Abstract
Background
Compared with serum potassium levels 4–5.5 mEq/L, those <4 mEq/L have been shown to increase mortality in chronic heart failure (HF). Expert opinions suggest that serum potassium levels >5.5 mEq/L may be harmful in HF. However, little is known about the safety of serum potassium 5–5.5 mEq/L.
Methods
Of the 7788 chronic HF patients in the Digitalis Investigation Group trial, 5656 had serum potassium 4–5.5 mEq/L. Of these, 567 had mild hyperkalemia (5–5.5 mEq/L) and 5089 had normokalemia (4–4.9 mEq/L). Propensity scores for mild hyperkalemia were used to assemble a balanced cohort of 548 patients with mild hyperkalemia and 1629 patients with normokalemia. Cox regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for association between mild hyperkalemia and mortality during a median follow-up of 38 months.
Results
All-cause mortality occurred in 36% and 38% of matched patients with normokalemia and mild hyperkalemia respectively (HR, 1.07; 95% CI, 0.90–1.26; P= 0.458). Unadjusted, multivariable-adjusted, and propensity-adjusted HRs for mortality associated with mild hyperkalemia were 1.33 (95% CI, 1.15–1.52; P<0.0001), 1.16 (95% CI, 1.01–1.34; P=0.040) and 1.13 (95% CI, 0.98–1.31; P=0.091) respectively. Mild hyperkalemia had no association with cardiovascular or HF mortality or all-cause or cardiovascular hospitalization.
Conclusion
Serum potassium 4–4.9 mEq/L is optimal and 5–5.5 mEq/L appears relatively safe in HF. Despite lack of an intrinsic association, the bivariate association of mild-hyperkalemia with mortality suggests that it may be useful as a biomarker of poor prognosis in HF.
Keywords: Mild hyperkalemia, heart failure, mortality, hospitalization
1. Introduction
According to the American College of Cardiology and American Heart Association 2005 guidelines for chronic heart failure (HF), many experts believe that in patients with chronic HF serum potassium levels between 4 and 5 mEq/L may be optimal [1]. However, other experts have suggested that serum potassium levels up to 5.5 may be beneficial and safe in chronic HF [2]. We have previously demonstrated that compared with serum potassium levels between 4 and 5.5 mEq/L, serum potassium levels <4 mEq/L are associated with increased risk of death in chronic HF [3]. However, there is little data concerning the safe upper limit for serum potassium in chronic HF. The objective of the current propensity-matched study was to compare outcomes of chronic HF patients with serum potassium 4–4.9 mEq/L with those with serum potassium 5–5.5 mEq/L.
2. Materials and methods
2.1. Source of data
We used public-use copies of the Digitalis Investigation Group (DIG) trial datasets obtained from the National Heart, Lung, and Blood Institute (NHLBI) for the current analysis. The rationale, design and results of the DIG trial have been previously published in detail [4, 5]. In brief, 7788 chronic HF patients (6800 with left ventricular ejection fraction ≤45%) were recruited from 302 centers (186 in the United States and 116 in Canada) during 1991–1993 and were randomized to digoxin or placebo.
2.2. Mild hyperkalemia
Of the 7788 DIG participants, 6857 had data on baseline serum potassium levels. We excluded 1189 patients with serum potassium <4 mEq/L and 12 patients with serum potassium >5.5 mEq/L. Of the 5656 patients included in the current analysis, 567 had mild hyperkalemia (5–5.5 mEq/L) and 5089 had normokalemia (4–4.9 mEq/L). Data on socio-demographic, clinical, sub-clinical and laboratory variables were collected at baseline.
2.3. Study outcomes
The primary outcome for the current study was all-cause mortality. Secondary outcomes were mortality due to cardiovascular causes and progressive HF, and hospitalization due to all causes, cardiovascular causes and worsening HF. Outcomes data were complete for 99% of the patients.
