Abstract
The C57BL/6J (B6/J) male mouse represents a standard for diet induced obesity (DIO) and is unique in expressing a loss-of-function Nicotinamide Nucleotide Transhydrogenase (Nnt) gene. This mutation was associated with a marked reduction in glucose-stimulated insulin secretion from B6/J islets in vitro and moderately impaired glucose clearance in vivo. To assess the contribution of this Nnt mutation, we compared DIO responsiveness of Nnt-mutant B6/J males to Nnt wildtype C57BL/6NJ (B6/NJ) males over a 14 week period of feeding a high fat (60% of calories) diet. Initial mean body weights at 6 weeks did not distinguish the substrains and both substrains were DIO sensitive. However, B6/J males outgained the B6/NJ males, with a significant 3g higher mean body weight at 20 weeks accompanied by significant increases in both lean and fat mass. Mean non-fasting serum glucose over time was also significantly higher in B6/J males, as was impairment of glucose tolerance assessed at 8 and 20 weeks of age. Serum leptin, but not insulin, was significantly higher in B6/J males over time. Potential contributions of the wildtype Nnt gene were demonstrable on a lower fat diet (10% of calories) where a significantly greater weight gain over time by B6/NJ males was correlated with a significantly higher serum insulin. In conclusion, DIO developed in response to 60% fat feeding regardless of Nnt allele status. Contribution of the B6/J-unique Nnt mutation was most evident in response to 10% fat feeding that resulted in reduced serum insulin and weight gain compared to B6/NJ males.
Keywords: Adiposity, Animal Models, Dietary Fat, Genetics, Insulin Secretion
INTRODUCTION
Male mice of certain inbred strains fed semi-defined diets containing a high percentage of energy (45–60%) derived from fat respond by development of diet induced obesity (DIO). DIO in mice recapitulates aspects of the metabolic syndrome associated with human obesity, notably insulin resistance, impaired glucose tolerance, and dyslipidemia (1). The C57BL/6J (B6/J) male mouse has served as a paradigm strain for DIO sensitivity (2). B6/J males fed a standard chow diet clear glucose less rapidly than males of many other inbred strains following an acute challenge in a glucose tolerance test (3). Studies indicating that B6/J beta cells exhibit an insulin secretory defect in their acute response to glucose challenge have been reviewed (4). B6/J mice have recently been differentiated from other C57BL strains tested by a null allele encoding nicotinamide nucleotide transhydrogenase (Nnt) on Chr. 13 (5). A missense (methionine to threonine) mutation in the mitochondrial leader sequence, coupled with an in-frame 5 exon deletion removing 4 putative transmembrane helices, produced markedly lower NNT protein expression in liver and islets (5). As NNT is an inner mitochondrial membrane proton translocase thought to be important in ox/redox balance, its reduced expression and function in B6/J beta cells would be expected to affect ATP production and thus, impair glucose stimulated insulin release (6–7)
In the present study, we evaluate the contribution of the B6/J unique Nnt mutation to the strain’s high DIO sensitivity by a side-by-side comparison with the B6/NJ substrain. B6/NJ is a NIH substrain separated at F32 from B6/J at The Jackson Laboratory in 1951 and re-introduced to The Jackson Laboratory in 2005. We have found that this substrain, like all other mouse strains yet reported (5), carries a wildtype Nnt allele.
METHODS AND PROCEDURES
A cohort of 40 B6/J (JAX #664) males and 39 B6/NJ (JAX#5304) males obtained from the production facility of The Jackson Laboratory at 4 weeks of age were maintained on an autoclaved chow diet (LabDiet® 5K52, 6% fat, PMI, Richmond, IN) in a specific pathogen-free research vivarium. Mice were caged in groups of 10 with food removal from the hopper of each cage monitored weekly. At 6 weeks of age, the cohorts were switched from chow diet to a semi-defined diet containing high fat (60% of calories, Research Diets, New Brunswick, NJ, diet D12492). A second set of 30 B6/J and 30 B6/NJ males were also received at 4 weeks and switched from chow diet at 6 weeks of age to a semi-defined low fat diet (10% of calories, diet D12450B) for comparison. Fat source in the high fat diet is primarily lard versus almost equal parts lard and soybean oil in the low fat diet. Mice were fed diets ad libitum and provided acidified drinking water for 14 weeks. Body weights were measured weekly. Glucose tolerance tests (GTT) were administered to a subset from each group following a 16 hr fast at 8 weeks of age (2 weeks on diet) and 20 weeks (14 weeks on diet) using an i.p. bolus of 2g glucose/kg body weight. Blood glucose was determined using a glucometer (One Touch Ultra) and insulin and leptin concentrations in a subset of each group were determined in serum obtained from the retro-orbital sinus blood of conscious mice at 8, 12, 16, and 20 weeks of age by ELISA (Crystal Chem). Lean and fat body mass were determined by dual emission X-ray absorptiometry (DEXA, Lunar Piximus) on a subset of the mice at 20 weeks of age. For longitudinally sampled phenotypes, linear mixed models with repeated measures were employed with SAS 9.1 software (SAS Systems, Inc., Cary, N.C.) to compare effects of substrain and age on body weight and blood analytes. Serum insulin and leptin values were log-transformed to make distributions normal. In the GTT, glucose values exceeding the 600 mg/dl high upper limit of glucometer sensitivity were assigned a value of 600, and accordingly, GTT values were right censored, and regression analysis with right censoring was applied for GTT analysis using proc LIFEREG in SAS9.1. Significance was set at P<0.05.
