Abstract
The purpose of this study was to explore changes in activation of the cortical network that serves auditory sentence comprehension in children in response to increasing demands of complex sentences. A further goal is to study how individual differences in children’s receptive language abilities are associated with such changes in cortical responses. Fourteen children, 10 to 16 years old, participated in an event-related functional magnetic resonance imaging experiment using a cross modal sentence-picture verification paradigm. We manipulated sentence difficulty and length in a 2 × 2 factorial design. Task-related activation covered large regions of the left and right superior temporal cortex, inferior parietal lobe, precuneous, cingulate, middle frontal gyrus and precentral gyrus. Sentence difficulty, independent of length, led to increased activation in the left temporal-parietal junction and right superior temporal gyrus. Changes in activation in frontal regions positively correlated with age-standardized receptive vocabulary scores and negatively correlated with reaction time on a receptive grammar test outside the scanner. Thus, individual differences in language skills were associated with changes in the network in response to changing task demands. These preliminary findings in a small sample of typically developing children suggest that the investigation of individual differences may prove useful in elucidating the underlying neural mechanisms of language disorders in children.
Keywords: language, sentence comprehension, children, functional magnetic resonance imaging (fMRI), individual differences, inter-subject variability
Functional magnetic resonance imaging (fMRI) has proven highly valuable in describing the neural substrate of language functions in healthy volunteers and in identifying differences in neural activity across age groups and clinical populations. Recent studies have begun to further explore the application of fMRI to capture individual differences in the neural substrate of complex functions such as language and reading (Ben-Shachar, Dougherty, Deutsch, & Wandell, 2007; Kherif et al., 2009; Prat, Keller, & Just, 2007; Seghier, Lee, Schofield, Ellis, & Price, 2008). Mapping the neural correlates of individual variation is important for developing clinical applications of fMRI, because diagnoses and treatment recommendations are made at the individual level. In this exploratory study we aimed to investigate inter-individual variation in the neural basis of language processing. In particular, we were interested in whether dynamic changes in patterns of neural activation as a function of task difficulty would be associated with individual differences in receptive language abilities.
Language comprehension is a complex skill, essential for mature social communication and for learning. However, whereas difficulties with language production are quickly apparent in everyday social situations, difficulties with language comprehension may be quite subtle, going unnoticed until a listener responds inappropriately, fails to comply with requests, violates social expectations, or in other ways demonstrates misunderstanding. Individuals vary in their skill and speed of language comprehension. Receptive language skills, and in particular, grammatical understanding, are often significantly below age expectations in children with language impairment (Bishop, 1982; Evans, 1996, 2002; Evans & MacWhinney, 1999; Friedmann & Novogrodsky, 2004; Law, Tomblin, & Zhang, 2008; Tomblin & Zhang, 2006). Children with receptive language difficulties that persist into school age are at long-term risk for academic underachievement and psychiatric disorders (Clegg, Hollis, Mawhood, & Rutter, 2005).
The cortical network that serves language comprehension in healthy adult volunteers has been well described using fMRI, though the functions of component regions are still under debate. Regardless of specific paradigms used, fMRI studies typically find robust activations within the superior and middle temporal lobes (Ben-Shachar, Hendler, Kahn, Ben-Bashat, & Grodzinsky, 2003; Ben-Shachar, Palti, & Grodzinsky, 2004; Cabeza & Nyberg, 2000; Friederici, Ruschemeyer, Hahne, & Fiebach, 2003; Humphries, Binder, Medler, & Liebenthal, 2007; Just, Carpenter, Keller, Eddy, & Thulborn, 1996; Keller, Carpenter, & Just, 2001; Marinkovic, 2004). Activations in these areas are usually bilateral for auditory stimuli and left lateralized for visual stimuli (Cabeza & Nyberg, 2000; Marinkovic, 2004). Many studies also find activation of the left inferior frontal gyrus, (Ben-Shachar et al., 2003; Ben-Shachar et al., 2004; Cabeza & Nyberg, 2000; Friederici et al., 2003; Hoen, Pachot-Clouard, Segebarth, & Dominey, 2006; Humphries et al., 2007; Love et al., 2006; Peelle, McMillan, Moore, Grossman, & Wingfield, 2004; Yokoyama et al., 2007), though they differ in terms of the specific anatomical areas activated. Beyond the temporal lobe and the inferior frontal gyrus (considered as the canonical language network), other regions, such as the angular gyrus (Caplan et al., 2002; Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004; Humphries et al., 2007) and dorsolateral prefrontal cortex (Reichle, Carpenter, & Just, 2000) are frequently activated during language comprehension.
Importantly, the neural network for sentence comprehension in adults has been found to be highly dynamic (Just & Varma, 2007), varying in relation to both sentence complexity and task demands. As syntactic complexity increases, the volumes of activation have been found to increase within left hemisphere perisylvian regions, particularly inferior frontal cortex (Caplan et al., 2002; Kaan & Swaab, 2002; Keller et al., 2001) and within right hemisphere homologues of left hemisphere language areas (Just et al., 1996). In addition, Love et al. (2006) have shown that the task of making thematic judgments about sentences recruits the inferior frontal gyrus extending to dorsolateral prefrontal cortex, regions that remained inactive during passive comprehension of the same sentences (Love et al., 2006). These findings highlight the flexibility of sentence comprehension neural processes and demonstrate that activation patterns can rapidly change to meet changing task demands. The increase in BOLD signal as a function of increased task difficulty has been termed neural adaptability (Just & Varma, 2007). Studies of reading suggest that response to task difficulty could be an important factor for individual differences in language abilities (Just & Varma, 2007; Prat et al., 2007).