2.4. Assembly of a balanced study cohort
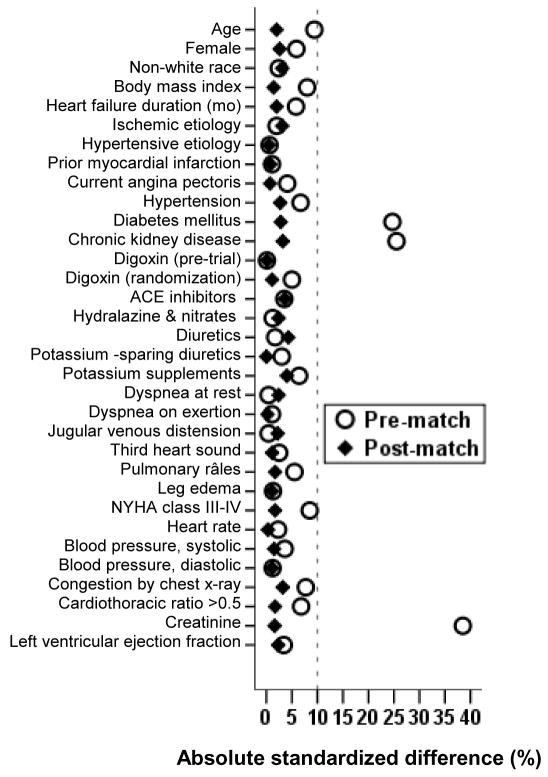
Propensity score for an exposure is the conditional probability of receiving that exposure given a set of measured baseline characteristics and can be used to assemble a matched cohort in which those exposed and unexposed would be balanced on all measured baseline characteristics [6–9]. Propensity scores for mild hyperkalemia were estimated for each of the 5656 patients using a non-parsimonious multivariable logistic regression model based on all measured baseline covariates displayed in Figure 1 [10–14]. Using a 1 to 3 greedy matching protocol described elsewhere [10–14], we were able to match all but 19 of the 567 patients with mild hyperkalemia with 1629 patients with normokalemia. Absolute standardized differences for baseline covariates were examined to assess pre-match imbalances and post-match balances with results presented as a Love plot [10–14]. An absolute standardized difference of 0% indicates no bias, with values under 10% considered to be inconsequential.
Figure 1.
Love plot displaying absolute standardized differences for baseline covariates for patients with normal potassium levels (4–4.9 mEq/L) and mild hyperkalemia (5–5.5 mEq/L), before and after propensity score matching (ACE=angiotensin converting enzyme, NYHA = New York Heart Association)
2.5. Statistical analysis
Matched Cox regression models were used to determine associations between mild hyperkalemia and outcomes during 38 months of median follow up. To assess the effect of loss of participants during matching, we repeated our analysis in all 5656 pre-match patients using three different statistical models: (1) unadjusted, (2) multivariable-adjusted, using all covariates used in the propensity score model, and (3) propensity score-adjusted. Considering significant imbalance in baseline prevalence of diabetes mellitus (DM) and chronic kidney disease (CKD) between patients with mild hyperkalemia and normal serum potassium, we separately analyzed the association of mild hyperkalemia and all-cause mortality adjusting for DM alone, CKD alone and both DM and CKD. CKD was defined as glomerular filtration rate <60 ml/min/1.73 m2 estimated using the Modified Diet in Renal Disease formula [15]. Subgroup analyses were conducted to determine the homogeneity of association between mild hyperkalemia and all-cause mortality. All statistical tests were two-tailed with a p-value <0.05 considered significant. All data analyses were performed using SPSS for Windows version 15 (SPSS Inc., Chicago, IL).
3. Results
3.1. Patient characteristics
Matched patients had a mean (±SD) age of 65 (±10) years, 21% were female and 13% were non-white. Before matching, the prevalence of DM and CKD were significantly higher among those with serum potassium 5–5.5 mEq/L compared to those with serum potassium 4–4.9 mEq/L. The prevalence of DM and CKD, along with other baseline characteristics was well balanced after matching (Table 1 and Figure 1). Post-match absolute standardized differences for all measured covariates were <5% suggesting substantial covariate balance across groups after matching (Figure 1).
Table 1.
