogenase in human lung cancer. Oncol Rep. 2006;15:1533–1539. [PubMed] [Google Scholar]
- 368.Arioka H, Nishio K, Ishida T, et al. Enhancement of cisplatin sensitivity in high mobility group 2 cDNA-transfected human lung cancer cells. Jpn J Cancer Res. 1999;90:108–115. doi: 10.1111/j.1349-7006.1999.tb00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Almeida GM, Duarte TL, Farmer PB, Steward WP, Jones GD. Multiple end-point analysis reveals cisplatin damage tolerance to be a chemoresistance mechanism in a NSCLC model: implications for predictive testing. Int J Cancer. 2008;122:1810–1819. doi: 10.1002/ijc.23188. [DOI] [PubMed] [Google Scholar]
- 370.Bergqvist M, Brattstrom D, Gullbo J, Hesselius P, Brodin O, Wagenius G. p53 status and its in vitro relationship to radiosensitivity and chemosensitivity in lung cancer. Anticancer Res. 2003;23:1207–1212. [PubMed] [Google Scholar]
- 371.Brattstrom D, Bergqvist M, Lamberg K, et al. Complete sequence of p53 gene in 20 patients with lung cancer: comparison with chemosensitivity and immunohistochemistry. Med Oncol. 1998;15:255–261. doi: 10.1007/BF02787209. [DOI] [PubMed] [Google Scholar]
- 372.Tsai CM, Chang KT, Wu LH, et al. Correlations between intrinsic chemoresistance and HER-2/neu gene expression, p53 gene mutations, and cell proliferation characteristics in non-small cell lung cancer cell lines. Cancer Res. 1996;56:206–209. [PubMed] [Google Scholar]
- 373.Safran H, King T, Choy H, et al. p53 mutations do not predict response to paclitaxel/radiation for nonsmall cell lung carcinoma. Cancer. 1996;78:1203–1210. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1203::AID-CNCR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 374.Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene. 1999;18:477–485. doi: 10.1038/sj.onc.1202314. [DOI] [PubMed] [Google Scholar]
- 375.Wang T, Xu J, Zhong NS. Relationship between the acquired multi-drug resistance of human large cell lung cancer cell line NCI-H460 by cisplatin selection and p53 mutation. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:102–107. [PubMed] [Google Scholar]
- 376.Han JY, Chung YJ, Park SW, et al. The relationship between cisplatin-induced apoptosis and p53, bcl-2 and bax expression in human lung cancer cells. Korean J Intern Med. 1999;14:42–52. doi: 10.3904/kjim.1999.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Cuddihy AR, Jalali F, Coackley C, Bristow RG. WTp53 induction does not override MTp53 chemoresistance and radioresistance due to gain-of-function in lung cancer cells. Mol Cancer Ther. 2008;7:980–992. doi: 10.1158/1535-7163.MCT-07-0471. [DOI] [PubMed] [Google Scholar]
- 378.Lai SL, Perng RP, Hwang J. p53 gene status modulates the chemosensitivity of non-small cell lung cancer cells. J Biomed Sci. 2000;7:64–70. doi: 10.1007/BF02255920. [DOI] [PubMed] [Google Scholar]
- 379.Osaki S, Nakanishi Y, Takayama K, Pei XH, Ueno H, Hara N. Alteration of drug chemosensitivity caused by the adenovirus-mediated transfer of the wild-type p53 gene in human lung cancer cells. Cancer Gene Ther. 2000;7:300–307. doi: 10.1038/sj.cgt.7700096. [DOI] [PubMed] [Google Scholar]
- 380.Breen L, Heenan M, Amberger-Murphy V, Clynes M. Investigation of the role of p53 in chemotherapy resistance of lung cancer cell lines. Anticancer Res. 2007;27:1361–1364. [PubMed] [Google Scholar]
- 381.Choi JH, Ahn KS, Kim J, Hong YS. Enhanced induction of Bax gene expression in H460 and H1299 cells with the combined treatment of cisplatin and adenovirus mediated wt-p53 gene transfer. Exp Mol Med. 2000;32:23–28. doi: 10.1038/emm.2000.5. [DOI] [PubMed] [Google Scholar]
- 382.Rohr UP, Wulf MA, Stahn S, et al. Non-small lung cancer cells are prime targets for p53 gene transfer mediated by a recombinant adeno-associated virus type-2 vector. Cancer Gene Ther. 2003;10:898–906. doi: 10.1038/sj.cgt.7700643. [DOI] [PubMed] [Google Scholar]
- 383.Riva CM. Restoration of wild-type p53 activity enhances the sensitivity of pleural metastasis to cisplatin through an apoptotic mechanism. Anticancer Res. 2000;20:4463–4471. [PubMed] [Google Scholar]
- 384.Inoue A, Narumi K, Matsubara N, et al. Administration of wild-type p53 adenoviral vector synergistically enhances the cytotoxicity of anti-cancer drugs in human lung cancer cells irrespective of the status of p53 gene. Cancer Lett. 2000;157:105–112. doi: 10.1016/s0304-3835(00)00480-8. [DOI] [PubMed] [Google Scholar]
- 385.Wang Y, Blandino G, Oren M, Givol D. Induced p53 expression in lung cancer cell line promotes cell senescence and differentially modifies the cytotoxicity of anti-cancer drugs. Oncogene. 1998;17:1923–1930. doi: 10.1038/sj.onc.1202113. [DOI] [PubMed] [Google Scholar]
- 386.Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang WW, Owen-Schaub LB, Roth JA. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 387.Ling YH, Zou Y, Perez-Soler R. Induction of senescence-like phenotype and loss of paclitaxel sensitivity after wild-type p53 gene transfection of p53-null human non-small cell lung cancer H358 cells. Anticancer Res. 2000;20:693–702. [PubMed] [Google Scholar]
- 388.He Y, Fan SZ, Jiang YG, et al. Effect of p73 gene on chemosensitivity of human lung adenocarcinoma cells H1299. Ai Zheng. 2004;23:645–649. [PubMed] [Google Scholar]
- 389.Corn PG, El-Deiry WS. Microarray analysis of p53-dependent gene expression in response to hypoxia and DNA damage. Cancer Biol Ther. 2007;6:1858–1866. doi: 10.4161/cbt.6.12.5330. [DOI] [PubMed] [Google Scholar]
- 390.Mori T, Okamoto H, Takahashi N, Ueda R, Okamoto T. Aberrant overexpression of 53BP2 mRNA in lung cancer cell lines. FEBS Lett. 2000;465:124–128. doi: 10.1016/s0014-5793(99)01726-3. [DOI] [PubMed] [Google Scholar]
- 391.Han JY, Lee GK, Jang DH, Lee SY, Lee JS. Association of p53 codon 72 polymorphism and MDM2 SNP309 with clinical outcome of advanced nonsmall cell lung cancer. Cancer. 2008;113:799–807. doi: 10.1002/cncr.23668. [DOI] [PubMed] [Google Scholar]
- 392.Wang JL, Jiao SC, Ye P, Li JY. P53 protein expression and chemosensitivity to cisplatin in patients with non-small cell lung cancer: a meta-analysis. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:770–773. [PubMed] [Google Scholar]
- 393.Kandioler-Eckersberger D, Kappel S, Mittlbock M, et al. The TP53 genotype but not immunohistochemical result is predictive of response to cisplatin-based neoadjuvant therapy in stage III non-small cell lung cancer. J Thorac Cardiovasc Surg. 1999;117:744–750. doi: 10.1016/S0022-5223(99)70295-3. [DOI] [PubMed] [Google Scholar]
- 394.Higashiyama M, Kodama K, Yokouchi H, et al. Immunohistochemical p53 protein status in nonsmall cell lung cancer is a promising indicator in determining in vitro chemosensitivity to some anticancer drugs. J Surg Oncol. 1998;68:19–24. doi: 10.1002/(sici)1096-9098(199805)68:1<19::aid-jso5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 395.Vogt U, Zaczek A, Klinke F, Granetzny A, Bielawski K, Falkiewicz B. p53 Gene status in relation to ex vivo chemosensitivity of non-small cell lung cancer. J Cancer Res Clin Oncol. 2002;128:141–147. doi: 10.1007/s00432-001-0305-2. [DOI] [PubMed] [Google Scholar]
- 396.Kandioler D, Stamatis G, Eberhardt W, et al. Growing clinical evidence for the interaction of the p53 genotype and response to induction chemotherapy in advanced non-small cell lung cancer. J Thorac Cardiovasc Surg. 2008;135:1036–1041. doi: 10.1016/j.jtcvs.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 397.Fijolek J, Wiatr E, Rowinska-Zakrzewska E, et al. p53 and HER2/neu expression in relation to chemotherapy response in patients with non-small cell lung cancer. Int J Biol Markers. 2006;21:81–87. doi: 10.1177/172460080602100203. [DOI] [PubMed] [Google Scholar]
- 398.Johnson EA, Klimstra DS, Herndon JE, 2nd, et al. Aberrant p53 staining does not predict cisplatin resistance in locally advanced non-small cell lung cancer. Cancer Invest. 2002;20:686–692. doi: 10.1081/cnv-120003537. [DOI] [PubMed] [Google Scholar]
- 399.Graziano SL, Tatum A, Herndon JE, 2nd, et al. Use of neuroendocrine markers, p53, and HER2 to predict response to chemotherapy in patients with stage III non-small cell lung cancer: a Cancer and Leukemia Group B study. Lung Cancer. 2001;33:115–123. doi: 10.1016/s0169-5002(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 400.Rusch V, Klimstra D, Venkatraman E, et al. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995;55:5038–5042. [PubMed] [Google Scholar]
