Abstract
Background & Aims
Abscisic acid (ABA) is effective in preventing insulin resistance and obesity-related inflammation through a PPAR γ-dependent mechanism. The objective of this study was to assess the efficacy ABA in improving glucose homeostasis and suppress inflammation when administered in combination with rosiglitazone (Ros) and to determine whether PPAR γ activation by ABA is initiated via cAMP/protein kinase A (PKA) signaling.
Methods
Obese db/db mice were fed high-fat diets containing 0, 10, or 70 mg/kg Ros with and without racemic ABA (100 mg/kg) for 60 days. Glucose tolerance and fasting insulin levels were assessed at 6 and 8 weeks, respectively, and adipose tissue macrophage (ATM) infiltration was examined by flow cytometry. Gene expression was examined on white adipose tissue (WAT) and stromal vascular cells (SVCs) cultured with ABA, Ros, or an ABA/Ros combination.
Results
Both Ros and ABA improved glucose tolerance, and ABA decreased plasma insulin levels while having no effect on Ros-induced weight gain. ABA in combination with low-dose Ros (10 mg/kg; Roslo) synergistically inhibited ATM infiltration. Treatment of SVCs with Ros, ABA or ABA/Ros suppressed expression of the M1 marker CCL17. ABA and Ros synergistically increased PPAR γ activity and pretreatment with a cAMP-inhibitor or a PKA-inhibitor abrogated ABA-induced PPAR γ activation.
Conclusions
ABA and Ros act synergistically to modulate PPAR γ activity and macrophage accumulation in WAT and ABA enhances PPAR γ activity through a membrane-initiated mechanism dependent on cAMP/PKA signaling.
Keywords: Diabetes, macrophages, inflammation, obesity, ABA, PPAR
Introduction
Over the past two decades the onset of obesity in the United States and worldwide has risen to epidemic proportions. According to recent estimates, 17.1% of US children and adolescents are overweigh and over 32.2% of adults are obese [1]. This high prevalence of obesity has led to an increase in obesity-related diseases, including type II diabetes, cardiovascular disease, and stroke, and, according to a recent meta-analysis, is responsible for 9.1% of all health care-related expenditures in the United States [2]. Thus, novel approaches for preventing obesity-related illnesses are both timely and urgently needed.
It is in this regard that the isoprenoid phytohormone abscisic acid (ABA) shows promise as a putative therapeutic against obesity-related inflammatory complications. ABA is a vitamin A derivative that is effective in improving glucose homeostasis and reducing obesity-related inflammation in obese and overweight mice [3]. Based on its ubiquitous presence in plants and the recent finding that it can be synthesized by mammalian cells [4], it may be well-tolerated by the human body. Our studies have shown that ABA increases the activity of the nuclear receptor peroxisome proliferator-activated receptor γ (PPAR γ) in 3T3-L1 preadipocytes and that its full antidiabetic effects are dependent on the presence of PPAR γ in immune cells [5,6]. PPAR γ is the molecular target of the thiazolidinedione (TZD) class of antidiabetic drugs, which includes rosiglitzone (Avandia™), pioglitazone (Actos™) and troglitazone which was removed from the market due to its hepatotoxicity [7,8]. Whereas TZD usage has been shown to be associated with significant side effects such as excessive fluid retention, liver damage and weight gain [9], there were no detectable side-effects in mice fed ABA at doses ranging from 100 to 800 mg/kg of diet [5].
Recent studies have shown that ABA also acts as an activator of the cAMP/PKA signaling pathway in pancreatic and immune cells [10,11]. Interestingly, PKA can activate PPAR γ indirectly through a phosphorylation-dependent mechanism [12], suggesting a possible link between proximal membrane-initiated signaling and PPAR γ activation. In light of these findings, the objective of this study was to more closely examine the similarities between ABA and rosiglitazone as treatments for obesity-related inflammation and diabetes, and to investigate a potential synergistic interaction between the two compounds in modulating glucose tolerance and inflammatory macrophage accumulation in WAT. In this study obese/diabetic db/db mice were fed high-fat diets containing 0, 15, or 70 mg/kg rosiglitazone maleate (control, Roslo, and Roshi respectively), each with or without racemic ABA (100 mg/kg). Our results show that ABA acts similar to Roslo in improving glucose tolerance in obese mice but also lowers insulin levels and, unlike Ros, it does not induce weight gain or fluid retention. Combining ABA with rosiglitazone showed significant synergistic activity in down-modulating macrophage accumulation in WAT and in increasing macrophage PPAR γ activity.
Research Design and Methods
Mice and Dietary Treatments
Twelve-fifteen week-old BKS.Cg -+Leprdb /+Leprdb /OlaHsd (db/db) mice were housed at the animal facilities at Virginia Polytechnic Institute and State University in a room maintained at 75° F, with a 12:12 h light-dark cycle starting from 6:00 AM. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Virginia Polytechnic Institute and State University and met or exceeded requirements of the Public Health Service/National Institutes of Health and the Animal Welfare Act.
Mice (n=40) weighing over 40 g were fed one of six experimental diets: a control high-fat diet (control), a high-fat diet containing low-dose rosiglitazone maleate (Roslo, 10 mg/kg diet), or a high-fat diet containing a high dose of rosiglitazone maleate (Roshi, 70 mg/kg diet), each with or without all racemic ABA (100 mg/kg, Sigma Aldrich, St. Louis, MS) for 60 days. The basal composition of these high saturated fat diets has been previously published [5,13,14]. Mice were weighed and fasting (12h) blood glucose levels were monitored weekly. Blood glucose levels were assessed with an Accu-Chek® Glucometer (Roche, Indianapolis, IN). An intraperitoneal glucose tolerance test (IPGTT, 1 g glucose/kg body weight) was conducted on fasted mice (12h) for 0, 30, 90, and 180 minute time points and insulin levels were measured on day 54. On day 60 mice were sacrificed by CO2 narcosis with secondary thoracotomy. Abdominal (epididymal) WAT, subcutaneous WAT, and liver were then excised and weighed. Abdominal (epididymal) and subcutaneous WAT were then digested and fractionated.