The Nnt allele expressed by the B6/NJ substrain was PCR genotyped using a three primer, two allele PCR assay that discriminates between the Nnt wild-type allele and the mutant allele lacking exons 7- 11 inclusive found in B6/J mice (7). Primers were designed using the mouse genome sequence of the Nnt locus available from the public Ensembl website. The primer sequences are (all 5’ – 3’):
Nnt-COM (GTAGGGCCAACTGTTTCTGCATGA)
Nnt-WT (GGGCATAGGAAGCAAATACCAAGTTG)
Nnt-MUT (GTGGAATTCCGCTGAGAGAACTCTT)
The “COM” primer participates in amplification of both the wild type and NntB6J mutant alleles, while the “WT” and “MUT” primers are specific to the wild-type and NntB6J mutant alleles respectively. The amplification products are 579 bp for the wild type allele and 743 bp for the mutant allele. Serendipitously, use of the primers with heterozygous mutant NntB6J template produces an additional faint product at 1kb that can assist in assignment of genotype. Amplification conditions used were initial melt 95°C, 5min; then 35 cycles of 95°C, 45 sec, 58°C, 30 sec, 72°C, 45 sec; followed by a final extension of 5 min at 72°C. Products were analyzed by electrophoresis through a 1% SeaKem agarose, 1x TBE gel followed by staining in ethidium bromide and visualized using a UV light box and a commercial imaging system (BioRad).
All procedures involving the use of animals were approved by the Animal Care and Use Committees of The Jackson Laboratory and The University of California, Irvine.
RESULTS
Substrain responsiveness to 60% fat diet
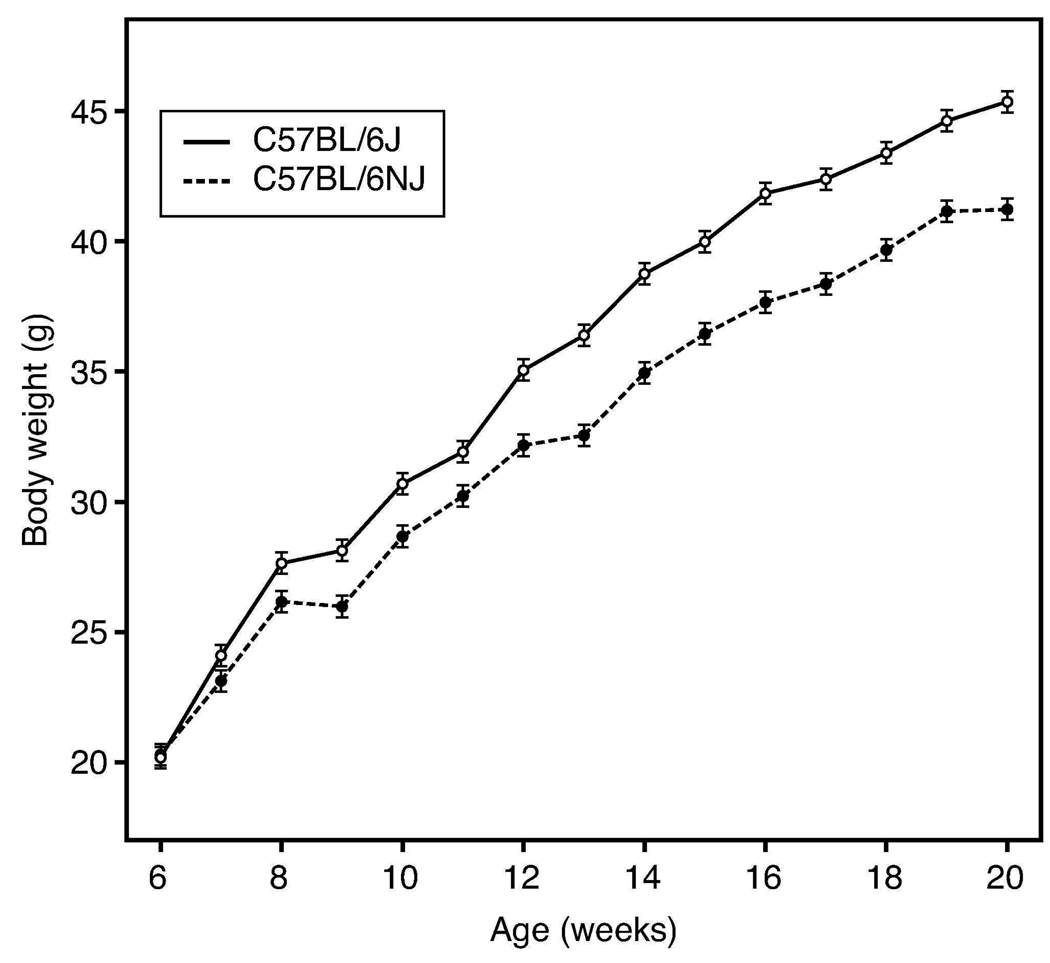
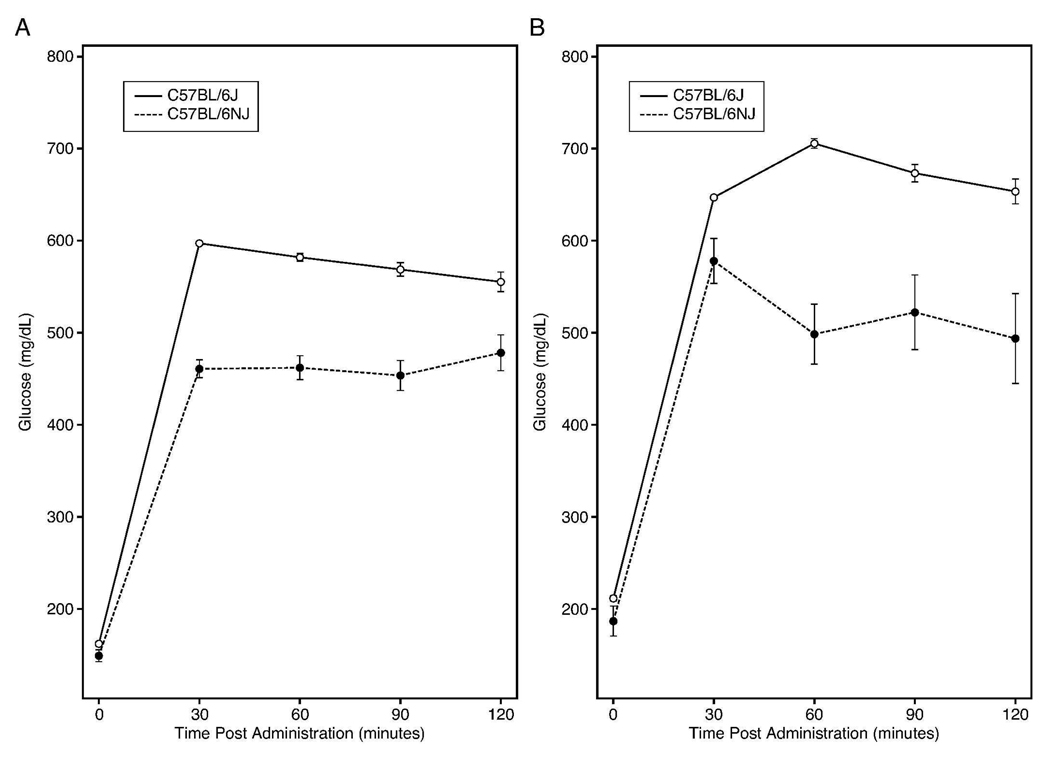
