Abstract
Aging is becoming a critical heath care issue and a burgeoning economic burden on society. Mechanotransduction is the ability of the cell to sense, process, and respond to mechanical stimuli and is an important regulator of physiologic function that has been found to play a role in regulating gene expression, protein synthesis, cell differentiation, tissue growth, and most recently, the pathophysiology of disease. Here we will review some of the recent findings of this field and attempt, where possible, to present changes in mechanotransduction that are associated with the aging process in several selected physiological systems, including musculoskeletal, cardiovascular, neuronal, respiratory systems and skin.
Keywords: Aging, Mechanotransduction, Musculoskeletal, Cardiovascular, Neuronal, Respiratory system, Skin
1. Introduction
The aged population now comprises the fastest growing segment of people living in the United States. Living longer is associated with higher morbidity. Indeed, health care spending per capita for the elderly population is more than three and five times higher than that of a working-age person and child, respectively (Hartman et al., 2008). It is thought that mechanical forces are a primary regulator of several biological functions. Indeed, mechanical signals have been shown to mediate the development of a variety of tissues (e.g. skeletal muscle, bone, cartilage, blood vessels and heart), and can affect diverse cellular processes including cell growth, differentiation, cellular migration, gene expression, protein synthesis, and apoptosis (Alenghat and Ingber, 2002; Ingber, 2003a). Given the potential importance that mechanical signaling functions in maintaining cellular homeostasis it is not surprising that changes in mechanotransduction may also play a role in the pathophysiology of disease (Ingber, 2003a). Recent data strongly supports this notion as it is becoming recognized that many aspects of sarcopenia, cardiovascular and respiratory disease may be related to alteration in cellular mechanotransduction (Blough and Linderman, 2000; Rice et al., 2007b; Pardo et al., 2008; Hwee and Bodine, 2009a). The study of age-associated alterations in cellular mechanotransduction has only recently begun to be appreciated for its potential role in mediating cellular function and dysfunction. Here we will investigate the possibility that the ability of the cell to sense, process, and respond to mechanical stimuli is altered with aging and that these changes may be involved in the etiology of aging-associated disease. In an order to focus our discussion, we have chosen to examine how aging may affect mechanotransduction processes in: 1. musculoskeletal system (including skeletal muscle, bone and joint), 2. cardiovascular system, 3. neuronal system, 4. respiratory system, and 5. skin.
2. Mechanotransduction processes
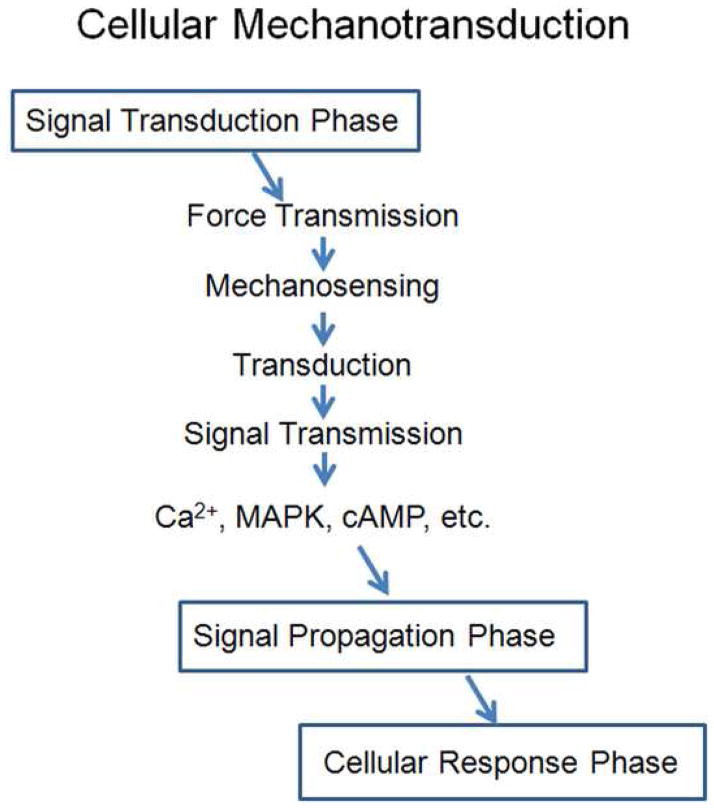
The process of converting an external mechanical force into an internal cellular response is termed mechanotransduction (Wang et al., 1993; Davies, 1995; Alenghat and Ingber, 2002; Jaalouk and Lammerding, 2009). This process consists of three distinct phases 1) signal transduction, 2) signal propagation, and 3) cellular response (Figure 1). Mechanical forces exerted on cell/tissue can result in alterations of cell membrane tension. The integration of external forces into a biochemical signaling event involves four distinct elements: force transmission, mechanosensing, transduction and signal transmission (Figure 1). Force transmission is the result of various molecular components working together to transmit external loading onto or across the cell membrane. Cells in a differentiated, quiescent tissue have several types of external complexes (e.g. integrins, costameres, desmasomes, etc.) that function to position the cell in a specific orientation within the tissue architecture and act to set a “baseline” level of cell tension. This concept is called tensegrity and was first popularized by Ingber and his colleagues at Harvard Medical School during the early 1990s (Ingber, 1991, 1993; Wang et al., 1993). Although beyond the scope of the present review, several excellent reviews on this topic exist (Ingber, 1991, 1993, 1997, 2003b, 2003c, 2008). Changes in this baseline tension result in force transmission to the cellular membrane. Mechanosensing occurs as the cell realizes these external forces. This event sets the stage for the third and fourth elements of the signal transduction phase to occur (Figure 1). These latter processes of transduction and signal transmission are poorly understood, but are likely to include the participation of several proteins and protein complexes including the cytoskeletal elements (e.g. F-actin, intermediate filament, and microtubules), mechanically-activated ion channels, focal adhesions, integrins, adhesion receptors, ATP release, autocrine factor release, and G-protein coupled mechanoreceptors (Ingber, 2006; Geiger et al., 2009; Jaalouk and Lammerding, 2009). Because of the complexity of cell loading stimuli, the diversity of potential cellular transducers involved, and the potential interplay between different elements the initial phase of the mechanotransductive response is extremely complicated and quite difficult to mimic in the laboratory. Similarly, the contextual importance of physical contact between different structural hierarchies makes it nearly impossible to fully represent all of the critical features of the mechanoregulatory response seen in living tissues. As a result of these difficulties, very little research has been done in the area of age-related mechanotransduction. Indeed, a review of the literature demonstrates that a great proportion of the work done in this field has been focused on the signal propagation phase.
Figure 1.
A simplified schematic of the mechanotransduction processes. The mechanotransductive responses consist of three distinct phases: signal transduction, signal propagation, and cellular response.
3. Effect of aging on skeletal muscle mechanotransduction
3.1. Aging is associated with declines in skeletal muscle mass and function
The age-related loss of skeletal muscle mass and muscle strength, known as sarcopenia, is a health problem that is expected to only increase in coming years as a greater portion of the population lives longer (Castillo et al., 2003; Dirks et al., 2006; Melov et al., 2007). The maintenance of muscle mass and function with increasing age is directly related to quality of life, disease prevention, and has profound socioeconomic significance. Sarcopenia is characterized by a loss of skeletal muscle mass that is greater in proportion than losses in fat-free mass. These alterations typically result in an increase in body fat content (Kyle et al., 2001). It is thought that the magnitude of these changes accelerate with aging (Kyle et al., 2001; Castillo et al., 2003; Lushaj et al., 2008). In humans in their eighth decade of life or older, lean body mass is significantly less than those age 70 to 79 (Kyle et al., 2001), and clinical studies have indicated that the prevalence of sarcopenia dramatically increases by more than 4-fold from ages 70–75 to 85 and older (Castillo et al., 2003). The loss of muscle mass is highly correlated to an age-related decrease in muscle strength. Population-based studies have demonstrated that sarcopenia is associated with decreases in balance, increased muscle weakness and fatigue, an increased incidence of falling and fracture, and a higher prevalence of disability (Castillo et al., 2003). Similar muscle-related changes have been observed in aged animals. For example, recent studies have shown that the Fischer 344/NNIaHSD × Brown Norway/BiNia (F344BN) rat accelerates age-associated decreases in muscle mass, muscle cross-sectional area, myosin and actin expression, and diminished muscle function after 30 months of age (Rice et al., 2005c; Lushaj et al., 2008; Wu et al., 2009b).
3.2. Aging decreases the ability of load-induced growth in skeletal muscle
Mechanical loading and contractile activity play a critical role in the regulation of muscle mass and altered loading can lead to structural remodeling of muscle. Although human and animal studies have demonstrated that aged muscles maintain the ability to undergo hypertrophy, the capacity to sense and subsequently respond to the mechanical stimuli is diminished (Carson et al., 1995; Blough and Linderman, 2000; Pehme et al., 2004; Kosek and Bamman, 2008; Hwee and Bodine, 2009b). In addition it is thought that the aged muscle cell membrane is also more prone to mechanically-induced damage (Rice et al., 2006). Whether these changes are due to alteration in the muscle dystrophin-associated glycoprotein complex (DGC) or change to other structures are currently unknown. Nevertheless, understanding the mechanism(s) of impaired mechanotransduction in aging muscle is likely to shed light on strategies designed to prevent and treat aging-associated skeletal muscle dysfunction. Here we will review the primary intramuscular signaling cascades thought to regulate muscle mass and comment, where possible, on how aging may affect these pathways (Figure 2).
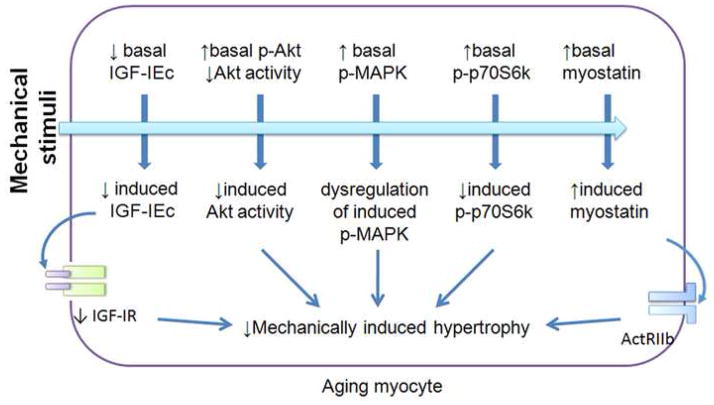
Figure 2.
Dysregulation of mechanotransduction in aging skeletal muscle. At rest the basal expression of mechano growth factor (MGF or IGF-IEc) and IGF-I receptor (IGF-IR) are decreased, while myostatin expression is increased. With aging, the basal phosphorylation of MAPK, Akt and p70S6k are higher, while Akt activity may be decreased. These alterations are thought to impair the ability of aged skeletal muscle to sense and respond to the mechanical stimuli, which may be associated with decreases in mechanically-induced hypertrophy. ActRIIb: activin receptor IIb.
