Abstract
Traditional approaches to the study of hormones and cognition have been primarily observational or correlational in nature. Because this work does not permit causal relationships to be identified, very little is known about the specific molecules and cellular events through which hormones affect cognitive function. In this review, we propose a new approach to study hormones and memory, where the systematic blocking of cellular events can reveal which such events are necessary for hormones to influence memory consolidation. The discussion will focus on the modulation of the hippocampus and hippocampal memory by estrogens, given the extensive literature on this subject, and will illustrate how the application of this approach is beginning to reveal important new information about the molecular mechanisms through which estrogens modulate memory consolidation. The clinical relevance of this work will also be discussed.
Keywords: Estradiol, hippocampus, memory, object recognition, cell signaling, gene expression, ERK, estrogen receptors, selective estrogen receptor modulator, ER knockout, progesterone
1. Introduction
Considerable attention has been paid recently to understanding how ovarian hormones influence cognition and the function of brain regions critical for cognitive function. Such information is of great clinical relevance, given that significantly more women than men develop psychiatric disorders, such as depression and anxiety [1], and neurodegenerative conditions, such as dementia and Alzheimer’s disease [2, 3]. In particular, the relationship between the onset of age-related cognitive decline and the massive loss of estrogens and progestagens that accompanies menopause [4, 5] has been of much recent interest, given the publication of data from the Women’s Health Initiative (WHI) indicating that treatment with conjugated equine estrogens, either alone or in combination with a synthetic progestin, increased the risk of global cognitive decline and dementia in post-menopausal women [6–8]. Although not designed primarily to examine effects of hormone therapy on cognitive function, the WHI studies have raised many important questions regarding hormones and cognition, not the least of which is whether ovarian hormones should be used to prevent age-related cognitive decline.
The side effects of current estrogen treatments reported by the WHI (e.g., breast and uterine cancer, heart disease, and stroke [9–11]) preclude their widespread use to treat age-related cognitive decline or psychiatric disorders. Nevertheless, the development of drugs that mimic the effects of estrogens and natural progestagens in the brain may prove critical to maintaining cognitive health, given that ovarian hormones in adult females are trophic factors for cognitive regions of the brain such as the hippocampus [12], and that the loss of these hormones at menopause may render hippocampal neurons vulnerable to deterioration or exacerbate emerging age-related cognitive deficits. Therefore, the development of novel treatments that provide the beneficial effects of hormones on cognition, but do not produce their peripheral side effects, could improve the quality of life for millions of women. Identifying potential targets for drug development will require the discovery of the underlying molecular mechanisms in the brain through which estrogens and progestagens modulate cognition. Because many of these targets would lie downstream from estrogen and progesterone receptors, novel drugs could be developed to modulate the downstream effectors of hormone receptors rather than the receptors themselves, thus, leading to new drug treatments that produce the cognitive benefits of hormones without the adverse side effects of current therapies.
To illustrate how this molecular approach may be applied to hormones and cognition, this review will focus on the effects of the potent estrogen, 17β-estradiol (E2), on the hippocampus and hippocampal-dependent memory. The hippocampus, a bilateral medial temporal lobe structure that mediates the formation of memories involving spatial, relational, contextual, and object information [13–15], is exceedingly sensitive to levels of E2. This fact was first demonstrated in the early 1990’s by seminal reports showing rapid effects of E2 on CA1 dendritic spine density in the hippocampus of young female rats [16–19]. Further, the hippocampus is extremely vulnerable to the detrimental effects of aging and Alzheimer’s disease [20, 21], which has made it of particular interest to the study of ovarian hormone loss and age-related cognitive decline. In the nearly two decades since the publication of the initial spine density data, thousands of studies have examined the effects of E2 on the hippocampus and on hippocampal-dependent memory. Nevertheless, because most previous research was correlational in nature, or examined either memory or hippocampal function independently, surprisingly little is known about the molecular and biochemical events in the hippocampus necessary for E2 to influence memory formation. However, recent studies in rodents have begun to shed light on these events, and this research has provided interesting new avenues for the development of future hormone-based therapies. The goal of this review is to discuss this work and introduce a conceptual model that provides the framework for future research in this area.
2. Relating E2-induced changes in memory and the hippocampus
2.1. The Traditional Approach
The traditional approach used in the field of hormones and cognition has been largely observational and correlational. That is, subjects (e.g., humans, non-human primates, or rodents) are treated with hormones such as estradiol or progesterone (most often, systemically) and then some aspects of hippocampal function or hippocampal memory are tested, with the resulting outcome that the treatments affect these variables or not. This approach has been invaluable to the field, as it has systemically documented the aspects of hippocampal function that are sensitive to hormones and the types of memory that can be modulated by hormones. Further, correlative studies that have measured both hippocampal function and memory have revealed important insights into the potential neural mechanisms underlying hormone effects on memory. However, after nearly 20 years of such work, we would argue that it is time to take this research to the next level, past observations and correlations, to more direct investigations that pinpoint the specific neurobiological alterations that are necessary for hormones to influence cognition.
Abundant evidence demonstrates that ovarian hormones such as E2 rapidly and profoundly affect hippocampal morphology and physiology in both young and aging female rodents. For example, exogenous E2 in young female rats and mice prevents the ovariectomy-induced decrease hippocampal CA1 dendritic spine density [18, 22], enhances hippocampal synaptic excitability and LTP [23, 24], increases release of growth factors and expression of synaptic proteins (e.g., synaptophysin and PSD-95) [25–27], and rapidly activates numerous cell signaling cascades including the extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways [28–31]. Similarly, numerous studies have shown that E2 can enhance the acquisition and consolidation of various types of hippocampal memory (e.g., spatial, contextual, object recognition) in young females (e.g., [32–52], see [53] for review), and in middle-aged and aged females (e.g., [26, 48, 54–65], see [66] for review).
However, although some of these studies have reported that improvements in hippocampal memory are associated with increases in some aspect of hippocampal function, such data cannot definitively link these changes in a causative manner. As one example, we have previously shown in aged female mice that chronic estradiol benzoate treatment significantly improves spatial memory in the Morris water maze and increases hippocampal levels of the pre-synaptic protein synaptophysin [26]. Given that synaptophysin levels have been correlated with memory in humans and rodents [67–70], these data could suggest that an estradiol-induced increase in synaptophysin levels is responsible, at least in part, for the observed memory improvement. This conclusion is further supported by the fact that a dose of estradiol that did not improve memory also did not increase synaptophysin levels [26]. However, despite this clear association between increased synaptophysin levels and improved memory, no causal relationship can be drawn to show that increased hippocampal synaptophysin is necessary for estradiol to improve spatial memory. In fact, although somewhat unlikely, the estradiol-induced increases in both variables could be completely unrelated. As such, the failure to demonstrate that the specific hippocampal alteration(s) must occur in order for memory to be improved prevents definitive conclusions about the neurobiological mechanisms underlying hormone-induced behavioral change.
2.2. The Blocking Approach
To this end, we propose borrowing an approach from the extensive literature examining the neurobiology of learning and memory, where techniques such as post-training intracranial infusions of inhibitor drugs, gene knockouts, and gene silencing have allowed investigators to identify molecules at the receptor, cell signaling, epigenetic, genetic, and protein synthesis levels that are critical to the formation of long-term memories. This so-called “Blocking” approach [71] allows investigators to determine if a given event (e.g., receptor activation, protein phosphorylation, gene expression, protein synthesis) is necessary for the expression of a behavioral phenomena through the use of techniques such as those described above that prevent the occurrence of the event. If the behavior of interest is disrupted by the blocking manipulation, then this result suggests that the targeted event was necessary for the behavior to occur.
