Abstract
The Hedgehog (Hh) pathway has been implicated in a wide variety of human tumors, and early clinical trials with pathway antagonists have validated Hh signaling as a bona fide anti-cancer target. Despite these encouraging results, several issues surrounding the basic biology of the Hh pathway in human cancers remain unclear. These include the influence of specific oncogenic events on Hh signal transduction, the precise mode of Hh signaling (i.e., autocrine or paracrine) that occurs within human tumors, and the best means to inhibit aberrant pathway activity in the clinical setting. The cancer stem cell (CSC) hypothesis may explain a number of clinical phenomena, such as unchecked self-renewal and the development of metastatic disease, and to some extent, the Hh signaling pathway has been implicated in all of these processes. Therefore, Hh pathway inhibitors may also represent some of the first agents to formally examine the CSC hypothesis in the clinical setting. The diverse nature of Hh signaling in human cancers suggests that disease-specific factors must be carefully considered to identify the optimal use of novel pathway inhibitors.
Keywords: Hedgehog signaling, cancer stem cells, developmental therapeutics
Development and Hh pathway signal transduction
The Hedgehog (Hh) signaling pathway was initially identified in Drosophila as a critical mediator of segmental patterning during embryonic development, and it regulates the proliferation, migration and differentiation of target cells in a spatial, temporal, and concentration dependent manner (1–3). Hh signaling is conserved in vertebrates and highly active during mammalian development, especially within the neural tube and skeleton, but subsequently silenced in most adult tissues. However, some post-natal organs, such as the central nervous system (CNS) and the lung, rely on continued Hh signaling for tissue homeostasis and repair following injury (4–6).
Pathway activation is initiated by binding of one of the three secreted and lipid-modified ligands found in mammals, Sonic (SHh), Desert (DHh), and Indian (IHh) Hedgehog, to Patched (Ptch1), a 12-pass transmembrane spanning receptor (Figs. 1A, B). In the absence of ligand, Ptch constitutively represses the activity of Smoothened (Smo), a 7-pass transmembrane spanning protein with homology to G protein coupled receptors. Following Hh ligand binding to Ptch, the repression of Smo is released and the expression and/or post-translational processing of the three Gli zinc-finger transcription factors is modulated. Gli1 acts as a transcriptional activator and Gli3 as a repressor whereas Gli2 can either activate or repress gene expression depending on post-transcriptional and post-translational modifications (7). The balance between the activating and repressive forms of the Glis results in the expression of target genes, including Ptch1 and Gli1 (8, 9).
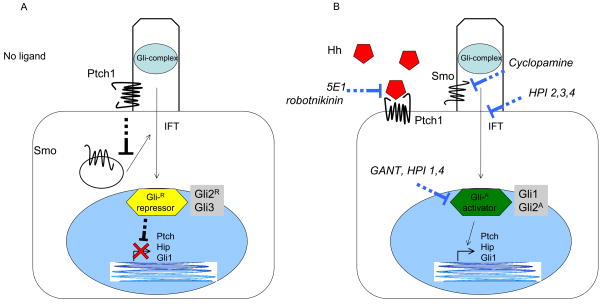
Figure 1. Hedgehog signaling.
A schematic of Hh pathway signal transduction derived from developmental and cancer models. (A) In the absence of Hh ligand, Ptch is located in the cilium and blocks Smo entry. Gli transcription factors exist in repressor forms that prevent transcription of target genes. (B) Three mammalian homologues of Hh (SHh, IHh, DHh) bind Ptch at the cell surface and allow it to move out of the primary cilium. Smo is derepressed and moves into the primary cilium where it can activate Gli transcription factors. During this process, the Gli transcription factors are processed to activator forms and translocated to the nucleus to induce the transcription of Hh target genes. Antibodies against the Hh ligands (5E1) and robotnikinin block pathway activation by preventing the interaction of Hh ligand with Ptch. Cyclopamine and novel antagonists of Smo directly bind and inhibit its function. Compounds such as HPI 2,3,4 block the transport of components in the signaling cascade. Direct Gli antagonists such as GANT block binding of Gli transcription factors to DNA.
Within this simplified schema, several other cellular components are required for Hh pathway activity. These include proteins involved in Hh ligand modification and cell surface binding (Hedgehog interacting protein (Hip), Hedgehog acyltransferase (Hhat), Growth arrest-specific 1 (Gas1), and CDO), and Gli processing and localization (Suppressor of fused (SuFu), Protein kinase A (PKA), Glycogen synthase kinase 3β (GSK3β), Casein kinase 1 (CK1), G protein-coupled receptor kinase 2 (GRK2) and βTRCP) (10–14). Moreover, increasing evidence has suggested that the sub-cellular localization of Hh pathway components is a major regulator of its activity. The examination of developmental defects arising in mice demonstrated that mutations within the intraflagellar transport proteins Kif3a and IFT88 produce patterning defects that mimic Hh loss-of-function mutations (15). These proteins are required for the assembly and maintenance of primary cilia that are present on most cells of the body during interphase and involved in a wide variety of cellular processes including mechanosensation and the transduction of several signaling pathways. A number of studies have subsequently demonstrated that pathway components translocate during activation, and in the absence of ligand, Ptch, but not Smo, is located within the primary cilia (16–20). Upon ligand binding, Ptch moves out and Smo moves into primary cilia to interact with Glis and their associated proteins that subsequently enter the nucleus to regulate gene expression (Fig. 1B).