Digestion of white adipose tissue
Abdominal and inguinal subcutaneous WAT was excised, weighed, minced into small <10 mg pieces and placed into digestion media (1XHBSS (Mediatech, Herndon, VA) supplemented with 2.5% HEPES (Mediatech) and 10% fetal bovine serum containing type II collagenase (0.2%, Sigma-Aldrich). Samples were incubated in a 37°C incubator for 30 minutes, filtered through a 100 μm nylon cell strainer to remove undigested particles, and centrifuged at 4°C at 1000 × g for 10 minutes. The pellet, consisting of stromal vascular cells (SVCs), was washed with 1XHBSS and centrifuged at 4°C at 1000 × g for 10 minutes. The supernatant was discarded and erythrocytes were lysed by incubating the SVCs in 2 mL erythrocyte lysis buffer for 2 minutes before stopping the reaction with 9 mL 1X PBS. Cells were then respun at 4°C at 1000 × g for 10 minutes, suspended in 1 ml of 1X PBS, and counted with a Coulter Counter (Beckman Coulter, Fullerton, CA).
Flow Cytometry
For immunophenotyping SVCs were seeded into 96-well plates (Costar) at 2 × 105 cell/well. For whole blood 10 μL of each sample was added per well. After an initial 20 minute incubation with FcBlock (20 μg/ml; BD Biosciencest — Pharmingen) to inhibit non-specific binding, cells were washed in PBS containing 5% serum and 0.09% sodium azide (FACS buffer) and stained with primary anti-mouse antibodies F4/80 PE-Cy5 (ebioscience, San Diego, CA), CD11b Alexa-fluor 700 (ebioscience), CD4 Alexa-Flour 700 (ebioscience), CD25 APC (BD), FoxP3 PE (ebioscience), or anti-human CCR2 PE (R&D systems, Minneapolis, MN) as previously shown [15]. Flow results were computed with a BD LSR II flow cytometer and data analyses was performed with FACS Diva software (BD).
LPS treatment of stromal vascular cells
Isolated cells from the stromal vascular fraction (SVF) of WAT from high-fat fed db/db mice were enumerated and seeded into 24-well plates at 2 × 106 cells/well. Cells were then treated for 6 hrs at 37°C with LPS (100 ng/mL) in addition to ABA (10 μM), Ros (1 μM), ABA and Ros, or vehicle alone (DMSO). After incubation cells were harvested with RLT lysis buffer and stored in −80°C for RNA isolation and gene expression analyses.
Real-time quantitative PCR
Total RNA was isolated from adipose tissue using the RNeasy Lipid Mini Kit (Qiagen) and from cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Total RNA was used to generate complementary DNA (cDNA) template using the qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD). The total reaction volume was 20 μL with the reaction incubated as follows in an MJ MiniCycler: 5 minutes at 25°C, 30 minutes at 52°, 5 minutes at 85°C, hold at 4°C. Each gene amplicon was purified with the MiniElute PCR Purification Kit (Qiagen) and quantitated on an agarose gel by using a DNA mass ladder (Promega). These purified amplicons were used to optimize real-time PCR conditions and to generate standard curves in the real-time PCR assay. Primer concentrations and annealing temperatures were optimized for the iCycler iQ system (Bio-Rad) for each set of primers using the system's gradient protocol. PCR efficiencies were maintained between 92 and 105% and correlation coefficients above 0.98 for each primer set during optimization and also during the real-time PCR of sample DNA as previously shown [6,15,16].
Complementary DNA (cDNA) concentrations for genes of interest were examined by real-time quantitative PCR using an iCycler IQ System and Sybr Green PCR master mix (Applied Biosystems, Foster City, CA). A standard curve was generated for each gene using 10-fold dilutions of purified amplicons starting at 5 pg of cDNA and used later to calculate the starting amount of target cDNA in the unknowns. SYBR green I is a general double-stranded DNA intercalating dye and, therefore, may detect nonspecific products and primer/dimers in addition to the amplicon of interest. In order to determine the number of products synthesized during the real-time PCR, a melting curve analysis was performed on each product. Real-time PCR was used to measure the starting amount of nucleic acid of each unknown sample of cDNA on the same 96-well plate. Results are presented as starting quantity of target cDNA (picograms) per microgram of total RNA.
Transfection and PPAR γ Reporter Activity Assays
A pCMX.PPAR γ expression plasmid and a pTK.PPRE3x luciferase reporter plasmid driven by the PPRE-containing Acyl-CoA oxidase promoter (kindly provided by Dr. R.M. Evans, The Salk Institute, San Diego, CA) were purified using Qiagen's Maxi kit (Valencia, CA). For transwell reporter assays 3T3-L1 pre-adipocytes and RAW 264.67 macrophages were seeded the lower and upper chambers, respectively, of 24-transwell plates in high glucose DMEM (Hyclone) containing 10% fetal bovine serum (FBS). After an overnight incubation each transwell was co-transfected with 0.6 μg plasmid DNA and 10 ng of pRL reporter control using F-2 transfection reagents (Targeting Systems, Santee, CA) according to the manufacturer's protocol. Media was changed after 24 hours and wells were treated with vehicle (DMSO), ABA (10 μM, Sigma), or Ros (1 or 10 μM, Cayman Chemicals, Ann Arbor, MI),). Cells were then placed in a 37°C incubator with 5% CO2 for 24 hours before being harvested in reporter lysis reagent.