This approach has the advantage of allowing investigators to test clearly defined hypotheses about the roles of specific molecules or cellular processes in hormonal modulation of cognition. As such, applying the blocking approach to the study of hormones and cognition can provide a powerful method of determining which molecules in the hippocampus and other brain regions are necessary for hormones to modulate cognitive processes like learning and memory. Further, this approach could be used more broadly, beyond the memory, towards the development of treatments for reducing mental illness (e.g., depression and anxiety disorders) in women, and potentially reducing the detrimental psychological effects of genetic disorders that predominantly affect females (e.g., Rett’s syndrome).
2.3. The Downstream Molecule Model
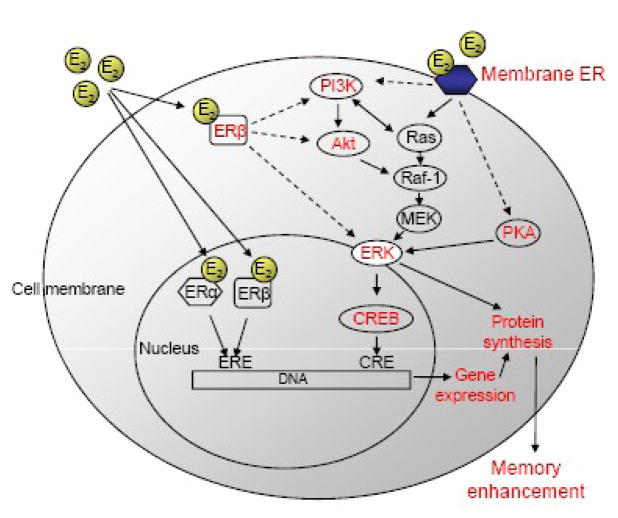
To frame the following discussion of the application of the blocking approach to estrogenic modulation of memory, we suggest a conceptual model for future studies of hormones and cognition. This “Downstream Molecule” model is built upon the framework provided by data from the blocking approach as applied to the neurobiology of learning and memory. Essentially, the model stipulates that the effects of hormones on cognition are mediated by alterations in cell signaling, epigenetic mechanisms, gene expression, and new protein synthesis (Fig. 1). For any hormone, the blocking approach can be used to block each process to determine which molecules at each step of the model are critical for effects on cognition. For E2 in the hippocampus, alterations in these molecules might lead to enhanced memory. Many effects of E2 on memory and hippocampal function are rapid (e.g., [28–31]), and likely involve either novel membrane-bound hormone receptors or non-traditional effects of nuclear hormone receptors [29, 31]. Studies using the blocking approach have already begun to determine which estrogen receptors and cell signaling pathways are critical to the effects of E2 on memory consolidation. This work will be discussed in detail in the following sections.
Fig. 1.
Schematic diagram of the “Downstream Molecule” model.
3. Practical application of the blocking approach to hormones and cognition
As an example of the application of the blocking approach to cognition, studies from the learning and memory literature have demonstrated that phosphorylation (i.e., activation) of the cell signaling molecule ERK in the hippocampus is necessary for rodents to form long-term hippocampal-dependent memories. ERK is one of a family of mitogen-activated kinases (MAPKs) that is phosphorylated as part of a G-protein initiated signal transduction cascade. Upon phosphorylation, ERK translocates to the cell nucleus where its subsequent phosphorylation of molecules like RSK (ribosomal S6 kinase) can lead to phosphorylation of the transcription factor cAMP response element binding protein (CREB) and gene transcription [72]. MAPK kinase (or MEK) is the exclusive upstream activator of ERK, and its actions can be inhibited using a number of compounds [72]. Thus, the necessity of ERK activation for any process can be tested using MEK inhibitors that prevent ERK activation. Indeed, studies in which the MEK inhibitors U0126 or PD098059 have been infused into the hippocampus or cerebral ventricles prior to training report impaired spatial memory, contextual fear conditioning, and object recognition 24–48 hours later [73–76]. Administering the MEK inhibitor immediately after training (i.e., post-training) also blocks long-term memory formation [77]. This finding is important because post-training inhibitor treatments can better pinpoint effects of ERK activation to the memory consolidation phase of memory processing, given that pre-training treatments will also affect memory acquisition. Consistent with the pharmacological data, mice with a knockdown or conditional knockout of the p42 isoform of ERK have impaired spatial memory and contextual fear conditioning, reduced neocortical thickness, and altered neocortical neurogenesis [78, 79], suggesting that p42 ERK is critical for memory and neural development. Collectively, this literature, which uses several blocking techniques to prevent ERK activation, provides strong evidence that ERK activation is necessary for long-term hippocampal memory formation. As such, similar experimental approaches could be useful for identifying molecules, such as ERK, that are necessary for hormone-induced memory enhancement.
Our initial attempts to use the blocking approach to pinpoint molecules underlying E2-induced memory modulation in female mice utilized three important elements: post-training treatments, rapidly metabolized E2, and a one-trial learning task. Post-training hormone and inhibitor treatments were used because, as previously mentioned, pre-training treatments affect both memory acquisition and consolidation, thus, obscuring molecular events associated with each phase of memory formation. In addition, hormones such as E2 affect performance factors (i.e., motivation, attention, and sensorimotor function) in addition to memory [80–82], thus, obscuring effects of E2 on memory specifically. As such, administering E2 immediately after training allows the effects of E2 be pinpointed specifically to the memory consolidation phase of memory processing, and thus greatly aids in the identification of molecules necessary for memory formation.
Next, we used a form of E2 encapsulated in 2-hydroxypropyl-β-cyclodextrin [29, 62, 83–86] to ensure that E2 was not in the circulation during retention testing. Cyclodextrin-encapsulated hormones are metabolized within hours [87], and because E2 is not in the circulation during training or testing, its specific effects on memory consolidation can be examined in the absence of non-mnemonic performance confounds. This form of E2 has been shown to enhance hippocampal-dependent memory when injected intraperitoneally (i.p.) in doses of 0.1–0.4 mg/kg [83, 86], which are high physiological/low pharmacological doses [88], and directly into the dorsal hippocampus and dorsal third ventricle in doses of 5–10 μg per infusion [29, 89].
Finally, we needed a hippocampal task that mice could learn in one trial and that involved minimal stress, given the well-documented interactions between gonadal and stress hormones (e.g., [90–92]). Because we had found that immediate post-training injection of 0.2 mg/kg E2 (i.p.) could enhance novel object recognition in young ovariectomized mice [83], we thought this task might be useful for these studies. Novel object recognition takes advantage of rodent’s natural affinity for novelty. In our testing protocol, mice first accumulate 30 seconds exploring two identical objects in an open arena [93]. Immediately after this training, mice are injected systemically or infused intracranially with E2. Retention is then tested 48 hours later by presenting mice with one novel and one familiar (identical to training) object. Mice who remember the familiar object spend more time than chance (15 seconds) exploring the novel object. Although there has been some controversy about the extent to which novel object recognition requires the hippocampus versus other brain regions (e.g., perirhinal cortex, frontal cortex) [94–96], hippocampal involvement in our testing protocol has been demonstrated with complete hippocampal lesions and dorsal hippocampal inactivation [97, 98]. Further, numerous studies from our lab have shown in young ovariectomized mice that immediate post-training intraperitoneal (i.p.) injection of 0.2 mg/kg E2 enhances object recognition tested 48 hours later (e.g., [83, 99]). Because the novel object recognition task involves rapid one-trial learning, we thought it might be an especially useful task in our efforts to identify the molecular mechanisms through which E2 modulates memory consolidation.
