Abstract
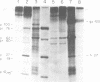
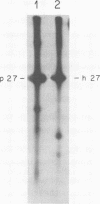
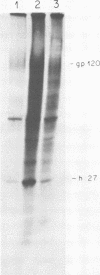
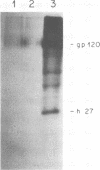
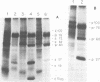
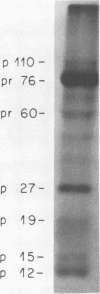
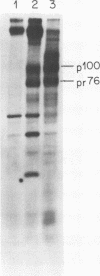
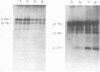
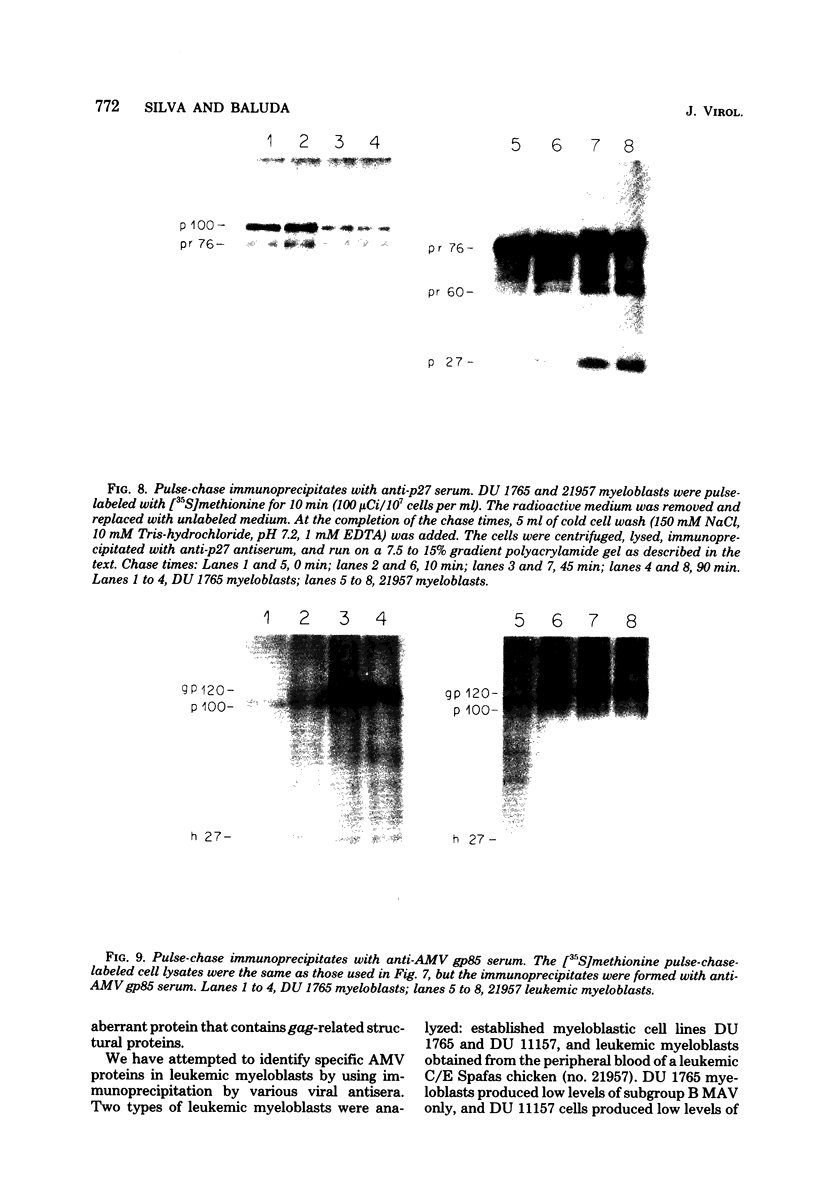
We have analyzed the avian myeloblastosis virus proteins in two types of leukemic myeloblasts: established myeloblastic cell lines (DU 1765 and DU 11157) and leukemic myeloblasts obtained from the peripheral blood of a leukemic C/E Spafas chicken (no. 21957). Using monospecific antisera for immunoprecipitation and polyacrylamide gel electrophoresis, we have detected gag gene-related proteins in the myeloblasts. The DU 1765 and DU 11157 cells contained a p100 protein which possessed antigenic determinants of the viral proteins p27, p19, p15, and p12. The p100 was not found in leukemic myeloblasts from Spafas chickens, and pulse-chase experiments showed that the p100 was not a precursor for the viral proteins. However, the p100 is present in uninfected line 15 chicken embryos. A pr76-like protein was identified in DU 1765 cells but migrated slightly further into gels than the pr76 of Spafas-derived leukemic myeloblasts. The Spafas-derived myeloblasts produced a pr60, whereas the DU 1765 cells contained instead a related protein of 62,000 daltons. Using anti-avian myeloblastosis virus gp85 sera, a glycoprotein of 120,000 daltons (gp120) was detected in all the tested leukemic myeloblasts. The gp120 was also present, in low amounts, in uninfected embyonic spleen and yolk sac cells. The anti-gp85 sera also precipitated a 27,000-dalton protein (h27) in these same cells. Both the gp120 and h27 could not be detected in either uninfected or myeloblastosis-associated virus-infected fibroblasts. Limited peptide hydrolysis revealed that h27 is different from the viral structural protein p27. In conclusion, monospecific antisera for gag and env gene products of avian myeloblastosis virus did not precipitate any unique or aberrant avian myeloblastosis virus protein from leukemic myeloblasts.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., GOETZ I. E. Morphological conversion of cell cultures by avian myeloblastosis virus. Virology. 1961 Oct;15:185–199. doi: 10.1016/0042-6822(61)90234-3. [DOI] [PubMed] [Google Scholar]
