Summary
It has been postulated that the cytoskeleton controls many aspects of T cell function, including activation, proliferation and apoptosis. Recent advances in our understanding of F-actin polymerization and the Ezrin-Radixin-Moesin (ERM) family of cytoskeleton signal proteins have provided new insights into immunological synapse formation during T cell activation. During aging there is a significant decline of T cell function largely attributable to declines in activation of CD4 T cells and defects in the formation of the immunological synapse. Here we discuss recent progress in the understanding of how aging alters F-actin and ERM proteins in mouse CD4 T cells, and the implications of these changes for the T cell activation process.
Keywords: T Lymphocytes, Aging, Cytoskeleton, Tyrosine Kinases, Signal Transduction, Cellular Activation, TCR signaling
Introduction
The cytoskeleton of T lymphocytes is composed of actin filaments, microtubules and intermediate filaments. The actin filaments (or F-actin) and the associated signaling machinery control many aspects of cell motility and provide the kinetic force that moves T cells (Samstag et al., 2003; Smith et al., 2007; Long et al., 2004; Pribila and Shimizu, 2003; Hogg et al., 2004); these systems also control the morphology and plasticity of T cells (Cogoli-Greuter et al., 2004; Dustin et al., 2004; Dustin, 2007; Krummel and Macara, 2006; Meiri, 2004; Miyamoto et al., 2003; Poenie et al., 2004; Pribila and Shimizu, 2003). The microtubule system is thought to regulate the polarized secretion of effector molecules and might contribute to receptor endocytosis as well as to the maintenance of F-actin dependent structures (Rey et al., 2007; Stradal et al., 2006; Bossi and Griffiths, 2005; Huse et al., 2008; Song et al., 2008; Gomez and Billadeau, 2008). The role of intermediate filaments is less well understood, but these are thought to provide architectural support and regulate the rigidity of T cells (Minin and Moldaver, 2008; Cai and Sheetz, 2009; Goldberg et al., 2008). Therefore, the cytoskeleton controls many aspects of T cell function and plays an essential role in cell homing, and in interactions with antigen presenting cells that lead to T cell activation (Dustin, 2005; Dustin, 2006; Dustin, 2007; Dustin, 2008b; Dustin, 2008a). With age, there is a significant decline in T cell function. Studies have shown that with age there is a significant decline in IL-2 production (Clise-Dwyer, 2007), while studies in our lab have shown defects in early TCR signals of CD4 T cells from old mice (for a review see Miller et al., 1997; Miller et al., 2005). In particular, CD4 T cells from old mice show defects in the translocation of talin during early phases of their interaction with APC, before the TCR starts to discriminate between agonist and antagonist peptide (Garcia and Miller, 2001) and defects in the translocation of many other key-signaling proteins to the area of APC-T cell interaction. These defects in translocation lead to a lack of immune synapse formation (Garcia and Miller, 2001; Garcia and Miller 2003).
Additional work showed that downstream pathways of the TCR are also affected by age, including Raf-1 and JNK signaling (Kirk and Miller, 1999; Kirk et al., 1999; Kirk and Miller, 1998) and revealed defects in NFAT nuclear translocation (Garcia and Miller, 2001; Garcia and Miller, 2003). The data suggest that defects in early aspects of TCR signaling may be in part responsible for the declines in cytokine production, including IL-2. In addition to our studies, other groups have shown that CD4 T cells from old mice show significant defects in proliferation (Haynes and Swain, 2006) and differentiation into memory or effectors cells (Vallejo, 2006; Hakim and Gress, 2007; Haynes and Eaton, 2005; Haynes, 2005; Haynes and Swain, 2006). The published data suggested a clear age-related decline in CD4 T cell function, but less is known about how age affects cytoskeleton structure and function, and how such changes might affect immunological synapse formation and later stages of T cell activation and function. Although it is likely that age could affect many aspects of the cytoskeletal structure and contribute at many stages in the defects in the TCR signaling, this review will focus on events related to activation of CD4 T cells immediately after encounter with antigen presenting cells (APC); defects at this early stage are likely to be rate-limiting for T cell transition from resting cell to activated effector. In addition, there are no studies in the effect of age on intermediate filaments of CD4 T cells that could help to clarify some of the age-related declines in CD4 function. We will in particular discuss two pathways: A) those that control F-actin formation and; B) those that control signals modulated by proteins in the Ezrin Radixin Moesin family (ERM) of cytoskeleton proteins.
A) Age-related defects in the T cell receptor signaling pathways leading to F-actin polymerization
Current models of interactions between T cells and APC suggest that integrins are involved in the earliest steps leading to recognition by the TCR of peptides presented by the Major Histocompatibility Complex (MHC) on the surface of APC (Burbach et al., 2007; Sechi and Wehland, 2004; Smith-Garvin et al., 2009; Ward and Marelli-Berg, 2009). If conditions are right, this interaction leads to a rapid increase in F-actin polymerization with microcluster formation and lammellopodia formation at the T cell surface interacting with the ACP and, eventually, to the formation of the immunological synapse. This multicomponent synapse includes concentric assemblies known as the p-SMAC (peripheral Supramolecular Activation Complex) and c-SMAC (central SMAC) (Davis et al., 1999; van der Merwe, 2002; Krummel and Davis, 2002; Dustin et al., 2001; Bromley et al., 2001). Actin dynamics and F-actin formation are instrumental in maintaining these structures and in generating efficient TCR activation signals (Gomez and Billadeau, 2008; Seminario and Bunnell, 2008; Billadeau and Burkhardt, 2006; Fuller et al., 2003; Cannon and Burkhardt, 2002). Detailed studies of the pathways leading to F-actin polymerization suggested that activation of the Zap70, lck and fyn tyrosine kinases, which takes place early in the signal cascade, can phosphorylate signaling targets that regulate F-actin status (van Leeuwen and Samelson, 1999; Smith-Garvin et al., 2009). For example, activation of Vav GTPase, a target of Zap70 and lck, is a necessary event for F-actin formation and cytoskeletal reorganization during TCR signaling (Salmond et al., 2009; Tybulewicz et al., 2003; Tybulewicz, 2005; Swat and Fujikawa, 2005; Wange, 2000). In addition, Vav effects on F-actin are, in part, mediated by members of the Rho family of GTPases, in particular the Rac1 GTPase, which in turn modulates proteins of the WAVE2 complex (Tybulewicz et al., 2003; Cantrell, 1998; Burkhardt et al., 2008; Huang and Burkhardt, 2007). Activation of Rac1 increases the recruitment of WAVE2 (WASP-family verprolin-homologous protein-2) to the ARP2/3 complex (Huang and Burkhardt, 2007; Burkhardt et al., 2008; Bustelo, 2002; Fischer et al., 1998; Fuller et al., 2003; Gomez and Billadeau, 2008; Swat and Fujikawa, 2005) leading to a direct increase in F-actin polymerization in the area of T cell-APC contact.
A-1) Aging decreases membrane fluidity and increases the level of F-actin in T cells
Membrane fluidity of T cells decreases with age in mice and in humans (Huber et al., 1991; Collins et al., 1991; Huber, 1989; Rivich et al., 1988; Traill et al., 1985; Rivnay et al., 1980; Rivnay et al., 1979). These initial studies attributed these effects to changes in the plasma membrane lipid composition. More recent investigations, however, using pharmacological agents that inhibit F-actin polymerization, such as lantrunculin and cytochalasin, have shown that decreases in F-actin polymerization can increase T cell membrane fluidity (Doherty and McMahon, 2008; Tooley et al., 2005; Blanchard and Hivroz, 2002a; Sheetz, 1993; Heath and Holifield, 1991; Groves, 2005; Blanchard and Hivroz, 2002b; de Pablo and varez de, 2000). This observation raises the possibility that increases in F-actin polymerization with age could be responsible for the decrease in membrane fluidity seen in T cells from old donors. Indeed, Brock et al. (Brock and Chrest, 1993) have shown that basal levels of F-actin are significantly higher in purified resting CD4 and CD8 T cells from aged C57BL/6 mice. In addition, these studies have shown that stimulation of the TCR using the mitogenic lectin Concanavalin-A induces rapid F-actin polymerization in T cells from young mice, but not in T cells from aged mice. The pharmacological agent Phorbol 12-myristate 13-acetate (PMA), which bypasses TCR to stimulate downstream signaling via protein kinase C (PK-C), induces actin polymerization in T cells from young and old animals to an equal extent, suggesting that the aging defects are at the level of TCR signaling. Similarly, Rao et al. (Rao et al., 1992) have shown that in resting human lymphocytes there is a significant age-related increase in the amount of F-actin. As in the mouse study, stimulation of human T cells by a lectin (Phytohaemagglutinin, or PHA) increased F-actin polymerization more strongly in young than in old T cells. Additional studies in mice of TCR mobility capping, an antibody-driven process that mimics TCR-MHC interaction, have shown that age diminishes both TCR mobility and capping (Cohen et al., 1991; Rao, 1982; Chiricolo et al., 1984; Brohee et al., 1982; Gilman et al., 1981; Noronha et al., 1980). All these early studies suggested that the age-related decrease in membrane fluidity may be due to alterations in cytoskeleton architecture with increases in F-actin polymerization, and also provided the first indication that age may alter the signaling machinery controlling actin polymerization.
