Abstract
Despite a half century of development, the orthodox monkey model of human amnesia needs improvement, in part because of two problems inherent in animal models of advanced human cognition. First, animal models are perforce comparative, but the principles of comparative and evolutionary biology have not featured prominently in developing the orthodox model. Second, no one understands the relationship between human consciousness and cognition in other animals, but the orthodox model implicitly assumes a close correspondence. If we treat these two difficulties with the deference they deserve, monkeys can tell us a lot about human amnesia and memory. Three future contributions seem most likely: (1) an improved monkey model, one refocused on the hippocampus rather than on the medial temporal lobe as a whole; (2) a better understanding of cortical areas unique to primates, especially the granular prefrontal cortex; and (3), taking the two together, insight into prefrontal-hippocampal interactions. We propose that interactions among the granular prefrontal areas create the kind of cross-domain, analogical and self-referential knowledge that underlies advanced cognition in modern humans. When these products of frontal-lobe function interact with the hippocampus, and its ancestral function in navigation, what emerges is the human ability to embed ourselves in scenarios — real and imagined, self-generated and received — thereby creating a coherent, conscious life experience.
Keywords: medial temporal lobe, memory systems, hippocampus, prefrontal cortex, animal models, consciousness
1. Introduction
Know then thyself, presume not God to scan;
The proper study of Mankind is Man….
He hangs between; in doubt to act, or rest,
In doubt to deem himself a God, or Beast
In doubt his Mind or Body to prefer,
Born but to die, and reas’ning but to err;
Alike in ignorance, his reason such,
Whether he thinks too little, or too much— Alexander Pope, The Proper Study of Mankind, 1870
The editors of this issue posed a seemingly simple question: What can research on monkeys tell us about human amnesia and the organization of memory? If, as the poet claims, “the proper study of mankind is man”, then the answer is clear: not much. Antivivisectionists, creationists, and proponents of “intelligent design” would certainly agree. So, too, would many neuroscientists.
Yet memory research on monkeys stands on an unshakeable biological foundation: monkeys and humans both descended from a common ancestor that lived 25–35 million years ago, much more recently than for other laboratory animals. Monkeys have memories, and the memory mechanisms of monkeys and humans have had only this limited time to diverge.
With many research methods out of the question for apes, monkey research offers the best opportunity for developing an animal model of human amnesia. So even if the “proper study” of humankind is humanity, to advance the poet into our gender-neutral modernity, the proper study of our simian relatives can make an important contribution, one based on the many research methods precluded for apes and humans but permitted for monkeys. For the study of monkey memory mechanisms, the main such method involves the use of anatomically selective lesions or inactivations combined with tests of memory. Passingham (2009) has expounded recently on this point and we subscribe to many of his views. The Journal of Comparative and Physiological Psychology was once a significant academic journal in the field, and its title summarizes the endeavor as well or better than current labels, such as experimental neuropsychology or behavioral neuroscience.
Using the methods of comparative and physiological psychology, by whatever label, the quest for a monkey model of human amnesia has continued for more than half a century. As is well known, the orthodox model holds that the medial temporal lobe (MTL), as a whole, contains the neural mechanisms of conscious memory. To avoid calling it conscious memory in monkeys, proxy terms such as declarative memory, explicit knowledge, and others have been pressed into service, but the use of surrogate terminology matters little. As Clark, Manns, & Squire (2002, p. 524) put it:
the fundamental distinction is between the capacity for conscious recollection of facts and events (declarative memory) and nondeclarative memory, which supports … forms of memory that are expressed through performance rather than recollection.
These definitions lead directly to a major problem for monkey models: monkeys express all of their memories through performance. Clark et al. (2002) meant to apply their definitions to humans, but when applied to monkeys they lead to two rather surprising conclusions: monkeys lack both declarative memory and conscious recollection.
But, of course, it is not as simple as that. Memory research in monkeys, including the orthodox monkey model of human amnesia, depends on precisely the opposite assumptions. This line of research has enjoyed a long, productive run, leading to important discoveries about the functions of the hippocampus, amygdala, entorhinal cortex, perirhinal cortex, and parahippocampal cortex, along with the other structures that compose the MTL. On reflection, however, this work has failed to achieve its initial goal of explaining the “dense amnesia” seen in certain human patients. Gaffan (2002) has made this point convincingly, and we have previously explained that viewing the MTL as a single entity subserving a single memory function accords poorly with empirical results on memory tests, as well as with the principles of brain organization and comparative neuroanatomy (Murray & Wise, 2004).
The history of this research underscores the obstacles encountered in developing animal models of advanced human cognition. The phrase “animal model”, itself, exposes these challenges and proves the poet’s point: we “hang … in doubt” about whether to treat our species as entirely apart from other animals. In the poet’s words, we often think “too little, or too much” about this issue. When we think too little, we ignore the differences between human and animal cognition and sometimes deny such differences altogether. When we think too much, even trivial distinctions appear to vitiate all animal models. Then we say, fallaciously, that humans and other animals are not exactly the same, so they differ completely. As already mentioned, monkeys and humans have had about 30 million years to diverge, which seems like a mere 30 million years from one perspective but a long 30 million years from another. A productive middle ground acknowledges both the differences between human and animal cognition and the similarities conveyed from our most recent common ancestor.
In what follows, we sketch a history of the monkey model of human amnesia from its roots in a celebrated clinical case (Section 2) to its current condition (Section 3). We then suggest some improvements to the model (Section 4) and address a key question in developing a monkey model of human amnesia: Can consciousness can be ignored (Section 5)? After presenting some examples of what monkey research can contribute without relying on assumptions regarding animal consciousness (Section 6), we imagine how human consciousness could have arisen (Section 7). A quick note about some convenient, but slightly erroneous, terminology used in this article: By animals we mean nonhuman animals, and by monkeys we mean macaque monkeys, usually rhesus monkeys, unless otherwise stated; we use the term ‘granular’ prefrontal cortex to refer to the homotypical areas of the frontal lobe, even though it has a homotypical cytoarchitecture rather than a granular one; in terms for memory, we use the term short-term memory to cover the related concepts of immediate memory and working memory; and we treat as equivalent declarative memory, explicit knowledge, conscious memory, recollection and recall. We define the hippocampal complex as the hippocampus proper (CA1-4 and dentate gyrus), subicular complex and entorhinal cortex, along with one of their principal axonal pathways, the fimbria-fornix.
2. The unforgettable Henry Molaison
The most celebrated case of human amnesia was that of Henry Molaison, known universally as H. M. Henry rose to prominence because of a conjunction of three events. First, he received an experimental medical treatment involving a circumscribed ablation of brain tissue. Second, this operation produced a striking alteration in his memory. And, third, psychologists documented and characterized this dramatic change in his behavior (Corkin, 1984, 2002; Milner, Corkin, & Teuber, 1968; Milner, 1972; Scoville & Milner, 1957). As a result, H. M. remains the textbook example of human amnesia, and to say that his case dramatically influenced memory research understates the case considerably.
The operation carried out in Henry, a bilateral ablation of the MTL, provided relief from epileptic seizures, which had proved debilitating and intractable to pharmacological treatment. Although the operation ameliorated his seizures, it did more than that.
2.1. What was Henry’s problem?
Psychologists studied Henry for over five decades, but within a few years of his operation the major findings had emerged. In essence, the surgery rendered Henry densely amnesic. Indeed, it was Henry who taught psychologists what it meant to be densely amnesic. As a result of his surgery, he had a devastating impairment in creating certain kinds of memories from the time of his operation in 1953, at age 27, until his recent death at age 82.
At the same time, Henry’s memory for events prior to surgery remained relatively intact. He did lose some memories stored prior to his operation, a phenomenon called retrograde amnesia. There remains an active debate regarding retrograde memory loss in amnesia (Moscovitch & Nadel, 1998; Nadel & Moscovitch, 1997), and Henry’s retrograde amnesia extended back more years and was of greater severity than initially supposed. But the loss of old memories did not propel Henry’s case to the prominence it attained, and we will not discuss retrograde amnesia further in this article. Despite some degree of retrograde amnesia, Henry’s spared remote memories allowed him to retain reasonably normal abilities in written and spoken language, social skills, and arithmetic, among other cognitive domains.
In addition to a relatively spared retrograde memory, Henry had a reasonably functional short-term memory. That is, he could remember and mentally manipulate a limited amount of information over intervals ranging from seconds to minutes, provided that nothing distracted him. In formal testing, given material that he could rehearse verbally (and consciously), H. M. could remember items without error for 40 seconds, the limit of one such test (Sidman, Stoddard, & Mohr, 1968). If he could encode the information verbally, Henry could remember it over longer periods, evidently through constant rehearsal (Milner, 2005). For example, he could remember a three-digit number for 15 minutes (Milner, 1959). To be clear, H. M. did have lower scores on certain tests aimed at assessing short-term memory (Sidman et al., 1968), and so do other amnesics (Aggleton, Nicol, Huston, & Fairbairn, 1988; Squire, Zola-Morgan, & Chen, 1988). Such tests, however, suffer from many interpretational problems. For example, scores on these tests can be affected by the recall, from long-term memory, of both the items to be remembered and the rules of the test, along with strategies for performing the feats of memory required. Unlike control subjects, amnesics cannot create explicit long-term strategy, rule or item memories for use during a given testing session or for subsequent sessions. Perhaps more importantly, scores on these tests often reflect some mixture of implicit familiarity judgments and explicit recollection (see Sections 3.2, 4.2 and 6.2). Accordingly, although control subjects get better scores on tests that attempt to measure short-term memory, these findings do not necessarily reflect a short-term-memory deficit in amnesics. More likely, such results reflect the confounding effects of familiarity judgments and the advantage that control subjects have in recalling and deploying useful rules and strategies from long-term memory. Taking his daily life activities into account, it is clear that H. M. had a reasonably functional, if sometimes subpar, short-term memory. Had this not been so, he could not have performed the psychological tests that were, for decades, his most notable occupation. What propelled H. M. to prominence —what we call his core deficit — involved his nearly complete inability to create long-term conscious memories.
Importantly, Henry’s problem did not lie in creating long-term memories per se. He could, for example, learn to draw within the borders of a two-dimensional figure while viewing his hand and the drawing in a mirror. This mirror-drawing task perturbs visual feedback, and the task takes a good deal of practice to master. Like healthy people, Henry learned this visuomotor skill in about three days (Milner, 1962). Unlike the healthy people, however, Henry denied having seen the test materials and also disclaimed any knowledge of having learned the task. The ability to learn new motor skills lasted for the rest of his life, with one particularly impressive demonstration at age 69 (Shadmehr, Brandt, & Corkin, 1998). Henry not only learned new motor skills, but he also learned new cognitive and perceptual skills such as mirror reading.