3T3-L1 pre-adipocytes grown in 25mm2 flasks in high glucose DMEM (Hyclone) containing 10% fetal bovine serum (FBS) until 60-70% confluence. Cells were co-transfected in the 25mm2 with 15 μg plasmid DNA and 25 ng of pRL reporter control using F-2 transfection reagents (Targeting Systems, Santee, CA) according to the manufacturer's protocol. After 24 hours, transfected cells were split from flasks with trypsin 1X (0.25%, Hyclone), enumerated with a cell counter (Coulter Counter, Beckman Coulter), and seeded into white, opaque 96-well plates (BD) at a concentration of 20,000 cells/well. Cells were then treated in replicates of 8 with vehicle (DMSO), Ros (1 μM), or ABA (10 μM) with and without 2’5’ dideoxyadenosine (10 μM; Sigma) and 14-22 myristolated PKA inhibitor fragment (PKAi, 30 μM; Sigma). Cells were then placed in a 37°C incubator with 5% CO2 for 6 hours and were then harvested in reporter lysis reagent.
Luciferase activity, normalized to pRL activity in the cell extracts was determined by using the Dual Luciferase II reporter assay system.(Promega, Madison, WI) using a Modulus luminometer (Turner Biosystems, Sunnyvale, CA). All values were normalized to control wells to calculate relative luciferase activity.
Statistical Analyses
Data were analyzed as a completely randomized design. To determine the statistical significance of the model, analysis of variance (ANOVA) was performed using the general linear model procedure of Statistical Analysis Software (SAS), and probability value (P) < 0.05 was considered to be significant. When the model was significant, ANOVA was followed by Fisher's Protected Least Significant Difference multiple comparison method.
Results
Effect of dietary ABA and rosiglitazone on obese db/db mice
Db/db mice were fed rosiglitazone at three different concentrations (0, 10, 70 mg/kg diet) with and without ABA (100 mg/kg diet). Both the low- and high-dose rosiglitazone treatments significantly increased the body weights of the mice during the 60-day dietary intervention (Table 1). There was significant edema in the interscapular region in mice fed Roshi that was not present in the other groups (data not shown).
Table 1.
Effect of rosiglitazone and abscisic acid (ABA) combination treatment on body and organ weights.a,b,c
| Diet | Initial body weight (g) | Final body weight (g) | Ab. WAT% Body Weight | Sc.WAT% Body Weight | Liver% Body Weight |
|---|---|---|---|---|---|
| No ABA | |||||
| Control | 42.6 ± 3.2 | 49.6 ± 1.8a | 7.2 ± 0.61c | 2.4 ± 0.48 | 6.2 ± 0.51a |
| Roslo | 42.0 ± 4.5 | 56.4± 2.4bc | 6.7 ± 0.61bc | 2.4 ± 0.43 | 9.2 ± 0.51b |
| Roshi | 40.6 ± 4.5 | 64.4 ± 2.4d | 4.6 ± 0.61a | 3.2 ± 0.43 | 6.7 ± 2.3a |
| With ABA | |||||
| Control | 41.3 ± 4.5 | 53.0 ± 2.7ab | 4.7 ± 0.68a | 2.8 ± 0.48 | 6.7 ± 0.56a |
| Roslo | 41.1 ± 4.5 | 51.6 ± 2.4ab | 5.4 ± 0.61ab | 2.6 ± 0.43 | 9.3 ± 0.51b |
| Roshi |
39.7± 4.5 |
62.6 ± 2.4cd |
4.5 ± 0.61a |
3.5 ± 0.43 |
6.7 ± 51a |
| P-value for rosiglitazone |
0.94 |
<0.0001 |
0.03 |
0.11 |
<0.0001 |
| P-value for ABA |
0.70 |
0.59 |
0.02 |
0.46 |
0.77 |
| P-value for interaction | 0.99 | 0.23 | 0.18 | 0.98 | 0.81 |
Organs were excised and weighed on day 60 of experiment.
Least squares means values in a column with a pound sign are significantly different (P < 0.05).
P-value of main effects of rosiglitazone treatments, ABA, and rosiglitazone and ABA combinations during the 60-day period. Data were analyzed as a completely randomized design.
At day 60 we compared weights of abdominal visceral white adipose tissue (Ab. WAT), inguinal subcutaneous white adipose tissue (SCAT), and livers among the different dietary treatments. ABA and Roshi significantly and independently reduced Ab. WAT as percent body weight, and there was little affect on inguinal SCAT weight. Liver weights were significantly increased by low dose Ros (Table 1).
Effect of ABA and rosiglitazone on glucose tolerance and fasting insulin
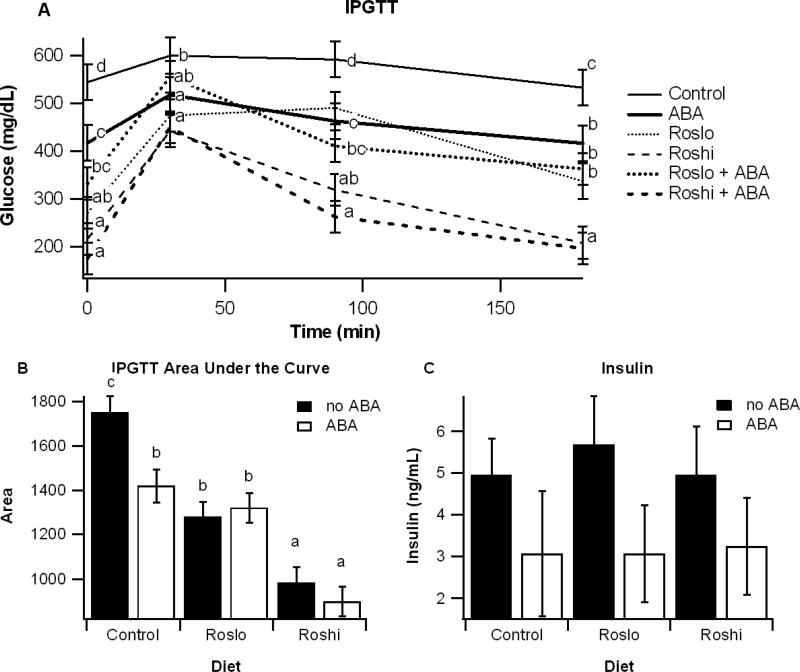
On day 42 of the dietary intervention an intraperitoneal glucose tolerance test (IPGTT) was performed, and insulin levels were assessed at the end of the study. All treatments, regardless of using ABA, Ros or both, significantly improved glucose tolerance relative to the control diet, though there were no significant effects resulting from a synergism between ABA and rosiglitazone on glucose tolerance (Figure 1). Dietary ABA supplementation significantly reduced fasting insulin levels (P=0.04; Figure 1).
