Abstract
Objective
To define relationships of androgen excesses to cancer risk.
Design
Retrospective cohort study.
Patients
Among 12,193 women evaluated for infertility between 1965-1988 and traced for cancer incidence through 1999, 2,560 had androgen excess or menstrual disorders; among these, 412 met established criteria for PCOS.
Main Outcome
Cancer incidence.
Methods
Derivation of standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for cancer risk comparisons with the general population and rate ratios (RRs) for comparisons with other infertility patients.
Results
Androgen excess/menstrual disorders patients showed significant SIRs for breast (1.31, 95% CIs 1.05-1.62) and uterine (2.02, 1.13-3.34) cancers and melanoma (1.96, 1.12-3.18). Significant associations for breast and uterine cancers were restricted to primary infertility patients (respective SIRs of 1.53 and 3.48). After adjustment for other cancer predictors, the only excess risk was for uterine cancer among primary infertility patients. Compared to women with secondary infertility and no androgen excess/menstrual disorder, those with primary infertility and a disorder had a RR of 1.88 (95% CI 0.82-4.32). Cancer risks among the women with PCOS or androgen excess disorders appeared similar to the more comprehensive group.
Conclusions
Previous findings linking androgen excess disorders to elevated uterine cancer risks may largely reflect underlying risk profiles.
Keywords: Androgen excess, PCOS, uterine cancer, breast cancer, ovarian cancer, risk
Introduction
Although the effects of estrogens on female cancers are well recognized, the role of other hormones is less clear. Recent interest has focused on the role of androgens (1), which may affect uterine and breast cancers as well as other cancers.
A variety of conditions are associated with androgen excess, the best known of which is polycystic ovary syndrome (PCOS), a condition affecting between 3-5% of women of reproductive ages (2;3). The clinical manifestations of PCOS are diverse and include menstrual dysfunction, infertility, hirsutism, acne, obesity and the metabolic syndrome. The condition is a frequent cause of hyperandrogenism, oligoanovulation, and abnormal insulin activity. The definition of PCOS has been controversial (4), although recently developed criteria (5) have allowed progress in understanding its etiology and clinical associations.
A number of clinical reports (6-7) have noted a link between PCOS and endometrial cancer, with additional support deriving from a limited number of epidemiologic investigations (8-12). However, most of these studies had limited numbers of patients or imprecise diagnostic criteria. Only a few studies have assessed relationships of androgen disorders to ovarian (13-14) or breast (12-13;15-18) cancers.
We took advantage of precise clinical workups among a large series of infertility patients to assess cancer risk associated with PCOS and androgen excess or menstrual disorders.
Materials and Methods
Study Subject Eligibility
This study was reviewed by institutional review boards at the National Cancer Institute and the participating institutions. We identified 12,193 patients who had sought advice for primary or secondary infertility at five large practices in Boston, MA; Chicago, IL; Detroit, MI; Palo Alto, CA; and New York City, NY between 1965 and 1988. Data on patient identifiers, infertility work-ups, drug information, menstrual and reproductive histories, and other factors affecting health (e.g., weight) were entered into computerized databases by trained medical abstractors.
