Abstract
The common food additive carrageenan predictably induces intestinal inflammation in animal models. Mechanisms of carrageenan-induced NFκB and IL-8 stimulation include an immune-mediated pathway involving toll-like receptor 4 (TLR4) and B-cell lymphoma/leukemia 10 (BCL10) and a reactive-oxygen species (ROS)-mediated pathway. To determine how the structure of CGN contributes to its initiation of inflammation by these two distinct mechanisms, we treated carrageenans with galactosidases and carrageenases, and determined the impact on IL-8 secretion and BCL10 production. Hydrolysis of carrageenan by the enzyme α-1→(3,6)-galactosidase significantly reduced the increases in IL-8 and BCL10, but other galactosidases tested, including α-1→6-, β-1→4-, and β-1→3,6-galactosidases had no effect. In contrast, specific κ- or ι-carrageenases, which hydrolyze the β-1,4-galactosidic bonds, produced increases in IL-8 and BCL10, attributable to increased exposure of the immunogenic α-1→3-galactosidic epitope of carrageenan to TLR4. These results were consistent with induction of the innate immune response by an interaction of TLR4 with the unusual α-D-Gal-(1→3)-D-Gal epitope that is present in carrageenan. Activation of the ROS-mediated pathway was unaffected by treatment of κ-CGN with either κ-CGNase (3 mg/L), α-1→(3,6)-galactosidase (20 mU/ml), or these enzymes in combination, indicating that the changes in IL-8 production were attributable to effects on the TLR4-BCL10-mediated innate immune pathway of induction of inflammation. These findings provide new information about the specificity of the carbohydrate-protein interaction between carrageenan and TLR4 and may help to devise treatments that modify the immune reactivity induced by carbohydrate antigens.
Keywords: carrageenan, inflammation, food additive, galactosidase, carrageenase
Introduction
The sulfated polysaccharide carrageenan (CGN) has a long history of use as a food additive in the Western diet [1,2]. CGN, obtained from several species of red algae, is frequently incorporated into processed foods to improve texture and solubility. CGN consumption has increased steadily in recent decades [3,4]. In finished food products, it exists predominantly as an undegraded sulfated polysaccharide of molecular weight over 100,000, although contamination by lower molecular weight forms is common [5]. Processes such as heating, acid treatment, mechanical effects, and bacterial digestion can all lead to the production of degraded, lower molecular weight forms of CGN [1,2,6,7].
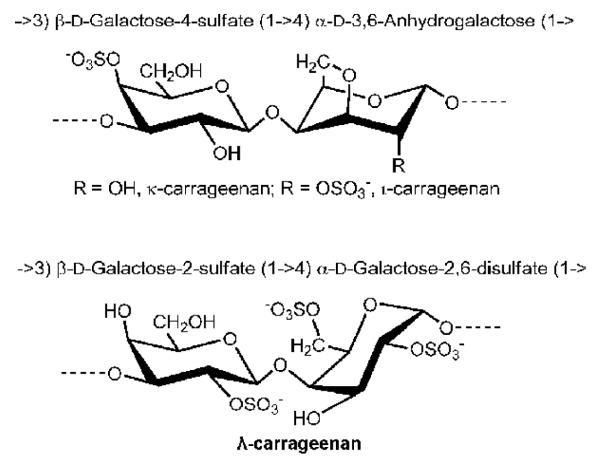
The major forms of CGN have in common their basic structure, consisting of an ideal disaccharide unit, composed of D-galactose residues alternatively linked by a β-1→4 and α-1→3 glycosidic linkages (G and D units, respectively). Carrageenans are classified according to the number and the position of sulfated ester (S) and by the occurrence of 3,6 anhydro-bridges in the α-linked residues (DA unit) Three major varieties are incorporated in food products, in various combinations: kappa- (κ, DAG4S), iota- (ι, DA2S-G4S), and lambda- (λ, D2S6S-G2S) carrageenans (Figure 1) [8,9]. κ- and ι-carrageenans form thermoreversible gels in aqueous solutions, the rigidity of which decreases strongly with the degree of sulfation. In contrast, λ-carrageenans do not feature 3,6-anhydro bridges and do not make physical gels, but highly viscous solutions [8,10]. These differences may confer biochemical reactivity and mimicry on the CGNs in ways not yet determined, since CGN resembles the naturally occurring sulfated glycosaminoglycans (sGAGs). In particular, CGN resembles the sGAGs chondroitin sulfate, dermatan sulfate, and keratan sulfate, which also contain sulfated galactose or modified galactose residues. Unlike the naturally occurring GAGs which have β-1→4 and β-1→3 linkages, CGN possesses the unusual α-1→3 galactosidic bond. This structure is recognized as an immune epitope, since it is foreign to humans and large apes which lack the enzyme α-1,3-galactosyltransferase. The anti-Gal antibody is an antibody that is found universally in human cells and recognizes the α-D-Gal-(1→3)-D-Gal linkage [11-15].
Figure 1. Sulfated di-galactose structure of carrageenan.
The repeating disaccharide structural unit of carrageenan is demonstrated. β-1→4- and α-1→3 galactosidic linkages connect galactose residues. Sites of sulfation differ among the three major types of carrageenan.
CGN exposure predictably induces increase in IL-8 secretion, in cells in tissue culture or tissues from human or animal colon [16-18]. Mechanisms of CGN-induced IL-8 activation require nuclear localization of NFκB, and proceed by at least two different pathways: a toll-like receptor-4 (TLR4)-BCL10-mediated pathway and a reactive oxygen species (ROS)-mediated pathway [17,18]. These pathways appear to activate different components of the IKK (Ikappa B kinase) signalosome, influencing NFκB either by effects predominantly on either IKKβ or IKKγ [19,20].
As we elucidate the biological effects of CGN, one of the essential considerations is how modification of specific structural features of CGN impacts on its biological reactivity. To evaluate the relationship between specific structural features of CGN and cellular responses, we performed experiments to determine how degradation of CGN by galactosidases and carrageenases alters the CGN-induced stimulation of IL-8 and BCL10. We measured IL-8 secretion and BCL10 production following pretreatment of CGNs by these enzymes and have documented the changes in CGN by gel electrophoresis, to better understand how the specific chemical structure of CGN contributes to the inflammatory response. We present these findings in this report.
Methods and Materials
Cell Culture
The NCM460 cell line is a normal human colonic mucosal epithelial cell line that is nontransfected and nonmalignant. It was originally derived from the normal colon mucosa of a 68-year-old Hispanic male [21]. Cells used in these experiments were grown in M3:10 medium (INCELL, San Antonio, TX) and maintained at 37°C in a humidified, 5% CO2 environment, with changes of medium twice weekly. Confluent cells in T-25 flasks were harvested by EDTA-trypsin and sub-cultured in six or twelve-well tissue culture plates. Cells were treated for 24 hours with λ(lambda)-, κ(kappa)-, κ8 (eight disaccharide fragment of κ-CGN) [22] or ι(iota)-CGN, at a concentration of (1 mg/L), either without pre-digestion or pre-digested × 24 hours with carrageenase or galactosidase, either singly or in combination. At the end of the treatment, spent media were collected from control and treated wells and stored at −80°C until further analysis. Cells were harvested by scraping, and total cell protein was measured by BCA™ protein assay kit (Pierce, Rockford, IL), using bovine serum albumin as standard.
