Abstract
Background
Directly observed therapy (DOT) for antiretroviral therapy (ART) may improve adherence, but there are limited data on its clinical effectiveness.
Methods
Adult patients initiating ART in a public clinic in Cape Town, South Africa, were randomized to treatment-supporter DOT-ART or self-administered ART. DOT-ART patients and supporters received baseline and follow up training and monitoring. The primary endpoints were the proportion of patients with HIV viral load (VL) <400copies/mL and change in CD4 cell counts at 12 and 24 months.
Results
274 patients enrolled (137 in each arm) and baseline characteristics were similar for both arms. The study was stopped early for futility by an independent Data and Safety Monitoring Board. In an intention-to-treat analysis, the proportions of patients with VL <400 copies/mL at 12 months were 72.8% in the DOT-ART arm and 68.4% in the Self-ART arm (p= 0.42). DOT-ART patients had greater median CD4 cell count (cells/µL) increases at 6 months (148 [IQR 84-222] vs. 111 [IQR 44-196]; p= 0.02) but similar results at all other time-points. Survival was significantly better in the DOT-ART arm (N=9, 6.6%) than in the Self-ART arm (N=21, 15.3%; log-rank p = 0.02). In Cox regression analysis, mortality was independently associated with study arm (DOT vs. self-ART; HR 0.38, 95% CI 0.17–0.86).
Conclusion
DOT-ART showed no effect on virologic outcomes but was associated with greater CD4 cell count increases at 6 month follow-up. Survival was significantly better for DOT-ART compared to Self-ART, but this was not explained by improved virologic or immunologic outcomes.
Keywords: HIV-1, antiretroviral therapy, adherence, randomized controlled trial, treatment social supporter, directly observed therapy
Introduction
Poor adherence to antiretroviral therapy (ART) is a major predictor of virologic failure [1, 2], emergence of drug resistance [3,4], disease progression [5], and death [6,7]. Although early studies suggested that rates of antiretroviral adherence in the developing world are as high or higher than those in the developed world [8], challenges to maintaining high treatment adherence rates remain in both settings [9,10]. Systematic reviews of randomized trials have shown that relatively few adherence interventions have been demonstrated to be effective and that more research is needed to identify efficacious interventions and optimal methods for implementation in real-world settings with limited resources [11–13].
Directly observed therapy (DOT) is a treatment strategy widely implemented in tuberculosis control programs. The clinic-based model of DOT is used by most tuberculosis programs, but it is not suitable for lifelong delivery of ART [14–15]. Studies from South Africa, Malawi and Nepal have shown that DOT for tuberculosis delivered by patient-nominated or community treatment supporters is effective and feasible [16–19]. Observational data from rural Haiti using community health workers to deliver AIDS care [20] showed that DOT-ART was acceptable and clinical outcomes were good. DOT for ART using patient-nominated treatment supporters is an attractive alternative as these individuals are acceptable to patients, generally do not expect remuneration, and often the greatest cost to the health sector is training supporters [20]. Randomized controlled trials of DOT strategies to enhance ART outcomes in the developed world have been conducted mainly in drug treatment clinics with mixed results [21–23]. A peer-delivered community randomized trial of DOT-ART in Mozambique showed that it was a feasible strategy to promote adherence, but did not show an intervention effect and was limited by lack of virologic outcomes [24].
We conducted a randomized controlled trial of patient-nominated treatment supporters providing partial DOT in HIV-infected South African adults initiating antiretroviral therapy. Our objective was to evaluate whether such a strategy over the initial twelve months of a first-line antiretroviral regimen would result in improved clinical, virological and immunological outcomes.
Methods
Study Population and Setting
The study site was a public sector ART clinic at the GF Jooste Hospital, a district level healthcare facility in Cape Town, Western Cape province of South Africa, serving a population of 1.2 million from several peri-urban townships. The HIV prevalence in pregnant women averages approximately 20% in this area. Patients initiating ART at the hospital clinic were eligible to enroll. No payment by patients is required for any component of the ART program. Of note, the standard of care in the Western Cape province is that patients are asked to choose a treatment supporter who attends a single pre-ART initiation counseling session on the importance of adherence and how to support patients’ adherence.
Study Design and Intervention Description
The study was an open-label, randomized, controlled trial comparing the impact of partial DOT administered by community-based, patient-nominated treatment supporters (DOT-ART arm) compared with self-administered ART (Self-ART arm) in ART naïve adult patients. Patients attending the ART clinic were eligible if they met the following criteria: 1) male or non-pregnant female 18 years of age or older; 2) HIV infection documented by two serologic tests; 3) eligible to start ART according to South African national guidelines of CD4 cell count ≤ 200 cells/µL or WHO Clinical Stage IV disease; 4) living in the study site catchment area at a stable address; 5) willing to disclose HIV status to a treatment supporter; and 6) signed informed consent. Individuals were excluded for: 1) documentation of prior ART use; 2) estimated life expectancy <6 months due to serious terminal conditions; 3) Karnofsky Performance Score <60; 4) serious liver disease (alanine aminotransferase > 5 times upper limit of normal); or 5) history of single dose nevirapine for prevention of mother to child transmission of HIV infection.
