Abstract
Background
People with Parkinson disease (PD) often fall while multi-tasking or walking backward, unavoidable activities in daily living. Dual tasks involving cognitive demand during gait and unfamiliar motor skills like backward walking could identify those with fall risk, but dual tasking while walking backward has not been examined in those with PD, those who experience Freezing of Gait (FOG), or healthy older controls.
Methods
Seventy-eight people with PD (mean age = 65.1±9.5 years, Female: 28%) and 74 age- and sex-matched controls (mean age = 65.0±10.0 years, Female: 23%) participated. A computerized walkway measured gait velocity, stride length, swing and stance percent, cadence, heel to heel base of support, functional ambulation profile, and gait asymmetry during forward and backward walking with and without a secondary cognitive task.
Results
Direction and task effects on walking performance were similar between healthy controls and those with PD. However, those with PD were more affected than controls, and freezers were more affected than non-freezers, by backward walking and dual tasking. Walking backward seemed to impact gait more than dual tasking in those with PD, although the subset of freezers appeared particularly impacted by both challenges.
Conclusion
People with PD are impaired while performing complex motor and mental tasks simultaneously, which may put them at risk for falling. Those with FOG are more adversely affected by both motor and mental challenges than those without. Evaluation of backward walking while performing a secondary task might be an effective clinical tool to identify locomotor difficulties.
Keywords: gait, dual task, backward, Parkinson disease, attention
Introduction
Falls are common among individuals with Parkinson disease (PD), a progressive neurodegenerative movement disorder affecting more than 1 million people in the United States 1, 2. Many falls occur while those with PD attempt to perform multiple tasks simultaneously 3 or are moving backward or perturbed in the backward direction 4. Some clinicians may recommend that those with PD are taught to avoid multi-tasking and walking backward, but these skills are unavoidable and necessary in activities of daily living (ADLs), such as when backing out of the closet after removing an item of clothing5.
Both dual tasking and walking backward may impair gait and balance in those with PD. Gait speed, stride length, and stability decrease when individuals with PD walk while concurrently performing another task such as mental arithmetic 6,7,8,9. Impaired multiple task performance may double the risk of sustaining a fall while performing an ADL 3. Backward gait of older individuals is characterized by lesser velocity, cadence, increased double support time, and shorter stride length and swing phase than younger people10, 11. Walking backward is further impaired in those with PD and particularly in those who experience freezing of gait (FOG) 12.
FOG is a debilitating phenomenon, affecting 53% of patients who have had PD more than 5 years 13. FOG correlates with balance and lower limb/gait-related symptoms at the onset of PD 14,15. Gait variability, asymmetry and dys-rhythmicity may contribute to FOG, as bilateral coordination appears to be impaired in those who experience FOG 16,–18. Dual tasking can elicit FOG in some individuals with PD, and affective and cognitive characteristics may be predisposing factors for FOG development, perhaps even playing an intrinsic role in its underlying mechanisms 17.
Because individuals with PD in general, and particularly those with FOG, may have difficulty modulating gait parameters according to task, gait analysis in PD should include functional locomotor tasks beyond simple forward walking 20(Morris et al 2001). Dual tasks involving mental operations during gait might be used as clinical tests to identify those at greater risk for falling 21,22. No study has examined dual tasking while walking backward in PD. This study aimed to quantify dual tasking while walking forward and backward in those with mild to moderate PD in comparison to a matched control group. A portion of the data for forward and backward walking in the absence of a secondary task has been published previously 12 and these data are included only as reference points for the dual task forward and dual task backward walking conditions. Dual task data have not been published previously in any form.
Methods
This work was approved by the Human Research Protection Office at Washington University in St. Louis. All participants provided written informed consent before participation.
Participants
All participants, both individuals with PD and healthy age-matched controls were recruited from the St. Louis community through advertisement at support groups and community events, from a database that follows approximately 2000 people with PD and from Volunteers for Health, a Washington University database of individuals interested in research. While some participants self-identified, most were directly recruited via telephone, and several were randomly asked to participate at a public site distant to the laboratory. Data files were coded for participant confidentiality. Participants were informally scrutinized for cognitive dysfunction during a health screening questionnaire in an interview process. All participants were personally able to answer the questions during the health screening and all participants with PD had been previously screened by their neurologists for cognitive dysfunction. None of the participants had been diagnosed with dementia. All participants signed their own consent forms and were fully cognizant of the study procedures to which they were agreeing.
Seventy-eight people with PD (mean age = 65.1±9.5 years, Female: 28%) and 74 age- and sex-matched controls (mean age = 65.0±10.0 years, Female: 23%) participated. Potential participants with PD were excluded if they had history or evidence of neurological deficit other than PD. All participants with PD had a diagnosis of idiopathic PD using criteria for clinically defined “definite PD” 23,24,25, demonstrated clear benefit from levodopa, were tested ON medications at a time of self-determined optimal performance, and could walk at least 3 meters without an assistive device. Participants were evaluated using the Unified Parkinson’s Disease Rating Scale Motor Subscale 3 (UPDRS) 26, 27 and classified according to Hoehn & Yahr stages 28. Freezing status was determined by the Freezing of Gait questionnaire 29. Participants were considered freezers if they had a score >1 on Item 3 on this questionnaire, indicating freezing frequency of more than once per week 30.