Toll-like receptor 4 (TLR4) blocking antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 20 mg/L was added to some of the NCM460 cell preparations for 24 hours, while incubated at 37°C in a humidified incubator with 5% CO2 , prior to treatment with ΚCGN that was pre-digested by enzymes (see below).
Digestion of κ-CGN and i-CGN
κ-carrageenase and i-carrageenase were gifts from Dr. Michel Gurvan [23-25]. Carrageenases, which cleave the β-1→4 linkages of the β-1→4-α-1→3 galactans, were used at a concentration of 3 mg/L to digest 1 mg/L carrageenan for 24 hours prior to exposure of the NCM460 cells. The κ-carrageenase (κ-CGNase) was prepared at a concentration of 2 g/L in a buffer of 50mM Tris-HCl pH 7.5, 100 mM NaCl. The ι-carrageenase (ι-CGNase) was at a concentration of 0.6 g/L in a buffer of 50 mM Tris-HCl pH 7.5, 100 mM NaCl, 5 mM CaCl2 and 5% glycerol. Enzymes were stored at 4°C. Carrageenase at a concentration of 3 mg/L was used to digest 1 mg of carrageenan. Subsequently, cells were treated with the digested CGN at a concentration of 1 mg/L, in 5% CO2 environment for 24 hours (unless stated otherwise).
The galactosidases, recombinant α-1→(3,6)-galactosidase from E. coli (10 - 200 U/L; Calbiochem, EMD Chemicals, Inc., Gibbstown, NJ); β-1→(3,6)-galactosidase (20 U/L, Calbiochem), α-1→6-galactosidase (20 U/L; Sigmal Chemical Co., St. Louis, MO) and β-1→4-galactosidase (6 - 20 U/L; Sigma) were obtained and combined with CGN for pre-digestion at 37°C, in 5% CO2 environment for 24 hours, and then the mixture was added to the NCM460 cells grown in microwells of 12-well tissue culture plates.
κ, κ8 and i-CGN were digested with galactosidase and/or carrageenase, either alone or in combination. Control wells received only the enzymes incubated under similar conditions for 24 hours. After 24 hours of exposure to the CGN-enzyme mixture, the spent media were collected and assayed for IL-8, and cells were harvested, lysed and assayed for either total cell protein or BCLl10.
ELISA for IL-8
The secretion of IL-8 in the spent media of control and treated NCM460 cells was determined by the DuoSet ELISA kit for human IL-8 (R&D Systems, Minneapolis, MN), as previously reported [16]. Microtiter plates were precoated with specific anti-IL-8 monoclonal antibody to capture the IL-8 in the spent media, and IL-8 bound to the antibody coated wells of microtiter plate was detected by biotin-conjugated secondary IL-8 antibody and streptavidin-horseradish peroxidase (HRP). Hydrogen peroxide-tetramethylbenzidine chromogenic substrate was used to develop the color and measure the bound-HRP. The intensity of color was read at 450 nm with a reference filter of 570 nm in an ELISA plate reader (SLT, Spectra), and was proportional to the HRP activity and IL-8 concentration in the particular sample. The IL-8 concentrations were extrapolated from a standard curve plotted by using known concentrations of IL-8. The sample values were normalized with total protein content (BCA™ Protein assay kit; Pierce) and expressed as picograms per milligram (ng/g) cellular protein.
ELISA for BCL10
BCL10 in control and treated NCM460 cells was determined by a solid-phase sandwich ELISA, the development of which was previously reported [26]. Control and treated cells were lysed in RIPA buffer (50 mM Tris-HCl containing 0.15 M NaCl, 1% Nonidet P40, 0.5% deoxycholic acid and 0.1% SDS, pH 7.4), and the cell extracts were stored at −80°C until assayed. BCL10 molecules in the samples or standards were captured in the wells of a microtiter plate precoated with rabbit polyclonal antibody to BCL10 (QED Bioscience, San Diego, CA). Immobilized BCL10 molecules were detected by a mouse monoclonal antibody to BCL10 (Novus Biologicals, Littleton, CO) and by goat anti-mouse IgG-HRP complex (Santa Cruz Biotechnology, Santa Cruz, CA). The peroxidase enzyme activity bound to BCL10 molecules was determined by chromogenic reaction with hydrogen peroxide-tetramethylbenzidine. Color development due to enzymatic activity was stopped by 2N sulfuric acid, and intensity of the color was measured at 450 nm in ELISA plate reader (SLT, Spectra). BCL10 concentrations were extrapolated using a standard curve that was derived from known concentrations of recombinant BCL10 (Calbiochem, EMD Bioscience, San Diego, CA). Sample values were normalized with the total cell protein concentrations determined by BCA™ protein assay kit (Pierce).
Polyacrylamide Gel Electroforesis following Digestion of κ-CGN by Galactosidases and/or Carrageenase
Heparin oligosaccharide standards and κ-CGN (100 mg/L) samples were run on 15% PAGE and stained with alcian blue. The five samples were: undigested κ-CGN, κ-CGN treated with α-1→(3,6)-galactosidase (20 U/L), κ-CGN treated with α-1→6-galactosidase (20 U/L), κ-CGN treated with κ-carrageenase (3 mg/L), and κ-CGN treated with κ-carrageenase (3 mg/L) and α-1→(3,6)-galactosidase (20 U/L).
Measurement of Reactive Oxygen Species (ROS)
The production of reactive oxygen species (ROS) by DSS was measured by hydroethidine fluorescence [27]. Cells were grown in a 96-well cell culture plate. After 24 hours, the medium was changed and treatment with κ-CGN (1 mg/L × 24 hours) or κCGN pre-digested × 24 hours with α-1→(3,6)-galactosidase (20 U/L and 100 U/L), or κ-CGNase (3 mg/L), separately, and in combination, was started. At the end of the treatment period, the medium in each well was removed, cells were washed with Hank’s Balanced Salt Solution (HBSS), and cells were covered with 200 μL of HBSS containing 10 μM dihydroethidine (Sigma). Cultures were incubated for 1 hour at 37°C, 5% CO2 then the medium was removed, and replaced with fresh HBSS (200 μL/well). The intracellular HE fluorescence was measured using a microplate fluorescence reader (FL600, Bio-Tek Instruments, Inc., Winooshi, VT) at 488 nm excitation wavelength with a 610-nm emission filter.
