Abstract
We report the immunopathological analysis of the brain and tumor of two patients who died of anti-NMDAR-associated encephalitis, and of the tumor of nine patients who recovered. Findings included prominent microgliosis and deposits of IgG with rare inflammatory infiltrates in the hippocampus, forebrain, basal ganglia, and spinal cord. Detection of cells expressing markers of cytotoxicity (TIA, granzyme B, perforin and Fas/Fas ligand) was extremely uncommon. All tumors showed NMDAR-expressing neurons and inflammatory infiltrates. All patients’ NMDAR antibodies were IgG1, IgG2, or IgG3. No complement deposits were observed in any of the central nervous system regions examined. Overall, these findings coupled with recently reported in vitro data showing that antibodies downregulate the levels of NMDA receptors suggest that the antibody immune-response is more relevant than cytotoxic T-cell mechanisms in the pathogenesis of anti-NMDAR-associated encephalitis.
Keywords: Paraneoplastic, Limbic encephalitis, NMDA, Antibodies, Teratoma
Introduction
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a severe but treatment responsive disorder that predominantly affects young individuals with or without tumor. Patients usually develop prodromal fever or headache followed in a few days by prominent psychiatric symptoms or less frequently short-term memory loss, then seizures, progressive unresponsiveness (catatonia-like stage), dyskinesias, autonomic instability, and hypoventilation. Previous studies, including brain biopsy or autopsy of 18 patients, showed perivascular lymphocytic cuffing, microglial activation, and IgG deposits as principal pathological features [4, 6, 10, 11]. However, a detailed analysis of the inflammatory infiltrates in the central nervous system (CNS) and tumors was not performed, and the IgG subtypes of NMDAR antibodies were not determined. We describe here a comprehensive immunopathological analysis of the brain and tumor of two patients who died of anti-NMDAR encephalitis, and of the tumor of nine patients who survived the disorder. In addition, we report the IgG subclass of anti-NMDAR antibodies in 13 patients.
Materials and methods
Patients
The clinical features of patients #1 and #2 have been previously reported [6]. Briefly, patient #1 was a 35-year-old woman, who presented with short-term memory deficits and generalized tonic-clonic seizures followed by decrease of level of consciousness and hypoventilation. She developed partial motor seizures in the left lower extremity, dystonic movements, and hyperthermia during the course of her disease. Brain MRI demonstrated medial temporal lobe FLAIR hyperintensity. CSF showed pleocytosis (189 WBC/ul), elevated protein concentration (68 mg/dl) and positive oligoclonal bands. The patient received immunotherapy (corticosteroids, plasma exchange, IVIg, and cyclophosphamide) and died 4 months after symptom onset. At autopsy a 3.5-cm mature teratoma was found in the left ovary.
Patient #2 was a 24-year-old woman presenting with paranoid thoughts, auditory hallucinations, agitation, and subsequently generalized seizures. She later developed severe autonomic instability and myoclonic and dyskinetic movements in the arms and face. Brain MRI showed T2 hyperintensity in the sulci of the parietal lobes with mild contrast enhancement of the overlaying meninges. CSF showed pleocytosis (219 WBC/ul), elevated protein concentration (129 mg/dl), and positive oligoclonal bands. She was treated with corticosteroids and died 3 months after symptom presentation. At autopsy a 1.5-cm mature teratoma was found in the right ovary.
Tissues
Tissues were obtained from autopsy of the two patients described above, and surgical tumor specimens (7 mature, 2 immature ovarian teratomas) of nine additional patients who survived the disorder. Nervous system samples were embedded in paraffin, including the spinal cord of one of the patients. Eight of 11 patients’ tumor samples were paraffin-embedded and three were kept frozen. Anti-NMDAR antibodies were found positive in sera and CSF of all patients, as reported previously. The antibody titers were significantly reduced following the surgical removal of the ovarian tumor in parallel with the amelioration of the symptoms suggesting that NMDAR antibodies are involved in the pathogenesis of the CNS symptoms [4, 6]. Control samples included paraffin-embedded tissue from multiple brain regions and lymph nodes obtained at autopsy of two neurologically normal individuals, and three ovarian teratomas (2 mature, 1 immature) and two endometrial cancer samples (with similar post mortem and fixation times) from patients without anti-NMDAR-associated encephalitis.