- 401.Gajra A, Tatum AH, Newman N, et al. The predictive value of neuroendocrine markers and p53 for response to chemotherapy and survival in patients with advanced non-small cell lung cancer. Lung Cancer. 2002;36:159–165. doi: 10.1016/s0169-5002(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 402.Kawasaki M, Nakanishi Y, Kuwano K, Yatsunami J, Takayama K, Hara N. The utility of p53 immunostaining of transbronchial biopsy specimens of lung cancer: p53 overexpression predicts poor prognosis and chemoresistance in advanced non-small cell lung cancer. Clin Cancer Res. 1997;3:1195–1200. [PubMed] [Google Scholar]
- 403.Berrieman HK, Cawkwell L, O'Kane SL, Smith L, Lind MJ. Hsp27 may allow prediction of the response to single-agent vinorelbine chemotherapy in non-small cell lung cancer. Oncol Rep. 2006;15:283–286. [PubMed] [Google Scholar]
- 404.Gregorc V, Darwish S, Ludovini V, et al. The clinical relevance of Bcl-2, Rb and p53 expression in advanced non-small cell lung cancer. Lung Cancer. 2003;42:275–281. doi: 10.1016/j.lungcan.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 405.Oshita F, Nishio K, Kameda Y, et al. Increased expression levels of p53 correlate with good response to cisplatin-based chemotherapy in non-small cell lung cancer. Oncol Rep. 2000;7:1225–1228. doi: 10.3892/or.7.6.1225. [DOI] [PubMed] [Google Scholar]
- 406.Higashiyama M, Miyoshi Y, Kodama K, et al. p53-regulated GML gene expression in non-small cell lung cancer. a promising relationship to cisplatin chemosensitivity. Eur J Cancer. 2000;36:489–495. doi: 10.1016/s0959-8049(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 407.Oshita F, Kameda Y, Hamanaka N, et al. High expression of integrin beta1 and p53 is a greater poor prognostic factor than clinical stage in small-cell lung cancer. Am J Clin Oncol. 2004;27:215–219. doi: 10.1097/01.coc.0000054894.64867.80. [DOI] [PubMed] [Google Scholar]
- 408.Shih CM, Chen K, Wang YC, Lee PJ, Wang YC. Elevated p53 and p21waf1 mRNA expression in blood lymphocytes from lung cancer patients with chemoresistance. Cancer Detect Prev. 2007;31:366–370. doi: 10.1016/j.cdp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 409.Yuan P, Miao XP, Zhang XM, et al. Association of the responsiveness of advanced non-small cell lung cancer to platinum-based chemotherapy with p53 and p73 polymorphisms. Zhonghua Zhong Liu Za Zhi. 2006;28:107–110. [PubMed] [Google Scholar]
- 410.Schuler M, Herrmann R, De Greve JL, et al. Adenovirus-mediated wild-type p53 gene transfer in patients receiving chemotherapy for advanced non-small-cell lung cancer: results of a multicenter phase II study. J Clin Oncol. 2001;19:1750–1758. doi: 10.1200/JCO.2001.19.6.1750. [DOI] [PubMed] [Google Scholar]
- 411.Okouoyo S, Herzer K, Ucur E, et al. Rescue of death receptor and mitochondrial apoptosis signaling in resistant human NSCLC in vivo. Int J Cancer. 2004;108:580–587. doi: 10.1002/ijc.11585. [DOI] [PubMed] [Google Scholar]
- 412.Checinska A, Giaccone G, Hoogeland BS, Ferreira CG, Rodriguez JA, Kruyt FA. TUCAN/CARDINAL/CARD8 and apoptosis resistance in non-small cell lung cancer cells. BMC Cancer. 2006;6:166. doi: 10.1186/1471-2407-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ferreira CG, Span SW, Peters GJ, Kruyt FA, Giaccone G. Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res. 2000;60:7133–7141. [PubMed] [Google Scholar]
- 414.Gassler N, Zhang C, Wenger T, et al. Dexamethasone-induced cisplatin and gemcitabine resistance in lung carcinoma samples treated ex vivo. Br J Cancer. 2005;92:1084–1088. doi: 10.1038/sj.bjc.6602453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Herr I, Ucur E, Herzer K, et al. Glucocorticoid cotreatment induces apoptosis resistance toward cancer therapy in carcinomas. Cancer Res. 2003;63:3112–3120. [PubMed] [Google Scholar]
- 416.Herr I, Wilhelm D, Meyer E, Jeremias I, Angel P, Debatin KM. JNK/SAPK activity contributes to TRAIL-induced apoptosis. Cell Death Differ. 1999;6:130–135. doi: 10.1038/sj.cdd.4400467. [DOI] [PubMed] [Google Scholar]
- 417.Tsuruta F, Sunayama J, Mori Y, et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Teraishi F, Zhang L, Guo W, et al. Activation of c-Jun NH2-terminal kinase is required for gemcitabine's cytotoxic effect in human lung cancer H1299 cells. FEBS Lett. 2005;579:6681–6687. doi: 10.1016/j.febslet.2005.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Chattopadhyay S, Machado-Pinilla R, Manguan-Garcia C, et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 2006;25:3335–3345. doi: 10.1038/sj.onc.1209364. [DOI] [PubMed] [Google Scholar]
- 420.Levresse V, Marek L, Blumberg D, Heasley LE. Regulation of platinum-compound cytotoxicity by the c-Jun N-terminal kinase and c-Jun signaling pathway in small-cell lung cancer cells. Mol Pharmacol. 2002;62:689–697. doi: 10.1124/mol.62.3.689. [DOI] [PubMed] [Google Scholar]
- 421.Yoshida S, Kawaguchi H, Sato S, Ueda R, Furukawa K, et al. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn J Cancer Res. 2002;93:816–824. doi: 10.1111/j.1349-7006.2002.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Zhao Y, El-Gabry M, Hei TK. Loss of Betaig-h3 protein is frequent in primary lung carcinoma and related to tumorigenic phenotype in lung cancer cells. Mol Carcinog. 2006;45:84–92. doi: 10.1002/mc.20167. [DOI] [PubMed] [Google Scholar]
- 423.Deng WG, Wu G, Ueda K, Xu K, Roth JA, Ji L. Enhancement of antitumor activity of cisplatin in human lung cancer cells by tumor suppressor FUS1. Cancer Gene Ther. 2008;15:29–39. doi: 10.1038/sj.cgt.7701094. [DOI] [PubMed] [Google Scholar]
- 424.Ikuta K, Takemura K, Kihara M, et al. Defects in apoptotic signal transduction in cisplatin-resistant non-small cell lung cancer cells. Oncol Rep. 2005;13:1229–1234. [PubMed] [Google Scholar]
- 425.Joseph B, Ekedahl J, Lewensohn R, Marchetti P, Formstecher P, Zhivotovsky B. Defective caspase-3 relocalization in non-small cell lung carcinoma. Oncogene. 2001;20:2877–2888. doi: 10.1038/sj.onc.1204402. [DOI] [PubMed] [Google Scholar]
- 426.Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Jr, Chou TC. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Cancer Res. 1999;59:6178–6184. [PubMed] [Google Scholar]
- 427.Krysan K, Dalwadi H, Sharma S, Pold M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res. 2004;64:6359–6362. doi: 10.1158/0008-5472.CAN-04-1681. [DOI] [PubMed] [Google Scholar]
- 428.Duarte ML, de Moraes E, Pontes E, et al. Role of p53 in the induction of cyclooxygenase-2 by cisplatin or paclitaxel in non-small cell lung cancer cell lines. Cancer Lett. 2009;279:57–64. doi: 10.1016/j.canlet.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 429.Duffy CP, Elliott CJ, O'Connor RA, et al. Enhancement of chemotherapeutic drug toxicity to human tumour cells in vitro by a subset of non-steroidal anti-inflammatory drugs (NSAIDs) Eur J Cancer. 1998;34:1250–1259. doi: 10.1016/s0959-8049(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 430.Fulzele SV, Shaik MS, Chatterjee A, Singh M. Anti-cancer effect of celecoxib and aerosolized docetaxel against human non-small cell lung cancer cell line, A549. J Pharm Pharmacol. 2006;58:327–336. doi: 10.1211/jpp.58.3.0006. [DOI] [PubMed] [Google Scholar]
- 431.Hida T, Kozaki K, Ito H, et al. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase 2 inhibitor, JTE-522, and conventional anticancer agents. Clin Cancer Res. 2002;8:2443–2447. [PubMed] [Google Scholar]
- 432.Hida T, Kozaki K, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 433.Kobayashi S, Okada S, Yoshida H, Fujimura S. Indomethacin enhances the cytotoxicity of VCR and ADR in human pulmonary adenocarcinoma cells. Tohoku J Exp Med. 1997;181:361–370. doi: 10.1620/tjem.181.361. [DOI] [PubMed] [Google Scholar]
- 434.Chen XJ, Xiao W, Qu X, Zhou SY. NS-398 enhances the efficacy of gemcitabine against lung adenocarcinoma through up-regulation of p21WAF1 and p27KIP1 protein. Neoplasma. 2008;55:200–204. [PubMed] [Google Scholar]
- 435.Hohla F, Schally AV, Szepeshazi K, et al. Synergistic inhibition of growth of lung carcinomas by antagonists of growth hormone-releasing hormone in combination with docetaxel. Proc Natl Acad Sci U S A. 2006;103:14513–14518. doi: 10.1073/pnas.0605309103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Mascaux C, Martin B, Paesmans M, et al. Has Cox-2 a prognostic role in non-small-cell lung cancer? A systematic review of the literature with meta-analysis of the survival results. Br J Cancer. 2006;95:139–145. doi: 10.1038/sj.bjc.6603226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Surowiak P, Pawelczyk K, Maciejczyk A, et al. Positive correlation between cyclooxygenase 2 and the expression of ABC transporters in non-small cell lung cancer. Anticancer Res. 2008;28:2967–2974. [PubMed] [Google Scholar]