So if Henry had reasonably functional short-term memory and could create some long-term memories, how should we characterize his problem? Despite his many intact mnemonic abilities, Henry could not create long-term memories that he could tell anyone about, with very few exceptions (Corkin, 2002). Thus his amnesia is best understood as a profound deficiency of conscious recollection, specifically an inability to encode and later recall the facts and events encountered in everyday life. Henry’s memory impairment was global in its involvement of all domains of conscious knowledge, dense in its severity, and anterograde in that the most dramatic effect of the surgery involved new memories as opposed to old (presurgical or premorbid) ones. Importantly, these characteristics do not apply to Henry, alone, but also to many other amnesics, some with damage or disease involving some of the structures removed in his surgery (Baddeley & Warrington, 1970; Brooks & Baddeley, 1976; Cohen & Squire, 1980).
Sadly, Henry’s inability to remember new people, names, places and events longer than his short-term memory span made it impossible for him to live independently. He described his state as “like waking from a dream … every day is alone in itself ….” (Milner, Corkin, & Teuber, 1968, p. 217). As this quotation shows, conscious memory is more that a simple record of facts and events; it permits us to embed ourselves in these facts and events — ordered in both space and time — thus providing the ‘feeling’ of a coherent, unified life experience. We return to this topic in Section 7.
2.2. What was Henry’s ablation?
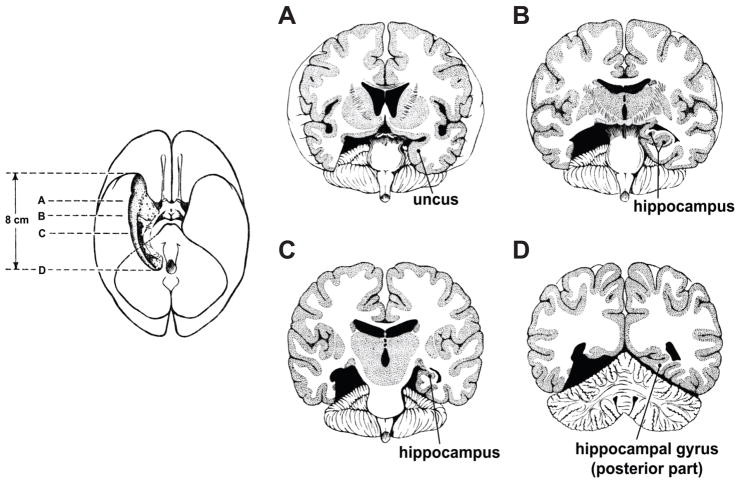
In 1953, no techniques could evaluate the extent of Henry’s lesion. Until the advent of magnetic resonance imaging (MRI), the assessment of his lesion relied only on the neurosurgeon’s notes (Scoville & Milner, 1957). The surgeon, Scoville, intended to remove the amygdala and hippocampus in both cerebral hemispheres, along with the cortex lying immediately ventral to these structures, which would include the entorhinal cortex and the parahippocampal cortex (Fig. 1).
Figure 1.
Depiction of the extent of the medial temporal lobe removal in patient H. M. A–D show sections at various anteroposterior levels, as indicated in the inset to the left. Although the neurosurgeon removed each structure bilaterally, the illustration shows the lesion extent in only one hemisphere. Reproduced from Scoville and Milner (1957).
Decades later, an MRI scan assessed Henry’s lesion (Corkin, Amaral, Gonzalez, Johnson, & Hyman, 1997). It revealed a bilaterally symmetric lesion, as intended, one that included the medial portion of the temporal pole cortex, most of the amygdala, most if not all the entorhinal cortex, and slightly more than half of the hippocampus, along with the nearby subicular complex. Scoville had entirely removed the anterior (temporal) part of the hippocampus, and the remaining, posterior (septal) portion had atrophied by the time of the scan (as had many other structures, such as the cerebellum). Although Corkin et al. (1997) reported that the perirhinal cortex and white matter of the temporal stem remained largely intact, this remains an open question (Gaffan, 2002; Goulet, Dore, & Murray, 1998). The integrity of a white matter bundle like the temporal stem is difficult to assess, and any damage to it would indirectly compromise the function of perirhinal cortex because its afferent and efferent projections pass through the temporal stem (Goulet et al., 1998; Muñoz, Mishkin, & Saunders, 2009). As we revise this article, anyone with an internet connection can view the sectioning of Henry’s brain in real time. So a detailed post-mortem assessment of his lesion remains for the future. Regardless of those final results, however, no doubt remains that the neurosurgeon removed much more than Henry’s hippocampus.
The fact that the neurosurgeon’s lesion included much more than the hippocampus, although recognized, did not dominate the early interpretations of H. M.’s condition. Milner (1959), for example, entitled an influential report on H. M. “The memory defect in bilateral hippocampal lesions”. Early investigators focused on the hippocampal complex because of several clinical observations. In their initial report, Scoville & Milner (1957) considered 10 patients with MTL removals of varying extent, and the amount of hippocampal damage seemed to provide the best prediction of postsurgical amnesia. For example, one patient with bilateral removal of the anterior MTL, sparing the hippocampus, showed little memory impairment. A patient with a unilateral inferior temporal lobectomy, one that included the hippocampus and underlying cortex, also had a fairly normal memory. Later, Penfield & Milner (1958) found severe memory impairment in only two of over 80 patients with unilateral temporal lobectomies. They suggested that these two patients had preexisting pathology of the contralateral hippocampus, and their idea later gained support from neuroanatomical analysis in similar patients (Penfield & Mathieson, 1974). Despite the initial focus on the hippocampus, contribution of the MTL, as a whole, has dominated most recent reviews on amnesia. In Section 4, we reconsider Milner’s original idea that damage to the hippocampal complex caused Henry’s core deficit.
2.3. What does Henry’s case tell us about human amnesia?
As we said earlier, Henry Molaison — who we call H. M. in the remainder of this article — taught psychologists what it meant to be densely amnesic, and his surgeon’s notes pointed to some of the brain structures involved in the disorder. But his amnesia is misunderstood if construed as an impairment in memory per se. Instead, H. M. had a selective impairment in one among many types of long-term memory: conscious memory. Only when people have impairments in long-term conscious memory do we call their disorder amnesia. H. M. and other amnesic patients have at their disposal vast amounts of memory, including knowledge acquired after their amnesia began. When people have impairments in these other kinds of memory, it goes by other names.
For example, technical knowledge — knowledge about tools and their use — remains intact in amnesia. The loss of such knowledge is called apraxia, not amnesia, and it depends on cortical areas outside the hippocampal complex and MTL. In humans, damage to the lateral temporal cortex selectively impairs tool recognition and naming. Functional neuroimaging studies of tool naming reveal increased regional blood-flow rates in visual motion areas and ventral premotor cortex (Chao, Haxby, & Martin, 1999; Chao & Martin, 2000; Martin & Chao, 2001). In the latter region, such activations occur for naming tools relative to naming other objects, viewing pictures of tools compared with viewing pictures of animals, faces, and houses, and also when subjects generate words associated with tool use (Martin & Chao, 2001). These and other findings support the idea that technical knowledge is represented in distributed networks of cortical regions that parallel the organization of other, less specialized sensory and motor systems, but not the hippocampal complex or other parts of the MTL.
To cite another example, social knowledge permits us to evaluate the mental states of other people, construed broadly to include emotions, intentions, thoughts, beliefs, and desires. Such knowledge is thought to be processed and represented in a network of regions composed of the amygdala, medial frontal cortex (including anterior cingulate cortex), and anterior insular cortex, along with the cortex of the temporal-parietal junction, posterior superior temporal sulcus, and inferior frontal gyrus (Frith & Frith, 2007; Blakemore, 2008). For example, thinking about the mental state of oneself or others engages portions of medial prefrontal cortex (Gilbert, Spengler, Simons, Steele, Lawrie, Frith, & Burgess, 2006). Failure to encode, retrieve, or process social knowledge is called autism or autism spectrum disorder, not amnesia. Social knowledge, like technical knowledge, remains intact in patients with global anterograde amnesia, including H. M. Note that social knowledge in this sense differs from identity knowledge based on the ability to remember a new face or voice, which amnesics lose.
Like individual identity knowledge, the ability to learn and remember new words and their meanings is lost in amnesia, but the underlying syntactical knowledge remains intact. The loss of knowledge about language is called aphasia, not amnesia. We could go on in this vein, delving into progressively more controversial areas. Hauser (2006), for example, has argued for an innate moral sense, one that subserves rapid moral judgments. Moral knowledge remains intact in human amnesia and its loss could lead to disorders such as sociopathy and psychopathy. Psychopaths report that what they have done is wrong and would be wrong for others, as well, so their problem does not appear to lie at a conscious level. No one would call someone an amnesic because he or she lost their moral sensibilities.
The understanding that human amnesia involves a deficiency in conscious memory has important implications for monkey models of human amnesia. As noted in Section 1, nondeclarative (i.e., subconscious) memories are expressed through performance (e.g., Clark et al., 2002), and monkeys express all their memories through performance. Because monkeys cannot speak, primate researchers have had to evaluate conscious memories with proxy tasks that invoke the concept of declarative memory or one of its equivalents. Next, we recount the history of this research.
3. History of the monkey model of human amnesia
3.1. Early efforts
Attempts to reproduce H. M.’s memory deficit in monkeys failed entirely at first. In the initial attempts, monkeys with bilateral lesions of the amygdala, hippocampus and underlying cortex could learn and remember the tested material without difficulty. For example, lesions of the MTL had little or no effect on the postoperative acquisition of object-discrimination problems, which measure the ability of monkeys to learn and remember which of two objects to choose in order to get food (Correll & Scoville, 1965a; Orbach, Milner, & Rasmussen, 1960). The lesions left retention of those postoperatively acquired memories intact, as well. In other tests of memory, monkeys with MTL lesions remembered single locations for up to 60 seconds, the same as intact monkeys (Correll & Scoville, 1967).
These early investigators did not know why their experiments failed. Early speculation focused on species differences or differences between the lesions in monkeys and humans, but these factors did not account for their results. Although the experimental lesions in monkeys did not match those in H. M. exactly — no experimental lesion could meet that standard — they included the homologous structures: the amygdala, hippocampus, subiculum, and underlying cortex. We now know that weaknesses in the behavioral tasks used, rather than lesion or species differences, caused the failure of these early attempts to model human amnesia. Murray (1996) has summarized this early work in detail. The lesions these early investigators made certainly produced a serious memory loss, as later research revealed. The memory tests used in the 1950s and 1960s simply did not assess the types of memories that the monkeys lost, a problem that persisted for many years.