3.3. Aging impairs the ability of skeletal muscle to activate Akt, mammalian target of rapamycin (mTOR) and ribosomal S6 kinase (p70S6k) signaling
Protein kinase B (Akt/PKB) is a serine/threonine protein kinase that plays a central role in integrating anabolic and catabolic responses. The Akt signaling pathway transduces signals emanating from growth factors, nutrients, cytokines and mechanical stimuli via changes in phosphorylation (Bodine et al., 2001; Funai et al., 2006). Activation of Akt stimulates protein synthesis, muscle hypertrophy and cell survival while it antagonizes muscle protein loss (Bodine et al., 2001; Funai et al., 2006; Miyazaki et al., 2008; Hwee and Bodine, 2009b). Akt is highly mechanosensitive, and its activity is thought to be mediated, at least in part, by the phosphatidylinositol-3-kinase (PI3K) (Xiong et al., 2009). It has been demonstrated that muscle loading increases Akt phosphorylation (activation) while unloading decreases Akt phosphorylation (Miyazaki et al., 2008; Hwee and Bodine, 2009b). Importantly, these alterations in Akt activation have been shown to be highly correlated with the addition (Hwee and Bodine, 2009b) or loss (Miyazaki et al., 2008) of muscle mass. Animal studies have demonstrated that the ability of mechanical loading to induce Akt signaling may become altered with age. For example, Hwee and Funai each demonstrated an age-associated uncoupling of Akt phosphorylation and the amount of Akt substrate phosphorylation following the increased muscle loading (Hwee and Bodine, 2009b) or a bout of electrically stimulated muscle contraction (Funai et al., 2006). These data are consistent with the work from our laboratory showing that aged skeletal muscle exhibits a mismatch between Akt kinase activity and the degree of Akt phosphorylation at Ser473 and Thr308 (Wu et al., 2009b). This impairment in Akt kinase activity is, in turn, associated with diminished insulin responsiveness, as higher levels of Akt phosphorylation (Ser474 and Thr308) were shown to be required to phosphorylate the Akt substrates glycogen synthase kinase (GSK)-3α and GSK3β (Wu et al., 2009b). Given the importance of Akt function in regulating muscle mass, it is likely that these changes in the ability of Akt to elicit downstream signaling could contribute to age-associated decrease in muscle mass. Similar to Akt, the mTOR signaling pathway has been shown to be involved in the regulation of load-induced skeletal muscle growth both in vitro and in vivo (Parkington et al., 2004; Hornberger and Chien, 2006). Up to a few years ago it was widely suggested that mTOR signaling was dependent, at least in part, on the activation of Akt (Bodine et al., 2001). However, recent studies using transgenic mice expressing a mutated Akt and pharmacological inhibitors specific to PI3K have demonstrated that the mechanical activation of mTOR can occur independently of the PI3K/Akt pathway (Hornberger et al., 2004). It is thought that the mechanical regulation of mTOR is mediated by activation of phospholipase D (PLD) (Hornberger et al., 2006). The regulation of PLD by mechanical stimuli is suggested by its localization at the Z-band (Hornberger et al., 2006), a site within the sarcomere that is particularly suited for the transmission of force (Goldstein et al., 1987). The PLD catalyzes the hydrolysis of phosphatidylcholine to form the lipid second messenger phosphatidic acid (PA) (Hornberger et al., 2006; Hornberger et al., 2007). Attesting to the role of mTOR in muscle growth, studies have demonstrated that mTOR inhibition by rapamycin can directly inhibit the load-induced activation of mTOR signaling and prevent mechanically-induced protein synthesis and muscle growth (Hornberger et al., 2004; Hornberger et al., 2006). In addition, it has also been demonstrated that PA can compete with rapamycin for binding to the FKBP-rapamycin-binding (FRB) domain of mTOR, and hence activate mTOR signaling (Hornberger et al., 2006; Hornberger et al., 2007). Mechanically-activated mTOR can further induce phosphorylation (activation) of ribosomal S6 kinase (p70S6k) (Morris et al., 2004; Parkington et al., 2004; Hornberger et al., 2007). Similar to that observed for Akt, aging has also been shown to negatively impact the ability of skeletal muscle to activate mTOR/p70S6k signaling. Indeed, Morris and others (2004), using a model of hindlimb suspension followed by muscle reloading, have clearly shown that the load-induced phosphorylation of p70S6k is lower in aged animals compared to adults (Morris et al., 2004). Interestingly, several reports have suggested that the basal phosphorylation of p70S6k is increased in older muscle (Parkington et al., 2004; Kinnard et al., 2005) and that this occurs at an age where muscle mass is actually decreasing. Although not clearly defined, this apparent contradiction may imply that p70S6k activity may become uncoupled from downstream ‘p70S6k signaling’. The existence of such dysfunction, if present, may contribute to the diminished capacity for mechanical stimuli to induce muscle hypertrophy with aging.
3.4. Load induced mitogen-activated protein kinases signaling may be impaired in aging muscle
The mitogen activated protein kinases (MAPK) are serine/threonine protein kinases that respond to growth factors, environmental stressors and inflammatory cytokines such as TNF-α, IL-1 and IL- 6 (Goodyear et al., 1996; Wretman et al., 2001; Zhan et al., 2007). MAPK signaling is involved in regulating gene expression, cellular metabolism, cell growth, and cell differentiation. In addition, the inappropriate activation of MAPK signaling can trigger inflammation, protein degradation and cellular apoptosis (Wretman et al., 2001; Keren et al., 2006; Wu et al., 2009a). MAPK proteins such as the extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK) and p38 MAPK are mechanically sensitive (Goodyear et al., 1996; Aronson et al., 1997; Williamson et al., 2003; Mylabathula et al., 2006). In muscle, the activation of MAPK have been shown to be involved in the regulation of glucose transport, gene expression, and muscle hypertrophy (Sakamoto and Goodyear, 2002; Gibala et al., 2009; Wu et al., 2009a). It is thought that the Ras protein plays an important role in the mechanical regulation of MAPK activation. Mechanical stimuli have been shown to activate Ras, which in turn stimulates the phosphorylation of Raf-1. This event is thought to lead to the activation of MAPK kinase (MEK) cascades resulting in phosphorylation of MAPK proteins (Lange-Carter et al., 1993; Aronson et al., 1997; Barton, 2006). In addition to the direct activation of MAPK proteins by stretch, muscle contraction can also induce MAPK phosphorylation by stimulating the release of the inflammatory cytokine TNF-α (Zhan et al., 2007) or through metabolic alterations such as increased reactive oxygen species (ROS) and acidosis (Wretman et al., 2001).
The effects of aging on the mechanical regulation of MAPK phosphorylation in skeletal muscle (Williamson et al., 2003; Parkington et al., 2004; Hornberger et al., 2005; Mylabathula et al., 2006; Kosek and Bamman, 2008; Ljubicic and Hood, 2009) appear to be MAPK isoform- and fiber type-specific (Table 1). Whether the differences observed between studies is due to differences in the age of the animals studied, experimental protocol or other factors remains to be determined. Nonetheless, compared to that observed in the young/adult animal, the basal phosphorylation of MAPK is higher in aged muscle from both human and animals (Williamson et al., 2003; Mylabathula et al., 2006; Wu et al., 2009a). The increase in MAPK basal phosphorylation appears to be associated with age-associated increases in oxidative stress (Hollander et al., 2000; Ji, 2002; Wu et al., 2009a), as interventions aimed to diminish ROS can effectively normalize age-associated MAPK hyper-phosphorylation (Wu et al., 2009a). It is possible that age-related muscle “stress” may impair the ability of aging skeletal muscle to sense and adapt to mechanical stimuli, resulting in dysregulation of the MAPK signaling pathways. Such changes, if present, could blunt anabolism and limited hypertrophic adaptation (Jozsi et al., 2000; Williamson et al., 2003; Kosek and Bamman, 2008). Additional studies to investigate this possibility will no doubt be useful in increasing our understanding of how aging may affect the ability of mechanical loading to activate MAPK signaling.
Table 1.
Response of MAPK phosphorylation to mechanical stimuli in aging skeletal muscle.
| isoform | Tissue(s) | Stimulation | Aged (vs. young) | Reference |
|---|---|---|---|---|
| p38 | Red TA | Electrical | Similar | (Ljubicic and Hood, 2009) |
| EDL | Stretch | Similar | (Hornberger et al., 2005) | |
| EDL | Stretch | Similar | (Mylabathula et al., 2006) | |
| Soleus | Stretch | Lower | (Mylabathula et al., 2006) | |
| VL | Exercise | Lower | (Williamson et al., 2003) | |
| VL | Resistance training | Higher | (Kosek and Bamman, 2008) | |
| JNK | EDL | Stretch | Similar | (Hornberger et al., 2005) |
| EDL | Stretch | Lower | (Mylabathula et al., 2006) | |
| Soleus | Stretch | Similar | (Mylabathula et al., 2006) | |
| VL | Exercise | Lower | (Williamson et al., 2003) | |
| ERK | EDL | Stretch | Higher | (Mylabathula et al., 2006) |
| Soleus | Stretch | Higher | (Mylabathula et al., 2006) | |
| VL | Exercise | Higher | (Williamson et al., 2003) | |
| VL | Resistance training | Similar | (Kosek and Bamman, 2008) | |
| TA | Electrical | Lower | (Parkington et al., 2004) | |
| Plantaris | Electrical | Lower | (Parkington et al., 2004) | |
TA: tibialis anterior; EDL: Extensor digitorum longus; VL: vastus lateralis.
3.5. Alteration of mechano growth factor (MGF) and its signaling pathway in aged skeletal muscle
Insulin-like growth factor-I (IGF-I) is an important regulator with anabolic properties for somatic growth and cellular metabolism. In skeletal muscle, at least two different IGF-I isoforms are expressed due to alternative splicing of the primary IGF-I transcript (Owino et al., 2001; Hameed et al., 2003; Cheema et al., 2005; Goldspink, 2007). Of these isoforms, one is quite similar to liver-derived IGF-I (IGF-IEa) and is important for the fusion of myoblasts to form myotubes (Owino et al., 2001; Hameed et al., 2003; Goldspink, 2004; Cheema et al., 2005; Goldspink, 2007). Expression of the other isoform, also known as mechano growth factor (MGF or IGF-IEc), is induced in response to physical activity. It is thought that MGF is required for secondary myotube formation and the activation of muscle satellite cells for local muscle repair. MGF is also likely to be involved in load-induced muscle hypertrophy (Owino et al., 2001; Hameed et al., 2003; Goldspink, 2004; Cheema et al., 2005; Goldspink, 2007). With aging, the basal level of MGF is decreased (Owino et al., 2001; Hameed et al., 2003). Although aged muscle retains the capacity to induce MGF expression in response to mechanical stimuli, the magnitude of load-induced MFG expression has shown to be diminished by about 50% of that seen in adult animals exposed to a similar degree of overload (Owino et al., 2001). Moreover, aged muscle has also been shown to exhibit a diminished ability to up-regulate expression of the IGF-I receptor following increased loading (Owino et al., 2001). Taken together, these data suggest that alterations in load-induced MGF expression or impairments in the MGF receptor signaling pathway may contribute to the attenuation of muscle growth and repair in aged subjects.
3.6. Myostatin expression is increased in aging muscle
Myostatin is a key negative regulator of the skeletal muscle growth (Grobet et al., 1997; McPherron and Lee, 1997). Myostatin gene inactivation and knockout studies have shown that mutated myostatin protein or reduced myostatin expression are coupled to marked increases in skeletal muscle mass (Grobet et al., 1997; McPherron and Lee, 1997). It is believed that myostatin signaling participates in the regulation of age-associated muscle atrophy as both serum myostatin and muscle myostatin expression are increased with age (Kawada et al., 2001; Yarasheski et al., 2002). These age-associated increases in myostatin are in turn associated with decreases in fat-free mass and diminished muscle mass (Kawada et al., 2001; Yarasheski et al., 2002). The expression of myostatin can be mechanically regulated with increased loading resulting in decreased myostatin expression (Kawada et al., 2001). Interestingly, it has been reported that inhibition of myostatin by PF-354 enhances aerobic performance in aged mice (Lebrasseur et al., 2009). This increase in muscle endurance was associated with decreased phosphorylation/activation of myostatin downstream molecule Smad3 (Lebrasseur et al., 2009) and impaired Smad4 translocation to the nucleus (Joulia-Ekaza and Cabello, 2007). Therefore, manipulation of myostatin signaling may be a useful strategy to ameliorate the aging-related defects in muscle mechanotransduction and muscle growth.
4. Effect of aging on bone mechanotransduction
4.1. Effects of aging on bone structure
Bone health and structure, like muscle, is regulated by mechanical loading. During growth, body weight and muscular forces increase the loading on the growing bone which in turn acts to augment bone strength and mass. In the third decade of life muscle strength generally begins to decrease which is associated with gradual decline in bone loading and bone mass (Sumner and Andriacchi, 1996). In addition to changes in bone loading, non-mechanical factors are also thought to contribute to age-related bone loss. Left unchecked, bone loss can approach decreases of up to 70% by the age of 70 (Frost, 1997).
The factors controlling bone mass are not fully understood. It is thought osteoblasts function to construct bone tissue while osteoclasts maintain mineral homeostasis by reabsorbing bone (Zaidi, 2007). Working together, these two cell types act to maintain the balance between bone deposition and resorption. Nonetheless, as we age the number of osteoblast cells tend to decrease, impairing the natural processes of bone tissue renovation and a reduction in bone mass (D’Ippolito et al., 1999).
