Abstract
Cancer of the exocrine pancreas is the fourth leading cause of cancer deaths in the United States. Currently, surgical resection is the only hope for cure. The majority of patients present with locally advanced or metastatic disease. The most common site for distant metastasis is the liver. We report here a modified auxotrophic strain of S. typhimurium that can target and control the growth of liver metastasis in a mouse model of pancreatic cancer. This strain of S. typhimurium is auxotrophic (Leucine-arginine dependent) but apparently receives sufficient nutritional support from tumor tissue. To increase tumor targeting ability and tumor killing efficacy, this strain was further modified by re-isolation from a tumor growing in a nude mouse termed A1-R. In the present study, we demonstrate the efficacy of locally- as well as systemically-administered A1-R on liver metastasis of pancreatic cancer. Mice treated with A1-R given locally via intrasplenic injections or systemically via tail-vein injections had a much lower hepatic and splenic tumor burden as compared to control mice. Systemic treatment with intravenous A1-R also increased survival time. All results were statistically significant. This study suggests the clinical potential of bacterial treatment of a critical metastatic target of pancreatic cancer.
INTRODUCTION
Coley observed more than a century ago that some cancer patients were cured of their tumors following post-operative bacterial infection (1). In middle part of the century, Malmgren et al showed that anaerobic bacteria had the ability to survive and replicate in necrotic tumor tissue with low oxygen content (2). Several approaches aimed at utilizing bacteria for cancer therapy have subsequently been described (3–15). Bifidobacterium longum has been shown to selectively grow in hypoxic regions of tumors following intravenous administration. This effect was demonstrated in 7,12-dimethylbenzanthracene induced rat mammary tumors by Yazawa et al (14, 15). Vogelstein et al. created a strain of Clostridium novyi, an obligate anaerobe, which was depleted of its lethal toxin (16). This strain of C. novyi was termed C. novyi NT. Following intravenous administration, the C. novyi NT spores germinated in the avascular regions of tumors in mice, causing damage to the surrounding viable tumor (16). Combined with conventional chemotherapy or radiotherapy, intravenous C. novyi NT spores caused extensive tumor damage within 24 hours (16).
Following attenuation by purine and other auxotrophic mutations, the facultative anaerobe S. typhimurium was used for cancer therapy (11, 17, 18). These genetically-modified bacteria replicated in tumors to levels more than 1,000 fold greater than in normal tissue (11). S. typhimurium was further modified genetically by disrupting the msbB gene to reduce the incidence of septic shock (11). The msbB mutant of S. typhimurium has been tested in a phase I clinical trial to determine its efficacy on metastatic melanoma and metastatic renal cell carcinoma (19). To raise the therapeutic index, S. typhimurium was further attenuated by deletion of the purI and msbB genes(19). The new strain of S. typhimurium, termed VNP20009, could then be safely administered to patients (19). More studies are needed to completely characterize the safety and efficacy of the bacteria and to improve its therapeutic index.
Mengesha et al. utilized S. typhimurium as a vector for gene delivery by developing a hypoxia-inducible promoter (HIP-1) to limit gene expression to hypoxic tumors. HIP-1 was able to drive gene expression in bacteria residing in human tumor xenografts implanted in mice (20). Genes linked to the HIP-1 promoter showed selective expression in tumors (20). Yu et al. used green fluorescent protein (GFP) labeled bacteria to visualize tumor targeting abilities of 3 pathogens: Vibrio cholerae, S. typhimurium and Listeria monocytogenes (21, 22).
We initially developed a strain of S. typhimurium, termed A1, which selectively grew in tumor xenografts. In contrast, normal tissue rapidly cleared infecting bacteria, even in immunodeficient athymic mice. S. typhimurium A1 is auxotrophic (leu/arg-dependent), but receives sufficient support from tumor tissue. In vivo, the bacteria was previously shown to cause PC-3 tumor growth inhibition and regression of subcutaneous xenografts (23). To increase the tumor-targeting capability of S. typhimurium A1, the strain was reisolated after infection of a human colon tumor growing in nude mice. We previously showed that the tumor isolated strain, termed A1-R, had increased tumor targeting ability both in vitro and in vivo. We also showed previously that A1-R demonstrated efficacy in the treatment of mouse models of orthotopic human breast cancer (24) and orthotopic mouse models of human prostate cancer (25). In the present study, we utilized a mouse model of pancreatic cancer liver metastasis to evaluate the therapeutic efficacy of S. typhimurium A1-R.
