Abstract
Gli1 is a transcription factor and oncogene with documented roles in the progression of several cancer types, including cancers of the skin and pancreas. The contribution of Gli1 to the progression of breast cancer is less established. In order to investigate the functional impact of Gli1 in breast cancer, expression of Gli1 and its contribution to cell growth was assessed in breast cancer cell lines. These in vitro results were compared to expression of Gli1, determined by immunohistochemistry, in 171 breast cancers. In these cancers, the association of Gli1 with expression of estrogen receptor α (ERα) and progesterone receptor (PR), ErbB2, p53, the rate of proliferation, and clinicopathologic parameters and outcome was assessed. Expression of Gli1 and ERα mRNA was strongly correlated in ERα-positive cell lines (r = 0.999). Treatment with estrogen increased expression of Gli1 in 2 of 3 ERα-positive cell lines; this increase was prevented by treatment with the ERα-specific antagonist MPP. Silencing of Gli1 by shRNA markedly reduced the survival of two ERα-negative cell lines, but caused only a modest reduction in ERα-positive cell lines. In breast cancer tissues, cancers with nuclear localization of Gli1 had a higher ERα (P=0.027) and lower p53 expression (P=0.017) than those without nuclear localization of Gli1. However, nuclear localization of Gli1 was predictive of a poorer cancer-specific survival in ERα-negative, including triple negative, cancers (P = 0.005), but not ERα-positive cancers. In conclusion, we demonstrate a positive association between expression of Gli1 and ERα; however, our data indicate a greater functional effect of Gli1 in ERα-negative cancers.
Keywords: Breast, Cancer, Gli1, Estrogen receptor α, Prognosis
Introduction
Gli1 is a zinc finger transcription factor, an established oncogene, and a member of the vertebrate Gli family, which also includes Gli2 and Gli3. The Gli transcription factors are members of the hedgehog signaling pathway, and have been most studied in this context [1]. The Gli transcription factors coordinately regulate Gli-mediated transcription [2]; however, Gli1 is exclusively a transcriptional activator, whereas Gli2 and Gli3 can function as either transcriptional activators or repressors [3, 4]. Hedgehog signaling activates Gli1 and Gli-mediated transcription, and because Gli1 is a target of Gli-mediated transcription, increases expression of Gli1. In addition to hedgehog signaling, expression of Gli1 and activation of Gli-mediated transcription are also induced by other signaling pathways, including TGFβ and Ras [5, 6]. The TGFβ stimulation of Gli1 transcription is SMAD3 mediated and independent of hedgehog signaling [5]. Oncogenic Ras also upregulates Gli1 expression and transcriptional activity independently of hedgehog signaling through MAPK/ERK and/or AKT/PI3K [7, 8]. Therefore, Gli1 and Gli-mediated transcription can be up-regulated by several signaling pathways known to be important in the development and progression of cancer.
Gli1 is transforming in rat kidney epithelial cells (i.e., RK3E cells) immortalized with adenovirus E1A [9]. In basal cell carcinomas, medulloblastomas and rhabdomyosarcomas, Gli1 is overexpressed as a result of constitutive activation of hedgehog signaling by mutations of hedgehog pathway members. This aberrant hedgehog signaling and resultant increase in Gli1 and Gli transcriptional activity drives the genesis of these cancers [10]. In other types of cancer, including carcinomas of the pancreas and prostate, mutations of hedgehog pathway members are rare; instead, Gli1 is up-regulated by either hedgehog ligand dependent or other hedgehog ligand-independent mechanisms [11-16]. In these cancers, Gli1 and Gli-mediated transcription have been shown to be important in cancer cell growth and survival both in vitro and in xenograft models [12, 13, 15, 17-19]. Gli1 is also reported to promote invasion and metastasis in prostate cancer and melanoma [12, 20].
In breast cancer, Gli1 is overexpressed in 40–100% of cancers [16, 21-23]. Gli1 is believed to be up-regulated by both hedgehog ligand dependent (i.e., overexpression of hedgehog ligand) and ligand independent (e.g., silencing of Ptch1 by promoter methylation) hedgehog signaling, as well as hedgehog independent mechanisms (e.g., Ras, TGFβ) [5, 16, 21-24]. Additionally, breast cancer stem cells, characterized by the cell surface phenotype CD44+CD24−/low and tumor initiating capacity, were found to express Gli1 at higher levels than other cancer cells [25].
The prognostic and functional significance of Gli1 in breast cancer has been less extensively explored. In this report, we identify a positive association between expression of Gli1 and estrogen receptor α (ERα) in breast cancer cell lines, as well as a differential effect of Gli1 on the growth of ERα-positive versus ERα-negative breast cancer cells. We then correlate these in vitro findings with expression of Gli1 in breast cancer tissues and report a prognostic role for Gli1 in breast cancer and specifically in ERα-negative breast cancers.
Methods
Cell line maintenance
The human breast cancer cell lines MDA-MB-361, T47D, MCF7, BT474, and Hs578T were obtained from the American Type Culture Collection (Manassas, VA). MDAMD-231 cells were a kind gift of Dr. Danny Welch. These breast cancer cell lines are classified into two major groups—ERα-positive (MDA-MB-361, T47D, MCF7, and BT474) and ERα-negative (MDA-MB-231 and Hs578T). MDA-MB-231, T47D, and MCF-7 were established from the pleural effusions of patients with metastatic breast cancer [26]. MDA-MB-361 was derived from a brain metastasis of breast cancer [27]. BT474 was isolated from a primary invasive ductal carcinoma [26]. Hs578T was derived from a carcinosarcoma of the breast [28]. MDAMB-231, MDA-MB-361, MCF7, T47D, and BT474 are all tumorigenic in nude mice, while Hs578T is non-tumorigenic [28, 29] Among the cell lines used in this study, MDA-MB-231 is the most invasive in vitro and the most metastatic in xenograft animal models [26]. Hs578T has a moderate capability for invasion without metastatic potential [26]. MDA-MB-361, MCF7, and T47D are weakly invasive and rarely shown to be metastatic as xenografts [26, 30-33].
MCF7, MDA-MB-231, Hs578T, and MDA-MB-361 were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS). T47D and BT474 were cultured in RPMI 1640 medium containing 10% FBS.