Studies from a variety of experimental systems have identified the major components involved in Hh signal transduction, but extension of these results to human cancers should be approached with caution for several reasons. Many genetic studies have determined the role of specific pathway components by examining the effects of mutations on normal developmental programs, but the precise similarities between development and carcinogenesis are not fully understood. Meanwhile, detailed biochemical and cell biology studies have typically studied immortalized and non-malignant embryonic fibroblasts (e.g., NIH 3T3 or CH310T1/2 cells) that exhibit robust pathway activity compared to most cancers. Additionally, these cells are mesenchymal in origin and can differentiate into mature cartilage, bone and adipose cells. Thus, their relevance to Hh signaling within cancers derived from epithelial or hematopoietic tissues is unclear. Both genetic and epigenetic events underlie the formation and progression of human cancers, but the influence of these factors on Hh pathway signaling is largely unknown either in general or within specific tumors. Activating mutations in KRAS are found in a wide variety of epithelial carcinomas and may cause Gli1 activation and Hh target gene expression in a Smo-independent manner (21, 22), therefore, oncogenic events may directly induce pathway activity. The Hh signaling pathway has also been found to interact with other signaling pathways commonly activated in human cancers, such as PI3K/AKT (23, 24), and it is possible those also alter classical Hh signal transduction. It is also unclear whether or not primarily cilia are absolutely required for Hh signaling in human cancers since they are absent in Drosophila cells that are fully capable of pathway activation and may or may not be found at a high frequency within primary human BCCs (25). Moreover, recent studies examining the role of primary cilia and aberrant Hh signaling in the formation of BCC and medulloblastoma have demonstrated that mutations within Smo are not tumorigenic in the absence of cilia (25, 26). However, a constitutively activated form of Gli2 paradoxically induced tumor formation when cilia formation was blocked. Therefore, Hh signaling appears to be complex in cancers, and although several aspects of Hh signal transduction remain unclear, a better understanding of the mechanisms mediating pathogenic pathway activity within human cancer cells may identify additional therapeutic strategies.
Hedgehog signaling in cancer
The identification of somatic PTCH1 mutations in patients with Gorlin syndrome and basal cell nevus syndrome first implicated a role for the Hh pathway in cancer as these individuals are highly predisposed to developing advanced basal cell carcinoma (BCC), medulloblastoma, and rhabdomyosarcoma (27, 28). Additional evidence that aberrant Hh pathway activity may play a causal role in human cancers was provided by the identification of PTCH1 and SMO mutations in sporadic BCCs and medulloblastomas (29–31). Definitive proof that aberrant Hh signaling can induce cancers has come from transgenic mouse studies in which conditional loss of function of Ptch or gain of function of Smo or the Glis can recapitulate medulloblastoma and BCC (reviewed in (32)). Other Hh pathway components may also be genetically altered in human cancers including SUFU mutations in medulloblastoma, GLI1 and GLI3 mutations in pancreatic adenocarcinoma as well as GLI1 gene amplification in glioblastoma (33–35).
In contrast to tumors harboring activating mutations, several human cancers display aberrant pathway activity in response to increased levels of HH ligand (Table 1). Within these ligand-dependent tumors, several modes of pathway activation have been described (Fig. 2). The simplest is autocrine and/or juxacrine signaling in which tumor cells both produce and respond to HH ligand (Fig 2B). This mode of pathogenic HH activity has been described in a wide variety of human tumors including small cell lung, pancreatic, colon, and metastatic prostate cancers as well as glioblastomas and melanomas (5, 24, 36–40). Increased HH pathway activity either from mutational activation or autocrine signaling may induce the expression of genes affecting proliferation (Cyclin D1, Cyclin D2, N-Myc, Insulin growth factor 2 (Igf2), Hes1), cell survival (Bcl2), angiogenesis (Vascular endothelial growth factor (Vegf)), and genetic instability (p53) (41–51). Moreover, HH ligand blocking antibodies (in the case of ligand-dependent signaling), pharmacologic SMO antagonists, or siRNA directed against SMO or GLI1 have been shown to inhibit tumor growth.
Table 1.
Cancers associated with Hedgehog signaling
A summary of selected references describing aberrant Hh signaling by tumor type, the mode of Hh signaling (mutational, ligand mediated (paracrine or autocrine), involvement of stroma, and activity in CSC), and the experimental systems examined (mouse models, preclinical (includes human cancer cells lines, xenografts, primary patient samples) and clinical trials).