3.2. Short-interval matching tasks
In the world of 1970s neuropsychology, a world in which, for decades, nothing anyone did produced a memory loss anything like H. M.’s, finally, at last, something did: the delayed matching- or nonmatching-to-sample task (Gaffan, 1974; Mishkin, 1978). For convenience, we refer to these tasks collectively as short-interval matching tasks. In these tasks, a monkey sees one or more sample objects on a test tray or two-dimensional stimuli on a video screen. Later, the monkey has to choose an object or stimulus according to one of two rules. According to the matching rule, the monkey must choose the sample object over a different object to obtain a food reward. According to the nonmatching rule, it must choose another object rather than the sample. Early attempts to use matching tasks had used a small set of stimuli, presented repeatedly from trial to trial, and obtained ambiguous results (Correll & Scoville, 1965b). As we discuss in Section 4.2, the number of items in the stimulus set and their frequency of appearance can affect the strategies used to solve the problem posed by short-interval matching tasks. The subsequent and more successful versions of the task employed novel stimuli on each trial or a very large set of stimuli, which, in combination with the requirement to remember either single or multiple stimuli over increasingly longer delay intervals, yielded a memory impairment in monkeys with MTL (Mishkin, 1978) or fornix lesions (Gaffan, 1974).
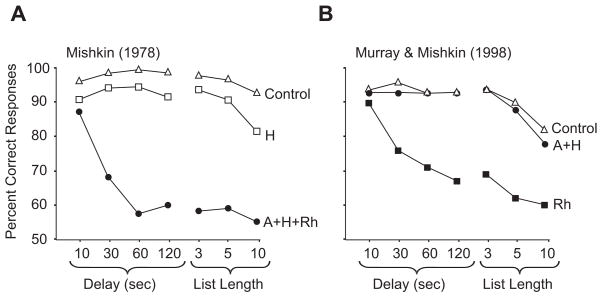
With this modified version of the matching task in hand, Mishkin (1978) concluded that combined lesions of the amygdala and hippocampus caused memory impairments like those of H. M. Monkeys with such lesions could remember objects for 10 s or so, but could not do so very well over longer periods or when several objects had to be remembered simultaneously (Fig. 2). Additional studies from laboratories headed by Mishkin and Squire seemed, at first, to verify Mishkin’s conclusion (Bachevalier, Parkinson, & Mishkin, 1985; Murray & Mishkin, 1984; Saunders, Murray, & Mishkin, 1984; Zola-Morgan, Squire, & Mishkin, 1982; Zola-Morgan & Squire, 1984, 1985). Lesions affecting the function of both the hippocampus and amygdala, like the MTL removal in H. M., produced deficits across sensory modalities, produced larger deficits when the lesion was larger, and spared certain abilities, such as skill learning. Lesions affecting either the hippocampus or the amygdala, alone, had little or no effect.
Figure 2.
Performance of monkeys on short-interval matching tasks. When only one sample object appeared, the delay varied from 10 to 120 seconds. Another way of taxing short-term memory involved the presentation of several samples to remember, called a “list”. Note that longer list lengths imposed additional delays. The chance level of performance was 50% correct. Abbreviations: A + H, a group of monkeys with combined, selective lesions of the amygdala (A) and hippocampus (H); A + H + Rh, a group of monkeys with combined lesions of the amygdala (A) and hippocampus (H) plus underlying parahippocampal cortex, which inadvertently disabled the subjacent rhinal cortex (Rh); H+, a group of monkeys with lesions of the hippocampal complex plus underlying parahippocampal cortex; Rh, a group of monkeys with lesions of the rhinal cortex. Control monkeys had no brain lesions. A modified from Mishkin (1978); B modified from Meunier et al. (1993) and Murray & Mishkin (1998)
Two enormous problems, however, lay hidden in this apparent advance. One involved the attribution of the memory deficit to lesions of the amygdala and hippocampus, an issue we take up later (Section 3.3). But an equally important problem involved the memory test used. Somehow, without anyone saying so explicitly, short-interval matching tasks became the principal proxy test for long-term, conscious memory in monkeys. In retrospect, it appears that the classification of the task in terms of “visual recognition”, “object recognition”, or “visual memory” took on greater influence than warranted, given what the task actually measured. Although short delays and a “list” of sample objects produced a deficit in monkeys with certain lesions (Fig. 2A), the short-interval matching task seems an unlikely candidate for a monkey model of human amnesia. As usually structured, the task measures memory loss over the short term (seconds and minutes), but H. M.’s core deficit involved an inability to create certain kinds of long-term memories (for recall days, weeks, months or years later). He had a reasonably functional short-term memory, as summarized in Section 2.1, but failed spectacularly at creating conscious long-term memories. In what follows, we call results from short-interval matching tasks H. M.-irrelevant. This designation does not imply that H. M. or other amnesics perform such tests as well as control subjects, but rather that such tests only indirectly address H. M.’s core deficit — his global deficit in creating conscious, long-term memories. Short-interval matching tasks were among the memory tests we discussed in Section 2.1, which measure a confounded combination of familiarity judgments, explicit recollections, and long-term memories of items, rules and strategies, along with their principal measure: short-term memory of the to-be-remembered items. The applicability of short-interval matching tasks to human amnesia has been challenged on other grounds, as well (Aggleton & Brown, 1999; Aggleton & Pearce, 2001). The distinction between H. M.’s core deficit in creating conscious long-term memories and other results plays a crucial role in the arguments we develop later. In Section 4.1, we argue that H. M.’s core deficit in anterograde memory resulted from damage to his hippocampal complex, and that damage elsewhere in the MTL did not contribute to his core deficit. In Section 6.2, we argue that familiarity judgments, which strongly affect performance on short-interval matching tasks, also have little to do with H. M.’s core deficit in conscious recollection.
The deficiencies of short-interval matching tasks would be unimportant if the orthodox monkey model of human amnesia relied on other memory tests, too. Indeed, in an initial formulation of the orthodox model, Squire and his colleagues proposed a battery of “MTL-dependent” tasks to measure memory, including: (1) object matching, (2) concurrent discrimination learning, (3) retention of rapidly learned object discriminations, and (4) spatial delayed response (Zola-Morgan & Squire, 1985). We described matching tasks above. The concurrent discrimination-learning task requires monkeys to learn which of two objects, when selected, produces a reward. In the concurrent procedure, the monkeys sees several pairs of objects before seeing any given pair again. In rapid object-discrimination learning, the monkey chooses between the same two objects on a number of consecutive trials, and memory for the correct (rewarded) item in the pair is tested by allowing the monkey to choose between the same two objects a day or more later. The spatial delayed-response task is much like a short-interval matching task, except that it requires the maintenance of spatial information — a cued location — over the short term, rather than nonspatial (object) information. Unfortunately, the battery of tests proposed by Zola-Morgan & Squire (1985) has failed the test of time. The concurrent discrimination-learning task depends largely on structures outside the MTL in monkeys (Buffalo, Stefanacci, Squire, & Zola, 1998; Gaffan & Murray, 1992; Malamut, Saunders, & Mishkin, 1984; Phillips, Malamut, Bachevalier, & Mishkin, 1988), and, likewise, monkeys with MTL lesions can perform the spatial delayed-response and related tasks as well as intact monkeys (Correll & Scoville, 1967; Murray & Mishkin, 1986; Waxler & Rosvold, 1970). The “retention of rapidly learned object discriminations” has some promise, but as developed to date it combines acquisition and retention in a way that does little to clarify the nature of the impairment. In the end, of the four-task battery proposed by Zola-Morgan & Squire (1985), only the short-interval matching tasks remains viable. And, as we have discussed here, that task does little to address H. M.’s core deficit.
3.3. Localization and mislocation
Research on monkeys should, in principle, allow investigators to narrow the possible causes of H. M.’s amnesia in neuroanatomical terms. Mishkin’s (1978) work seemed to have solved that problem, too. He concluded that lesions of the hippocampus needed to be combined with lesions of the amygdala to replicate H. M.’s amnesia. In support of his idea, recall that the surgeon’s notes indicated that H. M.’s amygdala had been removed bilaterally, along with his hippocampal complex, as summarized in Section 2.2.
Unfortunately, neither Mishkin’s original experiment nor any follow-up studies of the early 1980s (Bachevalier et al., 1985; Mahut, Zola-Morgan, & Moss, 1982; Mishkin, 1978; Murray & Mishkin, 1984; Saunders et al., 1984; Zola-Morgan et al., 1982, 1984, 1985) included a control group to test the possibility that damage to structures near the amygdala or the hippocampus had caused the impairment that they observed. As history later revealed, Mishkin’s results had nothing to do with the amygdala and only a little to do with the hippocampal complex (see Section 4.2). Figure 2 shows that the deficit resulted almost entirely from inadvertent damage to the cortex underlying the hippocampus and amygdala (Meunier, Bachevalier, Mishkin, & Murray, 1993; see also Eacott, Gaffan, & Murray, 1994). These underlying cortical areas included the perirhinal cortex and entorhinal cortex, together called the rhinal (Rh) cortex, which were either directly damaged (entorhinal cortex) or functionally compromised (perirhinal cortex) by the so-called “amygdala plus hippocampus” (A + H) lesion. Although this surgical procedure left the perirhinal cortex largely intact, it inadvertently cut many of its efferent and afferent axons. The lesion that Mishkin called “amygdala plus hippocampus” was therefore much more extensive, which is why Figure 2A designates it as A + H + Rh. Mishkin’s “amygdala” lesion included the rostral entorhinal cortex and a portion of the connections to and from the perirhinal cortex. Likewise, his “hippocampal” lesion took out the caudal entorhinal cortex and a different group of perirhinal cortex connections. Thus, only the combined removal of the amygdala and hippocampus involved all of the entorhinal cortex, as well as the lion’s share of perirhinal connections. We now know that removal of the ‘rhinal’ cortex, alone, causes nearly all of the memory loss seen in Mishkin’s original experiment (Rh in Fig. 2B). In contrast, combined lesions of the amygdala and hippocampal complex have no effect on the same task, provided that they are selective enough to preserve the underlying cortex (A + H in Fig. 2B). [Parahippocampal cortex (areas TF and TH), which was also included in the original amygdala plus hippocampal removal, likewise appears to make little or no contribution to the performance of short-interval matching tasks (Nemanic, Alvarado, & Bachevalier, 2004)].