Figure 1.
Effect of abscisic acid (ABA) and rosiglitazone (Ros) on glucose tolerance and fasting insulin. Obese db/db mice were fed high-fat diets containing 0, 15, or 70 mg/kg diet rosiglitazone maleate (control, Roslo, and Roshi, respectively) with and without racemic ABA (100 mg/kg diet). On day 42 mice underwent an intraperitoneal glucose tolerance test (IPGTT) (A). Areas under the curve (B) were calculated for each treatment. On day 55 fasting insulin levels were measured for mice on each diet (C). Data are represented as mean ± standard error. Points with different subscripts are significantly different from each other (P<0.05).
Effect of ABA and rosiglitazone on macrophage infiltration into adipose tissue
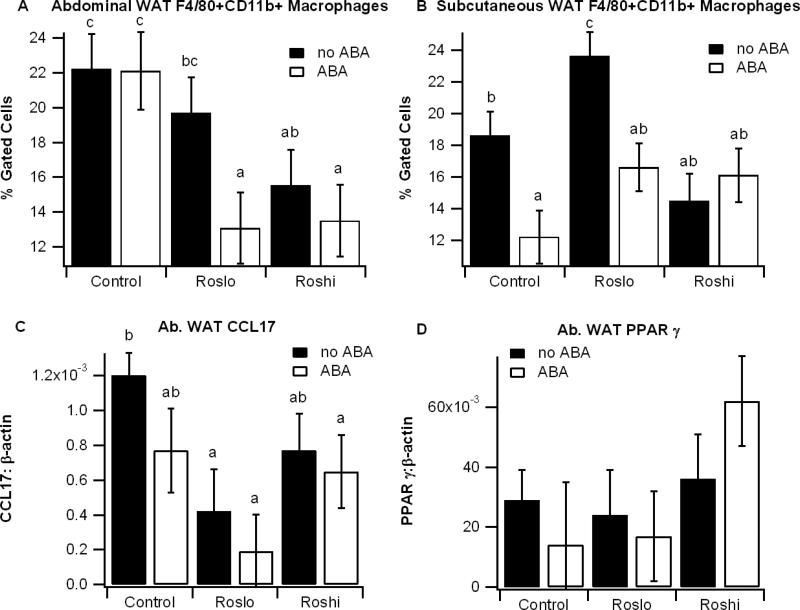
To determine the effect of ABA and Ros on obesity-related inflammation we assessed F4/80+CD11b+ macrophage into WAT with flow cytometry and performed gene expression analyses. Independently the ABA and Roslo treatments had little effect on macrophage infiltration into Ab. WAT, though there was a significant reduction in ATM infiltration when Roslo was administered in combination with ABA relative to the control diet (Figure 2). ABA significantly reduced ATM infiltration in SCAT in mice fed the control and Roslo diets. There was no added benefit in macrophage infiltration by adding ABA to Roshi. Ros significantly reduced expression of the M1 marker CCL17, and the combination of ABA and Roshi increased numerically, but not significantly, PPAR γ mRNA levels (Figure 2).
Figure 2.
Effect of abscisic acid (ABA) and rosiglitazone (Ros) on immune cell infiltration into white adipose tissue. Obese db/db mice were fed high-fat diets containing 0, 15, or 70 mg/kg diet rosiglitazone maleate (control, Roslo, and Roshi, respectively) with and without racemic ABA (100 mg/kg diet). On day 60 the percent of F4/80+CD11b+ in the stromal vascular fractions of abdominal white adipose tissue (Ab. WAT) (A) and subcutaneous WAT (B) were assessed by flow cytometry. The expressions of the M1 marker CCL17 (C) and peroxisome proliferator activated receptor γ (PPAR γ) (D) in Ab. WAT were calculated as a ratio to the housekeeping gene β-actin. Data are represented as mean ± standard error. Points with different subscripts are significantly different from each other (P<0.05).
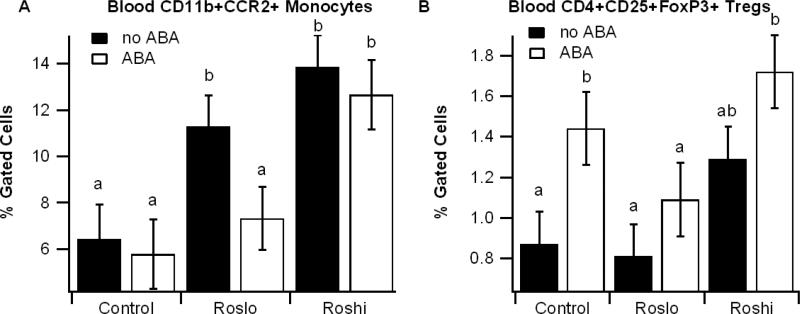
Effect of ABA and Ros on blood immune cell populations
Given that tissue macrophages are repopulated in part by infiltration of bone marrow-derived blood monocytes, we also examined the effect of the ABA and Ros dietary combinations on blood immune cells populations. We found that Ros significantly and dose-dependently increased blood CCR2+CD11b+ monocyte levels whereas ABA mitigated the Roslo-induced increase and increased the percentages of blood regulatory T cells (Tregs) independently of Ros (Figure 3).