Statistical analysis
Data are the mean ± standard deviation (SD) of at least three biological replicates and two technical duplicates of each determination, unless stated otherwise. Statistical significance was determined by one-way ANOVA with Tukey-Kramer post-test for multiple comparisons using Instat software, unless stated otherwise. Two-tail t-test ,performed using Microsoft Excel software, was used for some comparisons. P-values <0.05 are considered statistically significant. Correlation coefficient of BCL10 and IL-8 values following exposure to α-1→(3,6)-galactosidase of different concentrations was calculated by linear regression using Excel software and linear trend of Bcl10 and IL-8 with increasing concentrations of α-1→(3,6)-galactosidase was calculated using Instat software. In the figures, * represents p≤0.05; ** represents p≤0.01, and *** represents p≤0.001.
Results
IL-8 production by different CGNs
In a series of experiments testing different forms of CGN, including lambda, (λ) kappa (κ), and iota (ι) (Figure 1) of high molecular weight (>1 × 106 g/mol MW) and degraded forms of κ-CGN of lower molecular weight, we have detected differences in the CGN-induced secretion of IL-8 by NCM460 cells in culture. These results are presented in Table 1.
Table I.
IL-8 Secreted by NCM460 Cells in Response to ι-, κ-, and short κ-CGNs following pre-treatment by Carrageenase or Galactosidase
| EXPOSURE* | IL-8 [pg/mg cell protein ± (standard deviation)] |
|---|---|
| Control | 616 (17) |
| λ-CGN | 1273 (30) |
| ιCGN (4 h exposure) | 924 (47) |
| ιCGN | 950 (48) |
| ιCGNase | 748 (16) |
| ιCGN (4 h exposure) + ιCGNase | 1375 (88) |
| ιCGN + ιCGNase | 1509 (91) |
| ιCGN + α-1→(3,6)-galactosidase (20 U/L) | 795 (27) |
| ιCGN + ιCGNase + α-1→(3,6)-GAL (20 U/L) | 1340 (92) |
| κ-CGN | 1011 (52) |
| κ-CGN (3 mg/L) | 1060 (42) |
| κCGN + β-1→4-galactosidase | 1016 (70) |
| κCGN + α-1→6-galactosidase | 1014 (17) |
| κCGN + α-1→6-galactosidase + β-1→4-galactosidase | 1020 (64) |
| κCGN + α-1→(3,6)-galactosidase (20 U/L) | 896 (22) |
| κCGN + α-1→(3,6)-galactosidase (50 U/L) | 782 (27) |
| κCGN + α-1→(3,6)-galactosidase (100 U/L) | 698 (23) |
| κCGN + α-1→(3,6)-galactosidase (200 U/L) | 676 (50) |
| κCGN + α-1→(3,6)-galactosidase (20 U/L) + β-1→4-galactosidase | 891 (4) |
| κCGN + κ-CGNase | 1623 (54) |
| κCGN + κ-CGNase + β-1→4-galactosidase | 1629 (33) |
| κCGN + α-1→(3,6)-galactosidase (20 U/L) + κ-CGNase | 1431 (15) |
| κCGN + α-1→(3,6)-galactosidase (50 U/L) + κ-CGNase | 1245 (57) |
| κCGN + α-1→(3,6)-galactosidase (100 U/L) + κ-CGNase | 1105 (22) |
| κ-CGN (3 mg/L) ~1500 MW | 1596 (72) |
| κ-CGN (3 mg/L) ~4000 MW | 1737 (63) |
| κ-CGN (3 mg/L) ~8000 MW | 1605 (41) |
| κCGN8 | 1712 (68) |
| κCGN8 + β-1→4-galactosidase | 1744 (121) |
| κCGN8 + α-1→6-galactosidase | 1715 (74) |
| κCGN8 + α-1→6-galactosidase + β-1→4-galactosidase | 1732 (121) |
| κCGN8 + α-1→(3,6)-galactosidase (20 U/L) | 1408 (41) |
| κCGN8 α-1→(3,6)-galactosidase (20 U/L) + β-1→4-galactosidase | 1399 (49) |
| κCGN8 + κCGNase | 1822 (58) |
| κCGN8 + κCGNase + α-1→(3,6)-galactosidase (20 U/L) | 1514 (38) |
- Unless stated otherwise, the concentration of CGN was 1 mg/L, the exposure was for 24 hours, and data are the mean ± (S.D.) of at least 3 biological replicates with technical duplicates. Enzyme concentrations are expressed as units/liter (U/L), based on their established biological reactivity with their standard substrate.
IL-8 production by κ-CGNs of different disaccharide chain length, including 3, 8, and 16 disaccharide pairs (of MW ~1500, ~4000, and ~8000) was compared. The shorter, degraded CGNs induced a higher IL-8 response, consistent with increased exposure of the α-1→3-epitope.
The λCGN produced a higher IL-8 response than κ- (p<0.01) or ι-CGN (p<0.001), perhaps attributable to a more favorable structure with less internal bonding between C3 and C6 of the non-sulfated saccharide, producing the pyranose epimer.
Carrageenases increase IL-8 response
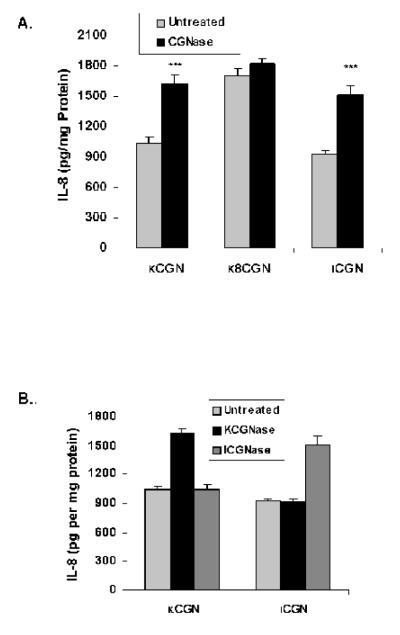
CGN (1 mg/L) was pre-treated for 24 hours with purified κ-carrageenase or ι-carrageenase (3 mg/L), enzymes highly specified for cleavage of the β-1→4 linkages of β-1→4-α-1→3 galactans that are present in κ-CGN or λ-CGN. NCM460 cells were treated with these preparations for 24 hours, and the secreted IL-8 was measured. Following treatment with carrageenase, IL-8 secretion increased significantly (Figure 2A; p<0.001, paired t-test). In the short, degraded κ-CGN, composed of 8 disaccharides, the baseline IL-8 secretion was higher, and the increase in IL-8 following treatment with carrageenase was less than with the undegraded κ-CGN. These differences suggest that there was greater exposure of the cells to the immune epitope in the degraded carrageenan at baseline, and that there was less of an increase in interaction between the immune epitope and the toll-like receptor 4 (TLR4) following carrageenase treatment of the already degraded CGN. The impact of the carrageenases was specific to the kappa or lambda form, with no evidence of cross-reactivity (Figure 2B).