Areas of cortical activation during sentence processing appear to be similar in school-aged children and adults, though there are far fewer child-focused than adult fMRI studies. Activations of the perisylvian regions and superior and middle temporal gyri are found in sentence and story comprehension tasks (Ahmad, Balsamo, Sachs, Xu, & Gaillard, 2003; Booth et al., 2000; Schmithorst, Holland, & Plante, 2006). A large study of children ages 5 to 18 years, using independent component analysis, found task-related components, including primary auditory cortex, mid-superior temporal gyrus, hippocampus, angular gyrus, and medial parietal lobe (Karunanayaka et al., 2007; Schmithorst et al., 2006). The degree of laterality for story and sentence comprehension in children varies across paradigms, with some studies finding left lateralized networks in the temporal and frontal lobes (Ahmad et al., 2003) and others finding primarily bilateral activation (Schmithorst et al., 2006). Booth and colleagues (2000) found that children showed greater left lateralization of temporal activations than did adults, and increased left lateralization was associated with poorer task performance (Booth et al., 2000). In a recent paper, Brauer & Friederici (2007) found higher activation in children compared to adults for syntactic and semantic violations, possibly due to performance differences also manifested in longer reaction times in the children. Inferior frontal activation passed statistical thresholds in children, but not in adults. The network for language comprehension has also been shown to vary as a function of age within childhood (Karunanayaka et al., 2007; Schmithorst & Holland, 2007; Schmithorst et al., 2006), gender (Schmithorst & Holland, 2007) and clinical population (Peterson et al., 2002).
Dynamic properties of children’s neural network for auditory sentence comprehension have not been previously well characterized. Based on the adult literature, we reasoned that the network would show greater activation during comprehension of more difficult sentences. We were particularly interested whether responses to task difficulty would be associated with individual differences in age or receptive language skills. Thus, the overall goal of the present exploratory study is to use fMRI examine the modulation of children’s cortical responses to auditory sentences by sentence difficulty and length, and to explore the relation between variations in the response of the network and individual differences in children’s receptive language skills.
Methods
Subjects
Fourteen healthy children between 10 and16 years of age (mean age = 12.5, standard deviation 1.9) took part in the experiment. Six were boys, one was African-American, four were Asian, and two were White-Hispanic. Five children were bilingual but all spoke English as their primary language. Participants served as controls in a larger study on language development in children with white matter injuries. All participants had no neurological, developmental, medical, or psychological disorders and no contraindications to obtaining an MRI scan. By report, they had no impairments of vision or hearing. The children were recruited from the local public and private schools. Given our location in Palo Alto CA, the group was weighted toward high socioeconomic status (SES). However, to explore individual differences in sentence comprehension, we chose children whose scores varied on standardized measures of language. The protocol was approved by the Institutional Review Board at Stanford University. Informed consent was obtained from parents and assent from study participants prior to initiation of the study.
Procedures
Behavioral measures
Participants underwent two testing sessions to assess language skills, cognitive abilities, and executive function. Measures included in this study are as follows:
The Wechsler Abbreviated Scale of Intelligence or WASI (The Psychological Corporation, 1999), is a widely used standardized test of intellectual ability, that measures both verbal and nonverbal cognitive ability and yields a Full Scale IQ composite score
Peabody Picture Vocabulary Test, Third Edition or PPVT-III (Dunn & Dunn, 1997) is a widely used test of receptive vocabulary that generates a standard score. Each item consists of four black-and-white drawings on a page. Participants are asked to identify which of the four illustrations best represents the stimulus word presented orally by the examiner.
Comprehensive Evaluation of Language Fundamentals-Fourth Edition or CELF -IV (Semel, Wiig, & Secord, 2005) is a norm-referenced test of expressive and receptive language skills. We used CELF-IV Receptive Language Index to measure the child’s listening and comprehension skills.
Test for Reception of Grammar-Second Edition or TROG-2 (Bishop, 2003) is a computerized measure of grammatical understanding. The TROG-2 assesses syntactic comprehension by presenting sentences in the auditory mode and using a four picture multiple choice format with lexical and grammatical foils. The vocabulary is simple and familiar to school aged children. Participants can press a button to hear the sentence as many times as necessary. The test consists of 80 items, organized into 20 blocks of four sentences of increasing syntactic difficulty. The level of difficulty of the items is designed to tap skills of school-aged children and adolescents. The test can be scored in terms of the total number of correct responses (out of 80), the number of blocks completed perfectly (out of 20), and the mean reaction time for correct responses.
fMRI Task
The sentence verification task consisted of auditory presentation of sentences followed by a picture. Participants were asked to judge with a button press if the picture accurately depicts the sentence or not. Sentences and pictures were derived from the TROG-2. In the scanner, we used a simple yes/no decision rather than the four-picture presentation to reduce the possibility of the child’s moving their head and to reduce potential confound activations from extensive eye movements. Sentences were divided into four conditions, each consisting of three different syntactic constructions: short/easy, short/hard, long/easy, long/hard (Table 1). The level of syntactic difficulty was assigned based on the classification of the sentence type within the TROG-2, which orders the presentation of sentences based on empirical data on the difficulty of that construction for children. Easy sentence constructions came from the first eight blocks on the test. Hard sentences came from the last ten blocks on the test. Minor changes in the auditory stimuli from the original sentences in the TROG-2 were included to allow the number of words to be consistent within the category. Short sentences were 5 to 7 words in length; long sentences were 8 to 9 words in length.
Table 1.
Examples of Stimuli used in the Sentence Verification Task during functional Magnetic Resonance Imaging
| Sentence condition | Syntactic constructions included, using terminology from the Test of Reception of Grammar-2 | Examples |
|---|---|---|
| Short/easy |
|
|
| Short/hard |
|
|
| Long/easy |
|
|
| Long/hard |
|
|
Subjects were instructed to indicate whether the sentence and the picture matched by pressing the “true” or “false” button on a button box. The words Right and Wrong were on the screen to the left and right of the picture to remind the subject of the side for each response. Each sentence was associated with one picture for a true response and one for a false response. True and false trials were randomized for each subject, ensuring an equal ratio and counterbalancing response type per item.