Spatiotemporal Gait Parameters
A 5m instrumented, computerized GAITRite walkway (CIR Systems, Inc., Havertown, PA) measured gait parameters. Participants began walking prior to reaching the mat and were requested to walk completely across and off the mat for several feet before stopping. First, participants performed “simple” conditions by walking at their normal or “comfortable” pace forward and then backward, performing three trials of each direction. Next, participants performed “dual” conditions by walking forward while performing a mental arithmetic task aloud. This procedure was repeated while participants walked backward. Participants were not given any practice attempts at the tasks. Tasks consisted of one trial each of counting backward from 100 by threes, from 50 by fours and from 75 by sixes. This order, i.e., beginning from 100, then from 50, etc., was observed for all participants for both forward and backward dual task conditions. Responses were categorized as “correct” or “error” if the given mathematical operation was conducted successfully or unsuccessfully. If participants erred once in calculation but were correct subsequently, only one error was noted. To compare performance across subjects, we calculated the average percentage of correct answers given on each trial, as well as the average numbers of correct and incorrect answers and the rate of answering.
Participants were given adequate rest time and allowed to sit between trials as needed. No participants reported fatigue, likely because of the short walking distance and limited number of trials. Results from trials of each condition were averaged. Primary variables of interest were gait velocity, stride length, swing and stance percent, cadence, heel to heel base of support (BOS), functional ambulation profile (FAP, a.k.a. Functional Ambulation Performance) and gait asymmetry (GA). GA reflects the bilateral lower extremity coordination of swing durations during gait and was calculated as:
as per the method of Yogev et al (2007) 31. Higher values of GA indicate more asymmetry. FAP values range from 0 to 100 and comprise the linear relationship of step length/leg length ratio to step time when velocity is normalized to leg length. A valid and reliable numerical representation of gait performance32, FAP aims to quantify variability in gait and distinguishes between people with and without PD 33. Higher values of FAP indicate less variable performance from stride to stride. For more specifics about FAP calculation, please see Hackney & Earhart (2009) 12.
Statistical Analyses
Two × two × two repeated measures ANOVAs (group (PD vs. Control or Freezer vs. Non-Freezer) × direction (forward vs. backward) × task (simple vs. dual)) determined statistical significance when comparing those with PD to Controls, and when comparing Freezers to Non-Freezers. Tukey-Kramer multiple-comparison tests were used to examine pair-wise differences between means. Values presented are means ± SD. The overall level of significance was set at p = 0.05, but was Bonferroni-corrected to account for multiple comparisons; therefore, the level of significance for any given test in the eight spatiotemporal gait parameters was p ≤ 0.00625, while the significance level set for the four variables related to the performance on the mental arithmetic task was p < 0.0125.
Results
Individuals with PD Versus Age- and Sex-Matched Controls
Hoehn & Yahr scale scores of those with PD ranged from 1–3, (2 at stage 1, 11 at stage 1.5, 49 at stage 2, 8 at stage 2.5 and 8 at stage 3). They had an average UPDRS motor subscale 3 score of 27.5 ± 9.2 and disease duration of 8.2 ± 5.0 years. Forty-five percent of those with PD were freezers. Those with PD and Controls did not differ significantly in age.
Performance on the Mental Arithmetic Task
Individuals with PD performed the mental arithmetic tasks with 83.4 ± 20% accuracy while walking forward (mean correct: 4.2 ± 2.3, errors: 0.6 ± 0.6, rate of answering: 0.71 ± 0.4 answers/second) and 85.1 ± 22% accuracy while walking backward (mean correct: 5.2 ± 3.2, errors: 0.6 ± 0.8, rate of answering: 0.50 ± 0.3 answers/second). Controls performed the mental arithmetic task with 85.5 ± 15% accuracy while walking forward (mean correct; 4.6 ± 1.9 errors: 0.7 ± 1.2, rate of answering: 0.84 ± 0.4 answers/second) and 89.9 ± 10% accuracy while walking backward (mean correct; 6.0 ± 2.5, errors: 0.6 ± 0.8, rate of answering: 0.76 ± 0.3 answers/second).
With respect to group, there were no significant differences in percentage of answers correctly given, average number of correct answers given or average number of errors made between those with PD and controls. Individuals with PD gave fewer answers per second than Controls (p < 0.001). With respect to direction, more correct answers were given, the percentage of correct answers was higher and fewer answers per second were given while walking backward as compared to walking forward (p < 0.001 for all).
Spatiotemporal Gait Parameters for PD Versus Control
There were significant main effects of group, direction and task, significant two-way group × task, direction × task, and group × direction interactions, and significant three-way interactions between group, direction and task (Table I). Only significant interactions are presented in the following paragraphs.
Table 1.