Figure 3.
Effect of abscisic acid (ABA) and rosiglitazone (Ros) on blood immune cells. Obese db/db mice were fed high-fat diets containing 0, 15, or 70 mg/kg diet rosiglitazone maleate (control, Roslo, and Roshi, respectively) with and without racemic ABA (100 mg/kg diet). The percent of CD11b+CCR2+ monocytes (A) and CD4+CD25+FoxP3+ Tregs (B) were assessed in whole blood. Data are represented as mean ± standard error. Points with different subscripts are significantly different from each other (P<0.05).
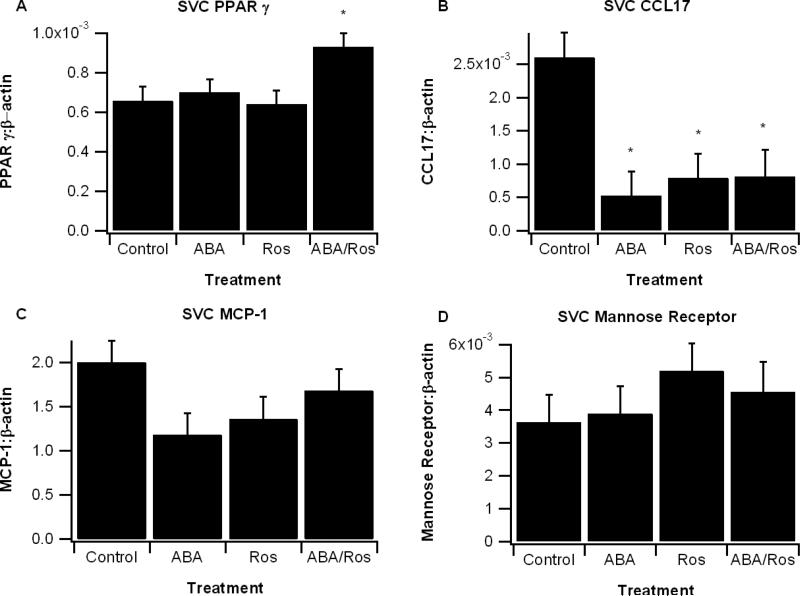
ABA and rosiglitazone effects on LPS-treated SVCs
SVCs from high-fat fed db/db mice were isolated and treated with LPS (100 ng/mL) with and without ABA (10 μM), rosiglitazone (1 μM), and their combination. After a 6-h treatment period we observed a significant increase in PPAR γ expression only in SVCs treated with both ABA and Ros. The M1 marker CCL17 was significantly reduced and all treatments and there were also a numerical reduction in MCP-1 (CCL2, P=0.12). Expression of the mannose receptor, an M2 marker, did not significantly differ among treatments (Figure 4).
Figure 4.
Effect of abscisic acid (ABA) and rosiglitazone combination on gene expression in lipopolysaccharide (LPS)-treated stromal vascular cells (SVCs). SVCs from abdominal white adipose tissue were isolated from db/db mice fed high-fat control diet for 60 days. Cells were seeded into 24-well plates at 1 × 106 cells/well and treated with LPS (100 ng/mL) with and without ABA (10 μM), Ros (1 μM), or ABA and Ros (ABA/Ros). The relative expressions of genes peroxisome proliferator activated receptor γ (PPAR γ) (A), CCL17 (B), monocyte chemoattractant protein 1 (MCP-1) (C), and mannose receptor (D) were calculated as a ratio to the housekeeping gene β-actin. Data are represented as mean ± standard error. Points with an asterisk are significantly different the control treatment (P<0.05).
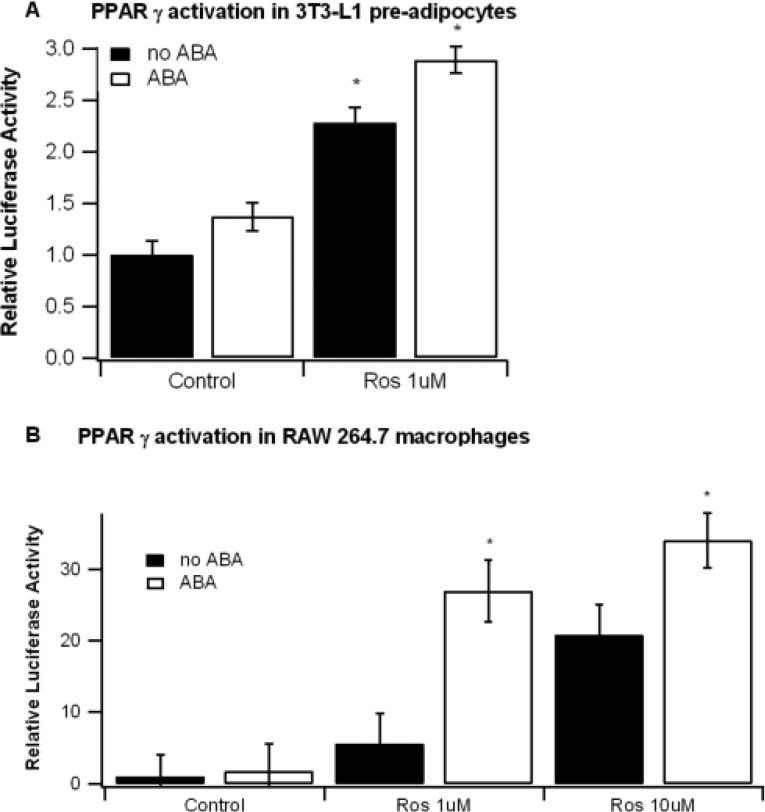
ABA and Ros increase PPAR γ synergistically in RAW 264.7 macrophages but not 3T3-L1 pre-adipocytes
To determine if a synergistic interaction exists between ABA and Ros in vitro we measured PPAR γ activity in ABA and Ros-treated 3T3-L1 pre-adipocytes and RAW 264.7 macrophages. In the 3T3-L1 pre-adipocytes both ABA (P=0.0007) and Ros (P<0.0001) independently augmented PPAR γ activation, though there was no interactive effect (P=0.37, Figure 5A). In the macrophage cell line the individual effects of ABA (P=0.006) and Ros (P<0.0001) were significant as well, but there was also a significant interaction effect suggesting synergistic activity between ABA and Ros (Figure 5B).