4.2. Mechanisms of load-induced signal transduction in bone
Calcium influx is considered to be one of the primary responses to changes in osteoblast and osteocyte loading (Mikuni-Takagaki, 1999; Chen et al., 2000; Ryder and Duncan, 2001; You et al., 2001). Although not well understood, it is thought that Ca2+ influx is regulated, at least in part, by fluid flow through the lacuno-canicular system. Attesting to this possibility, fluid flow has been shown to increase intracellular calcium in cultured osteoblastic cells and this response can be suppressed by gadolinium, a stretch-activated calcium channel blocker (Hung et al., 1996). In addition, voltage gated L-type calcium channels have also been show to play a role in bone mechanotransduction as changes in gene expression following calcium influx can be suppressed by incubating the cells with the L-type calcium channel blockers verapamil and nefedipine (Rawlinson et al., 1996; Li et al., 2002b; Li et al., 2003). Mechanical stimulation can also lead to the release of intracellular stores of calcium through a process that appears to be modulated by inositol 1, 4, 5-triphosphate (IP3) (Chen et al., 2000; You et al., 2001). Increased intracellular calcium levels in turn have been linked to the activation of the MAPK signaling pathway and an increased expression of osteopontin and other matrix proteins (You et al., 2001). In addition to the activation of calcium channels, fluid flow and mechanical loading has also been shown to result in the recruitment of integrins to focal adhesions and modulate fluid-flow induced gene expression (Pavalko et al., 1998). How focal adhesions regulate load-induced bone growth is not fully understood.
It is likely that paracrine and autocrine signaling may also contribute to the ability of bone to respond to mechanical signals. For example, recent reports have shown that certain non-steroidal anti-inflammatory drugs can suppress mechanically-induced bone formation by blocking the syntheses of prostaglandins (Forwood, 1996; Chow et al., 1998; Li et al., 2002a). Other data has suggested that skeletal remodeling can also be mediated through parathyroid hormone (PTH), the 1–34 PTH fragment, estrogen, and insulin-like growth factors (Sato et al., 2000; Zaman et al., 2000; Gross et al., 2002; Bakker et al., 2003; Li et al., 2003). To date, the molecular mechanisms that regulate bone mechanotransduction have not been fully elucidated. More research perhaps using cultured cells and molecular approaches will be invaluable to further our understanding how bone “health” is regulated by loading.
4.3. The effects of aging on bone mechanotransduction are poorly understood
In the mature bone, a third basic cell type, the osteocyte begins to emerge. As we age, osteocytes soon become the most abundant cell type. Osteocytes are mechanically sensitive and are able to initiate signaling processes in response to changes in sheer stress and pressure (Knothe Tate et al., 2004). In addition, osteocytes may help to anchor osteoclasts to the extracellular matrix (ECM) by producing osteopontins (Zaidi, 2007). Whether or how aging affects the function of osteoclasts is not well understood. Research directly examining how aging affects the response of bone cells to various stimuli is almost entirely lacking. Donahue et al (2001) and Cao et al (2007) are amongst a small number of researchers that have examined mechanotransduction in aging bone. Donahue and colleagues demonstrated that aging is associated with diminished calcium influx in osteoblasts following increased loading induced by fluid flow (Donahue et al., 2001). Similarly, Cao and co-workers have demonstrated that aged osteoblasts exhibit a reduced ability for IGF-I to stimulate osteoprogenitor formation (Cao et al., 2007). Further understanding these age-associated changes may provide better insight into developing pharmacological agents for the treatment of age related-bone disease.
5. Effect of aging on joint mechanotransduction
5.1. Effects of aging on joint structure
Joints can be classified into three basic groups based upon how the bones within the joint are connected: fibrous, cartilaginous, and synovial (Benjamin et al., 1995; Khan et al., 2007). It is well accepted that mechanical loading can influence joint structure and function. These alterations can occur during development and aging. For example, the fibrous suture joints in the skull are eventually replaced by bone in early childhood (Mao, 2002). Similarly, increased loading over a lifetime can also result in deleterious changes such as the development of arthritis which is commonly seen during the 5th and 6th decades of life.
5.2. Mechanisms of load-induced signal transduction in joints
Fibrous joints represent a group of joints in which the adjacent bones are joined with fibrous connective tissue (Mao, 2002). These joints can be further divided into three types; sutures, found in the scull, the syndesmosis type which is found between long bones such as the radius and ulna, and the gomphosis type such as that found in the tooth sockets. To our knowledge, little is known regarding the effects of aging on fibrous joint mechanotransduction. Nonetheless, recent data has demonstrated that this joint exhibits mechanosensitivity. For example, the expression of fibroblast growth factor-2 (FGF-2) is increased following the application of tensile stress to the rat coronal suture (Yu et al., 2001). Similarly, osteocalcin, collagen I and alkaline phosphatase gene expression is increased following mechanically induced rat tooth movement (Pavlin et al., 2001). Examining the cranial suture, Ikegame and others showed that increased tensile stresses induced the up-regulation of BMP-4 gene expression and Cbfa1/Osf-2, an osteoblast-specific transcription factor (Ikegame et al., 2001). Like other tissues, the up-regulation of genes and transcription factors in sutures is often accompanied by increased protein synthesis. Indeed, increased suture stress has been found to increase the synthesis of type III collagen (Meikle et al., 1984; Tanaka et al., 2000) and alkaline phosphatase activity (Miyawaki and Forbes, 1987).
Cartilaginous joints represent a group of joints in which the bones are joined with cartilage. Cartilaginous joints are found in the intervertebral discs of the spinal column and in growth regions of the long bones. Although mechanical factors have been posited to be involved in the pathology of the intervertebral disc little information exists regarding the mechanism(s) of mechanotransduction or whether aging affects this process (Hadjipavlou et al., 2008). Likewise, limited research exists regarding how mechanotransduction may take place at the growth plate and if this process may play a role in long bone development and health.
Synovial joints represent a group of joints in which the adjacent bones are not directly joined. These joints consist of an articular surface that is covered with hyaline cartilage (Silver and Bradica, 2002; Pacifici et al., 2005). Because both tensile and compressive forces occur at the joint surface due to translational and rotational motion the cartilage residing within these joints is exposed to a complex pattern of loading (Silver and Glasgold, 1995). As we age, these joints experience a heightened level of injury and tend to be affected by arthritis. Although not well understood it is thought that this disorder may be due, at least in part, to an inability of the chrondrocytes to properly regulate the expression of collagens and proteoglycans (Goldring and Marcu, 2009). In the articular cartilage, anabolic tissue responses appear to be initiated by mechanical loading, however the precise mechanism underlying mechanical modulated matrix synthesis is unknown (Vincent and Saklatvala, 2006). The most favored mechanism of tissue mechanotransduction associated with articular cartilage is integrin ligation, however integrins do not have intrinsic signaling capability (Millward-Sadler et al., 1999), leaving the exact mechanism of mechanocoupling unknown.
In general, static compression of articulate cartilage has been shown to decrease biosynthetic activity, while cyclic compression appears to increases protein synthesis (Wong et al., 1999). The amount of aggrecan and type II collagen mRNA has been shown to decrease with increasing compression over 24hr, however increases in aggregan and type II collagen mRNA have been reported with dynamic loading (Smith et al., 1996; Ragan et al., 1999; Millward-Sadler et al., 2000). Similarly, fibronectin and cartilage oliogomeric matrix protein have been shown to increase in response to cyclic compression (Steinmeyer et al., 1997; Wong et al., 1999). The signaling pathways regulating these changes are largely unknown however It has been demonstrated that the ERK-MAPK pathway is activated following cyclical loading and that this is most likely due to the release of basic fibroblast growth factor (bFGF) (Vincent et al., 2002; Vincent et al., 2004).
Although it is clear that joint loading is capable of eliciting a wide variety of different cellular responses the mechanisms responsible for these mechanically induced changes are, to date, largely unknown. Similarly, how aging may affect mechanotransductive processes within the different cells and tissues that comprise the joint have for the most part not been investigated. Additional research perhaps using cultured cells, tissue explants studies or molecular approaches will likely be invaluable to further our understanding how joint “health” is regulated by the interplay of loading and age.
6. Aging and cardiovascular mechanotransduction
6.1 Aging affects cardiovascular structure and function
Aging-associated heart diseases are major cause of death, accounting for about 29% of total death in the elderly (Heron et al., 2008). The cardiovascular system is exquisitely sensitive to mechanical stimuli. Indeed, mechanotransduction processes are important in the development of the heart and for the control of blood pressure and cardiac output. Like skeletal muscle and bone, increased cardiac loading can also result in cellular growth and hypertrophy. Diseases of mechanotransduction associated with the heart include angina, atherosclerosis, atrial fibrillation, heart failure, hypertension, intimal hyperplasia, and valvular disease (Ingber, 2003a).
Similar to other aging systems, aging in the heart is associated with a number of structural and functional alterations. The aged mammalian heart is characterized by diminished cardiomyocyte contractility (Harding et al., 1992), prolonged contraction and relaxation (Nair and Nair, 2001), and changes in calcium sensitivity (Lim et al., 2000; Nair and Nair, 2001). Although not well understood, these changes in cardiac function are thought to be mediated, at least in part, by age-associated changes in cardiac structure. Structural changes with aging include an increased deposition of ECM, increased sarcomere length and number, the loss of nuclear shape (Afilalo et al., 2007), lipofuscin accumulation, and cardiomyocyte hypertrophy (Terman and Brunk, 1998; Brunk and Terman, 2002; Nadal-Ginard et al., 2003; Terman and Brunk, 2005). It is also likely that cardiomyocytes number is decreased with aging (Bernhard and Laufer, 2008). The factor(s) responsible for these changes have not been fully elucidated but may include increased oxidative stress, reduced cellular repair, telomere shortening, altered cellular metabolism, the accumulation of post-translational modifications, increased apoptosis or necrosis, and inflammation (Bernhard and Laufer, 2008).
Aging in the vascular system is characterized by aging vessel elongation, torsion, enlargement of the lumen, and a thickening of the vascular walls (Ferrari et al., 2003). Under a normal lifetime of operation, the pulsatile nature of blood flow causes the vasculature to undergo a tremendous number of cyclic expansions and contractions. Given the tempestuous demands placed on the vessel walls, the contractile-elastic unit, elastin, begins to fatigue by the sixth decade of life (Franklin, 2006). Eventually, this fatigue is counteracted by changes in the ECM which may include the proliferation of collagen and calcium deposition (Franklin, 2006). How changes in the vessel ECM may affect the ability of the vasculature to respond to mechanical stimuli is not well understood.
6.2. Age-related changes to the ECM are associated with increased cardiac stiffness
The ECM acts to support and align cardiomyocytes and is involved in transmitting mechanical forces (Burgess et al., 2001). The composition of the ECM is dynamic and can change in response to environmental and mechanical stimuli. There is also evidence that the ECM changes with age. For example, the amount of fibronectin mRNA has been shown to be regulated in a developmental and age-specific manner (Mamuya et al., 1992). Boluyt and colleagues using captopril, demonstrated that fibronectin expression is increased in the senescent spontaneous hypertensive rat heart (Boluyt and Bing, 2000). Similarly, as the heart ages, collagen expression has also been shown to increase (Li et al., 1992; Orlandi et al., 2004; Lieber et al., 2008). It is thought that these changes in fibronectin and collagen may be associated with increased diastolic stiffness (Burgess et al., 2001; Merx et al., 2005). Aging has also been shown to be associated with increases protein cross-linking (Spinale et al., 1996), increased protein glycation and alterations in the expression of matrix metalloproteinases (MMPs). The MMPs are zinc-dependent endopeptidases that degrade proteins in the extracellular matrix and are inhibited by tissue inhibitor of metalloproteinases (TIMPs) (Malemud, 2006). In aged mouse, recent data has suggested that aging is associated with an increase in the amount of MMP-3, MMP-8, MMP-9, MMP-12, and MMP-14 and decreased levels of TIMP-3 and TIMP-4 (Lindsey et al., 2005). Changes of this type would be expected to favor ECM accumulation which may be associated with decreased left ventricular diastolic function (Bonnema et al., 2007). Whether these changes alone or others working in concert with them are responsible to age-related changes in cardiac stiffness has yet to be fully elucidated.