4. Using the blocking approach to identify receptors and cell signaling molecules necessary for E2 to enhance memory consolidation
4.1. Cell signaling
We turned to ERK as a potentially important molecule for the mnemonic effects if E2, given its importance for long-term hippocampal memory formation. An increasing number of studies have focused on rapid effects of E2 in neurons, and in vitro data supported a link between E2 and rapid effects on ERK activation and hippocampal function. For example, data from hippocampal cell culture studies had shown that E2 increases ERK phosphorylation within 10–20 min of application [100, 101], and that MEK inhibitors completely block not only this effect, but also E2-mediated neuroprotection [100–103] and E2-induced increases in synaptophysin protein levels [30]. In the intact rat, a single infusion of E2 into the lateral ventricle increased ERK phosphorylation throughout the hippocampus within 5 minutes [104]. As such, evidence clearly demonstrated that E2 could activate hippocampal ERK.
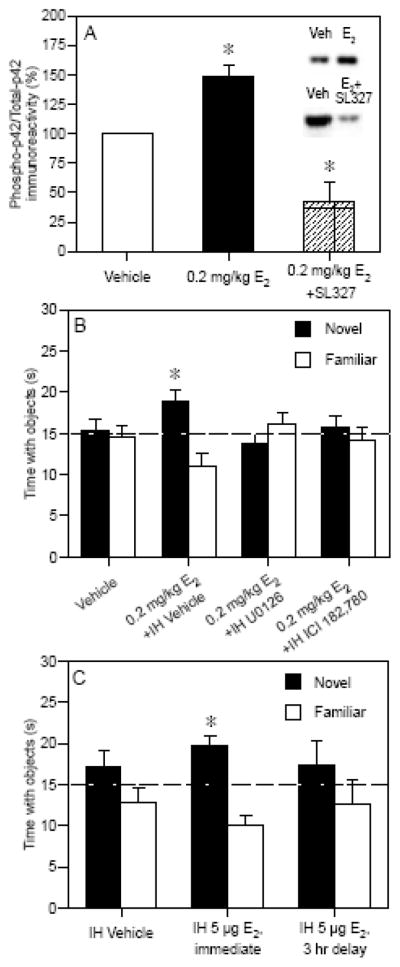
Based on these data, we hypothesized that the beneficial effects of 0.2 mg/kg E2 on novel object recognition were dependent on dorsal hippocampal ERK activation. We first set out to measure whether 0.2 mg/kg E2 increased ERK activation in the dorsal hippocampus of young ovariectomized mice. We found that 0.2 mg/kg E2 (i.p.) increased phosphorylation of the p42 isoform of ERK (Fig. 2A), but not the p44 isoform of ERK (data not shown), 60 minutes after injection [29]. This increase was blocked by concurrent i.p. injection of the MEK inhibitor, SL327 (30 mg/kg; Fig. 2A) [29]. Next, mice were implanted with bilateral infusion cannulae directed at the dorsal hippocampus and were trained in the object recognition task. Immediately after training, mice were injected with 0.2 mg/kg E2 and infused intrahippocampally (IH) with vehicle or the MEK inhibitor U0126 (0.5 μg/side of the hippocampus). U0126 blocked the beneficial effects of 0.2 mg/kg E2 on novel object recognition tested 48 hours after training (Fig. 2B) [29], demonstrating that dorsal hippocampal ERK activation is necessary for E2 to enhance object recognition. In addition, we found that infusion of E2 into the dorsal hippocampus (5 μg/side) immediately, but not 3 hours, after training could also significantly enhance object recognition (Fig. 2C), further localizing the behavioral effects of E2 to the dorsal hippocampus and demonstrating a relatively brief time window in which these effects occur [29]. We then wanted to see if IH infusion of U0126 would block the effects of intracranially infused E2. In order to prevent tissue damage from repeated infusions into the hippocampus, we infused E2 into the dorsal 3rd ventricle (ICV, 5 μg total), as a means of supplying E2 to the hippocampus, concurrently with IH infusion of U0126. We found that ICV-infused E2 increased phospho-p42 ERK levels within 5 minutes of infusion and enhanced 48-hour object recognition, and that these effects were blocked by U0126 (Z. Zhao, personal communication). Collectively, these data demonstrate that dorsal hippocampal ERK activation is necessary for systemically and intracranially administered E2 to enhance object memory consolidation in young ovariectomized female mice. Further, these studies demonstrate the feasibility of the blocking approach to understanding the molecular events underlying E2-induced memory modulation.
Fig. 2.
(A) Phospho-p42 ERK levels in the dorsal hippocampus 1 hr after 0.2 mg/kg E2. E2 significantly increased phospho-p42 ERK levels, and 30 mg/kg SL327 blocked this increase (*p < 0.05 relative to vehicle). Bars represent mean (± SEM) % change from vehicle. Inset: Representative Western blots. (B) Mice receiving 0.2 mg/kg E2 spent significantly more time with the novel object than chance (dashed line at 15 s, *p < 0.05), indicating memory for the familiar object. This effect was blocked by IH infusion of U0126 (0.5 μg/side) or ICI 182,780 (5 μg/side). (C) Mice receiving immediate, but not 3-hr delayed, IH infusions of E2 spent significantly more time than chance (dashed line at 15 s, *p < 0.05) with the novel object, suggesting that the effects of E2 on object recognition occur within 3 hours of infusion. Bars in (B) and (C) represent the mean (± SEM) time spent with each object in seconds. Adapted from [29].
We have also used this approach to examine signaling upstream from ERK, specifically NMDA receptor activation and activation of protein kinase A (PKA). Immediately after object recognition training, young ovariectomized mice were injected with 0.2 mg/kg E2 and infused IH with the NMDA antagonist APV (2.5 μg/side) or the PKA inhibitor Rp-cAMPS (18 μg/side). In addition to object recognition, ERK phosphorylation in the dorsal hippocampus was examined 1 hour after drug treatment. Both APV and Rp-cAMPS blocked the beneficial effects of 0.2 mg/kg E2 on 48-hour object recognition and dorsal hippocampal phospho-p42 ERK levels [84], suggesting that dorsal hippocampal NMDA receptor and PKA activation upstream from ERK activation are also involved in the mnemonic effects of E2. In addition, preliminary work from our lab shows that inhibition of PI3K or Akt also block the beneficial effects of E2 on object recognition (L. Fan, personal communication), demonstrating the utility of this approach in understanding the roles that various signaling molecules play in mediating the effects of E2 on memory consolidation.
4.2. Roles of nuclear estrogen receptors in mediating the effects of E2 on memory consolidation
One of the most critical issues to the development of more selective estrogen treatments is to determine which estrogen receptors mediate the memory enhancing effects of E2. Traditionally, the effects of E2 were thought to be mediated by the nuclear estrogen receptors (ERs) ERα and ERβ. ERα and ERβ are found throughout the hippocampus [105, 106], where they are located in the nucleus, dendritic spines, and axon terminals of pyramidal neurons [107]. In the classic (i.e., “genomic”) mechanism of estrogen action, estrogens pass through the outer cell membrane and bind to ERα and ERβ in the cytoplasm, which then causes the receptors to dimerize and translocate the estrogen-ER complex to the cell nucleus, and the complex to bind estrogen response elements (EREs) on target genes [108]. Because the responses linked to gene transcription were thought to be too slow to account for the rapid activation of cell signaling in the hippocampus, it was unclear if these receptors played a role in mediating rapid effects of E2 on hippocampal functioning. However, their presence in dendrites and axons [107]may suggest a potential mechanism for these effects. Such effects would be considered “non-genomic”, as they would not involve direct binding to an ERE.