- 438.Wilop S, Crysandt M, Bendel M, Mahnken AH, Osieka R, Jost E. Correlation of C-reactive protein with survival and radiographic response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Onkologie. 2008;31:665–670. doi: 10.1159/000165054. [DOI] [PubMed] [Google Scholar]
- 439.Mutter R, Lu B, Carbone DP, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res. 2009;15:2158–2165. doi: 10.1158/1078-0432.CCR-08-0629. [DOI] [PubMed] [Google Scholar]
- 440.Csiki I, Morrow JD, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–6640. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 441.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 442.Altorki NK, Keresztes RS, Port JL, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 443.Gasparini G, Meo S, Comella G, et al. The combination of the selective cyclooxygenase-2 inhibitor celecoxib with weekly paclitaxel is a safe and active second-line therapy for non-small cell lung cancer: a phase II study with biological correlates. Cancer J. 2005;11:209–216. doi: 10.1097/00130404-200505000-00007. [DOI] [PubMed] [Google Scholar]
- 444.Gadgeel SM, Wozniak A, Ruckdeschel JC, et al. Phase II study of docetaxel and celecoxib, a cyclooxygenase-2 inhibitor, in elderly or poor performance status (PS2) patients with advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:1293–1300. doi: 10.1097/JTO.0b013e31818b194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Schneider BJ, Kalemkerian GP, Kraut MJ, et al. Phase II study of celecoxib and docetaxel in non-small cell lung cancer (NSCLC) patients with progression after platinum-based therapy. J Thorac Oncol. 2008;3:1454–1459. doi: 10.1097/JTO.0b013e31818de1d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Nugent FW, Mertens WC, Graziano S, et al. Docetaxel and cyclooxygenase-2 inhibition with celecoxib for advanced non-small cell lung cancer progressing after platinum-based chemotherapy: a multicenter phase II trial. Lung Cancer. 2005;48:267–273. doi: 10.1016/j.lungcan.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 447.Aruajo AM, Mendez JC, Coelho AL, et al. Phase II study of celecoxib with cisplatin plus etoposide in extensive-stage small cell lung cancer. Cancer Invest. 2009;27:391–396. doi: 10.1080/07357900802232756. [DOI] [PubMed] [Google Scholar]
- 448.Gridelli C, Gallo C, Ceribelli A, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8:500–512. doi: 10.1016/S1470-2045(07)70146-8. [DOI] [PubMed] [Google Scholar]
- 449.Lilenbaum R, Socinski MA, Altorki NK, et al. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J Clin Oncol. 2006;24:4825–4832. doi: 10.1200/JCO.2006.07.4773. [DOI] [PubMed] [Google Scholar]
- 450.Wang L, Chanvorachote P, Toledo D, et al. Peroxide is a key mediator of Bcl-2 down-regulation and apoptosis induction by cisplatin in human lung cancer cells. Mol Pharmacol. 2008;73:119–127. doi: 10.1124/mol.107.040873. [DOI] [PubMed] [Google Scholar]
- 451.Chanvorachote P, Nimmannit U, Stehlik C, et al. Nitric oxide regulates cell sensitivity to cisplatininduced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 452.Wang J, Liu X, Wu M. Expression and reversion of drug resistance- and apoptosis-related genes of a DDP-resistant lung adenocarcinoma cell line A549DDP. Zhonghua Zhong Liu Za Zhi. 1999;21:422–426. [PubMed] [Google Scholar]
- 453.Zhang Y, Fujita N, Tsuruo T. p21Waf1/Cip1 acts in synergy with bcl-2 to confer multidrug resistance in a camptothecin-selected human lung-cancer cell line. Int J Cancer. 1999;83:790–797. doi: 10.1002/(sici)1097-0215(19991210)83:6<790::aid-ijc15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 454.Riedel RF, Porrello A, Pontzer E, et al. A genomic approach to identify molecular pathways associated with chemotherapy resistance. Mol Cancer Ther. 2008;7:3141–3149. doi: 10.1158/1535-7163.MCT-08-0642. [DOI] [PubMed] [Google Scholar]
- 455.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 456.Zangemeister-Wittke U, Schenker T, Luedke GH, Stahel RA. Synergistic cytotoxicity of bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small-cell lung cancer cell lines. Br J Cancer. 1998;78:1035–1042. doi: 10.1038/bjc.1998.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Kumar Biswas S, Huang J, Persaud S, Basu A. Down-regulation of Bcl-2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Mol Cancer Ther. 2004;3:327–334. [PubMed] [Google Scholar]
- 458.Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–146. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Huang Z, Lei X, Zhong M, Zhu B, Tang S, Liao D. Bcl-2 small interfering RNA sensitizes cisplatinresistant human lung adenocarcinoma A549/DDP cell to cisplatin and diallyl disulfide. Acta Biochim Biophys Sin (Shanghai) 2007;39:835–843. doi: 10.1111/j.1745-7270.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 460.Wang J, Liu X, Jiang W. Co-transfection of MRP and bcl-2 antisense S-oligodeoxynucleotides reduces drug resistance in cisplatin-resistant lung cancer cells. Chin Med J (Engl) 2000;113:957–960. [PubMed] [Google Scholar]
- 461.Linardopoulos S. Aurora-A kinase regulates NF-kappaB activity: lessons from combination studies. J Buon. 2007;12 Suppl 1:S67–S70. [PubMed] [Google Scholar]
- 462.Losert D, Pratscher B, Soutschek J, et al. Bcl-2 downregulation sensitizes nonsmall cell lung cancer cells to cisplatin, but not to docetaxel. Anticancer Drugs. 2007;18:755–761. doi: 10.1097/CAD.0b013e3280adc8c8. [DOI] [PubMed] [Google Scholar]
- 463.Hu Y, Bebb G, Tan S, et al. Antitumor efficacy of oblimersen Bcl-2 antisense oligonucleotide alone and in combination with vinorelbine in xenograft models of human non-small cell lung cancer. Clin Cancer Res. 2004;10:7662–7670. doi: 10.1158/1078-0432.CCR-04-1036. [DOI] [PubMed] [Google Scholar]
- 464.Krug LM, Miller VA, Filippa DA, Venkatraman E, Ng KK, Kris MG. Bcl-2 and bax expression in advanced non-small cell lung cancer: lack of correlation with chemotherapy response or survival in patients treated with docetaxel plus vinorelbine. Lung Cancer. 2003;39:139–143. doi: 10.1016/s0169-5002(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 465.Martin B, Paesmans M, Berghmans T, et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2003;89:55–64. doi: 10.1038/sj.bjc.6601095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Renouf DJ, Wood-Baker R, Ionescu DN, et al. BCL-2 expression is prognostic for improved survival in non-small cell lung cancer. J Thorac Oncol. 2009;4:486–491. doi: 10.1097/JTO.0b013e318199e03a. [DOI] [PubMed] [Google Scholar]
- 467.Mandziuk S, Dudzisz-Sledz M, Korszen-Pilecka I, Milanowski J, Wojcierowski J, Korobowicz E. Expression of p21 and bcl-2 proteins in paraffin-embedded preparations of non-small cell lung cancer in stage IIIA after Etoposide and Cisplatin induced chemotherapy. Ann Univ Mariae Curie Sklodowska [Med] 2003;58:149–153. [PubMed] [Google Scholar]
- 468.Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Kurdow R, Schniewind B, Zoefelt S, et al. Apoptosis by gemcitabine in non-small cell lung cancer cell line KNS62 is induced downstream of caspase 8 and is profoundly blocked by Bcl-xL over-expression. Langenbecks Arch Surg. 2005;390:243–248. doi: 10.1007/s00423-004-0531-6. [DOI] [PubMed] [Google Scholar]
- 470.Takemura A, Gemma A, Shibuya M, et al. Gemcitabine resistance in a highly metastatic subpopulation of a pulmonary adenocarcinoma cell line resistant to gefitinib. Int J Oncol. 2007;31:1325–1332. [PubMed] [Google Scholar]
- 471.Lei X, Huang Z, Zhong M, Zhu B, Tang S, Liao D. Bcl-XL small interfering RNA sensitizes cisplatinresistant human lung adenocarcinoma cells. Acta Biochim Biophys Sin (Shanghai) 2007;39:344–350. doi: 10.1111/j.1745-7270.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 472.Sonnemann J, Gekeler V, Ahlbrecht K, et al. Down-regulation of protein kinase Ceta by antisense oligonucleotides sensitises A549 lung cancer cells to vincristine and paclitaxel. Cancer Lett. 2004;209:177–185. doi: 10.1016/j.canlet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 473.Hajji N, Wallenborg K, Vlachos P, Nyman U, Hermanson O, Joseph B. Combinatorial action of the HDAC inhibitor trichostatin A and etoposide induces caspase-mediated AIF-dependent apoptotic cell death in non-small cell lung carcinoma cells. Oncogene. 2008;27:3134–3144. doi: 10.1038/sj.onc.1210976. [DOI] [PubMed] [Google Scholar]