Quantitative, reverse transcription PCR
After RNA extraction, all samples were subjected to DNase pretreatment prior to cDNA synthesis (High-Capacity cDNA Reverse Transcription kit, Applied Biosystems). Primer and probe sets for estrogen receptor (ER)α, Gli1, and Ribosomal protein large 0 (RPLP0) were purchased as proprietary TaqMan® Gene Expression Assays-on-Demand™ from Applied Biosystems (Hs01046816_m1, Hs00171790_m1, and Hs99999902_m1, respectively). Gene assays were performed using TaqMan® Universal PCR Master Mix (Applied Biosystems) and primer/probe sets for each target. Fluorescent signal data were collected by the ABI Prism 7700 Sequence Detection System and the log-linear phase of amplification was monitored to obtain CT values. RPLP0 was used as the internal reference. Relative expression values were calculated using the ΔΔCT method. Estrogen and ERα antagonist treatment of ERα-positive cell lines
For estrogen treatment, the maintenance medium was removed 2 days after plating and replaced with phenol red-free and serum-free DMEM for 24 or 48 h. The cells were then cultured in phenol red-free DMEM containing 0.5% charcoal-stripped FBS with 17β-estradiol (Steraloids Inc.) in ethanol for 48 h. For treatment with the ERα-antagonist methyl-piperidino-pyrazole (MPP) (Tocris Bioscience), 1.0 μM MPP in 95% ethanol was added to the medium 1 h before estrogen treatment. The cells were harvested for RNA extraction.
RNA interference
Lentiviral supernatants were prepared by transfection of 293T with pLKO.1-puro containing each of two shRNAs targeting human GLI1 (TRCN0000020484, TRCN0000020488; Mission shRNA plasmid DNA, Sigma–Aldrich) or a non-targeting control (NT) shRNA (Sigma–Aldrich) and the packaging plasmids pCMV-GagPol and pCMV-VSVg (gifts of Dr. Mike Ruppert). Lentiviral supernatants were collected at 48 h post-transfection and were used to transduce MCF7, MDA-MB-361, Hs578T, and MDA-MB-231 cells according to the vendor's recommended protocols followed by selection in puromycin (Cellgro) for at least 3 days. Silencing of Gli1 was confirmed by immunoblots and/or quantitative, reverse transcription PCR.
Western blot analysis
Cells were lysed in the presence of 50 mM Tris–HCl at pH 7.4, 2 mM dithiothreitol, 1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 7X protease inhibitor cocktail (Roche). The protein concentration was measured by the BCA Protein Assay (Pierce). Fifty micrograms of total protein from each sample was resolved on 4–15% gradient gels (Biorad Laboratories) and transferred to nitrocellulose membranes. Ten micrograms total protein of a lysate of F9 cells (Santa Cruz Biotechnologies), a teratocarcinoma cell line with very high Gli1 expression, was used as a positive control. Membranes were blocked with 5% dry milk in 0.1% PBS-T, followed by incubation with an immunoaffinity purified, rabbit, polyclonal antibody raised against residues 410–427 of human Gli1, a gift of Dr. Mike Ruppert [34], at 1:2,500 dilution at 4°C overnight. Secondary detection was achieved using an anti-rabbit horseradish peroxidase labeled secondary antibody (1:5,000 dilution, Biorad) and Supersignal West Femto ECL reagent (Pierce).
Measurement of cell growth
MCF7, MDA-MB-361, MDA-MB-231, and Hs578T cells with Gli1 silencing and their respective non-targeting control cells were plated in 96-well plates in maintenance media and allowed to grow until the NT control cell approached confluence. Growth was measured by MTT assay (Promega) according to the manufacturer's protocol.
Tissue microarrays
Tissue microarrays were constructed in collaboration with Applied Genomics Inc. (Huntsville, AL), after collecting formalin-fixed, paraffin-embedded infiltrating ductal carcinomas of the breast, acquired during 1988–1996, from the archives of the University of Alabama at Birmingham (UAB) Department of Pathology. Two tissue microarrays were constructed, each containing a single 1 mm diameter core from the same 171 infiltrating ductal carcinomas. Clinical data corresponding to each breast cancer were retrieved from the UAB Department of Surgery and the UAB Tumor Registry. Demographic data for the patients are summarized in Table 1. Tissue collection, tissue microarray construction, and retrieval of clinical data were performed after Institutional Review Board approval.
Table 1.
Clinical and demographic data
| Age range (years) | 25–83 |
| Race (%) | |
| Caucasian | 70 |
| African American | 23 |
| Other | 1 |
| Unknown | 6 |
| Clinical Stage (%) | |
| I | 2 |
| II | 37 |
| III/IV | 24 |
| Unknown | 15 |
| Mean follow-up (years) | 7.8 |
Immunohistochemical staining and assessment
For immunohistochemical staining of formalin-fixed, paraffin-embedded tissue microarrays, 5 μM sections were deparaffinized in xylene, followed by hydration in graded alcohols. The immunostaining procedure for Gli1 was similar to a previously described protocol [35]. In brief, 5 μM thick sections were incubated in 3% H2O2 in methanol for 30 min at room temperature, followed by incubation in primary goat polyclonal anti-Gli1 antibody (1:100; N-16, sc-6153; Santa Cruz Biotechnology) overnight at 4°C. Secondary detection was achieved by incubation in a polymer-based secondary detection reagent (Histofine Simple Stain Max PO, Nichirei Co., Ltd.) for 30 min at room temperature. Diaminobenzidine tetrachloride (Biogenex) was used as the chromogen. The specificity of the staining was verified by specific staining of pancreatic islets in formalin-fixed, paraffin-embedded pancreatic tissues. Pancreatic islets cells have more active hedgehog signaling and higher expression of several pathway members, including Gli1, than surrounding acinar and ductal cells [36, 37]. Negative controls consisted of histologic sections processed without the addition of primary antibody. After immunostaining, sections were counter stained with hematoxylin.
Immunostaining for ErbB2, p53, ER, PR, and Ki67 proceeded as previously described [38, 39]. Briefly, immunostaining for ER, PR, and Ki-67 required low temperature antigen retrieval with enzymatic pretreatment (0.1% trypsin) followed by incubation in 10 mM citrate buffer, pH 6, for 2 h at 80°C. No antigen retrieval was required for ErbB2 and p53. The antibodies used were anti-ErbB2 (clone 3B5, Oncogene Research Products, San Diego, CA; 0.25 μg/ml), anti-p53 (clone BP53.12, Oncogene Research Products, Inc., San Diego, CA; 0.25 μg/ml), anti-ER (clone ER88, Biogenex, San Ramon, CA, 0.33 mg/ ml total protein), anti-PR (clone PR88, Biogenex, San Ramon, CA, 0.33 mg/ml total protein), and anti-Ki67 (clone MIB-1, Biogenex, San Ramon, CA, 0.37 mg/ml total protein). Secondary detection was achieved using a biotinylated secondary antibody, a streptavidin–horseradish peroxidase complex (Ultra Streptavidin Detection System, Signet Laboratories) and diaminobenzidine tetrachloride as the chromogen.
The intensity of cancer cell membrane immunostaining for ErbB2 and intensity of cytoplasmic and nuclear staining for Gli1 were scored separately on a scale of 0 (no staining) to 4+ (strongest possible intensity), and the percentage of cells staining at each intensity was estimated. The proportion of cells at each intensity was multiplied by the corresponding intensity value and these products were added to obtain an immunostaining score (immunoscore) ranging from 0 to 4 [38, 39]. For ER, PR, p53, p27Kip1, Ki67, and Gli1, the percentage of cancer cells with nuclei that stained positively at any intensity was determined. The mean of the immunostaining values of replicate tissue samples for each cancer on the two tissue microarrays was used for data analysis.