Mishkin’s (1978) conclusions played a highly influential role in developing the orthodox monkey model of human amnesia, a scheme that dates from the 1980s and early 1990s (Mishkin, 1982; Squire & Zola-Morgan, 1991). His results seemed to point beyond the hippocampal complex to a larger group of structures in the MTL as the key substrates of conscious memory. Although based on H. M.-irrelevant results that he initially attributed to the wrong brain structures, Mishkin’s conclusions provided the foundation for today’s orthodoxy.
3.4. The legacies of history
The history of this line of research imparts many lessons about the challenges faced by animal models of advanced human cognition. A lot of things can go wrong — and they did. Once corrected, these mistakes would be mere historical curiosities, except that two of their legacies persist: (1) the concept of a reified MTL as a conscious-memory center and (2) the notion that short-interval matching tasks assess memory deficits like H. M.’s amnesia. Previously (Murray & Wise, 2004), we focused on problems with the first legacy, the concept of an MTL that operates as a single functional “thing” to support another “thing” called a “memory system”. We developed Gaffan’s (2002) analysis by arguing that dense amnesia results from the peculiar geometry of the primate brain, in which different neural pathways funnel diverse information through a tight spot, near the junction of the basal forebrain with the temporal lobe, so that a lesion there has catastrophic effects on memory. Here we focus on the second legacy: the domination of the field by short-interval matching tasks. Only by invoking the concept of a reified MTL do results from short-interval matching tasks enter the orthodox monkey model of human amnesia. Performance on these tasks depends on the perirhinal cortex, and if one excludes that area from the model then the matching tasks can leave with it. The reverse is equally true: if one excludes short-term matching tasks as H. M.–irrelevant, then the perirhinal cortex can leave the model. Accordingly, we propose that an improved model might dispense with both the perirhinal cortex and short-interval matching tasks, along with the concept of a reified MTL. Much the same can be said for the parahippocampal cortex and the tasks dependent on it. This new, improved model of human amnesia would then look a lot like Milner’s (1959) original one, which emphasized the hippocampal complex as the key to understanding H. M.’s amnesia, rather than the MTL as a whole. We emphasize that our previous analysis (Murray & Wise, 2004) does not differ all that much from the present one. Both advance the idea that multiple afferent and efferent pathways of the hippocampal complex need to be damaged to produce severe impairments in memory. They differ principally in whether to include afferent and efferent pathways of the amygdala, perirhinal cortex or parahippocampal cortex in a monkey model of amnesia. If any of these structures compose a “memory center” called the MTL, then the model must include their input and output pathways, too. Here we propose excluding them.
Recall that, originally, H. M.’s amnesia was attributed to the hippocampal complex (see Section 2.2, Milner, 1959; Penfield & Mathieson, 1974). More recently, Clark et al. (2002, p. 524) likewise concluded that “declarative memory depends on the integrity of the hippocampus and related structures. …” So the question is: what are the related structures? Not the MTL as a whole, many parts of which are no more “related” to the hippocampus than a host of cortical areas outside the MTL, except by an accident of evolutionary history that pushed the hippocampus into the temporal lobe of primates (as we explained in Murray & Wise, 2004). Perhaps the “related structures” include only the subicular complex and entorhinal cortex, rather than the long list of components included in the orthodox monkey model of human amnesia.
3.5. A way forward
To summarize the main points of this section, the orthodox monkey model of human amnesia assigns the MTL, as an entity, the role of encoding and later recollecting conscious memories. Yet the development of this model depended primarily on tasks that mainly measured memory on an inappropriate scale (short-interval matching tasks) and on impairments caused by unintended lesions (of axons going to and from the perirhinal cortex). Seemingly unconcerned with the frailty of its historical foundation or its dependence on a swarm of weakly consistent evidence, proponents of the model now consider it so well established that only evidence verging on disproof could (or should) dislodge it (e.g., Squire, Stark, & Clark, 2004; Mishkin, Suzuki, Gadian, & Vargha-Khadem, 1997; Suzuki, 2009; Suzuki & Baxter, 2009). Given its faulty foundation, however, it makes sense to reconsider the orthodox model in its entirety, and along with it the literature regarding amnesia in both monkeys and humans. Once we dispense with the concept of a reified MTL, the model no longer needs to include either the perirhinal cortex or any tasks that depend on the perirhinal cortex, such as short-interval matching tasks. What remains? More than one might expect. And what remains could someday lead to an improved monkey model of human amnesia, one focused more on the hippocampal complex than on a reified MTL, and one supported by tasks that measure long-term memory more rigorously than do short-interval matching tasks.
4. Toward an improved monkey model of human amnesia
4.1. Does hippocampal damage cause amnesia in humans?
A considerable body of evidence points to the hippocampal complex as the source of H. M.-like amnesia. As discussed earlier (Section 3.3), H. M. failed to store new facts and events that he could recall and express, hence the term declarative memory. At this point we need to distinguish between two main types of declarative memory: event memory, a term which is often used interchangeably with episodic memory, and fact memory, also known as semantic memory. We also need to distinguish between recollection and familiarity. Section 6.2 develops this distinction in more detail, but for now we can consider recollection as equivalent to declarative memory and familiarity as something else. Some evidence indicates that hippocampal lesions interfere with specific recollections and memories for events, but spares familiarity judgments (e.g., Aggleton & Shaw, 1996), and there have been claims that hippocampal damage also spares memories for facts, i.e., semantic memories (Vargha-Khadem et al., 1997, 2001). We need to consider these two claims separately.
The hippocampal complex subserves episodic memory
One line of evidence concerning episodic vs. semantic memory comes from a study of children with early hippocampal damage. Initially, they were reported to have profound impairments in episodic memory, with nearly complete sparing of semantic memory (Vargha-Khadem et al., 1997; 2001). MRI-based volume estimates in these select subjects indicated that they had a smaller hippocampus than controls: the only structure so affected. Subsequent studies of these and other patients with damage restricted to the hippocampus have shown, contrary to the initial reports of Vargha-Khadem et al., that these lesions do impair the acquisition of semantic memories (Gardiner, Brandt, Baddeley, Vargha-Khadem, & Mishkin, 2008; Holdstock, Mayes, Isaac, Gong, & Roberts, 2002; Manns, Hopkins, & Squire, 2003). Holdstock et al. (2002) emphasized that the hippocampus plays an especially important role in the rapid acquisition of semantic (factual) information, just as it does for the rapid acquisition of episodic (event) memory ( see also Kapur, 1994). Patients with hippocampal damage can acquire semantic knowledge only slowly, through repeated exposure to factual material (Gardiner et al., 2008; Holdstock et al., 2002). Thus, the functional distinction between the hippocampal cortex and most other cortical areas could relate to rapid versus slow learning (McClelland, McNaughton, & O’Reilly, 1995) rather than to episodic versus semantic memory per se. Of course, episodic memories require rapid learning because they often capture singular events. Even with these ideas in mind, we need to account for H. M.’s nearly complete incapacity to acquire semantic knowledge (Gabrieli, Cohen, & Corkin, 1988), compared to other amnesic patients, many who can acquire such information slowly. This discrepancy could have resulted from the fact that, in contrast to many other amnesic patients, H. M. had a complete surgical removal of the anterior (temporal) hippocampus or more extensive damage to the remainder of the hippocampal complex (i.e., the fimbria-fornix, subicular complex and entorhinal cortex).
Another study examined a series of 38 patients who had undergone colloid cyst removals. Colloid cysts typically form in the third ventricle, and their removal often results in damage to the fornix, which passes through the ventricle and to which the cyst may become attached. Indeed, the fornix may be compromised before the surgery. All the subjects in this study received structural MRI scans to measure the fornix, mammillary bodies and related structures. Strikingly, the volume of the mammillary bodies, which indirectly reflects the integrity of the fornix, significantly correlated with the scores for 13 of 14 tests of episodic memory (Tsivilis, Vann, Denby, Roberts, Mayes, Montaldi, & Aggleton, 2008). No other structures showed such a consistent relationship between size and memory scores. Because the fornix serves as the main afferent and efferent fiber bundle of the hippocampal complex, including the subicular complex, this finding, like that of Vargha-Khadem et al. (2001), points to a key role for these structures in episodic memory.
The hippocampal complex subserves recollection
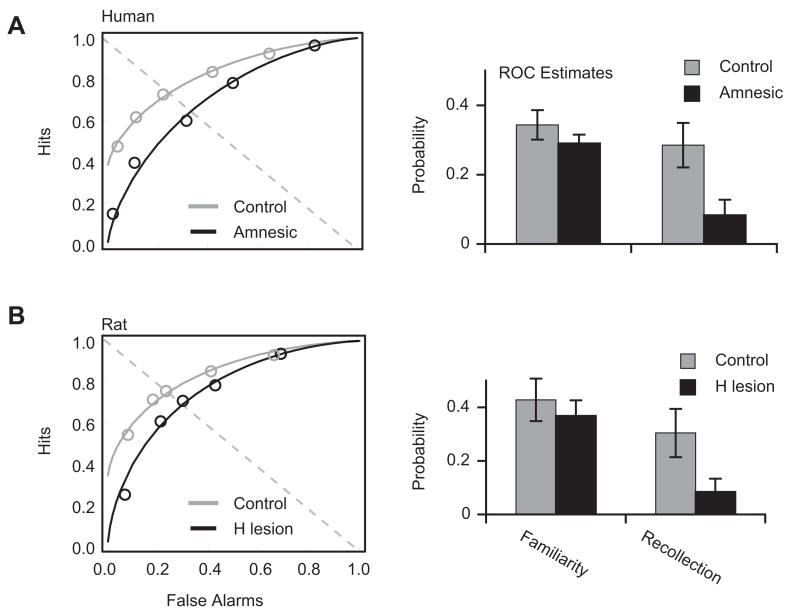
Regarding the issue of recollection vs. familiarity judgments, one issue involves whether they represent a single process or two distinct processes, both underlying recognition memory. Some have argued that preserved familiarity, in the face of recollection losses, might result from a single process, one with a lower threshold for familiarity judgments than for explicit recollection (Squire, Wixted, & Clark, 2007). Recent results indicate otherwise. Vann, Tsivilis, Denby, Quamme, Yonelinas, Aggleton, Montaldi, & Mayes (2009) studied the patients mentioned above, who had undergone surgical removal of a colloid cyst. Using three different experimental methods, they found that patients with small mammillary bodies had impairments in recollection memory relative to patients with large mammillary bodies; familiarity judgments did not differ between groups. Although several cases had earlier been reported to show this same pattern — impaired recollection with spared familiarity — there had also been reports to the contrary, with differing etiologies of amnesia complicating the interpretation within and across studies. The study by Vann et al. (2009) represents a significant advance because of three methodological strengths: the large number of patients with a single etiology of memory impairment; the use of a structural measure to identify subgroups (small vs. large mammillary bodies); and the use of several, complementary methods to assess familiarity and recollection. As such, it presents the strongest evidence to date in favor of the idea that the hippocampal complex subserves recollection rather than familiarity.