A-2) Age decreases lamellipodia formation
During APC-triggered activation, a T cell forms a lamellipodium, a flattening of the contact area with the APC, with a partial reorganization of the F-actin and the cytoskeleton prior to the formation of a complete functional immune synapse (Hogg et al., 2003; Hogg et al., 2004; Hyun et al., 2009; Nolz et al., 2006; Anvari et al., 2004; Tskvitaria-Fuller et al., 2003; Bunnell et al., 2001; Volkov et al., 1998; Otteskog and Sundqvist, 1983). This process can be mimicked and studied using T cells allowed to adhere to a cover glass coated with anti-CD3 antibody. Examples of this experimental system can found in data published by our laboratory (Garcia and Miller, 2002). In this model, (Figure 1) staining with phalloidin prior to analysis using confocal microscopy identifies F-actin. CD4+ T cells from young mice placed onto control surfaces, i.e. cover glass coated with antibodies against dinitrophenol hapten, form contact zones that are round and relatively small. Contact zones of CD4+ T cells from old mice are similarly round and homogeneous, but for unknown reasons are typically somewhat larger than those produced by T cells from young donors. In contrast, cover glass surfaces coated with anti-CD3 antibody induce CD4 T cells from young mice to spread onto the glass surface, with high levels of polymerized actin localized as a ring at the edge of the cell. However, CD4+ T cells from old mice contain two cellular populations: some of the cells do not form lamellipodia, while others spread nearly as well as T cells from young mice [see arrows in Figure 1 for contrasting examples]. The qualitative and quantitative analysis of lamellipodia formation confirm that CD4+ T cells from the old CB6F1 mice were indeed slightly larger than young cells on control slides with anti-DNP. In addition, incubation on anti-CD3-coated slides for 10 min can produce lammellopodia and cellular spreading behavior in 70 to 98 % of the CD4+T cells from young mice, but only 17 to 30 % of T cells from old mice can exhibit detectable membrane flattening (Garcia and Miller, 2002). The lack of lammellopodia formation during TCR stimulation suggests that T cells from old mice show defects in the process of cytoskeleton reorganization or in the signaling pathways controlling it. Other groups (Brock and Chrest, 1993; Rao et al., 1992) have reported that aging leads to an increase in the total levels of F-actin in resting T lymphocytes, accompanied by defects in the ability to form new actin filaments during TCR signaling. These observations are not incompatible with our own findings: age-related increases in F-actin, if confined to the cortical cytoskeleton just under the plasma membrane, could in principle lead to diminished membrane fluidity and lower T cell signaling. Poor TCR signaling could, in turn, decrease new F-actin polymerization at the site of T cell-APC contact. Therefore, defects in lammellopodia formation, in addition to previous studies showing increases in F-actin (Brock and Chrest, 1993; Rao et al., 1992), further support the idea that aging alters the cytoskeletal architecture of the CD4 T cells from old mice.
Figure 1. Age impairs lammellopodia formation in CD4+ T cells from old mice.
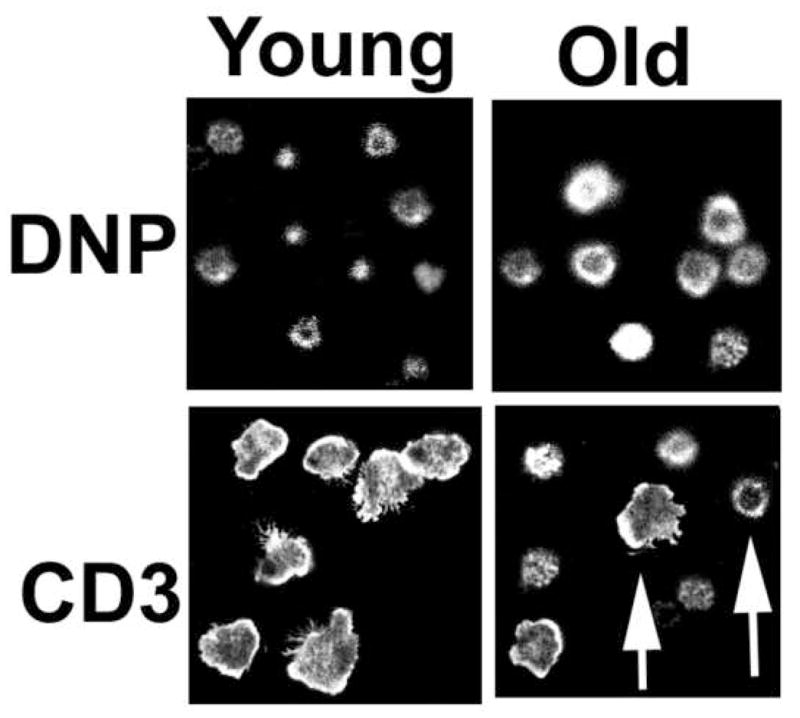
CD4+ T cells from young and old CB6F1 mice were incubated for 10 min on glass cover slips covered with anti-DNP (a negative control) or anti-CD3. The cells were fixed, stained with phalloidin to detect F-actin filaments, and analyzed by confocal microscopy. The images show a field of the Z-plane level at which the T cells are in contact with the glass slide. Nearly all of the T cells from the young mice show an extended zone of contact with the glass substrate, triggered by anti-CD3. The arrows indicate two types of cells from old mice; one (left arrow) resembling T cells from young mice with lammellopodia formation, and the other (right arrow) resembling unstimulated cells as in the anti-DNP control slides.
A-3) Age-related changes in the association of CD3ζ to the cytoskeleton
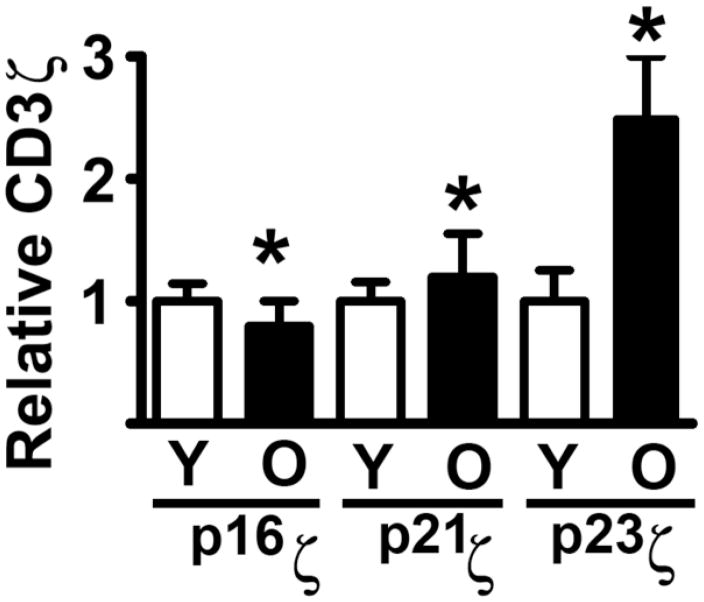
Additional evidence that age alters the T cell cytoskeleton came from analysis of CD3ζ sassociation with the cytoskeleton. It has been shown that phosphorylated forms of CD3ζ are associated with F-actin and that this association may be regulated by the tyrosine kinase lck (Garcia and Miller, 2002). This association between CD3ζ and F-actin suggested that age-related increases in F-actin might lead to increased association of CD3ζ with the cytoskeleton. We tested this hypothesis by measuring the amount of CD3ζ in a cellular fraction that contains polymerized F-actin and other cytoskeletal elements, comparing freshly isolated CD4 T cells from young and old mice (Garcia and Miller, 2002; Garcia and Miller, 2003). This “cytoskeletal fraction” is prepared on the basis of its resistance to solubilization by non-ionic detergents such as Brij-58. Figure 2 shows a summary of western blot analyses of CD3ζ in fractionated lysates from CD4+ T cells of young and old CB6F1 mice (Garcia and Miller, 2002). Three predominant CD3ζ forms can be detected: p16ζ (a relatively non-phosphorylated form of CD3ζ), and the tyrosine phosphorylated forms p21ζ and p23ζ. Interestingly, resting CD4 T cells from old mice show a two-fold increase in p23ζ association to the cytoskeleton fraction, and probably to F-actin, when compared to CD4 cells from young mice. These age-related increases are accompanied by a smaller, but statistically significant, increase in p21ζ and a decline in p16ζ association to the cytoskeleton (Garcia and Miller, 2002). Because CD3ζ phosphorylated isoforms are known to be preferentially associated to F-actin (Moran and Miceli, 1998; Peck et al., 1996; Geppert and Lipsky, 1991; Marano et al., 1989; Rozdzial et al., 1995; Rozdzial et al., 1998), our results support the idea that age-related increases in F-actin may lead to a higher association of phosphorylated CD3ζ isoforms to the cytoskeleton in resting T cells. In addition, these observations suggest that age may in turn constrain movement of TCR complexes needed for efficient T cell-APC interactions and immune synapse formation. In good agreement with this model, we have found that although TCR stimulation increases CD3ζ phosphorylation and association of all CD3ζ forms to the cytoskeleton in CD4 T cells from young mice, none of these effects are seen in CD4+ T cells from old mice (Garcia and Miller, 2002).