Data analysis
Correlation of expression of ERα and Gli1 in cell lines was assessed by Pearson's correlation co-efficient. Gli1 expression levels by quantitative, real-time PCR were compared between groups by Student's t-test with Welch's correction. Cell growth (i.e., absorbance values) was compared between groups by the Mann–Whitney test.
For immunohistochemical analysis, breast cancers with more than 2% of nuclei staining for Gli1 were designated as Gli1-positive. Those cancers with 2% or less of nuclei that were positive for Gli1 were designated as Gli1-negative. Cancers were considered ERα-positive or PR-positive with 10% or more positive nuclei and ErbB2-positive with 10% or more cells with strong (3+ or 4+) membrane staining, similar to the criterion for strong expression (3+) utilized by the Hercept-Test (Dako) [40]. For comparison of ERα with total cellular Gli1, the immunoscores for cytoplasmic and nuclear Gli1 were summed to arrive at a value for total Gli1. Comparison of the percentage of cells positive for ERα, PR, Ki-67 and p53, ErbB2 immuno-scores, and tumor size between Gli1-positive and Gli1-negative cancers was accomplished using the Mann–Whitney test. Numbers of cases with positive lymph nodes and clinical stage I disease were compared between Gli1-positive and Gli1-negative cancers using Fisher's exact test. Cause-specific overall survival was defined as the time from the date of diagnosis to the date of death due to breast cancer. Patients who were alive at the date of last contact or died from a disease other than breast cancer were censored at the date of last contact. The relationship between Gli1 nuclear expression and survival was examined by Kaplan–Meier curves [41]. Differences in overall survival functions were assessed using the log-rank test. Stepwise Cox regression was used to examine multivariate predictors of survival stratified by ERα status. For all analyses, a P value of <0.05 was deemed statistically significant.
Results
Expression of Gli1 correlates with expression of ERα in breast cancer cell lines
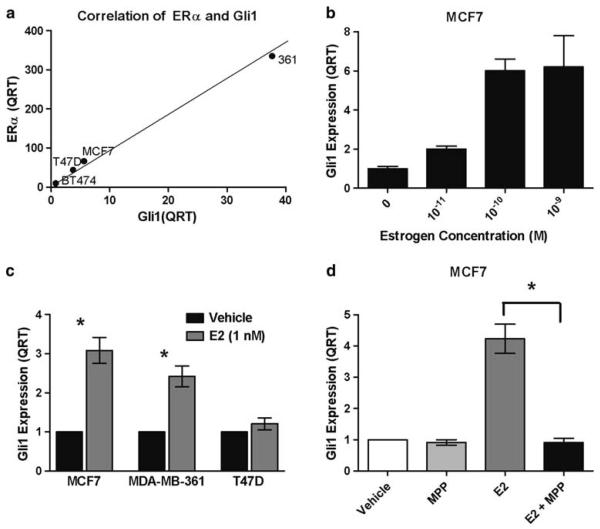
We have previously demonstrated that Gli1 is expressed at a higher level in breast cancer cell lines in comparison with MCF10A cells, which are derived from benign breast [21]. Next, we examined the relationship between the level of expression of ERα and Gli1 mRNA in four ERα-positive breast cancer cell lines, as measured by quantitative RT–PCR (QRT). There was a strong positive correlation (r = 0.999, P < 0.001) between expression of ERα and Gli1 mRNA (Fig. 1a). The Gli1 protein levels reflected the Gli1 mRNA levels in that Gli1 protein expression is highest in MDA-MB-361 cells and lower in the other ERα-positive cell lines (Supplemental Data, Figure S1), similar to the pattern found for Gli1 mRNA in these cell lines (Fig. 1a).
Fig. 1.
Estrogen, ERα, and Gli1 in breast cancer cell lines. a Expression of Gli1 and ERα were measured by quantitative RT–PCR (QRT) and expression was normalized to expression in MCF10A cells. A positive correlation was determined by Pearson's correlation co-efficient (r = 0.999, P < 0.001). b MCF7 cells were treated with a range of concentrations of 17β-estradiol for 48 h. Gli1 expression was measured by QRT and expression was normalized to the vehicle control. c MCF7, MDA-MB-361 and T47D ERα-positive cell lines were treated with 1 nM 17β-estradiol (E2) for 48 h and expression of Gli1 was measured by QRT. Expression is normalized to the vehicle control. The data are the mean and standard error of three experiments performed in triplicate. Asterisks indicate significance, P = 0.018 for MCF7 and P = 0.010 for MDA-MB-361. d MCF7 cells were treated with the ERα-specific antagonist, MPP, at 1 μM for 1 h prior to the addition of E2 (1 nM) for 48 h. Gli1 expression was measured by QRT. Expression is normalized to the vehicle control. Asterisks indicate significance, P = 0.021). The data are the mean and standard error of three experiments performed in triplicate
Estrogen up-regulates Gli1 expression via ERα
Estrogen has been reported to regulate Gli1 expression [42, 43]. Therefore, to investigate a possible explanation for the correlation between Gli1 and ERα expression, we treated several ERα-positive cell lines with estrogen and determined the effect on Gli1 expression by QRT. In MCF7 cells, treatment with 17β-estradiol for 48 h caused a dose-dependent increase in Gli1 mRNA (Fig. 1b). Treatment of MCF7, MDA-MB-361 (361), and T47D ERα-positive cell lines with 1 nM 17β-estradiol for 48 h resulted in a significant increase in Gli1 mRNA in MCF7 (3.1-fold, P = 0.018) and 361 cells (2.7-fold, P = 0.010), but not in T47D cells (Fig. 1c). Therefore, estrogen stimulates expression of Gli1 in some ERα-positive cell lines, but not others. In order to determine whether the estrogen-induced increase in Gli1 is mediated through ERα, MCF7 breast cancer cells were pretreated with an ERα specific inhibitor, MPP, prior to administration of 1 nM 17β-estradiol for 48 h. In the presence of MPP, estrogen no longer increased expression of Gli1 (P = 0.021, Fig. 1d). These results confirm and expand on a prior report indicating that estrogen induces expression of Gli1 through ERα in MCF7 cells [43].