6.3. Aging can alter the expression of cardiac mechanosensory molecules
The sarcomere is the basic contractile unit of the cardiomyocyte. Integrins are found in the sarcolemmal membrane next to the costameres (Sussman et al., 2002). Similar to skeletal muscle, integrins are thought to play a key role in regulating cardiac mechanotransduction (Ingber, 2003a). With advancing age, research has shown that the expression of integrin subunits in the heart is altered (Burgess et al., 2001). Whether these changes in integrin subunit expression lead to age-related changes in cardiac mechanotransduction has not, to our knowledge, been investigated. In addition to the integrins, the Z-discs of the sarcomere are considered to be mechanosensors (Goldstein et al., 1988, 1989). The Z-disc is linked to the sarcolemma by dystrophin (Pyle and Solaro, 2004). The organization and localization of dystrophin and other dystrophin associated proteins, such as utrophin, vinculin and talin, have been shown to be altered with aging (Mora et al., 1996). Although not well understood, it is possible that such changes could lead to change in cardiac stiffness or to load-induced signaling. Similarly, desmin is another protein that functions to connect different regions of the saromere. Whether or how aging affects desmin expression is unclear as different studies have shown to decrease (Diedrich et al., 2007; Lieber et al., 2008) or remain unaltered with aging (Diedrich et al., 2007). Like desmin, titin has also been implicated in sensing mechanical loading (Pyle and Solaro, 2004). Titin functions to connect the thick filament of the sarcomere to the Z-disc and is responsible for the passive tension of the cardiomyocytes (Pyle and Solaro, 2004). In addition to its structural role, titin also contains binding sites for other proteins such as telethonin, alpha actinin, calpain-3, obscurin, myosin-binding protein C, calmodulin 1, CAPN3, and MURF1. Titin isoforms have been shown to change during heart development (Warren et al., 2004). How changes in the Z-disc, dystrophin, desmin or titin might affect cardiac mechanotransduction during aging is not yet clear.
Stretch-activated channels are activated by forces acting upon the ECM, integral membrane proteins, cytoskeleton, or the membrane itself. The impact of aging on stretch-activated channels and their signaling pathways in the heart is not well understood. Recent data has suggested that senescent mouse myocytes exhibit impaired calcium handling with increasing pacing frequency (Lim et al., 2000). There is also evidence to suggest the molecules involved in calcium signaling may change with age. For example, the calmodulin-dependent protein kinase II, which is involved in Ca2+ uptake into the sarcoplasmic reticulum, was significantly increased in ventricular extracts from old mice (Kim et al., 1998). Conversely, ATP-dependent Ca2+-uptake activity of the sarcoplasmic reticulum and the relative amount of CaM kinase II was reduced significantly with aging (Xu and Narayanan, 1998). Likewise, the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) gene which is down-regulated in several models of cardiac hypertrophy also exhibits lower levels of mRNA in the senescent heart. How these changes may affect the ability of the aging heart to sense and respond to mechanical stimulation is not clear.
6.4. The effect of aging on vascular endothelial cells
The blood vessels of the vascular system are lined with endothelial cells (EC). The prominent mediator of EC function is fluid shear stress. Increases in blood flow stimulate EC in the arteries and this can induce relaxation of the surrounding vascular smooth muscle cells (VSMC). This process occurs as a result of hyperpolarization of VSMC, and is mediated through the production and release of nitric oxide (NO), arachidonic acid metabolites, and/or prostacyclin (Vanhoutte et al., 1995; Bittar et al., 2006; Campbell and Falck, 2007). Although we do not yet understand how the response to shear stress is orchestrated in EC, integrins, vascular endothelial (VE)-cadherin, platelet/endothelial cell-adhesion molecule 1 (PECAM1), G-proteins, receptor tyrosine kinases, integrins, and ion channels have all been identified as potential mechanical sensors (Li et al., 2005; Orr et al., 2006). Similar to other cell types, the cytoskeleton is also thought to play a role as the microtubules, actin, and intermediate filaments of the EC can act to transmit forces throughout the cell cytoskeleton (Davies, 1995). Likewise, there is also evidence to suggest that EC contain mechanosensitive ion channels that can be activated by shear force (Hoger et al., 2002) resulting in membrane hyperpolarization through the inward flux of potassium ion (Zaritsky et al., 2000). In general, aging in the vasculature is associated with loss of endothelial function as well as disruption of the endothelia lining. How aging may alter EC mechanotransduction has not fully investigated.
6.5. The effect of aging on vascular smooth muscle mechanotransductive signaling
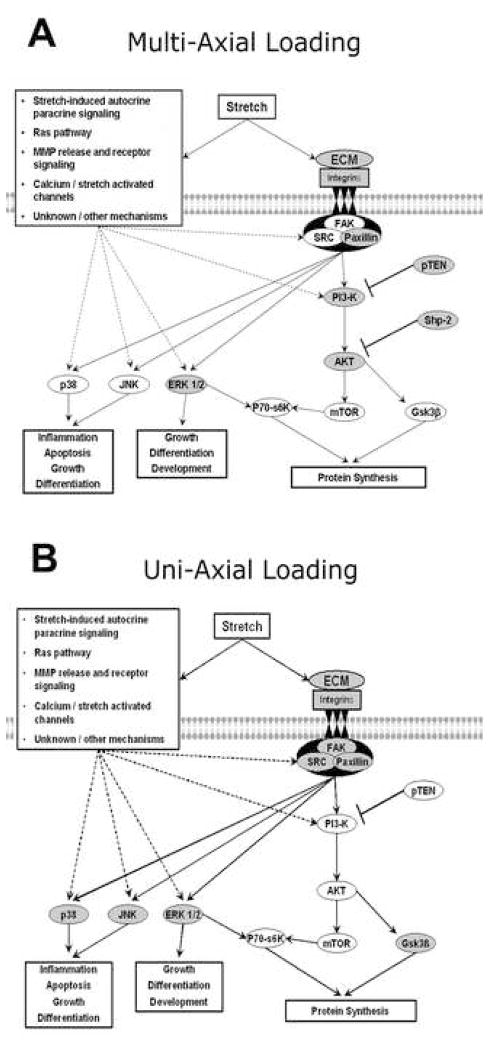
In addition to EC, VSMC are also capable of sensing and responding to mechanical loading. Recent data using multi-axial stretch and uni-axial stretch (Figure 3A and 3B) has demonstrated that aging is associated with alterations in how the aorta respond to mechanical loading (Rice et al., 2005a; Rice et al., 2005b; Rice et al., 2007a; Rice et al., 2008). For example, pressure-induced Fak-related non-kinase (FRNK p41) translocation increased with aging while RhoA translocation appeared to be unaffected (Rice et al., 2007a). In adult F344BN aorta, both increased aortic intraluminal pressure and uni-axial stretch caused increased activation of the ERK1/2, p38 MAPK, and JNK proteins (Rice et al., 2005a; Rice et al., 2007b). In contrast, aging diminished the response of p38 MAPK and JNK to increased aortic pressure but did not affect the response of the aorta to uni-axial stretch (Rice et al., 2005a; Rice et al., 2007b). With aging the contents of Akt, protein tyrosine phosphatase (SHP-2), and phosphatase and tensin homolog (PTEN) were significantly increased, while those of p70S6k and GSK3β were unchanged (Rice et al., 2005b). The pressure-induced phosphorylation of p70S6k in aged F344BN aorta was impaired compared to that observed in the adult aorta (Rice et al., 2005b). Together, these results suggest that VSMC can distinguish between different stretch types (multi-axial vs. uni-axial) and that the signaling response is altered with age. In addition, aging in the aorta of the F344BN has been shown to result in diminished contractile force generation and increased calcium-dependent stress relaxation (Blough et al., 2007). The reasons for differences with aging are not clear but may be related to increases in vascular stiffness (Blough et al., 2007). Indeed, an increase in vascular stiffness would be predicted to decrease the vessel deformation with loading. This decrease in deformation, in turn, may act to reduce mechanically-regulated signaling. In addition to changes in vascular stiffness, it is also possible that changes in the membrane composition may play a role in age-associated vasacular mechanotransduction. For example, high plasma membrane cholesterol levels have been shown to inhibit the function of stretch-activated ion channels (Fang et al., 2005). Similarly, age-associated reductions in the number of ion channel subunits or diminished nitric oxide synthase (NOS) activity and nitric oxide release could also play a role. Whether changes in vessel stiffness, alterations in membrane composition, or other factors are responsible for the attenuation of load-induced signaling with aging will require further investigation.
Figure 3.
Stress-induced signaling in aorta. A. Effect of aging on load-induced signaling following multi-axial stress in the aging F344BN aorta. B. Effect of aging on load-induced signaling following uni-axial stress in the aging F344BN aorta. Unfilled circles represent age-related differences (see text for details).
7. Effect of aging on neuronal mechanotransduction
7.1. Aging brain and Alzheimer’s disease
The aging brain is characterized by a shrinkage of brain mass, the degeneration of synaptic transmission, a loss of neurons, and a decrease in the abundance of chemical messengers (Dickstein et al., 2007). These age-associated alterations, if localized to the hippocampus and cerebral cortex, can result in memory impairment and the decline of cognitive function. Alzheimer’s disease (AD) is the most common progressive neurodegenerative disease, and is one of top five causes of death in the elderly (Heron et al., 2008). In Alzheimer’s disease, plaques of aggregated amyloid β peptide (Aβ) are formed around nerve cells (Li et al., 2004; Sun et al., 2006; Tesco et al., 2007). It is thought that this accumulation of Aβ interrupts synaptic transmission and alters synaptic plasticity. In addition, aging-related increase in the hyperphosphorylation of Tau proteins may be associated with an increase the formation of neurofibrillary tangles (Olesen, 1994; Deutsch et al., 2006). Currently, the link between neuronal mechanotransduction and brain aging/neurodegenerative disease is not well understood.
7.2. Dysregulation of ion channel/calcium homeostasis in Alzheimer’s disease development
Mechanosensitive cation channels, including stretch-activated cation channels (SACCs) and several transient receptor potential channels (TRPC), have been characterized both in sensory neurons and in non-specialized neurons (Erxleben, 1989; Jia et al., 2007). Mechanosensitive cation channels that conduct Ca2+ are present in neuronal dendrites, soma, and at the synaptic terminal. It is thought that these channels regulate neurite outgrowth, nerve growth cone motility, neurotransmitter release, synaptic plasticity, and in some cases, gene expression (Raza et al., 2007; Thibault et al., 2007; Bojarski et al., 2008; Wojda et al., 2008). Intracellular Ca2+ concentrations are modulated by Ca2+ influx from the extracellular environment via different types of channels, or by Ca2+ release from internal stores. Recent studies have demonstrated that aging increases the activity of voltage-gated Ca2+ channels in hippocampal neurons, and is associated with an elevation in intracellular Ca2+ concentration (Campbell et al., 1996; Landfield, 1996; Thibault and Landfield, 1996). Calcium influx through SACCs inhibits neurite outgrowth, whereas Ca2+ influx through other channels in the plasma membrane (e.g., TRPC) or release from internal stores through inositol-1,4,5-triphosphate receptor (IP3R) and ryanodine receptor (RyR) stimulation promotes neurite extension and steering (Jacques-Fricke et al., 2006). Although not well understood, it is thought that the dysregulation of intracellular Ca2+ levels is linked to the Aβ aggregation and changes in Tau phosphorylation (Pierrot et al., 2006; Bojarski et al., 2008).