Immunohistochemical analysis of inflammatory infiltrates and deposits of IgG in patients’ CNS and tumor
Paraffin-embedded brain and tumor tissues were deparaffinized and the antigens retrieved, as reported [3]. Tissue sections were serially incubated with 0.3% H2O2 for 20 min, 10% goat serum for 1 h, and the indicated primary antibodies (Table 1) diluted in 10% goat serum in phosphate buffered saline (PBS) overnight at 4°C. After using the appropriate secondary antibodies (all diluted 1:2,000), the reactivity was developed with the avidin–biotin–peroxidase method [5].
Table 1.
Antibodies and specificities
| Antibody/species | Cellular specificity | Dilution | Source |
|---|---|---|---|
| MAP-2a/chicken | Neurons | 1:20,000 | Covance, Berkeley, CA, USA |
| CD3/rabbit | T cells | 1:100 | Dako, Glostrup, Denmark |
| CD4/mouse | Helper T cells | 1:100 | Zymed, San Francisco, CA, USA |
| CD8/mouse | Cytotoxic T cells | 1:100 | Zymed, San Francisco, CA, USA |
| CD20/mouse | B cells | 1:50 | Dako, Glostrup, Denmark |
| CD68/mouse | Macrophages, microglia | 1:200 | Dako, Glostrup, Denmark, USA |
| CD79a/rabbit | Plasma cells | 1:300 | Novus Biologicals, Littleton, CO, USA |
| TIA-1/mouse | Cytotoxic T cells, NK cells | 1:100 | Beckman Coulter, Fullerton, CA, USA |
| Granzyme B/mouse | Cytotoxic T cells, NK cells | 1:20 | Chemicon, Temecula, CA, USA |
| Perforin/mouse | Cytotoxic T cells, NK cells | 1:30 | Biomeda, Foster City, CA, USA |
| Fas/mouse | Activated T and B cells | 1:20 | Chemicon, Temecula, CA, USA |
| Fas ligand/mouse | Activated T cells | 1:20 | Chemicon, Temecula, CA, USA |
| IgG/goat | Human IgG | 1:1,500 | Jackson Immunoresearch, West Grove, PA, USA |
| C9/mouse | Human complement | 1:50 | Cell Sciences, Canton, MA, USA |
| Human IgMb/mouse | Human IgM | 1:200 | Southern Biotech, Birmingham, AL, USA |
| Human IgG1-4b/mouse | Human IgG subclasses | 1:200 | Sigma, St. Louis, MO, USA IgG1 (F0767), IgG2 (F4516), IgG3 (F4641), IgG4 (F9890) |
MAP-2 microtubule associated protein-2
Used only on tumor samples
Used on HEK2t93 cells expressing NR1/NR2 heteromers of the NMDAR
The degree of inflammatory infiltrates was graded as follows: −, less than 1% of positive cells in microscopic field; +, 1–25%; ++, 26–50%; +++, 51–75%; and ++++, 76–100%. The degree of deposits of IgG and complement outside perivascular regions was performed by comparing the immunoreactivity in patients’ and control tissues and graded: −, negative; +, mild; ++, moderate; +++, intense; ++++, very intense.
Analysis of IgG subclass of anti-NMDAR antibodies
The distribution of anti-NMDAR immunoglobulin types (IgG, IgM) and IgG subclasses was examined in the CSF of 13 patients (11 patients whose nervous system and/or tumor samples were examined and additional two patients also with ovarian teratoma) using HEK293 cells ectopically expressing NR1/NR2B heteromers of the NMDAR, as reported [6]. Coverslips with these cells were incubated with patients CSF diluted (1:10) in 5% goat serum, overnight at 4°C. After washing with PBS, cells were incubated with mouse fluorescein-labeled monoclonal antibodies to human IgM (Southern Biotech, Birmingham, AL, USA) or to human IgG1, IgG2, IgG3 or IgG4 subclasses (Sigma, St. Louis, MO, USA) all diluted 1:200, for 1 h at room temperature (Table 1). After washing, results were photographed under a fluorescence microscope using Zeiss Axiovision software (Zeiss, Thornwood, NY, USA).