Figure 2. Age increases CD3ζ phosphorylated isoforms associated with the cytoskeleton of CD4+T cells.
Resting CD4+ T cells from young and old mice were solubilized with Brij-50, and the cytoskeletal fractions purified by centrifugation. The samples were resolved by SDS and analyzed for levels of the different CD3ζ isoforms (p16, p21 and p23) using anti-CD3ζ western blots. The bar graph represents the mean and SEM of each of the CD3ζ isoforms, normalized to the levels in young cells for each endpoint. Groups indicated by * are different at a significance level of p < 0.05.
A-4) Age-related defects in the dynamics of cytoskeletal reorganization during immune synapse formation
During T cell APC interaction there is a translocation of proteins and signaling molecules to the T cell-APC interface that results in the formation of an immunological synapse. Several groups (Davis et al., 1999; Tskvitaria-Fuller et al., 2003; Wulfing et al., 2000) have shown that F-actin assembly plays an essential role in the translocation of many of these synapse proteins. Confocal microscopy of F-actin polymerization at the interface can be performed using fluorochrome-labeled phalloidin, while translocation of proteins can be followed using fluorochrome-labeled antibody stains. Using this methodology, we have shown significant age declines in F-actin accumulation at the interface of CD4 T cells with APC (Tamir et al., 2000; Garcia and Miller, 2001; Garcia and Miller, 2003). However, these decreases are accompanied by a small but significant increase in the total F-actin in CD4 T cells from old mice; a result that corresponds well with published data from other groups (Brock and Chrest, 1993). Parallel results were also obtained using two stimulation systems: one in which polyclonal T cells of normal mice are activated by contact with a myeloma line, 145-2C11 (Tamir et al., 2000), that expresses anti-CD3 antibody on the surface, and one in which T cells from peptide-specific transgenic mice are activated by a B cell line (CH12) pulsed with the cognate peptide (Garcia and Miller, 2001) to mimic physiological stimulation by peptide-bearing APC. Examples of using the CH12 system can be found in several of our publication (Garcia and Miller, 2001 and 2003). In these studies, around 66 % of the CD4 T cells from young donors can accumulate F-actin in the T cells-ACP contact, but only 22 % of the cells from old donors show accumulation of F-actin. Apart from F-actin, a similar pattern of age-related declines can be seen in other proteins that are translocated to the immune synapse. For example, around 68 % of CD4 T cells from young mice are able to translocate the cytoskeletal protein talin, compared to only 20% of CD4 cells from old mice. Both systems were able to confirm a significant age-related decline in the induction of F-actin polymerization and a lack of immune synapse formation (Garcia and Miller, 2001; Garcia and Miller, 2002; Garcia and Miller, 2003; Tamir et al., 2000). Additional studies of key proteins in the upstream signal pathways leading to F-actin polymerization, such as Vav1, also showed an age-related decline in translocation to the immune synapse (see (Garcia and Miller, 2001; Garcia and Miller, 2003). Simultaneous evaluation of pairs of proteins in individual cells showed that CD4 T cells from old mice include two separate populations, one able to respond with full translocation of proteins to the immune synapse and other that does not respond at all (Garcia and Miller, 2001; Garcia and Miller, 2003). These results are reminiscent of the two populations, one responsive and one non-responsive, seen in studies of lammellopodia formation (see Figure 2, Garcia and Miller, 2002). We do not know the processes that create these two distinct populations of responsive and non-responsive CD4 T cells in old mice. However, studies by Laura Haynes and Susan Swain (Haynes and Swain, 2006; Eaton et al., 2008; Jones et al., 2008; Clise-Dwyer et al., 2007) have shown that those CD4 T cells of aged mice that are recent emigrants from the thymus cells can respond to antigen stimulation and proliferate as well as T cells from young mice. It is possible that the small percentage of CD4 T cells from old donors able to respond to stimulation may correspond to the set of relatively new thymus emigrant cells, in contrast to long lived, unresponsive cells.
A-5) The age related decline in F-actin polymerization and immune synapse formation can be reversed by enzymatic treatments that remove sialic acid residues from surface glycoproteins
The age-related alterations in cytoskeleton dynamics and interaction with CD3ζ may be responsible for some of the age-related defects in TCR signaling. However, CD4 T cells from old mice also show dramatic alteration in the glycosylation of surface proteins, including CD43 and CD45 (Garcia et al., 2005). Because the blocking of TCR signaling takes place very early in the process of T cell-APC interaction (Garcia and Miller, 2001; Garcia and Miller, 2003), we hypothesized that these glycosylation changes may contribute to age-related defects in TCR signaling. This hypothesis was supported by evidence that enzymatic treatments that alter surface glycosylation can improve T cell function (Garcia and Miller, 2003). The data show that treatment with one such enzyme, O-sialoglycoprotein endopeptidase (OSGE), which cleaves portions of the CD44, CD43 and CD45 molecules bearing O-linked sialyl-glycan chains, can restore the formation of immune synapses in CD4 T cells from old mice. Approximately 60% – 70% of the T cells from young mice were able to accumulate F-actin, and translocated other proteins to the immune synapse; the proportion of responsive cells diminished with aging to approximately 20–25 %. OSGE treatment did not increase the proportion of young T cells able to form immune synapse. In contrast, around 50–60 % of the CD4 T cells from old mice can form immune synapse after OSGE treatments. This is a dramatic effect on old T cells, restoring TCR all aspects of signaling, including F-actin accumulation and translocation of multiple proteins to the immune synapse. Further work showed that treatment with OSGE could also increase cytokine production and expression of activation antigens (Berger et al., 2006; Garcia and Miller, 2003; Sadighi Akha et al., 2006; Berger et al., 2005). Therefore, age-dependent changes in the glycosylation pattern of surface macromolecules could contribute to defects in APC-induced cytoskeleton rearrangements and F-actin formation. At the present, we do not know the biological reason for the changes in the glycosylation; but we hypothesize that those could be the result of alteration in the pattern of glycosyltransferases in the ER and golgi, or to alteration in the expression of surface proteins that regulate TCR signaling.