Main Effects and Two-way Interactions: PD vs. Control
| Main Effects | Velocity (m/s) |
Stride Length (m) |
Swing Percent |
Stance Percent |
Cadence (steps/min) |
Base of Support (m) |
Functional Ambulation Profile |
Gait Asymmetry |
|
|---|---|---|---|---|---|---|---|---|---|
| Group | Control: PD: |
0.9±0.3 0.8±0.4 |
1.12±0.27 0.93±0.36 |
34.5±3.3 31.4±5.0 |
65.5±3.5 68.7±5.1 |
92±23 98±25 |
0.15 ±0.06 0.15±0.06 |
78±19 71±21 |
6.7±11 8.3±14 |
| Task | Simple: Dual: |
1.0 ±0.3 0.7±0.3 |
1.08±0.32 0.97±0.31 |
34.1±3.5 31.8±5.1 |
66.0±3.6 68.3±5.3 |
108±17 82±24 |
0.14±0.06 0.15±0.07 |
81±19 68±19 |
5.5±11 9.6±14 |
| Direction | Forward: Backward: |
1.0±0.3 0.65±0.3 |
1.22±0.32 0.82±0.29 |
34.1±3.6 31.7±5.0 |
65.9±3.6 68.4± 5.2 |
95±22 95±27 |
0.10±0.04 0.19±0.05 |
86±16 62±16 |
6.5±10 8.6±15 |
| Group × Task | |||||||||
| Control simple | 1.0±0.3 | 1.16±0.27 | 35.3±2.5 | 64.8±2.3 | 106±12 | 0.15±0.06 | 86±15 | 3.8±5.1 | |
| PD simple | 0.9±0.4 | 1.00±0.37 | 33.0±3.9 | 67.1±4.2 | 110±20 | 0.14±0.06 | 77±20 | 7.1±15 | |
| Control dual | 0.7±0.3 | 1.09±0.27 | 33.8±3.9 | 66.3±4.2 | 79±23 | 0.15±0.07 | 71±19 | 9.7±15 | |
| PD dual | 0.6±0.3 | 0.86±0.34 | 29.9±5.4 | 70.2±5.5 | 86±25 | 0.15±0.07 | 65±19 | 9.6±13 | |
| Direction × Task | |||||||||
| Simple forward | 1.2±0.2 | 1.30±0.20 | 35.1±2.1 | 64.9±2.1 | 107±11 | 0.10±0.03 | 95±6.7 | 3.7±3.5 | |
| Dual forward | 0.8±0.3 | 1.15±0.28 | 33.2±4.4 | 66.8±4.5 | 83±23 | 0.10±0.04 | 78±18 | 9.4±14 | |
| Simple backward | 0.8±0.3 | 0.85±0.28 | 33.1±4.2 | 67.1±4.4 | 109±21 | 0.19±0.05 | 67±16 | 7.3±15 | |
| Dual backward | 0.5±0.2 | 0.79±0.28 | 30.4±5.4 | 69.8±5.6 | 82±25 | 0.20±0.05 | 57±14 | 9.9±14 | |
| Group × Direction | |||||||||
| Control forward | 1.0±0.3 | 1.29±0.22 | 35.3±2.6 | 64.7±2.6 | 92±21 | 0.10±0.04 | 89±16 | 6.4±12 | |
| PD forward | 1.0±0.3 | 1.16±0.27 | 33.0±4.0 | 67.0±4.1 | 98±22 | 0.11±0.04 | 84±16 | 6.6±8.6 | |
| Control backward | 0.7±0.3 | 0.95±0.24 | 33.8±3.8 | 66.4±4.0 | 92±24 | 0.20±0.04 | 68±15 | 7.1±11 | |
| PD backward | 0.6±0.3 | 0.69±0.29 | 29.8±5.3 | 70.3±5.5 | 99±29 | 0.19±0.06 | 57±15 | 10±17 | |
Values are means ± SDs. Shaded boxes indicate significant results.
Main Effects of Group, Task and Direction for PD Versus Control
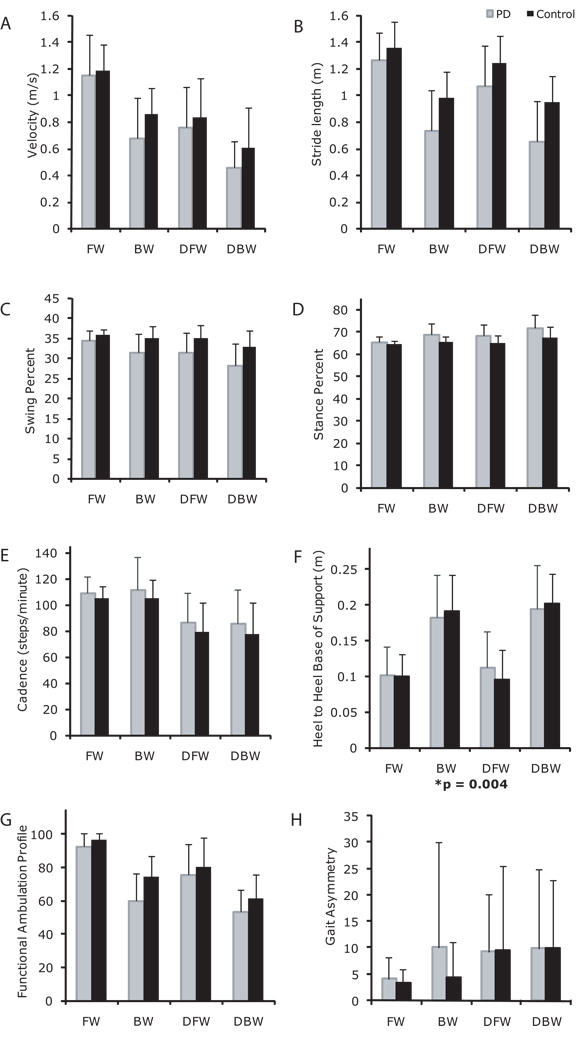
With respect to group, those with PD walked more slowly (Figure 1A), with shorter strides (Figure 1B), lesser swing percent (Figure 1C), greater stance percent (Figure 1D), and lower FAP values (Figure 1G) than Control. With respect to task, participants walked more slowly, with shorter strides, lesser swing percent, greater stance percent, lower cadence (Figure 1E), lower FAP values, wider BOS (Figure 1F) and greater asymmetry (Figure 1H) with the addition of a secondary cognitive task. With respect to direction, participants walked backward more slowly, with shorter strides, lesser swing percent, greater stance percent, wider BOS, and lower FAP values compared to forward walking.