MATERIALS AND METHODS
GFP Gene Transfection of S. typhimurium) (23)
S. typhimurium (ATCC 14028) was grown at 37°C to midlogarithmic phase in liquid LB and harvested at 4°C. Bacteria (2.0 × 108) in 40 μl of 10% glycerol were mixed with 2 μl of the pGFP (Clontech, Mountain View, CA) vector containing the hr-GFP gene (Stratagene, La Jolla, CA) and placed on ice for 5 min before electroporation with a Gene Pulser apparatus (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Electroporation was done at 1.8 kV with the pulse controller at 1,000- parallel resistance.
Induction of Bacterial Mutations with Nitrosoguanidine (NTG) and Selection for Auxotrophs (23)
Freshly prepared NTG (1 mg/ml in sterile water) was added to the washed culture to a final concentration of 100 μg/ml in Tris-maleic acid buffer (pH 6.0). The bacteria were incubated with NTG for 30 min. The NTG-treated cells were grown in nutrient broth to express any mutations that were induced. Bacterial colonies were replica-plated in supplemented minimal agar plates containing specific amino acids to identify the requirements of the auxotrophs. Auxotroph A1, which required leu and arg, was identified.
Re-isolation of S. typhimurium A1 (24)
S. typhimurium A1 auxotrophs expressing GFP were reisolated as follows: The A1 bacteria were injected into the tail vein of an HT-29 human colon tumor-bearing nude mouse. Three days after infection, the tumor tissue was removed from the infected mouse. The tumor tissue was then homogenized and diluted with PBS. The resulting supernatant of the tumor tissue was cultured in LB agar plates at 37°C overnight. The bacteria colony with the brightest green fluorescence was picked up and cultured in 5 ml of LB medium. This strain was termed “A1-R.”
Preparation of Bacteria (24)
The A1-R bacteria were grown overnight on LB medium and then diluted 1:10 in LB medium. Bacteria were harvested at late-log phase, washed with PBS, and then diluted in PBS. Bacteria were then injected into the tail vein of nude mice (5 × 107 cfu per 100 μl PBS).
Cell Culture
Low passage XPA-1 human pancreatic cancer cells were established at the Johns Hopkins University Baltimore, MD, USA. The cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine (Gibco-BRL, Life Technologies, Inc., Grand Island, NY, USA). The cell line was cultured at 37°C in a 5% incubator.
RFP vector production and transduction of XPA-1 human pancreatic cancer cell line (26)
The RFP (DsRed-2) gene (Clontech Laboratories, Mountain View, CA) was inserted in the retroviral-based mammalian expression vector pLNCX (Clontech) to form the pLNCX DsRed-2 vector as previously described (26). For RFP gene transduction, 20% confluent XPA-1 human pancreatic cancer cells were incubated with a 1:1 precipitated mixture of retroviral-containing supernatants of PT67 cells and RPMI 1640 or other culture medium (Life Technologies) containing 10% fetal bovine serum (Gemini Biological Products) for 72 hours as previously described ans selected using G1428 as previously described (26).
Selection of Highly Aggressive Subpopulations of XPA-1 RFP Human Pancreatic Cancer Cells
XPA-1 RFP human pancreatic cancer cells were serially passaged in the pancreas of nontransgenic nude mice. After the mice developed disseminated disease, including malignant ascites, they were sacrificed and 50 microliters of ascitic fluid was injected into the pancreas of another nontransgenic nude mouse. After five successive passages, the ascitic fluid from the final passage was transferred into a cell culture flask containing RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine (Gibco-BRL, Life Technologies, Inc., Grand Island, NY, USA) with 1% Penicillin-Streptomycin. The flasks were stored at 37°C in a 5% incubator. After the tumor cells were adherent, the cells were then maintained in culture as described above. This highly aggressive subpopulation of XPA-1 RFP Human Pancreatic Cancer Cells is henceforth referred to as XPA-1 RFP P5A. Mesenteric metastases were also harvested from the same fifth generation animal and were fragmented with surgical instruments and placed in a cell culture dish with RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM glutamine (Gibco-BRL, Life Technologies, Inc., Grand Island, NY, USA) with 1% Penicillin-Streptomycin. The flasks were stored at 37°C in a 5% incubator. Once adherent tumor cells were detected via microscopy, the cells were passaged and maintained in culture as described above. This second population of highly aggressive XPA-1 RFP Human Pancreatic Cancer Cells is henceforth referred to as XPA-1 RFP P5B.