Figure 2. Increased IL-8 response to CGN following treatment with carrageenase.
A. NCM460 cells were exposed to either κ-CGN, short κ8CGN, or ι-CGN that had been pre-treated with κ- or ι-carrageenase (3 mg/L) for 24 hours. Significant increases in IL-8 production occurred for κ-CGN and ι-CGN (p<0.001, paired t-test), but not for the short κ-carrageenan comprised of 8 disaccharides. These changes suggest that digestion with carrageenase led to increased exposure of the immunogenic epitope in the undegraded carrageenans, and that the impact of digestion by carrageenase was less in the already degraded, short κ-CGN. Data presented are the mean ± S.D. and are representative of 6 independent biological experiments with technical duplicates of each determination. (CGN=carrageenan)
B. Pre-treatment of κ-CGN with ι-carrageenase or of ι-CGN with κ-carrageenase produced no changes in the IL-8 responses, consistent with specificity of the carrageenases for specific forms of carrageenan. Data are the mean ± S.D. of 3 independent biological experiments with technical replicates of each.
Only α-1→(3,6)-galactosidase has effect on IL-8 secretion
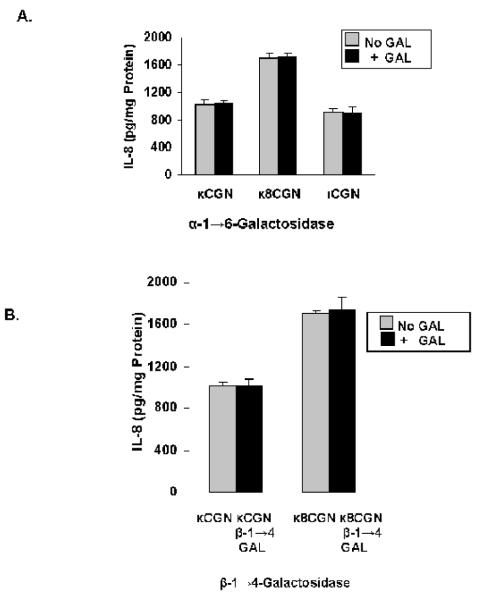
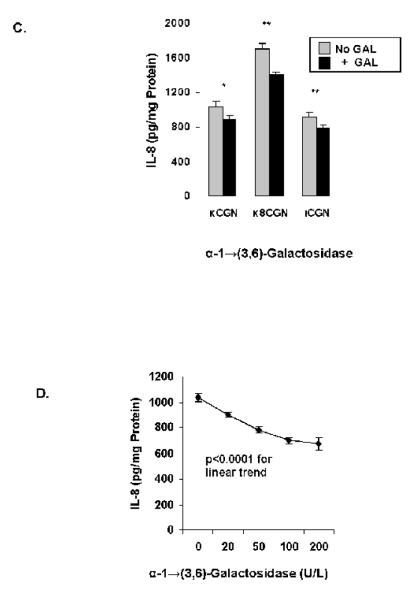
NCM460 cells were treated for 24 hours with κ-CGN or ι-CGN (1 mg/L) that had been combined with one of three different galactosidases, either α-1→6-galactosidase (20 U/L), β-1→4-galactosidase (20 U/L), β-1→(3,6)-galactosidase (20 U/L), or α-1→(3,6)-galactosidase for 24 hours (20 U/L). Results demonstrated no effect of either α-1→6-galactosidase (Figure 3A), β-1→4-galactosidase (Figure 3B), or β-1→(3,6)-galactosidase on the IL-8 response (not shown), in contrast to significant declines in association with exposure to α-1→(3,6)-galactosidase (Figure 3C). A dose-response effect was evident between the concentration of α-1→(3,6)-galactosidase and the secreted IL-8 (Figure 3D) (p<0.001, 1-way ANOVA with Tukey-Kramer post-test).
Figure 3. Only galactosidase that hydrolyzed the α-1→3-galactosidic bond produced decline in IL-8 production.
A. Pre-treatment of carrageenans by α-1→6-galactosidase (20 U/L) had no effect on IL-8 secretion in the NCM460 cells. Data are the mean ± S.D. and are representative of 9 independent biological experiments with technical replicates of each. (CGN=carrageenan)
B. Pre-treatment by β-1→4-galactosidase (20 U/L) of κ-carrageenan and short κ8 CGN had no effect on IL-8 secretion in the NCM460 cells. Data are the mean ± S.D. and are representative of 9 independent biological experiments with technical replicates of each. (Gal=galactosidase)
C. Pre-treatment of carrageenans by α-1→(3,6)-galactosidase (20 U/L) produced significant declines (p=0.03 for κCGN; p=0.003 for κ8CGN; p=0.008 for ιCGN by paired t-tests) in IL-8 secretion in the NCM460 cells, indicating the importance of the Gal-α-(1→3)-Gal epitope for the carrageenan-induced IL-8 response. Data are the mean ± S.D. and are representative of 14 independent biological experiments with technical duplicates for κ-CGN and n=6 for short κ8CGN and ι-CGN.
D. IL-8 secretion increased following exposure to increasing concentrations of α-1→(3,6)-galactosidase (20 U/L to 200 U/L) in the NCM460 cells (p<0.001, 1-way ANOVA with Tukey-Kramer post-test and p<0.0001 for linear trend). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
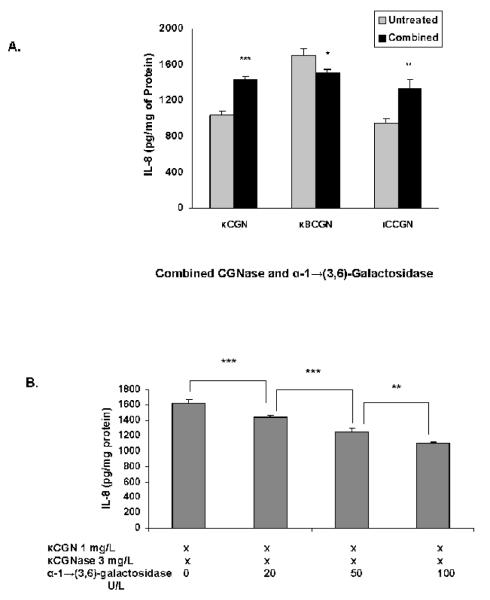
Effect of combined α-1→(3,6)-Galactosidase and Carrageenase
NCM460 cells were exposed to carrageenans that were pretreated with a combination of κ-CGNase (3 mg/L) and α-1→(3,6)-galactosidase (20 U/L) (Figure 4A). At this concentration of α-1→(3,6)-galactosidase, IL-8 secretion rose significantly following exposure to the undegraded κ- and ι-carrageenans, and declined following degraded κ8-carrageenan. As the concentration of α-1→(3,6)-galactosidase increased from 20 U/L to 100 U/L, the increase in the IL-8 secretion induced by exposure of the κ-CGN to carrageenase declined significantly (Figure 4B; p<0.001, 1-way ANOVA with Tukey-Kramer post-test). These changes were consistent with altered exposure to the α-Gal-(1→3)-Gal configuration by enzymatic digestion of the carrageeenan.