Figure 1.
Walking velocity (A), stride length (B), swing percent (C), stance percent (D), cadence (E), base of support (F) functional ambulation profile scores (G) and gait asymmetry (H) of individuals with PD (light gray bars), and Controls (black bars) in forward walking (FW), backward walking (BW), dual task forward walking (DT), and dual task backward walking (DTB). Values are means +/− SDs. *significant 3-way interaction among group, task and direction.
Interactions PD Versus Control
Group × Task: Both groups were similarly affected by the dual tasks, except that there was a greater decrement in stride length and swing percent for the PD dual task conditions compared to controls (Figures 1B, C).
Direction × Task: Both forward and backward walking were similarly affected by the dual tasks, except that there was a greater decrement in velocity, stride length, swing percent and FAP, and a greater increment in stance percent, for backward dual tasks conditions as compared to forward dual task conditions (Figure 1A-D, G).
Group × Direction: Both groups were similarly affected by direction, except that there was a greater decrement in velocity, stride length, swing percent, and FAP, and a greater increase in stance percent, for the PD backward walking conditions compared to Controls (Figures 1A–D, G). Both groups increased width of the base of support during backward walking conditions, but the increment in BOS was larger in the Controls than in PD (Figure 1F).
Group × Task × Direction: There was a significant three-way interaction of group × task × direction for base of support (Figure 1F).
Freezer Versus Non-Freezer Comparisons
This section compares the performance of those with PD who were classified as Freezers to those with PD who were classified as Non-Freezers. Freezers had PD for a greater duration than Non-Freezers (Freezers: 10.5±5.9 years, Non-freezers: 6.4±3.7 years, p=0.002), but did not differ from Non-Freezers with respect to disease severity (UPDRS Freezers: 29.2±9.6, Non-Freezers: 26.2±8.7, p=0.150).
Performance on the Mental Arithmetic Task: Freezers versus Non-Freezers
Freezers performed the mental arithmetic tasks with 81.9 ± 20% accuracy while walking forward (mean correct: 4.6 ± 2.3, errors: 0.7 ± 0.6, rate of answering: 0.62 ± 0.3 answers/second) and 80.6 ± 26% accuracy while walking backward (mean correct: 5.6 ± 3.6, errors: 0.8 ± 1.1, rate of answering: 0.46 ± 0.3 answers/second). Non-freezers performed the mental arithmetic task with 84.6 ± 21% accuracy while walking forward (mean correct: 3.9 ± 2.2, errors: 0.5 ± 0.5, rate of answering: 0.78 ± 0.4 answers/second) and 88.6 ± 19% accuracy while walking backward (mean correct: 4.9 ± 2.8, errors: 0.4 ± 0.4, rate of answering: 0.53 ± 0.4).
With respect to group, there were no significant differences in percentage of answers correctly given, or number of correct answers given between Freezers and Non-Freezers. Freezers made more errors than Non Freezers (p = 0.020) but this was not significant with the Bonferroni correction. With respect to direction, more correct answers were given and fewer answers per second were given while walking backward as compared to walking forward (p < 0.001 for both).
Spatiotemporal Gait Parameters: Freezers versus Non-Freezers
There were significant main effects of group, task and direction, significant two-way direction-task and group-task interactions and significant three-way interactions between group, task and direction (Table 2). Only significant interactions are presented in the following paragraphs.
Table 2.