Importantly, a single-case study of a patient receiving surgery for relief of intractable epilepsy has reported the opposite pattern of impairment. Surgeons removed left anterior temporal lobe structures from that patient, including much of the perirhinal cortex, entorhinal cortex and most of the amygdala. The hippocampus remained intact. After surgery, the investigators tested the patient on four separate neuropsychological measures, all of which revealed impaired familiarity judgments but preserved recollection (Bowles, Crupi, Mirsattari, Pigott, Parrent, Pruessner, Yonelinas, & Kohler, 2007). The pattern of impaired familiarity in the face of intact recollection is critical in distinguishing between one- and two-process models of recognition memory. Although several patients have now been reported to display impaired recall but preserved familiarity, these findings might result from a single process, one with a lower threshold for familiarity than for recollection judgments (Squire et al., 2007). Although only a single case, the patient with impaired familiarity but preserved recollection (Bowles et al., 2007) seems to rule out the one-process account and argues strongly that two distinct processes contribute to recognition memory: recollection, which depends on the hippocampal complex, and familiarity, which does not. We return to this topic in Section 6.2, when we consider the results of hippocampal lesions in rodents that support this idea.
Lesions of the hippocampal complex cause amnesia
Other patients, selected and studied because of their amnesia, have undergone intensive neuropsychological testing. and, in some cases, neuropathological analysis, as well (Rempel-Clower, Zola, Squire, & Amaral, 1996; Zola-Morgan, Squire, & Amaral, 1986). Neuropathological analysis often misses sites of brain damage, but taking these reports at face value, we review the findings with an eye to providing a complete picture of the neural substrates of amnesia. As in H. M., the memory impairment in these patients occurred in the absence of deficits in other cognitive domains. In the four patients with detailed neuropathological reports, moderate anterograde amnesia occurred with bilateral cell loss limited primarily to CA1 in patients R. B. and G. D., whereas a severe anterograde memory impairment occurred with bilateral cell loss in CA1, CA2, CA3, the dentate gyrus, the subiculum, and the entorhinal cortex (patient W. H.). W. H. also displayed marked atrophy of the mammillary bodies, but neither R. B. nor G. D. had such shrinkage. This finding agrees with neuroanatomical findings showing that efferent fibers from the subicular complex make up the bulk of the fornix and with results from patients with the removals of colloid cysts near the fornix, outlined above. Taken together, these findings point to a role for the hippocampal complex in conscious memory, rather than for the MTL as a whole.
Another amnesic patient with a detailed neuropathological report, patient E. P., suffered from viral encephalitis (Stefanacci, Buffalo, Schmolck, & Squire, 2000). E. P.’s extensive brain damage included the hippocampal complex, perirhinal cortex, parahippocampal cortex, and fusiform gyri, along with atrophy in temporal, parietal and insular cortex. Despite this massive extension of pathology beyond H. M.’s lesion (Fig. 1) and beyond W. H.’s anoxic damage (CA1, CA2, CA3, the dentate gyrus, the subiculum, and the entorhinal cortex), E. P.’s amnesia was about the same as theirs. All three patients had similar scores on the delay component of the revised Wechsler Memory Scale, which tests the memory for information presented 25–35 minutes earlier (Rempel-Clower et al., 1996; Stefanacci et al., 2000).. The finding that the extension of E. P.’s lesion beyond the hippocampal complex caused no more impairment than the two patients with damage confined to the hippocampal complex again points to the hippocampal complex, rather than the MTL, as the key to understanding conscious memory in humans.
Activity in the hippocampal complex correlates with self-referential event memory
Functional imaging studies have also pointed to a role for the hippocampal complex in functions fundamental to conscious memory. This work has revealed a network of structures that change blood-flow rates in relation to autobiographical memory retrieval (Conway, Pleydell-Pearce, Whitecross, & Sharpe, 2003; Maguire, 2001; Svoboda, McKinnon, & Levine, 2006), including episodic memory. Maguire and her colleagues have identified neural correlates of two separable components of episodic memory: scene construction and connection to self (Hassabis, Kumaran, & Maguire, 2007; Hassabis & Maguire, 2009). Their functional neuroimaging studies required subjects to recall recent episodic memories, to retrieve fictitious experiences constructed one week earlier, and to construct new fictitious experiences while being scanned. Analogous object-based tasks served as controls. When contrasting the three conditions involving personal experiences (imagined or real) relative to the three conditions involving objects, a network of brain regions showed task-related effects, including the hippocampus, parahippocampal gyrus, retrosplenial cortex, precuneus, posterior parietal cortex, and ventromedial prefrontal cortex. When contrasting real and fictitious events, three brain regions had higher blood flow for real memories: the precuneus, posterior cingulate cortex, and anterior medial prefrontal cortex (area 10). Because activation in area 10 and posterior cingulate cortex occurred only during episodic memory recall, Maguire and her colleagues identified these two regions as contributing to functions beyond scene construction and involving the subjects embedding themselves in the events. The posterior cingulate cortex and area 10 have also been implicated in self-reflection (Johnson, Baxter, Wilder, Pipe, Heiserman, & Prigatano, 2002), theory of mind (Amodio & Frith, 2006; Kumaran & Maguire, 2005), and thinking about future events (Addis, Moscovitch, & McAndrews, 2007; Hassabis et al., 2007). Thus, these two regions may contribute to episodic memory by supporting processing related to the self (Conway & Pleydell-Pearce, 2000) and mental time travel (Tulving, 2002; Wheeler, Stuss, & Tulving, 1997), a topic taken up again in Section 7.2. Studies emphasizing episodic memory as a constructive process bring to the fore an aspect of episodic memory not always appreciated. Episodic memory allows us not only to create a continuous record of life experience, with the self embedded, but also to recombine stored information in novel ways that permit us to evaluate the suitability of different potential courses of action (Hassabis & Maguire, 2009). The involvement of area 10 catches our attention for two reasons: this area, often called the frontal pole cortex, is the largest area in the prefrontal cortex of humans (Öngür, Ferry, & Price, 2003), and it expanded so much during human evolution (Semendeferi, Armstrong, Schleicher, Zilles, & Van Hoesen, 2001) that it dominates the geometry of the anterior brain and braincase. The initial study of neuronal activity in monkeys concluded that the likely homologue of the frontal pole cortex plays a role in monitoring or evaluating self-generated decisions (Tsujimoto, Genovesio, & Wise, 2009). This conclusion has some relevance to the ideas about self-reference and cross-domain knowledge presented in Section 7.
The hippocampal complex subserves trace conditioning
Another line of research linking the hippocampus with conscious memory comes from studies of Pavlovian eye-blink conditioning. Hippocampal lesions cause a deficit in one kind of Pavlovian learning called trace conditioning (McEchron, Bouwmeester, Tseng, Weiss, & Disterhoft, 1998; McEchron & Disterhoft, 1999; Solomon, Vander Schaaf, Thompson, & Weisz, 1986). In Pavlovian conditioning, an initially neutral stimulus, called the conditioned stimulus, precedes an unconditioned stimulus, which triggers a reflex response. With repeated exposure, the conditioned stimulus comes to elicit a similar response. In the standard conditioning paradigm, the conditioned stimulus remains present until the unconditioned stimulus occurs after a fixed delay. Hence the name for this paradigm: delay conditioning. When the conditioned stimulus occurs only briefly, then goes away before the unconditioned stimulus occurs, learning still takes place. The conditioned stimulus is thought to leave a “trace” behind, hence the name of this paradigm: trace conditioning. Hippocampal damage causes a deficit in trace conditioning but not delay conditioning. According to Clark et al. (2002), conscious awareness, not stimulus memory per se, affects trace conditioning. In their experiments on eyeblink conditioning, manipulations that increased awareness (e.g., explicit instructions) increased the speed of trace conditioning and those that decreased awareness (e.g., distraction) slowed it. In addition, when they manipulated expectancy for the unconditioned stimulus, an air puff, the probability of a conditioned response correlated with expectancy in trace conditioning but not in delay conditioning. These results point to the importance of conscious awareness in the functions of the hippocampal complex, even for the learning of low-order, conditioned reflexes.
4.2. Does hippocampal damage cause H. M.-relevant memory deficits in monkeys?
Section 4.1 points to the hippocampal complex, rather than the MTL as a whole, as the neural substrate for the kind of long-term memories lost in human amnesics like H. M. If so, then a monkey model of human amnesia should focus on the hippocampal complex, rather than the conglomeration of structures known as the MTL. Furthermore, the task used in such a model should yield a pattern of impaired and preserved memory functions similar to those seen in H. M. and similar patients. As we explained in Section 3, short-interval matching tasks do not fit the bill very well.
Given that H. M. and other amnesics have a reasonably functional short-term memory, it should not be surprising that complete bilateral removal of the hippocampal complex can leave performance on short-interval matching tasks unaffected in monkeys — under certain circumstances (Correll & Scoville, 1965b; Murray & Mishkin, 1984; Murray & Mishkin, 1998). Most straightforwardly, monkeys can solve the problem posed by short-interval matching tasks through focused, attentive maintenance of object representations in short-term (‘working’or ‘maintenance’) memory, and later applying either the matching or nonmatching rule. One can think of this algorithm as a strategy: one among many solutions to a given problem. However, as we explained in Section 3.2, short-interval matching tasks often measure more than short-term memory, and perhaps for this reason damage to the hippocampal complex (alone) or transection of the fornix can affect performance on certain versions of the task. We think that differences in the monkeys’ strategies could account for inconsistent results both within and between laboratories. Depending on the number of stimuli used and other factors, monkeys can solve the problem posed by matching tasks by using various strategies in addition to, or instead of, short-term maintenance memory. One such strategy involves choosing an object based on its familiarity, i.e., on the basis of whether the item has been encountered previously. This strategy amounts to a discrimination of novel from familiar items. Alternatively, monkeys might choose an object based on how recently it has been viewed, as opposed to its familiarity or novelty.