Like all other extant C57BL strains reported to date with the exception of B6/J, PCR genotyping established that the B6/NJ substrain carried a wildtype Nnt allele. Hence, the mutation in B6/J would have occurred after substrain separation. Fig. 1A shows that males of both substrains exhibit a strong DIO responsiveness to the high fat diet, but that the B6/J rate of weight gain is significantly greater (P<0.0001). Severely impaired glucose tolerance presented in males of both substrains after 2 weeks on diet (Fig. 2A) and became even more severe after 14 weeks on diet (Fig. 2B). At both time points, B6/J male glucose clearance was significantly worse (P<0.0001). Data in Table 1 compare differences in mean DIO phenotypes at 20 weeks. The mean 4.2 g body weight difference in B6/J males at 20 weeks correlated with a significantly higher mean lean and fat mass (P=0.03). Consistent with their greater adiposity, MANOVA analysis of log-transformed serum leptin and insulin sampled at the 8, 12, 16, and 20 week intervals showed significantly higher leptin concentrations in B6/J (P <0.01), but not insulin (P = 0.09) over time. It was shown previously that, in response to high fat diet, B6/J males can increase their first phase insulin secretory response to glucose (8), thus likely compensating for any Nnt associated impairment. Nevertheless, consistent with a role for a functional Nnt allele in promoting glucose tolerance, mean non-fasting serum glucose concentration was significantly lower in B6/NJ (P<0.002) over time. Thus, both substrains responded robustly to high fat diet, but with B6/J males showing greater adiposity and maintaining higher non-fasting serum glucose concentrations. No significant substrain differences in cage food disappearance were found on this diet.