Figure 5.
Effect of abscisic acid (ABA) and rosiglitazone (Ros) combination on PPAR γ transactivation in 3T3-L1 pre-adipocytes and RAW 264.7 macrophages. 3T3-L1 preadipocytes and RAW 264.7 macrophages were seeded into the lower and upper chambers, respectively, of a 24-transwell plate before being transfected with a pTK.PPRE3x luciferase reporter plasmid driven by the PPRE-containing Acyl-CoA oxidase promoter and a pRL control plasmid. Cells were treated for 24 with ABA (10 μM), Ros (1 or 10 μM), or their combination. Luciferase activity was normalized to pRL activity in the cell extracts and relative luciferase activity was calculated a ratio of the activity in the treatment wells to control wells. Data are represented as mean ± standard error. Points with an asterisk indicate that a treatment is significantly different from control, non-ABA treated well (P<0.05).
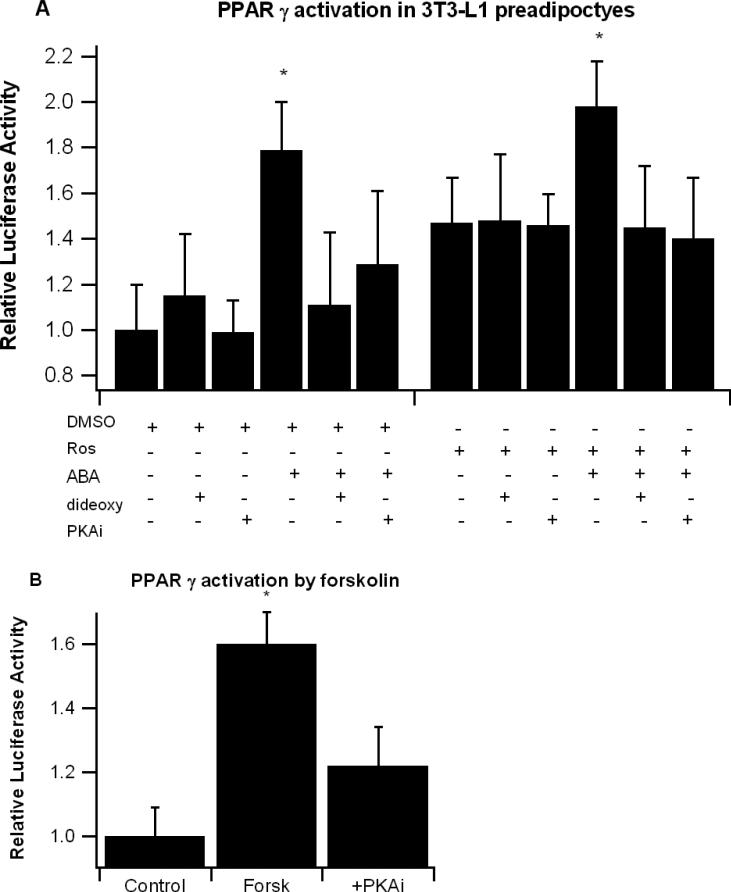
ABA-induced PPAR γ activation is inhibited by cAMP/PKA inhibition
To determine whether ABA-induced activation of PPAR γ is dependent on cAMP/PKA signaling, 3T3-L1 pre-adipocytes were treated for 6 hrs with or without Ros (1 μM) and ABA (10 μM), 2’5’-dideoxyadenosine (10 μM), and 14-22 myristoylated PKA inhibitor fragment (PKAi, 30 μM). Both ABA and Ros increased PPAR γ activity after the 6-hr treatment, and addition of either the cAMP-specific or PKA-specific inhibitor prevented ABA-induced PPAR γ activation without affecting Ros-induced activation (Figure 6A). We also examined whether the cAMP activator forskolin would increase PPAR γ activity in 3T3-L1 pre-adipocytes similar to ABA. After 6-h forskolin at 10 μM significantly enhanced PPAR γ activity, and the activation was inhibited with addition of PKAi. (Figure 6B).
Figure 6.
Effect of cAMP/PKA inhibition on abscisic acid (ABA)-induced PPAR γ activation in 3T3-L1 preadipocytes. Cells were transfected with a pTK.PPRE3x luciferase reporter plasmid driven by the PPRE-containing Acyl-CoA oxidase promoter and were treated for 6-h with vehicle (DMSO) or Ros (1 μM) with ABA (10 μM), the cAMP-inhibitor 2’5’ dideoxyadenosine (dideoxy, 10 μM), or 14-22 myristolated PKA inhibitor fragment (PKAi, 30 μM) (A). PPAR γ-transfected 3T3-L1 pre-adipocytes were also treated with cAMP activator forskolin (forks, 10 μM) with and without PKAi (B). Luciferase activity was normalized to pRL activity in the cell extracts and relative luciferase activity was calculated a ratio of the activity in the treatment wells to control wells. Data are represented as mean ± standard error. Points with an asterisk indicate that a treatment is significantly different from its respective ‘without ABA’ control (P<0.05).