B) Age-related changes in ERM signaling pathways
Lymphocytes, including T cells, express ezrin and moesin proteins, members of the Ezrin-Radixin-Moesin (ERM) family of cytoskeleton proteins. The ERM are proteins that link the cell cortex with membrane components and the actin cytoskeleton, and in particular with actin filaments ((Bretscher, 1999; Bretscher et al., 2000; Bretscher et al., 2002). In T cells, ERM proteins control cell shape, cytokinesis, and cell adhesion (Bretscher, 1999; Li et al., 2007; Mangeat et al., 1999; Lee et al., 2004) and participate in immune synapse formation (Makrogianneli et al., 2009; Nijhara et al., 2004; Faure et al., 2004; Cullinan et al., 2002; Itoh et al., 2002; del Pozo et al., 1998; Murphy, 2005). In addition, it has been suggested that the ERM family has an important function in maintaining lipid raft structures in T cells (Brdickova et al., 2001; Itoh et al., 2002; Tomas et al., 2002) and that they control some aspects of apoptosis signaling (Niggli and Rossy, 2008; Ramaswamy et al., 2007; Hebert et al., 2008). The ERM proteins are present in two conformations: a dephosphorylated form corresponding to a “dormant” or inactive state and a serine/threonine phosphorylated form that activates ERM function (Bretscher et al., 2002; Cullinan et al., 2002; Niggli and Rossy, 2008; Shaw, 2001).
When the proteins are in the active state, a sequence domain previously known as the B4.1 (band 4.1 or F domain) homology domain present in the Ezrin (E) Radixin (R) and Moesin (M), or FERM domain, can interact with membrane components and signaling molecules, including CD44, CD43 and EBP50, while the C-terminus can directly bind to actin and F-actin (Allenspach et al., 2001; Bretscher et al., 2000; Delon et al., 2001; Itoh et al., 2002; Lee et al., 2004; Martin et al., 2003; Niggli and Rossy, 2008). The kinases responsible for ERM phosphorylation are poorly defined (Ren et al., 2009; Auvinen et al., 2007; Larsson, 2006); but it is known that the Rho family of small GTPases, including Rac1 and RhoA, are upstream regulators of the ERM phosphorylation (Hebert et al., 2008; Lee et al., 2004; Makrogianneli et al., 2009; Yonemura et al., 2002; Doherty and McMahon, 2008; Nijhara et al., 2004; Otteskog and Sundqvist, 1983; Salojin et al., 1999). It has been shown that constitutively active mutants of Rac1 can induce ERM dephosphorylation leading to inactivation of ERM signaling and function (Nijhara et al., 2004). In addition, antigen stimulation induces a rapid, but transient, dephosphorylation of ERM proteins in which the activation of Vav1 is involved. Because the Rho family is under the control of the Vav proto-oncogene (Salojin et al., 1999; Cantrell, 1998; Fischer et al., 1998; Han et al., 1997; Romero and Fischer, 1996; Swat and Fujikawa, 2005; Tybulewicz et al., 2003; Wulfing et al., 2000), these results suggest a complex network of signaling pathways regulating the ERM proteins, including Vav1-Rac1 signaling pathways.
During immune synapse formation, one important function of the ERM proteins in T cells is the exclusion of CD43 and other glycoproteins from the T cell-APC interface (Allenspach et al., 2001; Delon et al., 2001). CD43 is an abundant, highly sialylated surface glycoprotein that plays a negative regulatory role in T cell activation (Faure et al., 2004). Thus, inhibition of ERM dephosphorylation and CD43 exclusion from the immune synapse impair T cell activation (Cannon et al., 2008; Mody et al., 2007; Tong et al., 2004). In addition, ERM proteins are thought to be a key component in the formation of the distal pole complex (Allenspach et al., 2001; Cullinan et al., 2002), a structure that removes many negative regulators of the TCR signaling, including CD43, from the immune synapse.
B-1) CD43 is not excluded from the immune synapse in CD4 T cells from old mice
CD43 exclusion from the immune synapse was analyzed in naïve CD4 T cells from young and old AND-strain mice by confocal microscopy using the peptide-pulsed CH12 system (Garcia and Miller, 2003). In this system, CD43 is excluded from the area of T cell APC contact as a result of early TCR signaling. Further quantitative analysis of CD43 exclusion in individual T cells shows that about 70% of CD4 T cells from young mice are able to exclude CD43, compared to only 30% of T cells from old mice. This disparity presumably reflects the lack of efficient TCR-MHC signaling in T cells from old mice. More surprising, however, is the observation that OSGE treatment, which can restore the translocation of proteins to the immune synapse in aged T cells, is not able to restore CD43 exclusion. In this system, after OSGE treatments only 30% of the CD4 T cells from old donors show exclusion of CD43, compared with 70 % of T cells from young donors (Garcia and Miller, 2003). These results provided the first indication that age not only affects pathways leading to F-actin polymerization but, in addition, leads to synapse independent changes in ERM signaling required for CD43 exclusion.
B-2) Age-related declines in the level of ERM phosphorylation in CD4 T cells
Phosphorylations at Thr558 in moesin and Thr567 in ezrin define the active form of these proteins (Lee et al., 2004; Li et al., 2007; Niggli and Rossy, 2008). We measured the levels of phospho-ERM (pERM) in resting and stimulated CD4 T cells from young and old CB6F1 donors. We found that CD4 T cells from old mice have a 50% decline in levels of pERM without significant changes in the expression of the ERM proteins (Garcia et al., 2007). Furthermore, in vitro stimulation by TCR crosslinking induces a significant dephosphorylation of ERM in CD4 T cells from young mice but has no such effect on samples from old donors, suggesting additional defects in ERM upstream signaling, possibly related to defects in TCR-dependent signals (Garcia and Miller, 2001; Miller et al., 1997). Because of the importance of ERM protein in linking cortical actin with the membrane and regulating interaction of surface proteins and membrane signaling molecules (Bretscher, 1999; Charrin and Alcover, 2006; Niggli and Rossy, 2008), it is plausible that declines in pERM in aged CD4 T cells could have important implications for T cell function.
B-3) Age alters the association of ERM proteins with surface molecules CD43, CD44 and the signaling adaptor EBP50
The current model of ERM function suggests that age-related declines in pERM should be accompanied by declines in ERM association to surface molecules (such as CD44 and CD43) and adaptor proteins (such as EBP50). We quantified the association of CD44 and EBP50 to ERM and found significant age-related declines in their intermolecular associations (Garcia et al., 2007). This declines correspond well with the present model of ERM function (Niggli and Rossy, 2008): age-related decline in pERM is accompanied by a loss in association with both CD44 and EBP50. However, analyses of CD43 association to the ERM revealed a more complex pattern: age increases the association of CD43 to moesin, despite the decline with age in pERM, and there was no evidence for CD43 association to ezrin (Garcia et al., 2007). In addition, as predicted by the ERM model, stimulation of the TCR leads to the expected significant declines in CD43 association to ERM in CD4 T cells from young mice, but does not produce these changes in the samples from old mice. The basis for the disparity between the effects on CD43 and those on CD44 and EBP50 is not yet clear, but it has been suggested that the CD43 cytoplasmic domain can form indirect associations with the cytoskeletal matrix that involve other TCR signaling molecules (Allenspach et al., 2001; Cullinan et al., 2002; Tong et al., 2004). Our data suggest that age-related increases in association of CD43 to moesin may be the result of complex interactions between CD43, ERM proteins, and unknown sets of adaptor and signaling molecules (Tong et al., 2004).
B-4) Age-dependent changes in RhoA and Rac GTPase activities
The declines in ERM phosphorylation suggest that upstream regulators of ERM phosphorylation could also be altered by age. At present, the kinases and phosphatases responsible for direct regulation of pERM are not well defined, but in lymphocytes ERM phosphorylation status is known to be controlled by the RhoA and Rac1 GTPases (Brdickova et al., 2001; Nijhara et al., 2004; Ramaswamy et al., 2007; Salojin et al., 1999). Increases in Rac1 GTPase activity, probably accompanied by declines in RhoA, have been shown to reduce ERM phosphorylation. Using specific assays to measure the activity of RhoA and Rac1 we found that CD4 T cells from old CB6F1 mice show significant enhancement in Rac1 GTPase activity and decline in RhoA activity (Garcia et al., 2007). The age-related increases in Rac1 activity accompanied by a decline in RhoA and pERM are consistent with the decline with age in baseline ERM phosphorylation in T cells. We do not know the basis for the changes Rac1/Rho GTPase activity, although we speculate that this involves alterations in upstream effectors such as Vav. Enhancement of Vav1 GTPase activity has been shown to increase Rac1 activity (Cantrell, 1998; Fischer et al., 1998; Salojin et al., 1999; Swat and Fujikawa, 2005; Tybulewicz et al., 2003). The Vav signaling pathway can control ERM phosphorylation status and ERM function (Faure et al., 2004). In this context we have recently found that age increases Vav activity (Garcia and Miller, 2009), consistent with a model in which altered Vav leads to augmented Rac1 action and in this way to changes in baseline ERM activity. Activation of Vav1 and Rac1 have also been shown to increase F-actin polymerization (Fischer et al., 1998; Hornstein et al., 2004; Tybulewicz et al., 2003; Wulfing et al., 2000), suggesting that increases in F-actin polymerization found in T cells from old mice, described in the first section of this review, could be also the result of increases in activity of theVav-Rac1 signaling pathway. Further work is needed to define upstream controls and downstream effects of altered Vav function in aged T cells, and to see whether alternative pathways that regulate Rho GTPases (for examples see Dovas and Couchman, 2005; DerMardirossian and Bokoch, 2005; Olofsson, 1999; Zalcman et al., 1999; Sasaki and Takai, 1998) may also play a role in the age-related declines of ERM function.