Animals
Nontransgenic nude mice between 4 to 6 weeks of age were used in this study. The animals were bred and maintained in a HEPA-filtered environment at AntiCancer Inc., (San Diego, CA) with cages, food and bedding sterilized by autoclaving. The animal diets were obtained from Harlan Tekland (Madison, WI). Ampicillin (5.0%, w/v; Sigma, St. Louis, MO) was added to the autoclaved drinking water. All animal studies were conducted in accordance with the principles and procedures outlined in the NIH guide for the Care and Use of Laboratory Animals under assurance number A3873-1.
Intrasplenic injection (27)
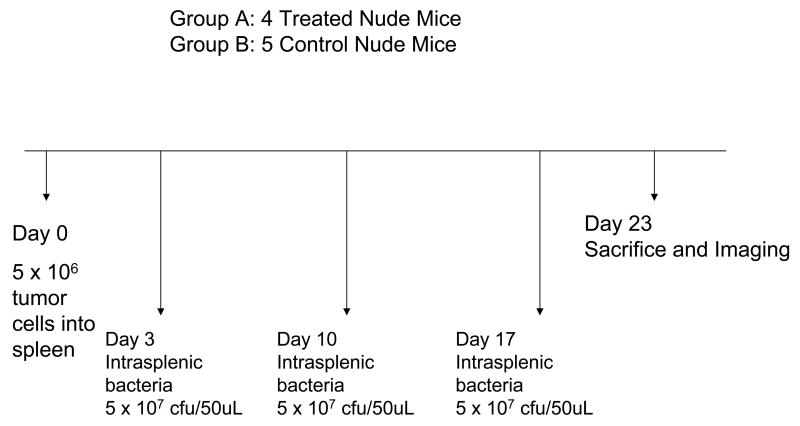
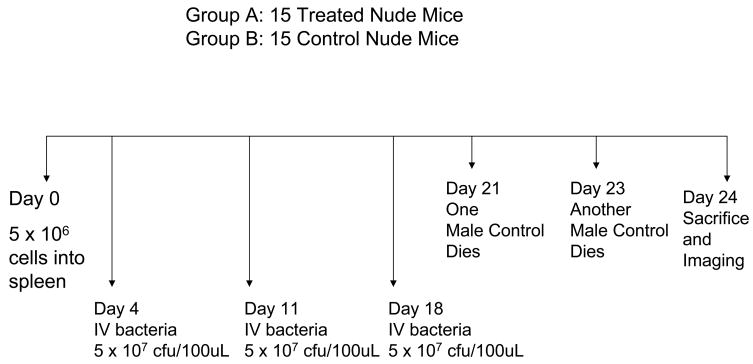
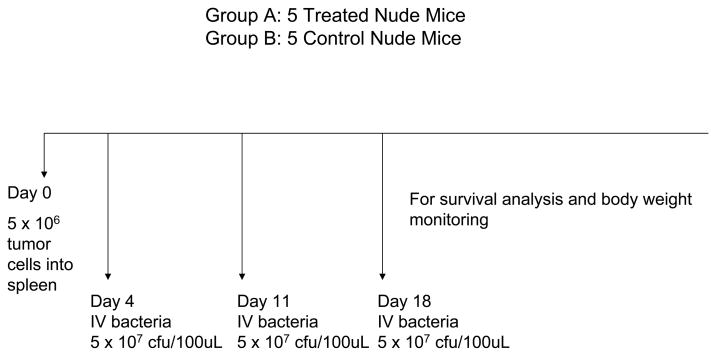
Nontransgenic nude mice were anesthetized with a ketamine mixture (10 μL ketamine HCl, 7.6 μL xylazine, 2.4 μL acepromazine maleate, and 10 μL H2O) injected subcutaneously. Human XPA-1 RFP P5 pancreatic cancer cells (5.0 × 106/50 μL Matrigel) were injected slowly as a cell suspension into the spleen of nude mice during open laparotomy for experiments. Hemostasis was then secured by gentle pressure using a surgical gauze for 2 minutes. The skin and peritoneum were then sutured in a single layer using 6-0 prolene suture. 3 separate experiments were performed in this study. Experiment (1): To study the effect of bacteria delivered via the intrasplenic route, 10 mice were prepared as described above using the XPA-1 RFP P5B cell line. Experiment (2): Another 30 mice were prepared separately using the XPA-1 RFP P5A cell line to study the effect of bacteria delivered via the intravenous route on tumor burden. Experiment (3): Lastly 10 mice were prepared as described above using the XPA-1 RFP P5A cell line to study the effect of intravenous bacterial therapy on animal survival as well as to document any potential adverse effects of treatment.
