Abstract
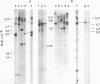
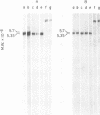
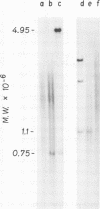
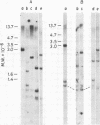
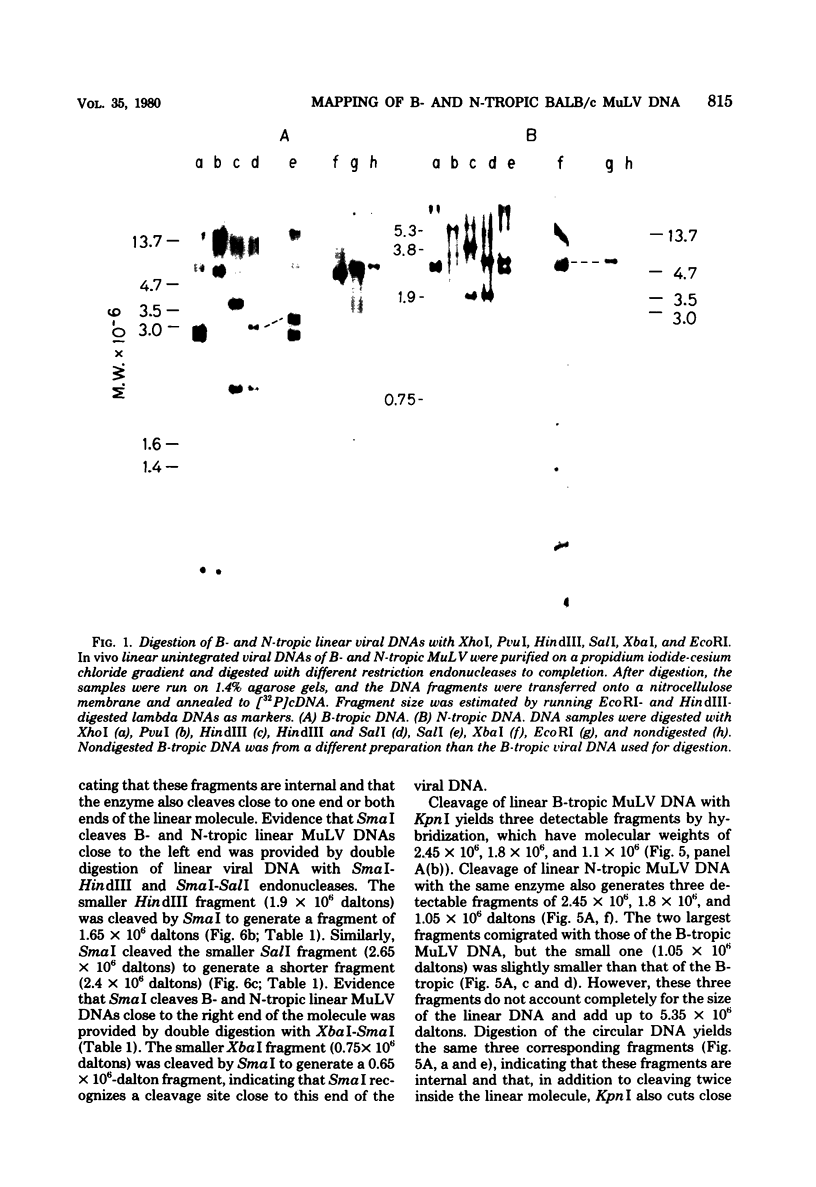
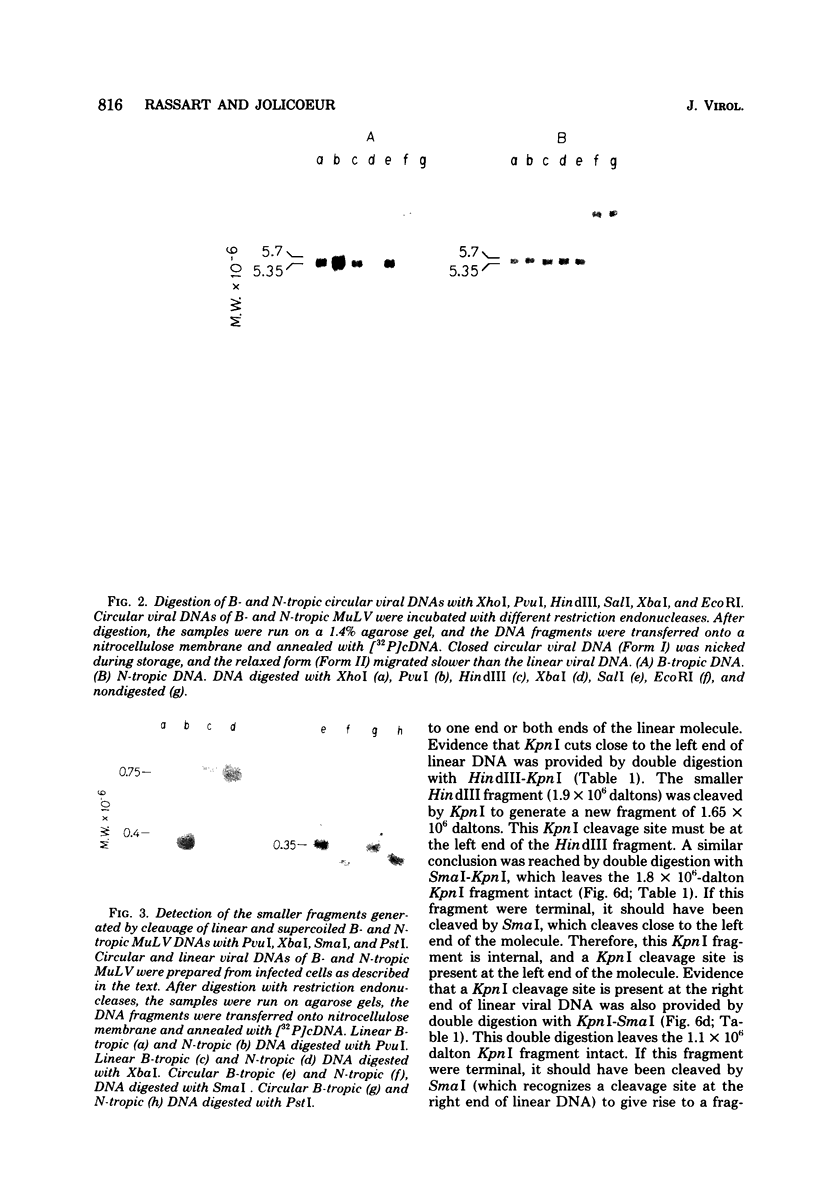
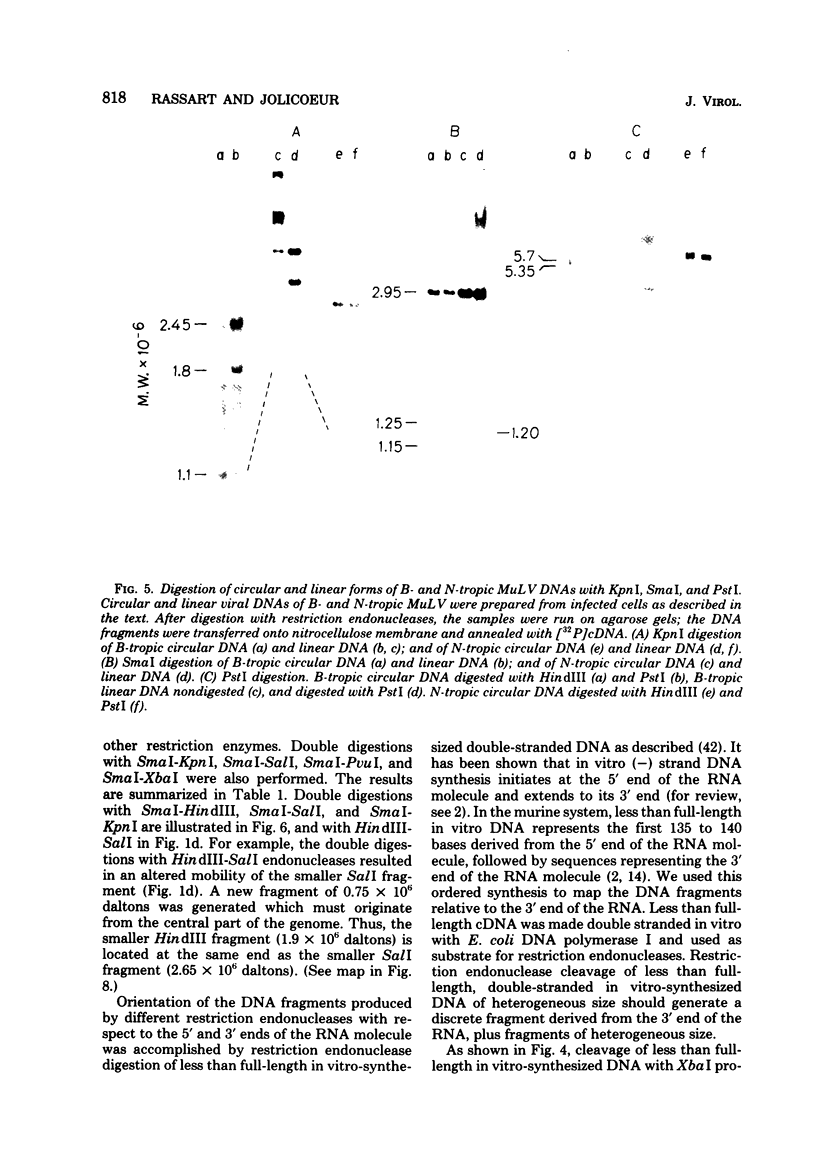
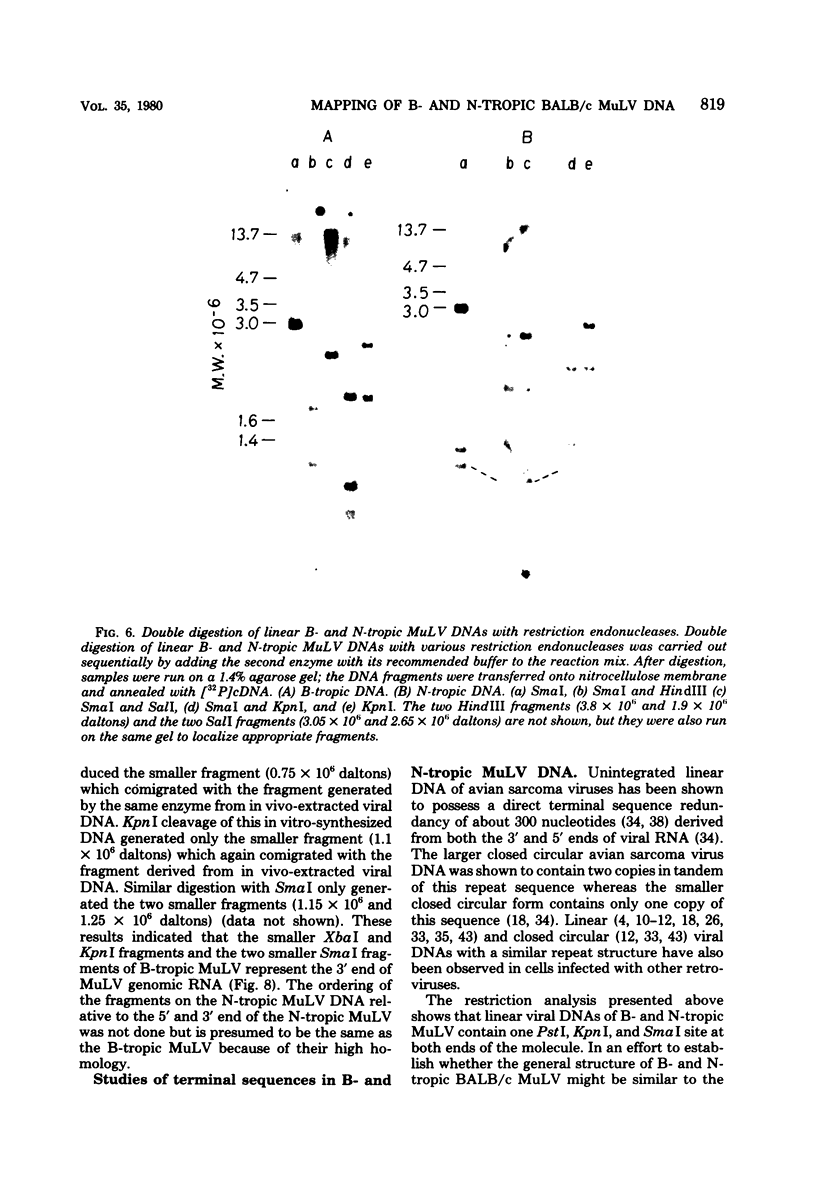
Unintegrated linear and closed circular DNAs of B- and N-tropic endogenous BALB/c murine leukemia virus (MuLV) were extracted from newly infected mouse cells and cleaved with EcoRI, XhoI, PvuI, HindIII, SalI, XbaI, KpnI, SmaI, and PstI restriction endonucleases. The DNA fragments were separated by electrophoresis and analyzed by the Southern blot hybridization procedure. EcoRI did not cleave the two genomes. A physical map of 15 cleavage sites on B- and N-tropic genomes was constructed with the other restriction endonucleases. Identical cleavage sites of B- and N-tropic MuLV DNAs were found with all these enzymes. However, the N-tropic linear genome was found to lack about 75 base pairs at each end of the molecule. PstI, KpnI, and SmaI recognize a cleavage site at both ends of the linear molecules. And sequences derived from the 5' end of the RNA genome were found in the third left end of the linear DNA and at its extreme right-end terminus, suggesting the presence of redundant sequences. Two species of closed circular viral DNA were observed. The larger species has the same size as the linear molecule and appears to be a circularized form of linear DNA. The smaller species contains sequences