Conclusions and Remarks
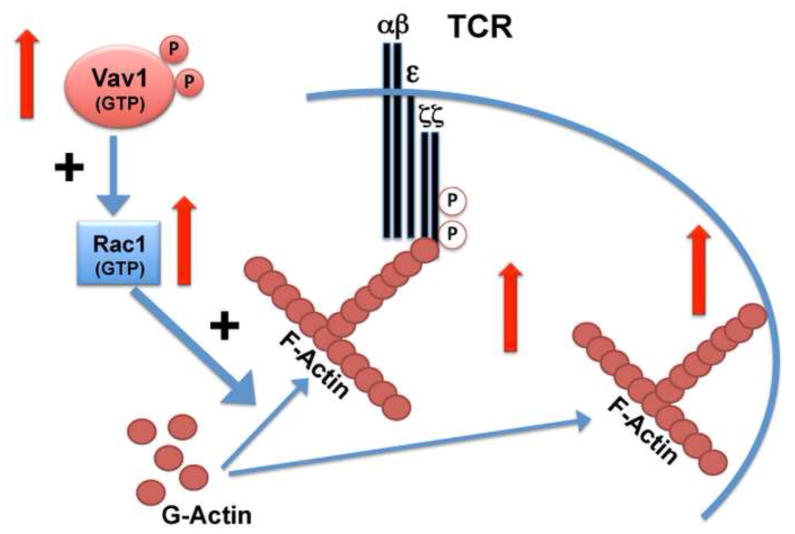
Immunosenescence, the age-related decline in protective immune function, reflects both a change in the proportions of different classes of T cells and also cell autonomous defects in the earliest stages of the activation process. The most striking and consistent changes are seen in CD4 T cells, including changes in baseline characteristics and responses to stimuli. Early studies suggested that changes in membrane fluidity may be responsible for some of the age related defect in T cell function, and more recently alterations in cytoskeletal architecture have been implicated in immunosenescence. However, most of the early studies were conducted using a mixture of CD4 and CD8 naïve and memory cells, making it difficult to disentangle changes due to alterations in cell proportions, cell interactions, and cell autonomous activation defects. More recent work, reviewed above, has shown that CD4 T cells from old mice show changes in their cytoskeletal architecture that are independent of defects in TCR signal transduction. These include the increases in F-actin polymerization, the lack of lammellopodia formation, increases in CD3ζ association to the cytoskeletal matrix and increases in Vav1-Rac1 signaling. From these results a model of the effect of age in resting CD4 T cells can be postulated, as presented in Figure 3. In this model, aging leads to increases in the GTPase activity of Vav that increases the activity of the Rac1 function. The increases in Rac1, in turn, lead to increases the F-actin levels of CD4 T cells from old donors. The higher F-actin may be responsible for the increases in CD3ζ association to the cytoskeleton matrix, which in turn diminish the fluidity of the membrane and impair efficient TCR capping. In addition, the increases in F-actin may also be responsible for increases in association of other signaling proteins to the F-actin cytoskeleton, perhaps including CD43, which can have a negative impact on TCR signaling.
Figure 3. General model of aging effects on F-actin signaling.
During aging there is a significant increase (indicated by the red arrows) in Vav phosphorylation and increases in GTPase activity. These increases lead to activation of Rac1function that in turn increases the polymerization of G-actin to the F-actin form. Higher F-actin present in the T cells results in increases in association of CD3ζ phosphorylated isoforms to the cytoskeleton. The overall result is a decrease in TCR mobility and membrane fluidity that may diminish TCR signaling. In addition, the age-related increases in F-actin may result in its association to other signaling molecules in the cytoskeleton or increases in the links between membrane and cytoskeleton, with unknown consequences for T cell function.
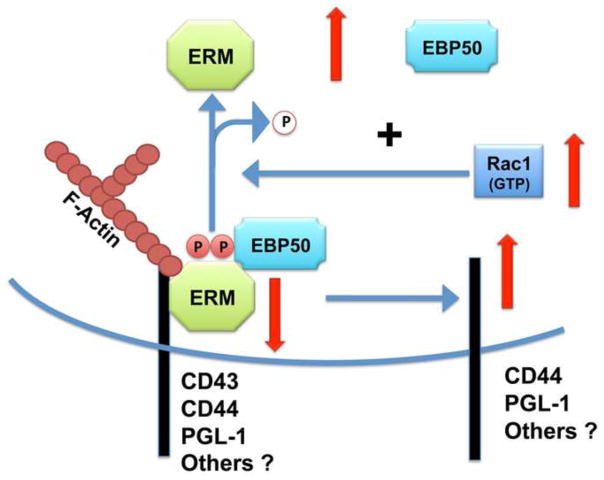
The reasons for the age-related increases in the Vav-Rac1 activity are not known; it is tempting to speculate that those changes may be the result of age-related alterations in the tyrosine kinases upstream of Vav (such as Zap-70 or lck) or changes in the phosphatases involved in regulating Vav phosphorylation. However, the increases in Vav-Rac1 activity with age may have other consequences in addition to those mediated by changes in F-actin polymerization. Rac1 and other Rho family members control the phosphorylation status of the ERM proteins (Niggli and Rossy, 2008; Nijhara et al., 2004). In this hypothetical model (see Figure 4), age-related increases in Rac1 induce a dephosphorylation of the ERM, which in turn increases the dissociation of the ERM from the membrane and dissociation of negative signaling molecules that regulate TCR signaling to the cytosolic environment, such as EBP50. In this context, it has been suggested that pERM proteins may help to maintain the distal pole complex, responsible for maintaining the polarization of the T cell during APC interaction, and may thus help to exclude negative regulators from the T cell-APC interface where TCR signaling is taking place. The age-related declines in ERM phosphorylation may weaken the cytoskeleton framework that maintains the formation of the distal pole complex and prevent exclusion of surface molecules that are ordinarily excluded from the immune synapse. Disruption of this membrane segregation process may allow these surface proteins, which may be heavily glycosylated in aged T cells, to interact with the TCR complex and inhibit TCR signaling.
Figure 4. Aging impairs ERM function.
The age-related increases in Rac1 also have other consequences. The higher threshold level of Rac1 activity induces dephosphorylation of the ERM, which in turn leads to dissociation of signaling molecules, such as EBP50 and CD44, from the ERM. Some of these molecules, such as EBP50, can diminish TCR signaling when released. In addition the lack of active phosphorylated ERM could prevent formation of the distal pole complex in CD4 T cells from old mice, increasing the access of TCR negative regulators (such as CD43, CD44 and others) to interfere with the formation of the immunological synapse.