Stimuli were presented in an event related design using E-Prime (Psychology Software Tools, Pittsburgh, PA). Each trial lasted 8.75 seconds (3.5 seconds for sentence presentation and 5.25 seconds for picture presentation and button press). Longer sentences were presented with shorter pauses between words to fit within the 3.5 second duration, which still reflected a natural rate of conversation. Trials were preceded and followed by a fixation cross that lasted a variable amount of time. Fixation periods allowed us to reliably estimate baseline BOLD signals and subtract those away in the general analysis of “task related activity”. However, the main contrasts of interest involved hard versus easy sentences, and short versus long sentences. Such comparisons are largely invariant to the sensory components as those exist in all 4 conditions, and therefore appropriately isolate the regions involved in aspects of language processing, not sensory processing. A total of 96 presentations were divided into 4 runs of 24 stimuli, each run lasting 4 minutes and 40 seconds. Ten 4-run task lists were generated with the sentences in random order and separated by randomized time intervals of fixation jittered in 1.75 seconds (from 0 to 5.25 seconds). The order of event types was counterbalanced using “optseq2” (http://surfer.nmr.mgh.harvard.edu/optseq/), to minimize bias that might result from carry over effects between successive events (Dale, 1999). Each subject was randomly assigned a stimulus list.
Within the scanner, the auditory stimulus was presented through an Avotec pneumatic audio presentation system. Pneumatic tubes were sealed directly on to earplug, successfully reducing scanner noise while allowing the subject to clearly hear the sentences. The pictures were projected onto a screen in front of the subject from an MR compatible projector. Button press responses were recorded for later analysis. The entire task was preceded and followed by 10.5 seconds of fixation to allow the signal to reach equilibrium and to obtain an accurate fixation baseline.
fMRI Data Acquisition
Data was acquired on a 3T, GE Signa 750 scanner (GE Healthcare Systems). The full session lasted about 40 minutes and started with a T1 weighted, 3-plane localizer. This image was used for subsequent prescriptions. We acquired a T1 weighted inplane anatomy with 26, 4 mm thick slices (GRE, TR = 34 ms, TE = 2 ms, flip angle 30 degrees, FOV = 22 cm, Matrix 256 × 192), aligned to the plane of the anterior and posterior comissures. Before collecting functional data we ran a high order shim (Kim, Adalsteinsson, Glover, & Spielman, 2002) to correct for inhomogeneities in the magnetic field. T2* weighted functional images were collected using a spiral in/out pulse sequence (Glover & Law, 2001) (TR = 1.75 seconds; TE = 30 ms; flip angle = 70 degrees; FOV = 22cm; Matrix = 64 × 64; slice thickness = 4 mm) and the same prescription as the inplane anatomy. A total of 160 volumes were collected in each sequence and the sequence was repeated four times. The first six volumes (10.5 seconds) were discarded. A high resolution T1 weighted IR-prep 3D FSPGR scan was collected following the functional scans (FOV = 24 x16.8 cm, Matrix = 256 × 192 × 130, 1.2mm slices, TI = 300ms, flip angle = 15 degrees, 1 NEX).
Data Analysis
Behavioral measures
Behavioral data were analyzed in MATLAB (The Mathworks, Natick, MA) and SPSS version 16.0 (SPSS inc., Chicago, Illinois). For each subject, we computed the number of correct responses in total and for each sentence type. We also computed mean reaction time to respond on correct trials only.
fMRI data pre-processing
Data were preprocessed and analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Images were corrected for slice acquisition timing then realigned to the mean functional image to correct for within-scan and between-scan motion. The inplane image was coregistered to the mean functional image to correct for any movement between collection of anatomical and functional data. The high resolution T1 weighted image was coregistered to the inplane and segmented into gray matter, white matter, and cerebral spinal fluid. These images were smoothed and used to generate a brain mask. The gray matter segmentation was used to estimate the non-linear transformation to the MNI template. This transformation was applied to the functional images and they were resampled to 3 × 3 × 3 mm3 voxels. The images then were smoothed with an 8 mm Gaussian kernel to reduce spatial noise. Statistical analysis was performed for each subject by calculating a general linear model for voxels within the cortical regions of the brain mask. The four sentence types were convolved with a canonical hemodynamic response function (HRF) and their temporal and dispersion derivatives were estimated. Regressors were included for the 6 motion parameters, for each run and for the overall mean. A highpass filter with a 128 second cutoff was used to remove low frequency drift and an AR(1) correction was used for serial autocorrelation. Contrasts were estimated for a 2 × 2 factorial design.
Voxelwise Group Analysis of the Distribution of Activation
Group level statistics were calculated using a random effects model with the contrast images from each subject. We set the statistical threshold for all analyses at p < 0.001 (uncorrected for multiple comparisons). Though a statistical threshold of p < 0.001 is common in imaging studies we recognize that uncorrected analyses must be interpreted cautiously. In light of the fact that this was an exploratory study with a limited sample size we elected not to use SPM’s conservative voxelwise corrections because of the high probability of type 2 errors. However, to reduce the risk of accumulating alpha errors, we analyzed clusters with a minimal cluster size of 10 voxels. The analysis procedure is similar to that used in studies with related objectives and similar populations (Brauer & Friederici, 2007; Prat et al., 2007).
Individual Difference Analysis
To investigate whether developmental changes in the cortical responses to sentence difficulty, we constructed a model in which age was a covariate in the random effects model comparing hard to easy sentences. To investigate changes in cortical responses to hard versus easy sentences in relation to the individual’s receptive language skills, we constructed three models which included PPVT-III standard score, CELF-IV standard score or TROG-2 mean reaction time as a covariate in the random effects model comparing hard sentences to easy sentences. These analyses thus identify regions where the difference in the BOLD response between hard and easy sentences is associated with the individual’s age, score or performance on the behavioral assessment of receptive language. (For PPVT-III and CELF-IV, higher scores indicate stronger performance whereas for TROG-2 reaction time, lower scores indicate stronger performance. We examined positive and negative correlations for all three measures.) For TROG-2, reaction time data was available for 13 out of 14 subjects. The other two models included all 14 subjects.