Following screening and provision of written informed consent, patients were randomized to one of the two treatment strategies DOT vs. Self-ART. Allocation of treatment assignment was concealed; treatment assignments were placed in opaque envelopes which were sequentially opened by the study coordinator at enrollment. Treatment supporters in the DOT-ART arm were selected using a personal network inventory instrument [25] that allowed patients to identify individuals who were aware of their HIV diagnosis, supportive of their needs, and who patients felt could support adherence. Treatment supporters in the DOT-ART arm underwent a 90-minute baseline training session on ART adherence and support techniques, and were asked to observe at least one medication dose daily and document it on a study adherence chart. In addition, DOT-ART treatment supporters and patients received 4 extra ART adherence training sessions at baseline and booster education sessions every 3 months for the initial 12 months after ART initiation. In the Self-ART group, patients selected a treatment supporter without the network instrument, and both patients and supporters were asked to attend a single 90-minute training session describing ART and the importance of adherence without DOT, as per standard care. After 12 months, treatment supporter training and partial DOT were tapered on a schedule left to the discretion of the patient and supporter. In the Self-ART arm, ART was self-administered. All patients were given pill boxes which were filled at monthly pharmacy visits, when pill counts of remaining pills were also performed.
All patients were screened for alcohol using the “CAGE”, a short, four-question screening test that diagnoses alcohol problems over a lifetime. A total score of 2 or higher (answering “yes” to two or more questions) indicates a problem with alcohol or a positive test [26]. Depression was assessed in both arms using the Brief Symptom Inventory (BSI), which includes seven questions targeting depression. The lowest possible score is 7 and the highest possible score is 35 [27].
The initial ART regimen consisted of stavudine 30 mg twice daily plus lamivudine 150 mg twice daily plus efavirenz 600 mg once daily. For women not willing or able to use contraception, nevirapine 200 mg twice daily following a 14-day once daily lead-in was substituted. Other alterations in the treatment regimen were permitted following South African national guidelines.
All patients were followed monthly for clinical evaluation, medication refills, and counselling as needed. Measurements of plasma HIV-1 viral load (Roche UltraSensitive HIV-1 Monitor assay), CD4 cell count were performed every six months and biochemistry, hematology and other safety laboratory tests were performed whenever clinically indicated.
Endpoints
The primary efficacy endpoints were defined as the proportion of participants with undetectable HIV viral load (VL <400 copies/mL) and the median change in CD4 cell counts at 12 and 24 months; viral load and CD4 counts were also determined at 6 and 18 months. Secondary endpoints included adherence as evaluated by pill count and development of a new or recurrent AIDS-defining illness and death.
Sample Size and Power Considerations
Based on published data on ART outcomes in Cape Town, South Africa [28], we assumed that the proportion of patients with undetectable viral load (VL<400 copies/mL) at 12 months in the self-ART arm would be 70%. With a total sample size of 240 patients, our study was powered to detect a 15% difference for the primary endpoint of the proportion of patients with HIV VL <400 copies/mL at 12 months after ART initiation in the DOT vs. Self-ART arms (alpha = 0.05, 2 tailed). The target sample size to be recruited was 274 patients to account for possible loss to follow-up (<15%).
Statistical Analysis
Initial descriptive analyses were conducted to compare baseline and follow up results. Unadjusted endpoint analyses were conducted on an intention to treat basis using all patients enrolled to compare outcomes in the DOT vs. Self-ART arm. For viral load analyses, missing values were considered detectable (missing = failure analysis). As-treated analyses were also conducted using patients remaining in the study with available data. Cross-sectional comparisons between study groups were conducted using two sample t-test, Wilcoxon rank-sum test, chi-square or Fisher's exact and Kaplan-Meier analysis as appropriate. Logistic regression analysis and Cox proportional hazards regression analyses to compare between intervention groups after adjusting for the relevant covariates, including study arm, age, sex, baseline CD4 cell counts and viral load and pill count adherence when appropriate. Analyses were performed using SAS version 9.1 (SAS Inc., Cary, NC).
Regulatory approvals
The study was approved by the committees on the protection of human subjects or ethics boards from Johns Hopkins University School of Medicine and the University of Cape Town. An independent Data Safety and Monitoring Board (DSMB) appointed by the study sponsors reviewed the study prior to initiation and annually.
