Abstract
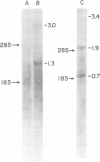
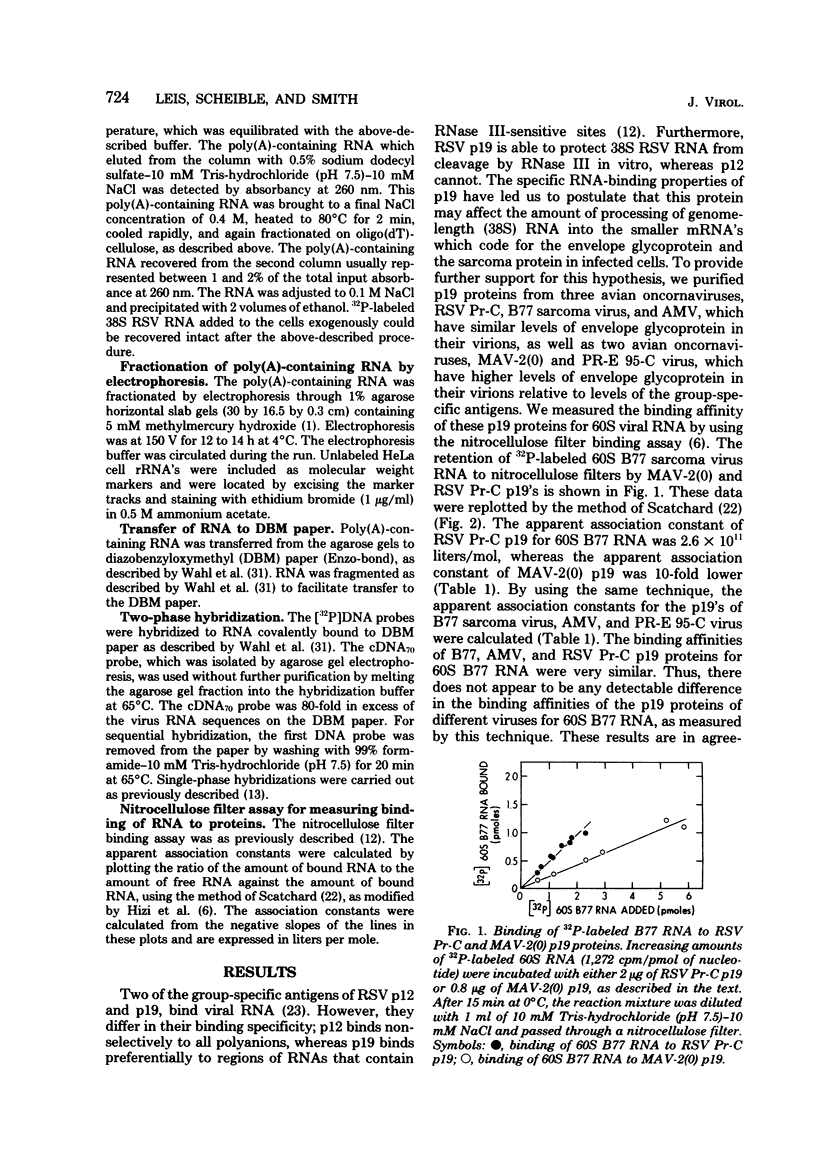
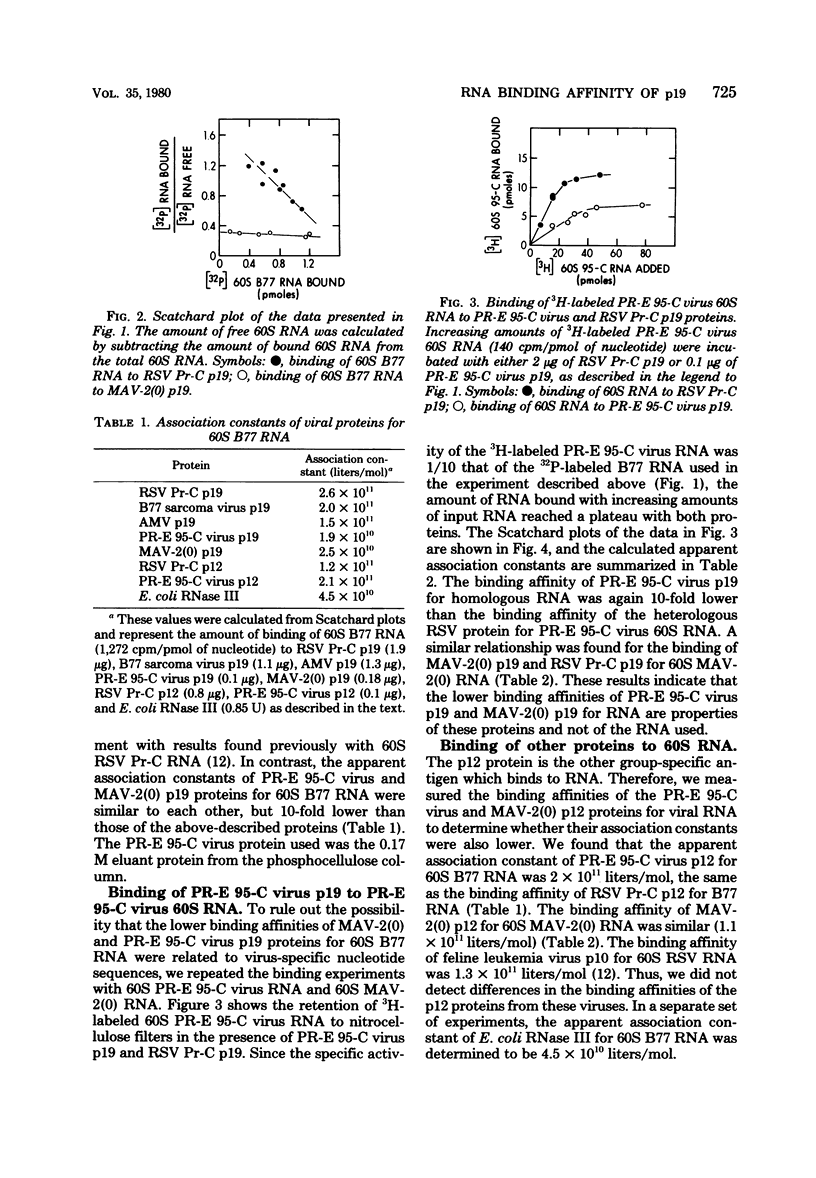
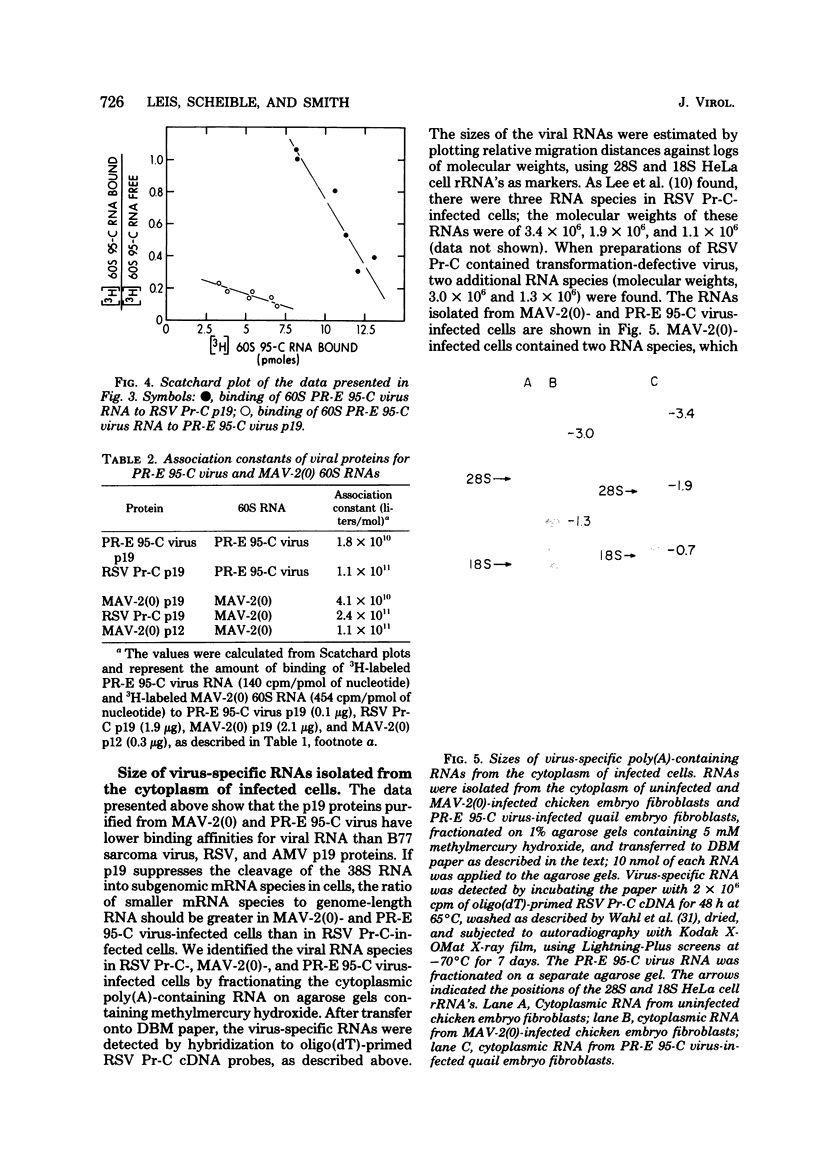
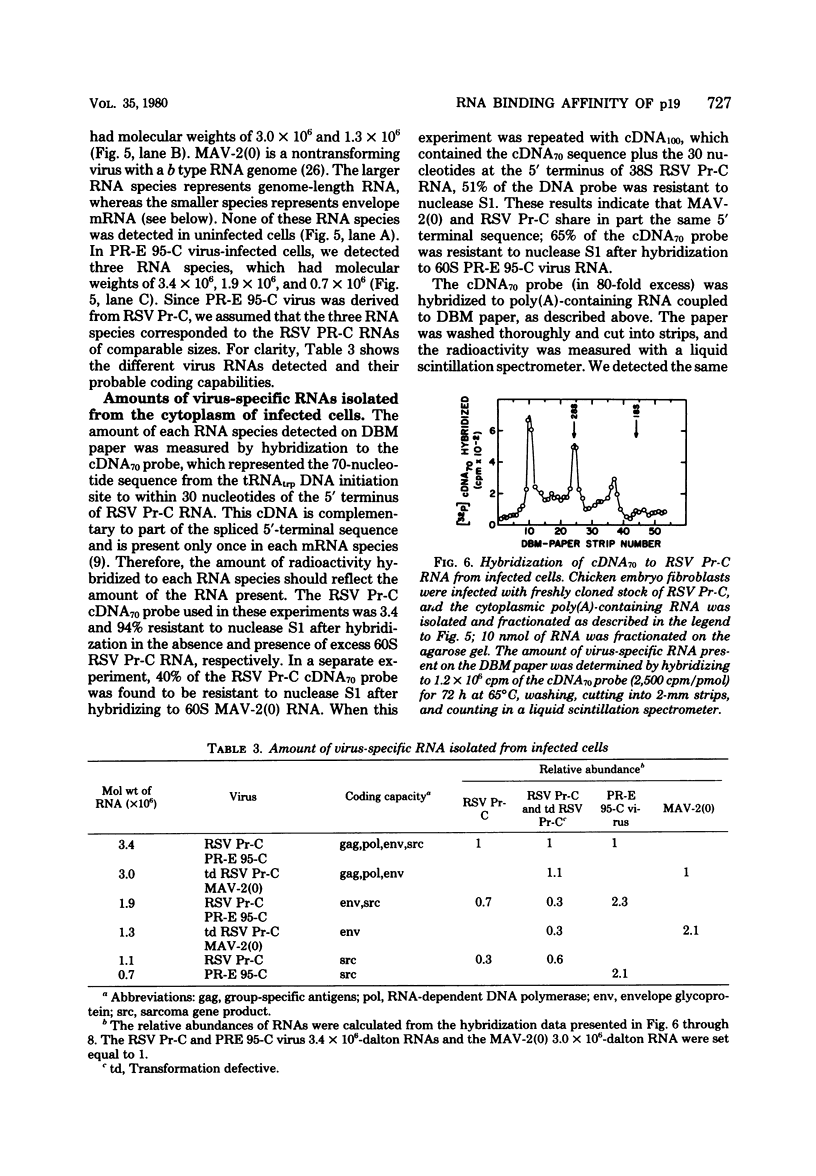
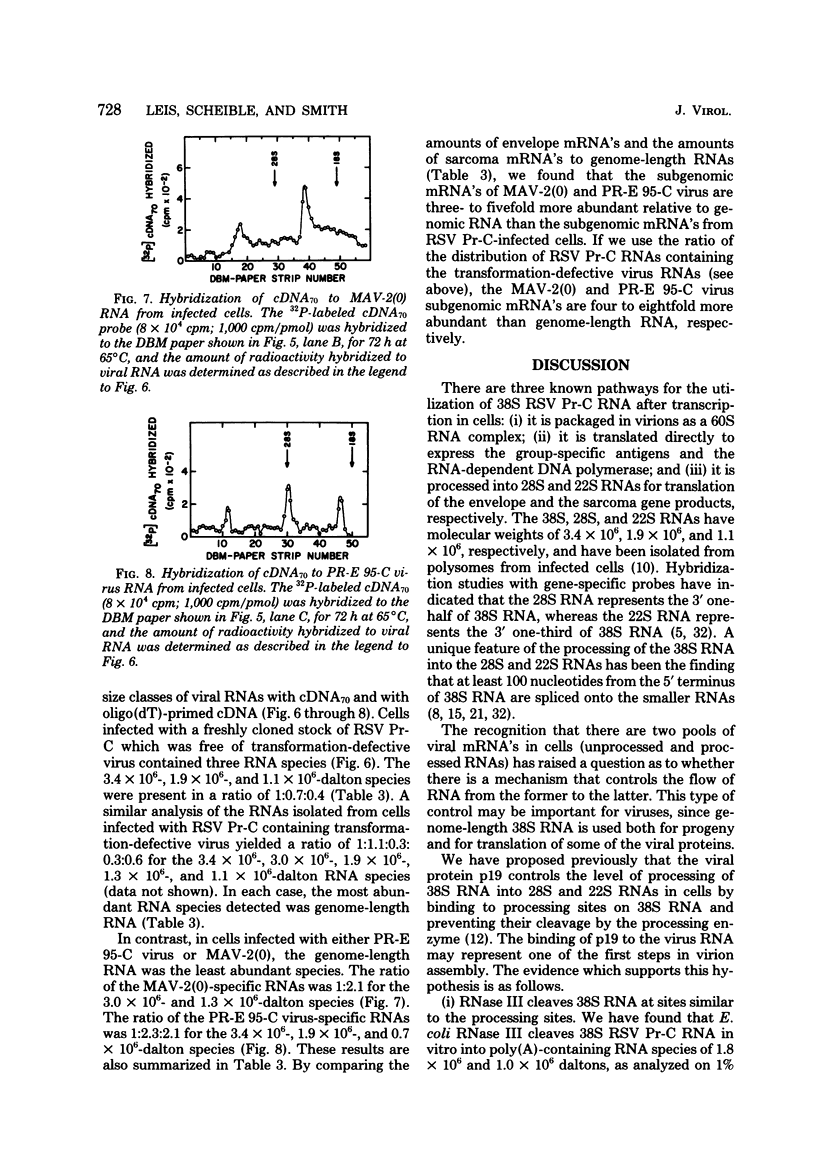
We purified the p19 proteins from the Prague C strain of Rous sarcoma virus, avian myeloblastosis virus, B77 sarcoma virus, myeloblastosis-associated virus-2(0), and PR-E 95-C virus and measured their binding affinities for 60S viral RNA by the nitrocellulose filter binding technique. The apparent association constants of the p19 proteins from Rous sarcoma virus Prague C, avian myeloblastosis virus, and B77 sarcoma virus for homologous and heterologous 60S RNAs were similar (1.5 x 10(11) to 2.6 x 10(11) liters/mol), whereas those of myeloblastosis-associated virus-2(0) and PR-E 95-C virus were 10-fold lower. The sizes and relative amounts of the virus-specific polyadenylic acid-containing RNAs in the cytoplasms of cells infected with Rous sarcoma virus Prague C, myeloblastosis-associated virus-2(0), and PR-E 95-C virus were determined by fractionating the RNAs on agarose gels containing methylmercury