Glil1 promotes cell growth and survival in ERα-negative cell lines
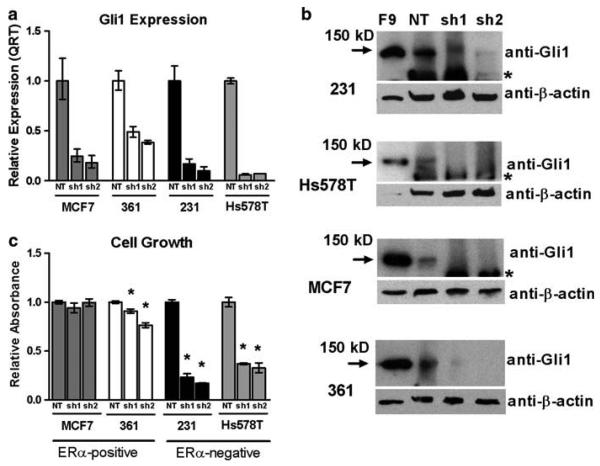
Gli1 has previously been reported to promote cell survival and growth in several cancer types [12, 19, 44, 45]. In order to assess its role in mediating the growth of ERα-positive and ERα-negative breast cancers, expression of Gli1 was silenced by RNA interference in two ERα-positive (MCF7 and 361) and two ERα-negative (MDA-MB-231 and Hs578T) breast cancer cell lines. The cells were transduced to express two different shRNAs recognizing Gli1 as well as an shRNA that does not recognize any human genes (non-targeting negative control (NT)). Decreased expression of Gli1 by at least 60% compared to the NT control was confirmed by QRT and WB in each cell line (Fig. 2a, b). The number of viable cells present was measured by MTT assay after the NT control cells approached confluence, which was at day 5 for the rapidly growing, ERα-negative MDA-MB-231 (231) and Hs578T cells, and day 7 and day 9 for the ERα-positive MCF7 and 361 cells, respectively. Reduction of Gli1 had no effect on the growth of MCF7 cells. There was a modest reduction of growth of 361 cells (9 and 24% reduction with two different shRNAs targeting Gli1, P = 0.003 and P < 0.001, respectively) (Fig. 2c). In contrast, silencing of Gli1 caused a marked reduction in growth in both 231 (77 and 83% reduction with two different shRNAs, P < 0.001 for each) and Hs578T (63 and 67% reduction with two different shRNAs, P = 0.002 for each) cells. These results suggest that Gli1 has a greater impact on cell growth and/or survival of ERα-negative than ERα-positive breast cancers.
Fig. 2.
Growth of Gli1-silenced breast cancer cells. a Gli1 expression was reduced by lentiviral transduction of shRNA directed to Gli1 (sh1, sh2). A non-targeting (NT) shRNA was a control. QRT was performed to confirm a reduction in Gli1 expression in the ERα-positive MCF7 and MDA-MB-361 (361) cells and the ERα-negative MDA-MB-231 (231) and Hs578T cells. The mean expression and standard deviation are presented. b Gli1 expression was also verified by western blot analysis using a rabbit polyclonal antibody. β-actin is a loading control. F9 cells are a teratocarcinoma cell line with a very high expression of Gli1 and are a positive control. The asterisks mark a non-specific immunoreactive band. The absence of an immunore-active band to β-actin in F9 cells in the blot for Hs578T cells is due to technical issues, but does not interfere in the interpretation of the results. c NT control and Gli1 silenced cells were grown until the NT cells approached confluence, which was 7 days for MCF7 cells, 9 days for MDA-MB-361 cells, and 5 days for both MDA-MB-231 and Hs578T cells. Growth was measured by MTT assay. Data are normalized to the NT control and are the mean and standard error of three experiments performed in triplicate. Significance is indicated by asterisks (P < 0.05)
Nuclear localization of Gli1 is present in 31% of breast cancers and is associated with ERα expression and absence of nuclear localization of p53
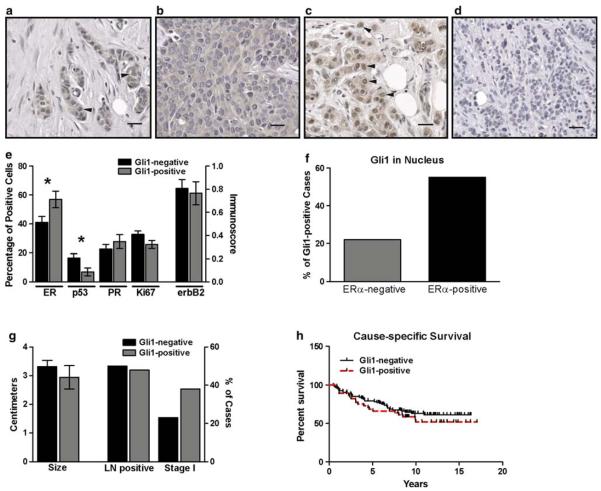
In order to determine whether our in vitro findings, specifically a relationship between ERα and Gli1 expression and function, are reflected in the expression patterns and behavior of breast cancers in vivo, we assessed Gli1 expression in breast cancer tissues and its impact on patient outcome. Two tissue microarrays containing tissue cores representing 171 infiltrating ductal carcinomas of the breast were immunostained for Gli1. Expression of Gli1 in cancer epithelial cells was primarily cytoplasmic, but there was also nuclear staining in a subset of breast cancers. Cytoplasmic expression was detected in 95% (162/171) of breast cancers. In 31% (53/171) of cancers, there was nuclear localization in addition to cytoplasmic expression of Gli1, suggesting Gli1 transcriptional activation in this subset. Nuclear localization ranged from relatively infrequent (present in 3% of cells) to extensive (present in 80% of cells) (Fig. 3a–d).
Fig. 3.