Figure 4. Effect of combined exposure to carrageenase and α-1→(3,6)-galactosidase.
A. Specific κ- or ι-carrageenase (3 mg/L) and α-1→(3,6)-galactosidase (20 U/L) were combined to pre-treat carrageenan for 24 hours. IL-8 secretion increased when NCM460 cells were exposed to undegraded κ- or ι- carrageenan, reflecting a predominant effect of the carrageenase. In contrast, in the short, degraded κ-carrageenan (κ8CGN), the combination resulted in decline in IL-8 secretion, indicating an increased effect of the α-1→(3,6)-galactosidase. Differences in IL-8 are statistically significant (p=0.0004 for κCGN; p=0.02 for κ8CGN; p=0.003 for ιCGN, by paired t-tests). Data are the mean ± S.D. of 9 independent biological experiments with technical duplicates of each determination for κ-CGN and n=6 for short κ8CGN and ι-CGN. (CGN=carrageenan)
B. Dose-response to α-1→(3,6)-galactosidase was evident, since secreted IL-8 declined with exposure of undegraded κ-carrageenan to increasing concentrations of α-1→(3,6)-galactosidase, including 20, 50, and 100 U/L in the presence of κ-carrageenase (3 mg/L) (p<0.001, for differences between 0 and 20 U/L and 20 and 50 U/L and p<0.01 for difference between 50 and 100 U/L,1-way ANOVA, with Tukey-Kramer post-test). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
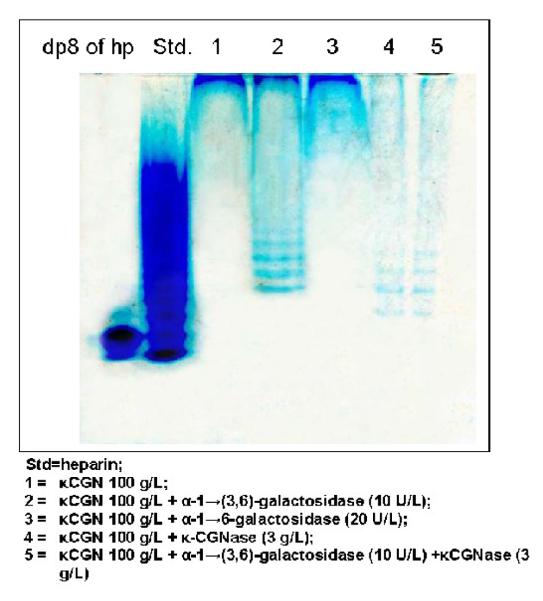
PAGE of κCGN digestion products
Samples of κ-CGN were exposed to galactosidases and/or κ-carrageenase for 24 hours, and digestion products were analyzed by PAGE. Undegraded oligosaccharides were present in samples 1 and 3, representing undegraded, untreated κ-CGN and κ-CGN exposed to α-1→6-galactosidase, respectively (Figure 5). Lower molecular weight oligosaccharides were observed in samples 2, 4, and 5, indicating degradation by enzymatic treatment. Sample 2, which was exposed to α-1→(3,6)-galactosidase, had polysaccharide remaining at the top of the gel, indicating incomplete degradation. In contrast, in samples 4 and 5, only a small amount of polysaccharide remained at the top of the gel, consistent with much more complete degradation than in sample 2. Lower molecular weight products were present in samples 4 and 5 than in sample 2. Sample 3 was resistant to degradation by the α-1→6-galactosidase.
Figure 5. Demonstration of κCGN degradation products following enzymatic digestion by PAGE.
Digestion products of heparin are presented as molecular weight standards (Std). κ-CGN of high molecular weight without exposure to enzymes is in lane 1. Lane 2 is κ-CGN digested by α-1→(3,6)-galactosidase; lane 3 is κ-CGN un-digested by α-1→6-galactosidase; lane 4 is κ-CGN digested by κ-carrageenase; and lane 5 is κCGN digested by a combination of κ-carrageenase and α-1→(3,6)-galactosidase. Lower molecular weight products are shown in lanes 4 and 5 than in lane 2. In the experiments with the NCM460 cells, κ-carrageenase was used at a ten-fold lower concentration relative to the amount of carrageenan than in this analysis.
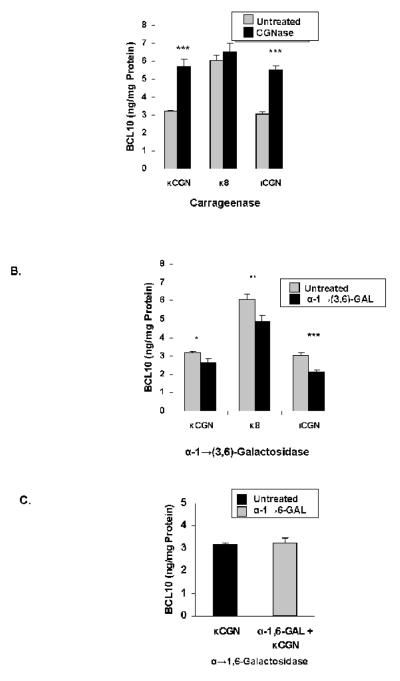
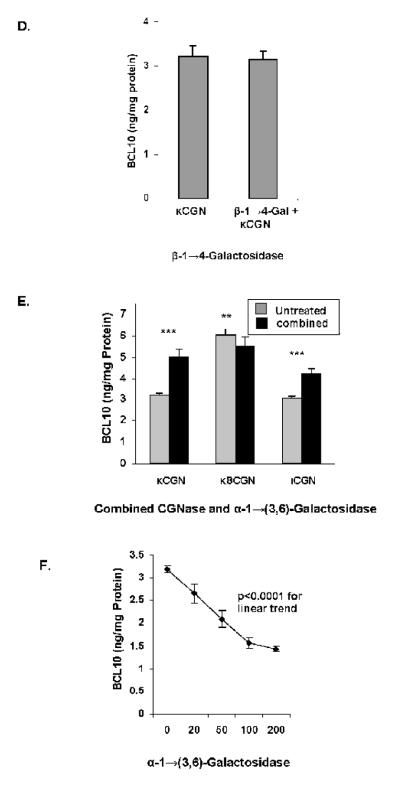
Effect of Enzymes on BCL10 production
When NCM460 cells were exposed to CGN in the presence of κCGNase (3 mg/L) (Figure 6A), significant increases in BCL10 occurred, consistent with an increase in immune stimulation when CGN was digested by CGNase. In contrast, pre-treatment with α-1→(3,6)-galactosidase (20 U/L) produced marked declines in the BCL10 response (Figure 6B), consistent with reduced exposure to the immunogenic epitope. Following pre-treatment with α-1→6-galactosidase (20 U/L; Figure 6C), β-1→4-galactosidase (20 U/L; Figure 6D), or β-1→(3,6)-galactosidase (not shown), no changes in the BCL10 protein occurred, indicating no effect of these enzymes on the immune reactivity of the CGN. When κCGNase and α-1→(3,6)-galactosidase (20 U/L) were combined, the increases in BCL10 produced by κCGNase alone were reduced, and BCL10 content was less in the degraded κ8CGN-exposed cells than when untreated (Figure 6E). The κCGN-induced increase in BCL10 declined with increasing concentrations of α-1→(3,6)-galactosidase (Figure 6F; p<0.001,1-way ANOVA with Tukey-Kramer post-test). The declines in κCGN-induced increases in IL-8 and in BCL10 shown with exposure to increasing concentrations of α-1→(3,6)-galactosidase were compared by linear regression and found to be highly correlated (r=0.9949), indicating the close association between decline in BCL10 and IL-8, and suggestive of a mechanistic link.