Results
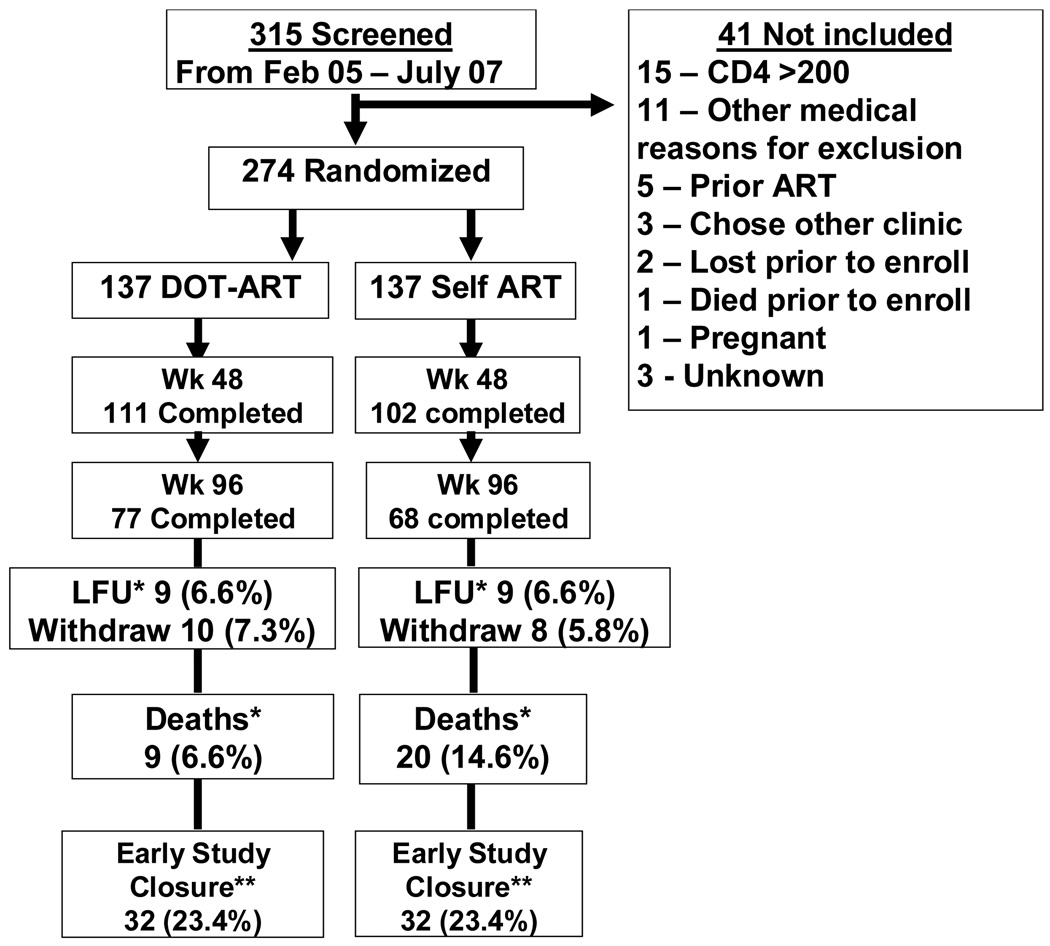
A total of 274 participants were randomized, 137 to Self-ART and 137 to DOT-ART. Enrollment began February 13, 2005, and ended on July 7, 2007, with the last follow-up occurring on July 25, 2008. Figure 1 shows participant flow through the study. There were no significant differences in baseline characteristics between the two study groups (Table 1). 100% of patients and 86% of supporters attended the baseline training session. Attendance at all 3 booster training sessions in the DOT-ART arm was 73% for patients and 53% for supporters. Patients in the DOT-ART arm were more likely than Self-ART arm to bring their treatment supporter to the clinic visit through month 12 (50% vs. 20%, p<0.001). Patients in the DOT-ART arm reported seeing their treatment supporters at least once daily an average of 90% of the time. Use of the study adherence chart by treatment supporters averaged 80% in the DOT-ART arm.
Figure 1.
Study Flow Diagram
*Deaths occurred uniformly in both arms and patients lost-to-follow up were defined as patients who did not return for follow-up appointments until the study closure.
**Early study closure applied for patients who did not complete 96 months of follow-up due to earlier closure of the study as recommended by the data safety monitoring board.
Table1.