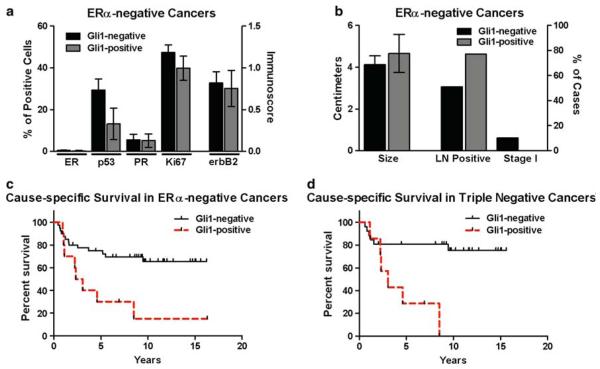
Gli1 in ERα-positive and ERα-negative breast cancers. a–d There was variability in the staining pattern of infiltrating ductal carcinomas of the breast for Gli1. In some cancers, expression was both cytoplasmic and nuclear (arrowheads) (a). In others, expression was primarily or exclusively cytoplasmic (b), or primarily nuclear (arrowheads) (c). In 8 cancers, there was no nuclear or cytoplasmic Gli1 expression (d). For a–d, magnification is 200×, scale bar = 40 μM. e Cancers with Gli1 nuclear staining (Gli1-positive, n = 53) and without nuclear staining (Gli1-negative, n = 118) were compared for the percentages of cells positive for ERα, PR, p53, Ki-67 and membrane expression of ErbB2 (immunoscore). Asterisks indicate statistical significance for ERα (P = 0.027) and p53 (P = 0.017). f A comparison of the percentage of cancers which were Gli1-positive among ERα-positive (n = 89) and ERα-negative (n = 60) breast cancers was significantly different (P = 0.033). g A comparison of tumor size and the number of cases with lymph node metastases or in clinical stage I at the time of diagnosis was not different between Gli1-positive and Gli1-negative cancers. h The Kaplan–Meier estimate was used to compare cause-specific survival (i.e., death due to cancer) in women with Gli1-positive (n = 45) and Gli1-negative (n = 94) cancers. The log-rank test indicated there was no significant difference
Most of these cancers were also immunostained for the presence of ERα (n = 149), PR (n = 149), p53 (n = 154), ErbB2 (n = 156), and Ki67 (n = 150). As nuclear localization of Gli1 is likely a better indicator of Gli1 transcriptional activity than cytoplasmic Gli1, we focused our analyses on nuclear staining. We compared the presence of these biomarkers in cancers with nuclear localization of Gli1 (hereafter referred to as Gli1-positive cancers) with those without nuclear localization of Gli1 (referred to as Gli1-negative cancers). Gli1-positive cancers had a higher expression of ERα (P = 0.027) and a lower level of nuclear localization of p53 (P = 0.017) (Fig. 3e). The rate of proliferation (i.e., percentage of cells with Ki67 labeling) was lower in Gli1 positive cancers, but this difference was of borderline significance (P = 0.060) (Fig. 3e). There was no significant difference in PR or membrane expression of ErbB2. When comparing ERα-positive (n = 89) and ERα-negative cancers (n = 60), ERα-positive cancers were more likely to also be Gli1-positive (P = 0.033) (Fig. 3f). The percentage of ERα positive cells was also higher in breast cancers with high total cellular Gli1 (i.e., the sum of the immunoscores for cytoplasmic and nuclear Gli1) than those with low total cellular Gli1 (using the mean of total cellular Gli1 as the cut-off for high vs. low) (55 vs. 40%, respectively, P = 0.035) (data not shown). This suggests that there is a positive association between ERα and total cellular Gli1 expression, and not just nuclear localization of Gli1.
The half-life of wild type p53 is usually too short to allow sufficient accumulation in the nucleus for detection by immunohistochemistry, and immunohistochemical detection of p53 is considered to be indicative of a mutant p53 protein with a longer half-life [46]. Therefore, our data indicate that Gli1-positive cancers are less likely to have a mutant p53 than are Gli1-negative cancers. There was no significant difference in mean tumor size, the number of cancers with lymph node metastases, or the number of cancers in clinical stage I at the time of diagnosis in Gli1-positive versus Gli1-negative cancers (Fig. 3g). We obtained sufficiently detailed survival information for 139 cancers to compare cause-specific survival (i.e., death due to cancer) in women with Gli1-positive (n = 45) versus Gli1-negative (n = 94) cancers (Fig. 3h), and no difference was observed. ERα-positive cancers have lower rates of proliferation, lower rates of p53 mutation, lower stage of disease and a better overall survival than ERα-negative cancers [47, 48]. Therefore, we considered the possibility that the relationship between Gli1 and ERα may explain the lower rate of p53 mutation and borderline lower rate of proliferation in Gli1-positive cancers and may be obscuring an effect of nuclear localization of Gli1 on survival. In order to address this, we analyzed nuclear localization of Gli1 in ERα-positive and ERα-negative cancers separately.
Nuclear localization of Gli1 predicts a poorer survival in ERα-negative, but not ERα-positive, breast cancers
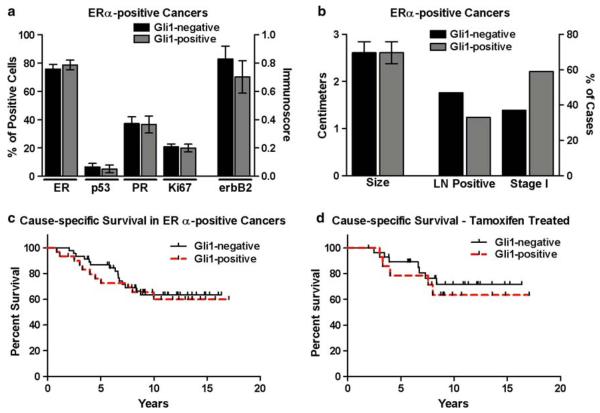
Among ERα-positive cancers, there was no significant difference in expression of ERα, PR, p53, erbB2, or Ki67 in Gli1-positive (n = 34) versus Gli1-negative (n = 55) cancers (Fig. 4a). There was also no significant difference in tumor size, lymph node status, the number of cancers in clinical stage I, or in cause-specific survival in Gli1-positive versus Gli1-negative cancers (Fig. 4b, c). It has been previously suggested that Gli1 may mediate resistance to tamoxifen or other estrogen antagonists in the treatment of ERα-positive breast cancers [49]. In order to assess a possible effect of Gli1 in mediating resistance to tamoxifen in this group of cancers, cause-specific survival was compared in those women with ERα-positive cancers treated with tamoxifen (n = 43) that were Gli1-positive versus Gli1-negative, and no significant difference was observed (Fig. 4d).
Fig. 4.
Nuclear localization of Gli1 in ERα-positive breast cancers. a In ERα-positive breast cancers, there was no significant difference in expression of ERα, PR, p53, Ki67 or ErbB2 in Gli1-positive (n = 34) and Gli1-negative (n = 55) cancers. b In ERα-positive breast cancers, a comparison of tumor size and the number of cases with lymph node metastases or in clinical stage I at the time of diagnosis was not different between Gli1-positive and Gli1-negative cancers. c In ERα-positive breast cancers, the Kaplan–Meier estimate was used to compare cause-specific survival (i.e., death due to cancer) in women with Gli1-positive (n = 30) and Gli1-negative (n = 46) cancers. The log-rank test indicated there was no significant difference. d Cause-specific survival was compare in only those ERα-positive breast cancers which were treated with tamoxifen. There was no difference in survival in Gli1-positive (n = 15) versus Gli1-negative cancers (n = 28)
Among ERα-negative cancers, there was also no significant difference in ERα, p53, PR, Ki67, ErbB2, or tumor size in Gli1-positive (n = 13) versus Gli1-negative (n = 47) cancers (Fig. 5a, b). Unlike ERα-positive cancers, those ERα-negative cancers with nuclear Gli1 were more likely to be lymph node positive, but this difference did not reach statistical significance (P = 0.122). In addition, none of the ERα-negative cancers with nuclear Gli1 were in clinical stage I at the time of presentation, whereas, 10% of ERα-negative, Gli1-negative cancers were clinical stage I (Fig. 5b). These lymph node and clinical stage data suggest a more aggressive clinical course for ERα-negative, Gli1-positive cancers than ERα-negative, and Gli1-negative cancers. In accordance with this, cause-specific survival was significantly poorer in the ERα-negative, Gli1-positive cancers (P = 0.005) (Fig. 5c).