| Tumor Type | Mutation/Ligand mediated (paracrine/auto crine) | Stroma involved in signaling? | Required for CSC? | Type of data: Mouse Model, Preclinical, Clinical Trial | Selected References |
|---|---|---|---|---|---|
| MM | Ligand-autocrine Paracrine (stroma tumor) |
Yes, stroma to tumor | Yes | Preclinical | 52, 61 |
| Medulloblastoma | Mutation Ligand |
NR | NR | Mouse model Preclinical Clinical trial |
26, 42, 82, 83, 96, 99 |
| Upper GI | Ligand | NR | NR | Preclinical | 36 |
| Pancreatic | Ligand-autocrine (100) Epithelial cell Intrinsic (101) Paracrine (to stroma) (22, 52, 53) |
Data support intrinsic requirement and paracrine signaling to singnaling to stroma | Required by CSC {Feldman} | Mouse model, Preclinical |
22, 53, 60, 97, 100–103 |
| Ewings Sarcoma | Non Smo, direct activation Gli1 | NR | NR | Preclinical | 86, 87 |
| Melanoma | Non-ligand, non-mutation. | NR | NR | Mouse Model Preclinical |
24, 104 |
| Glioma | Ligand-paracrine (exogenous to tumor) | Possibly | Yes | Preclinical | 39, 40, 105 |
| Breast | Ligand, both autocrine and paracrine | Possibly | Yes | Mouse Model Preclinical |
59, 106 |
| Ovarian | Ligand | NR | NR | Preclinical | 107, 108 |
| Prostate | Ligand--autocrine | NR | Yes | Mouse model Preclinical |
38, 109 |
| Small cell lung | Ligand | NR | No | Mouse model Preclinical |
5 |
| Lymphoma | Ligand-autocrine and paracrine | Stroma signals to tumor | NR | Preclinical | 52, 110, 111 |
| Leukemia/CML | Up-regulation of Smo. No mutation Ligand-NR |
No | Yes | Mouse model Preclinical |
56–58 |
| BCC | Mutations in Ptch and Smo. Ligand driven mouse model |
No | NR | Mouse Model, Preclinical, Clinical Trial |
25, 29, 30, 80 |
NR, not reported; CSC, cancer stem cell; CML, chronic myeloid leukemia; BCC, basal cell carcinoma
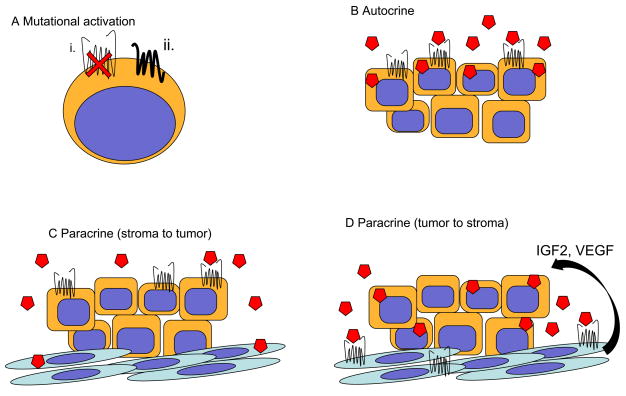
Figure 2. Models of Hh signaling in cancer.
(A) Non-ligand mediated signaling (mutational activation). Mutations in PTCH1, SMO, SUFU or amplification of GLI1 have all been reported in human cacners. i. Loss of PTCH1 activity may increase Hh pathway activity. ii. Overexpression or activating mutations in SMO are also depicted. (B) Ligand mediated autocrine signaling. Tumor cells produce Hh ligand that stimulates Hh pathway activity in tumor cells. (C) Ligand mediated paracrine signaling. Non-malignant stromal cells produces Hh ligand required by tumor cells for growth and survival. (D) Ligand mediated paracrine signaling. Tumor cells produce Hh ligand that activates Hh signaling in non-malignant stromal and endothelial cells. This results in the production of unknown factors within the microenvironment that ultimately supports tumor cell growth and survival as well as angiogenesis.
Hh signaling may act in a paracrine fashion during embryonic development in which cells secreting Hh ligands are distinct from those responding with pathway activation, and several recent studies have demonstrated that pathologic Hh signaling may also occur in a similar manner that involves the tumor microenvironment (52–54). In a recent report examining B cell lymphomas and multiple myeloma (MM), HH ligand was found to be primarily produced by stromal cells derived from the bone marrow, spleen or lymph nodes, rather than tumor cells (52) (Fig. 2C). However, the activation of HH signaling within tumor cells was found to act as an important survival factor since treatment with SMO antagonists induced cell death and decreased BCL2 expression in vitro as well as inhibted tumor growth in vivo. This effect was specifically mediated by HH signaling as the expression of GLI1 or a constitutively activated form of SMO abrogated these effects.
An alternative mode of paracrine Hh signaling has been recently described in which tumor cells secrete HH ligands that induce pathway activity within stromal cells (53–55) (Fig. 2D). In a study examining human pancreatic and colon primary tumors and cell lines grown as xenografts in mice, the expression of HH ligand by tumor cells did not correlate with their expression of target genes, but rather was associated with canonical pathway activity by tumor infiltrating stromal cells derived from the murine host (53). Decreased tumor growth was observed following administration of a pharmacologic SMO antagonist or HH neutralizing monoclonal antibody and correlated with decreased expression of mouse Gli1 expression, whereas human GLI1 expression remained unchanged. Further studies utilizing a transgenic mouse model of pancreatic cancer similarly failed to identify canonical Hh pathway activity within epithelial tumor cells despite expression of a constitutively activated form of Smo in these cells (54). These results strongly suggest that tumor derived HH ligand primarily induces pathway activity within infiltrating stromal cells that in turn influences tumor growth. Although the precise factors generated by stromal cells that modulate tumor growth are unknown, HH pathway activation may induce the secretion of soluble factors, such as IGF2 or VEGF that influence tumor cell proliferation and survival (43, 48, 49).
Conflicting data regarding the precise mode of Hh signaling in specific tumors, such as pancreatic and colon carcinoma (see below), may be due to differences in experimental systems. Given the spatial restriction of Hh signaling during normal development, the study of human tumors may require three-dimensional models to precisely determine the role HH plays in cancer biology and the anti-tumor effects of pathway inhibitors. It is also likely that the exact role of Hh signaling will be dependent on the specific tumor type as well as distinct clinical and biological factors, such as disease stage or genetic lesions. For example, intact Hh signaling, specifically Smo expression, is required for myeloid leukemia arising from the BCR-ABL, but not the MLL-AF9, fusion gene (56–58). Although delineating disease specific information will likely require extensive studies, it is likely required to optimize the clinical use of Hh pathway inhibitors.