Follow-up of Patients
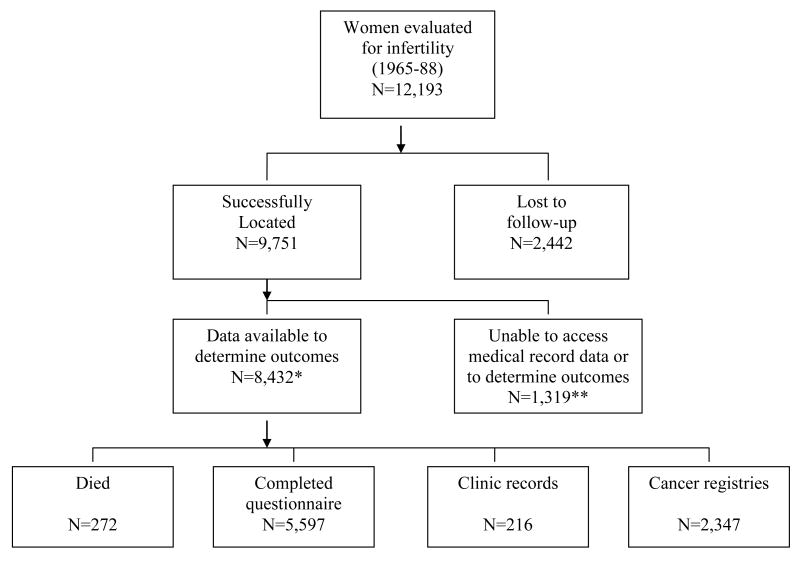
During 2000-2002, attempts were made to locate study subjects and ascertain updated health status (Figure 1). Various sources were used to trace patients and identify occurrences of cancer, with 2,442 (20.0%) lost to follow-up. Information on cancers was obtained through completed questionnaires as well as linkages against the National Death Index (NDI) and eight cancer registries in states where many patients resided (California, Florida, Illinois, Massachusetts, Michigan, New Jersey, New York, and Texas).
Figure 1.
Field and Analytic Status of Eligible Study Subjects, Women Evaluated for Infertility, 1965-1988
**Analyses excluded 10 patients who developed cancer within the first year of follow-up.
*Includes 1,151 patients who refused access to their medical record data plus 168 patients who did not reside in cancer registry states and who did not respond to our questionnaire.
A total of 1,151 (9.4%) of the located patients indicated that they did not want to participate in the study or allow use of their medical record data. Of the remaining patients, 168 patients did not live in cancer registry states and we were unable to determine updated health information through other sources, 272 patients had died, 5,597 completed questionnaires, 216 continued receiving care at the institutions where they were evaluated for infertility, and 2,347 were linked against state cancer registries.
In addition to updated health status, the questionnaires collected information on demographic factors and lifestyle factors that could affect health, including menstrual and reproductive history; use of exogenous hormones; anthropometric factors; cigarette smoking; alcohol consumption; and screening for breast and ovarian diseases.
Analytic Dataset
Person years were accrued beginning one year after the date of first evaluation for infertility and continuing through the earliest date of cancer occurrence, death, the date last known alive and free of cancer, or December 31, 1999. Patients whose vital status depended on linkage against a cancer registry were assigned variable study ending dates, depending on when each registry had complete information (range of 1997-1999). We eliminated 10 patients who were diagnosed with cancer during the first year of follow-up, leaving 8,422 analytic study subjects and 155,624 person-years of follow-up.
We used Rotterdam criteria (5) to define a subcohort of women with PCOS based on the presence of two or more of the following: 1) oligoovulation or anovulation, 2) clinical and/or biochemical signs of hyperandrogenism (not due to a specific disorder such as adrenal disease), or 3) polycystic ovaries.
Although large numbers of our patients were oligoovulatory or anovulatory (19), few had hyperandrogenism (as defined on the basis of hirsuitism or total testosterone levels >75 ng/dl or free testosterone levels >7.3 pg/ml) or polycystic ovaries (defined by laparoscopy or laparotomy rather than by ultrasound). Only 412 women met Rotterdam criteria for PCOS. We therefore expanded this cohort to include women who had hyperandrogenism or polycystic ovaries (androgen excess disorders). We also employed a wider definition in which we included all women whose records indicated that they had any one of the criterion (androgen excess or menstrual disorders).
Statistical Analyses
We calculated standardized incidence ratios (SIRs) and 95% confidence intervals (CI) comparing cancer incidence rates within each cohort to U.S. rates. SIRs were computed as the number of observed cancers among the patient subgroups divided by the expected events based on age, race and calendar year-specific incidence disease rates from the Surveillance Epidemiology and End Results (SEER) Program of the NCI.
