Abstract
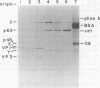
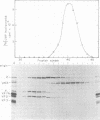
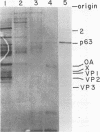
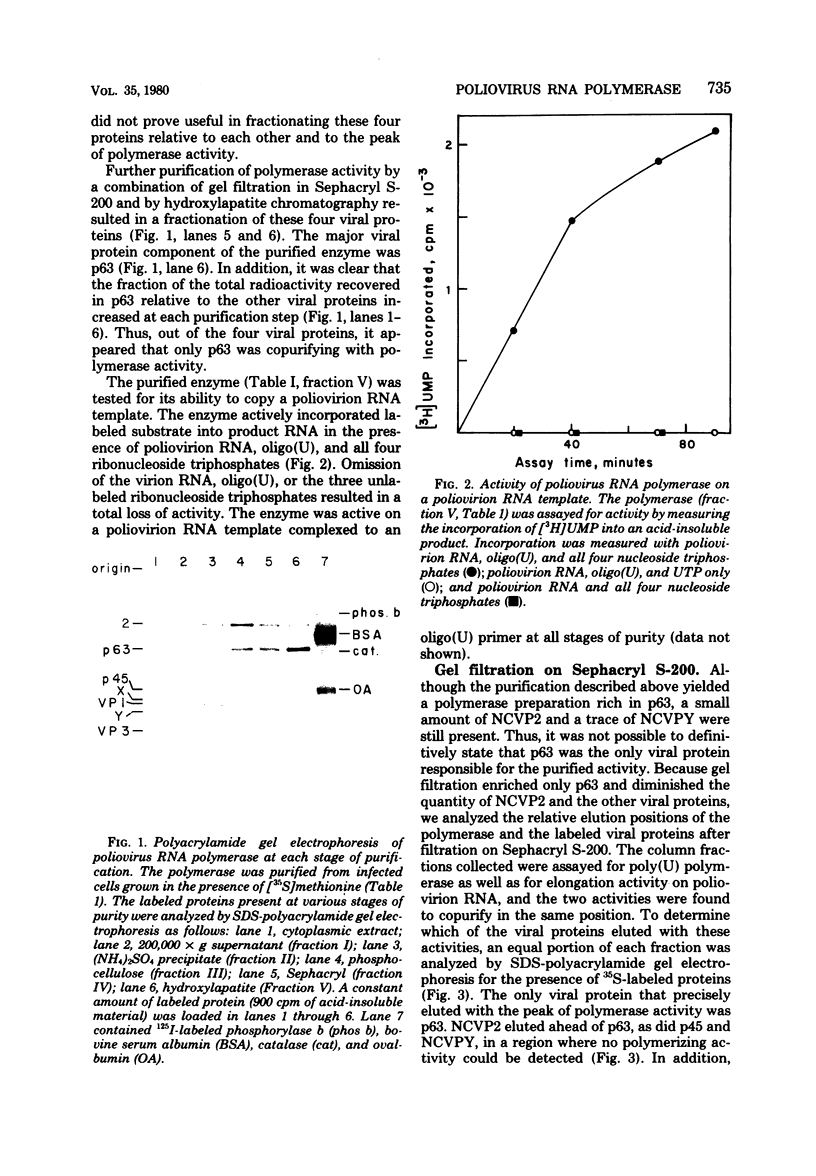
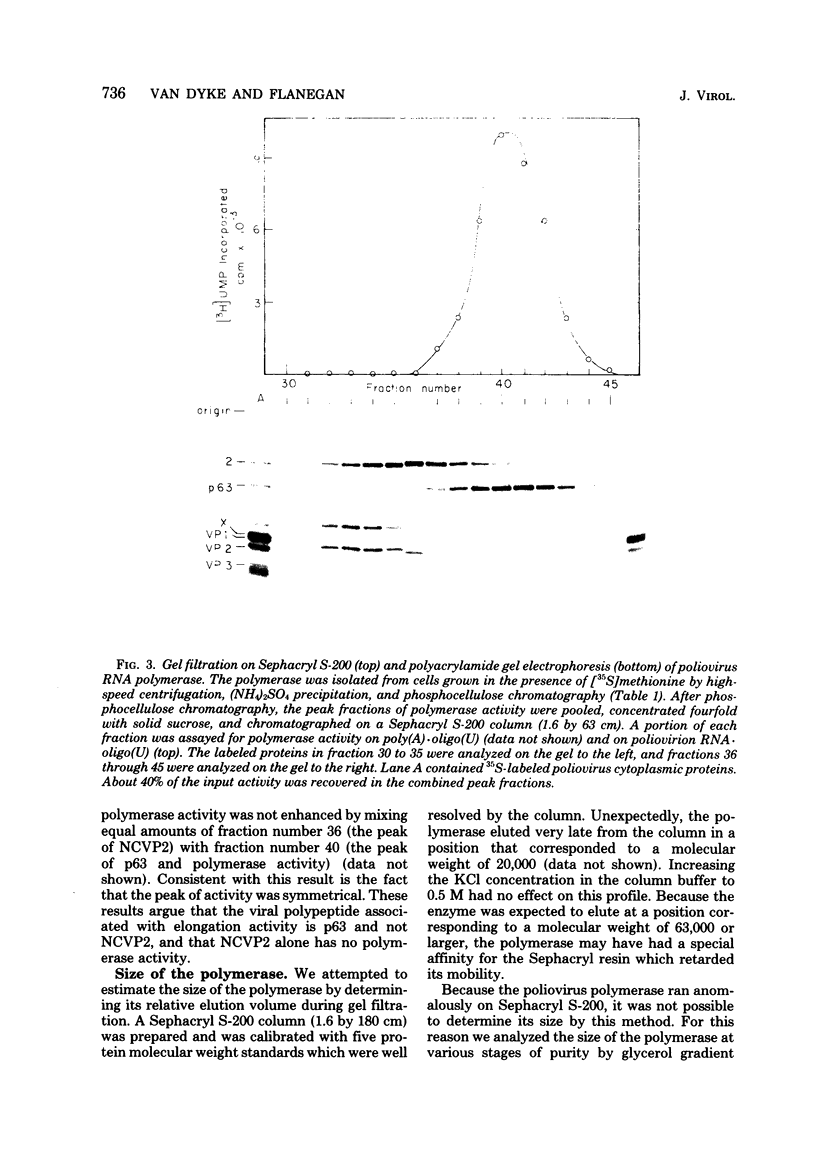
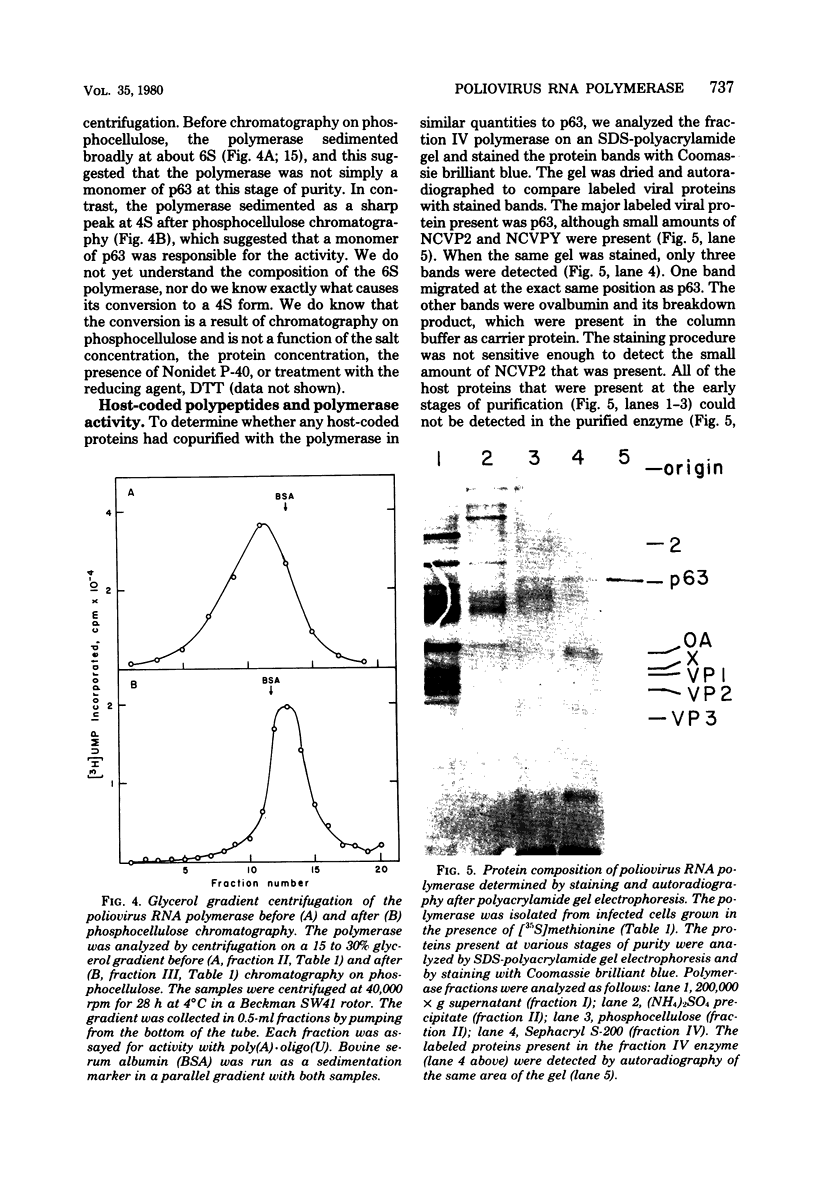
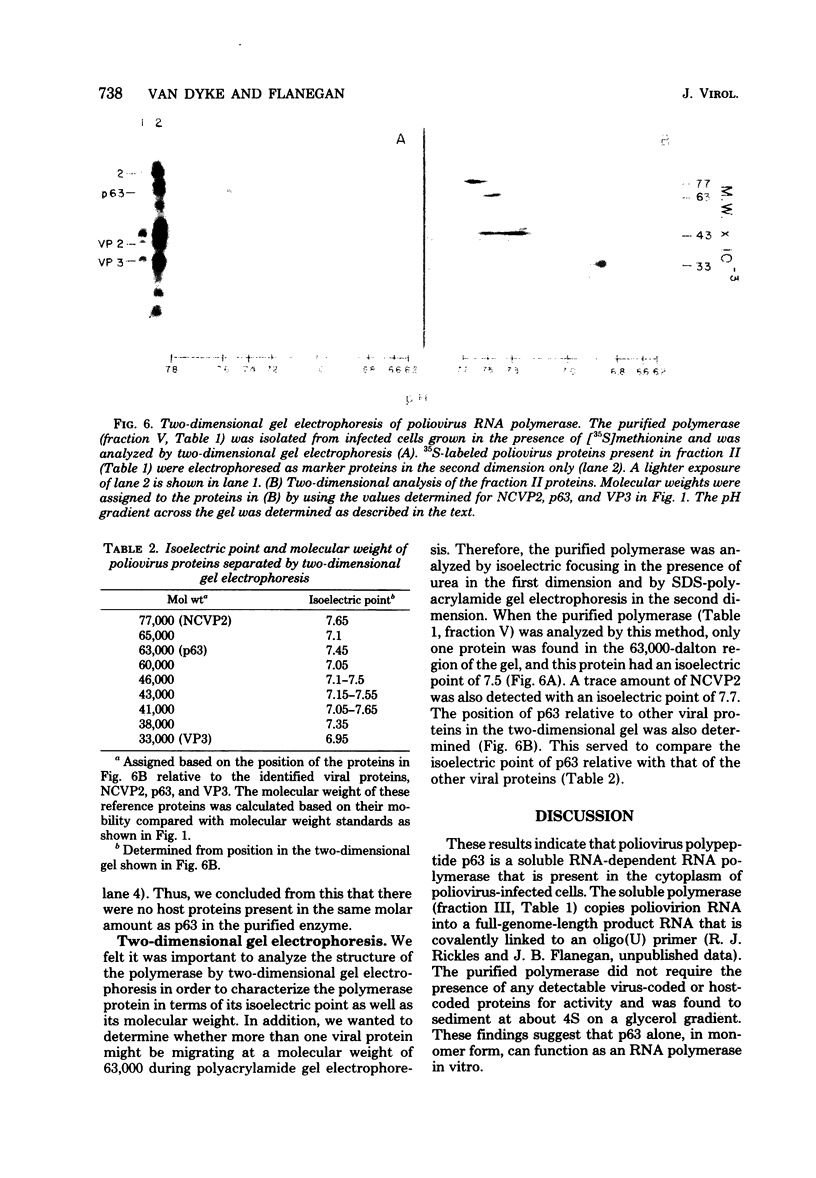
A poliovirus-specific RNA-dependent RNA polymerase was isolated from a cytoplasmic extract of infected HeLa cells and was shown to copurify with a single virus-specific protein. The polymerase was isolated from cells labeled with [35S]-methionine and was fractionated from other soluble cytoplasmic proteins by ammonium sulfate precipitation, phosphocellulose chromatography, gel filtration on Sephacryl S-200, and chromatography on hydroxylapatite. The activity of the enzyme was measured by using either polyadenylic acid or poliovirion RNA as a template in the presence of an oligouridylic acid primer. A single virus-specific protein that had an apparent molecular weight of 63,000 (p63) was found to copurify with this activity. Host-coded proteins were present in reduced molar amounts relative to p63. Noncapsid viral protein 2 (NCVP2) and other viral proteins were clearly separated from p63 by gel filtration on Sephacryl S-200. Polymerase