Baseline patient characteristics, by serum potassium, before and after propensity score matching
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| N (%) or mean (±SD) | Serum potassium 4–4.9 mEq/L (n= 5089) | Serum potassium 5–5.5 mEq/L (n = 567) | P value | Serum potassium 4–4.9 mEq/L (n = 1629) | Serum potassium 5–5.5 mEq/L (n = 548) | P value |
| Age (years) | 64 (±11) | 65 (±10) | 0.046 | 65 (±10) | 65 (±10) | 0.668 |
| Female | 1197 (24%) | 121 (21%) | 0.244 | 346 (21%) | 118 (22%) | 0.885 |
| Non-white | 674 (13%) | 71 (13%) | 0.630 | 214 (13%) | 70 (13%) | 0.827 |
| Body mass index, kg/m2 | 27 (±5) | 27 (±5) | 0.063 | 27 (±5) | 27 (±5) | 0.848 |
| Duration of heart failure (mo) | 29 (±36) | 31 (±39) | 0.160 | 29 (±38) | 30 (±38) | 0.973 |
| Primary cause of heart failure | ||||||
| Ischemic | 3574 (70%) | 407 (72%) | 0.832 | 1178 (72%) | 393 (72%) | 0.947 |
| Hypertensive | 474 (9%) | 53 (9%) | 156 (10%) | 50 (9%) | ||
| Idiopathic | 723 (14%) | 73 (13%) | 198 (12%) | 71 (13%) | ||
| Others | 318 (6%) | 34 (6%) | 97 (6%) | 34 (6%) | ||
| Prior myocardial infarction | 3251 (64%) | 369 (65%) | 0.573 | 1048 (64%) | 358 (65%) | 0.674 |
| Current angina pectoris | 1375 (27%) | 164 (29%) | 0.334 | 439 (30%) | 160 (29%) | 0.308 |
| Hypertension | 2326 (46%) | 278 (49%) | 0.132 | 805 (49%) | 264 (48%) | 0.615 |
| Diabetes mellitus | 1415 (28%) | 224 (40%) | <0.0001 | 642 (39%) | 208 (38%) | 0.546 |
| Chronic kidney disease | 2266 (45%) | 323 (57%) | <0.0001 | 892 (55%) | 304 (56%) | 0.770 |
| Medications | ||||||
| Pre-trial digoxin use | 2113 (42%) | 233 (41%) | 0.845 | 686 (42%) | 226 (41%) | 0.721 |
| Trial use of digoxin | 2528 (50%) | 294 (52%) | 0.326 | 848 (52%) | 282 (52%) | 0.809 |
| Angiotensin-converting enzyme inhibitors | 4747 (93%) | 536 (95%) | 0.254 | 1538 (94%) | 517 (94%) | 0.950 |
| Diuretics | 3912 (77%) | 431 (76%) | 0.646 | 1265 (78%) | 415 (76%) | 0.353 |
| Potassium-sparing diuretics | 354 (7%) | 34 (6%) | 0.391 | 108 (7%) | 32 (6%) | 0.514 |
| Potassium supplement | 1491 (29%) | 147 (26%) | 0.093 | 450 (28%) | 142 (26%) | 0.436 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 1099 (22%) | 124 (22%) | 0.881 | 354 (22%) | 117 (21%) | 0.851 |
| Dyspnea on exertion | 3835 (75%) | 429 (76%) | 0.874 | 1228 (75%) | 411 (75%) | 0.857 |
| Limitation of activity | 3873 (76%) | 432 (76%) | 0.964 | 1243 (76%) | 415 (76%) | 0.785 |