Figure 6. Cellular BCL10 production is modified by exposure to CGN-metabolizing enzymes.
A. Bcl10 production in the NCM460 cells increased following pre-treatment of κCGN, short, degraded κ8CGN, and ιCGN (1 mg/L) with specific carrageenase (3 mg/L), consistent with increased activation of a BCL10-mediated pathway following increased exposure to the α-Gal-(1→3)-Gal epitope. Results were statistically significant for κ- and ι-carrageenans (p<0.001, paired t-tests). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
B. Following exposure to α-1→(3,6)-galactosidase (20 U/L), BCL10 values declined, reflecting less exposure of the immunogenic epitope. Results were statistically significant (p=0.02 for κCGN; p=0.01 for κ8CGN; p=0.0009 for ιCGN, paired t-tests). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
C. Following exposure to α-1→,6-galactosidase (20 U/L), no changes in BCL10 content were found. Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
D. Following exposure to (20 U/L), no change in BCl10 content occurred. Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
E. When NCM460 cells were exposed to carrageenan pretreated with both specific carrageenase (3 mg/L) and α-1→(3,6)-galactosidase (20 U/L), cellular BCL10 content increased following exposure to κ-carrageenan and ι-carrageenan, but declined following exposure to the short, degraded κ-carrageenan, in a pattern similar to that observed with IL-8. Results are statistically significant with increases for undegraded κ- and ι-CGN (p<0.001, paired t-test) and decline for κ8CGN (p=0.01, paired t-test). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
F. With increasing concentrations of α-1→(3,6)-galactosidase (20, 50, and 100 U/L), increasing declines in BCL10 production were observed in the NCM460 cells, again similar to the changes observed in IL-8 (p<0.001, 1-way ANOVA with Tukey-Kramer post test, and p<0.0001 for linear trend; r2 = 0.94 for linear trend). Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
No effect of enzymes on ROS production
Baseline ROS production in the NCM460 cells was 912 ± 67, when measured by hydroethidine fluorescence. Following exposure to κ-CGN for 24 hours, ROS production increased to 8509 ± 367. No significant changes in ROS production were observed when κ-CGN was pre-treated with either κ-CGNase (3 mg/L), α-1→(3,6)-galactosidase (20 U/L), or these enzymes in combination. Previously, we demonstrated that CGN induced increases in IL-8 by a TLR4-BCL10-immune-mediated pathway and by an ROS-mediated pathway. The lack of change in ROS following exposure to carrageenase or α-1→(3,6)-galactosidase findings is consistent with a pathway of immune activation by CGN that is initiated by the α-Gal-(1→3)-Gal epitope.
TLR4 blocking antibody inhibits the impact of enzymes on IL-8 secretion
When NCM460 cells were exposed to TLR4 blocking antibody for 24 hours prior to treatment with κ-CGN that was pre-digested by κ-CGNase or by α-1→(3,6)-galactosidase, the previously detected changes in the secretion of IL-8 were inhibited (Figure 7). These results confirm that the modified CGN acts through the TLR4-BCL10 mediated pathway.
Figure 7. TLR4 blocking antibody inhibits changes in IL-8 secretion caused by enzymes.
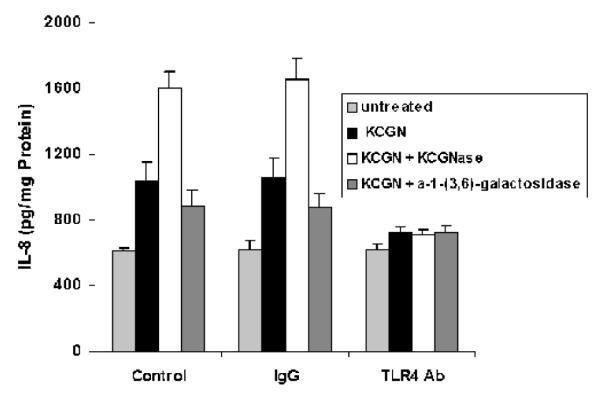
When NCM460 cells were exposed to TLR4 blocking antibody for 24 hours prior to treatment by κ-CGN (1 mg/L) that had been digested by κ-carrageenase or by α-1→(3,6)-galactosidase, the previously demonstrated changes in IL-8 secretion were inhibited. These findings support the interpretation that these enzymes affect the interaction of CGN with the TLR4. Data are the mean ± S.D. of 3 independent biological experiments with technical duplicates of each. (CGN=carrageenan)
Discussion
Carrageenan has been used for decades in food products to improve the texture of processed foods and to increase the solubility of other ingredients, such as casein found in milk products [28]. In the laboratory, CGN has also been used for decades, to induce inflammation and study the mediators of inflammation and the effectiveness of anti-inflammatory treatments [29-32]. Our recent work has identified two mechanisms by which carrageenan predictably induces inflammation in colonic epithelial cells [16-18]. The first of these involves the cell surface receptor TLR4, and includes MyD88, IRAK, and BCL10. BCL10 has been recognized as an important mediator of inflammation in immune cells, due to its association with IKKγ (also called NEMO), which is the regulatory subunit of the IKK signalosome. The activation of BCL10 appears to influence the ubiquitination of IKKγ, enabling the phosphorylation of IκBα by the catalytic components (IKKβ and IKKα) of the IKK complex [19,20]. This permits exposure of the nuclear localization signal of NFκB, leading to increased production of IL-8. In the MALT lymphomas, translocations involving BCL10 are associated with the constitutive activation of BCL10 [33,34]. Hence, the finding that specific enzymatic exposures to CGN alter production of BCL10 and of IL-8 provides a mechanistic insight into a basic pathway of immune activation through the α-Gal-(1→3)-Gal epitope. The variation in BCLl10 responsiveness by enzymatic modification of galactosyl bonds appears to signal whether or not there is activation of an immune-mediated mechanism of inflammation.
