Abstract
Objective
To investigate the expression of TG-interacting factor (TGIF), a Smad transcriptional corepressor, in leiomyoma and matched myometrial tissue samples and the effect of TGIF overexpression in myometrial cells.
Design
Experimental study.
Setting
Tertiary university hospital.
Patient(s)
Uterine leiomyoma and myometrial tissues from sixteen patients.
Intervention(s)
None.
Main Outcome Measure(s)
The distribution of TGIF in leiomyoma and myometrial tissues by immunohistochemistry stain. mRAN and protein expression levels by real-time quantitative polymerase chain-reaction (QPCR) and Western blot. Transcriptional regulation of TGIF in myometrial cells with over-expressed TGIF.
Result(s)
TGIF is present in the smooth muscle cells of the leiomyoma and the myometrium but not in the extracellular matrix. TGIF mRNA and protein expressions were significantly higher in the leiomyoma compared to the matched, unaffected myometrial tissues in both phases of the menstrual cycle. There were no differences in mRNA or protein expression throughout the menstrual cycle. Overexpression of TGIF protein in myometrial cells significantly suppressed up-regulation of plasminogen activator inhibitor (PAI-1) induced by TGF-β1 treatment.
Conclusion(s)
TGIF expression is increased in leiomyoma compared to myometrium. This increase in TGIF expression is not affected by endogenous ovarian hormones. TGIF is a potential repressor of TGF-β pathways in myometrial cells.
Keywords: fibrosis, leiomyoma, TGF-β, TGIF, PAI-1
INTRODUCTION
Leiomyoma consists of transformed smooth muscle cells and abundant extracellular matrix (ECM), which accounts for their fibrotic quality. Numerous growth factors and receptors are involved in leiomyoma growth (1). Of the many factors identified, transforming growth factor β (TGF-β) is the most potent cytokine, inducing the pathological growth of fibrotic tissue (2).
TGF-β stimulation has proven to increase ECM protein production and decrease proteolytic degradation of ECM in leiomyomata (3). Higher expression of TGF-β has been reported in leiomyoma compared to unaffected myometrium, consistent with its profibrotic effect (4). The role of TGF-β in the etiology of leiomyomata is further supported by increased expression of latent binding protein-1 (LTBP-1) and fibrillin-1 (FBN-1) in leiomyomata compared to myometrium (5). Both proteins are associated with TGF-β activation.
TG-interacting factor (TGIF) is a homeodomain protein of the three-amino-acid loop extension superfamily (6, 7). TGIF is essential for craniofacial development in humans; deletions or mis-sense mutations of TGIF gene are associated with holoprosencephaly, a congenital structural forebrain anomaly (8, 9). The major function of TGIF is to bind to a TGF-β-activated Smad2 protein and to act as a corepressor of transcription in the TGF-β signaling pathway (10). Recent studies demonstrate that TGIF is associated with TGF-β signaling control in ECM remodeling and is the pathogenesis of renal glomerulosclerosis (11, 12). Although leiomyoma is the most common uterine fibrotic disorder in premenopausal women, there is scant information on the role of TGIF in the pathogenesis of uterine leiomyoma.
In this study we sought to document and evaluate TGIF expression in leiomyoma compared to matched myometrium in premenopausal women. We also assessed the effect of endogenous ovarian hormones on TGIF expression, given that leiomyoma growth is associated with ovarian hormones. Because TGIF affects ECM synthesis and degradation, we determined whether over-expressed TGIF protein results in differential gene expression of plasminogen activator inhibitor (PAI-1), which is the major physiological inhibitor of tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA) and it plays an important role in determining net fibrinolytic activity in vivo.