Administration of Bacteria
Experiment (1)
4 days post tumor-cell injection, 1 animal died due to surgical complications. The remaining 9 were randomized into treatment and control groups. The 4 mice in the treatment group were treated with 3 doses of intrasplenic S. typhimurium A1-R (5 × 107 cfu/100uL PBS) on Day 3, Day 10 and Day 17 post tumor-cell injection. To administer intrasplenic bacteria, the mice were anesthetized as described above, the surgical site was re-opened and the bacteria were injected into the spleen under direct vision. 5 mice served as untreated controls.
Experiment (2)
All 30 mice which had been injected with XPA-1 RFP P5A cells as part of Experiment 2 survived the tumor cell injection and were randomized into treatment and control groups. The 15 mice in the treatment group were treated with 3 doses of intravenous S. typhimurium A1-R (5 × 107 cfu/100uL PBS) via tail vein injection on Day 4, Day 11 and Day 18 post tumor-cell injection. The remaining 15 mice served as untreated controls.
Experiment (3)
All 10 mice which had been injected with XPA-1 RFP P5A cells as part of Experiment 3 survived tumor cell injection and were randomized into treatment and control groups. The 5 mice in the treatment group were treated with 3 doses of intravenous S. typhimurium A1-R (5 × 107 cfu/100uL PBS) via tail vein injection on Day 4, Day 11 and Day 18 post tumor-cell injection. The remaining 5 mice served as untreated controls.
Figures 1a, 1b and 1c summarize the experimental protocols for the Experiments 1, 2, and 3, respectively.
Figure 1. Summary of experimental protocols.
Figures 1a, 1b and 1c summarize the experimental protocols for the Experiments 1, 2, and 3, respectively.
Analysis of Antitumor Efficacy
Experiment (1)
At Day 23 the 9 mice used in the intrasplenic bacteria experiment (4 treated, 5 controls) were sacrificed. Hepatic and splenic tumor burden of these mice were compared using fluorescent imaging.
Experiment (2)
Of the 30 mice included in this experiment, 2/15 untreated controls died on Day 21 and Day 23, respectively, prior to imaging. At Day 24, the remaining 13 untreated controls and 15 treated mice were sacrificed and hepatic and splenic tumor burden were compared using fluorescent imaging.
Analysis of Effect of Intravenous Treatment on Survival and Potential Adverse Effects from Systemic Therapy
Experiment (3)
The 10 animals included in this experiment 5 of the 10 mice were randomly selected to receive treatment with S. typhimurium A1-R (5 × 107 cfu/100uL PBS) intravenously via tail vein injection. A second and third dose of intravenous S. typhimurium A1-R (5 × 107 cfu/100uL PBS) was given on Day 11 and Day 18 post tumor-cell injection respectively. The remaining 5 mice served as untreated controls. The 10 animals were then observed to document survival time and body weight for 7 weeks (49 days) post tumor-cell injection.
Imaging in Live Mice (28)
The Olympus OV100 Small Animal Imaging System (Olympus) containing an MT-20 light source (Olympus) and DP70 CCD camera (Olympus) was used for imaging in live mice (28). High-resolution images were captured directly on a PC (Fujitsu Siemens, Munich, Germany). Images were processed for contrast and brightness and analyzed with the use of Paint Shop Pro 8 and Cell (Olympus).
Statistical analysis
All statistical analyses were performed using SPSS version 12.0 (SPSS, Inc. Chicago, IL).
RESULTS
Intrasplenic injection of XPA-1 RFP P5 human pancreatic cancer cells creates liver metastasis in nude mice
As described in the Materials and Methods, a total of 50 nude mice were each given an intrasplenic injection of XPA-1 RFP P5B or P5A cell suspension. Post-procedural survival was 98%. There was 1 perioperative death. Tumor take in the livers of the untreated controls was 100% at the conclusion of each experiment.