Main Effects and Two-way Interactions: Freezer vs. Non-Freezer
| Main Effects | Velocity (m/s) |
Stride Length (m) |
Swing Percent |
Stance Percent |
Cadence (steps/min) |
Base of Support (m) |
Functional Ambulation Profile |
Gait Asymmetry |
|
|---|---|---|---|---|---|---|---|---|---|
| Group | Non-freezer: Freezer: |
0.8±0.4 0.7±0.4 |
0.98±0.36 0.86±0.35 |
32.6±4.2 30.0±5.4 |
67.4±4.2 70.2±5.7 |
100±22 97±29 |
0.14±0.06 0.16 ±0.07 |
74±20 66±20 |
7.2±12 9.8±1 |
| Task | Simple: Dual: |
0.9 ±0.4 0.6±0.3 |
1.00±0.37 0.86±0.34 |
33.0±3.9 30.0±5.4 |
67.1±4.2 70.2±5.5 |
110±20 86±35 |
0.14±0.06 0.15±0.07 |
77±20 65±19 |
7.1±15 9.6±13 |
| Direction | Forward: Backward: |
1.0±0.3 0.6±0.3 |
1.16±0.27 0.69±0.30 |
33.0±4.0 29.8±5.3 |
67.0±4.1 70.3±5.5 |
98±22 99±29 |
0.11±0.04 0.19±0.05 |
84±16 57±15 |
6.6±9 10±17 |
| Group × Task | |||||||||
| Non-Freezer simple | 1.0±0.4 | 1.05±0.338 | 33.8±3.6 | 66.3±3.5 | 110±18 | 0.14±0.06 | 79±20 | 6.5±13 | |
| Freezer simple | 0.9±0.4 | 0.93±0.36 | 32.0±4.0 | 68.2±4.7 | 111±20 | 0.15±0.06 | 74±21 | 7.8±16 | |
| Non-Freezer dual | 0.7±0.3 | 0.92±0.34 | 31.5±4.5 | 68.6±4.5 | 90±22 | 0.14±0.06 | 69±20 | 7.8±12 | |
| Freezer dual | 0.5±0.2 | 0.78±0.33 | 27.9±5.8 | 72.2±6.0 | 82±27 | 0.17±0.07 | 59±16 | 12±14 | |
| Direction × Task | |||||||||
| Simple forward | 1.2±0.3 | 1.26±0.24 | 34.4±2.4 | 65.5±2.4 | 109±13 | 0.10±0.04 | 93±8.1 | 4.1±4.2 | |
| Dual forward | 0.8±0.3 | 1.06±0.30 | 31.6±4.7 | 68.5±4.8 | 87±23 | 0.11±0.05 | 76±18 | 9.2±11 | |
| Simple backward | 0.7±0.3 | 0.67±0.30 | 31.4±4.5 | 68.8±4.9 | 112±25 | 0.18±0.06 | 60±16 | 10±20 | |
| Dual backward | 0.5±0.2 | 0.65±0.27 | 28.2±5.5 | 71.9±5.6 | 86±26 | 0.20±0.06 | 53±13 | 10±15 | |
| Group × Direction | |||||||||
| Non-Freezer forward | 1.0±0.3 | 1.22±0.32 | 34.2±2.9 | 65.8±2.9 | 100±17 | 0.10±0.04 | 88±14 | 4.9±5.1 | |
| Freezer forward | 0.9±0.3 | 1.09±0.28 | 31.5±4.7 | 68.5±4.8 | 95±27 | 0.12±0.05 | 79±18 | 8.8±11 | |
| Non-Freezer backward | 0.6±0.3 | 0.75±0.30 | 31.0±4.7 | 69.1±4.7 | 99±27 | 0.18±0.05 | 60±16 | 9.5±16 | |
| Freezer backward | 0.5±0.3 | 0.62±0.26 | 28.4±5.5 | 71.9±6.1 | 99±31 | 0.20±0.06 | 53±19 | 11±19 | |
Values are means ± SDs. Shaded boxes indicate significant results.
Main Effects of Group, Direction and Task for Freezer Versus Non-Freezer Comparisons
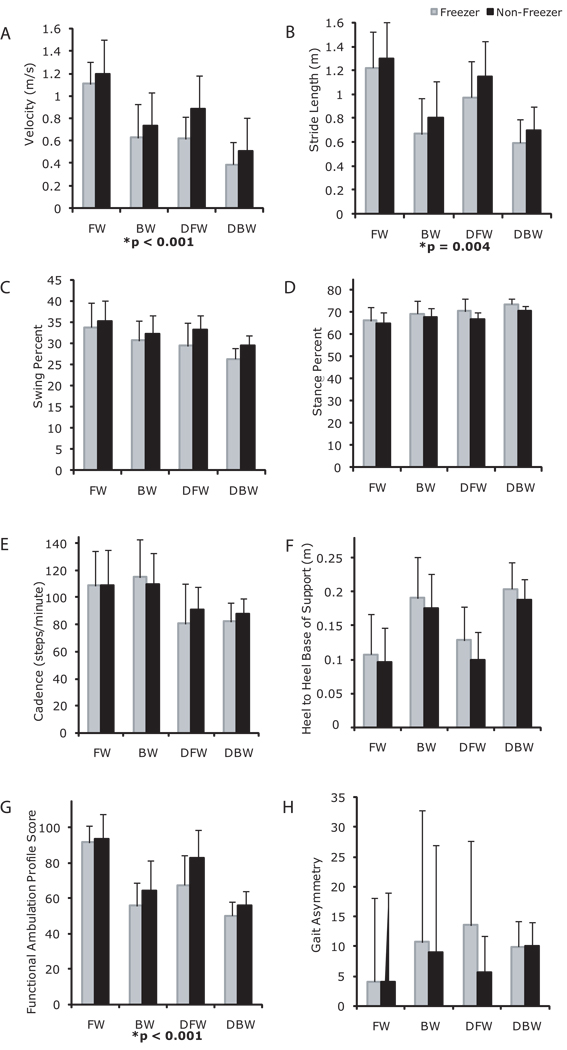
With respect to group, Freezers walked more slowly, had lesser swing percent (Figures 2A–C), greater stance percent (Figure 2D) and lower FAP values (Figure 2G) than Non-Freezers. With respect to task, participants walked more slowly, with shorter strides, lesser swing percent, greater stance percent, lower cadence (Figure 2E), wider BOS, and lower FAP values in dual task conditions as compared to simple conditions. With respect to direction, participants walked backward more slowly, with shorter strides, lesser swing percent, greater stance percent, wider BOS (Figure 2F) and lower FAP values as compared to forward walking.