In matching tasks that employ a small set of stimuli, it seems likely that monkeys keep a representation of the sample in short-term memory, a process that does not depend on the integrity of the hippocampal complex. The application of a short-term-memory strategy could explain the lack of effect of hippocampal lesions on matching tasks with small stimulus sets (see Section 3.2, e.g., Correll & Scoville, 1965b, who used two stimuli). In contrast, tasks employing large stimulus sets may lead to reliance on recency or familiarity strategies instead of, or in addition to, a short-term-memory strategy. Here we divide larger stimulus sets into those that use novel (trial-unique) stimuli (or a set sufficiently large, more than 1,000 items or so, to resemble novel stimuli) and those that use an intermediate number of stimuli (~300–400 stimuli). Repeated presentation of stimuli — as occurs with intermediate set sizes —would hamper familiarity judgements, because all stimuli become familiar, and thereby promote a recency strategy. This strategic bias might explain why hippocampal-lesion studies involving intermediate-sized stimulus sets have tended to show deficits on matching tasks (Beason-Held, Rosene, Killiany, & Moss, 1999; Zola et al., 2000; see also Gaffan, 1974), whereas those with larger stimulus sets (Murray & Mishkin, 1998; Nemanic et al., 2004) and small stimulus sets (Correll & Scoville, 1965b) have not. This interpretation implies that the hippocampal complex subserves recency judgments, an account supported by the finding that fornix transection causes a deficit in recency memory, but leaves novelty and familiarity judgments intact (Charles, Gaffan, & Buckley, 2004). Note that, to the extent that recency judgements depend on the order of event sequences, they can be related directly to the concept of episodic (event) memory. On the other hand, the use of a familiarity strategy — as likely occurs with large set sizes and trial-unique stimuli — could render performance immune from damage to the hippocampal complex (see Sections 4.1 and 6.2). Beyond the size of stimulus sets, other factors could also affect the strategy used by monkeys, such as having the monkeys learn the task postoperatively (Beason-Held et al., 1999; Zola et al., 2000) rather than preoperatively (Murray & Mishkin, 1998) and how often the memory interval changes during a block of trials (Gaffan, 1974; see Baxter & Murray, 2001 for additional discussion). Because of their complexity and dependence on several strategies, short-interval matching tasks seem to us to be a poor choice for improving the monkey model of human amnesia. So we need a different task, one for which hippocampal dysfunction causes a pattern of spared and impaired memory functions like those seen in human amnesia.
Of course, damage to the hippocampus causes many deficits in memory, and we cannot review the vast literature on this topic here. Note that our question is not, Does hippocampal damage cause memory deficits in monkeys?, but rather, Does hippocampal damage cause H. M.-relevant memory deficits in monkeys? As a further complication, many of the deficits in spatial memory that have been attributed to the hippocampus in monkeys (Angeli, Murray, & Mishkin, 1993; Mahut & Moss, 1986; Parkinson, Murray, & Mishkin, 1988) resulted instead from inadvertent damage to the underlying parahippocampal cortex (Malkova & Mishkin, 2003; Murray & Mishkin, 1998). Lesions confined to the hippocampal complex, alone, cause impairments in navigating within a large-scale environment (Hampton, Hampstead, & Murray, 2004; Lavenex, Amaral, & Lavenex, 2006), remembering a location within a scene (Murray, Baxter, & Gaffan, 1998), remembering two or more locations simultaneously (Beason-Held et al., 1999), and remembering the locations of objects in an array (Bachevalier & Nemanic, 2008). One synthesis of these data holds that spatial tasks requiring an extrinsic (allocentric) frame of reference depend on the integrity of the hippocampal complex (Banta Lavenex & Lavenex, 2009), which helps bring the monkey data into line with results from other species, including rodents, reptiles and teleost fishes. Taken together, these results and ideas suggest that the ancestral role of the hippocampal complex, one that evolved early in vertebrate history, involves navigation in an extrinsic reference frame (Rodriguez, Lopez, Vargas, Broglio, Gomez, & Salas, 2002). But deficits in spatial memory and navigation do not, on their face, resemble H. M.’s global anterograde amnesia very closely. Later, in Section 7, we consider a more general conceptualization of navigation, with special attention to self-referential navigation.
4.3. A way forward
A promising line of monkey research involves the object-in-place scene task devised by Gaffan (1994). This task requires monkeys to identify and touch the one of two foreground “objects” in a complex scene composed of several geometric forms of varying size, shape and color. Selection of the correct foreground object within a scene leads to delivery of food reward, and monkeys are required to learn several such scenes concurrently. It has been proposed that the object-in-place scenes task taxes episodic memory in monkeys (Gaffan, 1994), a topic we take up in more detail in Section 5.4. For now, we note that damage to separate parts of the extended hippocampal system, including the fornix, anterior thalamus, and mammillary bodies, yields a deficit on this task (Gaffan, 1994; Parker & Gaffan, 1997a, b). Because the deficits that follow the different lesions have the same magnitude, and because addition of a fornix transection to monkeys that have sustained a mammillary body lesion yields no greater impairment, it appears that these structures work together as a functional unit. Although the object-in-place scenes task provides clear evidence for the learning of object discriminations embedded in complex scenes, and clear evidence of being dependent on the hippocampal complex, it needs some development to serve as part of a compelling monkey model of human amnesia. Monkeys learn the individual discriminations over several trials, a learning rate that contrasts with the one-exposure event-capture that characterizes conscious, episodic memory in humans. Nevertheless, the recollection of remote memories seems to be mediated via corticocortical interactions involving the prefrontal cortex (Browning & Gaffan, 2008a, b), while new learning depends on the fornix (Buckley, Wilson, & Gaffan, 2008).
A variant of Gaffan’s object-in-place scenes task has been used in human neuropsychology, specifically, in patients who had sustained fornix transection as a consequence of surgical removal of colloid cysts (Aggleton et al., 2000). Although these patients showed impairments, they were mild ones. The fornix-damaged group performed significantly worse than the two control groups only on the first of four trials with a set of 20 object-in-place scenes. Thus, it seems likely that fornix damage must combine with other disruptions to produce a severe amnesia. And, indeed, Gaffan, Parker, & Easton (2001) found more severe effects on memory in monkeys, relative to that seen after fornix transection, when they combined fornix transection with section of the anterior temporal stem and amygdala lesions. They examined the effect of such combined lesions on several memory tasks, including the short-interval matching task and the concurrent object-discrimination task, along with the object-in-place scenes task. Gaffan et al. (2001) found severe impairments on all three tasks. For both object-in-place scenes and concurrent object-discrimination learning, combined lesions caused larger deficits than either fornix transection, alone, or damage to the temporal stem and amygdala, alone. For the matching task, section of the temporal stem and amygdala produced the full impairment; addition of fornix transection had no additional effect. The latter finding is consistent with the idea that perirhinal cortex, rather than the hippocampus, is essential for performance on matching tasks with large stimulus sets. One monkey with the full combined lesion retained a large number of preoperative learned concurrent-discrimination problems, which resembles H. M.’s preserved remote memories. Accordingly, Gaffan et al. (2001) concluded that the pattern of impairments resembled that seen in humans with dense amnesia. Some of these findings might seem inconsistent with the ideas propounded here. They seem more in line with the idea that many structures, including the hippocampus, need to be compromised in order to produce a severe, H. M.-like impairment. To the contrary, we think that these findings point to the multiple routes by which the hippocampal complex can communicate with other brain regions, such as the prefrontal cortex. Thus, to produce a severe amnesia, fiber-cutting lesions need to include more than the fornix. Interruption of the temporal stem and fornix could, together, eliminate most if not all of the routes through which the prefrontal cortex and hippocampal complex communicate. Accordingly, the object-in-place scenes task holds considerable promise for future development of the monkey model of human amnesia, especially when combined with a more refined analysis of the routes of information flow to and from the hippocampal complex.
Along with the object-in-place scenes task, a task involving the arbitrary mapping of symbolic information to actions holds some promise. This task, sometimes called conditional motor learning or simply the arbitrary mapping task, involves nothing more than learning and later retrieving simple stimulus-response (S-R) associations. A nonspatial visual cue instructs one action while other, similar cues instruct different actions. By “nonspatial” cue, we mean object-like stimuli with many features that distinguish one cue from another. In the typical training and testing procedure, a single stimulus appears on a video screen and the monkey must choose among several responses, only one of which will produce a reward. A computer randomly selects one stimulus from a set on each trial, so several trials might intervene between repetitions of a given stimulus, with different stimuli and responses, some correct and some incorrect. This feature of the task requires the monkeys to lay down long-term memories of the cue-action mappings. For example, monkeys sometimes take several testing sessions, extending over days, to learn a new set of cue-action mappings, especially during early phases of training. When that happens, they invariably begin each day near the level of performance reached at the end of the previous day. The arbitrary mapping task thus differs importantly from the short-interval matching task, which monkeys could solve by maintaining a representation of the sample stimulus in short-term memory and later applying the matching or nonmatching rule. It differs from the object-in-place scenes task in that monkeys can (although they do not always) learn the mappings from the experience of a single, successfully performed trial (Brasted, Bussey, Murray, & Wise, 2005), like the one-exposure event-capture that characterizes conscious, episodic memory in humans.
We and others have studied the neural substrates of arbitrary mapping. Damage to the hippocampal complex yields a pattern of deficits and preserved memory functions in the arbitrary mapping task that matches H. M.’s amnesia fairly closely. Like H. M., monkeys with lesions of the hippocampus and subjacent cortex have a dramatic deficit in new learning that depends on long-term memory, as explained above (Murray & Wise, 1996). As shown in Figure 3A (unfilled circles), bilateral ablation of the hippocampus and subjacent cortical areas causes substantial deficits in the learning of new arbitrary mappings (Murray & Wise, 1996; Wise & Murray, 1999, 2000). Also like H. M., the lesioned monkeys can recall memories that they had established prior to their surgery. They also had preserved knowledge of at least three specific response strategies, learned prior to surgery, which depended on an intact short-term memory (Wise & Murray, 1999). The results we observed for this simple S-R task thus matched H. M.’s pattern of amnesia and preserved mnemonic capacities much more closely than results from short-interval matching tasks and many other tasks used to probe hippocampal function.