| Jugular venous distension | 622 (12%) | 68 (12%) | 0.874 | 195 (12%) | 64 (12%) | 0.855 |
| Third heart sound | 1187 (23%) | 125 (22%) | 0.494 | 341 (21%) | 120 (22%) | 0.633 |
| Pulmonary râles | 793 (16%) | 99 (18%) | 0.245 | 273 (17%) | 93 (17%) | 0.909 |
| Lower extremity edema | 1047 (21%) | 114 (20%) | 0.794 | 322 (20%) | 108 (20%) | 0.976 |
| New York Heart Association class | ||||||
| Class I | 732 (14%) | 77 (14%) | 0.220 | 225 (14%) | 76 (14%) | 0.982 |
| Class II | 2785 (55%) | 291 (51%) | 854 (52%) | 284 (52%) | ||
| Class III | 1481 (29%) | 189 (33%) | 524 (32%) | 178 (33%) | ||
| Class IV | 91 (2%) | 10 (2%) | 26 (2%) | 10 (2%) | ||
| Heart rate (/minute), | 78 (±13) | 78 (±12) | 0.450 | 78 (±13) | 78 (±12) | 0.890 |
| Systolic blood pressure (mm Hg) | 127 (±20) | 128 (±21) | 0.558 | 128 (±21) | 128 (±21) | 0.896 |
| Diastolic blood pressure (mm Hg) | 75 (±11) | 75 (±11) | 0.950 | 75 (±11) | 75 (±11) | 0.634 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 692 (14%) | 61 (11%) | 0.059 | 181 (11%) | 58 (11%) | 0.733 |
| Cardiothoracic ratio >0.5 | 3045 (60%) | 321 (57%) | 0.138 | 953 (59%) | 310 (57%) | 0.428 |
| Serum creatinine (mg/dL) | 1.27 (±0.35) | 1.43 (±0.48) | <0.0001 | 1.39 (±0.41) | 1.39 (±0.43) | 0.966 |
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 64 (±20) | 58 (±27) | <0.0001 | 59 (±20) | 59 (±27) | 0.441 |
| Ejection fraction (%) | 32 (±12) | 31 (±13) | 0.301 | 31 (±12) | 31 (±13) | 0.890 |
3.2. Mild hyperkalemia and outcomes
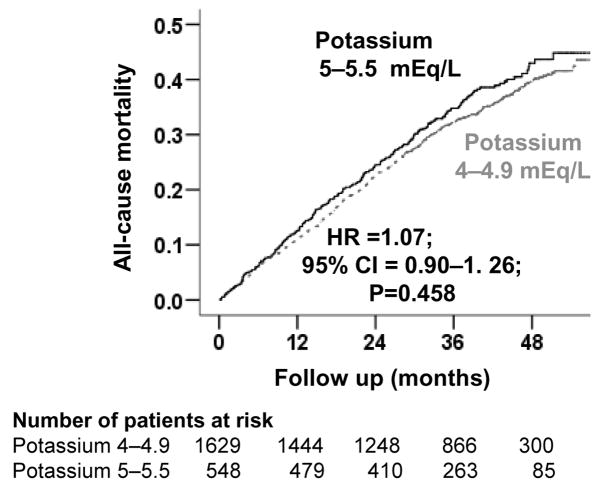
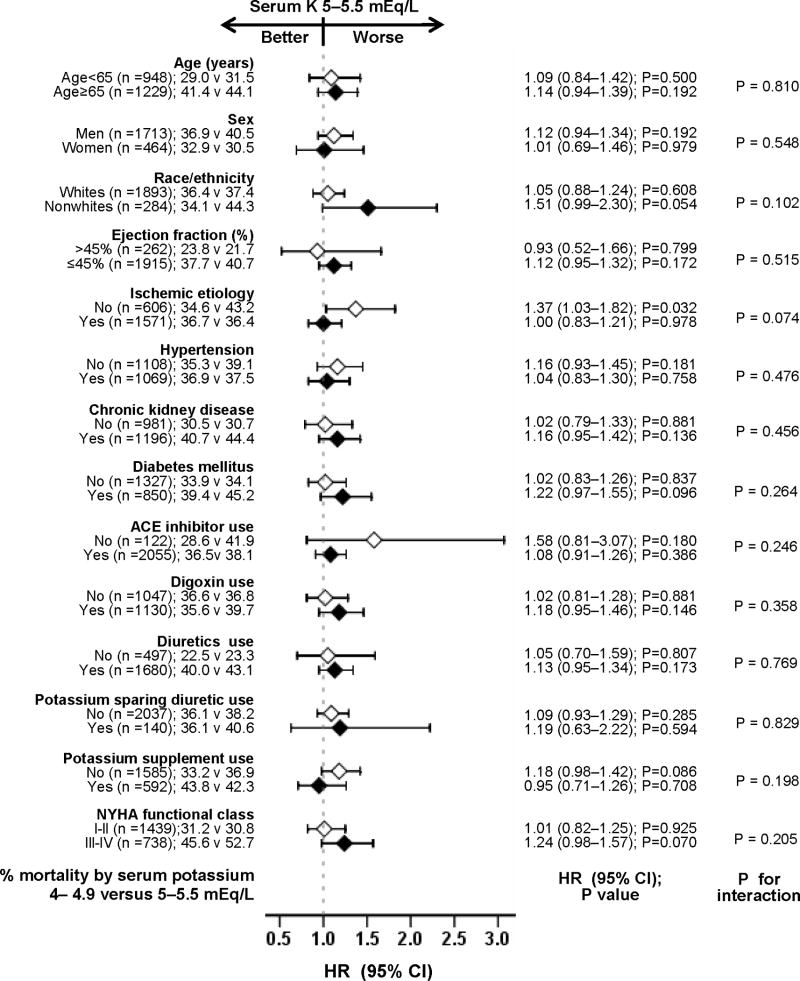
Among matched participants, 798 (37%) participants died from all causes during 6180 person-years of follow up. All-cause mortality occurred in 38% (rate 1387/10000 person-years) and 36% (rate, 1260/10000 person-years) of participants with mild hyperkalemia and normokalemia respectively (matched hazard ratio {HR}, 1.07; 95% confidence interval {CI}, 0.90–1.26; P = 0.458; Table 2 and Figure 2). When we used serum potassium as a continuous variable, each unit increase in serum potassium was associated with a non-significant 7% increase in risk of total mortality (HR, 1.07; 95% CI, 0.90–1.28; P = 0.423). Associations between mild hyperkalemia and all-cause mortality in various subgroups of patients are displayed in Figure 3.