Hedgehog signaling in cancer stem cells
Emerging data from many human tumors including glioblastoma, breast cancer, pancreatic adenocarcinoma, MM and chronic myeloid leukemia (CML) have suggested that Hh signaling regulates cancer stem cells (CSC) (39, 40, 56, 59–62). CSCs have been functionally defined by their capacity to undergo self-renewal and give rise to differentiated progeny that recapitulates the original tumor in an ectopic setting (63). Self-renewal is required for maintenance of the malignant clone, and reports examining mouse models of CML have provided evidence that Hh signaling regulates this property (56, 62). In these studies, the loss of Hh signaling by genetically disrupting Smo resulted in the inhibition of BCR-ABL expressing leukemic stem cells and prolonged survival. Active Hh signaling pathway has also been identified in glioblastoma CSCs, and pathway inhibition with cyclopamine or siRNA directed against pathway components results in the loss of tumorigenic potential (39, 40). In breast cancer, pathway activation in CSCs using Hh ligand and GLI1 or GLI2 expression or inhibition with cyclopamine or siRNA directed against GLI1 or GLI2 alters the expression of BMI-1, a central regulator of self-renewal in normal stem cells, and tumorigenic potential in vitro and in vivo (59). In MM, CSCs that phenotypically resemble normal memory B cells have been found to display relatively higher levels of Hh signaling than the mature plasma cells constituting the tumor bulk (61). Pathway activation with SHH ligand resulted in CSC self-renewal and expansion whereas the SMO antagonist cyclopamine or the ligand-neutralizing antibody 5E1 induced terminal differentiation and loss of clonogenic growth potential (Figs. 3A and B). Thus, HH signaling may dictate CSC fate decisions that include self-renewal and differentiation possibly by generation of a malignant niche (64). Data from MM also suggest that Hh signaling is likely to act through multiple modes and are involved in interactions between CSCs, differentiated tumor cells, and the microenvironment (Fig. 3C).
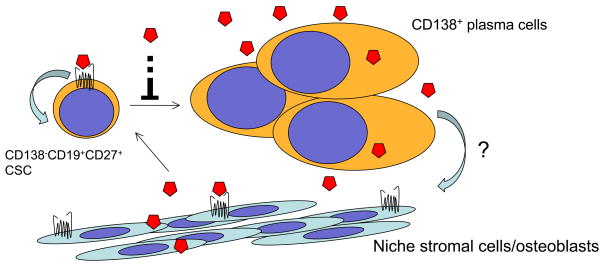
Figure 3. Hedgehog Signaling in Multiple Myeloma.
Regulation of MM CSC by the HH signaling pathway. The inhibition of HH signaling (A) inhibits MM CSC displaying the side population phenotype by (B) inducing terminal plasma cells differentiation of MM CSC as indicated by the expression of CD138. (C) Multiple modes of signaling appear to be active in MM. Experimental data suggest that differentiated plasma cells can produce the ligand necessary for CSC survival and proliferation. Blocking signaling leads to CSC differentiation. Normal bone marrow stromal cells can also produce ligand and signal to myeloma cells to support their growth and survival. A possible role for tumor-to-stoma paracrine signaling may also take occur.
In addition to tumor formation, CSCs have been implicated in disease progression and the development of metastasis in solid tumors (Fig. 4) (65, 66), and Hh signaling may play a critical role in this process similar to the Notch and Wnt pathways in cancer (67, 68). In colon carcinomas derived from primary clinical specimens, Hh signaling has been found to be preferentially activated within CSCs as evidenced by relatively higher expression of GLI1, GLI2, PTCH1, and HIP within this cellular compartment (37). Moreover, the relative expression of these pathway components as well as the target gene SNAIL1, which is associated with the epithelial to mesenchymal transition (EMT) and implicated in metastasis, increases in CSCs with disease stage. Inhibition of Hh pathway activity with cyclopamine or siRNA against SMO, GLI1, and GLI2, or expression of the repressive form of GLI3 reduced tumor cell proliferation and induced apoptosis. Moreover, cyclopamine reduced tumor regrowth in vivo and shRNA directed against SMO eliminated the formation of metastatic disease. The relationship between EMT and clonogenic growth potential has also been examined in pancreatic CSCs, and cyclopamine has been found to inhibit each of these functional properties and the formation of metastatic disease (60).
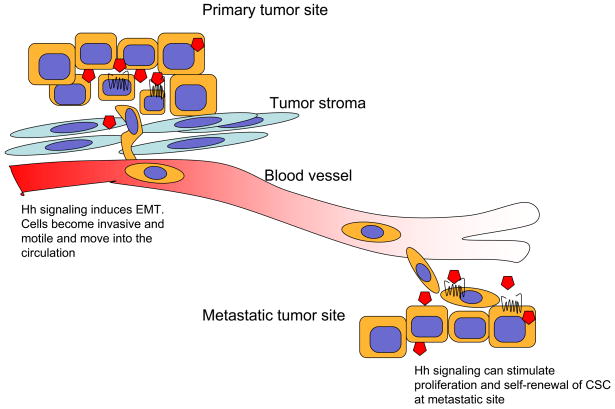
Figure 4. Hh signaling induces EMT and metastasis formation.
Cells undergoing EMT under the influence of Hh signaling and become more motile and invasive as they acquire mesenchymal cell properties. This allows cells to escape from the primary tumor and circulate to distant sites. Once established at a distant site, Hh may be required for the clonogenic growth and self-renewal.