activity coeluted from the column precisely with p63. NCVP2 was totally inactive as an RNA polymerase and did not stimulate the polymerase activity of p63. The purified enzyme sedimented at about 4S on a glycerol gradient and thus appeared to be a monomer of p63. Two-dimensional gel electrophoresis of the polymerase protein indicated that it had an isoelectric point of about 7.5. Thus, the viral polypeptide, p63, as defined by the above physical parameters, is an RNA-dependent RNA polymerase that can copy poliovirion RNA when oligouridylic acid is used as a primer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTIMORE D., EGGERS H. J., FRANKLIN R. M., TAMM I. Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proc Natl Acad Sci U S A. 1963 Jun;49:843–849. doi: 10.1073/pnas.49.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Girard M. An intermediate in the synthesis of poliovirus RNA. Proc Natl Acad Sci U S A. 1966 Aug;56(2):741–748. doi: 10.1073/pnas.56.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowles S. A., Tershak D. R. Proteolysis of noncapsid protein 2 of type 3 poliovirus at the restrictive temperature: breakdown of noncapsid protein 2 correlates with loss of RNA synthesis. J Virol. 1978 Aug;27(2):443–448. doi: 10.1128/jvi.27.2.443-448.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Shimshick E. J., Yin F. H. Association of the polioviral RNA polymerase complex with phospholipid membranes. J Virol. 1976 Aug;19(2):457–466. doi: 10.1128/jvi.19.2.457-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Baron M. H., Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Lund H. Untranslated vesicular stomatitis virus messenger RNA after poliovirus infection. Virology. 1977 Jul 15;80(2):297–308. doi: 10.1016/s0042-6822(77)80006-8. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Van Dyke T. A. Isolation of a soluble and template-dependent poliovirus RNA polymerase that copies virion RNA in vitro. J Virol. 1979 Oct;32(1):155–161. doi: 10.1128/jvi.32.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M. In vitro synthesis of poliovirus ribonucleic acid: role of the replicative intermediate. J Virol. 1969 Apr;3(4):376–384. doi: 10.1128/jvi.3.4.376-384.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst M. N., Roberts R. M. Analysis of polypeptide turnover rates in Chinese hamster ovary cell plasma membranes using two-dimensional electrophoresis. J Biol Chem. 1979 Jun 25;254(12):5000–5007. [PubMed] [Google Scholar]
- Lundquist R. E., Ehrenfeld E., Maizel J. V., Jr Isolation of a viral polypeptide associated with poliovirus RNA polymerase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4773–4777. doi: 10.1073/pnas.71.12.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R. E., Maizel J. V., Jr In vivo regulation of the poliovirus RNA polymerase. Virology. 1978 Sep;89(2):484–493. doi: 10.1016/0042-6822(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Lundquist R. E., Maizel J. V., Jr Structural studies of the RNA component of the poliovirus replication complex. I. Purification and biochemical characterization. Virology. 1978 Apr;85(2):434–444. doi: 10.1016/0042-6822(78)90450-6. [DOI] [PubMed] [Google Scholar]
- Meyer J., Lundquist R. E., Maizel J. V., Jr Structural studies of the RNA component of the poliovirus replication complex. II. Characterization by electron microscopy and autoradiography. Virology. 1978 Apr;85(2):445–455. doi: 10.1016/0042-6822(78)90451-8. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., Guttman N., Baltimore D., Lodishi H. F. Complete translation of poliovirus RNA in a eukaryotic cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4157–4161. doi: 10.1073/pnas.72.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]