common to both the linear and the larger circular viral DNA but seems to be deleted from sequences present at either one or both ends of the linear DNA. Therefore, the general structure of the linear and circular DNA species of these B- and N-tropic endogenous BALB/c MuLV appears analogous to the structure found with other retroviruses.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Gilboa E., Rothenberg E., Yoshimura F. Production of a discrete, infectious, double-stranded DNA by reverse transcription in virions of Moloney murine leukemia virus. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):869–874. doi: 10.1101/sqb.1979.043.01.093. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Gerwin B. I., Duran-Troise G., Gisselbrecht S., Rein A. Murine sarcoma virus pseudotypes acquire a determinant specifying N or B tropism from leukaemia virus during rescue. Nature. 1975 Jul 17;256(5514):223–225. doi: 10.1038/256223a0. [DOI] [PubMed] [Google Scholar]
- Benz E. W., Jr, Dina D. Moloney murine sarcoma virions synthesize full-genome-length double-stranded DNA in vitro. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3294–3298. doi: 10.1073/pnas.76.7.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Robbins K. C., Aaronson S. A. The transforming gene of Moloney murine sarcoma virus. Nature. 1979 Nov 22;282(5737):378–383. doi: 10.1038/282378a0. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotide maps of N-, B-, and B leads to NB-tropic murine leukemia viruses derived from BALB/c. J Virol. 