A second analytic approach involved internal analyses within the cohort of infertility patients, comparing cancer risks among women with different androgen excess disorders to those without these disorders. Person-years were truncated at the time of hysterectomy (self-reports via questionnaire) for the uterine cancer analyses and at the time of bilateral oophorectomy for the ovarian cancer analyses; 30 women who had had a hysterectomy and 60 women who had a bilateral oophorectomy within 1 year of their first clinic visit were not eligible for analyses of uterine and ovarian cancer, respectively.
Rate ratios (RRs) and their 95% CIs for cancer associated with differing definitions of androgen-related disorders were estimated by Poisson regression using standard likelihood ratio methods (20). Multivariate models were employed to adjust for age at risk, calendar year of follow-up, study site, nulligravity at entry (which related to increased risks of breast, uterine and ovarian cancer in this population) and clomiphene (which related to increased uterine cancer risk) (21-23). We also evaluated the impact on uterine cancer risk of additional adjustment for body mass index (BMI).
Results
A total of 2,560 of the patients met at least one of the Rotterdam criteria for PCOS and were classified with androgen excess or menstrual disorders (Table 1). Of these, 56 met all three criteria, 356 met two criteria, and 2,148 met only one. Our more restricted definition of androgen excess disorders consisted of 855 patients, comprised of 412 who met Rotterdam criteria for PCOS (two of three criteria), 109 patients who had polycystic ovaries alone, and 334 who had hyperandrogenism alone.
Table 1.
Classification of the 8,422 Analytic Study Patients According to Disorder Classification Criteria
| Number of Criteria | Oligoovulation or anovulation | Hyperandrogenism | Polycystic ovaries | Frequency | Disorder Classification1 |
|---|---|---|---|---|---|
| 3 | Yes | Yes | Yes | 56 | PCOS |
| 2 | No | Yes | Yes | 22 | PCOS |
| Yes | No | Yes | 163 | PCOS | |
| Yes | Yes | No | 171 | PCOS | |
| 1 | Yes | No | No | 1,705 | Menstrual disorder only |
| No | Yes | No | 334 | Androgen excess only | |
| No | No | Yes | 109 | Androgen excess only | |
| 0 | No | No | No | 5,862 | No androgen excess or menstrual disorder |
Patients who met defined criteria for PCOS according to Rotterdam criteria (n=412). Androgen excess disorder patients include those with PCOS plus those with androgen excesses (n=855). Androgen excess or menstrual disorder patients include all of the above mentioned patients plus those with menstrual disorders (n=2,560).
A total of 48,417 person-years were contributed by the 2,560 patients with androgen excess or menstrual disorders. Nearly 80% of the subjects were Caucasian. The median age at first evaluation was 30.9 years, while the median year of first evaluation was 1978.9. The median length of follow-up was 18.9 years. A total of 39% of the patients had been exposed to clomiphene, while 10% received gonadotrophins. Similar characteristics were seen among the patients with the more specific diagnoses of androgen excess disorders or PCOS had similar characteristics as well as among the total population of infertility patients.
Neither the patients with androgen excess or menstrual disorders nor those with specific androgen excess disorders had an overall increased risk of developing any noncutaneous cancer as compared with the general population (respective SIRs and 95% CIs of 0.99, 0.85-1.15 and 0.93, 0.70-1.22) (Table 2). However, statistically significant excess SIRs were observed for breast cancer (SIR=1.31, 95% CI 1.05-1.62), uterine cancer (2.02, 1.13-3.34) and melanoma (1.96, 1.12-3.18). Similar excess risks were seen for uterine cancer and melanoma among the patients who had androgen excess disorders, although the SIRs were based on small numbers and not statistically significant. Non-significant elevations in risk were also seen for thyroid cancer (SIR=2.68, 0.87-6.27, 5 cases) and lymphatic tumors (1.99, 0.64-4.64, 5 cases). No increased risk of breast cancer was seen for these patients.
Table 2.