Baseline Characteristics of Participants by Study Arm
| Variable | Total | DOT-ART | Self-ART |
|---|---|---|---|
| (n = 274) | (n =137) | (n =137) | |
| Age (yrs): Mean (sd) | 36.2. (9.1) | 35.7 (9.7) | 36.7 (9.2) |
| Sex (n, %) | |||
| Male | 116 (42.3%) | 58 (42.3) | 58 (42.3%) |
| Race | |||
| Black | 235 (85.7%) | 118 (86.1%) | 117 (85.4%) |
| Mixed-Race | 39 (14.2%) | 19 (13.9%) | 20 (14.6%) |
| Baseline Median CD4 | |||
| (IQR) (cells/µL) | 98 (43–148) | 92 (41–144) | 103 (51–150) |
| Baseline Median VL | |||
| log10(c/mL) | 5.01 (4.60–5.39) | 5 (4.55–5.41) | 5 (4,67–5.36) |
| WHO Stage III | 124 (45.2%) | 63 (46.0%) | 61 (44.5%) |
| WHO Stage IV | 126 (46.0%) | 65 (47.5%) | 61 (44.5%) |
| History of TB (n, %) | 183 (66.8%) | 98 (71.5%) | 85 (62.0%) |
| Mean Depression | 23.3 (10.1) | 23.1 (10.0) | 23.5(10.3) |
| Score (SD) | |||
| Alcohol Abuse | 47/152 (30.9%) | 23/76 (30.3%) | 47/152 (30.9%) |
| CAGE + (n, %) | |||
| Employed (n, %) | 72 (26.3%) | 37 (27.0%) | 35 (25.6%) |
The study was halted for futility upon the recommendation of the DSMB at its third annual review when interim analysis showed no significant differences in viral load suppression at any time point in the two treatment arms. At the time the study was halted, 114 participants (83%) the DOT-ART arm had reached the 12 month follow up point compared with 108 participants (79%) in the Self-ART arm. However primary endpoint data were available on 110 participants in the DOT-ART arm and for 103 participants in the Self-ART arm. The proportion of patients with plasma HIV-1 VL <400 copies/mL in the intention-to-treat analysis, where missing equals failure, at 12 and 24 months was 72.8% and 60.6% in the DOT-ART arm versus 68.4 and 59.6 in the Self-ART arm (Table 2). Somewhat lower but similar proportions of patients in both treatment arms had VL <50 copies/mL at both time points, as well. In as-treated analyses, 90% of patients in both arms had VL <400 at 12 months (p=0.94) and 85% vs 92% had VL <400 at 24 months (p=0.16). Of patients who achieved virologic suppression, there was no difference in the time to virological rebound (VL >400 copies/mL) following initial suppression of VL to <400 copies/mL by study arm (log-rank test p=0.468).
Table 2.
Proportion of Patients with HIV RNA Levels of <400 and <50 Copies/mL at 12 and 24 Months [Intention-to-treat (ITT), Missing=Failure and As Treated (AT)* analyses]
| Analysis | Total | DOT-ART | Self-ART | P valuea |
|---|---|---|---|---|
| (n) | (n)(%) | (n) (%) | ||
| 6 Months | ||||
| ITT, M=F | ||||
| <400copies/ml | 256 | 104/129 (80.6%) | 99/127 (77.9%) | 0.59 |
| ATT | ||||
| <400copies/ml | 227 | 104/115 (90.4%) | 99/112 (88.4%) | 0.61 |
| ITT, M=F | ||||
| <50copies/ml | 272 | 92/136(67.5%) | 85/136 (65.4%) | 0.69 |
| ATT | ||||
| <50copies/ml | 227 | 104/115(90.4%) | 99/112(88.3%) | 0.61 |
| 12 Months | ||||
| ITT, M=F | ||||
| <400copies/ml | 272 | 99/136 (72.8) | 93/136 (68.4%) | 0.42 |
| ATT | ||||
| <400copies/ml | 213 | 99/110 (90.0) | 93/103 (90.3) | 0.94 |
| ITT, M=F | ||||
| <50copies/ml | 272 | 88/136(64.7%) | 85/136 (62.5%) | 0.71 |
| ATT | ||||
| <50copies/ml | 213 | 88/110(80.0%) | 85/103(82.5%) | 0.64 |
| 24 Months | ||||
| ITT, M=F | ||||
| <400copies/ml | 208 | 63/104 (60.6) | 62/104 (59.6) | 0.89 |
| ATT | ||||
| <400copies/ml | 167 | 76/89 (85.4%) | 72/78 (92.3%) | 0.16 |
| ITT, M=F | 208 | 56/104 (53.9%) | 57/104 (54.8%) | 0.89 |
| <50copies/ml | ||||
| ATT | 167 | 67/89 (75.3%) | 65/78 (83.3%) | 0.20 |
| <50copies/ml | ||||
Based on the chi-square test (χ2) with one degree of freedom, comparing patients in the DOT vs. Self-ART Arm.
As treated analysis includes only those patients who initiated treatment after randomization.