Figure 2.
Walking velocity (A), stride length (B), swing percent (C), stance percent (D), cadence (E) base of support (F), functional ambulation profile scores (G) and gait asymmetry (H) of individuals with PD classified as Freezers (light gray bars), and Non-Freezers (black bars) in forward walking (FW), backward walking (BW), dual task forward walking (DT), and dual task backward walking (DTB). Values are means +/− SDs. *significant 3-way interaction among group, task and direction.
Interactions for Freezers Versus Non-Freezers
Group × Task: Freezers and non-freezers were similarly affected by dual task conditions. There were no significant interactions between group and task.
Direction × Task: Both forward and backward walking were similarly affected by the dual tasks, except that there was a greater decrement in velocity and stride length, and FAP for backward dual tasks conditions as compared to forward dual task conditions (Figure 2A, B, G).
Group × Direction: Both groups were similarly affected by direction, except that there was a greater decrement in velocity for the Freezer backward walking conditions compared to Non-Freezers (Figure 2A).
Group × Task × Direction: There were significant three-way interactions of group × task × direction for velocity, stride length, and FAP (Figure 2A, B, G).
Discussion
This is the first study to examine dual tasking while walking backward in people with PD and healthy older controls and the first to demonstrate differential effects of a secondary task on those with and without FOG. Healthy older controls show a similar pattern of impact of direction and task to those with PD. With respect to spatiotemporal gait parameters, those with PD were more impaired than Controls while Freezers were more impaired than Non-Freezers across all conditions.
Impact of Task
Dual tasking appeared to adversely impact all gait variables examined. With respect to gait variables, dual tasking affected cadence more than did backward direction and Freezers were more impacted by both challenges, i.e., backward direction and dual tasking, than Non-Freezers. Not only did individuals with PD walk more slowly while dual tasking, but they also gave answers at a slower rate than did Controls. Attention has profound effects on gait 38 and patients with PD may have limited attentional resources, defective central executive functioning, and less automaticity while performing in a dual task situation 34. While walking and performing a difficult cognitive task, gait performance may deteriorate in those with PD because of equal treatment of all elements of a complex task 3 (Bloem et al 2006).
Impact of Direction
Those with PD were generally more impacted by the backward direction than by the secondary task. In the present study notable gait asymmetry, which has been linked to deprived attentional resources allocated to gait 31 was demonstrated in those with PD in the simple backward direction, an effect not seen in Controls. Secondary tasks can be either motor or cognitive and can affect the gait and motor performance of those with PD adversely 9, 34. Being less habitual, walking backward is likely a motor task that requires additional attentional resources. Those with PD are impaired while walking backward 12, possibly because backward walking relies more heavily on proprioception than forward walking35. Proprioceptive disturbances in PD have been attributed to abnormal processing of proprioceptive signals in the basal ganglia 36,37.
Gait Variability, Asymmetry and Automaticity
In the present study, those with PD became more variable (as measured by the FAP) when performing backward walking or the secondary mental arithmetic task. Those with PD demonstrated gait asymmetry in all challenging conditions, i.e. backward walking or dual task conditions, and Freezers demonstrated more gait asymmetry (albeit non-significantly) in dual tasking than did Non-Freezers. Healthy gait coordination, a relatively automatic process in controls, may require attentional control in those with PD; therefore, their gait is particularly affected during performance of secondary tasks 39. Subjects with PD are increasingly variable in gait while performing a cognitive task, 38 and individuals with PD and elderly fallers demonstrate more gait asymmetry, exacerbated under dual task conditions 31. Dual tasking has been linked to gait dys-rhythmicity and asymmetry and may lower the threshold for FOG 17. Automaticity is greatly impaired in those who experience FOG 41, as their internal drivers of movement seem to be particularly impacted by deficient basal ganglia function. Dual tasks activate similar brain regions in those with PD and controls in the task training and motor adaptation stages that precede automaticity; however, those with PD probably achieve automaticity with more difficulty, as during task training and motor adaptation, greater activity is found in the cerebellum, premotor area, parietal cortex, precuneus and prefrontal cortex when compared to controls. Possibly, this compensates for basal ganglia dysfunction that impairs automatic movement 40.