Standardized Incidence Ratios (SIR) for Selected Cancer by Characterization of Androgen Excess or Menstrual Disorders, Comparison of Subcohorts of Infertile Women with the General Population1
| Androgen Excess or Menstrual Disorders (n=2,560)2 | Androgen Excess Disorders (n=855)3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Observed No.4 | Expected No.5 | SIR6 | 95% CI | Observed No.4 | Expected No. | SIR5 | 95% CI | |
| All sites | 179 | 180.15 | 0.99 | 0.85-1.15 | 54 | 57.82 | 0.93 | 0.70-1.22 |
| Colon | 6 | 4.76 | 1.26 | 0.46-2.75 | ||||
| Trachea, bronchus, lung | 5 | 8.55 | 0.58 | 0.19-1.36 | ||||
| Breast | 89 | 67.80 | 1.31 | 1.05-1.62 | 23 | 21.59 | 1.07 | 0.68-1.60 |
| Cervix | 5 | 7.13 | 0.70 | 0.23-1.64 | ||||
| Uterine corpus | 15 | 7.42 | 2.02 | 1.13-3.34 | 5 | 2.27 | 2.20 | 0.71-5.13 |
| Ovary | 12 | 6.82 | 1.76 | 0.91-3.07 | ||||
| Melanoma | 16 | 8.17 | 1.96 | 1.12-3.18 | 5 | 2.71 | 1.85 | 0.59-4.31 |
| Thyroid | 6 | 5.59 | 1.07 | 0.39-2.33 | 5 | 1.86 | 2.68 | 0.87-6.27 |
| Lymphatic tumors | 8 | 7.91 | 1.01 | 0.44-1.99 | 5 | 2.51 | 1.99 | 0.64-4.64 |
Based on data from the Surveillance, Epidemiology and End Results (SEER) Program.
Includes infertility patients who had either oligo- or anovulation, clinical and/or biochemical signs of hyperandrogenism, or polycystic ovaries.
Includes infertility patients who met Rotterdam criteria for PCOS (had 2 or the 3 conditions defined above) as well as those who had either clinical or biochemical signs of hyperandrogenism or polycystic ovaries.
Only cancers for which there were 5 or more observed cases are shown.
Expected rates were generated by applying age, race, and calendar year-specific SEER incidence rates to the person years of the cohort of interest.
Numbers of observed cancers divided by the expected number.
SIRs were also examined among the relatively small group of patients (n=412) who met established criteria for PCOS. The risk for uterine cancer was consistent with that among the patients with androgen excess or menstrual disorders (SIR=1.91, 95% CI 0.2-6.0, based on 2 cancers) (data not shown). There was no evidence of elevated breast cancer risk among the PCOS patients (SIR=0.90, 0.4-1.7, based on 9 breast cancers).
Significant increases in the risk of breast and uterine cancers were restricted to patients with primary infertility (no previous pregnancies) (SIR and 95% CI of 1.53, 1.10-2.08 for breast cancer and 3.48, 1.66-6.40 for uterine cancer). Ovarian cancer was elevated among patients with secondary infertility (SIR=2.18, 95% CI 0.99-4.14), but not among those with primary infertility. Approximately 2-fold non-significant increases in melanoma risk were seen among both primary and secondary infertility patients.
We also examined cancer incidence among the patients with androgen excess disorders, although number of events limited interpretation of most risks. Such patients with primary infertility demonstrated increased risks of both uterine cancer (SIR=3.03, 95% CI 0.61-8.85) and melanoma (2.47, 0.50-7.22).
In internal analyses, the highest, albeit non-significant, RRs for the patients with androgen excess or menstrual disorders were seen for uterine cancer (RR=1.33, 95% CI 0.69-2.58) and melanoma (1.27, 0.67-2.40) (Table 3). The increased risk of uterine cancer was somewhat attenuated after adjustment for BMI (1.17, 0.60-2.28). Similar RRs were seen for these two cancer sites (and for uterine cancer after adjustment for BMI) when only patients with androgen excess disorders were considered. These patients also demonstrated a statistically significantly elevated risk of thyroid cancer (RR=3.64, 1.24-10.63), although based on only 5 observed cancers.
Table 3.