MATERIALS AND METHODS
Subjects and Tissue Selection
With approval from the Institutional Review Board of Stanford University School of Medicine, we collected biopsies of intramural leiomyomata and matched unaffected myometrium from premenopausal women undergoing hysterectomies after informed consents were obtained. Exclusion criteria are: endometriosis; malignant diseases; pelvic inflammatory disease, inflammatory bowel disease or connective tissue disorders; or women who had received hormonal therapy within three months before surgery. The phase of the menstrual cycle was determined by the endometrial histology. Myometrial samples were obtained from the uterine fundus 1 cm away from the endometrium. Leiomyomata between 5 cm and 8 cm in diameter were chosen for sample collection. Specimens from two patients were fixed in 10% formaldehyde for immunohistochemistry stain. Tissues were plunged into liquid nitrogen immediately after excision and stored at −80°C for further processing.
Immunohistochemistry for TGIF
To localize the presence of TGIF in myometrium and leiomyoma, immunohistochemical staining was performed as described previously (13). Specimens fixed in 10% formaldehyde and embedded in paraffin were sliced, de-paraffinized and re-hydrated. After washing with Tris-buffered saline Triton-X100 (TBS-T, pH 7.5, 0.02% Triton-X100), endogenous peroxidases were blocked with 3% H2O2. The slides were incubated with goat anti-TGIF (1/40, Santa Cruz, CA) primary antibody overnight at 4°C and incubated with a secondary antibody, biotin conjugated rabbit anti-goat IgG (1/50, Sigma, MO) for 30 min at room temperature. The avidin-biotin alkaline phosphatase staining method (Vector Laboratory, Burlingame, CA) was applied. Levamisol was added to block endogenous alkaline phosphatase activity. Slides were counterstained and photographed with AxioCam (Zeiss, Oberkochen, Germany).
Total RNA Extraction and Real-Time Quantitative PCR
We extracted the total RNA and generated complementary DNA (cDNA) from total RNA as described previously (14). Expressions of TGIF mRNA were analyzed by real-time quantitative PCR (QPCR) performed on the Mx3005P Multiplex Quantification PCR System with MxPro QPCR software (Stratagene, La Jolla, CA). Primers used in QPCR to amplify TGIF were designed by the Primer3 website designer (15). The sequences were, forward: GCTGAGAAAGGATGGCAAAG and reverse: GGAATGAAATGGGGTCTCCT. For normalization of real-time quantification, hypoxanthine phosphoribosyl transferase 1 (HPRT1) was used as endogenous reference (16). We carried out QPCR by using Brilliant SYBR Green QPCR Master Mix (Stratagene) as previously described (17). Briefly, each well of Optical 96-Well Reaction Plate (Stratagene) was filled with 25 μl reaction solution containing 7.625μl of water, 12.5μl of 2X Master Mix, 1μl of forward primer, 1μl of reverse primer, 0.375μl of diluted reference dye (1/500, Stratagene), and 2.5μl of cDNA. After denaturation heating at 95°C, the amplification cycles were repeated 40 times with the following thermal conditions: 95°C for 30 s, 60°C for 1 min, and 72°C for 30 s. Finally, the melting curve program was carried out at 55−95°C to ensure that there was only PCR product amplified and no primer dimers. Electrophoresis of PCR products on 2% agarose gel confirmed their size at 139 bp. Subsequent PCR product sequencing ensured that the correct gene sequence was amplified. After normalization, relative quantification of the target gene was further divided by calibrator sample value. All of the real-time QPCR reactions were performed in duplicate.
Total Protein Extraction and Western Blot
The total protein was extracted as described previously (5). Samples were reduced with 5% 2-mercaptolethanol and separated by 10% sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes (Pierce, Rockford, IL). After blocking the membranes were incubated with rabbit anti-TGIF monoclonal antibody (1:1000, Abcam, Inc., Cambridge, MA) for 2 h and then in donkey anti-rabbit IgG antibody conjugated to horseradish peroxidase (HRP) (1:2000, GE Healthcare, Sunnyvale, CA) for one hour. The blots were re-probed with mouse anti-β-actin monoclonal antibody (1:5000, Sigma, MO), then 1/5000 dilution of sheep anti-mouse IgG antibody conjugated to HRP (Amersham, Buckinghamshire, UK). Densitometry of immunoreactive bands on Western blot was performed with Bio-Rad Quality One Software (Bio-Rad). All of the Western blot experiments were performed at least twice to confirm the reproducibility.