Implications
Competition for attention through challenging activities will increase gait difficulty in those with PD. A limitation of the present study is the lack of formal cognitive screening of participants; however, overall, this study demonstrates that those with PD are considerably more impaired than healthy controls in complex functional tasks that might be necessary to successfully complete ADLs. Therapeutic assessment should thus include evaluation of performance during complex functional activities 42. Motor and cognitive domains have functional coupling, in that challenges in one domain result in compromised performance in the other. This is clearly illustrated by the performance of those with PD in both the mental and motor tasks, which appeared to be impaired as compared to the performance of healthy, age-matched controls. In fact, executive function and neurocognitive speed could be distinct clinical markers of disease progression in PD 43. Potentially this could be evaluated while a person with PD performs multitasking involving motor and cognitive challenges, such as that provided by the dual task backward condition, which appeared to most adversely impact gait of all participants.
Clearly, new therapies are needed to address these deficits in complex motor and mental tasks. Deep brain stimulation, the premier surgical option for those with PD, appears not to benefit dual task performance 44 and the present study shows that dual tasking is impaired even when participants are on their prescribed medications. Therapeutic approaches may include multiple task training, which has been shown to increase gait velocity in those with PD 45. Practice in situations that require divided attention can improve dual task ability 46,34. In addition, those with PD appear to benefit from rhythmical cues during dual task situations. Cues may reduce attentional costs of walking by facilitating particular attentional allocation 47. Gait variability of individuals with PD, which has been correlated with fall risk, disease duration and severity, motor function, and cognitive function 38, was reduced with cues 48. Training with cues in multitask situations may be an effective form of training and may reduce FOG 30. FOG may be even more effectively addressed by adding a focus on training gait symmetry in the context of a cued dual task paradigm, as gait asymmetry may lower the threshold for FOG 16. Training with backward walking under dual task conditions may be advantageous, as gait asymmetry clearly increased during backward walking in those with PD.
Acknowledgements
We thank Josh Funk, Callie Mosiman, Minna Hong, Ruth Porter, Michael Falvo, Lauren Mehner, Tiffany Chung, Ba Huynh, Jeff Becket, Kyleen Albert, Laura Cohen, Patricia Engel, Callie Chen, Ryan Choi and Karen Stringer, for their assistance with this project. A grant from the American Parkinson Disease Association and NIH grant K01-HD048437 supported this work. The study sponsors played no role in study design, collection, analysis, or interpretation of data, writing of the manuscript or in the decision to submit the manuscript for publication.
References
- 1.Melton LJ, III, Leibson CL, Achenbach SJ, et al. Fracture risk after the diagnosis of Parkinson’s disease: influence of concomitant dementia. Mov Disord. 2006;21(9):1361–1367. doi: 10.1002/mds.20946. [DOI] [PubMed] [Google Scholar]
- 2.Bacon WE. Secular trends in hip fracture occurrence and survival rate: age and sex differences. J Aging Health. 1996;8:538–553. doi: 10.1177/089826439600800404. [DOI] [PubMed] [Google Scholar]
- 3.Bloem BR, Grimbergen YAM, van Dijk JG, Munneke M. The “posture second” strategy: A review of wrong priorities in Parkinson’s disease. J Neurol Sciences. 2006;248:196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Horak FB, Dimitrova D, Nutt JG. Direction specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;198(2):504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and Freezing of Gait in Parkinson’s Disease: A Review of Two Interconnected, Episodic Phenomena. Mov Disord. 2004;19(8):871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 6.Galletly R, Brauer SG. Does the type of concurrent task affect preferred and cued gait in people with Parkinson’s disease? Aust J Physiother. 2005;51:175–180. doi: 10.1016/s0004-9514(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 7.Canning CG. The effect of directing attention during walking under dual task conditions in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11:95–99. doi: 10.1016/j.parkreldis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Rochester L, Hetherington V, Jones D, et al. Attending to the task: interference effects of functional tasks on walking in Parkinson’s disease and the roles of cognition, depression, fatigue, and balance. Arch Phys Med Rehabil. 2004;85:1578–1585. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea S, Morris ME, Iansek R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. 2002;82:888–897. [PubMed] [Google Scholar]
- 10.Laufer Y. The Effect of Age on Characteristics of Forward and Backward Gait at Preferred and Accelerated Walking Speed. J Gerontol: Medical Sciences. 2005;60A(5):627–632. doi: 10.1093/gerona/60.5.627. [DOI] [PubMed] [Google Scholar]
- 11.Hackney ME, Earhart GM. Age and Gender Effects on Dual Task Forward and Backward Walking. Submitted. [Google Scholar]
- 12.Hackney ME, Earhart GM. Backwards Walking in Parkinson Disease. Mov Disord. 2009;24(2):218–223. doi: 10.1002/mds.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E. Abnormalitites of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord. 2001;16:1066–1075. doi: 10.1002/mds.1206. [DOI] [PubMed] [Google Scholar]