Rate Ratios for Selected Cancers by Characterization of Androgen Excess or Menstrual Disorders, Comparison Within the Cohort of Women with Infertility
| Androgen Excess or Menstrual Disorders | Androgen Excess Disorders | |||||
|---|---|---|---|---|---|---|
| No. of Cancers | RR1 | 95% CI | No. of Cancers | RR1 | 95% CI | |
| All sites | 179 | 1.04 | 0.87-1.24 | 54 | 0.98 | 0.74-1.30 |
| Breast | 89 | 1.02 | 0.79-1.31 | 23 | 0.81 | 0.53-1.25 |
| Uterine corpus2 | 15 | 1.33 | 0.69-2.58 | 5 | 1.28 | 0.49-3.35 |
| Uterine corpus (adjusted additionally for body mass index) | 15 | 1.17 | 0.60-2.28 | 5 | 1.13 | 0.43-2.97 |
| Ovary3 | 12 | 0.90 | 0.46-1.77 | 2 | 0.42 | 0.10-1.75 |
| Melanoma | 16 | 1.27 | 0.67-2.40 | 5 | 1.17 | 0.45-3.02 |
| Thyroid | 6 | 1.09 | 0.40-2.98 | 5 | 3.64 | 1.24-10.63 |
RRs adjusted for age at follow-up, calendar time, study site, gravidity at entry, and clomiphene usage.
Person-years for this cancer site censored at time of hysterectomy.
Person-years for this cancer site censored at time of removal of both ovaries.
We also calculated risks in reference to women who should have been at lowest risk, i.e., those with secondary infertility and no evidence of a disorder (Table 4). For uterine cancer, the RR among women with primary infertility and androgen excess or menstrual disorders was somewhat elevated, but not significantly so (RR=1.88, 95% CI 0.82-4.32). A similar increased risk was seen among patients with androgen excess disorders, although the RR of 1.78 was based on only 3 cases. Breast cancer risk was fairly uniform across the four exposure levels defined by type of infertility and endocrinologic abnormality, regardless of whether the focus was on patients with androgen excess or menstrual disorders or only those with androgen excess disorders.
Table 4.
Ratios for Uterine and Breast Cancers by Characterization of Androgen Excess or Menstrual Disorders and Type of Infertility, Comparison Within the Cohort of Women with Infertility
| Uterine Cancer | Breast Cancer | |||||
|---|---|---|---|---|---|---|
| No of cases | RR1 | 95% CI | No. of cases | RR1 | 95% CI | |
| Androgen Excess or Menstrual Disorders | ||||||
| Secondary infertility, no disorder | 14 | 1.00 | (referent) | 120 | 1.00 | (referent) |
| Secondary infertility, disorder | 5 | 0.59 | 0.20-1.67 | 48 | 0.87 | 0.62-1.23 |
| Primary infertility, no disorder | 10 | 0.90 | 0.40-2.04 | 83 | 0.92 | 0.69-1.22 |
| Primary infertility, disorder | 10 | 1.88 | 0.82-4.32 | 41 | 1.13 | 0.79-1.62 |
| Androgen Excess Disorders | ||||||
| Secondary infertility, no disorder | 17 | 1.00 | (referent) | 159 | 1.00 | (referent) |
| Secondary infertility, disorder | 2 | 0.96 | 0.21-4.22 | 9 | 0.56 | 0.28-1.10 |
| Primary infertility, no disorder | 17 | 1.39 | 0.71-2.74 | 110 | 0.96 | 0.76-1.23 |
| Primary infertility, disorder | 3 | 1.78 | 0.51-6.25 | 14 | 1.10 | 0.63-1.90 |
RRs were adjusted for age at follow-up, calendar time, study site, gravidity at entry, and clomiphene usage. Risks for uterine cancer were adjusted additionally for body mass index. Person-years for uterine cancer analyses were truncated at the time of hysterectomy.