Figure 1.
Greater rate of weight gain in a cohort of 40 B6/J males compared to 39 B6/NJ males fed 60% fat diet from 6–20 weeks of age (P<0.0001). Note comparable starting weights.
Figure 2.
Severe impairment of glucose tolerance in both substrains after 2 weeks (A) and 14 weeks (B) of feeding 60% fat diet, but with greater impairment in B6/J males (P<0.0001).
TABLE 1.
Substrain comparison of 20-week-old males fed 60% fat diet [mean ± SEM (n)]
| Substrain | SG a (mg/dl) |
BW (g) |
Lean Tissue (g) |
Fat Tissue (g) |
Percent Fat |
SI (ng/ml) |
SL (ng/ml) |
|---|---|---|---|---|---|---|---|
| B6/J | 237±5b (40) |
45.4±0.4 b (40) |
22.5±0.5c (12) |
20.6±1.1c (12) |
47.6 (12) |
8.9±1.3 (10) |
90.1±5.3 (10) |
| B6/NJ | 198±5 (39) |
41.2±0.4 (39) |
20.8±0.5 (12) |
16.3±1.1 (12) |
42.7 (12) |
10.7±3.1 (10) |
72.4±8.5 (10) |
SG=serum glucose; BW=Body Weight; SI=serum insulin; SL= serum leptin
Substrain differences significant at P<0.0001.
Substrain differences significant at P=0.03.
Substrain responsiveness to 10% fat diet
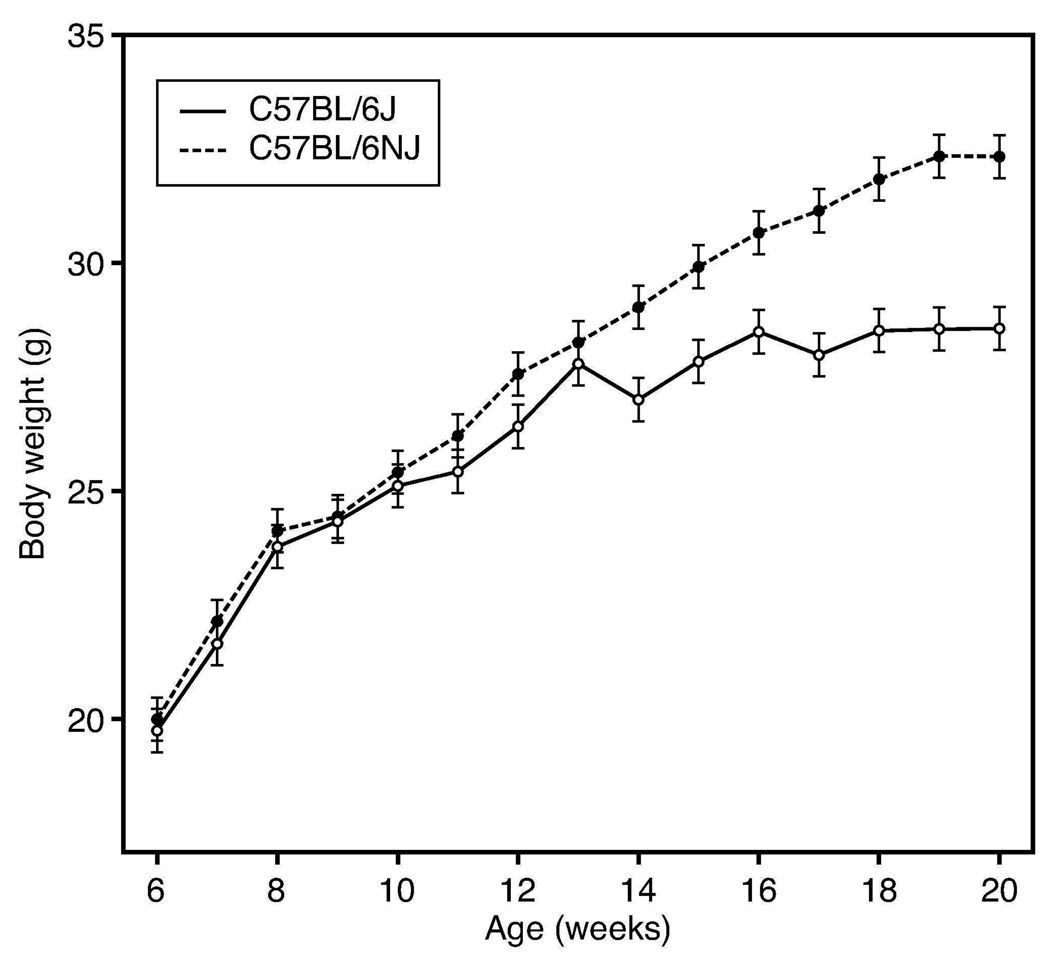
Whereas enhanced responsiveness to the adiposity-promoting action of the high fat diet differentiated the stronger responding Nnt-deficient B6/J males from wildtype B6/NJ, an entirely different pattern emerged on the 10% fat diet. Although peripubertal rates of body weight gain were comparable, B6/NJ males older than 12 weeks continued to gain while growth in B6/J males plateaued (Figure 3). Although lean mass after 14 weeks on diet was comparable, fat mass of B6/NJ had nearly doubled (10.8±1.2 g vs 6.0±1.2 g in B6/J). Paradoxically in view of the greater adiposity