Discussion
ABA is an endogenously produced isoprenoid phytohormone which, when supplemented into the diet, has shown to be effective in preventing obesity-related insulin resistance and inflammation in overweight and obese mice [5]. We have previously demonstrated that ABA activates PPAR γ in vitro and that its full antidiabetic effects are dependent on presence of immune cell PPAR γ [3,5]. There are also studies which show that ABA can function through a cAMP/PKA-dependent mechanism [10], and this system has also been shown to positively upregulate PPAR γ [11] by increasing the affinity of PPAR γ for endogenous and synthetic ligands. Our previous studies had shown that ABA could be used preventively to suppress inflammation and improve glucose tolerance [3,5]. The main objective of this study was to assess ABA's therapeutic efficacy against diabetes and inflammation alone or in combination with the synthetic PPAR γ ligand rosiglitazone. In contrast to previous ABA studies designed with a focus on prevention and that accordingly used healthy mice at the beginning of the study, this project used mice with pre-established obesity and diabetes, thereby examining for the first time dietary ABA's therapeutic efficacy in diabetes and inflammation.
Obese db/db mice were fed high-fat diets containing Ros (0, 15, 70 mg/kg) with and without racemic ABA (100 mg/kg) for 60 days. In total, the body and tissue weights showed no enhanced affect from the combined ABA and Ros treatments. We observed that Ros at both doses significantly increased body weights, and excessive fluid retention in the interscapular region was noted in both of the groups fed Roshi. Both doses of rosiglitazone increased liver weight, and only Roshi increased inguinal SCAT weight. Thus, the significant effect of Ros in body weight gain reported in Table 1, which was observed in spite of a small effect on adiposity, could be explained by increased fluid retention and/or increased liver weight. The finding that Ros-treatment augments liver weight is consistent with previous findings from transgenic obese mice [17], and the lower SCAT mass in the Roslo group in comparison to the Roshi group may account for the increased lipid deposition in the Roslo livers. More specifically, SCAT may have acted like a sink for fat and prevented hepatic fat accumulation. In contrast to the Ros treatments, ABA did not affect liver weight and independently decreased Ab. WAT mass, suggesting that this effect of ABA was PPAR γ–independent. Even though Ros did not lower insulin levels regardless of the presence of ABA in the diet, glucose tolerance was improved. These findings suggest that the beneficial effects of Ros and ABA on glucose tolerance were mediated by the anti-inflammatory actions of these compounds. In this regard, it is well established that insulin is less effective in improving glucose tolerance in an inflamed environment. For instance TNF-α impairs insulin signaling by increasing serine phosphorylation of the insulin receptor substrate IRS-1 [18]. Alternatively, the improved glucose tolerance could be mediated by increase adiponectin, a potent insulin-sensitizing adipocytokine [19] that can decrease hepatic glucose production [20] and increase oxidation of intramuscular lipids [21]. Of note, we demonstrated that ABA increased WAT adiponectin expression [5].
Obesity is associated with low-grade chronic inflammation, but it has only been in the past five years when it was discovered that macrophages play a major role in its development [22,23]. Macrophages are essential components of the innate immune system that contribute to fighting infections but they are also involved in the genesis of chronic diseases such as diabetes, obesity and atherosclerosis. Obesity and overweight are associated with macrophage infiltration into adipose tissue [15] and a phenotypic switch from an M2 anti-inflammatory phenotype to an M1, pro-inflammatory phenotype [24,25]. Interestingly, we discovered significantly enhanced benefits from the ABA/Ros combination therapy in regard to macrophage infiltration of visceral and subcutaneous adipose tissue. More specifically, ABA in combination with Roslo was significantly more effective than either treatment alone in reducing the accumulation of ATMs in the visceral abdominal adipose tissue depot, suggesting a synergistic effect between ABA and Roslo. Additionally, ABA mitigated an increase in adipose tissue macrophages induced by the Roslo treatment in subcutaneous adipose tissue.
We also found that ABA independently increased Tregs in the blood of db/db mice. Tregs play an important role in regulating the activation and proliferation of CD4+ lymphocytes, and the obesity-induced increases in circulating leptin and interleukin-6 (IL-6) is thought to contribute to decreased Treg function [26]. In two models of chronic inflammation, the obesity and experimentally-induced colitis model, we have shown that hepatic Tregs are decreased when compared to healthy control mice [27]. Given the central role of Tregs as down-regulators of inflammation, the maintenance of this population by ABA may account, in part, for its anti-inflammatory effects.
To gain a better understanding of the individual and combined affects of ABA and Ros we isolated SVCs from the WAT of high-fat fed db/db mice, treated them with LPS for 6 hr, and assessed gene expression. The combination treatment, but not ABA or Ros alone, significantly increased PPAR γ expression. TZDs have been shown to differentially increase or decrease PPAR γ expression while still enhancing PPAR γ activity [12,28], so the result showing increased PPAR γ expression by the combination may or may not correspond to enhanced PPAR activity. Expression of M1 markers were reduced similarly by ABA, Ros and ABA/Ros treatment, suggesting that both ABA and Ros can down-modulate M1 polarization or reduce macrophage pro-inflammatory production in SVCs. In addition to inhibiting inflammation PPAR γ activation is involved in the priming of monocytes towards the alternatively activated, anti-inflammatory phenotype [29,30]. We did not observe significant changes in the M2 marker mannose receptor in the SVF, though this was perhaps due to a limited number of monocytes in the SVF.
In line with the findings in ATMs, we found a synergistic interaction between ABA and Ros in their ability to activated PPAR γ in RAW 264.7 macrophages. Given that Ros saturates PPAR γ ligand-binding domain (LBD), the fact that a synergism was observed between ABA and Ros suggests differential mechanisms of action. While Ros is known to activate PPAR γ directly by binding its LBD, ABA may activate PPAR γ indirectly through ligand-independent mechanisms [12].