Cell Culture and Expression of TGIF Protein in SK-UT-1 Cells
SK-UT-1 human uterine leiomyosarcoma cells (ATCC, Manassas, VA) were maintained in high glucose Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetalbovine serum, and 1% penicillin/streptomycin at 37°C with 5% CO2. Calcium phosphate precipitation was performed to transfect the cells. The cells were transfected either with pCMV5 Flag TGIF plasmid (Addgene, Cambridge, MA) or pcDNA 3.1/V5-His-TOPO plasmid (Invitrogen), with the latter serving as the negative control. Briefly, 10μg of plasmid DNA was mixed with 500μl CaCl2 which was then transferred to 500μl of 2X BBS [280 mM NaCl, 50mM BES (n,n-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid), 1.5 mM Na2HPO4 , pH 6.95]. The mixture was allowed to incubate for 15 min at room temperature. 2.7 ml of transfection medium (low glucose DMEM) was added to the cells. After this, 300 μl of the plasmid DNA mixture was dropped slowly into the well. The cells were incubated at 37°C with 3% CO2 for 16 hours. The transfection medium was replaced with culture medium before further treatment with TGF-β1 (R&D system).
Statistical Analysis
Data are expressed as the mean + SEM. One-way ANOVA test, Student's t test and paired t test were used as appropriate. The difference was considered to be statistically significant at P < 0.05. Statistical analysis was performed with the JMPIN software (SAS Institute, Inc., v5.1, Cary, NC).
RESULTS
Identification and Localization of TGIF Expression in Myometrium and Leiomyoma
Our immunohistochemistry stains showed that TGIF was similarly distributed in the smooth muscle cells of both leiomyoma and myometrial tissues, but not in the ECM (data not shown).
TGIF mRNA Expression Levels in Myometrium and Leiomyoma
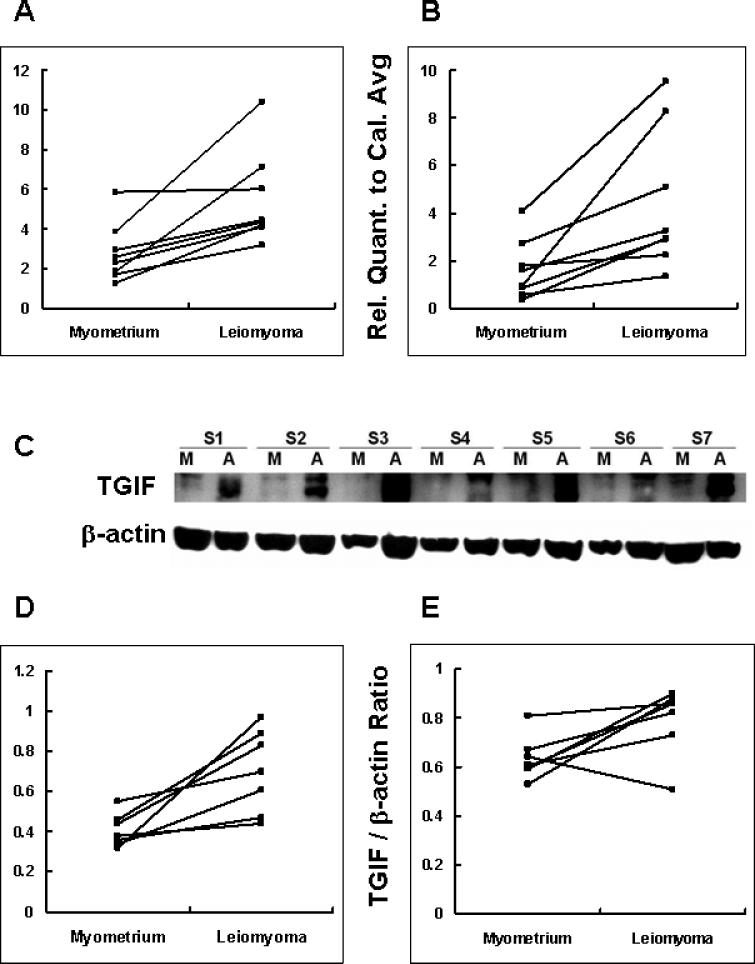
Samples were collected from patients in the proliferative phase (N = 8) and the secretory phase (N = 8) of the menstrual cycle in order to examine the effect of endogenous ovarian hormones. As shown in Figure 1A and B, the level of TGIF mRNA in the leiomyoma tissue is approximately 2-fold higher than that in the matched unaffected myometrial tissue during the secretory phase (P = 0.01). The expression level of TGIF mRNA in the leiomyoma tissue is approximately 3-fold higher than that in the matched unaffected myometrial tissue during the proliferative phase (P = 0.012). We also compared the expression levels of TGIF mRNA of leiomyoma tissues from patients in different phases using real-time QPCR in independent, duplicated experiments. No significant difference was found in leiomyoma tissues between the proliferative and secretory phases, suggesting that expression of TGIF is not modulated by endogenous ovarian hormones. Similarly, the TGIF mRNA expression levels did not vary during the menstrual cycle in unaffected myometrial tissues.