- 474.Shoemaker AR, Oleksijew A, Bauch J, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res. 2006;66:8731–8739. doi: 10.1158/0008-5472.CAN-06-0367. [DOI] [PubMed] [Google Scholar]
- 475.Pardo OE, Wellbrock C, Khanzada UK, et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. Embo J. 2006;25:3078–3088. doi: 10.1038/sj.emboj.7601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Song L, Coppola D, Livingston S, Cress D, Haura EB. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther. 2005;4:267–276. doi: 10.4161/cbt.4.3.1496. [DOI] [PubMed] [Google Scholar]
- 477.Li J, Viallet J, Haura EB. A small molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis and enhances cisplatin-induced apoptosis in non-small cell lung cancer cells. Cancer Chemother Pharmacol. 2008;61:525–534. doi: 10.1007/s00280-007-0499-3. [DOI] [PubMed] [Google Scholar]
- 478.Wesarg E, Hoffarth S, Wiewrodt R, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007;121:2387–2394. doi: 10.1002/ijc.22977. [DOI] [PubMed] [Google Scholar]
- 479.Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, et al. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int J Cancer. 2005;117:755–763. doi: 10.1002/ijc.21242. [DOI] [PubMed] [Google Scholar]
- 480.Zhang MC, Hu CP, Chen Q. Effect of down-regulation of survivin gene on apoptosis and cisplatin resistance in cisplatin resistant human lung adenocarcinoma A549/CDDP cells. Zhonghua Zhong Liu Za Zhi. 2006;28:408–412. [PubMed] [Google Scholar]
- 481.Yang H, Fu JH, Hu Y, et al. Influence of SiRNA targeting survivin on chemosensitivity of H460/cDDP lung cancer cells. J Int Med Res. 2008;36:734–747. doi: 10.1177/147323000803600416. [DOI] [PubMed] [Google Scholar]
- 482.Olie RA, Simoes-Wust AP, Baumann B, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 483.Zhang MC, Hu CP, Chen Q, Xia Y. Experimental study of antisense oligodeoxynucleotide targeting survivin gene for cisplatin resistant human lung adeno-carcinoma xenograft in nude mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:717–722. [PubMed] [Google Scholar]
- 484.Dasgupta P, Kinkade R, Joshi B, Decook C, Haura E, Chellappan S. Nicotine inhibits apoptosis induced by chemotherapeutic drugs by up-regulating XIAP and survivin. Proc Natl Acad Sci U S A. 2006;103:6332–6337. doi: 10.1073/pnas.0509313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Hodkinson PS, Elliott T, Wong WS, et al. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13:1776–1788. doi: 10.1038/sj.cdd.4401849. [DOI] [PubMed] [Google Scholar]
- 486.Crnkovic-Mertens I, Muley T, Meister M, et al. The anti-apoptotic livin gene is an important determinant for the apoptotic resistance of non-small cell lung cancer cells. Lung Cancer. 2006;54:135–142. doi: 10.1016/j.lungcan.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 487.Checinska A, Hoogeland BS, Rodriguez JA, Giaccone G, Kruyt FA. Role of XIAP in inhibiting cisplatin-induced caspase activation in non-small cell lung cancer cells: a small molecule Smac mimic sensitizes for chemotherapy-induced apoptosis by enhancing caspase-3 activation. Exp Cell Res. 2007;313:1215–1224. doi: 10.1016/j.yexcr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 488.Bartling B, Lewensohn R, Zhivotovsky B. Endogenously released Smac is insufficient to mediate cell death of human lung carcinoma in response to etoposide. Exp Cell Res. 2004;298:83–95. doi: 10.1016/j.yexcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 489.Ekedahl J, Joseph B, Grigoriev MY, et al. Expression of inhibitor of apoptosis proteins in small- and non-small-cell lung carcinoma cells. Exp Cell Res. 2002;279:277–290. doi: 10.1006/excr.2002.5608. [DOI] [PubMed] [Google Scholar]
- 490.Bandala E, Espinosa M, Maldonado V, Melendez-Zajgla J. Inhibitor of apoptosis-1 (IAP-1) expression and apoptosis in non-small-cell lung cancer cells exposed to gemcitabine. Biochem Pharmacol. 2001;62:13–19. doi: 10.1016/s0006-2952(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 491.Misawa M, Tauchi T, Sashida G, et al. Inhibition of human telomerase enhances the effect of chemotherapeutic agents in lung cancer cells. Int J Oncol. 2002;21:1087–1092. [PubMed] [Google Scholar]
- 492.Liu D, Kojima T, Ouchi M, et al. Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol Cancer Ther. 2009;8:980–987. doi: 10.1158/1535-7163.MCT-08-0901. [DOI] [PubMed] [Google Scholar]
- 493.Fujiwara T, Kagawa S, Kishimoto H, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- 494.Tsurutani J, Soda H, Oka M, et al. Antiproliferative effects of the histone deacetylase inhibitor FR901228 on small-cell lung cancer lines and drug-resistant sublines. Int J Cancer. 2003;104:238–242. doi: 10.1002/ijc.10921. [DOI] [PubMed] [Google Scholar]
- 495.Nikolova T, Christmann M, Kaina B. FEN1 is overexpressed in testis, lung and brain tumors. Anticancer Res. 2009;29:2453–2459. [PubMed] [Google Scholar]
- 496.Camps C, Sirera R, Bremnes RM, et al. Quantification in the serum of the catalytic fraction of reverse telomerase: a useful prognostic factor in advanced non-small cell lung cancer. Anticancer Res. 2006;26:4905–4909. [PubMed] [Google Scholar]
- 497.Lin X, Gu J, Lu C, Spitz MR, Wu X. Expression of telomere-associated genes as prognostic markers for overall survival in patients with non-small cell lung cancer. Clin Cancer Res. 2006;12:5720–5725. doi: 10.1158/1078-0432.CCR-05-2809. [DOI] [PubMed] [Google Scholar]
- 498.Zhu CQ, Cutz JC, Liu N, et al. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–1459. doi: 10.1038/sj.bjc.6603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Chen KY, Lee LN, Yu CJ, Lee YC, Kuo SH, Yang PC. Elevation of telomerase activity positively correlates to poor prognosis of patients with non-small cell lung cancer. Cancer Lett. 2006;240:148–156. doi: 10.1016/j.canlet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 500.Metzger R, Vallbohmer D, Muller-Tidow C, et al. Increased human telomerase reverse transcriptase (hTERT) mRNA expression but not telomerase activity is related to survival in curatively resected non-small cell lung cancer. Anticancer Res. 2009;29:1157–1162. [PubMed] [Google Scholar]
- 501.Frias C, Garcia-Aranda C, De Juan C, et al. Telomere shortening is associated with poor prognosis and telomerase activity correlates with DNA repair impairment in non-small cell lung cancer. Lung Cancer. 2008;60:416–425. doi: 10.1016/j.lungcan.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 502.Gryaznov SM, Jackson S, Dikmen G, et al. Oligonucleotide conjugate GRN163L targeting human telomerase as potential anticancer and antimetastatic agent. Nucleosides Nucleotides Nucleic Acids. 2007;26:1577–1579. doi: 10.1080/15257770701547271. [DOI] [PubMed] [Google Scholar]
- 503.Spalding AC, Jotte RM, Scheinman RI, et al. TRAIL and inhibitors of apoptosis are opposing determinants for NF-kappaB-dependent, genotoxin-induced apoptosis of cancer cells. Oncogene. 2002;21:260–271. doi: 10.1038/sj.onc.1205048. [DOI] [PubMed] [Google Scholar]
- 504.Liu X, Yue P, Khuri FR, Sun SY. Decoy receptor 2 (DcR2) is a p53 target gene and regulates chemosensitivity. Cancer Res. 2005;65:9169–9175. doi: 10.1158/0008-5472.CAN-05-0939. [DOI] [PubMed] [Google Scholar]
- 505.Lei W, Mayotte JE, Levitt ML. Enhancement of chemosensitivity and programmed cell death by tyrosine kinase inhibitors correlates with EGFR expression in non-small cell lung cancer cells. Anticancer Res. 1999;19:221–228. [PubMed] [Google Scholar]
- 506.Ando K, Ohmori T, Inoue F, et al. Enhancement of sensitivity to tumor necrosis factor alpha in nonsmall cell lung cancer cells with acquired resistance to gefitinib. Clin Cancer Res. 2005;11:8872–8879. doi: 10.1158/1078-0432.CCR-05-0811. [DOI] [PubMed] [Google Scholar]
- 507.Bai L, Zhu R, Chen Z, et al. Potential role of short hairpin RNA targeting epidermal growth factor receptor in growth and sensitivity to drugs of human lung adenocarcinoma cells. Biochem Pharmacol. 2006;71:1265–1271. doi: 10.1016/j.bcp.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 508.Rosetti M, Zoli W, Tesei A, et al. Iressa strengthens the cytotoxic effect of docetaxel in NSCLC models that harbor specific molecular characteristics. J Cell Physiol. 2007;212:710–716. doi: 10.1002/jcp.21067. [DOI] [PubMed] [Google Scholar]
- 509.Giovannetti E, Lemos C, Tekle C, et al. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol. 2008;73:1290–1300. doi: 10.1124/mol.107.042382. [DOI] [PubMed] [Google Scholar]
- 510.Li T, Ling YH, Goldman ID, Perez-Soler R. Schedule-dependent cytotoxic synergism of pemetrexed and erlotinib in human non-small cell lung cancer cells. Clin Cancer Res. 2007;13:3413–3422. doi: 10.1158/1078-0432.CCR-06-2923. [DOI] [PubMed] [Google Scholar]