A1-R delivered via Intrasplenic Injection Suppresses XPA-1 RFP P5B Tumor Growth in the Liver and Spleen
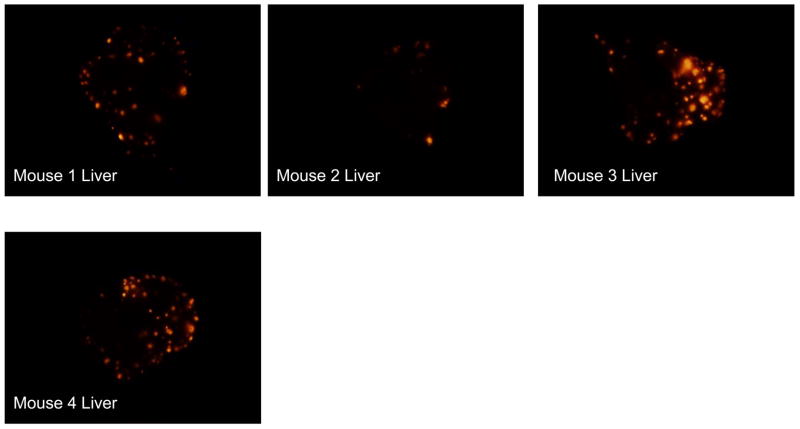
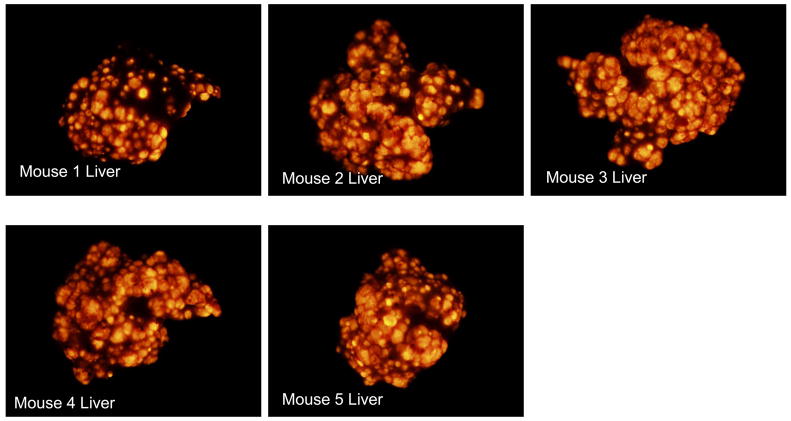
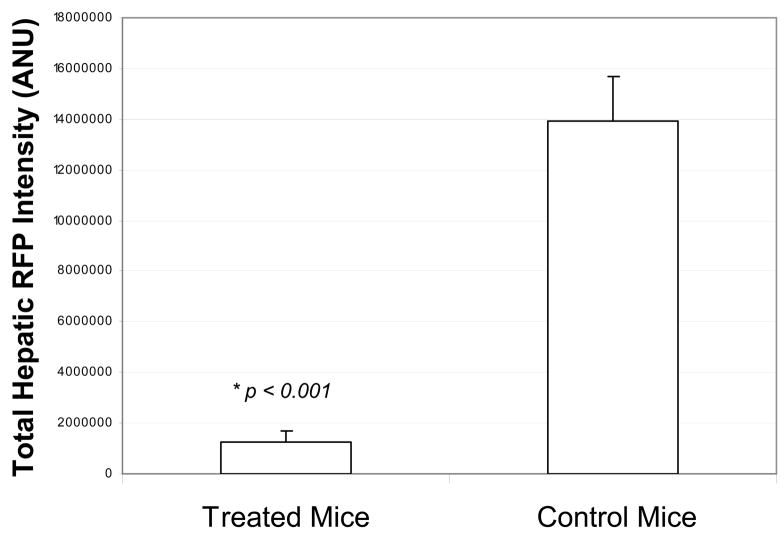
GFP-labeled S. typhimurium A1-R bacteria were delivered via operative intrasplenic injections as described above. The 4 Treated mice received weekly intrasplenic S. typhimurium for a total of 3 doses on Day 3, Day 10 and Day 17 post-tumor cell injection. The remaining 5 mice served as untreated controls. All 9 mice were sacrificed on Day 23 post tumor-cell injection. For the excised liver and spleen, the RFP intensities of all pixels were quantified and summed with the mean total RFP intensity computed for each group. The 4 mice which had been treated with intrasplenic S. typhimurium had a significantly lower mean total RFP intensity on the liver surface as compared with the 5 untreated controls (t test, p < 0.001). Liver weights were recorded for each mouse and the treated mice had a significantly lower mean liver weight (2.5 g +/− 0.0854) as compared to the control mice (4.7 g +/− 0.483) (t test, p = 0.009). Treated mice also had a significantly lower mean total RFP intensity on the splenic surface (t test, p = 0.001). Figure 2a shows the liver images from the 4 treated mice. Figure 2b shows the liver images from the 5 control mice. Figure 2c shows the mean total RFP intensity from the liver of the 4 control mice as compared to the mean total RFP intensity from the liver of the 5 treated mice.