Double labeling studies in patients’ tumors
To avoid reactivity with endogenous IgG, all immunohistochemical studies with tumor tissue utilized IgG purified from sera of two patients with antibodies to NR1/NR2 heteromers of the NMDAR and labeled with biotin [8]. For double labeling experiments, frozen or paraffin embedded tumor sections were simultaneously incubated with biotinylated patients’ IgG (1:30) and rabbit anti-NR1, anti-NR2A (Upstate, Lake Placid, NY, USA; 1:50) or anti-NR2B antibody (Zymed, San Francisco, CA, USA; 1:50) diluted in 10% goat serum in PBS, overnight at 4°C. Sections were then incubated with the appropriate Alexa Fluor secondary antibodies diluted 1:2,000 (Molecular Probes, Eugene, OR, USA) and avidin-FITC diluted 1:500 (Roche, Indianapolis, IN, USA).
Results
The general neuropathological findings of the autopsy of one of the patients (#1) have previously been reported [6]. In brief, the brain showed predominant atrophy of the temporal lobes and hippocampi. Microscopic studies revealed a remarkable loss of pyramidal neurons in hippocampus, predominantly in Sommer’s sector, with extensive gliosis and microglial proliferation. In other brain regions, the pathological findings were mild and included a few areas of neuronal degeneration and gliosis in the neocortex, and rare loss of Purkinje cells of the cerebellum. The brain of patient #2 did not have significant atrophy. Microscopic studies revealed minimal inflammatory infiltrates in the leptomeninges, severe reactive gliosis in the superior temporal gyrus and hippocampus (most prominent in CA4) and mild neuronal loss and gliosis in basal ganglia. Examination of cerebellum was unrevealing. The spinal cord showed microglial nodules mainly affecting motor neurons of the ventral horns and endoneural edema and Wallerian degeneration in some of the associated nerve roots.
CNS findings: extensive microgliosis, moderate inflammatory infiltrates, and deposits of IgG
All areas of the CNS of patients with anti-NMDAR encephalitis showed increased reactive microglia defined by the morphology of the cells and the immunoreactivity with the anti-CD68 antibody (Fig. 1a). The extent of microgliosis was highest in the hippocampus, basal forebrain, basal ganglia and the spinal cord (Table 2). In contrast, the presence of lymphocytic infiltrates (demonstrated by antibodies to CD3, CD4, CD8, CD20, and CD79a) was uncommon, and many of the sections examined did not contain lymphocytes (Fig. 1b–d). Rare T-cell infiltrates were noted in the perivascular and leptomeningeal regions or were scarcely distributed in the brain parenchyma (Fig. 1b). B cells (CD20) and plasma cells (CD79a) were predominantly identified in the perivascular space (Fig. 1c, d). Analysis of T-cell infiltrates with several markers of cytotoxicity, including granzyme B, perforin, and TIA-1 (T-cell intracytoplasmic antigen-1), showed very few reactive cells (~1%) and absence of Fas or Fas ligand-positive cells; in contrast, human lymph node and tumors used as control tissue for the reactivity of these markers showed positive staining with all of them (Supplementary Figure 1).
Fig. 1.
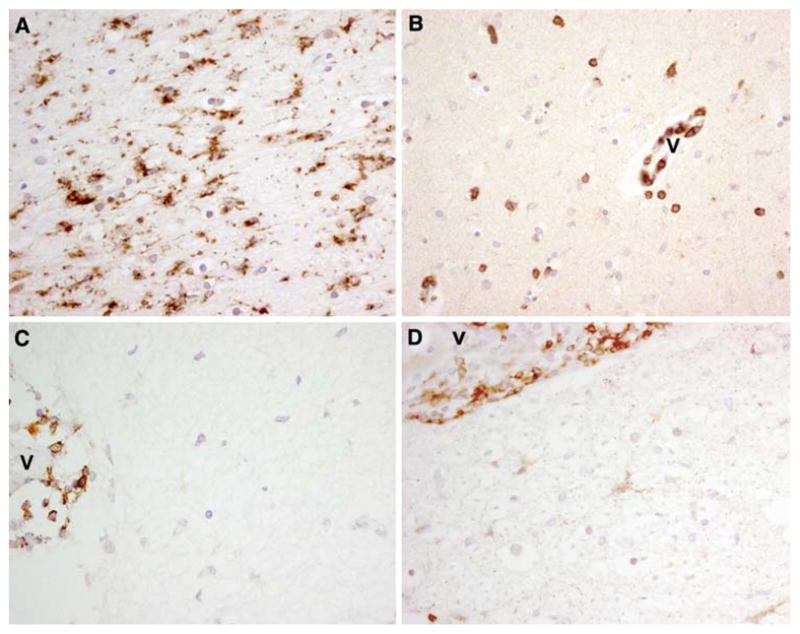
Microgliosis and rare infiltrates of T-cells in the hippocampus and spinal cord of patient #2. a–c Paraffin embedded sections of hippocampus immunolabeled with the microglial marker CD68 (a), the T-cell marker CD3 (b) and the B cell marker CD20 (c). d Paraffin embedded spinal cord section immunolabeled with the plasma cell marker CD79a. Note the perivascular location of CD3+, CD20+ and CD79a+ cells (v blood vessel). a–d ×400, avidin–biotin–peroxidase technique with mild hematoxylin counterstaining
Table 2.