- 511.Van Schaeybroeck S, Kyula J, Kelly DM, et al. Chemotherapy-induced epidermal growth factor receptor activation determines response to combined gefitinib/chemotherapy treatment in non-small cell lung cancer cells. Mol Cancer Ther. 2006;5:1154–1165. doi: 10.1158/1535-7163.MCT-05-0446. [DOI] [PubMed] [Google Scholar]
- 512.Sirotnak FM, Zakowski MF, Miller VA, Scher HI, Kris MG. Efficacy of cytotoxic agents against human tumor xenografts is markedly enhanced by coadministration of ZD1839 (Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res. 2000;6:4885–4892. [PubMed] [Google Scholar]
- 513.Mahaffey CM, Davies AM, Lara PN, Jr., et al. Schedule-dependent apoptosis in K-ras mutant nonsmall- cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clin Lung Cancer. 2007;8:548–553. doi: 10.3816/clc.2007.n.041. [DOI] [PubMed] [Google Scholar]
- 514.Steiner P, Joynes C, Bassi R, et al. Tumor growth inhibition with cetuximab and chemotherapy in nonsmall cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res. 2007;13:1540–1551. doi: 10.1158/1078-0432.CCR-06-1887. [DOI] [PubMed] [Google Scholar]
- 515.Raben D, Helfrich B, Chan DC, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11:795–805. [PubMed] [Google Scholar]
- 516.Tsai CH, Chen T, Chang K, Hsiao S. Combination effects of gefitinib plus cisplatin in non-small cell lung cancer (NSCLC): Why have phase III trials failed? J Clin Oncol. 2009;27 Abstract # 11022. [Google Scholar]
- 517.Meert AP, Martin B, Delmotte P, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002;20:975–981. doi: 10.1183/09031936.02.00296502. [DOI] [PubMed] [Google Scholar]
- 518.Cappuzzo F, Ligorio C, Toschi L, et al. EGFR and HER2 gene copy number and response to first-line chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2:423–429. doi: 10.1097/01.JTO.0000268676.79872.9b. [DOI] [PubMed] [Google Scholar]
- 519.Dziadziuszko R, Holm B, Skov BG, et al. Epidermal growth factor receptor gene copy number and protein level are not associated with outcome of non-small-cell lung cancer patients treated with chemotherapy. Ann Oncol. 2007;18:447–452. doi: 10.1093/annonc/mdl407. [DOI] [PubMed] [Google Scholar]
- 520.Hirsch FR, Varella-Garcia M, Dziadziuszko R, et al. Fluorescence in situ hybridization subgroup analysis of TRIBUTE, a phase III trial of erlotinib plus carboplatin and paclitaxel in non-small cell lung cancer. Clin Cancer Res. 2008;14:6317–6323. doi: 10.1158/1078-0432.CCR-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Lee KH, Han SW, Hwang PG, et al. Epidermal growth factor receptor mutations and response to chemotherapy in patients with non-small-cell lung cancer. Jpn J Clin Oncol. 2006;36:344–350. doi: 10.1093/jjco/hyl039. [DOI] [PubMed] [Google Scholar]
- 522.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 523.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 524.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 525.Bunn PA, Jr., Helfrich B, Soriano AF, et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res. 2001;7:3239–3250. [PubMed] [Google Scholar]
- 526.Tsai CM, Levitzki A, Wu LH, et al. Enhancement of chemosensitivity by tyrphostin AG825 in high-p185(neu) expressing non-small cell lung cancer cells. Cancer Res. 1996;56:1068–1074. [PubMed] [Google Scholar]
- 527.Tsai CM, Chang KT, Chen JY, Chen YM, Chen MH, Perng RP. Cytotoxic effects of gemcitabine-containing regimens against human non-small cell lung cancer cell lines which express different levels of p185neu. Cancer Res. 1996;56:794–801. [PubMed] [Google Scholar]
- 528.Tsai CM, Chang KT, Perng RP, et al. Correlation of intrinsic chemoresistance of non-small-cell lung cancer cell lines with HER-2/neu gene expression but not with ras gene mutations. J Natl Cancer Inst. 1993;85:897–901. doi: 10.1093/jnci/85.11.897. [DOI] [PubMed] [Google Scholar]
- 529.You XL, Yen L, Zeng-Rong N, Al Moustafa AE, Alaoui-Jamali MA. Dual effect of erbB-2 depletion on the regulation of DNA repair and cell cycle mechanisms in non-small cell lung cancer cells. Oncogene. 1998;17:3177–3186. doi: 10.1038/sj.onc.1202246. [DOI] [PubMed] [Google Scholar]
- 530.Zhang L, Hung MC. Sensitization of HER-2/neu-overexpressing non-small cell lung cancer cells to chemotherapeutic drugs by tyrosine kinase inhibitor emodin. Oncogene. 1996;12:571–576. [PubMed] [Google Scholar]
- 531.Meert AP, Martin B, Paesmans M, et al. The role of HER-2/neu expression on the survival of patients with lung cancer: a systematic review of the literature. Br J Cancer. 2003;89:959–965. doi: 10.1038/sj.bjc.6601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Graziano SL, Kern JA, Herndon JE, et al. Analysis of neuroendocrine markers, HER2 and CEA before and after chemotherapy in patients with stage IIIA non-small cell lung cancer: a Cancer and Leukemia Group B study. Lung Cancer. 1998;21:203–211. doi: 10.1016/s0169-5002(98)00063-4. [DOI] [PubMed] [Google Scholar]
- 533.Junker K, Stachetzki U, Rademacher D, et al. HER2/neu expression and amplification in non-small cell lung cancer prior to and after neoadjuvant therapy. Lung Cancer. 2005;48:59–67. doi: 10.1016/j.lungcan.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 534.Dong A, Kong M, Ma Z, Qian J, Cheng H, Xu X. Knockdown of insulin-like growth factor 1 receptor enhances chemosensitivity to cisplatin in human lung adenocarcinoma A549 cells. Acta Biochim Biophys Sin (Shanghai) 2008;40:497–504. doi: 10.1111/j.1745-7270.2008.00429.x. [DOI] [PubMed] [Google Scholar]
- 535.Warshamana-Greene GS, Litz J, Buchdunger E, Garcia-Echeverria C, Hofmann F, Krystal GW. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–1571. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 536.Karp DD, Paz-Ares LG, Novello S, et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27:2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 537.Cordes N, Beinke C, Plasswilm L, van Beuningen D. Irradiation and various cytotoxic drugs enhance tyrosine phosphorylation and beta(1)-integrin clustering in human A549 lung cancer cells in a substratum-dependent manner in vitro. Strahlenther Onkol. 2004;180:157–164. doi: 10.1007/s00066-004-1144-2. [DOI] [PubMed] [Google Scholar]
- 538.Hodkinson PS, Mackinnon AC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int J Radiat Biol. 2007;83:733–741. doi: 10.1080/09553000701570204. [DOI] [PubMed] [Google Scholar]
- 539.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 540.Rintoul RC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clin Sci (Lond) 2002;102:417–424. [PubMed] [Google Scholar]
- 541.Kraus AC, Ferber I, Bachmann SO, et al. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–8695. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 542.Gu T, Ohashi R, Cui R, et al. Osteopontin is involved in the development of acquired chemo-resistance of cisplatin in small cell lung cancer. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 543.Ohashi R, Takahashi F, Cui R, et al. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett. 2007;252:225–234. doi: 10.1016/j.canlet.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 544.Bartling B, Hofmann HS, Silber RE, Simm A. Differential impact of fibroblasts on the efficient cell death of lung cancer cells induced by paclitaxel and cisplatin. Cancer Biol Ther. 2008;7:1250–1261. doi: 10.4161/cbt.7.8.6264. [DOI] [PubMed] [Google Scholar]
- 545.Liu H, Zhang T, Li X, et al. Predictive value of MMP-7 expression for response to chemotherapy and survival in patients with non-small cell lung cancer. Cancer Sci. 2008;99:2185–2192. doi: 10.1111/j.1349-7006.2008.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Liu H, Zhang T, Wu B, Huang J, Zhou Y, Zhu J. Chronic exposure to exogenous matrilysin induces chemoresistance and enhances Bcl-2 expression in A549 lung adenocarcinoma cells. Mol Biol Rep. 2008 doi: 10.1007/s11033-008-9422-1. [DOI] [PubMed] [Google Scholar]
- 547.Camps C, Sirera R, Bremnes RM, et al. Analysis of c-kit expression in small cell lung cancer: prevalence and prognostic implications. Lung Cancer. 2006;52:343–347. doi: 10.1016/j.lungcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 548.Zhang P, Gao WY, Turner S, Ducatman BS. Gleevec (STI-571) inhibits lung cancer cell growth (A549) and potentiates the cisplatin effect in vitro. Mol Cancer. 2003;2:1. doi: 10.1186/1476-4598-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Chen JT, Huang CY, Chiang YY, et al. HGF increases cisplatin resistance via down-regulation of AIF in lung cancer cells. Am J Respir Cell Mol Biol. 2008;38:559–565. doi: 10.1165/rcmb.2007-0001OC. [DOI] [PubMed] [Google Scholar]