Results
Behavioral Results
Table 2 includes the mean standardized scores on the WASI, PPVT-III and CELF-IV for this sample of participants. As a group, the children were above average on these measures, though scores ranged from average to superior. To examine correlations among the measures, we used Spearman correlations because of small sample size. Examining correlations among receptive language tests, we found that scores on the PPVT-III and CELF-IV showed a modest correlation that did not reach statistical significance (r = 0.491, p = 0.075). Table 2 also shows the mean number of correct trials and reaction time for the TROG-2. The TROG-2 accuracy measurement was significantly correlated with CELF-IV scores (r = 0.699, p = 0.008) and showed a modest correlation with PPVT-III scores that approached significance (r = 0.513, p = 0.073). In contrast the TROG-2 reaction time measurement was not correlated with either of the standardized test scores. Percent of correct responses and reaction time on the TROG were not correlated with age.
Table 2.
Behavioral test scores and task performance
| Measure | Mean | SD | Range |
|---|---|---|---|
| Standardized Test Scores | |||
| WASI Full Scale Intelligence | 121.8 | 12.8 | 102.0 – 144.0 |
| PPVT-III | 116.2 | 9.3 | 103.0 – 134.0 |
| CELF-IV Receptive Language Index | 114.6 | 10.2 | 96.0 – 134.0 |
| TROG-2 Performance | |||
| Mean number correct (out of 80) | 76.8 | 1.6 | 73 – 79 |
| Mean reaction time in milliseconds | 2829 | 546 | 1987 – 3695 |
| In-Scanner Sentence Comprehension | |||
| Mean number correct (out of 24) | |||
| Short/Easy | 22.2 | 1.5 | 19.0 – 24.0 |
| Short/Hard | 20.5 | 1.4 | 18.0 – 23.0 |
| Long/Easy | 20.9 | 2.8 | 15.0 – 24.0 |
| Long/Hard | 19.0 | 2.0 | 14.0 – 22.0 |
| Mean reaction time in milliseconds | |||
| Short/Easy | 1851 | 377 | 1106 – 2428 |
| Short/Hard | 2003 | 408 | 1073 – 2466 |
| Long/Easy | 2169 | 422 | 1294 – 2613 |
| Long/Hard | 2670 | 500 | 1851 – 3349 |
CELF-IV = Clinical Evaluation of Language Fundamentals. Fourth Edition
PPVT-III = Peabody Picture Vocabulary Test, Third Edition
WASI= Wechsler Abbreviated Scale of Intelligence
TROG-2 = Test for Reception of Grammar, Second Edition
Table 2 also includes the number of correct responses and mean reaction times for each sentence type in the fMRI task. A repeated measures general linear model analysis revealed, as expected, lower accuracy (F(1, 12) = 7.69, p = 0.017) and longer reaction time (F(1,12) = 33.21, p < 0.001) for long sentences than for short sentences. Similarly, accuracy was lower (F(1, 12) = 11.12, p = 0.006) and reaction time longer (F(1,12) = 97.88, p < 0.001) for hard sentences than for easy sentences. Reaction time showed a significant interaction between length and difficulty (F(1, 12) = 21.03, p = 0.001) but accuracy did not (F(1, 12) = 0.05, p = 0.827). Using Spearman correlations, reaction times among all trial types were highly correlated (r > 0.8, p < 0.001). Age did not show a significant positive correlation with accuracy on the behavioral measures (r’s = − 0.133 to 0.513, p = 0.073 – 0.767) or a significant negative correlation with reaction time (r’s = −0.014 to 0.265, p = 0.382 – 0.986). Neither standardized measure of receptive language skills showed a significant correlation with task performance (r’s = −0.83 to 0.423, p = 0.149 to 0.861) or reaction time (r’s = −0.196 to 0.400, p = 0.176 to 0.979).
Distribution of Activations
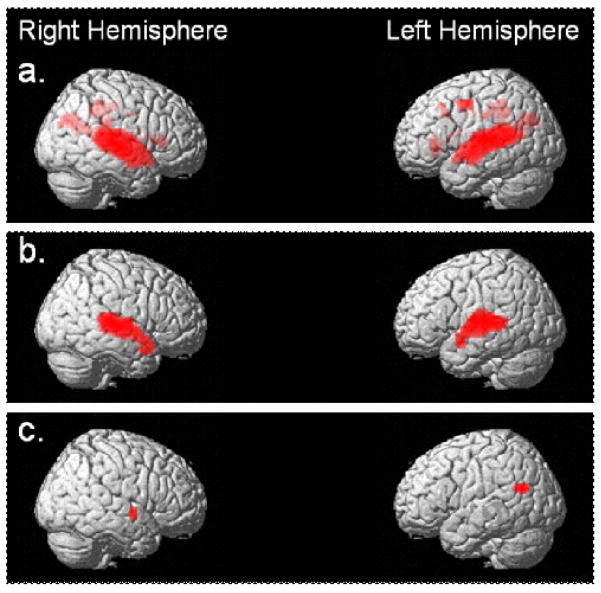
In order to characterize the entire network recruited to perform the auditory sentence comprehension task, we first generated a group statistical parametric map (SPM) comparing the BOLD response on language trials to BOLD signal at baseline. Figure 1a shows a three-dimensional rendering of the cortical surface. Regions with significantly greater BOLD signal on all sentence conditions compared to fixation are colored red (p < 0.001). Table 3a describes the areas of activation, peak z score, number of active voxels for the contrast, and MNI coordinates for the peak activations. For sentence comprehension in comparison to fixation, a large network of brain regions became active. Activation in the superior and middle temporal cortex was bilateral; the extent of activation was greater in the left hemisphere (Y coordinate = 9 to −60) than the right hemisphere (Y coordinate 15 to −45) but signal change was greater in the right hemisphere (z = 6.99) than left hemisphere (z = 6.17). Additional areas of activation included the insula, supramarginal and angular gyri, posterior cingulate and precuneus. Notably, we did not find task-related activation in the left or the right inferior frontal gyrus. To characterize network changes in response to sentence length, we generated an SPM for the main effect of sentence length (Figure 1b and Table 3b). Processing of long versus short sentences resulted in statistically greater activation bilaterally in the inferior, middle and superior temporal gyri and insula. To characterize network changes in response to increasing syntactic difficulty, we generated an SPM for the main effect of difficulty (Figure 1c and Table 3c). Two localized clusters of voxels showed significantly greater BOLD response to hard versus easy sentences, one in left middle temporal gyrus and one in right superior temporal gyrus and insula. The regions identified by these two contrasts were distinct and did not show any areas of overlap. We used a 2 × 2 ANOVA to identify regions showing an interaction of length by complexity. This analysis was limited to task-related regions identified by the proceeding three contrasts. No regions showed an interaction effect.