Patients in the DOT-ART arm had significantly higher median CD4 cell count changes between baseline and 6 months than patients in Self-ART (148 cells/µL [IQR 84-222] vs. 111 cells/µL [IQR44-196]; p= 0.02), but changes were similarly distributed at all other time-points beyond 6 months (Table 3). The median adherence assessed by pill counts was >95% at all time-points in both arms during follow-up. Baseline predictors of virologic suppression (VL<400 copies/mL) at 12 months in a multivariate logistic regression analysis were cumulative pill count adherence >90% (OR 12.4, 95% CI: 2.7–56.4; p=0.001), female sex (OR 3.8, 95% CI 1.2–12.6; p =0.028) and baseline CD4 cell count > 200 cells/µL (OR 4.76, 95% CI: 1.0–25; P=0.05).
Table 3.
Immunological Response: Median CD4 (IQR) Cell Count Increase From Baseline at 6, 12, 18 and 24 Months by Study Arm
| DOT-ART | Self-ART | P-Valuea | |
|---|---|---|---|
| 6 Months (n) | 110 | 107 | |
| Median CD4 (IQR) (cells/µL) Change from Baseline |
148 (84–222) | 111 (44–196) | 0.02 |
| 12 Months (n) | 105 | 100 | |
| Median CD4 (IQR) (cells/µL) Change from Baseline |
185 (108–276) | 160 (78–236) | 0.14 |
| 18 Months (n) | 95 | 94 | |
| Median CD4 (IQR) (cells/µL) Change from Baseline |
207 (116–366) | 185 (103–300) | 0.36 |
| 24 Months (n) | 84 | 77 | |
| Median CD4 (IQR) (cells/µL) Change from Baseline |
281 (144–403) | 262 (149–363) | 0.61 |
Wilcoxon Rank-Sum Test
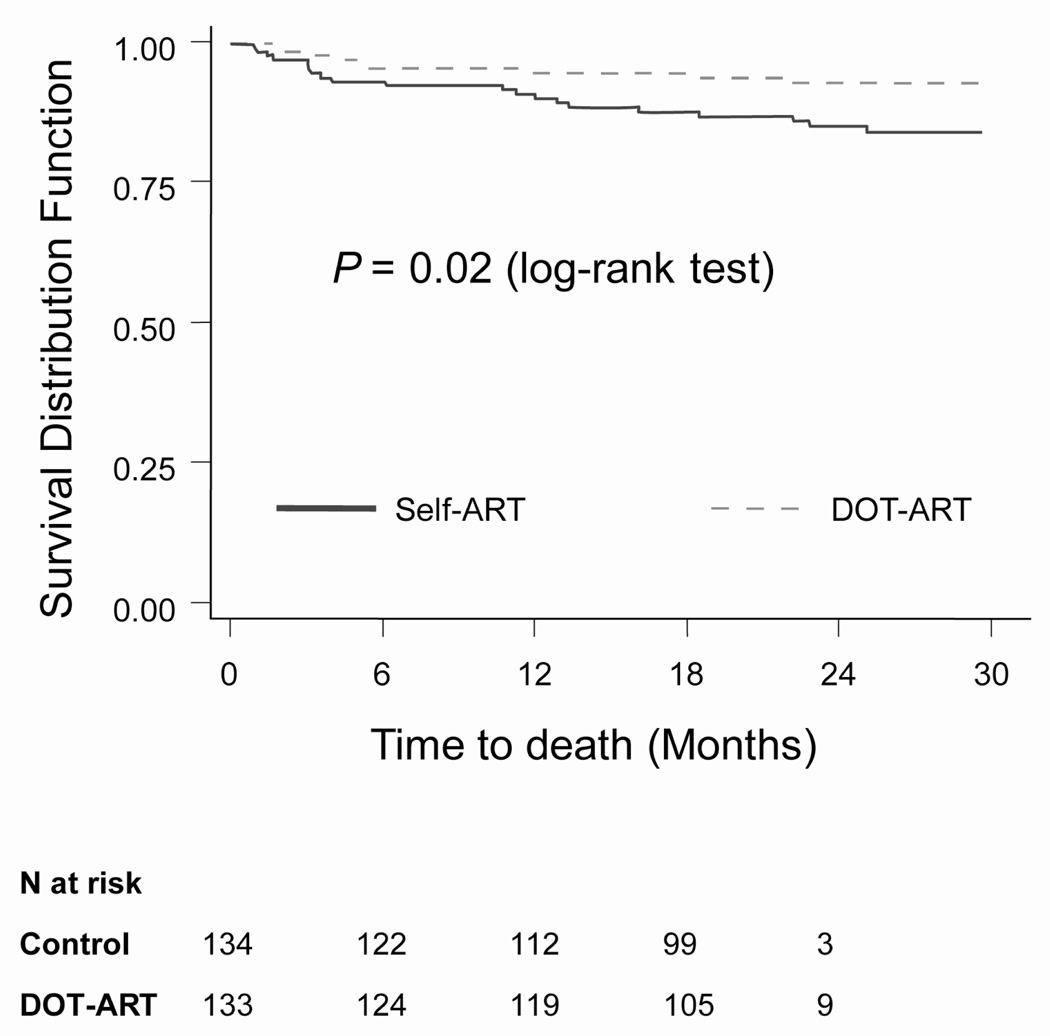
There were 30 deaths during the study, 9 in the DOT-ART arm (6.6%) and 21 (15.3%) in the Self-ART arm (Log-rank test from Kaplan-Meier analysis: p=0.02, Figure 2). Deaths occurred uniformly during follow-up time and were not clustered in the first several months of treatment, as shown in Figure 2. There was no difference in the time to new or recurrent AIDS-defining illness for the two study arms. In a Cox proportional hazards analysis controlling for covariates of age, sex and baseline CD4 cell counts, study arm was an independent predictor of death (DOT vs. Self-ART: HR 0.38, 95% CI 0.17–0.86; p-value: 0.02). Reported causes of death in the Self-ART arm were tuberculosis (n = 6); chronic diarrhea (n = 3); cryptococcal meningitis (n=3); Pneumocystis jirovecii pneumonia (n=2); encephalopathy (n=1); pulmonary embolism (n=1); and unknown (n=5). Reported causes of death in the DOT-ART arm were: isosporiasis (n=2); stroke (n=1); HIV-associated nephropathy (n=1); lactic acidosis (n=1); COPD or respiratory collapse (n=2); convulsions (n=1); and tuberculosis (n=1).
Figure 2.
Survival by Study Arm
Discussion
In this study, partial DOT-ART by patient-nominated treatment supporters had no effect on the primary endpoint, namely the proportion of patients with undetectable HIV viral load (VL <400 copies/mL) at 12 or 24 months. Participants in both arms had high adherence to treatment and virologic suppression was similar in both intent-to-treat and as-treated analyses. Patients in the DOT-ART arm had better early CD4 cell count increases, but these were not sustained beyond 6 months. Nonetheless, we documented a significant survival benefit in the DOT-ART arm after controlling for other factors associated with death.