- 550.Krystal GW, Sulanke G, Litz J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 551.Nguyen DM, Chen GA, Reddy R, et al. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg. 2004;127:365–375. doi: 10.1016/j.jtcvs.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 552.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 553.Hemstrom TH, Sandstrom M, Zhivotovsky B. Inhibitors of the PI3-kinase/Akt pathway induce mitotic catastrophe in non-small cell lung cancer cells. Int J Cancer. 2006;119:1028–1038. doi: 10.1002/ijc.21927. [DOI] [PubMed] [Google Scholar]
- 554.Yu K, Lucas J, Zhu T, et al. PWT-458, a novel pegylated-17-hydroxywortmannin, inhibits phosphatidylinositol 3-kinase signaling and suppresses growth of solid tumors. Cancer Biol Ther. 2005;4:538–545. doi: 10.4161/cbt.4.5.1660. [DOI] [PubMed] [Google Scholar]
- 555.Schmidt M, Hovelmann S, Beckers TL. A novel form of constitutively active farnesylated Akt1 prevents mammary epithelial cells from anoikis and suppresses chemotherapy-induced apoptosis. Br J Cancer. 2002;87:924–932. doi: 10.1038/sj.bjc.6600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 557.Hovelmann S, Beckers TL, Schmidt M. Molecular alterations in apoptotic pathways after PKB/Akt-mediated chemoresistance in NCI H460 cells. Br J Cancer. 2004;90:2370–2377. doi: 10.1038/sj.bjc.6601876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Lee MW, Kim DS, Min NY, Kim HT. Akt1 inhibition by RNA interference sensitizes human non-small cell lung cancer cells to cisplatin. Int J Cancer. 2008;122:2380–2384. doi: 10.1002/ijc.23371. [DOI] [PubMed] [Google Scholar]
- 559.Ueda Y, Igishi T, Hashimoto K, et al. Synergistic cell growth inhibition by the combination of amrubicin and Akt-suppressing tyrosine kinase inhibitors in small cell lung cancer cells: implication of c-Src and its inhibitor. Int J Oncol. 2009;34:689–696. doi: 10.3892/ijo_00000195. [DOI] [PubMed] [Google Scholar]
- 560.Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 2005;65:8423–8432. doi: 10.1158/0008-5472.CAN-05-0058. [DOI] [PubMed] [Google Scholar]
- 561.Wu C, Wangpaichitr M, Feun L, et al. Overcoming cisplatin resistance by mTOR inhibitor in lung cancer. Mol Cancer. 2005;4:25. doi: 10.1186/1476-4598-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Dhar R, Basu A. Constitutive activation of p70 S6 kinase is associated with intrinsic resistance to cisplatin. Int J Oncol. 2008;32:1133–1137. [PubMed] [Google Scholar]
- 563.Loprevite M, Favoni RE, De Cupis A, et al. In vitro study of farnesyltransferase inhibitor SCH 66336, in combination with chemotherapy and radiation, in non-small cell lung cancer cell lines. Oncol Rep. 2004;11:407–414. [PubMed] [Google Scholar]
- 564.Bartling B, Yang JY, Michod D, Widmann C, Lewensohn R, Zhivotovsky B. RasGTPase-activating protein is a target of caspases in spontaneous apoptosis of lung carcinoma cells and in response to etoposide. Carcinogenesis. 2004;25:909–921. doi: 10.1093/carcin/bgh075. [DOI] [PubMed] [Google Scholar]
- 565.Rodenhuis S, Boerrigter L, Top B, et al. Mutational activation of the K-ras oncogene and the effect of chemotherapy in advanced adenocarcinoma of the lung: a prospective study. J Clin Oncol. 1997;15:285–291. doi: 10.1200/JCO.1997.15.1.285. [DOI] [PubMed] [Google Scholar]
- 566.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Rosell R, Gonzalez-Larriba JL, Alberola V, et al. Single-agent paclitaxel by 3-hour infusion in the treatment of non-small cell lung cancer: links between p53 and K-ras gene status and chemosensitivity. Semin Oncol. 1995;22:12–18. [PubMed] [Google Scholar]
- 568.Cvetkovic G, Plavec G, Tomic I, et al. K-ras mutation predictive significance in platinum based chemotherapeutic protocols in patients with advanced non-small cell lung cancer. Vojnosanit Pregl. 2009;66:149–155. doi: 10.2298/vsp0902149c. [DOI] [PubMed] [Google Scholar]
- 569.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 570.Schiller JH, Adak S, Feins RH, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: a Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J Clin Oncol. 2001;19:448–457. doi: 10.1200/JCO.2001.19.2.448. [DOI] [PubMed] [Google Scholar]
- 571.Kim ES, Kies MS, Fossella FV, et al. Phase II study of the farnesyltransferase inhibitor lonafarnib with paclitaxel in patients with taxane-refractory/resistant nonsmall cell lung carcinoma. Cancer. 2005;104:561–569. doi: 10.1002/cncr.21188. [DOI] [PubMed] [Google Scholar]
- 572.Brognard J, Dennis PA. Variable apoptotic response of NSCLC cells to inhibition of the MEK/ERK pathway by small molecules or dominant negative mutants. Cell Death Differ. 2002;9:893–904. doi: 10.1038/sj.cdd.4401054. [DOI] [PubMed] [Google Scholar]
- 573.Nishio K, Arioka H, Ishida T, et al. Enhanced interaction between tubulin and microtubule-associated protein 2 via inhibition of MAP kinase and CDC2 kinase by paclitaxel. Int J Cancer. 1995;63:688–693. doi: 10.1002/ijc.2910630514. [DOI] [PubMed] [Google Scholar]
- 574.Morita M, Suyama H, Igishi T, et al. Dexamethasone inhibits paclitaxel-induced cytotoxic activity through retinoblastoma protein dephosphorylation in non-small cell lung cancer cells. Int J Oncol. 2007;30:187–192. [PubMed] [Google Scholar]
- 575.Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006;66:8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- 576.Vicent S, Garayoa M, Lopez-Picazo JM, et al. Mitogen-activated protein kinase phosphatase-1 is overexpressed in non-small cell lung cancer and is an independent predictor of outcome in patients. Clin Cancer Res. 2004;10:3639–3649. doi: 10.1158/1078-0432.CCR-03-0771. [DOI] [PubMed] [Google Scholar]
- 577.Basu A, Weixel K, Saijo N. Characterization of the protein kinase C signal transduction pathway in cisplatin-sensitive and -resistant human small cell lung carcinoma cells. Cell Growth Differ. 1996;7:1507–1512. [PubMed] [Google Scholar]
- 578.Ding L, Wang H, Lang W, Xiao L. Protein kinase C-epsilon promotes survival of lung cancer cells by suppressing apoptosis through dysregulation of the mitochondrial caspase pathway. J Biol Chem. 2002;277:35305–35313. doi: 10.1074/jbc.M201460200. [DOI] [PubMed] [Google Scholar]
- 579.Wang XY, Liu HT. Antisense expression of protein kinase C alpha improved sensitivity to anticancer drugs in human lung cancer LTEPa-2 cells. Zhongguo Yao Li Xue Bao. 1998;19:265–268. [PubMed] [Google Scholar]
- 580.Villalona-Calero MA, Ritch P, Figueroa JA, et al. A phase I/II study of LY900003, an antisense inhibitor of protein kinase C-alpha, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:6086–6093. doi: 10.1158/1078-0432.CCR-04-0779. [DOI] [PubMed] [Google Scholar]
- 581.Paz-Ares L, Douillard JY, Koralewski P, et al. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24:1428–1434. doi: 10.1200/JCO.2005.04.3299. [DOI] [PubMed] [Google Scholar]
- 582.Yang CP, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998;439:368–372. doi: 10.1016/s0014-5793(98)01354-4. [DOI] [PubMed] [Google Scholar]
- 583.Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2008;59:105–110. doi: 10.1016/j.lungcan.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 584.Kiura K, Watarai S, Ueoka H, et al. An alteration of ganglioside composition in cisplatin-resistant lung cancer cell line. Anticancer Res. 1998;18:2957–2960. [PubMed] [Google Scholar]
- 585.Ponnampalam AP, Rogers PA. Cyclic changes and hormonal regulation of annexin IV mRNA and protein in human endometrium. Mol Hum Reprod. 2006;12:661–669. doi: 10.1093/molehr/gal075. [DOI] [PubMed] [Google Scholar]
- 586.Han EK, Tahir SK, Cherian SP, Collins N, Ng SC. Modulation of paclitaxel resistance by annexin IV in human cancer cell lines. Br J Cancer. 2000;83:83–88. doi: 10.1054/bjoc.2000.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Van Waardenburg RC, Prins J, Meijer C, Uges DR, De Vries EG, Mulder NH. Effects of c-myc oncogene modulation on drug resistance in human small cell lung carcinoma cell lines. Anticancer Res. 1996;16:1963–1970. [PubMed] [Google Scholar]
- 588.Mizushima Y, Kashii T, Kobayashi M. Effect of cisplatin exposure on the degree of N-myc amplification in small cell lung carcinoma cell lines with N-myc amplification. Oncology. 1996;53:417–421. doi: 10.1159/000227598. [DOI] [PubMed] [Google Scholar]