7.3. Insulin resistance and Alzheimer’s disease
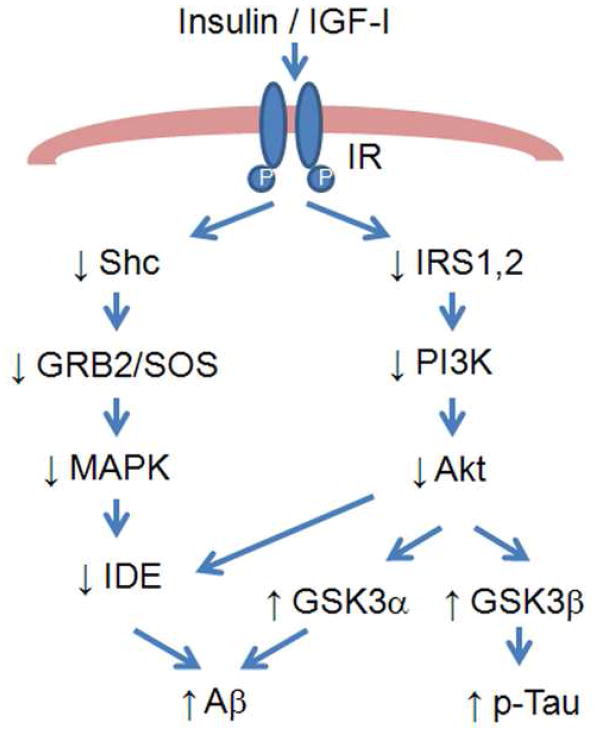
Insulin signaling is essential for neuronal development, memory formation, synaptic plasticity, and neuroprotection (Cole and Frautschy, 2007; Li and Holscher, 2007; Zhao and Townsend, 2009). It has been reported that the insulin signaling pathway is disturbed with aging and AD. For example, the expression of insulin and insulin receptor are reduced in AD and aging, with a greater decrease occurring in AD (Frolich et al., 1998; Hoyer, 1998). There is a dramatic decrease in the phosphorylation of Src homology adaptor protein (Shc), the association of Shc/growth factor receptor binding protein-2 (GRB2), and the expression of SH protein tyrosine phosphatase-2 (SHP2) in the forebrain cortex and the cerebellum of aged rats (Fernandes et al., 2001). This impairment in the Shc/GRB2 signaling may cause reduced synthesis of insulin-degrading enzyme (IDE), a protein responsible for the clearance of Aβ fibril, and this may be one mechanism why aging is oftentimes associated with an accumulation of Aβ (Figure 4). In addition, there is evidence that aging may reduce activation of the PI3K/Akt pathway which may be associated with a decreased ability of the aged brain to inhibit apoptosis (Eldar-Finkelman et al., 1999; Schubert et al., 2003). This alteration may also lead to decreased inhibition of the downstream kinase GSK-3 and increases in the Tau hyperphosphorylation which have been associated with increases in Aβ (Figure 4) (Phiel et al., 2003; Rickle et al., 2004). Additional studies using gene knockout or other molecular approaches to investigate insulin signaling may lead to new knowledge regarding how aging affects brain structure and function.
Figure 4.
An overview of the relationship between insulin/IGF-I desensitization and Alzheimer s disease. Insulin resistance is associated with diminished activation of Shc/GRB2/MAPK and IRS/PI3K/Akt signaling, which could lead to increased Tau phosphorylation and Aβ accumulation (see text for details).
8. Effect of aging on respiratory mechanotransduction
8.1. Age-associated changes in respiratory structure and function
Respiratory diseases cause approximately 6% of total deaths in people 65 years of age and older (Heron et al., 2008). Almost all components of the respiratory system, including lung parenchyma, pulmonary and bronchial vascular systems, and diaphragm, are affected by aging. As we age, the lung is thought to become less elastic, and muscles of the chest wall and diaphragm lose mass and strength (Mays et al., 1989). Therefore, the respiratory system experiences an increase in dead space and a decrease in arterial oxygen tension/concentration. Aging airways have also been shown to close more readily and tend to collapse, while the aging diaphragm is more susceptible to injury and atrophy (Cantillon and Bradford, 2000; Suzuki et al., 2009). As a result, the aged exhibit an increased possibility of developing respiratory failure, pneumonia, asthma, fibrosis, chronic bronchitis, chronic obstructive pulmonary disease (COPD), emphysema, aspiration, influenza, tuberculosis, and pleurisy (Rossi et al., 1996).
8.2. The effects of aging on the respiratory system in response to mechanical loading
Cells in the respiratory system are subjected to a variety of mechanical forces, including tensile and compressive forces, which can influence airway and alveolar epithelial cell function during the respiratory cycle. Aging is thought to negatively affect the ability of the respiratory system to respond to mechanical loading as the aging lung is more susceptible to pulmonary injury following mechanical ventilation (Nin et al., 2008). Aging also impairs the sensitivity and reactivity of airway smooth muscle to mechanical stimuli (An et al., 2007; Fabry and Fredberg, 2007). Likewise, the aging diaphragm not only exhibits a lower maximal isometric tension, but also an impaired ability to recover diaphragmatic force in response to unloading (Criswell et al., 2003). How these changes in the respiratory system respond to mechanical stimuli may affect load-induced signaling has to our knowledge not been investigated.
8.3 The effects of calcium channels on respiratory mechanotransduction
It is well accepted that the lung is sensitive to mechanical stimuli. For example, stretch can activate mechanosensitive cation channels to allow Ca2+ influx into lung cells (Bialecki et al., 1992; Winston et al., 1993; Boitano et al., 1994). This increase in cytoplasmic Ca2+ is associated with the activation of protein tyrosine kinase, which in turn activates phospholipase C-β and mediates the production of both IP3 and diacylglycerol (DAG). The IP3 induces Ca2+ release from intracellular stores, and along with DAG activates protein kinase C (PKC). PKC further activates transcriptional factors such as c-fos, which can bind to stretch response elements (SRE), shear stress-response elements (SSRE) or the shear 12-O-tetradecanoylphorbol 13-acetate-response element (TRE) to induce changes in gene expression (Resnick et al., 1993; Shyy et al., 1995; Garcia et al., 2006). How aging affects loading-induced Ca2+-dependent signaling in the lung is not clear, although it has been postulated that this process is negatively affected by increasing age (Papazafiri and Kletsas, 2003). Physical forces exerted on the smooth muscle of airways can induce oscillation of the cytosolic Ca2+ concentration, and this plays a key role in excitation-relaxation coupling. The balance between the phosphorylation of the myosin light chain (MLC) by Ca2+-activated calmodulin-dependent myosin light chain kinase (MLCK) and dephosphorylation of the MLC by myosin light chain phosphatase (MLCP) controls smooth muscle contraction and relaxation in the airway (Smith et al., 1995; Mehta et al., 2000; Totsukawa et al., 2000; Burridge and Doughman, 2006; Janssen and Killian, 2006; Fajmut and Brumen, 2008). Aging induces abnormal oscillation of the intracellular calcium concentration and dysregulation of the balance between MLCK and MLCP activities, which may be a cause of airway hypersensitivity and hyperreactivity (Smith et al., 1995; Ammit et al., 2000; Chitano et al., 2000; Mehta et al., 2000; Totsukawa et al., 2000; Parameswaran et al., 2002; Fajmut and Brumen, 2008).
Contraction-induced ROS generation in the diaphragm has been shown to be related to extracellular calcium concentration and is likely regulated in part by ROS production (Supinski et al., 1999). The oxidative challenge has also been linked to the impaired contractile function (Lawler et al., 1997b). These changes may be related to age-associated increases in diaphragmatic fatigability (Khawli and Reid, 1994; Lawler et al., 1997a). Further studies of the effects of aging on respiratory mechanotransduction may provide valuable insight regarding potential therapeutic treatments for age-associated respiratory dysfunction.
9. Effect of aging on skin mechanotransduction
9.1. Age-associated changes in skin structure and function
Although skin is a durable system, its structural character and physiological function undergo dramatic changes with age (Waller and Maibach, 2005; Farage et al., 2007; Puizina-Ivic, 2008; Robert et al., 2009). The decrease in epidermal thickness is accelerated with age, especially in exposed areas such as the face, neck, forearms, and hands, due to a slower renewal rate of epidermal cells (Boss and Seegmiller, 1981). Aged epidermis contains fewer melanocytes and immunocompetent Langerhans cells, resulting in uneven pigmentation and impaired immunity in aged skin (Waller and Maibach, 2005; Farage et al., 2007). The thickness of the dermis also decreases with age and this is accompanied by a decrease in number of mast cells and fibroblasts, and a decrease in the generation of collagen, elastin, glycosaminoglycans, and hyaluronic acid all of which are thought to contribute to the development of wrinkles and a loss of elasticity (Duncan and Leffell, 1997; Carrino et al., 2003; Sudel et al., 2005; Waller and Maibach, 2005). Aged skin is drier than younger skin due to lack of sebaceous glands in the dermal layer (McCullough and Kelly, 2006; Farage et al., 2007). The dermal-epidermal junction flattens with age resulting in a decrease in the transfer of nutrients and oxygen between the two layers, making the skin more fragile and susceptible to damage (Sudel et al., 2005; Farage et al., 2007). The subcutaneous fat in the hypodermis (the skin layer below the dermis) diminishes with age and causes the skin to wrinkle and sag (Hashizume, 2004; Farage et al., 2007). Taken together, aged skin has a reduced capability to repair and proliferate, is more susceptible to sunburn, trauma, and bruising, and has diminished perception of external stimuli such as pressure and temperature changes (Puizina-Ivic, 2008; Robert et al., 2009).
9.2. Age-related changes in mechanical properties of skin tissue
Type I collagen is the most abundant protein in skin, and constitutes the extracellular matrix of the dermis (Varani et al., 2006; Fisher et al., 2009). In normal skin, fibroblasts bind to intact collagen fibrils through integrin receptors resulting in the formation of an integrated structure that exhibits a basal level of load or tension within the dermal layer (Wang and Ingber, 1994; Grinnell, 2003). As we age, it is thought that the skin contains an increased amount of fragmented and disorganized collagen fibrils which in turn acts to dramatically reduce the amount of fibroblasts-collagen fibril linkages (Oba and Edwards, 2006; Varani et al., 2006; Fisher et al., 2009). This decrease in linkages in turn results in a weakened extracellular matrix, a reduction in the amount of mechanical loading experienced by the dermal fibroblasts and although not yet tested, the possibility that aging diminishes the ability of the skin to respond to changes in mechanical load (Fisher et al., 2002; Varani et al., 2006; Fisher et al., 2009). Though not well understood, it is thought that these alterations in the extracellular matrix of aged skin are due, at least in part, to increased ROS and ROS-induced matrix metalloproteinase 1 (MMP-1) expression. MMP-1 is an enzyme that degrades collagen, resulting in fragmentation of collagen and disorganization of fibroblast-collagen (Varani et al., 2000; Fisher et al., 2009). Indeed, research from Voorhee and colleagues has demonstrated that aging induces an elevated level of ROS, which is accompanied by increased expression of the transcriptional factors cJun, AP-1, and the α2β1 integrin, all key regulators of MMP-1 gene expression (Varani et al., 2006; Fisher et al., 2009). Studies on human subjects have also shown that MMP-1 expression and collagen fibril fragmentation is significantly increased in aged human dermal fibroblasts (Fisher et al., 2009). Age-associated increase of fragmented collagen in the dermis not only causes a loss of strength and a reduction of mechanical tension in aged human skin, but also further induces increases in ROS (Fisher et al., 2009).
Beside increased fragmentation of collagen in aged skin, collagen expression is also decreased with aging (Varani et al., 2006). To our knowledge whether changes in the amount of collagen, alterations in tissue ROS or decreases in the amount of fibroblast-collagen linkage result in a diminished ability of the skin to detect or propagate mechanical stimuli has not yet been investigated.
10. Conclusions and future directions
The ability of the cell to sense, process, and respond to mechanical stimuli appears to be altered with aging and these changes have been shown to be associated with increased susceptibility to mechanical damage, increased apoptosis, alterations in intracellular signaling, and a dysregulation of gene expression. These age-associated impairments appear to be ubiquitous, but are not well understood given the complexity of cellular mechanotransduction and the difficulty of studying mechanotransduction process in aged tissues. Understanding the cellular and molecular basis of physiological adaptation to mechanical loads may play a role in the frailty of aging. Additional research in this area will not only shed light on our understanding of mechanotransduction process in aging, but also may provide valuable clinical insight for the increasing aged population.