Figure 1.
The expressions of TGIF mRNA are compared between myometrium and leiomyoma. After normalizing with the internal control gene HPRT-1, relative quantification of the gene TGIF was divided by one calibrator sample value to generate the relative quantification to calibrator average (Rel. Quant. to Cal. Avg.). (A) The secretory phase (N = 8, P = 0.01). (B) The proliferative phase (N = 8, P = 0.012). Western blot of TGIF protein levels in myometrium and leiomyoma. (C) TGIF showed a double band of 30−35 kDa. ‘M’ represents myometrium, ‘A’ represents leiomyoma. (D) The secretory phase (N = 7, P = 0.01) (E) The proliferative phase (N = 7, P = 0.045).
* Comparisons between myometrium and leiomyoma, paired t-test.
TGIF Protein Levels Evaluated by Western Blot
In accordance with previous observations (18), the wild-type TGIF migrated as a double band of 30−35 kDa. Consistent with our mRNA data, Western blot showed that TGIF protein levels in the leiomyoma tissues were 1.73-fold higher than those in the unaffected myometrial tissues during the secretory phase (Figure 1D, P = 0.01). Similarly, the expression levels of TGIF protein were 1.25-fold higher in the leiomyoma tissue during the proliferative phase (Figure 1E, P = 0.045). The protein levels of TGIF in leiomyomata were similar between phases of menstrual cycles (N = 14, data not shown). Protein levels of TGIF in myometrium were not compared between phases of menstrual cycles due to the insufficient tissue samples for additional Western blot assays.
TGIF Protein Suppresses PAI-1 mRNA Up-regulation Induced by TGF-β1
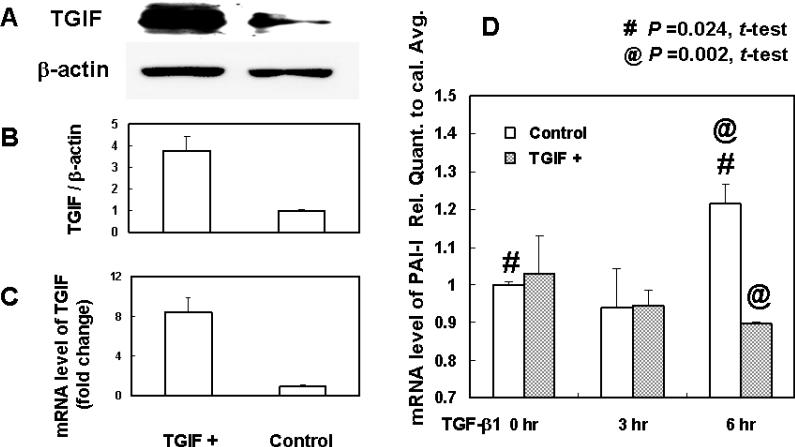
To explore a possible mechanism by which TGIF regulates ECM turnover, we investigated the effect of TGIF on the TGF-β signaling downstream product, PAI-1. Being primarily interested in ECM turnover in the human myometrial cell, we used leiomyosarcoma cell line SK-UT-1, which has been applied as a research model for myometrial cell (19) and ECM products (20). As shown in Figures 2A-C, TGIF protein and mRNA were markedly expressed in SK-UT-1 cells transfected with TGIF-containing plasmid compared to cells with the control plasmid. Subsequent treatment with TGF-β1 (1ng/ml) significantly up-regulated PAI-1 mRNA expression in the control group at 6 hours. On the contrary, this PAI-1 mRNA upregulation was inhibited in the TGIF-transfected cells at 6 hours (Figure 2D). All the experiments were duplicated in three independent experiments.