- 589.Van Waardenburg RC, Meijer C, Burger H, et al. Effects of an inducible anti-sense c-myc gene transfer in a drug-resistant human small-cell-lung-carcinoma cell line. Int J Cancer. 1997;73:544–550. doi: 10.1002/(sici)1097-0215(19971114)73:4<544::aid-ijc15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 590.Sawai A, Chandarlapaty S, Greulich H, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–596. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Ekedahl J, Joseph B, Marchetti P, et al. Heat shock protein 72 does not modulate ionizing radiation-induced apoptosis in U1810 non-small cell lung carcinoma cells. Cancer Biol Ther. 2003;2:663–669. [PubMed] [Google Scholar]
- 592.July LV, Beraldi E, So A, et al. Nucleotide-based therapies targeting clusterin chemosensitize human lung adenocarcinoma cells both in vitro and in vivo. Mol Cancer Ther. 2004;3:223–232. [PubMed] [Google Scholar]
- 593.Kim HR, Kim S, Kim EJ, et al. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer. 2008;60:47–56. doi: 10.1016/j.lungcan.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 594.Kuroda H, Takeno M, Murakami S, Miyazawa N, Kaneko T, Ishigatsubo Y. Inhibition of heme oxygenase-1 with an epidermal growth factor receptor inhibitor and cisplatin decreases proliferation of lung cancer A549 cells. Lung Cancer. 2009 doi: 10.1016/j.lungcan.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 595.Ohta T, Iijima K, Miyamoto M, et al. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res. 2008;68:1303–1309. doi: 10.1158/0008-5472.CAN-07-5003. [DOI] [PubMed] [Google Scholar]
- 596.Singh A, Boldin-Adamsky S, Thimmulappa RK, et al. RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy. Cancer Res. 2008;68:7975–7984. doi: 10.1158/0008-5472.CAN-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597.Wang XJ, Sun Z, Villeneuve NF, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Wang Q, Wang T, Wang Y, et al. VP-16 resistance in the NCI-H460 human lung cancer cell line is significantly associated with glucose-regulated protein78 (GRP78) induction. Anticancer Res. 2007;27:2359–2364. [PubMed] [Google Scholar]
- 599.Demarcq C, Bastian G, Remvikos Y. BrdUrd/DNA flow cytometry analysis demonstrates cis-diamminedichloroplatinum (II)-induced multiple cell-cycle modifications on human lung carcinoma cells. Cytometry. 1992;13:416–422. doi: 10.1002/cyto.990130412. [DOI] [PubMed] [Google Scholar]
- 600.Holdaway KM, Finlay GJ, Baguley BC. Relationship of cell cycle parameters to in vitro and in vivo chemosensitivity for a series of Lewis lung carcinoma lines. Eur J Cancer. 1992;28A:1427–1431. doi: 10.1016/0959-8049(92)90537-c. [DOI] [PubMed] [Google Scholar]
- 601.Prewitt TW, Matthews W, Chaudhri G, Pogrebniak HW, Pass HI. Tumor necrosis factor induces doxorubicin resistance to lung cancer cells in vitro. J Thorac Cardiovasc Surg. 1994;107:43–49. [PubMed] [Google Scholar]
- 602.Wahl AF, Donaldson KL, Mixan BJ, Trail PA, Siegall CB. Selective tumor sensitization to taxanes with the mAb-drug conjugate cBR96-doxorubicin. Int J Cancer. 2001;93:590–600. doi: 10.1002/ijc.1364. [DOI] [PubMed] [Google Scholar]
- 603.Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol. 2003;163:1109–1116. doi: 10.1016/S0002-9440(10)63470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Chen JG, Yang CP, Cammer M, Horwitz SB. Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res. 2003;63:7891–7899. [PubMed] [Google Scholar]
- 605.Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–1938. [PubMed] [Google Scholar]
- 606.Zhang P, Wang J, Gao W, Yuan BZ, Rogers J, Reed E. CHK2 kinase expression is down-regulated due to promoter methylation in non-small cell lung cancer. Mol Cancer. 2004:3–14. doi: 10.1186/1476-4598-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Kinzel B, Hall J, Natt F, Weiler J, Cohen D. Downregulation of Hus1 by antisense oligonucleotides enhances the sensitivity of human lung carcinoma cells to cisplatin. Cancer. 2002;94:1808–1814. doi: 10.1002/cncr.10383. [DOI] [PubMed] [Google Scholar]
- 608.Reed MF, Zagorski WA, Knudsen ES. RB activity alters checkpoint response and chemosensitivity in lung cancer lines. J Surg Res. 2007;142:364–372. doi: 10.1016/j.jss.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Shimizu E, Coxon A, Otterson GA, et al. RB protein status and clinical correlation from 171 cell lines representing lung cancer, extrapulmonary small cell carcinoma, and mesothelioma. Oncogene. 1994;9:2441–2448. [PubMed] [Google Scholar]
- 610.Fan T, Li R, Todd NW, et al. Up-regulation of 14-3-3zeta in lung cancer and its implication as prognostic and therapeutic target. Cancer Res. 2007;67:7901–7906. doi: 10.1158/0008-5472.CAN-07-0090. [DOI] [PubMed] [Google Scholar]
- 611.Ramirez JL, Rosell R, Taron M, et al. The Spanish Lung Cancer Group. 14-3-3sigma methylation in pretreatment serum circulating DNA of cisplatin-plus-gemcitabine-treated advanced non-small-cell lung cancer patients predicts survival. J Clin Oncol. 2005;23:9105–9112. doi: 10.1200/JCO.2005.02.2905. [DOI] [PubMed] [Google Scholar]
- 612.Oshita F, Kameda Y, Nishio K, et al. Increased expression levels of cyclin-dependent kinase inhibitor p27 correlate with good responses to platinum-based chemotherapy in non-small cell lung cancer. Oncol Rep. 2000;7:491–495. doi: 10.3892/or.7.3.491. [DOI] [PubMed] [Google Scholar]
- 613.Ishihara S, Minato K, Hoshino H, et al. The cyclin-dependent kinase inhibitor p27 as a prognostic factor in advanced non-small cell lung cancer: its immunohistochemical evaluation using biopsy specimens. Lung Cancer. 1999;26:187–194. doi: 10.1016/s0169-5002(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 614.Ishii T, Matsuse T, Masuda M, Teramoto S. The effects of S-phase kinase-associated protein 2 (SKP2) on cell cycle status, viability, and chemoresistance in A549 lung adenocarcinoma cells. Exp Lung Res. 2004;30:687–703. doi: 10.1080/01902140490517818. [DOI] [PubMed] [Google Scholar]
- 615.Filipits M, Pirker R, Dunant A, et al. Cell cycle regulators and outcome of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer: the International Adjuvant Lung Cancer Trial Biologic Program. J Clin Oncol. 2007;25:2735–2740. doi: 10.1200/JCO.2006.08.2867. [DOI] [PubMed] [Google Scholar]
- 616.Morero JL, Poleri C, Martin C, Van Kooten M, Chacon R, Rosenberg M. Influence of apoptosis and cell cycle regulator proteins on chemotherapy response and survival in stage IIIA/IIIB NSCLC patients. J Thorac Oncol. 2007;2:293–298. doi: 10.1097/01.JTO.0000263711.54073.fa. [DOI] [PubMed] [Google Scholar]
- 617.Roberson RS, Kussick SJ, Vallieres E, Chen SY, Wu DY. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res. 2005;65:2795–2803. doi: 10.1158/0008-5472.CAN-04-1270. [DOI] [PubMed] [Google Scholar]
- 618.Gautschi O, Hugli B, Ziegler A, et al. Cyclin D1 (CCND1) A870G gene polymorphism modulates smoking-induced lung cancer risk and response to platinum-based chemotherapy in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2006;51:303–311. doi: 10.1016/j.lungcan.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 619.Saijo T, Ishii G, Ochiai A, et al. Eg5 expression is closely correlated with the response of advanced non-small cell lung cancer to antimitotic agents combined with platinum chemotherapy. Lung Cancer. 2006;54:217–225. doi: 10.1016/j.lungcan.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 620.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 621.Denlinger CE, Rundall BK, Keller MD, Jones DR. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg. 2004;78:1207–1214. doi: 10.1016/j.athoracsur.2004.04.029. discussion -14. [DOI] [PubMed] [Google Scholar]
- 622.Jones DR, Broad RM, Madrid LV, Baldwin AS, Jr, Mayo MW. Inhibition of NF-kappaB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis. Ann Thorac Surg. 2000;70:930–936. doi: 10.1016/s0003-4975(00)01635-0. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 623.Osaki S, Nakanishi Y, Takayama K, Pei XH, Ueno H, Hara N. Transfer of IkappaBalpha gene increase the sensitivity of paclitaxel mediated with caspase 3 activation in human lung cancer cell. J Exp Clin Cancer Res. 2003;22:69–75. [PubMed] [Google Scholar]
- 624.Ni J, Takayama K, Ushijima R, et al. Adenovirus-mediated inhibitor kappaB gene transfer improves the chemosensitivity to anticancer drugs in human lung cancer in vitro and in vivo. Anticancer Res. 2008;28:601–608. [PubMed] [Google Scholar]
- 625.Oyaizu H, Adachi Y, Okumura T, et al. Proteasome inhibitor 1 enhances paclitaxel-induced apoptosis in human lung adenocarcinoma cell line. Oncol Rep. 2001;8:825–829. doi: 10.3892/or.8.4.825. [DOI] [PubMed] [Google Scholar]