Distribution of inflammatory infiltrates, IgG, and C9 complement in the CNS of two patients with anti-NMDAR encephalitis
| CD3 | CD4 | CD8 | CD20 | CD79a | CD68 | IgG | C9 | |
|---|---|---|---|---|---|---|---|---|
| Case #1 | ||||||||
| Hippocampus | + | + | + | + | ++ | ++++ | ++++ | − |
| Basal forebrain | + | − | + | − | − | ++++ | ++++ | − |
| Temporal cortex | − | − | − | − | − | +++ | ++ | − |
| Frontal cortex | − | − | − | − | − | ++ | + | − |
| Parietal cortex | − | − | − | − | − | ++ | + | − |
| Occipital cortex | − | − | − | − | − | +++ | + | − |
| Cerebellum | − | − | − | − | − | + | + | − |
| Case #2 | ||||||||
| Hippocampus | ++ | + | + | + | ++ | ++++ | ++++ | − |
| Amygdala | − | − | − | − | − | +++ | +++ | − |
| Basal forebrain | + | − | + | − | + | ++++ | ++++ | − |
| Temporal cortex | − | − | − | − | − | +++ | +++ | − |
| Frontal cortex | − | − | − | − | − | +++ | ++ | − |
| Occipital cortex | − | − | − | − | − | + | + | − |
| Basal ganglia | + | − | + | − | + | ++++ | ++++ | − |
| Cerebellum | − | − | − | − | − | + | + | − |
| Cervical spinal cord | ++ | + | + | + | ++ | ++++ | ++++ | − |
Cellular infiltrates: −, positive cells less than 1% of microscopic field; +, 1–25%; ++, 26–50%; +++, 51–75%; ++++, 76–100% IgG/C9 deposits: −, absent; +, mild; ++, moderate; +++, intense; ++++, very intense
All areas of the CNS examined showed deposits of IgG. These were most intense in the hippocampus, basal forebrain, basal ganglia, and cervical spinal cord (Table 2). The hippocampal deposits of IgG in one of the patients (#1) have been previously reported [6]. The other patient reported here showed similar IgG deposits mainly concentrated around the dentate gyri (data not shown), resembling the reactivity of patients’ anti-NMDAR antibodies with the neuropil of rat hippocampus [6]. No deposits of complement were identified in patients’ brain (Table 2), but positive complement reactivity was demonstrated in the control tissue substrate (human lymph node).
Anti-NMDAR antibodies are predominantly IgG1
Using HEK293 cells expressing NR1/NR2B as a substrate to determine antibody reactivity, the NMDAR antibodies of all 13 patients studied were IgG but not IgM. The subtypes of anti-NMDAR IgG were IgG1 in nine patients; IgG1 and IgG2 in one patient; IgG1 and IgG3 in one patient; IgG1, IgG2, and IgG3 in one patient; and IgG3 in one patient (data not shown). Overall, 12 of 13 patients had IgG1 NMDAR antibodies alone or associated with other IgG subclasses, and only one had pure IgG3 NMDAR antibodies. None of the patients had IgG4 antibodies.
Inflammatory infiltrates in the tumor
In all 11 tumor samples from patients with anti-NMDAR encephalitis, the presence of nervous tissue was demonstrated by the morphology of the cells and neuronal processes using microtubule associated protein 2 (MAP-2) antibody, a specific marker of neuronal dendritic processes (Fig. 2a). All samples exhibited NR1/NR2-expressing tumor cells in varying amounts. Patients’ serum IgG reactivity substantially co-localized with the reactivity of commercially available NR1, NR2A, and NR2B antibodies (Fig. 2b–d). Two of three ovarian teratomas from neurologically normal patients without anti-NMDAR antibodies also contained nervous tissue (demonstrated with MAP-2) that reacted with IgG from patients with NMDAR antibodies and co-localized with commercial NR1 and NR2 antibody reactivities (Fig. 2e–h).