Figure 2.
TGIF protein and mRNA are significantly increased by transfection with pCMV5 Flag TGIF plasmid (A, B, and C) compared to cells infected with control plasmid. (D) Overexpression of TGIF protein suppresses the PAI-1 up-regulation induced by TGF-β1. The transfected cells were cultured in serum-free medium for 16 hr before treatment with TGF-β1 (1ng/ml). TGF-β1 induces PAI-1 up-regulation in cells transfected with control plasmid at 6 hr (P = 0.024, #). PAI-1 up-regulation is significantly suppressed in cells transfected with TGIF plasmid (P = 0.002, @) compared to cells transfected with control plasmid. PAI-1 primer: forward: GTTACCCCCATGACTCCAGA, reverse: CGCAGACTTCTCACCAAACA.
DISCUSSION
In the present study, we documented the differential expression of TGIF in the leiomyoma and myometrial tissues of premenopausal women during the proliferative and secretory phases of the menstrual cycle. Our data consistently demonstrated that TGIF, a TGF-β signaling transcriptional corepressor, is highly expressed in leiomyoma tissues compared to matched myometrium. Secondly, TGIF appears to suppress the downstream gene product of TGF-β stimulation, PAI-1, which is a major regulator in ECM turnover. The expression of TGIF in smooth muscle cells suggests that there is an intracellular mechanism that may counteract the potent profibrotic cytokine TGF-β in uterine smooth muscle cells.
Expression of TGIF mRNA was documented only in a restricted number of human adult tissues (6). Although the original functional analysis revealed that TGIF has a specific binding ability to a retinoid response element from the rat cellular retinoic acid binding protein II (CRABP II) gene (21), TGIF is best known for its function as a co-repressor to Smad2, the mediator of TGF-β signaling. Recently, Seo et al. documented that TGIF can also associate with E3 ubiquitin ligase Tiul1 to target Smad2 degradation (22) and to interact with cytoplasmic pro-myelocytic leukemia protein, resulting in the inhibition of Smad2 phosphorylation (23). This suggests that TGIF represses TGF-β signaling through multiple mechanisms.
Numerous studies have placed emphasis on the profibrotic nature of TGF-β on leiomyomata. However, the negative regulation aspects – including intracellular negative feedback or ECM degradation mechanism in leiomyomata – are lacking. To date, two studies have examined TGIF gene expression in leiomyoma (24, 25). One study was designed to investigate differential gene expression patterns in untreated leiomyoma/myometrium compared to gonadotrophin-releasing hormone (GnRH)-treated leiomyoma/myometrium pairs. However, in a subanalysis of 3 pairs of matched-tissue groups, TGIF gene expression was found to be unchanged in the untreated leiomyoma compared to myometrium pairs (roughly 0.9-fold). These numbers were insufficient to confirm a true difference between the untreated tissues, especially when the fold change was small. Protein expression data were not available. Contrary to this report, our data consistently demonstrated that both TGIF mRNA and protein expression are up-regulated in leiomyoma compared to matched myometrium.