Figure 2. A1-R delivered via intrasplenic injection suppresses XPA-1 RFP P5B tumor growth in the liver.
GFP-labeled S. typhimurium A1-R bacteria were delivered via operative intrasplenic injections as described above. The 4 Treated mice received weekly intrasplenic S. typhimurium for a total of 3 doses on Day 3, Day 10 and Day 17 post-tumor cell injection. The remaining 5 mice served as untreated controls. All 9 mice were sacrificed on Day 23 post tumor-cell injection. For the excised liver and spleen, the RFP intensities of all pixels were quantified and summed with the mean total RFP intensity computed for each group. The 4 mice which had been treated with intrasplenic S. typhimurium had a significantly lower mean total RFP intensity on the liver surface as compared with the 5 untreated controls (t test, p < 0.001). Figure 2a shows the liver images from the 4 mice treated with intrasplenic injection of S. typhimurium A1-R. Figure 2b shows the liver images from the 5 control mice. Figure 2c shows the mean total RFP intensity from the liver of the 4 control mice as compared to the mean total RFP intensity from the liver of the 5 treated mice.
S. typhimurium delivered systemically via tail vein injection displays ability to target and grow within liver metastasis
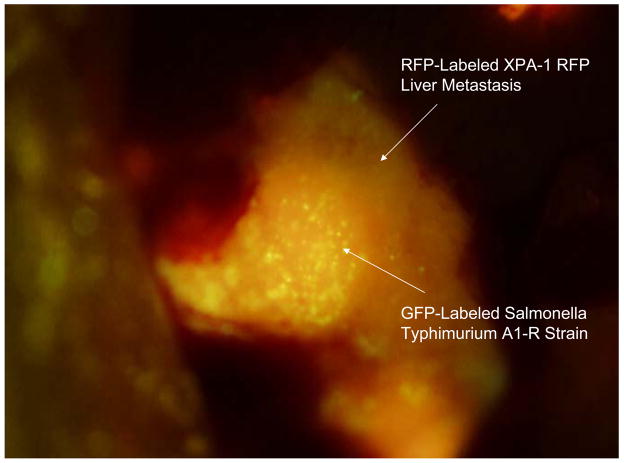
Figure 3 shows colonies of GFP-expressing S. typhimurium within an XPA-1 RFP liver metastasis 6 days after intravenous administration.
Figure 3. S. typhimurium delivered systemically via tail vein injection displays ability to target and grow within liver metastasis.
Figure 3 shows colonies of GFP-expressing S. typhimurium within an XPA-1 RFP liver metastasis 6 days after intravenous administration.
Intravenous delivered S. typhimurium A1-R suppresses XPA-1 RFP P5A tumor growth in the liver and spleen
GFP-labeled S. typhimurium A1-R bacteria were inoculated in the tail-vein in 15 of 30 nude mice which had previously received an intrasplenic injection of XPA-1 RFP P5A human pancreatic cancer cells. Treated mice received weekly intravenous S. typhimurium for a total of 3 doses on Day 4, Day 11 and Day 18 post tumor-cell injection. The remaining 15 mice served as untreated controls. Hepatic and splenic tumor burden was determined by fluorescent imaging at Day 24.
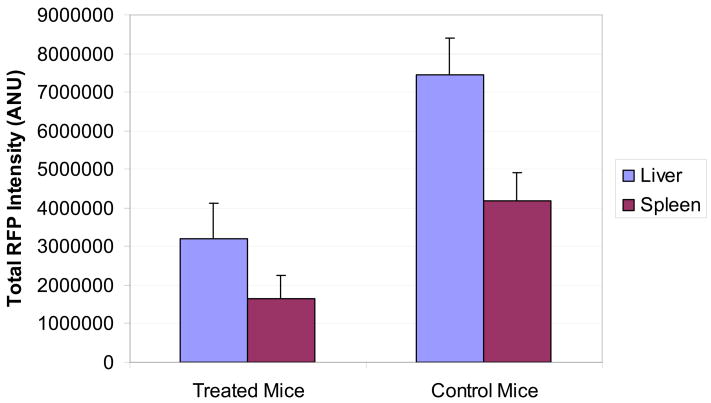
Two untreated control mice died at Day 21 and Day 23, respectively. At Day 24, the surviving mice were sacrificed and the livers and spleens were harvested and imaged. For each image, the RFP intensities of all pixels were quantified and summed with the mean total RFP intensity computed for each group. The 15 mice which had been treated with intravenous S. typhimurium had a significantly lower mean RFP signal from the liver as compared with the remaining 13 untreated controls (t test, p = 0.003). Liver weights were recorded for each mouse and the treated mice had a significantly lower mean liver weight (2.3 g +/− 0.150) as compared to the control mice (3.5 g +/− 0.324) (t test, p = 0.004). The treated mice also showed significantly less tumor in the spleen as determined by the mean total splenic RFP intensity (t test, p = 0.012).