Clinically targeting aberrant Hh signaling
Initial evidence that Hh signaling could be pharmacologically inhibited arose from the identification of cyclopamine, a steroidal alkyloid derived from Veratrum californicum, as an active compound capable of producing congenital defects in sheep (69). Recognition that SHh loss-of-function mutations recapitulate the neural defects seen with Veratrum teratogenicity led to findings that cyclopamine inhibits pathway activity by directly binding and inactivating Smo (70–72). Several pathway antagonists have been subsequently identified in compound screens that inhibit Gli-dependent reporter activity (73–77). Despite the unbiased nature of these screens against any one component of the Hh signaling pathway, the vast majority of compounds have been found to bind to and inhibit Smo despite being structurally distinct from one another and cyclopamine (reviewed in (78)). It is possible that these whole cell screens have primarily identified SMO antagonists because of its location on the cell surface, the central role it plays in Hh signal transduction, and the relative ease with which inhibitors of other 7-pass transmembrane proteins have been identified. An alternative approach to identifying novel Hh inhibitors has involved chemically modifying cyclopamine to improve its specificity, bioavailability, and pharmacokinetics (79).
The first clinical data reported using a SMO antagonist has involved GDC-0449 that was originally identified in a chemical compound screen and further modified and developed through a collaborative agreement by Curis and Genentech (74). The initial phase I trial examining the safety and tolerability of GDC-0449 enrolled a total of 68 solid tumor patients (80). Early data suggested efficacy in patients with advanced BCC, presumably because of the high frequency of aberrant Hh signaling, and the trial was expanded to further examine this group of patients. Of a total of 33 patients with locally advanced or metastatic basal cell carcinoma, 55% demonstrated clinical responses that included 2 complete remissions. Although efficacy data have not yet been reported from the other 35 patients without BCC enrolled in the trial, a published abstract indicated that a single patient with adenocystic carcinoma experienced stable disease (81). Reported toxicities were mild, and the most common events were mild loss of taste, hair loss, weight loss and hyponatremia. Importantly, biopsies of non-involved skin demonstrated in vivo inhibition of HH signaling evidenced by down regulation of GLI1 expression following drug administration. A transient subjective tumor response was also been reported in a single medulloblastoma patient treated with GDC-0449 under compassionate use (82). Early results from another SMO antagonist, BMS-833923 (XL139), co-developed by Exelixis and Bristol-Myers Squibb has been recently reported and demonstrated clinical benefit in patients with medulloblastoma and basal cell nevus syndrome (83). Based upon the safety and efficacy data generated in the early phase clinical trial, multiple phase II trials examining GDC-0449 in combination with standard agents in solid tumors have been initiated (Table 2). Moreover, early phase clinical testing of alternative SMO antagonists have been undertaken by several other companies including Exelixis/Bristol-Myers Squibb, Novartis, Infinity, and Pfizer (Table 2). Although all these agents target SMO, they are chemically distinct and no comparative studies have been carried out. It is possible that differences in SMO binding may lead to efficacy against some mutations that may arise during treatment (82), or that differences in pharmacokinetic properties may influence tissue distribution that provides more favorable inhibition of specific tumors, such as those in the CNS, or attenuation of side effects.
Table 2.
Novel Hedgehog pathway antagonists in clinical testing.
A summary of Hedgehog pathway antagonists currently undergoing clinical testing in humans. For each compound the company involved in development, the tumor types being tested (and the phase of testing), and associated references are listed.
| Compound | Company | Disease (Phase)* | Reference |
|---|---|---|---|
| GDC-0449 | Genetech/Curis | BCC (II), Pancreatic Cancer (I), SCLC (II), Medulloblastoma (II), Glioblastoma (II), Colorectal Ca (II), Ovarian Ca (II), Breast Ca, Gastric Ca (II), Gastroesphageal Ca (II) | 74, 80, 112 |
| IPI-926 | Infinity Pharmaceuticals | Solid tumors (I) | 79, 97, 112 |
| PF-04449913 | Pfizer | Myeloid Malignancies (I/II) | 112 |
| XL139/BMS833923 | Exelixis/Bristol-Myers Squibb | Solid Tumors (I), Multiple Myeloma (I/II), SCLC (I), Gastric Ca (I), Esophageal Ca (I), | 83, 112 |
| LDE225 | Novartis | Solid tumor (I), Medulloblastoma (I), BCC (I) | 112 |
BCC, basal cell carcinoma; SCLC, small cell lung cancer;
Theses early results provide critical proof-of-concept that inhibiting HH pathway activity has anti-tumor activity in humans, but they also generate several questions. For example, the majority of BCC tumor specimens from patients treated with GDC-0449 displayed increased levels of GLI1 expression consistent with the nature of the disease, but just over half of the patients experienced clinical responses despite circulating levels of drug that were well above the concentration required to inhibit Hh signaling in vitro (74, 80). Additionally, the examination of pretreatment specimens for the presence of primary cilia or HH pathway activity within tumor stromal cells may have correlated with responses and validated the importance of these factors in the clinical setting. Similarly, serial tumor biopsies were not required, but it would have been interesting to correlate the degree of intra-tumoral pathway inhibition with clinical responses to draw a conclusive relationship between pathway activity and human cancers. Finally, the rapid loss of response and finding of an acquired mutation in SMO in the medulloblastoma patient treated with GCD-0449 suggests that loss of response in BCC patients may have occur in a similar manner (82, 84), and isolation of tumor tissue at disease progression could have explored this possibility.