1978 Apr;26(1):143–152. doi: 10.1128/jvi.26.1.143-152.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotides that segregate with tropism and with properties of gp70 in recombinants between N- and B-tropic murine leukemia viruses. J Virol. 1978 Apr;26(1):153–158. doi: 10.1128/jvi.26.1.153-158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni A. M., Smotkin D., Weinberg R. A. Murine leukemia virus: detection of unintegrated double-stranded DNA forms of the provirus. Proc Natl Acad Sci U S A. 1975 Feb;72(2):447–451. doi: 10.1073/pnas.72.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E., Goff S., Shields A., Yoshimura F., Mitra S., Baltimore D. In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell. 1979 Apr;16(4):863–874. doi: 10.1016/0092-8674(79)90101-6. [DOI] [PubMed] [Google Scholar]
- Goldfarb M. P., Weinberg R. A. Physical map of biologically active Harvey sarcoma virus unintegrated linear DNA. J Virol. 1979 Oct;32(1):30–39. doi: 10.1128/jvi.32.1.30-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Gottlieb P. D. Evidence for recombination between N- and B-tropic murine leukemia viruses. J Virol. 1977 Mar;21(3):1074–1078. doi: 10.1128/jvi.21.3.1074-1078.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Hynes R. Six-NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977 Jan;21(1):309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. W., Sabran J. L., Mark G. E., Guntaka R. V., Taylor J. M. Analysis of unintegrated avian RNA tumor virus double-stranded DNA intermediates. J Virol. 1978 Dec;28(3):810–818. doi: 10.1128/jvi.28.3.810-818.