Fig. 5.
Nuclear localization of Gli1 in ERα-negative breast cancers. a In ERα-negative breast cancers, there was no significant difference in expression of ERα, PR, p53, Ki67 or ErbB2 in Gli1-positive (n = 13) and Gli1-negative (n = 47) cancers. b In ERα-negative breast cancers, a comparison of tumor size and the number of cases with lymph node metastases or in clinical stage I at the time of diagnosis was not different between Gli1-positive and Gli1-negative cancers. c In ERα-negative breast cancers, the Kaplan–Meier estimate was used to compare cause-specific survival (i.e., death due to cancer) in women with Gli1-positive (n = 10) and Gli1-negative (n = 41) cancers. The log-rank test indicated a significant difference (P = 0.005). d Cause-specific survival was compare in triple negative breast cancers. There was a statistically significant difference in survival in Gli1-positive (n = 7) versus Gli1-negative cancers (n = 27) (P = 0.003)
In a multivariable model controlling for stage at diagnosis and ErbB2 expression, those women with ERα-negative, Gli1-positive cancers had a significantly poorer cause-specific survival (hazard ratio = 6.89; 95% confidence interval = 1.97, 24.14) compared to ERα-negative, Gli1-negative cancers (Table 2). Among ERα-positive cancers, there was no significant relationship between cause-specific survival and Gli1-positivity (P = 0.58) (Table 2). Additionally, pathologic stage was predictive of survival in both ERα-positive and ERα-negative cancers, and ErbB2 expression was predictive only in ERα-negative cancers (Table 2).
Table 2.
Cox proportional hazard models for ERα-negative and ERα-positive cancers
| Predictor | Hazard ratio (95% CIa) | P-value |
|---|---|---|
| ERα-negative cancers | ||
| Gli1-positive (nuclear) | 6.89 (1.97, 24.14) | 0.0026 |
| ErbB2 immunoscore | 1.89 (1.04, 3.44) | 0.0376 |
| Clinical stage (I, II, III, IV) | 4.12 (1.22, 13.98) | 0.0230 |
| ERα-positive cancers | ||
| Gli1-positive (nuclear) | 1.62 (0.66, 4.00) | 0.2952 |
| ErbB2 immunoscore | 0.66 (0.33, 1.34) | 0.2543 |
| Clinical stage (I, II, III, IV) | 2.26 (1.25, 4.09) | 0.0069 |
Confidence interval
Those ERα-negative cancers that were also triple negative [i.e., ER-negative, PR-negative (<10% positive cells) and without over-expression of ErbB2 (<10% of cells with strong membrane staining)] were identified (n = 34). In women with triple negative cancers, those with Gli1-positive cancers also exhibited a poorer cause-specific survival than those with Gli1-negative cancers (P = 0.003) (Fig. 5d). These data suggest that nuclear localization or activity of Gli1 in ERα-negative breast cancers, including triple negative cancers, is detrimental to clinical outcome and has a greater impact than in ERα-positive cancers.
Discussion
Our finding that nuclear localization of Gli1 is higher in ERα-positive breast cancers is in accordance with a prior analysis of 52 breast cancers immunostained for Gli1 [23]. We also provide data indicating that estrogen induces expression of Gli1 in ERα-positive breast cancer cell lines, which may contribute to the positive association between ERα and Gli1 observed in both breast cancer cell lines and tissues. Our results expand on a prior study, which was limited to MCF7 cells [43], by demonstrating that estrogen induces Gli1 expression in several, but not all, ERα-positive breast cancer cell lines. In support of this, not all ERα-positive breast cancers in our study demonstrated nuclear localization of Gli1 (62% of cancers without nuclear localization of Gli1) or Gli1 in the cytoplasm (5% of cancers without cytoplasmic or nuclear Gli1). In the prior study of MCF7 cells, estrogen-induced expression of both Gli1 and sonic hedgehog ligand (Shh), and treatment with Shh stimulated Gli1 expression, suggesting that estrogen induced the expression of Gli1 by ligand-dependent activation of autocrine hedgehog signaling [43]. Other investigators, however, have found that most breast cancer cell lines tested, including MCF7, do not respond to Shh ligand by activation of hedgehog signaling or an increase in Gli1 expression [22]. This raises the possibility that estrogen may increase Gli1 through a hedgehog ligand-independent mechanism, such as estrogen-induced activation of MAPK signaling [50]; MAPK has been shown to stabilize Gli1 protein and increase Gli1 activity and expression [6, 8].
It also has been reported that Gli1 induces estrogen-independent proliferation and promotes G1/S phase transition in ERα-positive breast cancer cell lines, raising the possibility that Gli1 may promote estrogen-independent growth and resistance of ERα-positive breast cancers to anti-estrogen therapies [49]. Our data do not support such a role for Gli1 in ERα-positive breast cancers. In our cohort of ERα-positive breast cancers, there was no difference in cancer-specific survival in Gli1-positive versus Gli1-negative cancers. Among ERα-positive breast cancers that were treated with tamoxifen, nuclear localization of Gli1 also failed to predict a poorer cancer-specific survival, suggesting that Gli1 has no impact on response to anti-estrogen therapy.
Although nuclear localization of Gli1 is higher in ERα-positive than ERα-negative breast cancers, nuclear Gli1 predicted a poor prognosis only in ERα-negative cancers in our cohort, suggesting that cell context and concurrent signaling activity direct the functional effect of Gli1-mediated transcription. In addition, the ERα-negative breast cancer cell lines that we tested are more dependent on the presence of Gli1 for growth and survival in vitro than are the ERα-positive cell lines. However, in the breast cancer tissues, we found no evidence that Gli1 promoted the growth of either ERα-negative or ERα-positive breast cancers in that there was no difference in tumor size or the rate of proliferation in Gli1-positive versus Gli1-negative cancers. Our prognostic data suggest that, rather than influence the growth of the primary tumor, Gli1 is most important for those processes that result in death, i.e., cancer recurrence, invasion, and metastasis, in ERα-negative breast cancers. Our findings require confirmation in additional breast cancer cohorts.
Although the molecular mechanisms underlying a potential difference in Gli1 function in ERα-positive and ERα-negative breast cancers remains to be elucidated, differences in Gli1 expression and function have been demonstrated previously in different breast cancer cell populations—those cancer cells with a cancer stem cell phenotype versus those lacking this phenotype. Gli1 expression is higher in breast cancer stem cell populations, characterized by the cell surface phenotype CD44+CD24−/low, than in tumor cell populations lacking this marker profile [25, 51]. Additionally, breast cancer cells with the CD44+CD24−/low phenotype are more dependent upon Gli1-mediated transcription for cell growth and survival than those cells without the CD44+CD24−/low phenotype [25, 51]. Cancer stem cells are a subpopulation of cancer cells that are believed to be responsible for the development, recurrence, and heterogeneity of tumors [52]. Additionally, increasing evidence indicates that cancer cells with the capability of metastasizing have a stem cell phenotype [53-55]. Therefore, the role of Gli1 in breast cancer may be in maintaining the viability and function of cells with a stem cell phenotype and thereby promoting the ability of these cells to generate tumor recurrence and metastases. In support of this, silencing of Gli1 expression in ERα-negative breast cancer cell lines was shown to reduce their invasiveness [45].