Alternative strategies to inhibit Hh signaling
Initial results with SMO antagonists are encouraging, but the development of alternative means to inhibit Hh signaling may be desirable for several reasons. First, several genetic lesions acting downstream of SMO have been detected in human cancers, such as mutations or loss of heterozygosity of the negative pathway regulators SUFU or RENKCTD11 or amplifications in GLIs (33, 35, 85). Moreover, GLI1 activity may be induced in a SMO-independent manner by transforming events, such as EWS-FLI in Ewing sarcoma or mutant KRAS in pancreatic cancer (21, 22, 86, 87). Since Hh signal transduction ultimately activates the GLI transcription factors, inhibitors modulating GLI may be useful similar to therapeutic strategies being developed to target Wnt/β-catenin signaling in cancer (68). A screen for compounds capable of inhibiting Gli-mediated transcription identified Gli-antagonist (GANT)-58 and GANT-61 (88). Both of these compounds act downstream of Smo and SuFu and inhibit tumor growth both in vitro and in vivo. The precise inhibitory mechanism of these compounds is unclear, but GANT-61 limits Gli1 DNA binding. A screen designed to identify molecules capable of inhibiting Gli1 transcriptional activity without targeting Smo identified 4 distinct Hh pathway inhibitors (HPIs) (89). Each of these compounds inhibits Gli at a level epistatic to SuFu, but their precise mechanisms are unknown. HPI-1 inhibits both Gli1 and Gli2, perhaps by altering their post-translational modification, HPI-4 appears to partially inhibit ciliogenesis, and HPI-2 and 3 may inhibit the conversion of Gli2 from a repressive to an activated form. Although less specific, GLI activity may also be inhibited by modulating the activity of other pathways known to interact with Hh signaling, such as PI3K/AKT, MAPK, and TGFs (23, 24, 90–92).
Mutations in Hh pathway components have not been described in most cancers displaying aberrant signaling. Therefore, the inhibition of ligand binding may inhibit pathway activation, and the ligand neutralizing monoclonal antibody 5E1 has been shown to have clear anti-tumor activity in several diseases (see above). More recently, robotnikinin, was identified as a compound that binds to SHh and blocks its ability induce pathway activity at the level of Ptch (93). It is also possible that specific on-target side effects attributable to the inhibition of Hh pathway activity in some cells, such as defects in bone growth seen in young mice treated with a Smo antagonist (94), may be avoided by blocking the activity of a specific Hh ligand.
Clinical Translation of Hedgehog Inhibitors
It is likely that the effective clinical use of Hh inhibitors will require a precise understanding of the role that the pathway plays in a specific tumor. In cancers with mutational pathway activation affecting all tumor cells, pathway inhibition may impact the growth and survival of all tumor cells evident as reduction in disease burden as observed in BCC patients receiving GDC-0449 (80). Alternatively, Hh may be required early in carcinogenesis or in the formation of pre-cancerous lesions, but once tumors are established, it may not be needed for tumor maintenance or progression. In these cases, Hh inhibitors may be active chemo-preventative agents in high-risk patients, but would be ineffective in treating established disease. Alternatively, the Hh pathway may be activated in response to chemotherapy or during re-growth of minimal residual disease, perhaps by regulating CSCs (95). In this case Hh inhibitors would be most effective as maintenance therapy following tumor debulking with surgery or cytotoxic chemotherapy. Hh signaling has also been implicated in mediating chemoresistance (96, 97), therefore, inhibitors may act as potent chemo-sensitizers and require the simultaneous administration of cytotoxic chemotherapy.
Given the diverse biological effects of Hh activity, accurately measuring the activity of Hh inhibitors may also require context dependent indicators of clinical response. The Hh pathway may regulate EMT and the formation of metastatic disease (37, 38, 60). In this case, treatment with Hh inhibitors may not result in clinically significant inhibition of the primary tumor, but could prevent the formation of new metastasis and prolong progression free survival. Similarly, if Hh signaling is primarily active in CSCs, clinical responses to Hh inhibitors may be delayed in patients with extensive disease. Effective inhibition of CSCs should lead to improved overall survival, but most early phase trials are not designed or powered to detect differences in long-term outcomes (98). Therefore, reliable biomarkers that accurately quantify CSC may be useful endpoints within early clinical testing. Finally, patient selection is likely to be a critical factor in establishing the clinical efficacy of Hh pathway antagonists similar to most targeted therapies. Since mutations appear to drive only a minority of Hh related cancers, selection of patients will not be possible by simply genotyping tumor specimens, but better understanding the specific role the Hh signaling pathway plays in each tumor type is likely to be valuable during the development of these novel agents. It is clear that several issues regarding the precise role of the Hh signaling pathway in human cancer remained unresolved at this point, including the precise mechanisms of signal transduction, the exact mode of signaling between tumor cells and the microenvironment, and its role in the regulation of CSCs. However, it is clear that the Hh pathway can play an important role in tumor cell growth and survival, and these functions are likely to be broadly applicable across a variety of malignancies. As several novel inhibitors have entered clinical testing, precise correlative studies may allow many of these conflicts to be resolved within the most relevant system, the patients themselves.
Acknowledgments
Grant Support: National Institutes of Health R01CA127574, P01CA15396, K23CA107040, the Gabrielle’s Angel Foundation for Cancer Research, the Sidney Kimmel Foundation for Cancer Research, the Multiple Myeloma Research Foundation and the Leukemia and Lymphoma Society.