- Bergmann D. G., Souza L. M., Baluda M. A. Characterization of avian myeloblastosis-associated virus DNA intermediates. J Virol. 1980 May;34(2):366–372. doi: 10.1128/jvi.34.2.366-372.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosch V., Kurth R., Smart J. E. The detection of glycoproteins immunologically related to RSV gp85 in uninfected avian cells and in sera from uninfected birds. Virology. 1978 May 1;86(1):226–240. doi: 10.1016/0042-6822(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins J. J., Montelaro R. C., Denny T. P., Ishizaki R., Langlois A. J., Bolognest D. P. Normal chicken cells (chf-) express a surface antigen which cross-reacts with determinants of the major envelope glycoprotein (gp85) of avian myeloblastosis virus. Virology. 1978 May 1;86(1):205–216. doi: 10.1016/0042-6822(78)90021-1. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Shaikh R., Mason W. S. Identification of an avian oncovirus polyprotein in uninfected chick cells. Cell. 1978 May;14(1):89–104. doi: 10.1016/0092-8674(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Viral polyproteins in chick embryo fibroblasts infected with avian sarcoma leukosis viruses. Virology. 1978 Mar;85(1):241–252. doi: 10.1016/0042-6822(78)90428-2. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Langlois A. J., Ishizaki R., Beaudreau G. S., Kummer J. F., Beard J. W., Bolognesi D. P. Virus-infected avian cell lines established in vitro. Cancer Res. 1976 Nov;36(11 Pt 1):3894–3904. [PubMed] [Google Scholar]
- Langlois A. J., Sankaran S., Hsiung P. H., Beard J. W. Massive direct conversion of chick embryo cells by strain MC29 avian leukosis virus. J Virol. 1967 Oct;1(5):1082–1084. doi: 10.1128/jvi.1.5.1082-1084.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Ogura H., Friis R. Further evidence for the existence of a viral envelope protein defect in the Bryan high-titer strain of Rous sarcoma virus. J Virol. 1975 Aug;16(2):443–446. doi: 10.1128/jvi.16.2.443-446.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Silva R. F., Moscovici C. Spontaneous regression of leukemia in chickens infected with avian myeloblastosis virus. Proc Soc Exp Biol Med. 1973 Jul;143(3):604–611. doi: 10.3181/00379727-143-37376. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]