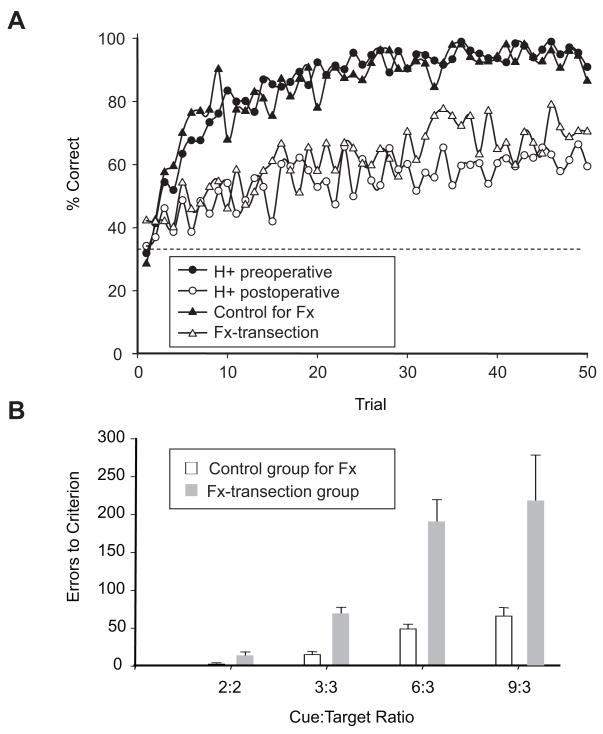
Figure 3.
The effects of removing the hippocampal complex and transecting the fornix on the arbitrary mapping task. A. Learning curves for two kinds of arbitrary mapping tasks. One study (triangles) involved a comparison of learning rates in a control group of monkeys (filled triangles) with a group of monkeys that had underdone fornix-transection (unfilled triangles). In this experiment, both the cues and the responses differed along nonspatial dimensions. The other study (circles) used similar cues but spatial responses. It involved a comparison between preoperative (filled circles) and postoperative (unfilled circles) performance of a single group of monkeys, which had undergone bilateral removals of the hippocampal complex, as well as underlying areas of cortex. Abbreviations: H+, lesions of the hippocampal complex plus subjacent cortex; Fx, fornix. B. Average number of errors to criterion (+S.E.M.) for novel mapping problems, which used different numbers of cues and response targets, denoted as the cue:target ratio. A reproduced from Brasted et al. (2005); B reproduced from Brasted et al. (2003).
Not only does the overall pattern of impaired and preserved memory functions seem to match those of H. M. and similar amnesics, but the deficit appears to be “global” in the sense that it does not depend on anything having to do with spatial factors such as stimulus location or the response being spatially differentiated (Brasted, Bussey, Murray, & Wise, 2003). Brasted et al. (2003) used a temporally differentiated response set in which monkeys had to either repeatedly tap a touch screen or maintain contact with it for about 4 seconds or 8 seconds. As shown in Figure 3A, we obtained nearly identical results for spatially and temporally (nonspatially) differentiated responses. We found little difference between the effects of fornix transections (Figure 3A, unfilled triangles) and aspiration lesions of the hippocampus plus subjacent cortical areas (Fig. 3A, unfilled circles). The results also did not depend on whether we compared preoperative vs. postoperative performance, as opposed to postoperative performance in lesioned vs. control monkeys (Fig. 3A). Figure 3B shows an effect of task difficulty. In the easiest version of the mapping task, such as when monkeys need only to map two cues onto two responses, fornix transection causes a small and only marginally significant deficit. When the task becomes more difficult, such as when monkeys need to map six or more stimuli onto three responses, large and highly significant deficits emerge.
Although the arbitrary mapping task has its advantages, the impairment in new learning in monkeys with hippocampal-complex lesions does not reach the severity of amnesia in H. M. or similar patients. Lesioned monkeys eventually learn new mappings, and they improved with experience in doing so. This fact limits the applicability of the task to a monkey model of human amnesia, probably because there are other, slower ways to learn new mappings, which depend on structures outside the hippocampal complex. We also know that extensive (but incomplete) damage to the hippocampus proper does not cause any deficit on this task (Brasted et al., 2005), much like the patients described above with damage to parts of the hippocampal complex (Section 4.1). Recall that damage restricted to CA1 produced mild anterograde amnesia, whereas more extensive damage to the hippocampal complex produced severe symptoms (Rempel-Clower et al., 1996). The fast mapping of visual stimuli onto other visual stimuli (also known as visual-visual paired associate learning) could provide yet further improvements in the behavioral tests used for a monkey model of human amnesia. Note that we avoid the circular reasoning that often characterizes discussions of the present kind. We do not classify the arbitrary mapping task as a test of conscious memory, by whatever label. Instead, we make two points: (1) the arbitrary mapping task could serve as a potentially useful component of a battery of tests probing hippocampal function, and (2) it enables us to study many attributes of interest (rapid, sometimes one-trial learning, preserved remote memories, etc.). In Sections 6.1 and 6.2, we take up these two ideas again and call them the ablation-correlation approach and the attribute approach, respectively.
Even if it is agreed that an improved monkey model of human amnesia should focus more on the hippocampal complex than on the MTL as a whole, and tasks that produce a pattern of impairments more like those of H. M. and other amnesics than short-interval matching tasks can manage, a major problem remains: monkeys still cannot tell us what they remember. The traditional approach to this problem is to dismiss it as intractable (or inconsequential) and to use proxy tasks and proxy terms for conscious memory, instead. The next section addresses whether the issue of consciousness can be avoided in an attempt to develop improved animal models of human amnesia.
5. Can the issue of consciousness be avoided?
5.1. Declarative memory
There have been several attempts to deal with the problem of animal consciousness. Squire and his colleagues have long held that any memory test in animals disrupted by damage to one or more of the structures composing the MTL can be considered a declarative (i.e., conscious) memory (Zola-Morgan, Squire, & Ramus, 1994). This approach ensures that any kind of memory dependent on the hippocampal complex, entorhinal, perirhinal and/or parahippocampal cortex automatically receives the same conscious-memory status typically applied to memory loss in human amnesics, except for the use of the proxy term declarative memory instead of conscious memory. As noted by others, however, this approach amounts to little more than circular reasoning (Morris, 1984; Nadel, 1992).
Another flaw in the orthodox model concerns a different aspect of logic. Just because a deficit in conscious memory is the most conspicuous result of bilateral MTL removal does not imply that other functions are unaffected. Indeed, there is evidence for a disruption of implicit (as opposed to explicit) spatial memory (Chun & Phelps, 1999) and implicit spatial perception (Lee et al., 2005a, b) after hippocampal damage in humans, as well as evidence for disrupted object and face perception (as opposed to memory) after brain damage that includes perirhinal cortex (Barense et al., 2005; Lee et al., 2005b). Accordingly, the conclusion that a kind of memory is conscious memory because MTL lesions cause deficits in it fails on two counts: circular logic and affirming the consequent. [Symbolically: p implies q; q is true (thus affirming the consequent); therefore (fallaciously) p is true. In practice it goes like this: If the MTL functions specifically in declarative memory, then we should see deficits in declarative-memory tasks. We see deficits in declarative-memory tasks (thus affirming the consequent), therefore (fallaciously) the MTL functions specifically in declarative memory.] Formal logic aside, note that issues regarding the perirhinal cortex and its functions go away once we exclude the perirhinal cortex and short-interval matching tasks from the monkey model of human amnesia.
In recognition of these problems, there have been other attempts to invoke the concept of conscious memory, as distinct from other types of memory, without using the word consciousness. Inherent in all of these schemes is the implication that some kind of memory in animals corresponds to conscious memory in humans, regardless of the proxy term used for it. Like the declarative – procedural dichotomy or the declarative – nondeclarative dichotomy, the use of terms that indirectly connote the attribute of conscious knowledge serves to reinforce the idea that MTL damage leads to the disruption of conscious memory in animals without having to say so explicitly. Next, we take up three of these attempts.
5.2. Goal-directed behavior
Another proxy term for conscious memory is goal-directed behavior, as defined by Balleine & Dickinson (1998). According to these authorities, goal-directed behavior can be identified by two main characteristics: (1) sensitivity to the value of the goal and (2) knowledge of the relationship between actions and the goal or outcome of those actions. In practice, these two characteristics are measured using reinforcer devaluation (to assess the behavioral effects of manipulating the value of the goal) and contingency degradation (to assess the behavioral effects of disrupting the relationship between action and goal/outcome). According to Balleine (2005), the memory guiding goal-directed behavior is the same as declarative memory, which is, as we have seen, the unstated equivalent of conscious memory. Equating conscious memory with “goal-directed behavior” depends on the dubious assumption that there is only one kind of goal-directed behavior. If there are two or more kinds, with one conscious and the others subconscious, then reinforcer devaluation or contingency degradation procedures do not help us very much. Indeed, a considerable amount of goal-directed behavior in humans is mediated subconsciously. During sleepwalking, for example, people behave in a goal-directed manner although they are not in a conscious state. Many goal-directed reaching movements proceed entirely without conscious control, a well-characterized phenomenon called autopilot control (Milner, Karnath, & Desmurget, 2003; Prablanc, Desmurget, & Grea, 2003; Pisella et al., 2000), and this phenomenon extends to locomotion and other movements, as well. Koch & Crick (2001, p. 893) refer to the control of human behavior by “the zombie within”. As they point out:
brain systems perform complex yet routine tasks without direct conscious input…. Such systems can deal with certain commonly encountered situations automatically, which is why we call them ‘zombie’ agents. One can become conscious of the actions of one’s own zombie, but usually only in retrospect.
The best evidence comes from studying dissociation of ‘vision for perception’ and ‘vision for action’ in both healthy humans and patients.
Wegner (2002) has likewise pointed out that much of human behavior runs automatically, without conscious control. In retrospect, our consciousness generates reasons for our actions, along with the illusion that they were consciously controlled.
5.3. Voluntary behavior
Passingham (1993) attempted to deal with the issue of animal consciousness by focusing on decision-making. In his view, when an animal has a choice between two or more alternative actions, the decision to choose one of them can be characterized as voluntary; otherwise it is simply “motivated” behavior. Motivated behavior depends on associating stimuli with rewards, the availability of food, and so forth. This approach has several advantages, not the least of which is the ease of application to both human and monkey research. But this operational definition of voluntary action cannot substitute for the deeper and more difficult concept of consciousness. The problem, as explained above, is that many human behaviors that qualify as voluntary in Passingham’s sense of the word, are mediated subconsciously.
5.4. Episodic-like memory
Other stand-ins for conscious, human memory include the flexible association of items learned as paired associates (Eichenbaum & Bunsey, 1995), among others, but we would like to focus on studies that attempt to model episodic memory, because we touched upon this line of research in Section 4.1 and it comes up again in Section 7. Gaffan (1994) has argued for the learning of complex scenes (object-in-place scenes) as an assay of episodic memory in monkeys. In his view, the fornix operates as part of a hippocampus-fornix-mammillary body-anterior thalamic circuit that contributes to the representation of complex scenes, including the spatial arrangement of objects in scenes, and this type of memory prevents confusion between memories of similar events. Taken on its own terms, this approach has promise. However, if one seeks to equate such episodic memories, if they are that, with conscious memories, the problem that arises is the same as with goal-directed or voluntary behavior. Furthermore, these tasks and ideas deal only with the aspect of episodic memory involving scenes, not, as explained in Section 4.1, with embedding oneself in an event.