hydroxide, transferring them to diazobenzyloxymethyl paper and hybridizing them to a 70-nucleotide complementary DNA probe. In cells infected with Rous sarcoma virus Prague C we detected 3.4 x 10(6)-, 1.9 x 10(6)-, and 1.1 x 10(6)-dalton RNAs, in PR-E 95-C virus-infected cells we detected 3.4 x 10(6)-, 1.9 x 10(6)- and 0.7 x 10(6)-dalton RNAs, and in cells infected with myeloblastosis-associated virus-2(0) we detected 3 x 10(6)- and 1.3 x 10(6)-dalton RNAs. Each of these RNA species contained RNA sequences derived from the 5' terminus of genome-length RNA, as evidenced by hybridization with the 5' 70-nucleotide complementary DNA. The ratios of subgenomic mRNA's to genome-length RNAs in cells infected with myeloblastosis-associated virus-2(0) and PR-E 95-C virus were three- to five-fold higher than the ratio in cells infected with Rous sarcoma virus Prague C. These results suggest that more processing of viral RNA in infected cells is correlated with lower binding affinities of the p19 protein for viral RNA, and they are consistent with the hypothesis that the p19 protein controls processing of viral RNA in cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizi A., Leis J. P., Joklik W. K. The RNA-dependent DNA polymerase of avian sarcoma virus B77. Binding of viral and nonviral ribonucleic acids to the alpha, beta2, and alphabeta forms of the enzyme. J Biol Chem. 1977 Oct 10;252(19):6878–6884. [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Varmus H. E., Bishop J. M. Virus-specific messenger RNAs in permissive cells infected by avian sarcoma virus. J Biol Chem. 1979 Aug 25;254(16):8015–8022. [PubMed] [Google Scholar]
- Leis J. P., McGinnis J., Green R. W. Rous sarcoma virus p19 binds to specific double-stranded regions of viral RNA: effect of p19 on cleavage of viral RNA by RNase III. Virology. 1978 Jan;84(1):87–98. doi: 10.1016/0042-6822(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Leis J. P. RNA-dependent DNA polymerase activity of RNA tumor virus. VI. Processive mode of action of avian myeloblastosis virus polymerase. J Virol. 1976 Sep;19(3):932–939. doi: 10.1128/jvi.19.3.932-939.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Smith R. E., Dierks P., Parsons J. T., Collett M. S., Faras A. J. In vitro transcription of reconstituted 35s RNA.tRNAtrp template.primer complexes by the avian oncornavirus DNA polymerase. Effect of temperature on the size of the DNA transcripts. Virology. 