Figure 1.
Three dimensional rendering of the cortical surface displaying regions of significant activity in red for: (a) Sentence Comprehension vs. Fixation, (b) Long vs. Short Sentences, (c) Hard vs. Easy Sentences. p < 0.001 uncorrected, minimum cluster size > 10 voxels.
Table 3.
Cortical Regions of Activation (cluster size > 10 contiguous voxels, p < 0.001 uncorrected) on the following contrasts (a) Sentence Comprehension vs. Fixation, (b) Long vs. Short Sentences, (c) Hard vs. Easy Sentences
| Cortical Region | Brodmann’s Areas | Peak Z- Score | Number Active Voxels | MNI Coordinates for Peak Activation | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
|
a. Sentence Comprehension vs. Fixation | ||||||
| Right Superior/Middle Temporal Gyri/Insula | 13, 21, 22, 38, 41, 42 | 6.99 | 1084 | 57 | −18 | 3 |
| Left Superior/Middle Temporal Gyri/Insula/Supramarginal Gyrus/Angular Gyrus | 13, 21, 22, 38, 39, 40, 41, 42 | 6.17 | 1114 | −57 | −24 | 9 |
| Posterior Cingulate/Precuneus | 7, 31 | 4.69 | 389 | −9 | −54 | 15 |
| Middle Frontal/Precentral Gyrus | 6 | 4.23 | 88 | −39 | 0 | 48 |
| Cingulate/Paracentral Lobule | 5, 31 | 4.04 | 223 | −3 | −36 | 39 |
| Cingulate | 23 | 3.52 | 33 | 0 | −15 | 33 |
| Left Middle Frontal Gyrus | 8 | 3.40 | 10 | −24 | 21 | 42 |
|
b. Long vs. Short Sentences | ||||||
| Right Inferior/Middle/Superior Temporal Gyri/Insula | 13, 20, 21, 22, 41, 42 | 5.13 | 487 | 51 | −24 | 0 |
| Left Inferior/Middle/Superior Temporal Gyri/Insula | 13, 20, 21, 22, 41, 42 | 4.47 | 460 | −54 | −12 | 6 |
|
c. Hard vs. Easy Sentences | ||||||
| Left Middle/Superior Temporal Gyri | 19, 39, 22 | 4.09 | 29 | −42 | −60 | 21 |
| Right Superior Temporal Gyrus/Insula | 13, 22 | 3.89 | 16 | 48 | −3 | −3 |
Individual difference in the cortical language network
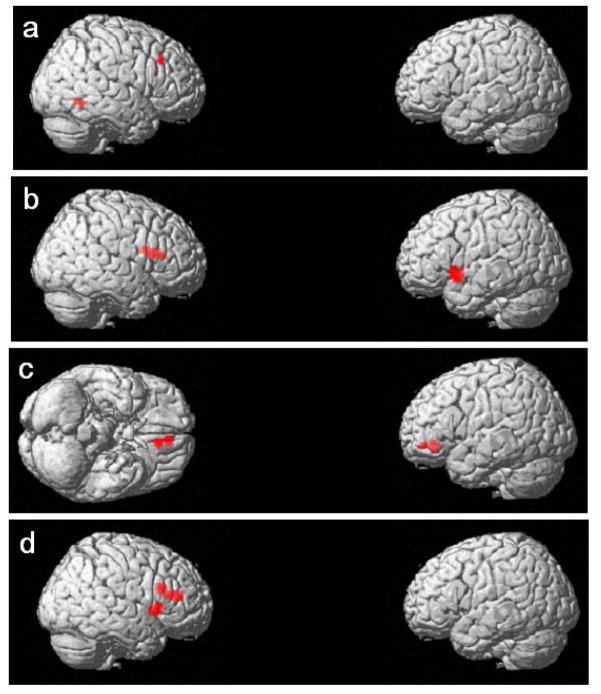
Figure 2a depicts cortical regions where the difference between the magnitudes of the BOLD response to hard sentences compared to easy sentences is negatively associated with age. Table 4a describes areas of activation, peak t score, number of active voxels for the contrast, and MNI coordinates for the peak activations for the same model. The two significant clusers were in the right hemisphere, one cluster of 16 voxels in the fusiform gyrus and one of 15 voxels in the middle frontal gyrus. The areas in which increased signal was associated with length and difficulty did not overlap. We found no regions where changes in the network were positively associated with age.
Figure 2.
Three dimensional rendering of the cortical surface displaying in red regions where cortical responses to increasing sentence complexity was significantly associated with (a) age (negative correlation); (b) Peabody Picture Vocabulary Test-III (PPVT-III) standard scores (positive correlation); (c) Mean reaction time on the Test of the Reception of Grammar 2nd edition (TROG-2) (negative correlation); (d) Mean reaction time on TROG-2 (positive correlation). All p < 0.001, minimum cluster size > 10 voxels.
Table 4.