Table 2.
Outcomes by baseline serum potassium in the matched cohort
| Rate per 10000 person-years (events/total follow up in years) |
|||||
|---|---|---|---|---|---|
| Outcomes | Serum potassium 4–4.9 mEq/L (n=1629) | Serum potassium 5–5.5 mEq/L (n=548) | Absolute rate difference (per 10000 person-years)* | Hazard ratio when potassium 5–5.5 mEq/L is compared with potassium 4–4.9 mEq/L (95% confidence interval)** | P value |
| All-cause mortality | 1260 (588/4666) | 1387 (210/1514) | + 127 | 1.07 (0.90–1.26) | 0.458 |
| Cardiovascular mortality | 986 (460/4666) | 1090 (165/1514) | + 104 | 1.08 (0.89–1.30) | 0.455 |
| Heart failure mortality | 420 (196/4666) | 555 (84/1514) | + 135 | 1.28 (0.97–1.69) | 0.079 |
| All-cause hospitalization | 4493 (1148/2555) | 4561 (379/831) | + 68 | 0.98 (0.85–1.12) | 0.727 |
| Cardiovascular hospitalization | 2888 (900/3116) | 3231 (315/975) | + 342 | 1.05 (0.91–1.22) | 0.515 |
| Heart failure hospitalization | 1344 (519/3862) | 1488 (189/1270) | + 144 | 1.10 (0.92–1.33) | 0.287 |
Absolute differences in event rates per 10,000 person-year of follow up were calculated by subtracting the event rates in the serum potassium 4 – 4.9 mEq/L group from the event rates in the serum potassium 5 – 5.5 mEq/L group (before values were rounded).
Post-match hazard ratios (95% confidence intervals) are adjusted for matching.
Figure 2.
Kaplan-Meier plots for all-cause mortality by serum potassium levels
Figure 3.
Hazard ratio (HR) and 95% confidence interval (CI) for all-cause mortality associated with mild hyperkalemia in subgroups of patients with chronic heart failure (ACE=angiotensin-converting enzyme, K=potassium, NYHA=New York Heart Association)
In the full pre-match cohort of 5656 patients, all-cause mortality occurred in 40% and 31% of patients with mild hyperkalemia and normokalemia respectively (unadjusted HR, 1.33; 95% CI, 1.15–1.52; P<0.0001). When we used serum potassium as a continuous variable, each unit increase in serum potassium was associated with a significant 29% increase in risk of total mortality (unadjusted HR, 1.29; 95% CI, 1.13–1.47; P <0.0001). Multivariable-adjusted and propensity-adjusted HRs were respectively 1.16 (95% CI, 1.01–1.34; P=0.040) and 1.13 (95% CI, 0.98–1.31; P=0.091). Post-match associations of mild hyperkalemia with other outcomes are displayed in Table 2. Associations of mild hyperkalemia and all-cause mortality among 5656 pre-match patients after adjustment for DM alone, CKD alone and both DM and CKD are displayed in Table 3.
Table 3.