in the B6/NJ males, food removal in the B6/J cages was slightly, but significantly (P<0.05) greater than in the B6/NJ cages. MANOVA analysis of log-transformed serum insulin and leptin sampled at the 8, 12, 16, and 20 week intervals showed significantly higher concentrations of insulin but not leptin (P<0.01) in B6/NJ. The significantly lower plasma insulin content over time in 10% fat-fed B6/J males was correlated with a significantly higher plasma glucose (Table 2), likely reflecting, in part, the impairment of glucose stimulated insulin secretion contributed by the Nnt mutation. Thus, if the primary impact of the Nnt mutation is to blunt beta cell glucose-stimulated insulin secretion, this phenotype is most readily detected on the low but not the high fat diet.
Figure 3.
Greater weight gain of a cohort of 30 B6/NJ males compared to 30 B6/J males fed the 10% fat diet from 6–20 weeks of age (P<0.0001). Note the reciprocal nature of the substrain response compared to that on the 60% fat diet shown in Figure 1.
Table 2.
Substrain comparison of 20-week-old males fed 10% fat diet [mean ± SEM (n)]
| Substrain | SG a (mg/dl) |
BW (g) |
Lean Tissue (g) |
Fat Tissue (g) |
Percent Fat |
SI (ng/ml) |
SL (ng/ml) |
|---|---|---|---|---|---|---|---|
| B6/J | 200±6b (30) |
28.6±0.3b (30) |
21.9±0.5 (9) |
6.0±0.4b (9) |
21.3b (9) |
1.8±0.2c (10) |
19.7±2.7c (10) |
| B6/NJ | 166±6 (30) |
32.3±0.4 (30) |
20.5±0.8 (9) |
10.8±0.9 (9) |
34.4 (9) |
5.7±1.0 (10) |
41.6±6.5 (10) |
SG=serum glucose; BW=Body Weight; SI=serum insulin; SL= serum leptin
Substrain differences significant at P<0.0001;
Substrain differences significant at P=0.03.