We thank Craig Peacock, Karen McGovern, and Sarah Brennan for helpful comments and critical review of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
W. Matsui, commercial research grant, Merck; patent interest, Infinity Pharmaceuticals; consultant, Bristol-Myers Squibb, Pfizer.
References
- 1.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 3.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22:2454–72. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 4.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic Hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 5.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–7. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 6.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–31. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126:3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–47. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stecca B, Ruiz I, Altaba A. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and Non-HEDGEHOG signals. Journal of Molecular Cell Biology. 2010 doi: 10.1093/jmcb/mjp052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buglino JA, Resh MD. Hhat is a palmitoylacyltransferase with specificity for N-palmitoylation of Sonic Hedgehog. J Biol Chem. 2008;283:22076–88. doi: 10.1074/jbc.M803901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen BL, Tenzen T, Mcmahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–57. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Ren X, Nelson C, et al. Activity-dependent internalization of smoothened mediated by {beta}-Arrestin 2 and GRK2. Science. 2004;306:2257. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 13.Pearse R, Collier L, Scott M, Tabin C. Vertebrate homologs of Drosophila suppressor of fused interact with the gli family of transcriptional regulators. Dev Biol. 1999;212:323–36. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci USA. 2006;103:33–8. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 16.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–21. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 18.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates Hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 19.Wilson CW, Chen M-H, Chuang P-T. Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS ONE. 2009;4:e5182. doi: 10.1371/journal.pone.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA. 2009;106:21666–71. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates Hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–55. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 22.Nolan-Stevaux O, Lau J, Truitt ML, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and AKT are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci USA. 2006;103:4505–10. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stecca B, Mas C, Clement V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong SY, Seol AD, So P-L, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–61. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Kim H, Dlugosz A, Ellison D, Gilbertson R, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009 doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 29.Xie J, Johnson RL, Zhang X, et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 1997;57:2369–72. [PubMed] [Google Scholar]
- 30.Xie J, Murone M, Luoh SM, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–2. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 31.Pietsch T, Waha A, Koch A, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–8. [PubMed] [Google Scholar]
- 32.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 33.Taylor MD, Liu L, Raffel C, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 34.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA. 1987;84:6899–903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 37.Varnat F, Duquet A, Malerba M, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. Embo Mol Med. 2009:1. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 39.Clement V, Sanchez P, de TN, Radovanovic I, Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated Hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417:299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 42.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by Hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 43.Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- 44.Ingram WJ, McCue KI, Tran TH, Hallahan AR, Wainwright BJ. Sonic Hedgehog regulates Hes1 through a novel mechanism that is independent of canonical Notch pathway signalling. Oncogene. 2008;27:1489–500. doi: 10.1038/sj.onc.1210767. [DOI] [PubMed] [Google Scholar]
- 45.Wall D, Mears A, McNeill B, et al. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic Hedgehog/Hes1 activity. J Cell Biol. 2009 doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regl G, Kasper M, Schnidar H, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–31. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 47.Kenney AM, Rowitch DH. Sonic Hedgehog promotes G1 cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–67. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pola R, Ling L, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 49.Kanda S, Mochizuki Y, Suematsu T, Miyata Y, Nomata K, Kanetake H. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278:8244–9. doi: 10.1074/jbc.M210635200. [DOI] [PubMed] [Google Scholar]
- 50.Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009;28:663–76. doi: 10.1038/emboj.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abe Y, Oda-Sato E, Tobiume K, et al. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA. 2008;105:4838–43. doi: 10.1073/pnas.0712216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dierks C, Grbic J, Zirlik K, et al. Essential role of stromally induced Hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–51. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 53.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for Hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 54.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Theunissen J-W, de Sauvage FJ. Paracrine Hedgehog signaling in cancer. Cancer Res. 2009;69:6007–10. doi: 10.1158/0008-5472.CAN-09-0756. [DOI] [PubMed] [Google Scholar]
- 56.Dierks C, Beigi R, Guo GR, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–49. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Zhao C, Blum J, Chen A, et al. Loss of [beta]-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–41. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann I, Stover EH, Cullen DE, et al. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Stem Cell. 2009;4:559–67. doi: 10.1016/j.stem.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–96. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA. 2007;104:4048–53. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–9. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien C, Kreso A, Jamieson C. Cancer stem cells and self-renewal. Clin Cancer Res. 2010:16. doi: 10.1158/1078-0432.CCR-09-2824. in press. [DOI] [PubMed] [Google Scholar]