Acknowledgments
Support: This work was supported by NIH grants AG019619 and AG030828
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15 (5):739–750. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- Anvari B, Torres JH, McIntyre BW. Regulation of pseudopodia localization in lymphocytes through application of mechanical forces by optical tweezers. J Biomed Opt. 2004;9 (5):865–872. doi: 10.1117/1.1778178. [DOI] [PubMed] [Google Scholar]
- Auvinen E, Kivi N, Vaheri A. Regulation of ezrin localization by Rac1 and PIPK in human epithelial cells. Exp Cell Res. 2007;313 (4):824–833. doi: 10.1016/j.yexcr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Berger SB, Sadighi Akha AA, Miller RA. A glycoprotein endopeptidase enhances calcium influx and cytokine production by CD4+ T cells of old and young mice. Int Immunol. 2005;17 (8):983–991. doi: 10.1093/intimm/dxh279. [DOI] [PubMed] [Google Scholar]
- Berger SB, Sadighi Akha AA, Miller RA, Garcia GG. CD43-independent augmentation of mouse T-cell function by glycoprotein cleaving enzymes. Immunology. 2006;119 (2):178–186. doi: 10.1111/j.1365-2567.2006.02419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billadeau DD, Burkhardt JK. Regulation of cytoskeletal dynamics at the immune synapse: new stars join the actin troupe. Traffic. 2006;7 (11):1451–1460. doi: 10.1111/j.1600-0854.2006.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Hivroz C. The immunological synapse: the more you look the less you know. Biol Cell. 2002b;94 (6):345–354. doi: 10.1016/s0248-4900(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Blanchard N, Hivroz C. The immunological synapse: the more you look the less you know. Biol Cell. 2002a;94 (6):345–354. doi: 10.1016/s0248-4900(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17 (1):87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Brdickova N, Brdicka T, Andera L, Spicka J, Angelisova P, Milgram SL, Horejsi V. Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton. FEBS Lett. 2001;507 (2):133–136. doi: 10.1016/s0014-5793(01)02955-6. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11 (1):109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3 (8):586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Brock MA, Chrest F. Differential regulation of actin polymerization following activation of resting T lymphocytes from young and aged mice. J Cell Physiol. 1993;157 (2):367–378. doi: 10.1002/jcp.1041570221. [DOI] [PubMed] [Google Scholar]
- Brohee D, Kennes B, Neve P. Increased stability of E-rosettes and restricted capping of sheep erythrocytes by lymphocytes of aged humans. Mech Ageing Dev. 1982;18 (1):47–52. doi: 10.1016/0047-6374(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14 (3):315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- Bustelo XR. Regulation of Vav proteins by intramolecular events. Front Biosci. 2002;7:d24–d30. doi: 10.2741/A766. [DOI] [PubMed] [Google Scholar]
- Cai Y, Sheetz MP. Force propagation across cells: mechanical coherence of dynamic cytoskeletons. Curr Opin Cell Biol. 2009;21 (1):47–50. doi: 10.1016/j.ceb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JL, Burkhardt JK. The regulation of actin remodeling during T-cell-APC conjugate formation. Immunol Rev. 2002;186:90–99. doi: 10.1034/j.1600-065x.2002.18609.x. [DOI] [PubMed] [Google Scholar]
- Cannon JL, Collins A, Mody PD, Balachandran D, Henriksen KJ, Smith CE, Tong J, Clay BS, Miller SD, Sperling AI. CD43 regulates Th2 differentiation and inflammation. J Immunol. 2008;180 (11):7385–7393. doi: 10.4049/jimmunol.180.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. Lymphocyte signalling: a coordinating role for Vav? Curr Biol. 1998;8 (15):R535–R538. doi: 10.1016/s0960-9822(07)00341-7. [DOI] [PubMed] [Google Scholar]
- Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987–1997. doi: 10.2741/1940. [DOI] [PubMed] [Google Scholar]
- Chiricolo M, Minelli L, Licastro F, Tabacchi P, Zannotti M, Franceschi C. Alterations of the capping phenomenon on lymphocytes from aged and Down’s syndrome subjects. Gerontology. 1984;30 (3):145–152. doi: 10.1159/000212622. [DOI] [PubMed] [Google Scholar]
- Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178 (3):1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- Cogoli-Greuter M, Lovis P, Vadrucci S. Signal transduction in T cells: an overview. J Gravit Physiol. 2004;11 (2):53–56. [PubMed] [Google Scholar]
- Cohen HJ, Boland KM, Rao KM. Age-related studies of SIg, Leu-4 and concanavalin A receptor densities and capping in human lymphocytes. Mech Ageing Dev. 1991;59 (3):253–262. doi: 10.1016/0047-6374(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Collins JM, Scott RB, McClish DK, Taylor JR, Grogan WM. Altered membrane anisotropy gradients of plasma membranes of living peripheral blood leukocytes in aging and Alzheimer’s disease. Mech Ageing Dev. 1991;59 (1–2):153–162. doi: 10.1016/0047-6374(91)90081-a. [DOI] [PubMed] [Google Scholar]
- Cullinan P, Sperling AI, Burkhardt JK. The distal pole complex: a novel membrane domain distal to the immunological synapse. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- Davis MM, Wulfing C, Krummel MF, Savage PA, Xu J, Sumen C, Dustin ML, Chien YH. Visualizing T-cell recognition. Cold Spring Harb Symp Quant Biol. 1999;64:243–251. doi: 10.1101/sqb.1999.64.243. [DOI] [PubMed] [Google Scholar]
- de Pablo MA, varez de CG. Modulatory effects of dietary lipids on immune system functions. Immunol Cell Biol. 2000;78 (1):31–39. doi: 10.1046/j.1440-1711.2000.00875.x. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Nieto M, Serrador JM, Sancho D, Vicente-Manzanares M, Martinez C, Sanchez-Madrid F. The two poles of the lymphocyte: specialized cell compartments for migration and recruitment. Cell Adhes Commun. 1998;6 (2–3):125–133. doi: 10.3109/15419069809004468. [DOI] [PubMed] [Google Scholar]
- Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15 (5):691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15 (7):356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008;37:65–95. doi: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390 (Pt 1):1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. A dynamic view of the immunological synapse. Semin Immunol. 2005;17 (6):400–410. doi: 10.1016/j.smim.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Dustin ML. Impact of the immunological synapse on T cell signaling. Results Probl Cell Differ. 2006;43:175–198. doi: 10.1007/400_019. [DOI] [PubMed] [Google Scholar]
- Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol. 2007;19 (5):529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008a;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- Dustin ML. Visualization of cell-cell interaction contacts-synapses and kinapses. Adv Exp Med Biol. 2008b;640:164–182. doi: 10.1007/978-0-387-09789-3_13. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Allen PM, Shaw AS. Environmental control of immunological synapse formation and duration. Trends Immunol. 2001;22 (4):192–194. doi: 10.1016/s1471-4906(01)01872-5. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5 (4):363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J Immunol. 2008;181 (7):4825–4831. doi: 10.4049/jimmunol.181.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VL, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5 (3):272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Tedford K, Penninger JM. Vav links antigen-receptor signaling to the actin cytoskeleton. Semin Immunol. 1998;10 (4):317–327. doi: 10.1006/smim.1998.0124. [DOI] [PubMed] [Google Scholar]
- Fuller CL, Braciale VL, Samelson LE. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol Rev. 2003;191:220–236. doi: 10.1034/j.1600-065x.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Berger SB, Sadighi Akha AA, Miller RA. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 2005;35 (2):622–631. doi: 10.1002/eji.200425538. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related changes in lck-Vav signaling pathways in mouse CD4 T cells. Cell Immunol. 2009 doi: 10.1016/j.cellimm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166 (5):3151–3157. doi: 10.4049/jimmunol.166.5.3151. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169 (9):5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol. 2003;33 (12):3464–3472. doi: 10.1002/eji.200324310. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Sadighi Akha AA, Miller RA. Age-related defects in moesin/ezrin cytoskeletal signals in mouse CD4 T cells. J Immunol. 2007;179 (10):6403–6409. doi: 10.4049/jimmunol.179.10.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert TD, Lipsky PE. Association of various T cell-surface molecules with the cytoskeleton. Effect of cross-linking and activation. J Immunol. 1991;146 (10):3298–3305. [PubMed] [Google Scholar]