Indeed, data from both in vitro and in vivo experiments demonstrate importance of rapid effects on cell signaling in mediating effects of E2 on memory and the hippocampus. In hippocampal cell lines transfected with ERα or ERβ, E2 can increase p42 ERK phosphorylation within 15 minutes [100, 101], suggesting that either receptor may mediate effects of E2 on phospho-p42 ERK activation. These effects are blocked by MEK inhibition [100, 101] and by the ERα/ERβ antagonist ICI 182,780 [100, 101], which blocks E2-induced ER dimerization [109] and translocation into the cell nucleus [110]. In hippocampal cell culture or in vivo, E2 can activate hippocampal ERK and PI3K 5–15 minutes after exposure, effects that are blocked by inhibitors of ERK and PI3K activation [29–31, 104, 111]. Further, E2-induced ERK activation is also necessary for this hormone to increase hippocampal synaptic protein levels [30] and CA1 dendritic spine density [112], linking rapid effects of E2 on hippocampal cell signaling to classic morphological alterations in this structure. The fact that E2-induced increases in ERK activation and in both morphological alterations can be blocked by ICI 182,780 suggests that one or both nuclear ERs are critically involved in these effects. Consistent with these findings, we also found that the effects of 0.2 mg/kg E2 on dorsal hippocampal phospho-p42 ERK levels and object recognition (Fig. 2B) were blocked by immediate post-training IH infusion of ICI 182,780 [29]. However, the rapidity of E2’s effects on cell signaling and memory suggests that these effects result from “non-genomic” effects of nuclear ERs that do not directly involve EREs. Recent findings suggest that ERβ can translocate to the plasma membrane upon exposure to E2 [113], which would provide a means for this receptor to interact with proteins that activate cell signaling pathways.
If nuclear ERs do play a role in mediating rapid effects of E2 on hippocampal function, then is one more important than the other? In vivo, two approaches may be especially useful in examining the relative contributions of nuclear ERs in mediating memory. The first approach employs ER knockout (ERKO) mice that lack functional copies of the gene for either nuclear ERα or ERβ. Previous studies using ERKO females treated with E2suggest that E2may enhance memory primarily through activation of ERβ. For example, ERαKO females receiving E2 exhibit improved spatial memory and inhibitory avoidance relative to untreated ERαKO mice [114, 115]. In contrast, ERβKO mice females receiving E2 show no improvement in object recognition or object placement tasks [116]. ERα activation may impair hippocampal memory, as administration of E2 in ERβKO females either impairs or fails to affect spatial and object memory relative to wild-type mice receiving E2[117–119].
The second approach to examine the role of nuclear ERs in memory employs pharmacological manipulation of ERα and ERβ activation. Two selective estrogen receptor modulator (SERM) drugs often used for this purpose are propylpyrazole-triol (PPT), which has a 410-fold greater affinity for ERα over ERβ[120], and diarylpropionitrile (DPN), which has a 70-fold greater affinity for ERβ over ERα[121]. PPT and DPN protect cultured hippocampal neurons from excitotoxicity, an effect that is blocked by MEK inhibition, but not by ICI 182,780 [31]. Both drugs have also been shown in culture to increase hippocampal phospho-p42 ERK levels within 10 minutes of application [31] and to increase CA1 dendritic spines within 2 hours of application [112]. Despite the fact that both PPT and DPN appear to promote hippocampal function in vitro, spatial memory in the Morris water maze is only improved by DPN among young ovariectomized rats tested after systemic post-training subcutaneous (s.c.) injections of 10 μg PPT or DPN [122]. However, 0.9 mg/kg of both drugs enhanced novel object recognition [123] and object placement [124] in other studies of young ovariectomized rats, suggesting that effects of PPT and DPN on memory may be task- or dose-dependent.
Because both the knockout and SERM approaches have critical shortcomings (e.g., potential compensatory mechanisms for life-long gene knockouts and a lack of absolute receptor specificity of the SERMs), the combined use of both approaches using the same behavioral paradigm may provide important converging evidence about the roles of nuclear ERs in memory consolidation. Therefore, we recently used the same novel object recognition task to test the involvement of nuclear ERs in memory using both knockout mice and SERMs. First, vehicle or 0.2 mg/kg E2 were given immediately post-training to young ovariectomized mice lacking either ERα (ERαKO, [125]) or ERβ (ERβKO, [126]). E2 enhanced 48-hour object recognition in ERαKO, but not ERβKO mice (Fig. 3A). Next, wild-type C57BL/6 mice were injected immediately post-training with 0.2 mg/kg E2 (i.p.) or with 0.05 mg/kg (s.c.) of PPT or DPN. E2 and DPN, but not PPT, enhanced object recognition 48 hours later (Fig. 3B). Together, these data suggest a role for ERβ, but not ERα, in mediating effects of E2 on object memory, which is consistent with previous studies on this subject.
Fig. 3.
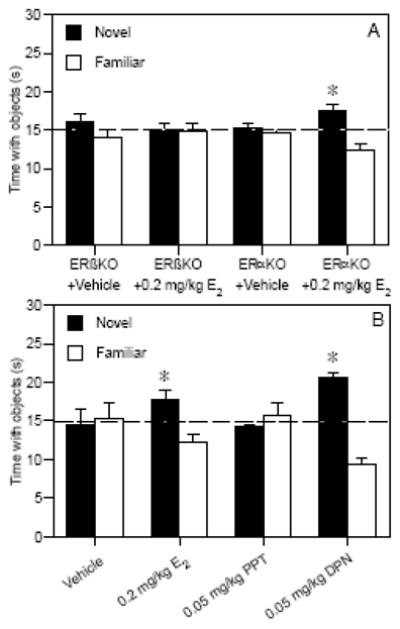
(A) 0.2 mg/kg E2 (Sigma, St. Louis, MO) significantly increased the time spent with the novel object relative to chance (dashed line at 15 s, *p < 0.05) in ovariectomized ERαKO mice (t(9) = 3.20, p < 0.05), but not in ERβKO mice (n=10/genotype), suggesting that only E2-treated mice with ERβ could remember the familiar object. (B) In ovariectomized wild-type C57BL/6 mice, 0.2 mg/kg E2 (t(8) = 2.43, p < 0.05) and 0.05 mg/kg DPN (t(9) = 7.06, p < 0.05), but not 0.05 mg/kg PPT, significantly increased the time spent with the novel object relative to chance (dashed line at 15 s, *p < 0.05). ER knockout and wild-type mice were obtained from Taconic Farms (Germantown, NY) and PPT and DPN were obtained from Tocris (Ellisville, MO). Bars in (A) and (B) represent the mean (± SEM) time spent with each object in seconds. Together, these data suggest a role for ERβ, but not ERα, in mediating beneficial effects of E2 on object recognition.
However, many questions remain, and the blocking approach could be especially useful in revealing the answers. For example, direct intracranial infusions of PPT, DPN, and cell signaling inhibitors would shed light on whether the mnemonic effects of these drugs are dependent on hippocampal cell signaling. Further, conditional knockouts restricting ERα and ERβ deletion to the dorsal hippocampus in adulthood would greatly facilitate our understanding the importance of hippocampal ERα and ERβ to E2-induced memory modulation.