The presence of the 3,6-anhydro bridge in D-galactose in CGN is also unique to red algae and is absent in humans, suggesting possible involvement of this conformation in the immune response, as well. The action of the carrageenases releases CGN oligosaccharides from κCGN with 3,6-anhydro-D-galactose and from ιCGN with 3,6-andydro-D-galactose-2-sulfate residues as the non-reducing end. The immune response may therefore be activated by the presence of the 3,6-anhydro-D-Gal-α-(1,3)-D-Gal-4-sulfate epitope of κCGN, or of the 3,6-anhydro-D-galactose-2-sulfate-α-(1,3)-D-Gal-4-sulfate epitope of ιCGN, in contrast to λCGN, in which the 3,6-anhydro linkage does not form. The IL-8 response to λCGN is significantly greater than to either ι- or κCGN, perhaps attributable to increased exposure of the α-Gal-(1→3)-Gal epitope. Also, the IL-8 response to ιCGN is always significantly less than to either κ- or λ-CGN; this difference may be attributable to the presence of the ester-sulfate at the C2 of the 3,6-anhydro-D-galactose unit, reducing to some extent the immune reactivity.
A second mechanism by which CGN activates an inflammatory cascade is mediated through reactive oxygen species (ROS), leading to decline in phosphorylation of Hsp27, which is involved in reciprocal phosphorylation with IKKβ by PP2A [18]. The study findings demonstrate that changes in ROS production are not responsible for the variation in IL-8 found after treatment of CGN with α-1→(3,6)-galactosidase or carrageenase. Additional effects of CGN that we have reported in colonic epithelial cells include changes in the BMP4/wnt9a axis and cell-cycle arrest [35,36]. These may or may not be mediated independently of the inflammatory cascades. We have not yet evaluated the impact of exposure to galactosidases and carrageenases on other responses to CGN. Other effects may arise from other structural features of CGN, including mimicry of the naturally occurring glycosaminoglycans.
Since variation in the bacterial composition of the colonic microflora may be associated with variation in the types of galactosidases and the presence or absence of carrageenases, the metabolism of CGN may vary from individual to individual or over time in the same individual. The propensity for development of intestinal inflammation from exposure to carrageenan may be related to the colonic sub-population of specific organisms that produce these enzymes. Differences in the colonic microflora may contribute to development of inflammatory bowel disease, colonic polyps, or colonic neoplasms, making some individuals more or less susceptible to harmful sequelae from exposure to dietary CGN.
It is pertinent to note that the α-Gal(1→3)Gal epitope is present in some bacterial lipopolysaccharides that are known to be toxic. These include two serogroups of E. coli that express lipopolysaccharide (LPS) with the α-D-Gal-(1→3)-D-gal epitope, 020 and 086 [37-39]. These have been associated with pathogenicity, and the 020 cross-reacts with Klebsiella pneumoniae 04 and the 086 crossreacts with Salmonella 043. The overlap between CGN and epitopes of these pathogenic bacteria demonstrates that this specific configuration found in CGN is likely to evoke the immune responses to CGN that are manifested by increased BCL10 and IL-8. The α-Gal-(1→3)-Gal epitope has also been associated with Cetuximab-induced anaphylaxis involving an IgE response [40]. This epitope resembles to some extent the epitope of the B-blood group antigen, but lacks the associated fucose residue. A different α-galactosyltransferase enzyme is required to generate the α-Gal-(1→3)-Gal epitope and produces a different configuration.
Additional experiments are required to determine whether the α-Gal-(1→3)-Gal structure directly interacts with TLR4, or if MD-2, CD14, or LBP are required to properly position the carrageenan, as occurs with LPS. The leucine-rich regions within the extracellular domain (ECD) of TLR4 may provide the backbone for direct interaction with carrageenan, but a specific structural conformation within the ECD that recognizes the α-1,3-galactosidic bond has not yet been identified. Determination of the crystal structure of TLR4 and MD-2 with eritoran showed that LPS required interaction with MD-2, in order to bind with TLR4 [41]. Phe126 and His155 residues of MD-2 were required for LPS-induced dimerization of the TLR4-MD-2 mouse complex. Subsequent experiments will help to elucidate whether or not MD-2 is required for the CGN-induced activation of the TLR4-mediated pathway.
The study findings suggest that treatment of CGN by an enzyme with α-1→3-galactosidase activity may lead to reduced inflammatory response to CGN. These results have the potential to ameliorate harmful effects of CGN by establishing colonic colonization with bacteria that produce this enzyme. However, since the marked biological reactivity of CGN may arise from more than this epitope, reduction of human exposure to CGN remains a more reliable means to reduce the harmful effects of CGN.
Acknowledgments
The authors acknowledge the contributions of Drs. Uri Galili and of Roland Stenutz to discussion about the α-D-Gal-(1→3)-D-Gal epitope.
Funding: VA Merit Review to JKT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imeson AP. Chapter 5. Carrageenan. In: Phillips GO, Williams PO, editors. Handbook of Hydrocolloids. Woodhead Publishing Limited; Cambridge, England: 2000. pp. 87–102. [Google Scholar]
- 2.Tobacman JK. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ Health Perspect. 2001;109:983–94. doi: 10.1289/ehp.01109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somogyi LP, Miller S, Kishi A. Food Additives. Specialty Chemicals, SRI Consulting; San Francisco, CA: 2005. 2005. [Google Scholar]
- 4.Tobacman JK, Wallace R, Zimmerman B. Consumption of carrageenan and other water-soluble polymers used as food additives and incidence of mammary carcinoma in the United States in the twentieth century. Med Hypotheses. 2001;56:589–98. doi: 10.1054/mehy.2000.1208. [DOI] [PubMed] [Google Scholar]
- 5.Marinalg Working Group on Molecular Weight Determination Technical Position on measurements related to meeting the EC molecular weight distribution specification for carrageenan and PEC. 2006 www.marinalg.org/papers/papers-inf.htm.