DISCUSSION
Although statistically higher adiposity and serum glucose elevations distinguished the DIO response of B6/J males compared to B6/NJ males after 14 weeks of high fat diet feeding, it should be noted that both substrains were highly DIO-sensitive. This was evidenced in part by the markedly impaired GTT exhibited by both substrains after only two weeks of high fat diet feeding, and the >40% body fat exhibited by both substrains at the 20 week termination point. The semi-defined high fat diet contains less enriched carbohydrates in comparison to the low fat diet (26.3g% in the former versus 67.3 g% in the latter). The markedly greater adiposity development in the B6/NJ males on the 10% fat diet was discordant with the observation of their slightly reduced disappearance of food per cage over time compared to the less adipose B6/J males. Food removal from the hopper is only a crude surrogate for food consumption. The small differences in food disappearance observed may therefore represent differences in food wastage rather than ingestion; we have noted behaviorial differences between the two substrains, including more robust barbering activity by B6/J males. The most likely explanation for the greater adiposity development in B6/NJ males is the significantly higher insulin secretion over time. Future indirect calorimetric analysis will be required to establish the degree to which the mutant Nnt allele, through its effects on glucose stimulated insulin secretion contributes to differential nutrient partitioning. Pancreatic islet NNT activity directly influences mouse beta cell insulin secretion at elevated glucose concentrations (7–9). Hence contributions of the B6/J Nnt null allele to phenotype would be anticipated to be most evident in a refined carbohydrate-enriched/fat-reduced dietary environment. In fact, the B6/J males fed the higher carbohydrate/lower fat diet secreted significantly less insulin over the 14 week study duration than did B6/NJ. This significant reduction in circulating insulin in B6/J males on the low fat diet correlated with this substrain’s lower weight gain over the post-pubertal period and its significantly lower adiposity and higher serum glucose at the study termination at 20 week. The B6/J substrain-unique Nnt mutation is the most likely explanation for this differential responsiveness, but it does not represent the only difference. It has been estimated that at least 3 recessive mutations are fixed to homozygosity at each inbreeding generation (10) of which the Nnt mutation represents but one example. B6/J differ from B6/NCrl mice in alcohol consumption and preference, differences associated with copy number variations on Chromosomes 4, 14, and X (11). Single nucleotide polymorphisms distinguishing B6/J from other substrains have also been identified on every autosome-(Drs. Gary Churchill, The Jackson Laboratory and Fernando Pardo-Manuel de Villena, University of North Carolina, personal communication). Regulation of insulin secretion is complex and entails multiple metabolic signals generated by glucose catabolism. Thus, it is not possible to attribute to allelic variation at Nnt alone all the significant differences in substrain DIO responsiveness reported herein.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the skilled technical support of Phil Russ, Adam O’Neill, Rob Wilpan and Lucy Rowe. Supported in part by The Jackson Laboratory and an NIH grant (HD-45913) to GRM. Institutional shared services at The Jackson Laboratory were supported by National Cancer Institute Center Support Grant CA34196.
Footnotes
Some of the results reported herein have been previously published in JAX® NOTES Issue 511, Fall 2008.
DISCLOSURES
The Jackson Laboratory is a non-profit research institution that distributes both B6 substrains described in this article. CAL and GRM have nothing to disclose.
REFERENCES
- 1.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 2.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to dietinduced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Kaku K, Fiedorek F, Province M, Permutt M. Genetic analysis of glucose tolerance in inbred mouse strains. Diabetes. 1988;37:707–713. doi: 10.2337/diab.37.6.707. [DOI] [PubMed] [Google Scholar]
- 4.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev. 2007;28:48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 5.Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 6.Freeman H, Shimomura K, Horner E, Cox RD, Ashcroft FM. Nicotinamide nucleotide transhydrogenase: a key role in insulin secretion. Cell Metab. 2006;3:35–45. doi: 10.1016/j.cmet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of Nicotinamide Nucleotide Transhydrogenase: A New Quantitive Trait Locus Accounting for Glucose Intolerance in C57BL/6J Mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 8.Andrikopoulos S, Massa CM, Aston-Mourney K, et al. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J Endocrinol. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- 9.Aston-Mourney K, Wong N, Kebede M, et al. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia. 2007;50:2476–2485. doi: 10.1007/s00125-007-0814-x. [DOI] [PubMed] [Google Scholar]
- 10.Bailey D. How pure are inbred strains of mice. Immunol Today. 1982;3:210–214. doi: 10.1016/0167-5699(82)90093-7. [DOI] [PubMed] [Google Scholar]
- 11.Mulligan MK, Ponomarev I, Boehm SL, 2nd, et al. Alcohol trait and transcriptional genomic analysis of C57BL/6 substrains. Genes Brain Behav. 2008;7:677–689. doi: 10.1111/j.1601-183X.2008.00405.x. [DOI] [PubMed] [Google Scholar]