Acknowledgments
This work was supported in part by NIH Grant AG-027103-1 to E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afilalo J, Sebag IA, Chalifour LE, Rivas D, Akter R, Sharma K, Duque G. Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;3:H1451–1456. doi: 10.1152/ajpheart.01194.2006. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002;119:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;1:257–263. doi: 10.1164/ajrccm.161.1.9901005. [DOI] [PubMed] [Google Scholar]
- An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, Fabry B, Fairbank NJ, Ford LE, Fredberg JJ, Gerthoffer WT, Gilbert SH, Gosens R, Gunst SJ, Halayko AJ, Ingram RH, Irvin CG, James AL, Janssen LJ, King GG, Knight DA, Lauzon AM, Lakser OJ, Ludwig MS, Lutchen KR, Maksym GN, Martin JG, Mauad T, McParland BE, Mijailovich SM, Mitchell HW, Mitchell RW, Mitzner W, Murphy TM, Pare PD, Pellegrino R, Sanderson MJ, Schellenberg RR, Seow CY, Silveira PS, Smith PG, Solway J, Stephens NL, Sterk PJ, Stewart AG, Tang DD, Tepper RS, Tran T, Wang L. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;5:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997;6:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AD, Joldersma M, Klein-Nulend J, Burger EH. Interactive effects of PTH and mechanical stress on nitric oxide and PGE2 production by primary mouse osteoblastic cells. Am J Physiol Endocrinol Metab. 2003;3:E608–613. doi: 10.1152/ajpendo.00501.2002. [DOI] [PubMed] [Google Scholar]
- Barton ER. Impact of sarcoglycan complex on mechanical signal transduction in murine skeletal muscle. Am J Physiol Cell Physiol. 2006;2:C411–419. doi: 10.1152/ajpcell.00192.2005. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J Anat. 1995:625–633. [PMC free article] [PubMed] [Google Scholar]
- Bernhard D, Laufer G. The aging cardiomyocyte: a mini-review. Gerontology. 2008;1:24–31. doi: 10.1159/000113503. [DOI] [PubMed] [Google Scholar]
- Bialecki RA, Kulik TJ, Colucci WS. Stretching increases calcium influx and efflux in cultured pulmonary arterial smooth muscle cells. Am J Physiol. 1992;5(Pt 1):L602–606. doi: 10.1152/ajplung.1992.263.5.L602. [DOI] [PubMed] [Google Scholar]
- Bittar J, Cepeda P, de la Fuente J, Douthat W, de Arteaga J, Massari PU. Renal transplantation in diabetic patients. Transplant Proc. 2006;3:895–898. doi: 10.1016/j.transproceed.2006.02.054. [DOI] [PubMed] [Google Scholar]
- Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 x Brown Norway rats. J Appl Physiol. 2000;4:1265–1270. doi: 10.1152/jappl.2000.88.4.1265. [DOI] [PubMed] [Google Scholar]
- Blough ER, Rice KM, Desai DH, Wehner P, Wright GL. Aging alters mechanical and contractile properties of the Fisher 344/Nnia X Norway/Binia rat aorta. Biogerontology. 2007;3:303–313. doi: 10.1007/s10522-006-9074-2. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;11:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Boitano S, Sanderson MJ, Dirksen ER. A role for Ca(2+)-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. J Cell Sci. 1994:3037–3044. doi: 10.1242/jcs.107.11.3037. [DOI] [PubMed] [Google Scholar]
- Bojarski L, Herms J, Kuznicki J. Calcium dysregulation in Alzheimer’s disease. Neurochem Int. 2008;4–5:621–633. doi: 10.1016/j.neuint.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Boluyt MO, Bing OH. Matrix gene expression and decompensated heart failure: the aged SHR model. Cardiovasc Res. 2000;2:239–249. doi: 10.1016/s0008-6363(00)00043-2. [DOI] [PubMed] [Google Scholar]
- Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J Card Fail. 2007;7:530–540. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med. 1981;6:434–440. [PMC free article] [PubMed] [Google Scholar]
- Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;8:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- Burgess ML, McCrea JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001;15:1739–1756. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Burridge K, Doughman R. Front and back by Rho and Rac. Nat Cell Biol. 2006;8:781–782. doi: 10.1038/ncb0806-781. [DOI] [PubMed] [Google Scholar]
- Campbell LW, Hao SY, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J Neurosci. 1996;19:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;3:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- Cantillon D, Bradford A. Effects of age and gender on rat upper airway muscle contractile properties. J Gerontol A Biol Sci Med Sci. 2000;8:B396–400. doi: 10.1093/gerona/55.8.b396. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;8:1271–1279. doi: 10.1359/jbmr.070506. [DOI] [PubMed] [Google Scholar]
- Carrino DA, Onnerfjord P, Sandy JD, Cs-Szabo G, Scott PG, Sorrell JM, Heinegard D, Caplan AI. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem. 2003;19:17566–17572. doi: 10.1074/jbc.M300124200. [DOI] [PubMed] [Google Scholar]
- Carson JA, Yamaguchi M, Alway SE. Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J Appl Physiol. 1995;1:293–299. doi: 10.1152/jappl.1995.78.1.293. [DOI] [PubMed] [Google Scholar]
- Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;3:226–231. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Cheema U, Brown R, Mudera V, Yang SY, McGrouther G, Goldspink G. Mechanical signals and IGF-I gene splicing in vitro in relation to development of skeletal muscle. J Cell Physiol. 2005;1:67–75. doi: 10.1002/jcp.20107. [DOI] [PubMed] [Google Scholar]
- Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;5:C989–997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- Chitano P, Wang J, Cox CM, Stephens NL, Murphy TM. Different ontogeny of rate of force generation and shortening velocity in guinea pig trachealis. J Appl Physiol. 2000;4:1338–1345. doi: 10.1152/jappl.2000.88.4.1338. [DOI] [PubMed] [Google Scholar]
- Chow JW, Fox SW, Lean JM, Chambers TJ. Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res. 1998;6:1039–1044. doi: 10.1359/jbmr.1998.13.6.1039. [DOI] [PubMed] [Google Scholar]
- Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer’s Disease. Exp Gerontol. 2007;1–2:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Criswell DS, Shanely RA, Betters JJ, McKenzie MJ, Sellman JE, Van Gammeren DL, Powers SK. Cumulative effects of aging and mechanical ventilation on in vitro diaphragm function. Chest. 2003;6:2302–2308. doi: 10.1378/chest.124.6.2302. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;7:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;3:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Lakshman RM. Dysregulation of tau phosphorylation is a hypothesized point of convergence in the pathogenesis of alzheimer’s disease, frontotemporal dementia and schizophrenia with therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;8:1369–1380. doi: 10.1016/j.pnpbp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;3:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich M, Tadic J, Mao L, Wacker MA, Nebrich G, Hetzer R, Regitz-Zagrosek V, Klose J. Heart protein expression related to age and sex in mice and humans. Int J Mol Med. 2007;6:865–874. [PubMed] [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;2:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;5:C1635–1641. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- Duncan KO, Leffell DJ. Preoperative assessment of the elderly patient. Dermatol Clin. 1997;4:583–593. doi: 10.1016/s0733-8635(05)70468-x. [DOI] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999;8:1662–1666. doi: 10.2337/diabetes.48.8.1662. [DOI] [PubMed] [Google Scholar]
- Erxleben C. Stretch-activated current through single ion channels in the abdominal stretch receptor organ of the crayfish. J Gen Physiol. 1989;6:1071–1083. doi: 10.1085/jgp.94.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry B, Fredberg JJ. Mechanotransduction, asthma, and airway smooth muscle. Drug Discov Today Dis Models. 2007;3:131–137. doi: 10.1016/j.ddmod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajmut A, Brumen M. MLC-kinase/phosphatase control of Ca2+ signal transduction in airway smooth muscles. J Theor Biol. 2008;3:474–481. doi: 10.1016/j.jtbi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Fang Y, Schram G, Romanenko VG, Shi C, Conti L, Vandenberg CA, Davies PF, Nattel S, Levitan I. Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am J Physiol Cell Physiol. 2005;5:C1134–1144. doi: 10.1152/ajpcell.00077.2005. [DOI] [PubMed] [Google Scholar]
- Farage MA, Miller KW, Elsner P, Maibach HI. Structural characteristics of the aging skin: a review. Cutan Ocul Toxicol. 2007;4:343–357. doi: 10.1080/15569520701622951. [DOI] [PubMed] [Google Scholar]
- Fernandes ML, Saad MJ, Velloso LA. Effects of age on elements of insulin-signaling pathway in central nervous system of rats. Endocrine. 2001;3:227–234. doi: 10.1385/endo:16:3:227. [DOI] [PubMed] [Google Scholar]
- Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol. 2003;6:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;11:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;1:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- Franklin SS. Hypertension in older people: part 1. J Clin Hypertens (Greenwich) 2006;6:444–449. doi: 10.1111/j.1524-6175.2006.05113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm. 1998;4–5:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;10:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;4:R1080–1086. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- Garcia CS, Prota LF, Morales MM, Romero PV, Zin WA, Rocco PR. Understanding the mechanisms of lung mechanical stress. Braz J Med Biol Res. 2006;6:697–706. doi: 10.1590/s0100-879x2006000600001. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;1:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;3:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;3:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink G. Age-related muscle loss and progressive dysfunction in mechanosensitive growth factor signaling. Ann N Y Acad Sci. 2004:294–298. doi: 10.1196/annals.1297.050. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Loss of muscle strength during aging studied at the gene level. Rejuvenation Res. 2007;3:397–405. doi: 10.1089/rej.2007.0597. [DOI] [PubMed] [Google Scholar]
- Goldstein MA, Michael LH, Schroeter JP, Sass RL. Z band dynamics as a function of sarcomere length and the contractile state of muscle. Faseb J. 1987;2:133–142. doi: 10.1096/fasebj.1.2.3609610. [DOI] [PubMed] [Google Scholar]
- Goldstein MA, Michael LH, Schroeter JP, Sass RL. Structural states in the Z band of skeletal muscle correlate with states of active and passive tension. J Gen Physiol. 1988;1:113–119. doi: 10.1085/jgp.92.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MA, Michael LH, Schroeter JP, Sass RL. Two structural states of Z-bands in cardiac muscle. Am J Physiol. 1989;2(Pt 2):H552–559. doi: 10.1152/ajpheart.1989.256.2.H552. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;2(Pt 1):E403–408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;5:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;1:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. J Bone Miner Res. 2002;3:493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;10:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;(Pt 1):247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SE, Jones SM, O’Gara P, del Monte F, Vescovo G, Poole-Wilson PA. Isolated ventricular myocytes from failing and non-failing human heart; the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992;5:549–564. doi: 10.1016/0022-2828(92)91843-t. [DOI] [PubMed] [Google Scholar]
- Hartman M, Catlin A, Lassman D, Cylus J, Heffler S. U.S. Health spending by age, selected years through 2004. Health Aff (Millwood) 2008;1:w1–w12. doi: 10.1377/hlthaff.27.1.w1. [DOI] [PubMed] [Google Scholar]
- Hashizume H. Skin aging and dry skin. J Dermatol. 2004;8:603–609. doi: 10.1111/j.1346-8138.2004.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Heron MP, Hoyert DL, Xu J, Scott C, Tejada-Vera B. Deaths: preliminary data for 2006. Natl Vital Stat Rep. 2008;16:31. [Google Scholar]
- Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci U S A. 2002;11:7780–7785. doi: 10.1073/pnas.102184999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander J, Bejma J, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression in skeletal muscle: fiber-specific effect of age. Mech Ageing Dev. 2000;1:33–45. doi: 10.1016/s0047-6374(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;6:1207–1216. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;12:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol. 2005;4:1562–1566. doi: 10.1152/japplphysiol.00870.2004. [DOI] [PubMed] [Google Scholar]