- Gilman SC, Woda BA, Feldman JD. T lymphocytes of young and aged rats. I Distribution, density, and capping of T antigens. J Immunol. 1981;127 (1):149–153. [PubMed] [Google Scholar]
- Goldberg MW, Fiserova J, Huttenlauch I, Stick R. A new model for nuclear lamina organization. Biochem Soc Trans. 2008;36 (Pt 6):1339–1343. doi: 10.1042/BST0361339. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can’t have one without the other. Adv Immunol. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- Groves JT. Molecular organization and signal transduction at intermembrane junctions. Angew Chem Int Ed Engl. 2005;44 (23):3524–3538. doi: 10.1002/anie.200461014. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70 (3):179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- Han J, Das B, Wei W, Van AL, Mosteller RD, Khosravi-Far R, Westwick JK, Der CJ, Broek D. Lck regulates Vav activation of members of the Rho family of GTPases. Mol Cell Biol. 1997;17 (3):1346–1353. doi: 10.1128/mcb.17.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17 (5):476–479. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100 (25):15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201 (6):845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24 (6):663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JP, Holifield BF. Cell locomotion: new research tests old ideas on membrane and cytoskeletal flow. Cell Motil Cytoskeleton. 1991;18 (4):245–257. doi: 10.1002/cm.970180402. [DOI] [PubMed] [Google Scholar]
- Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181 (9):5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- Hogg N, Laschinger M, Giles K, McDowall A. T-cell integrins: more than just sticking points. J Cell Sci. 2003;116 (Pt 23):4695–4705. doi: 10.1242/jcs.00876. [DOI] [PubMed] [Google Scholar]
- Hogg N, Smith A, McDowall A, Giles K, Stanley P, Laschinger M, Henderson R. How T cells use LFA-1 to attach and migrate. Immunol Lett. 2004;92 (1–2):51–54. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004;16 (1):1–11. doi: 10.1016/s0898-6568(03)00110-4. [DOI] [PubMed] [Google Scholar]
- Huang Y, Burkhardt JK. T-cell-receptor-dependent actin regulatory mechanisms. J Cell Sci. 2007;120 (Pt 5):723–730. doi: 10.1242/jcs.000786. [DOI] [PubMed] [Google Scholar]
- Huber LA. The immunology of aging: immunoregulatory role of lipoproteins. Wien Klin Wochenschr. 1989;101 (13):440–446. [PubMed] [Google Scholar]
- Huber LA, Xu QB, Jurgens G, Bock G, Buhler E, Gey KF, Schonitzer D, Traill KN, Wick G. Correlation of lymphocyte lipid composition membrane microviscosity and mitogen response in the aged. Eur J Immunol. 1991;21 (11):2761–2765. doi: 10.1002/eji.1830211117. [DOI] [PubMed] [Google Scholar]
- Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9 (10):1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun YM, Chung HL, McGrath JL, Waugh RE, Kim M. Activated integrin VLA-4 localizes to the lamellipodia and mediates t cell migration on VCAM-1. J Immunol. 2009;183 (1):359–369. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sakakibara M, Yamasaki S, Takeuchi A, Arase H, Miyazaki M, Nakajima N, Okada M, Saito T. Cutting edge: negative regulation of immune synapse formation by anchoring lipid raft to cytoskeleton through Cbp-EBP50-ERM assembly. J Immunol. 2002;168 (2):541–544. doi: 10.4049/jimmunol.168.2.541. [DOI] [PubMed] [Google Scholar]
- Jones SC, Clise-Dwyer K, Huston G, Dibble J, Eaton S, Haynes L, Swain SL. Impact of post-thymic cellular longevity on the development of age-associated CD4+ T cell defects. J Immunol. 2008;180 (7):4465–4475. doi: 10.4049/jimmunol.180.7.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk CJ, Freilich AM, Miller RA. Age-related decline in activation of JNK by TCR- and CD28-mediated signals in murine T-lymphocytes. Cell Immunol. 1999;197 (2):75–82. doi: 10.1006/cimm.1999.1567. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Miller RA. Age-sensitive and -insensitive pathways leading to JNK activation in mouse CD4(+) T-cells. Cell Immunol. 1999;197 (2):83–90. doi: 10.1006/cimm.1999.1568. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Miller RA. Analysis of Raf-1 activation in response to TCR activation and costimulation in murine T-lymphocytes: effect of age. Cell Immunol. 1998;190 (1):33–42. doi: 10.1006/cimm.1998.1382. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Davis MM. Dynamics of the immunological synapse: finding, establishing and solidifying a connection. Curr Opin Immunol. 2002;14 (1):66–74. doi: 10.1016/s0952-7915(01)00299-0. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7 (11):1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18 (3):276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lee JH, Katakai T, Hara T, Gonda H, Sugai M, Shimizu A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J Cell Biol. 2004;167 (2):327–337. doi: 10.1083/jcb.200403091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178 (3):1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- Long A, Mitchell S, Kashanin D, Williams V, Prina MA, Shvets I, Kelleher D, Volkov Y. A multidisciplinary approach to the study of T cell migration. Ann N Y Acad Sci. 2004;1028:313–319. doi: 10.1196/annals.1322.035. [DOI] [PubMed] [Google Scholar]
- Makrogianneli K, Carlin LM, Keppler MD, Matthews DR, Ofo E, Coolen A, meer-Beg SM, Barber PR, Vojnovic B, Ng T. Integrating receptor signal inputs that influence small Rho GTPase activation dynamics at the immunological synapse. Mol Cell Biol. 2009;29 (11):2997–3006. doi: 10.1128/MCB.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat P, Roy C, Martin M. ERM proteins in cell adhesion and membrane dynamics. Trends Cell Biol. 1999;9 (5):187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- Marano N, Holowka D, Baird B. Bivalent binding of an anti-CD3 antibody to Jurkat cells induces association of the T cell receptor complex with the cytoskeleton. J Immunol. 1989;143 (3):931–938. [PubMed] [Google Scholar]
- Martin TA, Harrison G, Mansel RE, Jiang WG. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol. 2003;46 (2):165–186. doi: 10.1016/s1040-8428(02)00172-5. [DOI] [PubMed] [Google Scholar]
- Meiri KF. Membrane/cytoskeleton communication. Subcell Biochem. 2004;37:247–282. doi: 10.1007/978-1-4757-5806-1_8. [DOI] [PubMed] [Google Scholar]
- Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Minin AA, Moldaver MV. Intermediate vimentin filaments and their role in intracellular organelle distribution. Biochemistry (Mosc) 2008;73 (13):1453–1466. doi: 10.1134/s0006297908130063. [DOI] [PubMed] [Google Scholar]
- Miyamoto YJ, Andruss BF, Mitchell JS, Billard MJ, McIntyre BW. Diverse roles of integrins in human T lymphocyte biology. Immunol Res. 2003;27 (1):71–84. doi: 10.1385/IR:27:1:71. [DOI] [PubMed] [Google Scholar]
- Mody PD, Cannon JL, Bandukwala HS, Blaine KM, Schilling AB, Swier K, Sperling AI. Signaling through CD43 regulates CD4 T-cell trafficking. Blood. 2007;110 (8):2974–2982. doi: 10.1182/blood-2007-01-065276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M, Miceli MC. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9 (6):787–796. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- Murphy KM. Fate vs choice: the immune system reloaded. Immunol Res. 2005;32 (1–3):193–200. doi: 10.1385/IR:32:1-3:193. [DOI] [PubMed] [Google Scholar]
- Niggli V, Rossy J. Ezrin/radixin/moesin: versatile controllers of signaling molecules and of the cortical cytoskeleton. Int J Biochem Cell Biol. 2008;40 (3):344–349. doi: 10.1016/j.biocel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Nijhara R, van Hennik PB, Gignac ML, Kruhlak MJ, Hordijk PL, Delon J, Shaw S. Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J Immunol. 2004;173 (8):4985–4993. doi: 10.4049/jimmunol.173.8.4985. [DOI] [PubMed] [Google Scholar]
- Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16 (1):24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha AB, Antel JP, Roos RP, Arnason BG. Changes in concanavalin A capping of human lymphocytes with age. Mech Ageing Dev. 1980;12 (4):331–337. doi: 10.1016/0047-6374(80)90066-4. [DOI] [PubMed] [Google Scholar]
- Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11 (8):545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Otteskog P, Sundqvist KG. Characterization of the spreading process in human T lymphocytes. Exp Cell Res. 1983;149 (1):201–213. doi: 10.1016/0014-4827(83)90392-0. [DOI] [PubMed] [Google Scholar]