Bruzzone et al recently showed that ABA significantly enhances insulin secretion in INS-1 and MIN-6 pancreatic cells through a cAMP-dependent mechanism [10]. Wantanabe has also linked the cAMP/PKA pathway to enhancing PPAR γ activity [12]. We show that ABA decreases fasting insulin levels independent of TZD and also that the ABA-induced activation of PPAR γ is inhibited by cAMP and PKA inhibition. PPAR γ activity is also increased by the cAMP activator forskolin in vitro. These findings suggest that ABA's effects on PPAR γ may be initiated though a membrane mechanism involving a G protein-coupled receptor, increased intracellular cAMP levels and PKA activation. The finding that ABA synergistically enhances PPAR γ activity in macrophages but not pre-adipocytes in vitro indicates that the synergistic effects are limited to certain types of cells. While the specific requirements behind this limitation are unclear, differential expression of co-activators and co-repressors may account for this cell type-dependency.
In conclusion, our findings demonstrate that ABA can ameliorate glucose tolerance and obesity-related inflammation in mice with pre-existing obesity/diabetes. Combination therapies containing a low-dose TZD with ABA may be more effective and safer than TZDs alone in ameliorating chronic inflammation associated with obesity. We also provide some data highlighting the possible importance of the cAMP/PKA/PPAR γ axis in mediating the beneficial effects of ABA.
Acknowledgements
Supported by a grant Supported by award number 5R01AT4308 of the National Center for Complementary and Alternative Medicine at the National Institutes of Health awarded to J.B.-R., European Commission grant number 224836, the Ramon y Cajal Program and funds from the Nutritional Immunology and Molecular Nutrition Laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No conflicts
Statement of authorship
JBR and RH designed the experiments, interpreted the results, contributed to write the paper and directed the project. AJG conducted the animal studies, contributed to write the paper and generated the laboratory results.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the united states, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: Payer- and service-specific estimates. Health Aff (Millwood) 2009 doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 3.Bassaganya-Riera J, Skoneczka J, Kingston DGJ, et al. Mechanisms of action and medicinal applications of abscisic acid. Current Medicinal Chemistry. 2009 doi: 10.2174/092986710790226110. In press. [DOI] [PubMed] [Google Scholar]
- 4.Bruzzone S, Moreschi I, Usai C, et al. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic adp-ribose as second messenger. Proc Natl Acad Sci U S A. 2007;104:5759–64. doi: 10.1073/pnas.0609379104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–16. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Guri AJ, Hontecillas R, Ferrer G, et al. Loss of ppar gamma in immune cells impairs the ability of abscisic acid to improve insulin sensitivity by suppressing monocyte chemoattractant protein-1 expression and macrophage infiltration into white adipose tissue. J Nutr Biochem. 2008;19:216–28. doi: 10.1016/j.jnutbio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (ppar gamma). J Biol Chem. 1995;270:12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 8.Marcy TR, Britton ML, Blevins SM. Second-generation thiazolidinediones and hepatotoxicity. Ann Pharmacother. 2004;38:1419–23. doi: 10.1345/aph.1E072. [DOI] [PubMed] [Google Scholar]
- 9.Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the american heart association and american diabetes association. October 7, 2003. Circulation. 2003;108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzone S, Bodrato N, Usai C, et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic adp ribose as second messenger. J Biol Chem. 2008;283:32188–97. doi: 10.1074/jbc.M802603200. [DOI] [PubMed] [Google Scholar]
- 11.Magnone M, Bruzzone S, Guida L, et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 2009 doi: 10.1074/jbc.M809546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe M, Inukai K, Katagiri H, Awata T, Oka Y, Katayama S. Regulation of ppar gamma transcriptional activity in 3t3-l1 adipocytes. Biochem Biophys Res Commun. 2003;300:429–36. doi: 10.1016/s0006-291x(02)02860-7. [DOI] [PubMed] [Google Scholar]
- 13.Hontecillas R, Diguardo M, Duran E, Orpi M, Bassaganya-Riera J. Catalpic acid decreases abdominal fat deposition, improves glucose homeostasis and upregulates ppar alpha expression in adipose tissue. Clin Nutr. 2008;27:764–72. doi: 10.1016/j.clnu.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Hontecillas R, O'Shea M, Einerhand A, Diguardo M, Bassaganya-Riera J. Activation of ppar gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr. 2009;28:184–95. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- 15.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. Ppar gamma is highly expressed in f4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol. 2009;258:138–46. doi: 10.1016/j.cellimm.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassaganya-Riera J, Reynolds K, Martino-Catt S, et al. Activation of ppar gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–91. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 17.Walczak R, Tontonoz P. Pparadigms and pparadoxes: Expanding roles for ppargamma in the control of lipid metabolism. J Lipid Res. 2002;43:177–86. [PubMed] [Google Scholar]
- 18.Zick Y. Insulin resistance: A phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 2001;11:437–41. doi: 10.1016/s0962-8924(01)02129-8. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial nf-kappab signaling through a camp-dependent pathway. Circulation. 2000;102:1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 20.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 21.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kda adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang K, Reilly SM, Karabacak V, et al. Adipocyte-derived th2 cytokines and myeloid ppardelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–95. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative m2 activation of kupffer cells by ppardelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: Does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–13. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 27.Bassaganya-Riera J, Ferrer G, Casagran O, et al. F4/80hiccr2hi macrophage infiltration into the intra-abdominal fat worsens the severity of experimental ibd in obese mice with dss colitis. e-SPEN, The European e-Journal of Clinical Nutrition and Metabolism. 2009;4:90–7. [Google Scholar]
- 28.Suzuki A, Yasuno T, Kojo H, Hirosumi J, Mutoh S, Notsu Y. Alteration in expression profiles of a series of diabetes-related genes in db/db mice following treatment with thiazolidinediones. Jpn J Pharmacol. 2000;84:113–23. doi: 10.1254/jjp.84.113. [DOI] [PubMed] [Google Scholar]
- 29.Bouhlel MA, Derudas B, Rigamonti E, et al. Ppargamma activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific ppargamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]