Fig. 2.
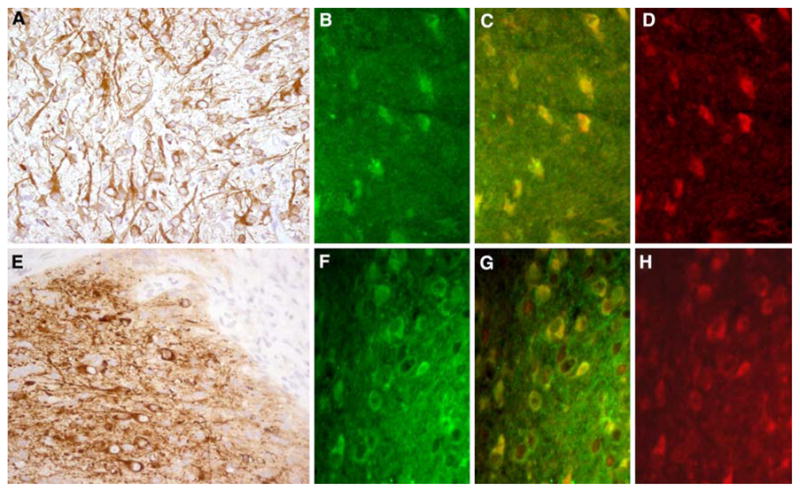
Nervous tissue in tumor sections of anti-NMDAR encephalitis and neurologically normal patients. a Ovarian teratoma of a patient with anti-NMDAR encephalitis immunolabeled with MAP-2 (brown staining), a marker specific for neurons and dendritic processes. b–d The same tumor immunolabeled with patient’s antibodies (b, green) and a specific antibody for NR2B (d, red); note that there is co-localization of reactivities (c, yellow), indicating that the patient’s antibodies react with NR2B-containing heretomers expressed in the tumor (similar findings were observed with NR1 and NR2A antibodies, not shown). e Ovarian teratoma of a patient without anti-NMDAR encephalitis immunolabeled with MAP-2 (brown staining). f–h The same tumor immunolabeled with antibodies from a patient with anti-NMDAR encephalitis (f, green) and anti-NR2B (h, red). g The co-localization of reactivities (yellow); similar findings were observed with NR1 and NR2A antibodies (not shown). a and e ×200, avidin–biotin–peroxidase technique with mild hematoxylin counterstaining, b–d and f–h ×400, immunofluorescence. All studies were performed with frozen sections
The presence of inflammatory infiltrates was examined in five ovarian teratomas from patients with encephalitis and NMDAR antibodies and from three patients without this disorder. All tumors from patients with NMDAR antibodies had extensive infiltrates of T lymphocytes and macrophages/monocytes, and less-abundant B lymphocytes and plasma cells (Fig. 3a, b; Table 3). The three tumors from patients without NMDAR associated encephalitis showed similar infiltrates of T cells and macrophages/monocytes but few or absence of B lymphocytes and plasma cells (Fig. 3c, d; Table 3). All examined cell sub-populations were detected both in the perivascular and interstitial regions. Inflammatory infiltrates were not confined to the regions with nervous tissue, and one control tumor sample with no neuronal cells (#6 in Table 3) also had inflammatory cells.
Fig. 3.