In the second study, Luo et al. reported that TGF-β stimulation of cultured leiomyoma smooth muscle cells may induce a transient TGIF mRNA expression (25). Thus, the increase in TGIF mRNA and protein expression in the leiomyoma tissue in our study may be a response to TGF-β and may serve as an intracellular negative feedback mechanism. It is also possible that the up-regulation in TGIF protein level in the leiomyoma is enhanced by a decrease in degradation of TGIF. For example, Dai and Liu documented that hepatocyte growth factor, a potent antifibrotic cytokine, is able to increase TGIF protein level through protein stabilization rather than new synthesis (11). Therefore, the cytokines that stimulate the accumulation of TGIF in smooth muscle cells will require further investigation.
The growth of leiomyoma is associated with sex steroid hormones, namely estrogen, progesterone and androgen. Because the menstrual cycle induces ovarian steroid hormone fluctuations, we specifically divided the patients according to their menstrual phase to assess the effect of endogenous hormones on TGIF expression. Our data show that the expression of TGIF mRNA and protein are not modulated by the menstrual phase, implying that TGIF expression may not be regulated by steroid hormones.
Although TGIF protein theoretically antagonizes the function of TGF-β, its increased expression in leiomyomata does not appear to block the fibrogenetic effect of TGF-β in leiomyomata, since leiomyomata demonstrate increased deposition and decreased degradation of ECM. To study the negative-regulation effects of TGIF, we over-expressed TGIF protein in smooth muscle cells, followed by TGF-β1 treatment in vitro. We observed that PAI-1 mRNA upregulation was suppressed in TGIF-over-expressed cells when treated with TGF-β1. This implies that TGIF expression in leiomyomata may be insufficient to suppress ECM deposition caused by TGF-β1 stimulation. Alternatively, suppression of PAI-1 expression may translate into increased fibrinolytic activity or ECM turnover and thus, leiomyoma growth.
In summary, we demonstrated that the expressions of TGIF mRNA and protein are higher in leiomyoma compared to matched unaffected myometrium, and TGIF expression levels are not affected by endogenous steroid hormones. The TGIF protein is potentially able to suppress the profibrotic effect of TGF-β by attenuating the downstream gene, PAI-1, in myometrial cells. Although TGIF's negative regulation on TGF-β in leiomyomata appears to be inadequate to suppress ECM deposition, the associated mechanisms may be important in developing treatment modalities and in understanding the pathogenesis of this complex fibrotic disorder.
Acknowledgements
The authors thank Lorna Groundwater for her invaluable editorial assistance with this manuscript.
Financial support:
This work was funded by NIH grant #AG17907 and the Mary Lake Polan Transition Fund from Stanford University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Where the work was done:
Department of Obstetrics and Gynecology, Stanford University School of Medicine, Stanford, California
Commercial product mentioned in title: None
Presented at a meeting: None
Capsule
TGIF expression is significantly increased in leiomyoma compared to unaffected myometrium. Over-expression of TGIF in cultured myometrial cells inhibits TGF-β1 induced PAI expression.
References
- 1.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–8. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 2.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. Faseb J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 3.Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertility and sterility. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 4.Dou Q, Zhao Y, Tarnuzzer RW, Rong H, Williams RS, Schultz GS, et al. Suppression of transforming growth factor-beta (TGF beta) and TGF beta receptor messenger ribonucleic acid and protein expression in leiomyomata in women receiving gonadotropin-releasing hormone agonist therapy. The Journal of clinical endocrinology and metabolism. 1996;81:3222–30. doi: 10.1210/jcem.81.9.8784073. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Wen Y, Polan ML, Qiao J, Chen BH. Increased expression of latent TGF-beta binding protein-1 and fibrillin-1 in human uterine leiomyomata. Molecular human reproduction. 2007;13:343–9. doi: 10.1093/molehr/gam007. [DOI] [PubMed] [Google Scholar]