Adherence, assessed by pill counts, was high and not significantly different between the two study arms at 6, 12, 18 and 24 months. The intriguing finding that mortality was lower in the DOT-ART arm despite the absence of virologic benefit might be explained by better virologic suppression before 6 months, but virologic data were not recorded for this time period. The better early CD4 cell count responses in the DOT-ART arm suggest that this might be the case. In addition, trained treatment supporters and the resources they provided to patients in the DOT-ART arm may have facilitated patients’ better use of medical services for treatment or preventive care, which could reduce morbidity and mortality, but we have no data to support this hypothesis. Finally, the finding that mortality was lower in the DOT-ART arm might be due to chance.
Several other community-based randomized trials testing DOT-ART interventions have been conducted. Wohl et al. in the United States studied a mixed ART-naïve and -experienced population without selection for risk factors for non-adherence in whom community workers administered DOT-ART for six months [22], while the ACTG A5073 study by Gross et al. evaluated DOT-ART strategies delivered by a health professional (pharmacist, nurse, etc.) in unselected ART-naïve patients in the United States, Caribbean and one site in South Africa [29]. Both of these studies found that DOT did not confer a significant advantage for either virologic suppression or adherence. Macalino et al. compared a community-based once-daily DOT delivered by outreach workers versus self-administered therapy in a randomized trial of 87 active substance users followed for only three months in United States [23]. These authors found a benefit to DOT-ART, although secondary analyses showed that the entire benefit was limited to ART-experienced patients who had failed prior antiretroviral regimens.
Our study did not include ART-experienced patients. Failure to show a benefit suggests that implementing DOT-ART with patient-nominated community treatment supporters in an unselected treatment-naïve population may be unnecessary in settings where average ART adherence has been shown to be high. However, HIV-infected individuals at greater risk for poorer adherence may benefit from such interventions. For example, Altice et al. conducted a 6-month community-based trial of DOT-ART in injection drug users and found an advantage with respect to both virologic suppression and CD4 cell count increases [21], however a 6-month post intervention analysis failed to show the persistence of the intervention effect at improving virological outcomes [30]. Our study population had no injection drug users, as is usual for African cohorts.