Cortical regions that show a Significant Correlation (cluster size > 10 voxels, p < 0.001 uncorrected) between Hard > Easy Sentences Contrast and (a) Age (negative correlation); (b) Peabody Picture Vocabulary Test-III (PPVT-III) (positive correlation), (c) Test of the Reception Of Grammar-2 (TROG-2) Mean Reaction Time (negative correlation), (d) TROG-2) Mean Reaction Time (positive correlation).
| Cortical Region | Brodmann’s Areas | Peak Z- Score | Number Active Voxels | MNI Coordinates for Peak Activation | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
|
a. Age negatively correlates with Hard > Easy contrast | ||||||
| Right Fusiform Gyrus | 37 | 3.50 | 16 | 39 | −54 | −9 |
| Right Middle Frontal Gyrus | 8, 9 | 3.40 | 15 | 48 | 30 | 36 |
|
b. PPVT-III correlates with. Hard > Easy contrast | ||||||
| Right Frontal Operculum/Claustrum | 4.01 | 44 | 27 | 21 | 15 | |
| Left Superior Temporal Gyrus/Inferior Frontal Gyrus/Insula | 13, 22, 38, 47 | 3.47 | 38 | −51 | 9 | −12 |
|
c. TROG-2 mean reaction time negatively correlates with Hard > Easy contrast | ||||||
| Left Anterior Cingulate/Left Medial Frontal Gyrus | 10, 11, 32 | 3.54 | 30 | −9 | 36 | −9 |
| d. TROG-2 mean reaction time positively correlates with Hard > Easy contrast | ||||||
| Right Inferior Frontal Gyrus/Right Precentral Gyrus/Right Insula | 13, 44, 45, 47 | 4.02 | 29 | 42 | 15 | 3 |
| Right Middle Frontal Gyrus/Right Inferior Frontal Gyrus/Right Insula | 10, 46 | 3.50 | 42 | 39 | 39 | 15 |
We repeated the random effect models of hard versus easy sentences, using three measures of individual differences as covariates—PPVT-III standard score, CELF-IV standard score, and mean reaction time on the TROG-2. Figure 2b depicts cortical regions where the difference between the magnitudes of the BOLD response to hard sentences compared to easy sentences was positively associated with PPVT-III score. Table 4b describes areas of activation, peak t score, number of active voxels for the contrast, and MNI coordinates for the peak activations for the same model. In the left hemisphere, 38 voxels showed this positive association with PPVT-III standard score, extending from the superior temporal gyrus to the insula and the inferior frontal gyrus. In the right hemisphere, there were 44 significant voxels, which are located in the frontal operculum and claustrum (also depicted in figure 2b). We found no regions of the cortex in which changes in the cortical responses were negatively associated with PPVT-III standard scores. We also found no regions of the cortex in which changes in the cortical response were positively or negatively associated with CELF-IV scores.
Figure 2c depicts cortical regions where the difference between the magnitudes of the BOLD response to hard sentences compared to easy sentences is negatively associated with reaction time on the TROG-2. Table 4c describes areas of activation, peak t score, number of active voxels for the contrast, and MNI coordinates for the peak activations for the same model. We found 30 voxels in the left hemisphere positioned near the midline, extending from the anterior cingulate to medial frontal gyrus, with a negative association with reaction time on the TROG-2. Figure 2d depicts cortical regions where the difference between the magnitudes of the BOLD response to hard sentences compared to easy sentences is positively associated with reaction time on the TROG-2. Table 4d describes areas of activation, peak t score, number of active voxels for the contrast, and MNI coordinates for the peak activations for the same model. We found 29 voxels in the right inferior frontal gyrus and precentral gyrus and 42 voxels in the near the juncture of the right middle frontal gyrus, right inferior frontal gyrus, and insula with the positive association with reaction time on the TROG-2.
Discussion
Results of this exploratory study suggest that the dynamic neural network for auditory sentence comprehension in typically developing children and adolescents changes in distinct ways as a function of sentence length and syntactic complexity. Increased bilateral temporal activation was associated with sentence length. Increased activation of the left posterior temporal lobe, right superior temporal gyrus and right insula was associated with syntactic complexity, though this effect was small and must be considered tentative until confirmed with a larger investigation. Our results further suggest that regions of the frontal cortex become more active in response to increasing task demands in children with superior receptive language skills than in children with average language abilities, when receptive language abilities are indexed by either standard scores on formal tests or processing speed on a computerized comprehension task outside of the scanner.
The sentence-verification task was designed to characterize dynamic properties of the language system and identify neural responses associated with individual differences in functional communications skills. This task, popular in the adult aphasia literature, is more natural and less meta-linguistic than other candidate tasks, such as grammaticality judgment. It is useful for demonstrating individual differences on a trial-by-trial basis because it allows for performance monitoring in terms of both accuracy and reaction time. We used three different syntactic structures in each of the stimulus categories. By extending the range of constructions we are likely to extend the range of behavioral and neural responses. Our paradigm was not designed to map specific cognitive functions to specific regions; rather it was designed to detect changes in the overall network response to varying stimulus and subject characteristics.
Sentence comprehension (compared to fixation) activated a bilateral network of cortical regions. Left lateralization for language processing tends to be greater in verbal fluency, verbal working memory and reading tasks than it is in auditory sentence and story comprehension (Gaillard et al., 2004; Gaillard et al., 2000; Holland et al., 2001; Schmithorst et al., 2006). These findings suggest that under naturalistic circumstances, the right hemisphere is actively involved, possibly equally to the left, in language comprehension. What is as yet unclear is to what extent the two hemispheres are redundant or processing in distinctive manners.
Though still an area of considerable debate, left lateralization, particularly in the inferior frontal gyrus, is often found in comprehension tasks that examine the processing of specific complex syntactic constructions (Ben-Shachar et al., 2003; Ben-Shachar et al., 2004), require detection of syntactic violations,(Friederici et al., 2003) or place high demands on verbal memory (Fiebach, Schlesewsky, Lohmann, von Cramon, & Friederici, 2005). In general the inferior frontal gyrus is implicated in language tasks with high processing demands. Though we did not find inferior frontal gyrus activation at the group level, the individual differences analysis demonstrated that children with better receptive language skills showed a greater increase in activation in the inferior frontal gyrus on complex sentences than children with average receptive language skills. This finding suggests that the children with the strongest language abilities are activating higher order brain functions when performing a more complex task.