Discussion
Patients with PCOS have been shown to have an increased risk of type 2 diabetes and cardiovascular diseases, but effects on other chronic diseases, including cancer, remain unresolved (24,25). Our study demonstrates the difficulty of studying the relationship of PCOS to cancer, given that only 4.9% of our patients met Rotterdam criteria (5), a rate lower than some might expect among infertility patients. Although we expanded the PCOS group to include patients with hyperandrogenism or polycystic ovaries, this only slightly increased numbers. We therefore additionally included patients with oligoovulation or anovulation, recognizing that this may have led to an inclusion of endocrinologic abnormalities that are not androgen-related. Nonetheless, this approach undoubtedly is superior to previous epidemiologic studies which have relied on patients reports of PCOS.
Many clinical studies have noted high rates of endometrial cancer among PCOS patients (6-7;26-30), with particularly high risks often noted among younger women (26;28;29). Few epidemiologic investigations, however, have evaluated relationships. Coulam and others (8) found a 3.1-fold increased risk of endometrial cancer associated with chronic anovulation, while Wild and others (12) found a 5.3-fold risk among women with medically documented polycystic ovaries or ovarian dysfunction. In one study, a relationship was restricted to younger women (31). Recently, dos Santos Silva (13) found 2-3-fold risks associated with WHO type II ovulatory disorders (mainly PCOS). Elevated risks have also been noted in case-control studies that have involved self-reports of PCOS (9;11;32).
Comparisons based on the general population are difficult to interpret, given that patients with ovulatory disorders often have risk factors that place them at inherently high cancer risks (33). Our internal analyses, which allowed for adjustment for many of these factors, including obesity, found only a non-significant 33% elevated uterine cancer risk. Differences in the magnitude of risk between the external and internal analyses suggest that the apparent excess uterine cancer risk previously ascribed to PCOS or androgen excess disorders may be due to shared risk factors. In fact, given that we had only limited information on obesity (including no changes in weight over time), we cannot rule out that all of the increased risk was attributable to obesity. However, it was of interest that the highest, albeit non-significant, risk of uterine cancer was among patients with primary infertility, possibly indicating that only the more severe forms of these disorders predispose to uterine cancer.
Although a high risk of breast cancer mortality has been noted among patients with polycystic ovaries (18), we did not find breast cancer incidence to be elevated among any of our subcohorts. Results from previous epidemiologic studies have ranged from increased risk (34) to no association (15;17) to possible reductions in risk (16), possibly reflecting divergent disease definitions employed. In one cohort study the self-reported prevalence of Stein-Leventhal syndrome was 1.35% (15), while in a case-control study 4.9% of the breast cancer cases reported having PCOS (16). In this latter study, PCOS was associated with a significantly reduced risk, possibly reflecting the widespread use of wedge resections for treatment of PCOS in the 1970s (35), a procedure which could have altered ovarian hormone secretion. Chance findings are also a possibility given small numbers involved.
A potential role for androgen disorders in ovarian carcinogenesis has been postulated (36), although few epidemiologic studies have been undertaken. Our initial findings of elevated ovarian cancer risk when comparisons involved the general population were not supported by more detailed internal analyses. One study noted a positive association between acne or hirsutism and risk, although the finding was based on small numbers (37). Two more recent studies have also reported positive relationships, although in one the increased risk was restricted to non-users of oral contraceptives or thin women (14) and the other pertained only to the development of borderline serous cancers (38). The results must also be cautiously interpreted given that self reports of polycystic ovarian disease were involved. The possibility of confounding by infertility treatment has also been acknowledged (14).
Our study had some limitations. This included the fact that disorders were determined during a time when newer classification modalities (e.g., androgen assays) were generally unavailable and we had no ability to update information beyond the time of the initial infertility evaluation. Whether our results are generalizable to women other than infertility patients must also be questioned. Further, our results may have been affected by residual confounding, either by factors that we were unable to completely measure (e.g., obesity) or by other factors for which we had no information, such as diabetes and metabolic syndrome.
In summary, we only observed an increased risk of uterine cancer associated with androgen excess/menstrual disorders, but much of the excess appeared to reflect shared characteristics between the two diseases. Although we were unable to extensively consider cancer risks in patients meeting strict criteria for PCOS, it appears from our findings that their cancer risks should not substantially exceed those of patients with broader definitions of androgen excess disorders.