Our study has a number of possible implications. First, the lack of benefit of the DOT intervention on the primary endpoint of virologic suppression should limit enthusiasm for wide implementation in unselected HIV-infected populations starting ART. At the very least, a general requirement for DOT-ART cannot be supported by the results of this trial. However, as shown by Amico and colleagues in a meta-analysis of randomized controlled trials, studies targeting groups with poor ART adherence had stronger effects than those targeting groups with mixed adherence levels pre-intervention [12]. Second, social capital provided by a trusted patient-nominated treatment supporter may contribute to survival through a mechanism unrelated to viral load and should be assessed in future studies. Indeed, qualitative data by our group [31] and others [32] supports this hypothesis. In the latter study seeking to explain high adherence rates found in a sub-Saharan Africa, Ware and colleagues argue that, in the face of extreme poverty, individuals rely heavily on social capital - “the use of relationships to obtain benefits and achieve desired ends”- to obtain basic resources, such as food or transportation. Their social capital and patients’ interests in preserving their support ties and minimizing inordinate dependency on them enhanced and motivated their adherence to ART.
Therefore, even without extensive additional interventions, HIV-infected patients in resource-limited settings such as South Africa may rely heavily on social capital to obtain needed resources to adhere to their ART, and that the availability of treatment supporters is likely based on patients’ existing social capital. A model of potential pathways linking social support and health proposed by Uchino illustrates how randomization to DOT-ART may have contributed to improved survival [33]. According to this model, social support can influence either or both behavioral processes (health promoting behaviors, such as care-seeking, in addition to medication adherence) and psychological processes (e.g., decreased depression, heightened sense of control and self-efficacy), which, in turn, contribute to biological processes that may ultimately lead to decreased morbidity and mortality. Our data are intriguing in this regard, but we have no evidence of a direct antiviral effect as a mechanism for the survival benefit.
Our study has several strengths. First, this is believed to be the first randomized controlled trial evaluating patient-nominated, community-based treatment supporters for DOT-ART with a report of robust biological and clinical endpoints in a resource-limited setting. Second, this is among the few reports documenting relatively long-term (up to 24-month) high level of ART adherence in sub-Saharan Africa regardless of intervention.
This study also has limitations. The DOT-ART intervention was stopped at 12 months and the transition phase did not ensure that participants developed a set of adherence strategies to replace DOT-ART, although patients and their treatment supporters might have continued DOT-ART on their own after 12 months. Therefore, the results of a longer intervention are not known, which is relevant considering that ART adherence does tend to decrease with time. In addition, the relatively low incidence of AIDS-defining illness and death limit our ability to make final conclusions about clinical benefits of DOT-ART with nominated treatment supporters.
In summary, this randomized, controlled trial of partial DOT compared to standard of care did not improve virologic outcomes, but was associated with significantly better 6-month CD4 cell count increases and survival which was not explained by improved virologic or immunologic outcomes. The overall proportions of patients who had undetectable viral loads at 24 months in the intent-to-treat analysis was disappointingly low at <60%. This finding underscores the importance of identifying additional interventions to improve the outcomes of ART for patients in resource-poor settings. Additional community-based programs to support treatment adherence to improve clinical outcomes are needed, and studies of such interventions should be sufficiently large to detect clinical endpoints and focus on populations with poorer adherence. Finally, more research attention is needed to examine characteristics of treatment supporters nominated by patients in each condition and ways in which they may or may not have affected patients’ adherence or survival.
Acknowledgements
The authors wish to thank the study participants, treatment supporters, students, interns and the clinical staff at GF Jooste Hospital, Manenberg, Cape Town, South Africa, for their contributions to this study; and Joanna Downer, PhD, for critical reading and editing of this manuscript.
Financial Support: This work was supported by the U.S. National Institute of Allergy and Infectious Diseases grants AI 5535901, AI 016137 (REC) and AI 068582-01(JBN); JBN also was supported by a European Developing Countries Clinical Trial Partnership Senior Fellowship Award TA-08-40200-021.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Consultant, J.B.N. (Pfizer), R.E.C. (Bristol-Myers Squibb). Honoraria, J.B.N. (GlaxoSmithKline, Merck-Sharp-Dohme for continuing medical education lectures) and G.M. (Merck-Sharp-Dohme and Abbott). Other, J.B.N. (Aspen Pharmaceuticals, GlaxoSmithKline for conferences and travel grants).
Trial Registration: Clinicaltrials.gov registration number: NCT00076804
Author Contributions:
Study design (JBN, AE, REC, ARK, GM); drafting and writing of the manuscript (JBN, GM, REC); data management (CM, MR); data analysis (MAC, JBN); data interpretation (JBN, REC, MAC, HS, GM, ARK); implementation, monitoring and patient follow up (AE, RG, JBN, GM); editing and final approval of the manuscript (All); administrative and logistic support (AE, RG, GM, REC).