Main effects of both length and difficulty were present in both hemispheres suggesting that increasing task demands draw upon greater resources from both the left and right hemisphere. Many fMRI studies of syntactic complexity hold sentence length constant (Friederici et al., 2003; Just et al., 1996; Keller et al., 2001). In this study we included the sentence length manipulation as a comparison condition for syntactic complexity to determine whether sentence length, in and of itself, would be associated with increased activations in superior and middle temporal lobes and/or parietal and frontal regions, relating to increased processing or memory requirements. We found long sentences increased activation only within the temporal lobe, probably due to increased auditory, linguistic and semantic processing. We found no overlap between areas associated with length and areas associated with complexity. Wernicke’s area showed increased activation in response to complex versus easy sentences, suggesting that irrespective of individual differences in strategy that likely arise from this multimodal paradigm, the left posterior superior temporal lobe is important for the comprehension of syntactically complex sentences in children. Along with Wernicke’s area, a right hemisphere homologue showed a significant effect of sentence complexity at the group level, highlighting that language comprehension in our task required both hemispheres to dynamically adapt to the stimuli.
We found significant negative associations between age and responses to task difficulty in the right fusiform gyrus and right middle frontal gyrus, even though we did not find age effects in the analyses of behavioral data in the laboratory or in the scanner. Age effects on patterns of activation in sentence or narrative processing have been found in some small studies (Booth et al., 2000; Karunanayaka et al., 2007; Schmithorst, Holland, & Plante, 2007; Szaflarski et al., 2006) though none have specifically examined the relationship between age and adaptability. Our results suggest that as the task demands increase younger children rely more heavily on the fusiform gyrus and middle frontal gyrus to handle the increased difficulty. Increased responses in the fusiform gyrus could indicate that younger children in comparison to older children rely more heavily on visual and semantic information for comprehension as the sentences become more difficult (Schmithorst, Holland, Plante et al., 2007). This interpretation is consistent with Booth et al (2000) in which increased activation of visual association areas in children compare to adults was attributed to children relying more heavily on visualization to comprehend language. Given that task performance was not associated with age, the negative association between age and adaptability appears to reflect that the alternative strategy that younger children use is not incompatible with accurate comprehension.
In this sentence comprehension paradigm, we found that two of the measures—PPVT-III and reaction time on TROG-2--were associated with change in the extent of activation for hard versus easy sentences. The regions where changes were related to receptive language abilities included a left hemisphere perisylvian and a right hemisphere deep gray matter area. Processing speed was negatively associated with changes in response to task difficulty in the anterior cingulate and medial frontal gyrus. These were not regions identified in the general task-related maps probably because the participants with relatively weaker language abilities were not activating them differentially in comprehending hard versus easy sentences. Interestingly, these frontal lobe regions are often associated with memory, error monitoring and attention (Badre & Wagner, 2002, 2007; Love et al., 2006; Rushworth, Walton, Kennerley, & Bannerman, 2004; van Veen & Carter, 2002). Processing speed was positively associated changes in response to task difficulty in the right hemisphere, including right inferior frontal gyrus (BA44, 45). This result is analogous with findings in the reading literature; poor readers show greater activation in right hemisphere BA 44, 45 than do good readers (Simos et al., 2002) Thus, slow language processors, like poor readers, may be recruiting superfluous, suboptimal or compensatory regions in response to increasing task difficulty (Shaywitz & Shaywitz, 2008). We recognize that the associations between skill level and activation patterns do not necessarily establish a cause-effect relationship; both processes may be related to another underlying process. To elucidate the underlying mechanisms that explain individual differences in children’s language abilities it will be important to measure variations in the neural response to difficulty on a variety of linguistic tasks using ROIs defined on individual brains.
This study was limited by its small sample size and the restricted range of cognitive abilities and relatively high socioeconomic status of the participants. Because we were unable to use corrected p-values these exploratory results should be interpreted cautiously. We plan to attempt to confirm these findings in a larger sample of typically developing children with a greater range of cognitive and language abilities. However, the implications of this study provide an intriguing account of the relationship between individual differences in brain activity and individual differences in receptive language abilities. Based on these findings, future studies employing individual brain analysis methods and larger samples will allow for the investigation of the specific brain regions in which neural responses to task difficulty is related to language skills.
In summary, we have shown that sentence comprehension in children and adolescents is served by a dynamic and distributed network that varies as a function of stimulus demands, receptive language abilities and age. In future work we will extend the methods to include clinical populations, particularly children with language disorders, to determine the degree of network adaptability and sources of individual variation. We also recognize that a dynamic network must rely on rapid communication among brain regions. Though we found bilateral activations, we do not know to what extent the left and right hemisphere are functioning in parallel and to what extent information is being sent from one hemisphere to the other. In the future, we will study children with white matter lesions to begin to address this issue. Finally, we are intrigued to learn if the sentence comprehension network changes as a function of education or interventions for children with poor receptive language skills.
Acknowledgments
The authors gratefully acknowledge Dorothy V. M. Bishop, PhD for providing us with pre-publication copies of pictures from TROG-2 from which stimuli for the scanning task were derived. We also acknowledge Eliana Lee for assistance in data collection; Ben Hutchinson for analytic scripts; and Irene M. Loe, Lynne C. Huffman, Eliana Lee, and James L. McClelland for helpful comments on earlier versions. This work was supported by a grant from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, RO1 HD046500 to Heidi M. Feldman and by a grant from National Institutes of Health NCRR, P41RR09784 Center for Advanced MR Techniques at Stanford to Gary H Glover.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason D. Yeatman, Email: jyeatman@stanford.edu.
Michal Ben-Shachar, Email: michal.benshachar@gmail.com.
Gary H. Glover, Email: gary.glover@stanford.edu.
Heidi M. Feldman, Email: hfeldman@stanford.edu.