- Hornberger TA, Stuppard R, Conley KE, Fedele MJ, Fiorotto ML, Chin ER, Esser KA. Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism. Biochem J. 2004;(Pt 3):795–804. doi: 10.1042/BJ20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Sukhija KB, Wang XR, Chien S. mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr(389) in p70(S6k) FEBS Lett. 2007;24:4562–4566. doi: 10.1016/j.febslet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. Risk factors for Alzheimer’s disease during aging. Impacts of glucose/energy metabolism. J Neural Transm Suppl. 1998:187–194. doi: 10.1007/978-3-7091-7508-8_18. [DOI] [PubMed] [Google Scholar]
- Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J Biomech. 1996;11:1411–1417. doi: 10.1016/0021-9290(96)84536-2. [DOI] [PubMed] [Google Scholar]
- Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009a;6:618–628. doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwee DT, Bodine SC. Age-Related Deficit in Load-Induced Skeletal Muscle Growth. J Gerontol A Biol Sci Med Sci. 2009b doi: 10.1093/gerona/glp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame M, Ishibashi O, Yoshizawa T, Shimomura J, Komori T, Ozawa H, Kawashima H. Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res. 2001;1:24–32. doi: 10.1359/jbmr.2001.16.1.24. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest. 1991;3(Suppl):34S–40S. [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993:613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003a;8:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003b;(Pt 7):1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003c;(Pt 8):1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;7:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;2–3:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;1:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;21:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen LJ, Killian K. Airway smooth muscle as a target of asthma therapy: history and new directions. Respir Res. 2006:123. doi: 10.1186/1465-9921-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci. 2002:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;5:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Joulia-Ekaza D, Cabello G. The myostatin gene: physiology and pharmacological relevance. Curr Opin Pharmacol. 2007;3:310–315. doi: 10.1016/j.coph.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Jozsi AC, Dupont-Versteegden EE, Taylor-Jones JM, Evans WJ, Trappe TA, Campbell WW, Peterson CA. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev. 2000;1–3:45–56. doi: 10.1016/s0047-6374(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Kawada S, Tachi C, Ishii N. Content and localization of myostatin in mouse skeletal muscles during aging, mechanical unloading and reloading. J Muscle Res Cell Motil. 2001;8:627–633. doi: 10.1023/a:1016366409691. [DOI] [PubMed] [Google Scholar]
- Keren A, Tamir Y, Bengal E. The p38 MAPK signaling pathway: a major regulator of skeletal muscle development. Mol Cell Endocrinol. 2006;1–2:224–230. doi: 10.1016/j.mce.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Khan IM, Redman SN, Williams R, Dowthwaite GP, Oldfield SF, Archer CW. The development of synovial joints. Curr Top Dev Biol. 2007:1–36. doi: 10.1016/S0070-2153(06)79001-9. [DOI] [PubMed] [Google Scholar]
- Khawli FA, Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol. 1994;1:317–324. doi: 10.1152/jappl.1994.77.1.317. [DOI] [PubMed] [Google Scholar]
- Kim SO, Katz S, Pelech SL. Expression of second messenger- and cyclin-dependent protein kinases during postnatal development of rat heart. J Cell Biochem. 1998;4:506–521. doi: 10.1002/(sici)1097-4644(19980615)69:4<506::aid-jcb11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kinnard RS, Mylabathula DB, Uddemarri S, Rice KM, Wright GL, Blough ER. Regulation of p70S6k, GSK-3beta, and calcineurin in rat striated muscle during aging. Biogerontology. 2005;3:173–184. doi: 10.1007/s10522-005-7953-6. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;1:1–8. doi: 10.1016/s1357-2725(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Bamman MM. Modulation of the dystrophin-associated protein complex in response to resistance training in young and older men. J Appl Physiol. 2008;5:1476–1484. doi: 10.1152/japplphysiol.00708.2007. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Genton L, Hans D, Karsegard VL, Michel JP, Slosman DO, Pichard C. Total body mass, fat mass, fat-free mass, and skeletal muscle in older people: cross-sectional differences in 60-year-old persons. J Am Geriatr Soc. 2001;12:1633–1640. doi: 10.1046/j.1532-5415.2001.t01-1-49272.x. [DOI] [PubMed] [Google Scholar]
- Landfield PW. Aging-related increase in hippocampal calcium channels. Life Sci. 1996;5–6:399–404. doi: 10.1016/0024-3205(96)00318-9. [DOI] [PubMed] [Google Scholar]
- Lange-Carter CA, Pleiman CM, Gardner AM, Blumer KJ, Johnson GL. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993;5106:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Cline CC, Hu Z, Coast JR. Effect of oxidant challenge on contractile function of the aging rat diaphragm. Am J Physiol. 1997a;2(Pt 1):E201–207. doi: 10.1152/ajpendo.1997.272.2.E201. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Cline CC, Hu Z, Coast JR. Effect of oxidative stress and acidosis on diaphragm contractile function. Am J Physiol. 1997b;2(Pt 2):R630–636. doi: 10.1152/ajpregu.1997.273.2.R630. [DOI] [PubMed] [Google Scholar]
- Lebrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin Inhibition Enhances the Effects of Exercise on Performance and Metabolic Outcomes in Aged Mice. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- Li J, Burr DB, Turner CH. Suppression of prostaglandin synthesis with NS-398 has different effects on endocortical and periosteal bone formation induced by mechanical loading. Calcif Tissue Int. 2002a;4:320–329. doi: 10.1007/s00223-001-1025-y. [DOI] [PubMed] [Google Scholar]
- Li J, Duncan RL, Burr DB, Gattone VH, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology. 2003;4:1226–1233. doi: 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002b;10:1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;2:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;10:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cooley BC, Gould JS. Influence of topical heparin on stasis-induced thrombosis of microvascular anastomoses. Microsurgery. 1992;2:72–75. doi: 10.1002/micr.1920130205. [DOI] [PubMed] [Google Scholar]
- Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;10:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Lieber SC, Qiu H, Chen L, Shen YT, Hong C, Hunter WC, Aubry N, Vatner SF, Vatner DE. Cardiac dysfunction in aging conscious rats: altered cardiac cytoskeletal proteins as a potential mechanism. Am J Physiol Heart Circ Physiol. 2008;2:H860–866. doi: 10.1152/ajpheart.00146.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CC, Apstein CS, Colucci WS, Liao R. Impaired cell shortening and relengthening with increased pacing frequency are intrinsic to the senescent mouse cardiomyocyte. J Mol Cell Cardiol. 2000;11:2075–2082. doi: 10.1006/jmcc.2000.1239. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;2:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- Lushaj EB, Johnson JK, McKenzie D, Aiken JM. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci. 2008;9:921–927. doi: 10.1093/gerona/63.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Mamuya W, Chobanian A, Brecher P. Age-related changes in fibronectin expression in spontaneously hypertensive, Wistar-Kyoto, and Wistar rat hearts. Circ Res. 1992;6:1341–1350. doi: 10.1161/01.res.71.6.1341. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;12:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- Mays PK, McAnulty RJ, Laurent GJ. Age-related changes in lung collagen metabolism. A role for degradation in regulating lung collagen production. Am Rev Respir Dis. 1989;2:410–416. doi: 10.1164/ajrccm/140.2.410. [DOI] [PubMed] [Google Scholar]
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann N Y Acad Sci. 2006:323–331. doi: 10.1196/annals.1354.044. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;23:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Tang DD, Wu MF, Atkinson S, Gunst SJ. Role of Rho in Ca(2+)-insensitive contraction and paxillin tyrosine phosphorylation in smooth muscle. Am J Physiol Cell Physiol. 2000;2:C308–318. doi: 10.1152/ajpcell.2000.279.2.C308. [DOI] [PubMed] [Google Scholar]
- Meikle MC, Heath JK, Reynolds JJ. The use of in vitro models for investigating the response of fibrous joints to tensile mechanical stress. Am J Orthod. 1984;2:141–153. doi: 10.1016/0002-9416(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Melov S, Tarnopolsky MA, Beckman K, Felkey K, Hubbard A. Resistance exercise reverses aging in human skeletal muscle. PLoS One. 2007;5:e465. doi: 10.1371/journal.pone.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merx MW, Schafer C, Westenfeld R, Brandenburg V, Hidajat S, Weber C, Ketteler M, Jahnen-Dechent W. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol. 2005;11:3357–3364. doi: 10.1681/ASN.2005040365. [DOI] [PubMed] [Google Scholar]
- Mikuni-Takagaki Y. Mechanical responses and signal transduction pathways in stretched osteocytes. J Bone Miner Metab. 1999;1:57–60. doi: 10.1007/s007740050065. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Davies LW, Nuki G, Salter DM. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;9:2091–2099. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Wright MO, Lee H, Nishida K, Caldwell H, Nuki G, Salter DM. Integrin-regulated secretion of interleukin 4: A novel pathway of mechanotransduction in human articular chondrocytes. J Cell Biol. 1999;1:183–189. doi: 10.1083/jcb.145.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki S, Forbes DP. The morphologic and biochemical effects of tensile force application to the interparietal suture of the Sprague-Dawley rat. Am J Orthod Dentofacial Orthop. 1987;2:123–133. doi: 10.1016/0889-5406(87)90367-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Noguchi M, Takemasa T. Intermittent reloading attenuates muscle atrophy through modulating Akt/mTOR pathway. Med Sci Sports Exerc. 2008;5:848–855. doi: 10.1249/MSS.0b013e318163275f. [DOI] [PubMed] [Google Scholar]
- Mora M, Di Blasi C, Barresi R, Morandi L, Brambati B, Jarre L, Cornelio F. Developmental expression of dystrophin, dystrophin-associated glycoproteins and other membrane cytoskeletal proteins in human skeletal and heart muscle. Brain Res Dev Brain Res. 1996;1:70–82. doi: 10.1016/0165-3806(95)00169-7. [DOI] [PubMed] [Google Scholar]
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J Appl Physiol. 2004;1:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- Mylabathula DB, Rice KM, Wang Z, Uddemarri S, Kinnard RS, Blough ER. Age-associated changes in MAPK activation in fast- and slow-twitch skeletal muscle of the F344/NNiaHSD X Brown Norway/BiNia rat model. Exp Gerontol. 2006;2:205–214. doi: 10.1016/j.exger.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;2:139–150. doi: 10.1161/01.res.0000053618.86362.df. [DOI] [PubMed] [Google Scholar]
- Nair RR, Nair P. Age-dependent variation in contractility of adult cardiac myocytes. Int J Biochem Cell Biol. 2001;2:119–125. doi: 10.1016/s1357-2725(00)00077-7. [DOI] [PubMed] [Google Scholar]
- Nin N, Lorente JA, De Paula M, Fernandez-Segoviano P, Penuelas O, Sanchez-Ferrer A, Martinez-Caro L, Esteban A. Aging increases the susceptibility to injurious mechanical ventilation. Intensive Care Med. 2008;5:923–931. doi: 10.1007/s00134-007-0960-0. [DOI] [PubMed] [Google Scholar]
- Oba A, Edwards C. Relationships between changes in mechanical properties of the skin, wrinkling, and destruction of dermal collagen fiber bundles caused by photoaging. Skin Res Technol. 2006;4:283–288. doi: 10.1111/j.0909-752X.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Olesen OF. Tau, amyloid and Alzheimer’s disease. Ugeskr Laeger. 1994;8:1116–1117. 1121–1114. [PubMed] [Google Scholar]
- Orlandi A, Francesconi A, Marcellini M, Ferlosio A, Spagnoli LG. Role of ageing and coronary atherosclerosis in the development of cardiac fibrosis in the rabbit. Cardiovasc Res. 2004;3:544–552. doi: 10.1016/j.cardiores.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;1:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;2:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;3:237–248. doi: 10.1002/bdrc.20050. [DOI] [PubMed] [Google Scholar]
- Papazafiri P, Kletsas D. Developmental and age-related alterations of calcium homeostasis in human fibroblasts. Exp Gerontol. 2003;3:307–311. doi: 10.1016/s0531-5565(02)00201-2. [DOI] [PubMed] [Google Scholar]
- Parameswaran K, Janssen LJ, O’Byrne PM. Airway hyperresponsiveness and calcium handling by smooth muscle: a “deeper look”. Chest. 2002;2:621–624. doi: 10.1378/chest.121.2.621. [DOI] [PubMed] [Google Scholar]