4.3. Roles of non-nuclear estrogen receptors in mediating the effects of E2 on memory consolidation
Although ICI 182,780 blocks many of the effects of E2 and the SERMs on hippocampal neurons, it does not block all of their effects, including those on neuroprotection, synaptogenesis, or growth factor release [25, 31]. As a result, it has been postulated that rapid non-genomic effects of E2 on hippocampal neurons may also be mediated by membrane-bound ERs in the outer cell membrane, the precise identities of which are currently unknown [127, 128]. Although these receptors are not yet cloned, their function has been examined using a membrane-impermeable form of E2 called BSA-E2, in which E2 is covalently linked to bovine serum albumin. Unlike E2, BSA-E2 does not activate ERE-mediated gene transcription [129]. Infusion of BSA-E2 into the dorsal hippocampus or cerebral ventricles promotes ERK phosphorylation in vivo [29, 104] and enhances hippocampal-dependent memory [29, 50]. We recently showed for the first time that dorsal hippocampal ERK activation is necessary for BSA-E2 to enhance novel object recognition in young ovariectomized mice. In this experiment, immediate post-training infusions of BSA-E2 into the dorsal hippocampus (intrahippocampal, IH) or dorsal third ventricle (intracerebroventricular, ICV) significantly enhanced 48-hour object recognition (Fig. 4A) and robustly increased dorsal hippocampal phospho-p42 ERK activation 5 minutes after infusion (Fig. 4B) [29]. The importance of ERK activation in mediating the effects of BSA-E2 on novel object recognition was demonstrated by the fact that the effect of ICV BSA-E2 infusion on object recognition was completely blocked by IH infusion of U0126 (Fig. 4A) [29]. Interestingly, unlike with E2, the beneficial effects of ICV BSA-E2 on object recognition (Fig. 4A) or phospho-p42 ERK activation (Fig. 4B) were not blocked by IH infusion of ICI 182,780 [29]. Further, the beneficial effects of ICV BSA-E2 on object recognition were also not blocked by hippocampal inactivation with an IH infusion of the GABAA agonist Muscimol (data not shown) [29]. Together, these data suggest that the effects of BSA-E2 on object recognition depend on hippocampal activity, but not on ERα or ERβ. Although these data demonstrate that the beneficial effects of E2 on object memory consolidation can be mediated solely by putative membrane-bound ERs, the fact that ICI 182,780 blocks the beneficial effects of traditional E2 on object recognition (Fig. 2B) [29] suggests an additional role for a nuclear ER. More work will be needed to understand how each ER contributes to E2-induced memory enhancement.
Fig. 4.
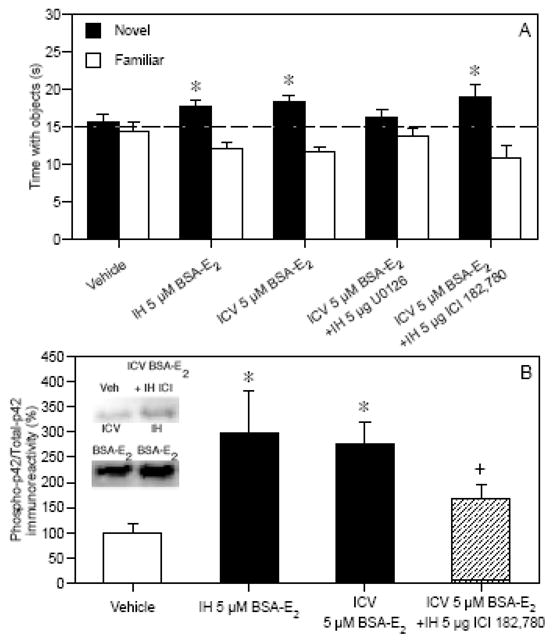
(A) IH and ICV BSA-E2 significantly increased the time spent with the novel object relative to chance (dashed line at 15 s, *p < 0.05), suggesting that BSA-E2 enhanced memory for the familiar object. This effect was blocked by IH infusions of U0126, but not IH ICI 182,780. Bars represent the mean (± SEM) time spent with each object in seconds. (B) Phospho-p42 ERK levels in dorsal hippocampus 5 min after BSA-E2 infusion; ICI 182,780 did not significantly reduce this effect. Bars are mean (± SEM) % change from vehicle (*p < 0.05 relative to vehicle; +p > 0.05 relative to all other groups). Inset: Representative Western blots. Adapted from [29].
4.4. Effects of progesterone on E2-induced alterations in hippocampal cell signaling and memory
Very little is known about the effects of natural progestagens, such as progesterone, or synthetic progestins (such as those commonly used in hormone therapy) on memory and hippocampal function, despite the fact that levels of progesterone also plunge at menopause. The few studies that have examined effects of progesterone on hippocampal function report some similar effects of E2 and progesterone on hippocampal function, although their effects are not identical. For example, progesterone has a biphasic effect on CA1 dendritic spine density, at first increasing spine density within the first 6 hours of treatment and then decreasing density below baseline over the next 18 hours [19]. These effects are likely mediated by the two nuclear progesterone receptors (PRA and PRB), which, like the ERs, are located in dendritic spines, nuclei, and axon terminals throughout the hippocampus [130]. The PR antagonist RU-486 blocks progesterone-induced increases in CA1 dendritic spine density [19], suggesting that one or both of the nuclear PRs mediate this effect. However, progesterone also rapidly activates both the ERK and PI3K/Akt signaling pathways in the hippocampus [111, 131, 132], and studies from mouse neocortex show that RU-486 does not prevent progesterone from phosphorylating ERK and Akt [133]. These data suggest non-genomic mechanisms similar to those of E2, and indeed, multiple membrane-bound PRs have been identified in vertebrates and invertebrates [134, 135]. Further, studies from my lab in which progesterone was administered systemically or into the dorsal hippocampus immediately after training show that progesterone can improve object recognition in young, middle-aged, and aged ovariectomized mice [65, 136, 137].
Although progesterone can facilitate memory and hippocampal functioning when given alone, numerous studies report that progesterone given in combination with E2 reverses the beneficial effects of E2. Indeed, in the WHI, the synthetic progestin medroxyprogesterone acetate appears to increase the risks of global cognitive decline and dementia in post-menopausal women more than conjugated equine estrogens alone [6–8]. In rodents, progesterone blocks E2’s neuroprotective effects in the hippocampus [138] and neurotrophic effects in the entorhinal cortex [139]. Further, acute or chronic progesterone reverses the beneficial effects of E2 on spatial memory tested in middle-aged ovariectomized rats [57] and aged ovariectomized mice [63]. However, the literature on E2 plus progesterone treatment is quite inconsistent, and other studies in ovariectomized aging rats report beneficial effects of chronically administered E2 plus progesterone treatment on spatial memory tested in aging rats [56, 60].