Mild-hyperkalemia* and all-cause mortality in the pre-match cohort
| Outcomes | Hazard ratio when potassium 5–5.5 mEq/L is compared with potassium 4–4.9 mEq/L (95% confidence interval) | P value |
|---|---|---|
| Unadjusted | 1.33 (1.15–1.52) | <0.0001 |
| Adjusted for diabetes mellitus | 1.27 (1.10–1.46) | 0.001 |
| Adjusted for chronic kidney disease | 1.27 (1.10–1.46) | 0.001 |
| Adjusted for both diabetes mellitus and chronic kidney disease | 1.22 (1.06–1.41) | 0.006 |
| Multivariable adjusted (forward model) | 1.16 (1.01–1.34) | 0.040 |
| Multivariable adjusted (backward model) | 1.16 (1.01–1.34) | 0.043 |
| Multivariable adjusted (forced entry model) | 1.16 (1.01–1.34) | 0.040 |
| Propensity score adjusted | 1.13 (0.98–1.31) | 0.091 |
Mild-hyperkalemia is defined as serum potassium level 5–5.5 mEq/L
4. Discussion
There are several important findings of the current study. First, chronic HF patients with normokalemia and mild hyperkalemia had rather similar baseline characteristics except for a significantly higher prevalence of DM and CKD in those with mild hyperkalemia. Second, mild hyperkalemia had a significant bivariate association with all-cause mortality, suggesting that it can be used as an early biomarker to identify chronic HF patients at increased risk of death. Third, mild hyperkalemia had no intrinsic association with all-cause mortality suggesting that serum potassium levels between 5 and 5.5 mEq/L may be relatively safe in these patients. Taken together with our previous finding of an increased mortality associated with serum potassium <4 mEq/L [3, 13], these findings suggest that the optimal level of serum potassium in chronic HF may be between 4 and 5.5 mEq/L, with levels between 4 and 5 mEq/L being the most optimal, which is also in keeping with expert opinion [2].
The substantial imbalance in the distribution of DM and CKD between patients with mild hyperkalemia and normokalemia explains in part the significant bivariate association between mild hyperkalemia and all-cause mortality. Both DM and CKD are known to be associated with increased mortality in chronic HF [16, 17]. Yet, these patients may be deprived of therapy with life-saving drugs such as an angiotensin-converting enzyme inhibitor, as they are also more prone to develop hyperkalemia. Interestingly, the association between mild hyperkalemia and all-cause mortality remained significant despite adjustment for DM, CKD, or both (Table 3). This suggests possible confounding by other covariates and/or residual confounding by DM and CKD, despite adjustment in a regression model. The persistence of a significant association between mild hyperkalemia and all-cause mortality after multivariable risk adjustment indicates that the use of the traditional regression-based multivariable risk adjustment models would have led us to conclude that mild hyperkalemia had an independent association with all-cause mortality in chronic HF. However, the association lost significance after adjustment for propensity scores in the pre-match cohort and in the propensity-matched cohort highlighting the conservative nature of propensity score methods.
Despite their popular use for risk adjustment, multivariable regression models are often limited by improper assumptions and imbalances on measured baseline covariates between exposed and unexposed groups. The issue of covariate imbalance is particularly important as in the presence of such imbalance regression adjustments are based on extrapolations beyond data and may not be trustworthy [18]. Propensity scores, on the other hand, may be used to assemble cohorts in which exposed and unexposed patients are well-balanced on all measured baseline covariates. Perhaps more importantly, risk adjustment using propensity methods is done during study design and investigators remain blinded to study outcomes, thus mimicking a key feature of randomized clinical trials [9]. This is even more important for studies of non-drug exposures as patients cannot be randomized to non-drug exposures such as mild hyperkalemia.