- 6.Bertolino E, Reimund B, Wildt-Perinic D, Clerc RG. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. The Journal of biological chemistry. 1995;270:31178–88. doi: 10.1074/jbc.270.52.31178. [DOI] [PubMed] [Google Scholar]
- 7.Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic acids research. 1997;25:4173–80. doi: 10.1093/nar/25.21.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallis D, Muenke M. Mutations in holoprosencephaly. Human mutation. 2000;16:99–108. doi: 10.1002/1098-1004(200008)16:2<99::AID-HUMU2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, et al. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nature genetics. 2000;25:205–8. doi: 10.1038/76074. [DOI] [PubMed] [Google Scholar]
- 10.Wotton D, Lo RS, Lee S, Massague J. A Smad transcriptional corepressor. Cell. 1999;97:29–39. doi: 10.1016/s0092-8674(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 11.Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–12. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- 12.Wen X, Li Y, Hu K, Dai C, Liu Y. Hepatocyte growth factor receptor signaling mediates the anti-fibrotic action of 9-cis-retinoic acid in glomerular mesangial cells. The American journal of pathology. 2005;167:947–57. doi: 10.1016/S0002-9440(10)61185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen B, Wen Y, Yu X, Polan ML. Elastin metabolism in pelvic tissues: is it modulated by reproductive hormones? American journal of obstetrics and gynecology. 2005;192:1605–13. doi: 10.1016/j.ajog.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Wen Y, Zhang Z, Wang H, Warrington JA, Polan ML. Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. American journal of obstetrics and gynecology. 2003;189:89–97. doi: 10.1067/mob.2003.373. [DOI] [PubMed] [Google Scholar]
- 15.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–86. [DOI] [PubMed] [Google Scholar]
- 16.Wen Y, Polan ML, Chen B. Do extracellular matrix protein expressions change with cyclic reproductive hormones in pelvic connective tissue from women with stress urinary incontinence? Human reproduction (Oxford, England) 2006;21:1266–73. doi: 10.1093/humrep/dei485. [DOI] [PubMed] [Google Scholar]
- 17.Wen Y, Man WC, Sokol ER, Polan ML, Chen BH. Is alpha2-macroglobulin important in female stress urinary incontinence? Human reproduction (Oxford, England) 2008;23:387–93. doi: 10.1093/humrep/dem370. [DOI] [PubMed] [Google Scholar]
- 18.Ferrand N, Demange C, Prunier C, Seo SR, Atfi A. A mechanism for mutational inactivation of the homeodomain protein TGIF in holoprosencephaly. Faseb J. 2006;21:488–96. doi: 10.1096/fj.06-6423com. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht JL, Atal NS, Tadros PN, Orsino A, Lye SJ, Sadovsky Y, et al. Rat uterine myometrium contains the gap junction protein connexin45, which has a differing temporal expression pattern from connexin43. American journal of obstetrics and gynecology. 1996;175:853–8. doi: 10.1016/s0002-9378(96)80012-3. [DOI] [PubMed] [Google Scholar]
- 20.Ungefroren H, Gellersen B, Krull NB, Kalthoff H. Biglycan gene expression in the human leiomyosarcoma cell line SK-UT-1. Basal and protein kinase A-induced transcription involves binding of Sp1-like/Sp3 proteins in the proximal promoter region. The Journal of biological chemistry. 1998;273:29230–40. doi: 10.1074/jbc.273.44.29230. [DOI] [PubMed] [Google Scholar]
- 21.Bartholin L, Powers SE, Melhuish TA, Lasse S, Weinstein M, Wotton D. TGIF inhibits retinoid signaling. Molecular and cellular biology. 2006;26:990–1001. doi: 10.1128/MCB.26.3.990-1001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo SR, Lallemand F, Ferrand N, Pessah M, L'Hoste S, Camonis J, et al. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. The EMBO journal. 2004;23:3780–92. doi: 10.1038/sj.emboj.7600398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo SR, Ferrand N, Faresse N, Prunier C, Abecassis L, Pessah M, et al. Nuclear retention of the tumor suppressor cPML by the homeodomain protein TGIF restricts TGF-beta signaling. Molecular cell. 2006;23:547–59. doi: 10.1016/j.molcel.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Ding L, Xu J, Williams RS, Chegini N. Leiomyoma and myometrial gene expression profiles and their responses to gonadotropin-releasing hormone analog therapy. Endocrinology. 2005;146:1074–96. doi: 10.1210/en.2004-1384. [DOI] [PubMed] [Google Scholar]
- 25.Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146:1097–118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]