- Peck MD, Li Z, Jy W, Chu AJ, Bourguignon LY. Association of murine splenocyte CD3 complex to the cytoskeleton: absence of modulation by exogenous fatty acids. Cell Biol Int. 1996;20 (8):531–537. doi: 10.1006/cbir.1996.0069. [DOI] [PubMed] [Google Scholar]
- Poenie M, Kuhn J, Combs J. Real-time visualization of the cytoskeleton and effector functions in T cells. Curr Opin Immunol. 2004;16 (4):428–438. doi: 10.1016/j.coi.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Pribila JT, Shimizu Y. Signal transduction events regulating integrin function and T cell migration: new functions and complexity. Immunol Res. 2003;27 (1):107–128. doi: 10.1385/ir:27:1:107. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M, Dumont C, Cruz AC, Muppidi JR, Gomez TS, Billadeau DD, Tybulewicz VL, Siegel RM. Cutting edge: Rac GTPases sensitize activated T cells to die via Fas. J Immunol. 2007;179 (10):6384–6388. doi: 10.4049/jimmunol.179.10.6384. [DOI] [PubMed] [Google Scholar]
- Rao KM. Age-related differential effects of zinc on concanavalin A-induced capping of human lymphocytes. Exp Gerontol. 1982;17 (3):205–211. [PubMed] [Google Scholar]
- Rao KM, Currie MS, Padmanabhan J, Cohen HJ. Age-related alterations in actin cytoskeleton and receptor expression in human leukocytes. J Gerontol. 1992;47 (2):B37–B44. doi: 10.1093/geronj/47.2.b37. [DOI] [PubMed] [Google Scholar]
- Ren L, Hong SH, Cassavaugh J, Osborne T, Chou AJ, Kim SY, Gorlick R, Hewitt SM, Khanna C. The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene. 2009;28 (6):792–802. doi: 10.1038/onc.2008.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M, Sanchez-Madrid F, Valenzuela-Fernandez A. The role of actomyosin and the microtubular network in both the immunological synapse and T cell activation. Front Biosci. 2007;12:437–447. doi: 10.2741/2073. [DOI] [PubMed] [Google Scholar]
- Rivich DA, Rosen GM, Cohen HJ. Membrane protein organization of peripheral blood lymphocytes from healthy young and aged adults. Mech Ageing Dev. 1988;45 (1):65–74. doi: 10.1016/0047-6374(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Rivnay B, Bergman S, Shinitzky M, Globerson A. Correlations between membrane viscosity, serum cholesterol, lymphocyte activation and aging in man. Mech Ageing Dev. 1980;12 (2):119–126. doi: 10.1016/0047-6374(80)90088-3. [DOI] [PubMed] [Google Scholar]
- Rivnay B, Globerson A, Shinitzky M. Viscosity of lymphocyte plasma membrane in aging mice and its possible relation to serum cholesterol. Mech Ageing Dev. 1979;10 (1–2):71–79. doi: 10.1016/0047-6374(79)90071-x. [DOI] [PubMed] [Google Scholar]
- Romero F, Fischer S. Structure and function of vav. Cell Signal. 1996;8 (8):545–553. doi: 10.1016/s0898-6568(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Rozdzial MM, Malissen B, Finkel TH. Tyrosine-phosphorylated T cell receptor zeta chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3 (5):623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- Rozdzial MM, Pleiman CM, Cambier JC, Finkel TH. pp56Lck mediates TCR zeta-chain binding to the microfilament cytoskeleton. J Immunol. 1998;161 (10):5491–5499. [PubMed] [Google Scholar]
- Sadighi Akha AA, Berger SB, Miller RA. Enhancement of CD8 T-cell function through modifying surface glycoproteins in young and old mice. Immunology. 2006;119 (2):187–194. doi: 10.1111/j.1365-2567.2006.02420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228 (1):9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Salojin KV, Zhang J, Delovitch TL. TCR and CD28 are coupled via ZAP-70 to the activation of the Vav/Rac-1-/PAK-1/p38 MAPK signaling pathway. J Immunol. 1999;163 (2):844–853. [PubMed] [Google Scholar]
- Samstag Y, Eibert SM, Klemke M, Wabnitz GH. Actin cytoskeletal dynamics in T lymphocyte activation and migration. J Leukoc Biol. 2003;73 (1):30–48. doi: 10.1189/jlb.0602272. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245 (3):641–645. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- Sechi AS, Wehland J. Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol. 2004;25 (5):257–265. doi: 10.1016/j.it.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Seminario MC, Bunnell SC. Signal initiation in T-cell receptor microclusters. Immunol Rev. 2008;221:90–106. doi: 10.1111/j.1600-065X.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- Shaw AS. FERMing up the synapse. Immunity. 2001;15 (5):683–686. doi: 10.1016/s1074-7613(01)00237-0. [DOI] [PubMed] [Google Scholar]
- Sheetz MP. Glycoprotein motility and dynamic domains in fluid plasma membranes. Annu Rev Biophys Biomol Struct. 1993;22:417–431. doi: 10.1146/annurev.bb.22.060193.002221. [DOI] [PubMed] [Google Scholar]
- Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5 (4):239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stradal TE, Pusch R, Kliche S. Molecular regulation of cytoskeletal rearrangements during T cell signalling. Results Probl Cell Differ. 2006;43:219–244. doi: 10.1007/400_022. [DOI] [PubMed] [Google Scholar]
- Swat W, Fujikawa K. The Vav family: at the crossroads of signaling pathways. Immunol Res. 2005;32 (1–3):259–265. doi: 10.1385/IR:32:1-3:259. [DOI] [PubMed] [Google Scholar]
- Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165 (3):1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- Tomas EM, Chau TA, Madrenas J. Clustering of a lipid-raft associated pool of ERM proteins at the immunological synapse upon T cell receptor or CD28 ligation. Immunol Lett. 2002;83 (2):143–147. doi: 10.1016/s0165-2478(02)00075-5. [DOI] [PubMed] [Google Scholar]
- Tong J, Allenspach EJ, Takahashi SM, Mody PD, Park C, Burkhardt JK, Sperling AI. CD43 regulation of T cell activation is not through steric inhibition of T cell-APC interactions but through an intracellular mechanism. J Exp Med. 2004;199 (9):1277–1283. doi: 10.1084/jem.20021602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley AJ, Jacobelli J, Moldovan MC, Douglas A, Krummel MF. T cell synapse assembly: proteins, motors and the underlying cell biology. Semin Immunol. 2005;17 (1):65–75. doi: 10.1016/j.smim.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Traill KN, Schonitzer D, Jurgens G, Bock G, Pfeilschifter R, Hilchenbach M, Holasek A, Forster O, Wick G. Age-related changes in lymphocyte subset proportions, surface differentiation antigen density and plasma membrane fluidity: application of the eurage senieur protocol admission criteria. Mech Ageing Dev. 1985;33 (1):39–66. doi: 10.1016/0047-6374(85)90108-3. [DOI] [PubMed] [Google Scholar]
- Tskvitaria-Fuller I, Rozelle AL, Yin HL, Wulfing C. Regulation of sustained actin dynamics by the TCR and costimulation as a mechanism of receptor localization. J Immunol. 2003;171 (5):2287–2295. doi: 10.4049/jimmunol.171.5.2287. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol. 2005;17 (3):267–274. doi: 10.1016/j.coi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Vav1: a key signal transducer downstream of the TCR. Immunol Rev. 2003;192:42–52. doi: 10.1034/j.1600-065x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- Vallejo AN. Age-dependent alterations of the T cell repertoire and functional diversity of T cells of the aged. Immunol Res. 2006;36 (1–3):221–228. doi: 10.1385/IR:36:1:221. [DOI] [PubMed] [Google Scholar]
- van der Merwe PA. Formation and function of the immunological synapse. Curr Opin Immunol. 2002;14 (3):293–298. doi: 10.1016/s0952-7915(02)00350-3. [DOI] [PubMed] [Google Scholar]
- van Leeuwen JE, Samelson LE. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11 (3):242–248. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- Volkov Y, Long A, Kelleher D. Inside the crawling T cell: leukocyte function-associated antigen-1 cross-linking is associated with microtubule-directed translocation of protein kinase C isoenzymes beta(I) and delta. J Immunol. 1998;161 (12):6487–6495. [PubMed] [Google Scholar]
- Wange RL. LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE 2000. 2000;(63):RE1. doi: 10.1126/stke.2000.63.re1. [DOI] [PubMed] [Google Scholar]
- Ward SG, Marelli-Berg FM. Mechanisms of chemokine and antigen-dependent T-lymphocyte navigation. Biochem J. 2009;418 (1):13–27. doi: 10.1042/BJ20081969. [DOI] [PubMed] [Google Scholar]
- Wulfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc Natl Acad Sci U S A. 2000;97 (18):10150–10155. doi: 10.1073/pnas.97.18.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Matsui T, Tsukita S, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115 (Pt 12):2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- Zalcman G, Dorseuil O, Garcia-Ranea JA, Gacon G, Camonis J. RhoGAPs and RhoGDIs, (His)stories of two families. Prog Mol Subcell Biol. 1999;22:85–113. doi: 10.1007/978-3-642-58591-3_5. [DOI] [PubMed] [Google Scholar]