- 14.Giladi N, McMahon D, Przedborski S, et al. Motor blocks in Parkinson’s disease. Neurology. 1992;42:333–339. doi: 10.1212/wnl.42.2.333. [DOI] [PubMed] [Google Scholar]
- 15.Giladi N, McDermott MP, Fahn S, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–1721. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- 16.Plotnik M, Giladi N, Balash Y, Perez C, Hausdorff JM. Is Freezing of Gait in Parkinson’s Disease related to Asymmetric Motor Function? Ann Neurol. 2005;57:656–663. doi: 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- 17.Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson’s disease. J Neurol Sciences. 2006;248:173–176. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur J Neurosci. 2008;27:1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- 19.Okuma Y. Freezing of gait in Parkinson’s disease. J Neurol. 2006;253(Suppl 7) doi: 10.1007/s00415-006-7007-2. VII/27–VII/32. DOI 10.1007/s00415-006-7007-2. [DOI] [PubMed] [Google Scholar]
- 20.Morris ME, Huxham F, McGinley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech. 2001;16:459–470. doi: 10.1016/s0268-0033(01)00035-3. [DOI] [PubMed] [Google Scholar]
- 21.Yogev-Selgimann G, Hausdorff JM, Giladi N. The Role of Executive Function and Attention in Gait. Mov Disord. 2008;23(3):329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melzer I, Oddsson LIE. The effect of a cognitive task on Voluntary step execution in Healthy elderly and young individuals. J Am Geriatr Soc. 2004;52:1255–1262. doi: 10.1111/j.1532-5415.2004.52353.x. [DOI] [PubMed] [Google Scholar]
- 23.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88(5):539–543. [PubMed] [Google Scholar]
- 24.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, Daniels SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahn S, Elton RL. UPDRS program members. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 27.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 29.Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat Disord. 2000;6:165–170. doi: 10.1016/s1353-8020(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 30.Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2007;177:336–346. doi: 10.1007/s00221-006-0676-3. [DOI] [PubMed] [Google Scholar]
- 32.Nelson AJ. Functional Ambulation Profile. Phys Ther. 1974;54(10):1059–1065. doi: 10.1093/ptj/54.10.1059. [DOI] [PubMed] [Google Scholar]
- 33.Nelson AJ, Zwick D, Brody S, et al. The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson Gait. NeuroRehabilitation. 2002;17(3):255–262. [PubMed] [Google Scholar]
- 34.Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2008;79:760–766. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- 35.Thomas MA, Fast A. One step forwards and two steps back: the dangers of walking backward in therapy. Am J Phys Med Rehabil. 2000;79(5):459–461. doi: 10.1097/00002060-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP. Impaired vertical postural control and proprioceptive integration deficits in Parkinson’s disease. Neuroscience. 2007;146(2):852–863. doi: 10.1016/j.neuroscience.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 37.Grimbergen YAM, Munneke M, Bloem BR. Falls in Parkinson’s Disease. Curr Opin Neurol. 2004;17:405–415. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- 38.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson's disease. J Geriatr Psychiatry Neurol. 2003;16(1):53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 39.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 40.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 41.Hallett M. Intrinsic and extrinsic aspects of freezing of gait. Mov Disord. 2008;23(Suppl 2):S439–S443. doi: 10.1002/mds.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rochester L, Hetherington V, Jones D, et al. Attending to the Task: Interference Effects on Functional Tasks on Walking in Parkinson’s disease and the Roles of Cognition, Depression, Fatigure and Balance. Arch Phys Med Rehabil. 2004;85:1578–1585. doi: 10.1016/j.apmr.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 43.De Frias CM, Dixon RA, Fisher N, Camicioli R. Intraindividual variability in neurocognitive speed: A comparison of Parkinson’s disease and normal older adults. Neuropsychologia. 2007;45:2499–2507. doi: 10.1016/j.neuropsychologia.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Page D, Jahanshahi M. Deep Brain Stimulation of the Subthalamic Nucleus Improves set shifting but does not affect Dual Task Performance in Parkinson’s Disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15(2):198–206. doi: 10.1109/TNSRE.2007.897074. [DOI] [PubMed] [Google Scholar]
- 45.Canning CG, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s disease: a pilot study. Clin Rehabil. 2008;22:226–233. doi: 10.1177/0269215507082341. [DOI] [PubMed] [Google Scholar]
- 46.Silsupadol P, Siu KC, Shumway-Cook A, Woolacott MH. Training of balance under single- and dual-task conditions in older adults with balance impairment. Phys Ther. 2006;86:269–281. [PubMed] [Google Scholar]
- 47.Rochester L, Nieuwboer A, Baker K, Hetherington V, Willems AM, Chavret F, et al. The Attentional cost of external rhythmical cues and their impact on gait in Parkinson’s disease: effect of cue modality and task complexity. J Neural Transm. 2007;114:1243–1248. doi: 10.1007/s00702-007-0756-y. [DOI] [PubMed] [Google Scholar]
- 48.Baker K, Rochester L, Nieuwboer A. The effect of cues on gait variability – reducing the attentional cost of walking in people with Parkinson’s disease. Parkinsonism Relat disord. 2008;14:314–320. doi: 10.1016/j.parkreldis.2007.09.008. [DOI] [PubMed] [Google Scholar]