References
- 1.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000 Jul 4;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 3.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005 Feb 1;191(3):339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 4.Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007 May 11;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001 Jun 15;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002 May 3;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 7.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, Chaisson RE, Maartens G. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African Adults. J Acquir Immune Defic Syndr. 2006 Sep;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 8.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006 Aug 9;296(6):679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 9.Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. The Lancet. 2007 Dec 8;370(9603):1885–1887. doi: 10.1016/S0140-6736(07)61790-5. [DOI] [PubMed] [Google Scholar]
- 10.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006 Nov;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006 Dec 1;43 Suppl 1:S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006 Mar;41(3):285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 13.Rueda S, Park-Wyllie LY, Bayoumi AM, Tynan AM, Antoniou TA, Rourke SB, Glazier RH. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006 Jul 19;3 doi: 10.1002/14651858.CD001442.pub2. CD001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden? AIDS Patient Care STDS. 2002;16:527–535. doi: 10.1089/108729102761041083. [DOI] [PubMed] [Google Scholar]
- 15.Bangsberg DR, Mundy LM, Tulsky JP. Expanding directly observed therapy: tuberculosis to human immunodeficiency virus. Am. J. Med. 2001;110(8):664–666. doi: 10.1016/s0002-9343(01)00729-x. [DOI] [PubMed] [Google Scholar]
- 16.Clarke M, Dick J, Zwarenstein M, et al. Lay health worker intervention with choice of DOT superior to standard TB care for farm dwellers in South Africa: a cluster randomized control trial. Int J Tuberc Lung Dis. 2005;9:673–679. [PubMed] [Google Scholar]
- 17.Manders AJ, Banerjee A, van den Borne HW, et al. Can guardians supervise TB treatment as well as health workers? A study on adherence during the intensive phase. Int J Tuberc Lung Dis. 2001;9:838–842. [PubMed] [Google Scholar]
- 18.Wilkinson D, Davies GR, Connolly C. Directly observed therapy for tuberculosis in rural South Africa, 1991 through 1994. Am J Public Health. 1996;86:1094–1097. doi: 10.2105/ajph.86.8_pt_1.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newell JN, Baral SC, Pande SB, Bam DS, Malla P. Family-member DOTS and community DOTS for tuberculosis control in Nepal: cluster-randomised controlled trial. The Lancet. 2006 Mar 18;367(9514):903–909. doi: 10.1016/S0140-6736(06)68380-3. [DOI] [PubMed] [Google Scholar]
- 20.Farmer P, Léandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79(12):1145–1151. [PMC free article] [PubMed] [Google Scholar]
- 21.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007 Sep 15;45(6):770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohl AR, Garland WH, Valencia R, et al. A randomized trial of directly administered antiretroviral therapy and adherence case management intervention. Clin Infect Dis. 2006 Jun 1;42(11):1619–1627. doi: 10.1086/503906. [DOI] [PubMed] [Google Scholar]
- 23.Macalino GE, Hogan JW, Mitty JA, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007 Jul 11;21(11):1473–1477. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- 24.Pearson CR, Micek MA, Simoni JM, Hoff PD, Matediana E, Martin DP, Gloyd SS. Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique. J Acquir Immune Defic Syndr. 2007 Oct 1;46(2):238–244. doi: 10.1097/QAI.0b013e318153f7ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knowlton AR. Informal HIV caregiving in a vulnerable population: toward a network resource framework. Soc Sci Med. 2003;56(6):1307–1320. doi: 10.1016/s0277-9536(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 26.Ewing JA. 'Detecting Alcoholism: The CAGE Questionaire'. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 27.Valverde EE, Purcell DW, Waldrop-Valverde D, Malow R, Knowlton AR, Gomez CA, Farrell N, Latka MH for the INSPIRE Team. Correlates of depression among HIV-positive women and men who inject drugs. J Acquir Immune Defic Syndr. 2007;46 Suppl 2:S96–S100. doi: 10.1097/QAI.0b013e318157683b. [DOI] [PubMed] [Google Scholar]
- 28.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003 Jun 13;17(9):1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 29.Gross R, Tierney C, Andrade A, Lalama C, Rosenkranz S, Eshleman SH, Flanigan T, Santana J, Salomon N, Reisler R, Wiggins I, Hogg E, Flexner C, Mildvan D AIDS Clinical Trials Group A5073 Study Team. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009 Jul 13;169(13):1224–1232. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009 Feb 1;50(2):176–181. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nachega JB, Knowlton AR, Deluca A, Schoeman JH, Watkinson L, Efron A, Chaisson RE, Maartens G. Treatment supporter to improve adherence to antiretroviral therapy in HIV-infected South African adults. A qualitative study. J Acquir Immune Defic Syndr. 2006 Dec 1;43 Suppl 1:S127–S133. doi: 10.1097/01.qai.0000248349.25630.3d. [DOI] [PubMed] [Google Scholar]
- 32.Ware NC, Idoko J, Kaaya S, Biraro IA, Wyatt MA, et al. Explaining adherence success in sub-Saharan Africa: An ethnographic study. PLoS Med. 2009;6(1):e. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchino B. Social support and health: A review of physiologic processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]