The lack of an intrinsic association between mild hyperkalemia and all-cause mortality may also be due to lack of an effect of a mild elevation of serum potassium levels on cardiac rhythm. As opposed to hypokalemia, mild hyperkalemia may be considered less harmful in chronic HF and levels up to 5.5 mEq/L have been recommended to be safe in these patients [2]. The effect of serum potassium levels between 5.5 and 6.5 mEq/L without electrocardiographic evidence of hyperkalemic cardiac arrhythmias is currently unknown [2]. However, because mild hyperkalemia is a harbinger of more severe hyperkalemia, patients with serum potassium between 5.5 and 6.5 mEq/L may quickly develop more severe hyperkalemia, especially in the presence of DM and CKD [19–21]. Therefore, serum potassium in patients with chronic HF should be kept between 4 and 5.5 mEq/L and preferably between 4 and 5 mEq/L. HF patients with serum potassium levels between 5 and 5.5 mEq/L should be closely monitored, especially in those with DM and CKD. This caution is also supported by the late separation of Kaplan-Meier plots after the first two years of follow-up in our matched patients. The development of more severe hyperkalemia in patients with mild hyperkalemia may potentially explain the late increase in mortality in those patients, which may be significant during a longer follow-up and/or in a larger sample size. The progression to more severe hyperkalemia may also in part be mediated via the progression of DM and CKD during follow-up. Although the baseline prevalence of DM and CKD was similar in our matched patients with mild hyperkalemia and normokalemia, it is possible that DM and CKD in those with mild hyperkalemia were more severe and/or advanced, and may have progressed at a faster rate during follow-up.
Over 90% of the DIG participants were receiving angiotensin-converting enzyme inhibitors, a life-saving neurohormonal antagonist known to raise serum potassium. There is no need to discontinue the use of these and other neurohormonal antagonists such as angiotensin receptor blockers and aldosterone inhibitors in chronic HF patients with serum potassium between 5 and 5.5 mEq/L. However, patients with serum potassium levels between 5 and 5.5 mEq/L may require long-term serial monitoring of serum potassium for early identification of progression to more severe hyperkalemia, in which case, it may be prudent to reduce the dose of the offending drug. Eplerenone, a selective aldosterone receptor inhibitor, in 25 to 50 mg/day dosages, has been shown to reduce mortality in post-acute myocardial infarction patients with systolic HF treated with standard therapy without causing severe hyperkalemia (serum potassium ≥6.0 mEq/L) when serum potassium was periodically monitored [21]. In that study, DM and CKD were also strong predictors of severe hyperkalemia, but the presence of these conditions did not neutralize the mortality benefit of eplerenone.
A few limitations of this study must be acknowledged. Our study was based on trial-eligible, young, predominantly male HF patients in normal sinus rhythm from the pre-beta-blocker era of HF therapy. Therefore, these findings may need to be replicated in more contemporary cohorts of HF patients. We had no data on serum potassium during follow-up and underestimation of true associations due to regression dilution is possible [22].
In conclusion, serum potassium levels between 4 and 5 mEq/L are optimal in patients with chronic HF. Although serum potassium levels 5–5.5 mEq/L appear to be relatively safe, considering the risk of development of more severe hyperkalemia, serum potassium levels should be closely monitored in these patients. Despite the lack of an intrinsic effect of mild-hyperkalemia on mortality, its bivariate association with mortality suggests that it may be a useful biomarker of poor prognosis in HF.
Acknowledgments
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [23].
Footnotes
Conflict of Interest Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Endorsed by the Heart Rhythm Society. Published online before print September 13, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J Am Coll Cardiol. 2004;43:155–61. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A, Zannad F, Love TE, et al. A propensity-matched study of the association of low serum potassium levels and mortality in chronic heart failure. Eur Heart J. 2007;28:1334–43. doi: 10.1093/eurheartj/ehm091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 8.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 9.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 10.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–9. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed A, Pitt B, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–46. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–53. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alper AB, Campbell RC, Anker SD, et al. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekundayo OJ, Dell’italia LJ, Sanders PW, et al. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed A, Aban IB, Vaccarino V, et al. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–90. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed A, Rich MW, Sanders PW, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–8. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzmaurice G. Confounding: regression adjustment. Nutrition. 2006;22:581–3. doi: 10.1016/j.nut.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 19.de Denus S, Tardif JC, White M, et al. Quantification of the risk and predictors of hyperkalemia in patients with left ventricular dysfunction: a retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials. Am Heart J. 2006;152:705–12. doi: 10.1016/j.ahj.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Desai AS, Swedberg K, McMurray JJ, et al. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol. 2007;50:1959–66. doi: 10.1016/j.jacc.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R. Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) Circulation. 2008;118:1643–50. doi: 10.1161/CIRCULATIONAHA.108.778811. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–53. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 23.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]