One category of ERα-negative breast cancers, the basal-like subtype, are enriched in CD44+CD24−/low stem cells [56, 57]. Other ERα-negative subtypes may also be enriched in Gli1 dependent cancer stem cells and this may contribute to the greater functional impact of Gli1 in ERα-negative breast cancers. We also evaluated the prognostic role of Gli1 in triple negative breast cancers, which are those cancers that are negative for ERα, PR, and ErbB2. We found that nuclear localization of Gli1 also predicts a poor prognosis in this ERα-negative subgroup. Triple negative and basal-like breast cancers are distinct molecular classes of breast cancer with a high degree of overlap. Basal-like breast cancers are also negative for ERα, PR, and ErbB2 but are additionally characterized by expression of markers of basal and myoepithelial cells, such as basal cytokeratins and vimentin [58]. The identification of molecules, such as Gli1, that may promote progression and serve as therapeutic targets in the triple negative or basal-like subtypes is particularly important as the usual therapies targeted to ERα and ErbB2 are not effective.
Additional evidence that Gli1 is functionally important in breast cancers with an ERα-negative, basal-like pheno-type is the finding that Gli1 induces ERα-negative, basal-like breast cancers. Transgene expression of Gli1 in the mouse mammary gland, under the regulation of the mouse mammary tumor virus promoter, resulted in the development of mammary gland cancers that are ERα-negative and express vimentin and basal keratins indicative of a basal-like subtype breast cancer [59]. These Gli1-induced cancers also contained a population of stem/progenitor cells that expressed the progenitor cell markers keratin 6 and Bmi-1.
Our data also indicate an inverse relationship between nuclear localization of Gli1 and p53, which when detectable by immunohistochemistry suggests the presence of a mutated p53 protein. This inverse relationship may be simply a result of the positive association between nuclear localization of Gli1 and ERα-positive breast cancers, which commonly lack mutated p53. However, it has been demonstrated that in neural progenitor cells, wild type p53 inhibits the activity, nuclear localization and levels of Gli1 and in turn, Gli1 represses p53, establishing an inhibitory loop [60]. Gli1 was also shown to inhibit the nuclear accumulation and transcriptional activity of wild type p53 in murine embryonic fibroblasts [61]. Therefore, an inverse relationship between wild type p53 and Gli1 exists. Whether such a relationship also exists between mutated p53 and Gli1 and contributes to the inverse relationship identified in the breast cancer tissues is not known.
In summary, our data indicate that Gli1 expression and nuclear localization is greater in ERα-positive breast cancers. However, they also indicate that Gli1 has a greater functional effect in ERα-negative cancers, where it is important for cell growth or survival and its nuclear localization predicts a poorer clinical outcome.
Supplementary Material
Acknowledgments
This study was funded by the American Cancer Society (RSG-05-207-01-TBE), the Susan G. Komen Foundation (BCTR0707453) and the National Institutes of Health/National Cancer Institute (R03CA130057). We thank Drs. Mike Ruppert, Susan Lobo-Ruppert, Danny R. Welch, and Doug Hurst for their helpful advice and discussion. We also thank Dr. Mike Ruppert for the use of the Gli1 rabbit antibody and viral packaging vectors.
References
- 1.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Zapico ME. Primers on molecular pathways GLI: more than just hedgehog? Pancreatology. 2008;8:227–229. doi: 10.1159/000134271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipinski RJ, Gipp JJ, Zhang J, Doles JD, Bushman W. Unique and complimentary activities of the Gli transcription factors in hedgehog signaling. Exp Cell Res. 2006;312:1925–1938. doi: 10.1016/j.yexcr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 6.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M, Aberger F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Z, Mei FC, Xie J, Cheng X. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J Biol Chem. 2007;282:14048–14055. doi: 10.1074/jbc.M611089200. [DOI] [PubMed] [Google Scholar]
- 8.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz IAA. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruppert JM, Vogelstein B, Kinzler KW. The zinc finger protein GLI transforms primary cells in cooperation with adenovirus E1A. Mol Cell Biol. 1991;11:1724–1728. doi: 10.1128/mcb.11.3.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasca di Magliano M, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev. 2003;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 11.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 12.Karhadkar SS, Steven Bova G, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 13.Kayed H, Kleeff J, Keleg S, Guo J, Ketterer K, Berberat PO, Giese N, Esposito I, Giese T, Buchler MW, Friess H. Indian hedgehog signaling pathway: expression and regulation in pancreatic cancer. Int J Cancer. 2004;110:668–676. doi: 10.1002/ijc.20194. [DOI] [PubMed] [Google Scholar]
- 14.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 15.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf I, Bose S, Desmond JC, Lin BT, Williamson EA, Karlan BY, Koeffler HP. Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res Treat. 2007;105(2):139–155. doi: 10.1007/s10549-006-9440-4. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Chen K, Huang S, Zhang X, Adegboyega PA, Evers BM, Zhang H, Xie J. Frequent activation of the hedgehog pathway in advanced gastric adenocarcinomas. Carcinogenesis. 2005;26:1698–1705. doi: 10.1093/carcin/bgi130. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, Sheng T, Zhang Y, Zhang X, He J, Huang S, Chen K, Sultz J, Adegboyega PA, Zhang H, Xie J. Hedgehog signaling is activated in subsets of esophageal cancers. Int J Cancer. 2005;118(1):139–148. doi: 10.1002/ijc.21295. [DOI] [PubMed] [Google Scholar]