- 6.Marrs WM. The stability of carrageenan to processing. In: Williams PA, Phillips GO, editors. Gums and Stabilizers for the Food Industry. The Royal Society of Chemistry; Cambridge, England: 1998. [Google Scholar]
- 7.Michel G, Nyval-Collen P, Barbeyron T, Czjzek M, Helbert W. Bioconversion of red seaweed galactans: a focus on bacterial agarases and carrageenases. Appl Microbiol Biotechnol. 2006;71:23–33. doi: 10.1007/s00253-006-0377-7. [DOI] [PubMed] [Google Scholar]
- 8.Rees D. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]
- 9.Knutsen S, Myslabodski D, Larsen B, Usov A. A modified system of nomenclature for red algal galactans. Bot Mar. 1994;37:163–169. [Google Scholar]
- 10.Guibet M, Kervarec N, Genicot S, Chevolot Y, Helbert W. Complete assignment of (1)H and (13)C NMR spectra of Gigartina skottsbergii lambda-carrageenan using carrabiose oligosaccharides prepared by enzymatic hydrolysis. Carbohydr Res. 2006;341:1859–1869. doi: 10.1016/j.carres.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. Reactivity of human natural antibodies to endothelial cells from Galpha(1,3)Gal-deficient pigs. Transplantation. 2007;83:193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 12.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 13.Sandrin MS, Osman N, McKenzie IF. Transgenic approaches for the reduction in expression of GALα(1,3)GAL for xenotransplantation. Front Biosci. 1997;2:e1–11. doi: 10.2741/a220. [DOI] [PubMed] [Google Scholar]
- 14.Osman N, McKenzie IF, Ostenried K, Ioannou YA, Desnick RJ, Sandrin MS. Combined transgenic expression of alpha-galactosidase and alpha 1,2 fucosyltransferase leads to optimal reduction in the major xenoepitope Gal-alpha(1,3)Gal. Proc Natl Acad Sci USA. 1997;94:14677–14682. doi: 10.1073/pnas.94.26.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanemura M, Yin D, Chong AS, Galili U. Differential immune responses to alpha-gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J Clin Invest. 2000;105:301–10. doi: 10.1172/JCI7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borthakur A, Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan induces Interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G829–38. doi: 10.1152/ajpgi.00380.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya S, Gill R, Chen ML, Zhang F, Linhardt RJ, Dudeja PK, Tobacman JK. Toll-like Receptor 4 Mediates Induction of Bcl10-NFκB-IL-8 Inflammatory Pathway by Carrageenan in Human Intestinal Epithelial Cells. J Biol Chem. 2008;283:10550–8. doi: 10.1074/jbc.M708833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008;1780:973–82. doi: 10.1016/j.bbagen.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, Eby M, Xiao W, Dixit VM. Bcl10 activates the NFκB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 20.Drew D, Shimada E, Huynh K, Bergqvist S, Talwar R, Karin M, Ghosh G. Inhibitor kappaB kinase beta binding by inhibitor kappaB kinase gamma. Biochemistry. 2007;46:12482–90. doi: 10.1021/bi701137a. [DOI] [PubMed] [Google Scholar]
- 21.Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32:315–7. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 22.Yu G, Guan H, Ioanoviciu AS, Sikkander SA, Thanawiroon C, Tobacman JK, Toida T, Linhardt RJ. Structural Studies on κ-CGN Derived Oligosaccharides. Carbohydrate Res. 2002;337:433–440. doi: 10.1016/s0008-6215(02)00009-5. [DOI] [PubMed] [Google Scholar]
- 23.Michel G, Chantalat L, Fanchon E, Henrissat B, Kloareg B, Dideberg O. The iota-carrageenase of Alteromonas fortis. A beta-helix fold-containing enzyme for the degradation of a highly polyanionic polysaccharide. J Biol Chem. 2001;276:40202–9. doi: 10.1074/jbc.M100670200. [DOI] [PubMed] [Google Scholar]
- 24.Michel G, Chantalat L, Duee E, Barbeyron T, Henrissat B, Kloareg B, Dideberg O. The kappa-carrageenase of P. carrageenovora features a tunnel-shaped active site: a novel insight in the evolution of Clan-B glycoside hydrolases. Structure. 2001;9:513–25. doi: 10.1016/s0969-2126(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 25.Guibet M, Colin S, Barbeyron T, Genicot S, Kloareg B, Michel G, Helbert W. Degradation of lambda-carrageenan by Pseudoalteromonas carrageenovora lambda-carrageenase: a new family of glycoside hydrolases unrelated to kappa- and iota-carrageenases. Biochem J. 2007;404:105–14. doi: 10.1042/bj20061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhattacharyya S, Pant N, Dudeja, Tobacman JK. Development, evaluation, and application of a highly sensitive microtiter plate ELISA for human Bcl10 protein. J Immunoassay Immunochem. 2007;28:173–88. doi: 10.1080/15321810701454573. [DOI] [PubMed] [Google Scholar]
- 27.Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–93. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- 28.Spagnuolo PA, Dalgleish DG, Goff HD, Morris ER. Kappa-carrageenan interactions in systems containing casein micelles and polysaccharide stabilizers. Food Hydrocolloids. 2005;19:371–377. [Google Scholar]
- 29.Mottet KN. Carrageenan ulceration as a model for human ulcerative colitis. Lancet. 1970;2:1361. doi: 10.1016/s0140-6736(70)92385-8. [DOI] [PubMed] [Google Scholar]
- 30.Moyana TN, Lalonde JM. Carrageenan-induced intestinal injury in the rat - a model for inflammatory bowel disease. Ann Clin Lab Sci. 1990;20:420–426. [PubMed] [Google Scholar]
- 31.Onderdonk AB. The carrageenan model for experimental ulcerative colitis. Prog Clin Biol Res. 1985;186:237–245. 1985. [PubMed] [Google Scholar]
- 32.Oohashi Y, Ishioka TT, Wakabayashi K, Kuwabara H. A study of carcinogenesis induced by degraded carrageenan arising from squamous metaplasia of the rat colorectum. Cancer Lett. 1981;14:267–272. doi: 10.1016/0304-3835(81)90153-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Siebert R, Yan M, Hinzmann B, Cui X, Xue L, Rakestraw KM, Naeve CW, Beckmann G, Weisenburger DD, Sanger WG, Nowotny H, Vesely M, Callet-Bauchu E, Salles G, Dixit VM, Rosenthal A, Schlegelberger B, Morris SW. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32) Nat Genet. 1999;22:63–68. doi: 10.1038/8767. [DOI] [PubMed] [Google Scholar]
- 34.Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, Pan L, Crook T, Hamoudi R, Isaacson PG, Dyer JF. Bcl10 is involved in t(1;14)(p22;q32) of MALT B Cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:46–56. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya S, Borthakur A, Dudeja PK, Tobacman JK. Carrageenan reduces bone morphogenetic protein-4 (BMP4) and activates Wnt/β-Catenin pathway in normal human colonic epithelial cells. Dig Dis Sci. 2007;52:2766–74. doi: 10.1007/s10620-006-9531-4. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Borthakur A, Dudeja PK, Tobacman JK. Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro. J Nutr. 2008;138:469–75. doi: 10.1093/jn/138.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng L, Han W, Wang Q, Bastin D, Wang L. Characterization of Escherichia coli O86 O-antigen gene cluster and identification of O86-specific genes. Veterinary Microbiol. 2005;106:241–248. doi: 10.1016/j.vetmic.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Stenutz R, Weintraub A, Widmalm G. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev. 2006;30:382–403. doi: 10.1111/j.1574-6976.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 39. http://www.casper.organ.su.se/ECODAB/
- 40.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ, Lee JL. Crystal structure of the TLR4-MD-2 complex with bound endotoxoin antagonist Eritoran. Cell. 2007;130(5):906–17. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]