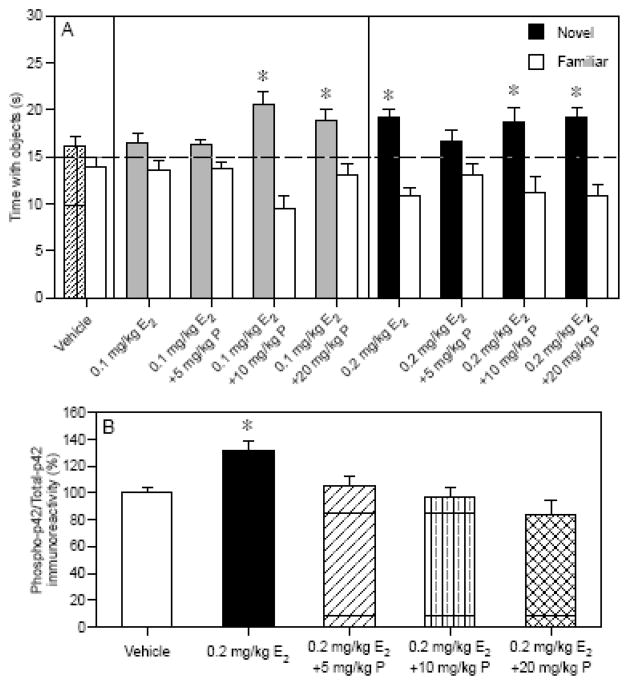
Despite these discrepant results, we thought that the post-training approach employed for E2 alone could be used to examine how progesterone interacts with E2, given that both E2 and progesterone increase ERK phosphorylation in vitro, and that our post-training treatments of each hormone separately improved memory in females throughout the adult lifespan. We examined the effects of combined E2 plus progesterone treatment on object recognition and dorsal hippocampal ERK activation using different dose combinations of behaviorally effective and sub-effective doses of both hormones. Previous studies had determined that 0.2 mg/kg E2 and 10 or 20 mg/kg progesterone enhanced 48-hour object recognition, whereas 0.1 mg/kg E2 and 5 mg/kg progesterone did not [83, 136]. In the combination study [140], mice were injected with 0.1 or 0.2 mg/kg E2 (i.p.) plus 5, 10, or 20 mg/kg progesterone (i.p.) immediately post-training, and then object recognition was tested after 48 hours and dorsal hippocampal phospho-ERK levels were measured after 1 hour. Ten and 20 mg/kg progesterone enhanced 48-hr object recognition regardless of the dose of E2 with which it was paired (Fig. 5A) [140]. Conversely, 5 mg/kg progesterone did not enhance memory, and blocked the beneficial effects of 0.2 mg/kg E2 on object recognition (Fig. 5A) [140]. Although 0.2 mg/kg E2 significantly increased dorsal hippocampal phospho-p42 ERK levels (Fig. 5B) [140], 0.1 mg/kg E2 had no such effect (data not shown). Interestingly, despite the fact that 10 and 20 mg/kg progesterone enhanced object recognition, all doses of progesterone blocked the 0.2 mg/kg E2-induced increase in dorsal hippocampal phospho-p42 ERK levels (Fig. 5B) [140]. These data may suggest that the effects of progesterone or combined E2 plus progesterone treatment influence ERK activation in a different time frame from E2 alone (e.g., an increase in ERK activation may take place earlier than 1 hour after injection) or enhance memory through different mechanisms from E2 alone (e.g., through signaling pathways such as PI3K/Akt).
Fig. 5.
(A) 0.2 mg/kg E2, but not 0.1 mg/kg E2, significantly increased the time spent with the novel object relative to chance (dashed line at 15 s). The 10 and 20 mg/kg doses of progesterone also significantly increased time spent with the novel object relative to chance regardless of the concomitant dose of E2. Bars represent the mean (± SEM) time spent with each object in seconds (*p < 0.05 relative to chance). (B) All doses of progesterone blocked the increase in phospho-p42 levels induced by 0.2 mg/kg E2 (*p < 0.05 relative to vehicle). Bars represent mean (± SEM) % change from vehicle. Adapted from [83, 140].
The fact that so little is known about how E2 and progesterone interact to influence memory and hippocampal function underscores the need for considerably more work on this subject. Understanding how estrogens and progestins affect cognitive function is of tremendous clinical importance, given that co-administration is recommended for treatment of menopausal women with a uterus because of the protection afforded by progesterone against uterine cancer [141]. Future work should aim to determine which hormone receptors and cell signaling pathways in the hippocampus mediate the mnemonic effects of E2 plus progesterone treatment, and whether dose-dependent interactions result from differential receptor and cell signaling activation. Such information would greatly advance our understanding of how E2 and progesterone together influence memory and hippocampal function, and would shed important light on how their interactions during the natural cycle may influence cognitive function.
5. Effects of E2 on gene expression
Although the effects of E2 on cell signaling do not appear to involve ERE-mediated gene transcription, the activation of cell signaling pathways can ultimately lead to gene transcription mediated by factors like CREB, c-Fos, and Elk-1. Certainly, ERK activation can lead to gene transcription [72], and the fact that ERK activation is necessary for E2 to enhance object recognition suggests that ERK-induced gene alterations may play an important role in the mnemonic effects of E2. The blocking approach has not yet been used to determine which genes are necessary for the effects of E2 on hippocampal-dependent memory, but two recent DNA microarray studies have provided several interesting candidates.
In one study [85], we treated young ovariectomized mice with a single injection of vehicle or 0.2 mg/kg E2 and then collected the dorsal hippocampus 60 minutes later. This dose of E2 enhances both spatial memory and object recognition in young ovariectomized mice [83], so gene alterations could not be specifically associated with improvements in one type of memory. Genes were considered altered by E2 if they showed a greater than 2-fold change in RNA expression levels compared to controls; 111 genes were up-regulated and 93 were down-regulated [85]. Of these, 17 up-regulated and 6 down-regulated genes have previously been implicated in learning and memory. mRNA expression changes in five genes were confirmed by quantitative real-time PCR (qRT-PCR) 1 hour after treatment, and resulting protein changes were confirmed with Western blotting 3–4 hours after treatment. The five genes were as follows: Hsp70, a heat shock protein known to be estrogen responsive; Igfbp2, an IGF-I binding protein; Actn4, an actin binding protein involved in protein trafficking; Tubb2a, the major component of microtubules; and Snap25, a synaptosome-specific protein required for neurotransmitter release [85]. mRNA and protein for all genes were significantly up-regulated by E2, with the exception of Igfbp2, which was down-regulated. Hsp70 is an integral part of the cytosolic estrogen receptor protein complex that keeps nuclear ERs in an inactive state until they bind to estrogens and translocate to the nucleus [142]. Hsp70 expression is also increased by E2 in various cell types, including in the brain, where its expression is influenced by sex [143]. Hsp70-1 mRNA and protein levels have also been implicated in spatial learning and memory [144], so the Hsp70 gene may be an interesting candidate for future studies linking gene expression to E2-induced memory modulation. Igfbp2 binds IGF-I in the serum and prevents it from activating the IGF-I receptor and initiating intracellular signaling cascades such as PI3K and ERK [145, 146]. Thus, an E2-induced down-regulation of Igfbp2 may lead to greater IGF-I availability, and subsequently to increased PI3K and ERK activation. The remaining genes, Actn4, Tubb2a, and Snap25, may be important in facilitating hormone effects on protein trafficking within cells [147], shuttling ERs to various locations within cells [148], or neurotransmitter release [149], respectively. Together, these data indicate that E2 has numerous effects on the expression of various genes that potentially underlie E2’s beneficial effects on cognition.
Another recent microarray study examined hippocampal gene expression after chronic estradiol treatment in young and middle-aged ovariectomized mice [150]. In this study, mice were given cyclic injections of oil vehicle or estradiol benzoate (5 μg) for 3 weeks prior to cued and spatial memory testing in the Morris water maze. Whole hippocampi were collected bilaterally after 5 weeks of treatment and analyzed with cDNA microarrays. Expression of a subset of genes was confirmed using oligonucleotide arrays and qRT-PCR. Behaviorally, middle-aged females treated with vehicle exhibited significant spatial learning and memory impairments relative to the other groups tested. Interestingly, more estradiol-induced alterations in gene expression were observed among middle-aged females (244 gene differentially expressed) compared to young females (58 genes differentially expressed) [150], which may be indicative of mechanisms in the middle-aged brain to counteract degenerative changes due to age and/or hormone loss. The oligonucleotide analysis of selected genes of interest found that estradiol reversed age-related increases gene expression in 5 genes (Ldb2, Foxo3a, Hdac2, Lass2, and Kctd3) and age-related decreases in 2 genes (Fbxw8 and cbini) [150]. mRNA expression in two of these genes was confirmed with qRT-PCR (Hdac2 and Lass2). Hdac2, histone deacetylase 2, may be of particular interest for studies of hormones and cognition due to recent evidence that this gene negatively regulates hippocampal-dependent memory and hippocampal synaptic plasticity [151].