- 626.Lee CT, Seol JY, Lee SY, et al. The effect of adenovirus-IkappaBalpha transduction on the chemosensitivity of lung cancer cell line with resistance to cis-diamminedichloroplatinum(II)(cisplatin) and doxorubicin(adriamycin) Lung Cancer. 2003;41:199–206. doi: 10.1016/s0169-5002(03)00227-7. [DOI] [PubMed] [Google Scholar]
- 627.Igarashi T, Izumi H, Uchiumi T, et al. Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene. 2007;26:4749–4760. doi: 10.1038/sj.onc.1210289. [DOI] [PubMed] [Google Scholar]
- 628.Tanabe M, Izumi H, Ise T, et al. Activating transcription factor 4 increases the cisplatin resistance of human cancer cell lines. Cancer Res. 2003;63:8592–8595. [PubMed] [Google Scholar]
- 629.Miyamoto N, Izumi H, Noguchi T. Tip60 is regulated by circadian transcription factor clock and is involved in cisplatin resistance. J Biol Chem. 2008;283:18218–18226. doi: 10.1074/jbc.M802332200. [DOI] [PubMed] [Google Scholar]
- 630.Zhuo WL, Wang Y, Zhuo XL, Zhang YS, Chen ZT. Short interfering RNA directed against TWIST, a novel zinc finger transcription factor, increases A549 cell sensitivity to cisplatin via MAPK/mitochondrial pathway. Biochem Biophys Res Commun. 2008;369:1098–1102. doi: 10.1016/j.bbrc.2008.02.143. [DOI] [PubMed] [Google Scholar]
- 631.Zhuo W, Wang Y, Zhuo X, Zhang Y, Ao X, Chen Z. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer. 2008 doi: 10.1016/j.lungcan.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 632.Oguri T, Katoh O, Takahashi T, et al. The Kruppel-type zinc finger family gene, HKR1, is induced in lung cancer by exposure to platinum drugs. Gene. 1998;222:61–67. doi: 10.1016/s0378-1119(98)00464-8. [DOI] [PubMed] [Google Scholar]
- 633.Ikuta K, Takemura K, Kihara M, et al. Overexpression of constitutive signal transducer and activator of transcription 3 mRNA in cisplatin-resistant human non-small cell lung cancer cells. Oncol Rep. 2005;13:217–222. [PubMed] [Google Scholar]
- 634.Hsu DS, Acharya CR, Balakumaran BS, et al. Characterizing the developmental pathways TTF-1, NKX2–8, and PAX9 in lung cancer. Proc Natl Acad Sci U S A. 2009;106:5312–5317. doi: 10.1073/pnas.0900827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Kim HJ, Hwang JY, Kim HJ, et al. Expression of a peroxisome proliferator-activated receptor gamma 1 splice variant that was identified in human lung cancers suppresses cell death induced by cisplatin and oxidative stress. Clin Cancer Res. 2007;13:2577–2583. doi: 10.1158/1078-0432.CCR-06-2062. [DOI] [PubMed] [Google Scholar]
- 636.Ma Y, Freeman SN, Cress WD. E2F4 deficiency promotes drug-induced apoptosis. Cancer Biol Ther. 2004;3:1262–1269. doi: 10.4161/cbt.3.12.1239. [DOI] [PubMed] [Google Scholar]
- 637.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol. 2008;26:2883–2889. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Levina V, Marrangoni A, DeMarco R, Gorelik E, Lokshin A. Drug-selected human lung stem-like cancer cells: tumorigenicity, metastatic properties and cytokine profile. Proc AACR. 2008;49:A#4616. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Chen YC, Hsu HS, Chen YW, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–4833. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 645.Basak S, Veena M, Oh S, et al. Evaluation of intratumoral heterogeneity of malignant pleural effusion and detection of lung cancer initiating cells. Proc AACR. 2009;50:A#175. [Google Scholar]
- 646.Sullivan J, Dodge M, Spinola M, Gao B, Minna J. Aldehyde dehydrogenase activity selects for non small cell lung cancer stem cells. Proc AACR. 2009;50:A#178. [Google Scholar]
- 647.Serrano D, Fernandez-Garcia I, Gonzalez-Moreno O, Montuenga L, Ortiz-de Solorzano C, Calvo A. The aldehyde dehydrogenase-positive cancer stem-line cell population maintains telomere length and is highly sensitive to telomerase inhibitors. Proc AACR. 2009;50:A#1057. [Google Scholar]
- 648.Lagenfeld J, Langenfeld E, Deen M. Lung cancers contain more than one cancer stem cell population. Proc AACR. 2009;50:A#177. [Google Scholar]
- 649.Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 650.Sheridan C, Kishimoto H, Fuchs RK, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Garcia Bueno JM, Ocana A, Castro-Garcia P, et al. An update on the biology of cancer stem cells in breast cancer. Clin Transl Oncol. 2008;10:786–793. doi: 10.1007/s12094-008-0291-9. [DOI] [PubMed] [Google Scholar]
- 652.Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51:191–200. doi: 10.1136/mp.51.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 654.Runz S, Mierke CT, Joumaa S, Behrens J, Fabry B, Altevogt P. CD24 induces localization of beta1 integrin to lipid raft domains. Biochem Biophys Res Commun. 2008;365:35–41. doi: 10.1016/j.bbrc.2007.10.139. [DOI] [PubMed] [Google Scholar]
- 655.Lu Z, Li H, Zhang H, Fan M, Shen X, He X. Expression and significance of CD44(+) ESA (+) CD24 (−/l0w) stem cell markers for breast cancer, in non-small-cell lung carcinoma. Ai Zheng. 2008;27:575–579. [PubMed] [Google Scholar]
- 656.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 657.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 658.Katoh M. Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol. 2007;31:461–466. [PubMed] [Google Scholar]
- 659.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 660.Blumenberg M, Gao S, Dickman K, Grollman AP, Bottinger EP, Zavadil J. Chromatin structure regulation in transforming growth factor-beta-directed epithelial-mesenchymal transition. Cells Tissues Organs. 2007;185:162–174. doi: 10.1159/000101317. [DOI] [PubMed] [Google Scholar]
- 661.Konishi J, Kawaguchi KS, Vo H, et al. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 662.Prudkin L, Liu D, Tchinda J, et al. NOTCH3/JAGGED1 pathway is involved in non-small cell lung cancer pathogenesis and interacts with EGFR pathway. Proc AACR. 2008;49:A#2168. [Google Scholar]
- 663.Chen Y, De Marco MA, Graziani I, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67:7954–7959. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- 664.Hassan K, Kalemkerian G, Wicha M. Aldehyde dehydrogenase activity identifies lung cancer cell line population that is more sensitive to Notch inhibition. Proc AACR. 2008;49:A#5409. [Google Scholar]
- 665.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 666.Luistro L, Smith M, Packman K, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting Notch singaling with in vivo efficacy and pharmacodynamic properties. Proc AACR. 2009;50:A#4759. doi: 10.1158/0008-5472.CAN-09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667.Lin L, Mernaugh R, Blum D, Carbone D, Dang T. Inhibition of Notch3 signaling pathway using small interfering peptides induces apoptosis in lung cancer cell lines. Proc AACR. 2008;49:A#5534. [Google Scholar]
- 668.Sunaga N, Kohno T, Kolligs FT, Fearon ER, Saito R, Yokota J. Constitutive activation of the Wnt signaling pathway by CTNNB1 (beta-catenin) mutations in a subset of human lung adenocarcinoma. Genes Chromosomes Cancer. 2001;30:316–321. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1097>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 669.Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 670.Nakashima T, Liu D, Nakano J, et al. Wnt1 overexpression associated with tumor proliferation and a poor prognosis in non-small cell lung cancer patients. Oncol Rep. 2008;19:203–209. [PubMed] [Google Scholar]
- 671.Chi S, Huang S, Li C, et al. Activation of the hedgehog pathway in a subset of lung cancers. Cancer Lett. 2006;244:53–60. doi: 10.1016/j.canlet.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 672.Vestergaard J, Pedersen MW, Pedersen N, et al. Hedgehog signaling in small-cell lung cancer: frequent in vivo but a rare event in vitro. Lung Cancer. 2006;52:281–290. doi: 10.1016/j.lungcan.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 673.Clague J, Chang J, Hildebrandt M, et al. Genetic variation in sonic hedgehog pathway genes as predictors of survival in patients with non-small cell lung cancer. Proc AACR. 2009;50:A#3843. [Google Scholar]
- 674.Cai L, Li JH, Xin Y, Niu LL, Zang JL, Sui GJ. Analysis of genes related to sensitivity to navalbine and docetaxel in 10 lung cancer cell lines. Zhonghua Zhong Liu Za Zhi. 2006;28:253–256. [PubMed] [Google Scholar]
- 675.Oshita F, Sekiyama A, Saito H, Yamada K, Noda K, Miyagi Y. Genome-wide cDNA microarray screening of genes related to the benefits of paclitaxel and irinotecan chemotherapy in patients with advanced non-small cell lung cancer. J Exp Ther Oncol. 2006;6:49–53. [PubMed] [Google Scholar]