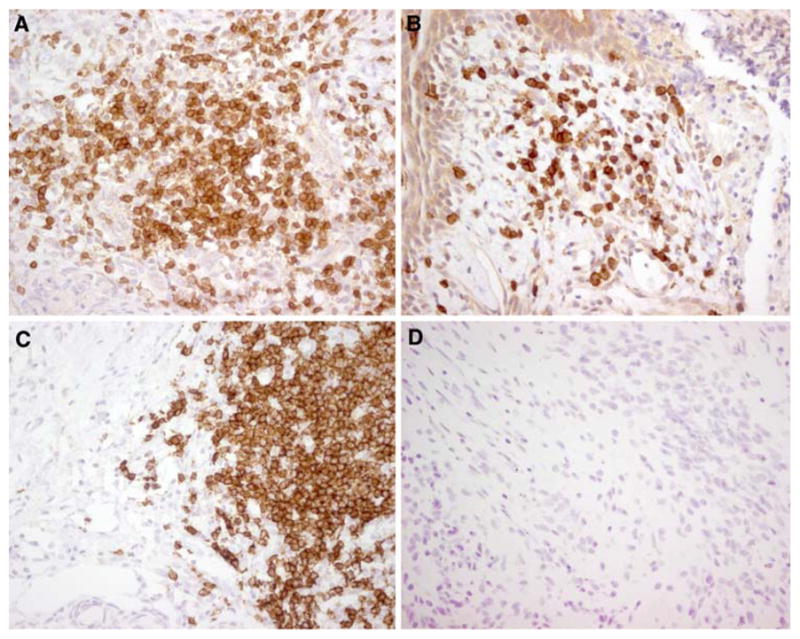
Inflammatory infiltrates in ovarian teratomas. a, b Ovarian teratoma of a patient with anti-NMDAR encephalitis immunolabeled for the presence of CD3+ cells (a) and CD20+ cells (b). c, d Ovarian teratoma of a patient without anti-NMDAR encephalitis immunolabeled for the presence of CD3+ cells (c) and CD20+ cells (d). While CD3+ cells (T cells) are present in both tumors, only the tumor of the patient with anti-NMDAR encephalitis shows infiltrates of CD20+ cells (B cells). a–d ×400, avidin–biotin–peroxidase technique with mild hematoxylin counterstaining. All studies were performed with frozen sections
Table 3.
Inflammatory infiltrates in ovarian teratomas
| CD3 | CD20 | CD79a | CD68 | |
|---|---|---|---|---|
| Patients’ tumor samples | ||||
| #1 | ++ | + | + | ++ |
| #2 | ++ | + | ++ | ++ |
| #3 | ++ | + | + | +++ |
| #4 | ++ | ++ | ++ | ++ |
| #5 | ++ | ++ | + | +++ |
| Control tumor samples | ||||
| #6 | ++ | + | − | +++ |
| #7 | ++ | − | − | ++ |
| #8 | + | − | − | + |
Cellular infiltrates: −, positive cells less than 1% of microscopic field; +, 1–25%; ++, 26–50%; +++, 51–75%; ++++, 76–100%
Discussion
Encephalitis associated with NMDAR antibodies is a recently described disorder that usually affects young adults and children, and can occur with or without tumor association. The disorder differs from other autoimmune paraneoplastic encephalitides in several ways; it results in a highly characteristic syndrome, associates with tumors that are usually teratomas of the ovary, and is treatment-responsive [4, 6]. Moreover, the current study indicates that the immunopathological findings are also different from most paraneoplastic encephalitides. While previous studies demonstrated that paraneoplastic anti-Hu or Ma2 encephalitis associate with extensive infiltrates of T cells expressing markers of cytotoxicity [1, 2, 5]; the current study shows that the main immunopathological features in patients who die of anti-NMDAR encephalitis are extensive microglial infiltrates and deposits of IgG, predominantly involving hippocampus, basal forebrain, basal ganglia and cervical spinal cord. Cells expressing cytotoxic T cell markers (TIA-1, granzyme B, perforin, Fas/Fas-ligand) were infrequent or absent in all areas of the CNS examined.
These immunopathological findings coupled with data from clinical studies, showing CSF pleocytosis in approximately 95% of the patients, frequent presence of CSF oligoclonal bands, and intrathecal synthesis of NMDAR antibodies [4, 6, 7], suggest an important role of the humoral immune response in the pathogenesis of this disorder. Support for this hypothesis comes from studies demonstrating that the epitopes targeted by patients’ antibodies are in the extracellular domain of NR1 (and therefore accessible to circulating antibodies) and that application of patients’ antibodies (or purified IgG without complement) into cultures of rat hippocampal neurons produce a substantial decrease of the levels of NMDA receptors [4]. The current study shows no detectable complement in patients’ brain regions with deposits of IgG, suggesting that the potential pathogenic effects of antibodies can occur without complement-mediated mechanisms. Our study, however, does not rule out the possibility that minimal amounts of complement, missed by immunohistochemical studies, could enhance the antibody effects.