Acknowledgments
We thank Jerome Mabie of IMS, Inc. of Rockville, MD for his assistance with computer analyses.
This study was supported in part by funds from the intramural research program of the National Cancer Institute, National Institutes of Health.
Footnotes
Capsule: After accounting for such risk factors as parity and obesity, infertility patients with PCOS or androgen excess/menstrual disorders showed only modest uterine cancer increases and no alterations of other cancers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Myers E. Surrogate end-points or primary outcomes in clinical trials in women with polycystic ovary syndrome? Hum Reprod. 2004;19(8):1697–1704. doi: 10.1093/humrep/deh322. [DOI] [PubMed] [Google Scholar]
- 5.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 6.Jafari K, Javaheri G, Ruiz G. Endometrial adenocarcinoma and the Stein-Leventhal syndrome. Obstet Gynecol. 1978;51(1):97–100. [PubMed] [Google Scholar]
- 7.Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med. 2008;26(1):62–71. doi: 10.1055/s-2007-992926. [DOI] [PubMed] [Google Scholar]
- 8.Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61(4):403–407. [PubMed] [Google Scholar]
- 9.Escobedo LG, Lee NC, Peterson HB, Wingo PA. Infertility-associated endometrial cancer risk may be limited to specific subgroups of infertile women. Obstet Gynecol. 1991;77(1):124–128. [PubMed] [Google Scholar]
- 10.Iatrakis G, Zervoudis S, Saviolakis A, Troulos M, Antoniou E, Sarantaki A, et al. Women younger than 50 years with endometrial cancer. Eur J Gynaecol Oncol. 2006;27(4):399–400. [PubMed] [Google Scholar]
- 11.Niwa K, Imai A, Hashimoto M, Yokoyama Y, Mori H, Matsuda Y, et al. A case-control study of uterine endometrial cancer of pre- and post-menopausal women. Oncol Rep. 2000;7(1):89–93. [PubMed] [Google Scholar]
- 12.Wild S, Pierpoint T, Jacobs H, McKeigue P. Long-term consequences of polycystic ovary syndrome: results of a 31 year follow-up study. Hum Fertil (Camb) 2000;3(2):101–105. doi: 10.1080/1464727002000198781. [DOI] [PubMed] [Google Scholar]
- 13.dos Santos Silva I, Wark PA, McCormack VA, Mayer D, Overton C, Little V, et al. Ovulation-stimulating drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer. 2009;100:1824–31. doi: 10.1038/sj.bjc.6605086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88(4 Pt 1):554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]
- 15.Anderson KE, Sellers TA, Chen PL, Rich SS, Hong CP, Folsom AR. Association of Stein-Leventhal syndrome with the incidence of postmenopausal breast carcinoma in a large prospective study of women in Iowa. Cancer. 1997;79(3):494–499. [PubMed] [Google Scholar]
- 16.Gammon MD, Thompson WD. Polycystic ovaries and the risk of breast cancer. Am J Epidemiol. 1991;134(8):818–824. doi: 10.1093/oxfordjournals.aje.a116156. [DOI] [PubMed] [Google Scholar]
- 17.Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, La Vecchia C. Selected medical conditions and risk of breast cancer. Br J Cancer. 1997;75(11):1699–1703. doi: 10.1038/bjc.1997.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS. Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol. 1998;51(7):581–586. doi: 10.1016/s0895-4356(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 19.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82(2):405–414. doi: 10.1016/j.fertnstert.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 20.Breslow NE, Day NE. Statistical methods in cancer research. Lyon, France: International Agency for Research on Cancer; 1987. [Google Scholar]