- 19.Qualtrough D, Buda A, Gaffield W, Williams AC, Paraskeva C. Hedgehog signalling in colorectal tumour cells: induction of apoptosis with cyclopamine treatment. Int J Cancer. 2004;110:831–837. doi: 10.1002/ijc.20227. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Harris LG, Metge BJ, Liu S, Riker AI, Samant RS, Shevde LA. The hedgehog pathway transcription factor, GLI1 promotes malignant behavior of cancer cells by upregulating osteopontin. J Biol Chem. 2009;284(34):22888–22897. doi: 10.1074/jbc.M109.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Frolova N, Sadlonova A, Novak Z, Steg A, Page GP, Welch DR, Lobo-Ruppert SM, Ruppert JM, Johnson MR, Frost AR. Hedgehog signaling and response to cyclopamine differs in epithelial and stromal cells in benign breast and breast cancer. Cancer Biol Ther. 2006;5:674–683. doi: 10.4161/cbt.5.6.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Harrington N, Moraes RC, Wu MF, Hilsenbeck SG, Lewis MT. Cyclopamine inhibition of human breast cancer cell growth independent of smoothened (Smo) Breast Cancer Res Treat. 2009;115(3):505–521. doi: 10.1007/s10549-008-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 24.Hatsell S, Frost AR. Hedgehog signaling in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2007;12:163–173. doi: 10.1007/s10911-007-9048-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cailleau R, Olive M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 28.Hackett AJ, Smith HS, Springer EL, Owens RB, Nelson-Rees WA, Riggs JL, Gardner MB. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (hs578t) and the diploid myoepithelial (hs578bst) cell lines. J Natl Cancer Inst. 1977;58:1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- 29.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 30.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–642. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 31.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97:779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 32.Yoneda T, Michigami T, Yi B, Williams PJ, Niewolna M, Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88:2979–2988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 33.Zhang RD, Fidler IJ, Price JE. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis. 1991;11:204–215. [PubMed] [Google Scholar]
- 34.Li X, Deng W, Nail CD, Bailey SK, Kraus MH, Ruppert JM, Lobo-Ruppert SM. Snail induction is an early response to Gli1 that determines the efficiency of epithelial transformation. Oncogene. 2006;25:609–621. doi: 10.1038/sj.onc.1209077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanai K, Nagai S, Wada J, Yamanaka N, Nakamura M, Torata N, Noshiro H, Tsuneyoshi M, Tanaka M, Katano M. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol. 2007;95:55–62. doi: 10.1002/jso.20606. [DOI] [PubMed] [Google Scholar]
- 36.Kayed H, Kleeff J, Keleg S, Buchler MW, Friess H. Distribution of Indian hedgehog and its receptors patched and smoothened in human chronic pancreatitis. J Endocrinol. 2003;178:467–478. doi: 10.1677/joe.0.1780467. [DOI] [PubMed] [Google Scholar]
- 37.Thomas MK, Rastalsky N, Lee JH, Habener JF. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–2047. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- 38.Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal women. Int J Cancer. 2002;98:118–127. doi: 10.1002/ijc.10171. [DOI] [PubMed] [Google Scholar]
- 39.Talley L, Chhieng DC, Bell WC, Grizzle WE, Frost AR. Immunohistochemical detection of egfr, p185(erbb-2), bcl-2 and p53 in breast carcinomas in pre-menopausal and post-menopausal women. Biotech Histochem. 2008;83:5–14. doi: 10.1080/10520290701822436. [DOI] [PubMed] [Google Scholar]
- 40.Tapia C, Glatz K, Novotny H, Lugli A, Horcic M, Seemayer CA, Tornillo L, Terracciano L, Spichtin H, Mirlacher M, Simon R, Sauter G. Close association between HER-2 amplification and overexpression in human tumors of non-breast origin. Mod Pathol. 2007;20:192–198. doi: 10.1038/modpathol.3800729. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 42.Katayama S, Ashizawa K, Gohma H, Fukuhara T, Narumi K, Tsuzuki Y, Tatemoto H, Nakada T, Nagai K. The expression of hedgehog genes (ihh, dhh) and hedgehog target genes (ptc1, gli1, coup-tfii) is affected by estrogenic stimuli in the uterus of immature female rats. Toxicol Appl Pharmacol. 2006;217:375–383. doi: 10.1016/j.taap.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Koga K, Nakamura M, Nakashima H, Akiyoshi T, Kubo M, Sato N, Kuroki S, Nomura M, Tanaka M, Katano M. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res. 2008;28:731–740. [PubMed] [Google Scholar]
- 44.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kameda C, Tanaka H, Yamasaki A, Nakamura M, Koga K, Sato N, Kubo M, Kuroki S, Tanaka M, Katano M. The hedgehog pathway is a possible therapeutic target for patients with estrogen receptor-negative breast cancer. Anticancer Res. 2009;29:871–879. [PubMed] [Google Scholar]
- 46.Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92:434–444. doi: 10.1038/sj.bjc.6602358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne SJ, Bowen RL, Jones JL, Wells CA. Predictive markers in breast cancer—the present. Histopathology. 2008;52:82–90. doi: 10.1111/j.1365-2559.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 48.Helin ML, Helle MJ, Helin HJ, Isola JJ. Proliferative activity and steroid receptors determined by immunohistochemistry in adjacent frozen sections of 102 breast carcinomas. Arch Pathol Lab Med. 1989;113:854–857. [PubMed] [Google Scholar]
- 49.Zhao J, Chen G, Cao D, Li Y, Diao F, Cai H, Jin Y, Lu J. Expression of gli1 correlates with the transition of breast cancer cells to estrogen-independent growth. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0323-3. doi:10.1007/s10549-009-0323-3. [DOI] [PubMed] [Google Scholar]
- 50.Keshamouni VG, Mattingly RR, Reddy KB. Mechanism of 17-beta-estradiol-induced Erk1/2 activation in breast cancer cells. A role for HER2 and PKC-delta. J Biol Chem. 2002;277:22558–22565. doi: 10.1074/jbc.M202351200. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka H, Nakamura M, Kameda C, Kubo M, Sato N, Kuroki S, Tanaka M, Katano M. The Hedgehog signaling pathway plays an essential role in maintaining the cd44+cd24− /low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 52.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartkowiak K, Wieczorek M, Buck F, Harder S, Moldenhauer J, Effenberger KE, Pantel K, Peter-Katalinic J, Brandt BH. Two-dimensional differential gel electrophoresis of a cell line derived from a breast cancer micrometastasis revealed a stem/progenitor cell protein profile. J Proteome Res. 2009;8(4):2004–2014. doi: 10.1021/pr8009758. [DOI] [PubMed] [Google Scholar]
- 54.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurt EM, Farrar WL. Cancer stem cells: the seeds of metastasis? Mol Interv. 2008;8:140–142. doi: 10.1124/mi.8.3.7. [DOI] [PubMed] [Google Scholar]
- 56.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. The cd44+/cd24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakshatri H, Srour EF, Badve S. Breast cancer stem cells and intrinsic subtypes: controversies rage on. Curr Stem Cell Res Ther. 2009;4:50–60. doi: 10.2174/157488809787169110. [DOI] [PubMed] [Google Scholar]
- 58.Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology. 2009;41:40–47. doi: 10.1080/00313020802563510. [DOI] [PubMed] [Google Scholar]
- 59.Fiaschi M, Rozell B, Bergstrom A, Toftgard R. Development of mammary tumors by conditional expression of GLI1. Cancer Res. 2009;69:4810–4817. doi: 10.1158/0008-5472.CAN-08-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009;28:663–676. doi: 10.1038/emboj.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abe Y, Oda-Sato E, Tobiume K, Kawauchi K, Taya Y, Okamoto K, Oren M, Tanaka N. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA. 2008;105:4838–4843. doi: 10.1073/pnas.0712216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.