References
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behav Cogn Neurosci Rev. 2002;1(3):206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Contrast responsivity in MT+ correlates with phonological awareness and reading measures in children. Neuroimage. 2007;37(4):1396–1406. doi: 10.1016/j.neuroimage.2007.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar M, Hendler T, Kahn I, Ben-Bashat D, Grodzinsky Y. The neural reality of syntactic transformations: evidence from functional magnetic resonance imaging. Psychological Science. 2003;14(5):433–440. doi: 10.1111/1467-9280.01459. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Palti D, Grodzinsky Y. Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage. 2004;21(4):1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Comprehension of spoken, written and signed sentences in childhood language disorders. Journal of Child Psychology & Psychiatry & Allied Disciplines. 1982;23(1):1–20. doi: 10.1111/j.1469-7610.1982.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Test for Reception of Grammar (TROG-2) Oxford, United Kingdom: Pearson Assessment; 2003. [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Developmental Neuropsychology. 2000;18(2):139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- Brauer J, Friederici AD. Functional neural networks of semantic and syntactic processes in the developing brain. Journal of Cognitive Neuroscience. 2007;19(10):1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberg G, West C, Waters G, Greve D, et al. Vascular responses to syntactic processing: event-related fMRI study of relative clauses. Human Brain Mapping. 2002;15(1):26–38. doi: 10.1002/hbm.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J, Hollis C, Mawhood L, Rutter M. Developmental language disorders--a follow-up in later adult life. Cognitive, language and psychosocial outcomes. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2005;46(2):128–149. doi: 10.1111/j.1469-7610.2004.00342.x. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test, Third Edition. San Antonio, TX: Pearson Assessments; 1997. [Google Scholar]
- Evans JL. SLI subgroups: Interaction between discourse constraints and morphosyntactic deficits. Journal of Speech & Hearing Research. 1996 Jun;39(3):655–660. doi: 10.1044/jshr.3903.655. [DOI] [PubMed] [Google Scholar]
- Evans JL. Variability in comprehension strategy use in children with SLI: a dynamical systems account. International Journal of Language & Communication Disorders. 2002;37(2):95–116. doi: 10.1080/13682820110116767. [DOI] [PubMed] [Google Scholar]
- Evans JL, MacWhinney B. Sentence processing strategies in children with expressive and expressive-receptive specific language impairments. International Journal of Language & Communication Disorders. 1999;34(2):117–134. doi: 10.1080/136828299247469. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca’s area in sentence processing: syntactic integration versus syntactic working memory. Human Brain Mapping. 2005;24(2):79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cerebral Cortex. 2003;13(2):170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friedmann N, Novogrodsky R. The acquisition of relative clause comprehension in Hebrew: a study of SLI and normal development. Journal of Child Language. 2004;31(3):661–681. doi: 10.1017/s0305000904006269. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Hoen M, Pachot-Clouard M, Segebarth C, Dominey PF. When Broca experiences the Janus syndrome: an ER-fMRI study comparing sentence comprehension and cognitive sequence processing. Cortex. 2006;42(4):605–623. doi: 10.1016/s0010-9452(08)70398-8. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36(3):924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter P, Keller T, Eddy W, Thulborn K. Brain Activation Modulated by Sentence Comprehension. Science. 1996;274(5284):114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Just MA, Varma S. The organization of thinking: what functional brain imaging reveals about the neuroarchitecture of complex cognition. Cognitive, Affective & Behavioral Neuroscience. 2007;7(3):153–191. doi: 10.3758/cabn.7.3.153. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends in Cognitive Sciences. 2002;6(8):350–356. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, Szaflarski JP, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34(1):349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: a fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11(3):223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Kherif F, Josse G, Seghier ML, Price CJ, Kherif F, Josse G, et al. The main sources of intersubject variability in neuronal activation for reading aloud. Journal of Cognitive Neuroscience. 2009;21(4):654–668. doi: 10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magnetic Resonance in Medicine. 2002;48(4):715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Law J, Tomblin JB, Zhang X. Characterizing the growth trajectories of language-impaired children between 7 and 11 years of age. Journal of Speech Language & Hearing Research. 2008;51(3):739–749. doi: 10.1044/1092-4388(2008/052). [DOI] [PubMed] [Google Scholar]
- Love T, Haist F, Nicol J, Swinney D, Love T, Haist F, et al. A functional neuroimaging investigation of the roles of structural complexity and task-demand during auditory sentence processing. Cortex. 2006;42(4):577–590. doi: 10.1016/s0010-9452(08)70396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K. Spatiotemporal dynamics of word processing in the human cortex. Neuroscientist. 2004;10(2):142–152. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain & Language. 2004;91(3):315–325. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Kane MJ, Whalen DH, Schneider KC, Katz KH, et al. A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics. 2002;110(6):1153–1162. doi: 10.1542/peds.110.6.1153. [DOI] [PubMed] [Google Scholar]
- Prat CS, Keller TA, Just MA. Individual differences in sentence comprehension: a functional magnetic resonance imaging investigation of syntactic and lexical processing demands. Journal of Cognitive Neuroscience. 2007;19(12):1950–1963. doi: 10.1162/jocn.2007.19.12.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle ED, Carpenter PA, Just MA. The neural bases of strategy and skill in sentence-picture verification. Cognitive Psychology. 2000;40(4):261–295. doi: 10.1006/cogp.2000.0733. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8(9):410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Sex differences in the development of neuroanatomical functional connectivity underlying intelligence found using Bayesian connectivity analysis. Neuroimage. 2007;35(1):406–419. doi: 10.1016/j.neuroimage.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2006;29(1):254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Development of effective connectivity for narrative comprehension in children. Neuroreport. 2007;18(14):1411–1415. doi: 10.1097/WNR.0b013e3282e9a4ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E, Schmithorst VJ, Holland SK, Plante E. Object identification and lexical/semantic access in children: a functional magnetic resonance imaging study of word-picture matching. Human Brain Mapping. 2007;28(10):1060–1074. doi: 10.1002/hbm.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals® - Fourth Edition (CELF® - 4) San Antonio, TX: 2005. [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: The neurobiology of reading and dyslexia. Development and Psychopathology. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, et al. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58(8):1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Annals of Neurology. 2006;59(5):796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) manual. San Antonio TX: The Psychological Corporation; 1999. [Google Scholar]
- Tomblin JB, Zhang X. The dimensionality of language ability in school-age children. Journal of Speech Language & Hearing Research. 2006;49(6):1193–1208. doi: 10.1044/1092-4388(2006/086). [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Watanabe J, Iwata K, Ikuta N, Haji T, Usui N, et al. Is Broca’s area involved in the processing of passive sentences? An event-related fMRI study. Neuropsychologia. 2007;45(5):989–996. doi: 10.1016/j.neuropsychologia.2006.09.003. [DOI] [PubMed] [Google Scholar]