Although purely descriptive, these new studies provide the foundation for future work examining the roles that the aforementioned genes may play in mediating the beneficial effects of E2 on object and spatial memory. Here again, the blocking approach can be used to test the importance of each gene in mediating the effects of E2. Numerous techniques could be used to prevent transcription or translation of these genes, including RNA interference (RNAi), oligodeoxynucleotides directed against specific mRNAs, genetic manipulations (e.g., gene knockouts) and epigenetic manipulations (e.g., increasing DNA methylation). In the coming years, the application of these techniques to the study of hormones and cognition will undoubtedly reveal critical insights at the genomic and proteomic levels about how E2-induced alterations in the hippocampus modulate memory processes.
6. Conclusions
In this review, we have proposed the application of a blocking approach to a Downstream Molecule model of hormones and cognition in order to identify molecules in the hippocampus through which E2 modulates memory formation. Thus far, this approach has provided important insights into the cellular events in the dorsal hippocampus that are critical for acute E2 treatments to enhance one type of hippocampal memory, object recognition, in young ovariectomized mice (Fig. 6). To date, our data regarding effects of E2 alone demonstrate that phosphorylation of the p42 isoform of ERK is necessary for post-training E2 treatment to enhance object recognition in young females [29]. This phosphorylation may be mediated by upstream activation of PKA, PI3K, and NMDA receptors [84]. Effects of E2 on dorsal hippocampal ERK activation and object recognition can be mediated entirely by BSA-E2[29], suggesting a critical role of membrane-bound ERs, although the nuclear ERs, specifically ERβ, may also be involved through direct interactions with cell signaling cascades. Because alterations in gene transcription and protein translation will also likely be critical for long-term memory enhancements produced by E2, future work should apply the blocking approach to understand which genes and proteins, perhaps starting with those already identified through microarray studies, are most critical to the mnemonic effects of E2.
Fig. 6.
Hypothetical model of the molecular mechanisms in the dorsal hippocampus underlying E2-induced enhancement of object memory consolidation. Solid lines represent events for which evidence exists, whereas dashed lines are hypothetical relationships. Terms in red indicate molecules or cellular processes that may be critical for the rapid effects of E2 on object memory consolidation. E2 binding to a membrane ER or ERβ may activate Ras, PI3K, Akt, and PKA, which can then activate ERK, which could stimulate epigenetic alterations, gene expression, and protein synthesis, and thereby improve memory.
One important issue regarding the application of these data to the development of new drug treatments is whether the effects will generalize to other types of memory or to treatments given chronically. If common neurobiological principles govern the formation of all types of hippocampal memories regardless of type (e.g., spatial, contextual, object) and length of training (e.g. one-trial or multiple-trial), then the findings generated from acute post-training treatments should generalize more broadly. The fact that processes like ERK activation are critically necessary in the hippocampus for long-term memory formation in the spatial Morris water maze, contextual fear conditioning, and novel object recognition tasks [73–76] suggests that this might be the case. Such rapid processes likely serve as triggers that lead to the morphological and physiological changes (e.g. spine formation, enhanced LTP) responsible for long-term memory. Maintenance of these structural and physiological alterations may require chronic treatment with drugs that stimulate cell signaling, and so mechanisms identified through acute treatments may be pivotal to the success of chronic treatments.
Although this review has focused primarily on the modulation of hippocampal function and hippocampal memory by E2, the Downstream Molecule model and blocking approach can be easily applied to any hormone, behavior, and brain region. E2 and the hippocampus have been emphasized here simply because the blocking approach has not been widely applied to this area of study. The application of this approach to study the neurobiological mechanisms though which other hormones influence cognition will undoubtedly provide a much more complete understanding of how these hormones affect cognitive processes, and may ultimately lead to safer and more effective treatments for reducing the detrimental effects of aging, mental illness, and neurodegenerative disease on cognitive function.
Acknowledgments
This work described in this article was sponsored by NIH grant R01 AG022525 to KMF, an American Psychological Association Diversity Program in Neuroscience fellowship (NIMH T32 MH18882) to SMF, a Samuel K. Bushnell Dissertation Fellowship from Yale Graduate School of Arts and Sciences to LLH, and Yale University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- 2.Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- 3.Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JRM, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Rates and risk factors for dementia and Alzheimer’s disease. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 4.Wolf OT, Kirschbaum C. Endogenous estradiol and testosterone levels are associated with cognitive performance in older women and men. Horm Behav. 2002;41:259–266. doi: 10.1006/hbeh.2002.1770. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. The Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 7.Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 8.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1801–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperbert C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women. The Women’s Health Initiative: A randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 12.Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: Recent insights and remaining challenges. Learn Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- 13.Eichenbaum H. Declarative memory: Insights from cognitive neurobiology. Annu Rev Psychol. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- 14.Eichenbaum H. The Cognitive Neuroscience of Memory. Oxford University Press; New York, NY: 2002. [Google Scholar]
- 15.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 16.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll I, Sutherland RJ. The aging hippocampus: Navigating between rat and human experiments. Rev Neurosci. 2005;16:87–121. doi: 10.1515/revneuro.2005.16.2.87. [DOI] [PubMed] [Google Scholar]
- 21.deToledo-Morrell L, Stoub TR, Wang C. Hippocampal atrophy and disconnection in incipient and mild Alzheimer’s disease. Prog Brain Res. 2007;163:741–753. doi: 10.1016/S0079-6123(07)63040-4. [DOI] [PubMed] [Google Scholar]
- 22.Frick KM, Fernandez SM, Bennett JC, Prange-Kiel J, MacLusky NJ, Leranth C. Behavioral training interferes with the ability of gonadal hormones to increase CA1 spine synapse density in ovariectomized female rats. Eur J Neurosci. 2004;19:3026–3032. doi: 10.1111/j.1460-9568.2004.03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17β-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 24.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–120. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 26.Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–558. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- 27.Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: An examination of synaptic proteins. Endocrinology. 2001;142:1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- 28.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23:2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Brinton RD. Estrogen receptor a and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res. 2007;1172:48–59. doi: 10.1016/j.brainres.2007.06.092. [DOI] [PubMed] [Google Scholar]
- 32.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 33.Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- 35.Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- 36.O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 37.Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- 38.Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 39.Bohacek J, Daniel JM. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav. 2007;52:237–243. doi: 10.1016/j.yhbeh.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- 41.Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- 42.Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- 43.Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- 45.Holmes MM, Wide JK, Galea LAM. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- 46.Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res. 2004;155:45–53. doi: 10.1016/j.bbr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Rissanen A, Puoliväli J, van Groen T, Riekkinen PJ. In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task. NeuroReport. 1999;10:1369–1372. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- 48.Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KBJ. Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging. 2002;23:87–95. doi: 10.1016/s0197-4580(01)00250-0. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frye CA, Rhodes ME. Enhancing effects of estrogen on inhibitory avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Res. 2002;956:285–293. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- 52.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daniel JM. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez SM, Frick KM. Chronic oral estrogen affects memory and neurochemistry in middle-aged female mice. Behav Neurosci. 2004;118:1340–1351. doi: 10.1037/0735-7044.118.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 56.Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- 57.Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- 58.Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heikkinen T, Puoliväli J, Liu L, Rissanen A, Tanila H. Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- 60.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 61.Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gresack JE, Kerr KM, Frick KM. Life-long environmental enrichment differentially affects the mnemonic response to estrogen in young, middle-aged, and aged female mice. Neurobiol Learn Mem. 2007;88:393–408. doi: 10.1016/j.nlm.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 64.Frye CA, Rhodes ME, Dudek B. Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks. Brain Res. 2005;1036:101–108. doi: 10.1016/j.brainres.2004.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008;54:455–462. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sze CI, Troncoso JC, Kawas C, Mouton P, Price DL, Martin LJ. Loss of the presynaptic vesicle protein synaptophysin in hippocampus correlates with cognitive decline in Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:933–944. doi: 10.1097/00005072-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 68.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 69.Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, Ingram DK, Jucker M. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging. 1998;19:599–606. doi: 10.1016/s0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 71.Klann E, Sweatt JD. Altered protein synthesis is a trigger for long-term memory formation. Neurobiol Learn Mem. 2008;89:247–259. doi: 10.1016/j.nlm.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 73.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;12:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O’Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]
- 76.Bozon B, Kelly A, Josselyn SA, Silva AJ, Davis S, Laroche S. MAPK, CREB and zif268 are all required for the consolidation of recognition memory, Philos Trans R Soc Lond, B. Biol Sci. 2003;358:805–814. doi: 10.1098/rstb.2002.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, Hiramoto T, Imamura O, Kobayashi Y, Watanabe Y, Itohara S, Takishima K. Extracellular signal-regulated kinase 2 (ERK2) knockdown mice show deficits in long-term memory; ERK2 has a specific function in learning and memory. J Neurosci. 2007;227:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samuels IS, Karlo JC, Faruzzi AN, Pickering K, Herrup K, Sweatt JD, Saitta SC, Landreth GE. Deletion of ERK2 mitogen-activated protein kinase identifies its key roles in cortical neurogenesis and cognitive function. J Neurosci. 2008;28:6983–6895. doi: 10.1523/JNEUROSCI.0679-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 81.Pfaff D, Frohlich J, Morgan M. Hormonal and genetic influences on arousal--sexual and otherwise. TINS. 2002;25:45–50. doi: 10.1016/s0166-2236(00)02084-1. [DOI] [PubMed] [Google Scholar]
- 82.McGaughy J, Sarter M. Effects of ovariectomy, 192 IgG-saporin-induced cortical cholinergic deafferentation, and administration of estradiol on sustained attention performance in rats. Behav Neurosci. 1999;113:1216–1232. doi: 10.1037//0735-7044.113.6.1216. [DOI] [PubMed] [Google Scholar]
- 83.Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–119. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 84.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pechenino AS, Frick KM. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol Learn Mem. 2009;91:315–322. doi: 10.1016/j.nlm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: Cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- 87.Pitha J, Pitha J. Amorphous water soluble derivatives of cyclodextrins: Nontoxic dissolution enhancing excipients. J Pharmacol Sci. 1985;74:987. doi: 10.1002/jps.2600740916. [DOI] [PubMed] [Google Scholar]
- 88.Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 89.Packard MG, Teather LA. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. NeuroReport. 1997;8:3009–3013. doi: 10.1097/00001756-199709290-00004. [DOI] [PubMed] [Google Scholar]
- 90.Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 94.Mumby DG. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behav Brain Res. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 95.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baker KB, Kim JJ. Effects of stress and hippocampal NMDA receptor antagonism on recognition memory in rats. Learn Mem. 2002;9:58–65. doi: 10.1101/lm.46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128:459–471. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 101.Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against β-amyloid toxicity requires expression of estrogen receptor α or β and activation of the MAPK pathway. J Neurochem. 2002;82:674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 102.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 103.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- 105.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 106.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 107.Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelson RJ. An introduction to behavioral endocrinology. 3. Sinauer Associates, Inc; Sunderland, MA: 2005. [Google Scholar]
- 109.Weatherman RV, Chang CY, Clegg NJ, Carroll DC, Day RN, Baxter JD, McDonnell DP, Scanlan TS, Schaufele F. Ligand-selective interactions of ER detected in living cells by fluorescence resonance energy transfer. Mol Endocrinol. 2002;16:487–496. doi: 10.1210/mend.16.3.0813. [DOI] [PubMed] [Google Scholar]
- 110.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 111.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto Y, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2008;57:363–375. doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induced rapid translocation of estrogen receptor β, but not estrogen receptor a, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fugger HN, Foster TC, Gustafsson JA, Rissman EF. Novel effects of estradiol and estrogen receptor αand β on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- 115.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 116.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rissman EF, Heck AL, Leonard JE, Shupnik MA, Gustafsson JA. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc Natl Acad Sci U S A. 2002;99:3996–4001. doi: 10.1073/pnas.012032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 119.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 2008;89:513–521. doi: 10.1016/j.nlm.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 121.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 122.Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 123.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Toran-Allerand CD. Novel sites and mechanisms of oestrogen action in the brain. Novartis Foundation Symposia. 2000;230:56–69. doi: 10.1002/0470870818.ch6. [DOI] [PubMed] [Google Scholar]
- 128.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERa and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 129.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 130.Waters EM, Torres-Reveron A, McEwen BS, Milner TA. Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: Synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 132.Guerra-Ariaza C, Amorim MAR, Pinto-Almazan R, Gonzales-Arenas A, Campos MG, Garcia-Segura LM. Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res. 2009;87:470–481. doi: 10.1002/jnr.21848. [DOI] [PubMed] [Google Scholar]
- 133.Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- 134.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behav Brain Res. 2008;194:174–180. doi: 10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharmacol Biochem Behav. 2009;93:177–182. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–210. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- 139.Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 140.Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post-training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Persson I, Yuen J, Bergkvist L, Schairer C. Cancer incidence and mortality in women receiving estrogen and estrogen-progestin replacement therapy--long-term follow-up of a Swedish cohort. Int J Cancer. 1996;67:327–332. doi: 10.1002/(SICI)1097-0215(19960729)67:3<327::AID-IJC4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 142.Whitesell L, Lindquist SL. Hsp90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5 doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 143.Olazabal UE, Pfaff DW, Mobbs CV. Sex differences in the regulation of heat shock protein 70 kDa and 90 kDa in the rat ventromedial hypothalamus by estrogen. Brain Res. 1992;596:311–314. doi: 10.1016/0006-8993(92)91563-t. [DOI] [PubMed] [Google Scholar]
- 144.Pizarro JM, Haro LS, Barea-Rodriguez EJ. Learning associated increase in heat shock cognate 70 mRNA and protein expression. Neurobiol Learn Mem. 2003;79:142–151. doi: 10.1016/s1074-7427(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 145.Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: implications in multiple sclerosis. Cytokine Growth Factor Rev. 2007;18:267–278. doi: 10.1016/j.cytogfr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 146.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal. 2006;18:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hosie M, Adamson M, Penny C. Actin binding protein expression is altered in uterine luminal epithelium by clomiphene citrate, a synthetic estrogen receptor modulator. Theriogenology. 2008;69:700–713. doi: 10.1016/j.theriogenology.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 148.Manavathi B, Acconcia F, Rayala SK, Kumar R. An inherent role of microtubule network in the action of nuclear receptor. Proc Natl Acad Sci USA. 2006;103:15981–15986. doi: 10.1073/pnas.0607445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y, Tang BL. SNAREs in neurons-beyond vesicle exocytosis. Mol Membr Biol. 2006;23:377–384. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- 150.Aenlle KK, Kumar A, Cui L, Jackson TC, Foster TC. Estrogen effects on cognition and hippocampal transcription in middle-aged mice. Neurobiol Aging. 2009;30:932–945. doi: 10.1016/j.neurobiolaging.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]