1978 Mar;85(1):28–42. doi: 10.1016/0042-6822(78)90409-9. [DOI] [PubMed] [Google Scholar]
- McGinnis J., Hizi A., Smith R. E., Leis J. P. In vitro translation of a 180,000-dalton Rous sarcoma virus precursor polypeptide containing both the DNA polymerase and the group-specific antigens. Virology. 1978 Feb;84(2):518–522. doi: 10.1016/0042-6822(78)90267-2. [DOI] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Brown S., Sen A., Eisenman R. Recombinant avian oncoviruses. II. Alterations in the gag proteins and evidence for intragenic recombination. Virology. 1979 Jan 30;92(2):463–481. doi: 10.1016/0042-6822(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Davids L. J., Neiman P. E. Comparison of an avian osteopetrosis virus with an avian lymphomatosis virus by RNA-DNA hybridization. J Virol. 1975 Jan;17(1):160–167. doi: 10.1128/jvi.17.1.160-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Nebes S., Leis J. Production of large amounts of 35S RNA and complementary DNA from avian RNA tumor viruses. Anal Biochem. 1977 Jan;77(1):226–234. doi: 10.1016/0003-2697(77)90308-6. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Allfrey V. G., Hanafusa H. Microinjection analysis of envelope-glycoprotein messenger activities of avian leukosis viral RNAs. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1614–1618. doi: 10.1073/pnas.74.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Smith R. E. Isolation and characterization of the envelope glycoprotein of an avian osteopetrosis virus: effect of host cell on antigenic reactivity. Virology. 1978 Oct 1;90(1):80–89. doi: 10.1016/0042-6822(78)90335-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Davis N. L. RNase III cleaves vesicular stomatitis virus genome-length RNAs but fails to cleave viral mRNA's. J Virol. 1979 Apr;30(1):108–115. doi: 10.1128/jvi.30.1.108-115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaane D. V., Gielkens A. L., Hesselink W. G., Bloemers H. P. Identification of Rauscher murine leukemia virus-specific mRNAs for the synthesis of gag- and env-gene products. Proc Natl Acad Sci U S A. 1977 May;74(5):1855–1859. doi: 10.1073/pnas.74.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]