Figure 4a shows the mean total RFP intensity from the liver of 13 control mice as compared to the mean total RFP intensity from the liver of the 15 treated mice. Figure 4b shows the mean total RFP intensity from the spleen of the 13 control mice as compared to the mean total RFP intensity from the spleen of the 15 treated mice. Error bars represent the standard error of the mean.
Figure 4. Intravenous delivered S. typhimurium A1-R suppresses XPA-1 RFP P5A tumor growth in the liver and spleen.
GFP-labeled S. typhimurium A1-R bacteria were inoculated in the tail-vein in 15 of 30 nude mice which had previously received an intrasplenic injection of XPA-1 RFP P5A human pancreatic cancer cells. Treated mice received weekly intravenous S. typhimurium for a total of 3 doses on Day 4, Day 11 and Day 18 post tumor-cell injection. The remaining 15 mice served as untreated controls. Hepatic and splenic tumor burden was determined by fluorescent imaging at Day 24. The 15 mice which had been treated with intravenous S. typhimurium had a significantly lower mean RFP signal from the liver as compared with the remaining 13 untreated controls (t test, p = 0.003). The treated mice also showed significantly less tumor in the spleen as determined by the mean total splenic RFP intensity (t test, p = 0.012).
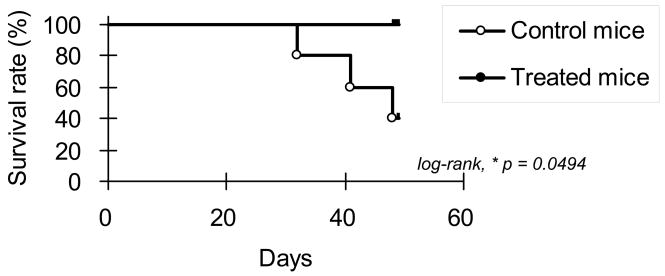
S. Typhimurium A1-R Increases Survival Time of Mice receiving an intrasplenic injection of XPA-1 RFP P5 human pancreatic cancer cells
10 nude mice were given an intrasplenic injection of XPA-1 RFP P5A cells. The 10 mice were randomized into 2 groups of 5 mice each. One group was treated with intravenous S. typhimurium A1-R according to the protocol described above, and the remaining 5 mice served as untreated controls. The animals were then kept under sterile conditions, and survival time was documented. Figure 5 shows the Kaplan-Meier survival graph for this experiment (log-rank, p = 0.0494).
Figure 5. S. Typhimurium A1-R Increases Survival Time of Mice receiving an intrasplenic injection of XPA-1 RFP P5 human pancreatic cancer cells.
10 nude mice were given an intrasplenic injection of XPA-1 RFP P5A cells. The 10 mice were randomized into 2 groups of 5 mice each. One group was treated with intravenous S. typhimurium A1-R according to the protocol described above, and the remaining 5 mice served as untreated controls. There was a statistically significant difference in survival between the 2 groups as shown in the Kaplan-Meier survival graph for this experiment (log-rank, p = 0.0494).
Host Safety
After intravenous injection with S. typhimurium A1-R, the body weights of the treated mice were monitored 3 times a week and compared with the body weights of the untreated controls. The treated mice initially showed a loss in body weight after the first dose of bacteria. However, this was transient and eventually there was no significant difference in body weights between the treated and control mice. No other significant adverse effects were noted, and the treated mice tolerated the treatment well and appeared healthy at the conclusion of the experiment. Furthermore, none of the mice receiving intrasplenic or intravenous injections of bacteria died prematurely, demonstrating high tolerance to bacterial therapy with S. typhimurium A1-R.
DISCUSSION
From the results described above, it is clear that S. typhimurium A1-R is effective in slowing down the growth and development of pancreatic cancer in nude mice. The most common metastatic site of pancreatic cancer is the liver. The present study showed that both intrasplenic and intravenous S. typhimurium A1-R had a dramatic effect on liver metastasis in our mouse model of pancreatic cancer. The intrasplenic delivery of bacteria seemed to achieve a more consistent result. The greater variation in response to intravenous treatment may be attributed to the fact that intravenous treatment is a systemic delivery as opposed to the loco-regional delivery via the spleen.