- 21.Althuis MD, Scoccia B, Lamb EJ, Moghissi KS, Westhoff CL, Mabie JE, et al. Melanoma, thyroid, cervical, and colon cancer risk after use of fertility drugs. Am J Obstet Gynecol. 2005;193(3 Pt 1):668–674. doi: 10.1016/j.ajog.2005.01.091. [DOI] [PubMed] [Google Scholar]
- 22.Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, et al. Ovarian cancer risk after the use of ovulation-stimulating drugs. Obstet Gynecol. 2004;103(6):1194–1203. doi: 10.1097/01.AOG.0000128139.92313.74. [DOI] [PubMed] [Google Scholar]
- 23.Brinton LA, Scoccia B, Moghissi KS, Westhoff CL, Althuis MD, Mabie JE, et al. Breast cancer risk associated with ovulation-stimulating drugs. Hum Reprod. 2004;19(9):2005–2013. doi: 10.1093/humrep/deh371. [DOI] [PubMed] [Google Scholar]
- 24.Hart R, Norman R. Polycystic ovarian syndrome--prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol. 2006;20(5):751–778. doi: 10.1016/j.bpobgyn.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Med Online. 2009 July 29; doi: 10.1016/s1472-6483(10)60175-7. www.rbmonline.com/Article/4404. [DOI] [PubMed]
- 26.Colafranceschi M, Taddei GL, Scarselli G, Branconi F, Tinacci G, Savino L. Clinico-pathological profile of endometrial carcinoma in young women (under 40 years of age) Eur J Gynaecol Oncol. 1989;10(5):353–356. [PubMed] [Google Scholar]
- 27.Gadducci A, Gargini A, Palla E, Fanucchi A, Genazzani AR. Polycystic ovary syndrome and gynecological cancers: is there a link? Gynecol Endocrinol. 2005;20(4):200–208. doi: 10.1080/09513590400021201. [DOI] [PubMed] [Google Scholar]
- 28.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64(3):417–420. [PubMed] [Google Scholar]
- 29.Honore LH, Davey SJ. Endometrial carcinoma in young women. A report of four cases. J Reprod Med. 1989;34(10):845–849. [PubMed] [Google Scholar]
- 30.Konishi I, Koshiyama M, Mandai M, Kuroda H, Yamamoto S, Nanbu K, et al. Increased expression of LH/hCG receptors in endometrial hyperplasia and carcinoma in anovulatory women. Gynecol Oncol. 1997;65(2):273–280. doi: 10.1006/gyno.1997.4656. [DOI] [PubMed] [Google Scholar]
- 31.Pillay OC, Te Fong LF, Crow JC, Benjamin E, Mould T, Atiomo W, et al. The association between polycystic ovaries and endometrial cancer. Hum Reprod. 2006;21(4):924–929. doi: 10.1093/humrep/dei420. [DOI] [PubMed] [Google Scholar]
- 32.Shu XO, Brinton LA, Zheng W, Gao YT, Fan J, Fraumeni JF., Jr A population-based case-control study of endometrial cancer in Shanghai, China. Int J Cancer. 1991;49(1):38–43. doi: 10.1002/ijc.2910490108. [DOI] [PubMed] [Google Scholar]
- 33.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 34.Baron JA, Weiderpass E, Newcomb PA, Stampfer M, Titus-Ernstoff L, Egan KM, et al. Metabolic disorders and breast cancer risk (United States) Cancer Causes Control. 2001;12(10):875–880. doi: 10.1023/a:1013796112348. [DOI] [PubMed] [Google Scholar]
- 35.Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril. 1995;63(3):439–463. doi: 10.1016/s0015-0282(16)57408-1. [DOI] [PubMed] [Google Scholar]
- 36.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90(23):1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 37.Wynder EL, Dodo H, Barber HR. Epidemiology of cancer of the ovary. Cancer. 1969;23(2):352–370. doi: 10.1002/1097-0142(196902)23:2<352::aid-cncr2820230212>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Olsen C, Green A, Nagle C, Jordan S, Whiteman D, Bain C, et al. Epithelial ovarian cancer: testing the androgens hypothesis. Endocr Relat Cancer. 2008 doi: 10.1677/ERC-08-0075. [DOI] [PubMed] [Google Scholar]