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto A., Hartley J. W., Rowe W. P. Fv-1 restriction of xenotropic and amphotropic murine leukemia virus genomes phenotypically mixed with ecotropic virus. Virology. 1979 Feb;93(1):215–225. doi: 10.1016/0042-6822(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2236–2240. doi: 10.1073/pnas.73.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of Fv-1 gene product on synthesis of N-tropic and B-tropic murine leukemia viral RNA. Cell. 1976 Jan;7(1):33–39. doi: 10.1016/0092-8674(76)90252-x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Baltimore D. Effect of the Fv-1 locus on the titration of murine leukemia viruses. J Virol. 1975 Dec;16(6):1593–1598. doi: 10.1128/jvi.16.6.1593-1598.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., O'Rear J. J., Temin H. M. DNA of noninfectious and infectious integrated spleen necrosis virus (SNV) is colinear with unintegrated SNV DNA and not grossly abnormal. Cell. 1979 Jan;16(1):51–61. doi: 10.1016/0092-8674(79)90187-9. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Kashmiri S. V., Bassin R. H., Gerwin B. L., Duran-Troise G. Phenotypic mixing between N- and B-tropic murine leukemia viruses: infectious particles with dual sensitivity to Fv-1 restriction. Cell. 1976 Mar;7(3):373–379. doi: 10.1016/0092-8674(76)90166-5. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Cabradilla C. D., Stephenson J. R., Aaronson S. A. Segregation of genetic information for a B-tropic leukemia virus with the structural locus for BALB:virus-1. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2953–2957. doi: 10.1073/pnas.74.7.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Donis-Keller H., Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979 Jan;16(1):43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Hsu T. W., Lai M. M. Restriction enzyme sites on the avian RNA tumor virus genome. J Virol. 1978 May;26(2):479–484. doi: 10.1128/jvi.26.2.479-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Deng C. T., Bishop J. M. Synthesis, structure and function of avian sarcoma virus-specific DNA in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):987–996. doi: 10.1101/sqb.1974.039.01.113. [DOI] [PubMed] [Google Scholar]
- Verma I. M. Genome organization of retroviruses. III. Restriction endonuclease cleavage maps of mouse sarcoma virus double-stranded DNA synthesized in vitro. Nucleic Acids Res. 1979;6(5):1863–1867. doi: 10.1093/nar/6.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., McKennett M. A. Genome organization of RNA tumor viruses II. Physical maps of in vitro-synthesized Moloney murine leukemia virus double-stranded DNA by restriction endonucleases. J Virol. 1978 Jun;26(3):630–645. doi: 10.1128/jvi.26.3.630-645.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F. K., Weinberg R. A. Restriction endonuclease cleavage of linear and closed circular murine leukemia viral DNAs: discovery of a smaller circular form. Cell. 1979 Feb;16(2):323–332. doi: 10.1016/0092-8674(79)90009-6. [DOI] [PubMed] [Google Scholar]