Ovarian teratomas not only express neuronal antigens but also contain mature or immature neurons that express NMDAR and react with patients’ antibodies. Furthermore, inflammatory infiltrates, including T cells, macrophages, B cells, and plasma cells, were identified in patients’ tumors samples, although B cells and plasma cells were absent or minimally present in teratomas from patients without NMDAR antibodies. These findings suggest that the tumor plays a role in triggering the anti-NMDAR immune response. However, not all patients with anti-NMDAR antibodies have teratomas. Two recent series comprising 181 patients showed that the detection of a tumor was age-and gender-dependent [4, 7]. While 55% of women older than 18 years had an ovarian teratoma only 9% of girls younger than 14 years had a teratoma. Male patients of any age rarely had a detectable tumor [4, 7]. Although one can argue that some of these patients may have an occult tumor [9], the large number of cases without a detectable tumor after a long-term follow-up suggests that this disorder often occurs as an autoimmune, non-paraneoplastic syndrome. In this regard anti-NMDA receptor encephalitis is similar to the Lambert–Eaton myasthenic syndrome (LEMS), a disorder of the neuromuscular synapse that can occur with or without cancer association. In non-paraneoplastic LEMS a tendency to autoimmunity has been reported [12]. Interestingly, a recent study showed that some patients with non-teratoma-associated anti-NMDA receptor encephalitis have evidence of additional autoimmunity (ANA, thyroid-peroxidase antibodies) despite the young age of the patients [7]. Therefore, while the presence of an NMDAR expressing tumor probably contributes to breaking immune tolerance, other unknown immunological triggers are likely involved. In addition to a potentially genetic background favoring autoimmunity, the adjuvant effect of a prodromal viral-like illness that typically occurs in most patients with anti-NMDAR encephalitis [4, 6, 10] is likely another factor.
Further studies, including frozen brain and tumor tissue from patients and controls (not available in the current study), will help to determine the NMDAR specificity of the deposits of IgG in the brain, and whether antibodies are also specifically targeting the NMDAR expressed by the teratomas.
Supplementary Material
Acknowledgments
This work was supported in part by RO1CA89054, RO1CA107192 (JD).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00401-009-0582-4) contains supplementary material, which is available to authorized users.
References
- 1.Bernal F, Graus F, Pifarre A, Saiz A, Benyahia B, Ribalta T. Immunohistochemical analysis of anti-Hu-associated paraneoplastic encephalomyelitis. Acta Neuropathol. 2002;103:509–515. doi: 10.1007/s00401-001-0498-0. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal DT, Salzman KL, Digre KB, Jensen RL, Dunson WA, Dalmau J. Early pathologic findings and long-term improvement in anti-Ma2-associated encephalitis. Neurology. 2006;67:146–149. doi: 10.1212/01.wnl.0000223647.83708.20. [DOI] [PubMed] [Google Scholar]
- 3.Cattoretti G, Pileri S, Parravicini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 4.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalmau J, Gultekin SH, Voltz R, et al. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain. 1999;122:27–39. doi: 10.1093/brain/122.1.27. [DOI] [PubMed] [Google Scholar]
- 6.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florance NR, Davis RL, Lam C, et al. Anti-NMDA receptor encephalitis in children and adolescents. Ann Neurol. 2009 doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furneaux HM, Rosenblum MK, Dalmau J, et al. Selective expression of Purkinje-cell antigens in tumor tissue from patients with paraneoplastic cerebellar degeneration. N Engl J Med. 1990;322:1844–1851. doi: 10.1056/NEJM199006283222604. [DOI] [PubMed] [Google Scholar]
- 9.Iizuka T, Sakai F, Ide T, et al. Anti-NMDAR encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504–511. doi: 10.1212/01.wnl.0000278388.90370.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sansing LH, Tuzun E, Ko MW, Baccon J, Lynch DR, Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein-Wexler R, Wootton-Gorges SL, Greco CM, Brunberg JA. Paraneoplastic limbic encephalitis in a teenage girl with an immature ovarian teratoma. Pediatr Radiol. 2005;35:694–697. doi: 10.1007/s00247-005-1402-1. [DOI] [PubMed] [Google Scholar]
- 12.Wirtz PW, Willcox N, van der Slik AR, et al. HLA and smoking in prediction and prognosis of small cell lung cancer in autoimmune Lambert-Eaton myasthenic syndrome. J Neuroimmunol. 2005;159:230–237. doi: 10.1016/j.jneuroim.2004.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