- 64.LaBarge M. Pinning down cancer stem cell niches. Clin Cancer Res. 2010:16. doi: 10.1158/1078-0432.CCR-09-2933. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mani S, Guo W, Liao M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–51. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pannuti A, Foreman K, Rizzo P, et al. Targeting cancer stem cells through notch signaling. Clin Cancer Res. 2010:16. doi: 10.1158/1078-0432.CCR-09-2823. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi-Yanaga F, Kahn M. Cancer stem cell therapeutics WNT. Clin Cancer Res. 2010:16. doi: 10.1158/1078-0432.CCR-09-2943. in press. [DOI] [PubMed] [Google Scholar]
- 69.Binns W, Keeler RF, Balls LD. Congenital deformities in lambs, calves, and goats resulting from maternal ingestion of Veratrum californicum: hare lip, cleft palate, ataxia, and hypoplasia of metacarpal and metatarsal bones. Clin Toxicol. 1972;5:245–61. doi: 10.3109/15563657208991003. [DOI] [PubMed] [Google Scholar]
- 70.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–7. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 71.Taipale J, Chen JK, Cooper MK, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–9. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 72.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449-a potent inhibitor of the Hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–81. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 75.Gendreau SB, Hawkins D, Ho C-P, et al. Preclinical characterization of BMS-833923 (XL139), a Hedgehog (HH) pathway inhibitor in early clinical development. Mol Cancer Ther. 2009;8 (12 Suppl):B192. [Google Scholar]
- 76.Frank-Kamenetsky M, Zhang XM, Bottega S, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. Journal of Biology 2002 1:10. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams JA, Guicherit OM, Zaharian BI, et al. Identification of a small molecule inhibitor of the Hedgehog signaling pathway: effects on basal cell carcinoma-like lesions. Proc Natl Acad Sci USA. 2003;100:4616–21. doi: 10.1073/pnas.0732813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahindroo N, Punchihewa C, Fujii N. Hedgehog-Gli signaling pathway inhibitors as anticancer agents. J Med Chem. 2009;52:3829–45. doi: 10.1021/jm801420y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tremblay M, Nevalainen M, Nair S, et al. Semisynthetic cyclopamine analogues as potent and orally bioavailable Hedgehog pathway antagonists. J Med Chem. 2008;51:6646–9. doi: 10.1021/jm8008508. [DOI] [PubMed] [Google Scholar]
- 80.Von Hoff D, Lorusso P, Rudin C, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009 doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 81.Von Hoff D, Rudin C, LoRusso P, et al. Efficacy data of GDC-0449, a systemic Hedgehog pathway antagonist, in a first-in-human, first-in-class Phase I study with locally advanced, multifocal or metastatic basal cell carcinoma patients. AACR Meeting Abstracts 2008. 2008:LB-138. [Google Scholar]
- 82.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siu LL, Papadopoulos KP, Alberts SR, et al. A first-in-human, phase I study of an oral Hedgehog pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors. Mol Cancer Ther. 2010;8:A55. [Google Scholar]
- 84.Yauch RL, Dijkgraaf GJP, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Marcotullio L, Ferretti E, De Smaele E, et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci USA. 2004;101:10833–8. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zwerner JP, Joo J, Warner KL, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. 2008;27:3282–91. doi: 10.1038/sj.onc.1210991. [DOI] [PubMed] [Google Scholar]
- 87.Beauchamp E, Bulut G, Abaan O, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–82. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–60. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hyman JM, Firestone AJ, Heine VM, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci U S A. 2009;106:14132–7. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kasper M, Schnidar H, Neill G, et al. Selective modulation of Hedgehog/GLI target gene expression by epidermal growth factor signaling in human keratinocytes. Mol Cell Biol. 2006;26:6283. doi: 10.1128/MCB.02317-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dennler S, Andre J, Alexaki I, et al. Induction of Sonic Hedgehog mediators by Transforming Growth Factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 92.Dennler S, Andre J, Verrecchia F, Mauviel A. Cloning of the human GLI2 Promoter: transcriptional activation by Transforming Growth Factor-beta via SMAD3/beta-Catenin cooperation. J Biol Chem. 2009;284:31523–31. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stanton BZ, Peng LF, Maloof N, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–6. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kimura H, Ng JMY, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–60. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 95.Travaglione V, Peacock C, MacDougall J, et al. A novel HH pathway inhibitor, IPI-926, delays recurrence post-chemotherapy in a primary human SCLC xenograft model. AACR Meeting Abstracts 2008. 2008:4611. [Google Scholar]
- 96.Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog Signaling Promotes Medulloblastoma Survival via BclII. American Journal of Pathology. 2007;170:347–55. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huff CA, Matsui W, Smith BD, Jones RJ. The paradox of response and survival in cancer therapeutics. Blood. 2006;107:431–4. doi: 10.1182/blood-2005-06-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–40. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 100.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–73. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 103.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–25. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das S, Harris LG, Metge BJ, et al. The Hedgehog pathway transcription factor GLI1 promotes malignant behavior of cancer cells by up-regulating Osteopontin. J Biol Chem. 2009;284:22888–97. doi: 10.1074/jbc.M109.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ehtesham M, Sarangi A, Valadez JG, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–61. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 106.Fiaschi M, Rozell B, Bergstrom A, Toftgard R. Development of mammary tumors by conditional expression of GLI1. Cancer Res. 2009;69:4810–7. doi: 10.1158/0008-5472.CAN-08-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhattacharya R, Kwon J, Ali B, et al. Role of Hedgehog signaling in ovarian cancer. Clin Cancer Res. 2008;14:7659–66. doi: 10.1158/1078-0432.CCR-08-1414. [DOI] [PubMed] [Google Scholar]
- 108.Liao X, Siu MKY, Au CWH, et al. Aberrant activation of Hedgehog signaling pathway in ovarian cancers: effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–40. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchez P, Hernandez AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101:12561–6. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh RR, Cho-Vega JH, Davuluri Y, et al. Sonic Hedgehog signaling pathway Is activated in ALK-positive anaplastic large cell lymphoma. Cancer Res. 2009;69:2550–8. doi: 10.1158/0008-5472.CAN-08-1808. [DOI] [PubMed] [Google Scholar]
- 111.Hegde GV, Munger CM, Emanuel K, et al. Targeting of Sonic Hedgehog-GLI signaling: a potential strategy to improve therapy for mantle cell lymphoma. Mol Cancer Ther. 2008;7:1450–60. doi: 10.1158/1535-7163.MCT-07-2118. [DOI] [PubMed] [Google Scholar]
- 112.National Institutes of Health. Clinical Trials website. http://clinicaltrials.gov/