- Pardo PS, Lopez MA, Boriek AM. FOXO transcription factors are mechanosensitive and their regulation is altered with aging in the respiratory pump. Am J Physiol Cell Physiol. 2008;4:C1056–1066. doi: 10.1152/ajpcell.00270.2007. [DOI] [PubMed] [Google Scholar]
- Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;1:243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;6(Pt 1):C1591–1601. [PubMed] [Google Scholar]
- Pavlin D, Zadro R, Gluhak-Heinrich J. Temporal pattern of stimulation of osteoblast-associated genes during mechanically-induced osteogenesis in vivo: early responses of osteocalcin and type I collagen. Connect Tissue Res. 2001;2:135–148. doi: 10.3109/03008200109014255. [DOI] [PubMed] [Google Scholar]
- Pehme A, Alev K, Kaasik P, Seene T. Age-related changes in skeletal-muscle myosin heavy-chain composition: effect of mechanical loading. J Aging Phys Act. 2004;1:29–44. doi: 10.1123/japa.12.1.29. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;6938:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem. 2006;52:39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- Puizina-Ivic N. Skin aging. Acta Dermatovenerol Alp Panonica Adriat. 2008;2:47–54. [PubMed] [Google Scholar]
- Pyle WG, Solaro RJ. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;3:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- Ragan PM, Badger AM, Cook M, Chin VI, Gowen M, Grodzinsky AJ, Lark MW. Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;6:836–842. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts’ and osteocytes’ early responses to mechanical strain. Bone. 1996;6:609–614. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- Raza M, Deshpande LS, Blair RE, Carter DS, Sombati S, DeLorenzo RJ. Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neurosci Lett. 2007;1:77–81. doi: 10.1016/j.neulet.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Jr, Gimbrone MA., Jr Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci U S A. 1993;10:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KM, Desai DH, Kinnard RS, Harris R, Wright GL, Blough ER. Load-induced focal adhesion mechanotransduction is altered with aging in the Fischer 344/NNiaHSd x Brown Norway/BiNia rat aorta. Biogerontology. 2007a;3:257–267. doi: 10.1007/s10522-006-9066-2. [DOI] [PubMed] [Google Scholar]
- Rice KM, Desai DH, Preston DL, Wehner PS, Blough ER. Uniaxial stretch-induced regulation of mitogen-activated protein kinase, Akt and p70 S6 kinase in the ageing Fischer 344 x Brown Norway rat aorta. Exp Physiol. 2007b;5:963–970. doi: 10.1113/expphysiol.2007.037275. [DOI] [PubMed] [Google Scholar]
- Rice KM, Kinnard RS, Harris R, Wright GL, Blough ER. Effects of aging on pressure-induced MAPK activation in the rat aorta. Pflugers Arch. 2005a;3:192–199. doi: 10.1007/s00424-005-1383-9. [DOI] [PubMed] [Google Scholar]
- Rice KM, Kinnard RS, Wright GL, Blough ER. Aging alters vascular mechanotransduction: pressure-induced regulation of p70S6k in the rat aorta. Mech Ageing Dev. 2005b;11:1213–1222. doi: 10.1016/j.mad.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005c;5:335–343. doi: 10.1007/s10522-005-4808-0. [DOI] [PubMed] [Google Scholar]
- Rice KM, Preston DL, Neff D, Norton M, Blough ER. Age-related dystrophin-glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. J Gerontol A Biol Sci Med Sci. 2006;11:1119–1129. doi: 10.1093/gerona/61.11.1119. [DOI] [PubMed] [Google Scholar]
- Rice KM, Wu M, Blough ER. Aortic Aging in the Fischer 344/NNiaHSd x Brown Norway/BiNia Rat. J Pharmacol Sci. 2008;4:393–398. doi: 10.1254/jphs.08r02cp. [DOI] [PubMed] [Google Scholar]
- Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF. Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport. 2004;6:955–959. doi: 10.1097/00001756-200404290-00005. [DOI] [PubMed] [Google Scholar]
- Robert L, Labat-Robert J, Robert AM. Physiology of skin aging. Pathol Biol (Paris) 2009;4:336–341. doi: 10.1016/j.patbio.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Rossi A, Ganassini A, Tantucci C, Grassi V. Aging and the respiratory system. Aging (Milano) 1996;3:143–161. doi: 10.1007/BF03339671. [DOI] [PubMed] [Google Scholar]
- Ryder KD, Duncan RL. Parathyroid hormone enhances fluid shear-induced [Ca2+]i signaling in osteoblastic cells through activation of mechanosensitive and voltage-sensitive Ca2+ channels. J Bone Miner Res. 2001;2:240–248. doi: 10.1359/jbmr.2001.16.2.240. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;1:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Sato M, Westmore M, Clendenon J, Smith S, Hannum B, Zeng GQ, Brommage R, Turner CH. Three-dimensional modeling of the effects of parathyroid hormone on bone distribution in lumbar vertebrae of ovariectomized cynomolgus macaques. Osteoporos Int. 2000;10:871–880. doi: 10.1007/s001980070047. [DOI] [PubMed] [Google Scholar]
- Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;18:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyy JY, Lin MC, Han J, Lu Y, Petrime M, Chien S. The cis-acting phorbol ester “12-O-tetradecanoylphorbol 13-acetate”-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc Natl Acad Sci U S A. 1995;17:8069–8073. doi: 10.1073/pnas.92.17.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver FH, Bradica G. Mechanobiology of cartilage: how do internal and external stresses affect mechanochemical transduction and elastic energy storage? Biomech Model Mechanobiol. 2002;3:219–238. doi: 10.1007/s10237-002-0017-9. [DOI] [PubMed] [Google Scholar]
- Silver FH, Glasgold AI. Cartilage wound healing. An overview. Otolaryngol Clin North Am. 1995;5:847–864. [PubMed] [Google Scholar]
- Smith PG, Tokui T, Ikebe M. Mechanical strain increases contractile enzyme activity in cultured airway smooth muscle cells. Am J Physiol. 1995;6(Pt 1):L999–1005. doi: 10.1152/ajplung.1995.268.6.L999. [DOI] [PubMed] [Google Scholar]
- Smith RL, Rusk SF, Ellison BE, Wessells P, Tsuchiya K, Carter DR, Caler WE, Sandell LJ, Schurman DJ. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;1:53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- Spinale FG, Zellner JL, Johnson WS, Eble DM, Munyer PD. Cellular and extracellular remodeling with the development and recovery from tachycardia-induced cardiomyopathy: changes in fibrillar collagen, myocyte adhesion capacity and proteoglycans. J Mol Cell Cardiol. 1996;8:1591–1608. doi: 10.1006/jmcc.1996.0150. [DOI] [PubMed] [Google Scholar]
- Steinmeyer J, Ackermann B, Raiss RX. Intermittent cyclic loading of cartilage explants modulates fibronectin metabolism. Osteoarthritis Cartilage. 1997;5:331–341. doi: 10.1016/s1063-4584(97)80037-4. [DOI] [PubMed] [Google Scholar]
- Sudel KM, Venzke K, Mielke H, Breitenbach U, Mundt C, Jaspers S, Koop U, Sauermann K, Knussman-Hartig E, Moll I, Gercken G, Young AR, Stab F, Wenck H, Gallinat S. Novel aspects of intrinsic and extrinsic aging of human skin: beneficial effects of soy extract. Photochem Photobiol. 2005;3:581–587. doi: 10.1562/2004-06-16-RA-202. [DOI] [PubMed] [Google Scholar]
- Sumner DR, Andriacchi TP. Adaptation to differential loading: comparison of growth-related changes in cross-sectional properties of the human femur and humerus. Bone. 1996;2:121–126. doi: 10.1016/8756-3282(96)00166-4. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;49:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski G, Nethery D, Stofan D, DiMarco A. Extracellular calcium modulates generation of reactive oxygen species by the contracting diaphragm. J Appl Physiol. 1999;6:2177–2185. doi: 10.1152/jappl.1999.87.6.2177. [DOI] [PubMed] [Google Scholar]
- Sussman MA, McCulloch A, Borg TK. Dance band on the Titanic: biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;10:888–898. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Maruyama A, Sugiura T, Machida S, Miyata H. Age-related changes in two- and three-dimensional morphology of type-identified endplates in the rat diaphragm. J Physiol Sci. 2009;1:57–62. doi: 10.1007/s12576-008-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Miyawaki Y, Tanaka M, Watanabe M, Lee K, del Pozo R, Tanne K. Effects of tensile forces on the expression of type III collagen in rat interparietal suture. Arch Oral Biol. 2000;12:1049–1057. doi: 10.1016/s0003-9969(00)00083-2. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Lipofuscin: mechanisms of formation and increase with age. Apmis. 1998;2:265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;3:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;5:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;3:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;5264:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Totsukawa G, Yamakita Y, Yamashiro S, Hartshorne DJ, Sasaki Y, Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;4:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Boulanger CM, Mombouli JV. Endothelium-derived relaxing factors and converting enzyme inhibition. Am J Cardiol. 1995;15:3E–12E. [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;6:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;3:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. Proc Natl Acad Sci U S A. 2002;12:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent T, Saklatvala J. Basic fibroblast growth factor: an extracellular mechanotransducer in articular cartilage? Biochem Soc Trans. 2006;(Pt 3):456–457. doi: 10.1042/BST0340456. [DOI] [PubMed] [Google Scholar]
- Vincent TL, Hermansson MA, Hansen UN, Amis AA, Saklatvala J. Basic fibroblast growth factor mediates transduction of mechanical signals when articular cartilage is loaded. Arthritis Rheum. 2004;2:526–533. doi: 10.1002/art.20047. [DOI] [PubMed] [Google Scholar]
- Waller JM, Maibach HI. Age and skin structure and function, a quantitative approach (I): blood flow, pH, thickness, and ultrasound echogenicity. Skin Res Technol. 2005;4:221–235. doi: 10.1111/j.0909-725X.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;5111:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;6:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mech Dev. 2004;11:1301–1312. doi: 10.1016/j.mod.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;(Pt 3):977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston FK, Thibault LE, Macarak EJ. An analysis of the time-dependent changes in intracellular calcium concentration in endothelial cells in culture induced by mechanical stimulation. J Biomech Eng. 1993;2:160–168. doi: 10.1115/1.2894116. [DOI] [PubMed] [Google Scholar]
- Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;9:575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;4:391–399. doi: 10.1016/s0945-053x(99)00029-3. [DOI] [PubMed] [Google Scholar]
- Wretman C, Lionikas A, Widegren U, Lannergren J, Westerblad H, Henriksson J. Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle. J Physiol. 2001;(Pt 1):155–164. doi: 10.1111/j.1469-7793.2001.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Desai DH, Kakarla SK, Katta A, Paturi S, Gutta AK, Rice KM, Walker EM, Jr, Blough ER. Acetaminophen prevents aging-associated hyperglycemia in aged rats: effect of aging-associated hyperactivation of p38-MAPK and ERK1/2. Diabetes Metab Res Rev. 2009a;3:279–286. doi: 10.1002/dmrr.932. [DOI] [PubMed] [Google Scholar]
- Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of akt/protein kinase B: s-nitrosylation and acetaminophen intervention. PLoS One. 2009;7:e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhou Y, Jarrett HW. Dystrophin glycoprotein complex-associated Gbetagamma subunits activate phosphatidylinositol-3-kinase/Akt signaling in skeletal muscle in a laminin-dependent manner. J Cell Physiol. 2009;2:402–414. doi: 10.1002/jcp.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol. 1998;6(Pt 2):H2087–2094. doi: 10.1152/ajpheart.1998.275.6.H2087. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;5:343–348. [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;16:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- Yu JC, Lucas JH, Fryberg K, Borke JL. Extrinsic tension results in FGF-2 release, membrane permeability change, and intracellular Ca++ increase in immature cranial sutures. J Craniofac Surg. 2001;4:391–398. doi: 10.1097/00001665-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;7:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- Zaman G, Cheng MZ, Jessop HL, White R, Lanyon LE. Mechanical strain activates estrogen response elements in bone cells. Bone. 2000;2:233–239. doi: 10.1016/s8756-3282(00)00324-0. [DOI] [PubMed] [Google Scholar]
- Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;2:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- Zhan M, Jin B, Chen SE, Reecy JM, Li YP. TACE release of TNF-alpha mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J Cell Sci. 2007;(Pt 4):692–701. doi: 10.1242/jcs.03372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;5:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]