The present study suggests that bacterial treatment could be used as an adjunct to surgery to treat micrometastasis which may be present but undetectable at the time of surgery. Bacterial treatment can also be potentially useful in the palliation of advanced pancreatic cancer to control metastatic disease. Currently, the mainstay of treatment for advanced pancreatic cancer is single agent chemotherapy. However, pancreatic cancer is relatively refractive even to active single agents and response rates remain low. The combination of chemotherapy with bacterial treatment should also be tried in the future for advanced pancreatic cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coley WB. Late results of the treatment of inoperable sarcoma by the mixed toxins of erysipelas and Bacillus prodigiosus. Am J Med Sci. 1906;131:375–430. [PMC free article] [PubMed] [Google Scholar]
- 2.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 3.Gericke D, Engelbart K. Oncolysis by Clostridia. Ii. Experiments on a Tumor Spectrum with a Variety of Clostridia in Combination with Heavy Metal. Cancer Res. 1964;24:217–221. [PubMed] [Google Scholar]
- 4.Moese JR, Moese G. Oncolysis by Clostridia. I. Activity of Clostridium Butyricum (M-55) and Other Nonpathogenic Clostridia against the Ehrlich Carcinoma. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 5.Thiele EH, Arison RN, Boxer GE. Oncolysis by Clostridia. Iii. Effects of Clostridia and Chemotherapeutic Agents on Rodent Tumors. Cancer Res. 1964;24:222–233. [PubMed] [Google Scholar]
- 6.Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- 7.Kimura NT, Taniguchi S, Aoki K, Baba T. Selective localization and growth of Bifidobacterium bifidum in mouse tumors following intravenous administration. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 8.Fox ME, Lemmon MJ, Mauchline ML, Davis TO, Giaccia AJ, Minton NP, Brown JM. Anaerobic bacteria as a delivery system for cancer gene therapy: in vitro activation of 5-fluorocytosine by genetically engineered clostridia. Gene Ther. 1996;3:173–178. [PubMed] [Google Scholar]
- 9.Lemmon MJ, van Zijl P, Fox ME, Mauchline ML, Giaccia AJ, Minton NP, Brown JM. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997;4:791–796. doi: 10.1038/sj.gt.3300468. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 11.Low KB, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, Lin SL, Luo X, Miller SI, Zheng L, King I, Pawelek JM, Bermudes D. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 12.Clairmont C, Lee KC, Pike J, Ittensohn M, Low KB, Pawelek J, Bermudes D, Brecher SM, Margitich D, Turnier J, Li Z, Luo X, King I, Zheng LM. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 13.Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for cancer gene therapy: selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000;7:269–274. doi: 10.1038/sj.cgt.7700122. [DOI] [PubMed] [Google Scholar]
- 15.Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, Taniguchi S. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [DOI] [PubMed] [Google Scholar]
- 16.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 19.Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, Freezer LJ, Morton KE, Seipp C, Haworth L, Mavroukakis S, White D, MacDonald S, Mao J, Sznol M, Rosenberg SA. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengesha A, Dubois L, Lambin P, Landuyt W, Chiu RK, Wouters BG, Theys J. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biol Ther. 2006;5:1120–1128. doi: 10.4161/cbt.5.9.2951. [DOI] [PubMed] [Google Scholar]
- 21.Yu YA, Timiryasova T, Zhang Q, Beltz R, Szalay AA. Optical imaging: bacteria, viruses, and mammalian cells encoding light-emitting proteins reveal the locations of primary tumors and metastases in animals. Anal Bioanal Chem. 2003;377:964–972. doi: 10.1007/s00216-003-2065-0. [DOI] [PubMed] [Google Scholar]
- 22.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Yang M, Li XM, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 25.Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji K, Yang M, Jiang P, Maitra A, Kaushal S, Yamauchi K, Katz MH, Moossa AR, Hoffman RM, Bouvet M. Common bile duct injection as a novel method for establishing red fluorescent protein (RFP)-expressing human pancreatic cancer in nude mice. Jop. 2006;7:193–199. [PubMed] [Google Scholar]
- 27.Bouvet M, Tsuji K, Yang M, Jiang P, Moossa AR, Hoffman RM. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 2006;66:11293–11297. doi: 10.1158/0008-5472.CAN-06-2662. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]