We emphasize that we do not question the importance of episodic memory in understanding human amnesia. No one doubts that H. M.’s surgery cost him the ability to encode and retrieve event memories, in terms of what happened, where it happened, and when. The problem involves studying episodic memory in animal models. A series of experiments examined food-caching behavior in scrub jays to test whether they expressed knowledge of what foods they had cached, where they had cached it, and when (Clayton & Dickinson, 1998, 1999). On the basis of these experiments, it appears that scrub jays know what, where, and when they cached a particular type of food, which has been taken as evidence that scrub jays have episodic memory. It has been especially surprising that despite several efforts to address this issue in rats and monkeys, little evidence for what-where-when memory has turned up. In some cases, the when component of a putative what-where-when memory can be explained away as circadian (time-of-day) or recency (passage-of-time) effects, rather than a true ordering of an event in time (see Eacott & Easton, 2009, this issue; Hampton & Schwartz, 2004 for reviews). One attempt to deal with this problem has involved the use of a variant of spontaneous tests of object recognition in rodents. Eacott and her colleagues have argued that which rather than when is the important factor in episodic memory because it provides the context, beyond location per se, in which an event occurred. Eacott and her colleagues (Eacott & Norman, 2004; Easton, Zinkivskay, & Eacott, 2009) showed that rats can reliably remember that a particular object (what) was located in a particular arm of the maze (where) in a specific context (which), and that this ability depends on the fornix. The same fornix-lesioned rats performed tests of object recognition normally. Together, the findings have been taken as evidence that rats with fornix lesions have impaired recall (based on results from the what-where-which test), but intact familiarity judgments (based on results from object-recognition tests) (Eacott & Easton, 2009). Another attempt involved rapid acquisition of food location (Day, Langston & Morris, 2003). Cued recall of recently acquired food-place pairs, but not remotely learned food-place pairs, depended on activity in the hippocampus. Although Day et al. (2003) linked their findings to episodic memory in humans, we note that they refrained from claiming their rats possessed the same ability. Does the existence of what-where-when memory in scrub jays or what-where-which memory in rats mean that they have conscious memories? As has been noted convincingly by others, what-where-when memory is neither necessary nor sufficient evidence of the ability to consciously recollect an event (Suddendorf & Corballis, 2007a, b). Humans can have what-where-when knowledge without recollecting an event, and conversely, can recall an event without full knowledge about what, where and when something happened. The same objections apply to what-where-which memory. The findings on scrub jays and rodents have spawned unnecessary controversy, mainly revolving around whether the what-where-when memory observed in birds really “corresponds” to the human experience of conscious episodic memories. The proxy term “episodic-like memory” has been used in an attempt to avoid this problem (Clayton, Bussey, Emery, & Dickinson, 2003), but evading the problem does nothing to resolve it.
5.5. A way forward
To summarize the main points of this section, we can neither ignore the problem of animal consciousness nor resolve it. What, in that seemingly forbidding context, can monkeys tell us about conscious memories? The next section explores how an improved monkey model of human amnesia can provide useful insights without depending upon assumptions about monkey consciousness.
6. What monkeys can tell us about human amnesia
If an improved monkey model of human amnesia cannot cope with the concept of consciousness (Section 5) and dispenses with the concept of a reified MTL, results from short-interval matching tasks, and tasks that depend on perirhinal cortex (Sections 3 and 4), how can it help us understand human amnesia? At least three approaches have been used to surmount the fact that monkeys cannot tell us what they remember. We call them the ablation-correlation approach, the attribute-correlation approach, and the report-based approach. The ablation-correlation approach focuses on the brain structures that contribute to conscious memory in humans; the attribute-correlation approach deals with the characteristics of conscious memory; and the report-based approach relies on actions that reveal the contents of memory. Although all three approaches can adopt a weak form that depends on circular reasoning about animal consciousness, they all can be developed in ways that accord with the principles of comparative and evolutionary biology. Sections 6.1, 6.2 and 6.3 address, respectively, how these three approaches to studying memory in animals can provide insight into human amnesia and memory without depending on assumptions about animal consciousness. Sections 6.4 and 6.5 elaborate an important aspect of the ablation-correlation approach, the contribution of the prefrontal cortex and its interaction with the hippocampal complex.
6.1. Ablation-correlation approach
The ablation-correlation approach aims at understanding the function of homologues, in monkeys, of structures that subserve conscious memory in humans. Even if, as some experts believe, consciousness emerged in humans (Section 7), we can nevertheless understand the neural underpinnings of conscious knowledge, and how it is stored, by studying the homologous structures in monkeys. We do not need to make any assumptions about monkey consciousness to pursue this line of research or to apply its results to human memory. Of the large body of work on hippocampal function, we focus on a couple of key, illustrative contributions.
MTL reification theory
One tenet of the orthodox monkey model of human amnesia is that the many structures composing the MTL make more-or-less the same contribution. An alternative view holds that different parts of the MTL perform different functions, such as the idea that the hippocampal complex mediates episodic memory. Likewise, as reviewed above (Section 4.1), several lines of evidence indicate that only one portion of the MTL in humans, the hippocampal complex, plays an important role in recollection (Aggleton & Brown, 2005; Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007). Evidence suggests that a different circuit, one that includes the perirhinal cortex and medial dorsal nucleus of the thalamus (Aggleton and Brown, 1999), but not the hippocampal complex (or its principal subcortical targets), mediates familiarity judgments.
The question of whether the various components of the MTL function cooperatively or in a specialized manner can be tested in monkeys without making assumptions about monkey consciousness, simply by examining the functional organization of homologous structures in the two species. The finding that the various structures composing the MTL in monkeys have several dissociable functions (Murray, Bussey, & Saksida, 2007; Murray & Wise, 2004), in addition to the familiarity-recollection dichotomy just mentioned, supports the division-of-labor side of the argument. These findings illustrate how a monkey model of human amnesia can help resolve controversies from the human literature. It would be a strange circumstance, indeed, if the perirhinal cortex, entorhinal cortex, parahippocampal cortex, hippocampus proper performed different functions in monkeys, but lost all of these specializations in humans. On grounds of parsimony, we can reject such a notion.
The multiple memory-trace theory
To cite another example, monkey research can test another tenet of the orthodox monkey model of human amnesia: that the MTL is essential for only a limited period of time, during which newly acquired memories are consolidated elsewhere in the brain, an idea that has been disputed in the multiple memory-trace theory (Moscovitch & Nadel, 1998; Nadel & Moscovitch, 1997). Yanike, Wirth, & Suzuki (2004), for example, studied the neurophysiology of the hippocampus in an arbitrary mapping task like that described in Section 4.3. They found that the hippocampus represents established, highly familiar stimulus-response mappings, along with novel ones. As elaborated in Section 4.3, previous lesion studies had shown that lesions of the hippocampal complex (including the fornix) cause a large deficit in learning such mappings, with preserved ability to perform according to preoperatively learned ones (Brasted et al., 2003; Wise & Murray, 1999). The most parsimonious account of the findings from lesion studies is that brain regions outside the hippocampal complex store the familiar mappings, and there is evidence that these outside locations include parts of the prefrontal cortex and premotor cortex (Bussey, Wise, & Murray, 2001). Lesion studies, however, cannot address whether familiar mappings are represented in the hippocampal complex, along with novel ones. The neurophysiological findings of Yanike et al. (2004) resolve this question: the hippocampal complex represents both novel and familiar mappings. These findings from monkeys, therefore, support the multiple-trace theory and further illustrate how we can test ideas about human amnesia and memory without making assumptions about monkey consciousness. (Note that the phrase familiar mappings, in the sense used for this task, bears no relation to the concept of familiarity, as discussed above.)
In summary, learning as much as we can about the function of the hippocampal complex will provide insight into human amnesia without making any assumptions about the existence of conscious memories in monkeys. Recall that in addition to losing the ability to encode new conscious memories, human patients with damage to the hippocampus have deficits on spatial learning. So by studying the other things that the hippocampus does, and how it does what it does, indirect knowledge can be gained about hippocampal function in conscious memory. As Gaffan (1998, 2002) has argued forcefully, what the hippocampus does depends on the inputs it receives. If it gets inputs in humans that reflect our conscious mental operations, then it will do with those inputs what it does with its other ones, whatever that might be. We develop this idea further in Section 7.2.
6.2. Attribute-correlation approach
A second approach in developing and improving animal models of human amnesia focuses on the characteristics of conscious memory. This approach builds on the ablation-correlation approach and, like it, requires no assumption that the memories in monkeys (or other animals) are conscious ones, only that that have a particular property in common.
One- vs. two-process models of recognition memory
As discussed above in Section 4.1, clinical evidence points to two components of human recognition memory — familiarity and recollection. Evidence for the distinction between familiarity and recollection comes from several sources, including neuropsychological studies, investigations with event-related potentials, and functional neuroimaging studies. One relevant method involves a particular attribute of conscious memory in humans, the shape of ROC curves. ROC stands for receiver operating characteristic and it measures the trade-off between selectivity and sensitivity during attempts to decode a given signal. In this case, the signal involves successful performance of a memory task. With maximal sensitivity, a target signal will always be detected (a “hit”), but other signals will be mistaken for the target signal (a “false alarm”). With minimal sensitivity, false alarms can be prevented, but so too will detection of the target signal. In the context of signal detection, ROC plots involve hits as a function of false alarms (Fig. 4). In tests of recognition memory in amnesic patients, the ROC curve has a mirror symmetry with respect to the major diagonal (the gray dashed line in Fig. 4A). Yonelinas, Kroll, Quamme, Lazzara, Sauvé, Widaman, & Knight (2002) have interpreted this result as reflecting a spared capacity for familiarity judgments. The ROC curve in control subjects shows a marked asymmetry (Fig. 4A), which they have interpreted as reflecting a combination of the recollection and familiarity components of recognition (for review see Eichenbaum et al., 2007). A study by Fortin, Wright & Eichenbaum (2004) showed that intact rats given a test of odor memory, with response criteria biased by using different levels of reward magnitude, displayed similarly shaped, asymmetrical ROC curves. Rats with hippocampal lesions displayed symmetrical ROC curves and whereas their ROC measures of familiarity matched that of control animals, their recollection measure was reduced, suggesting that familiarity mediates odor recognition in the rats with hippocampal lesions (Fig. 4B). Taken together with the findings from humans, these data support the idea that the hippocampal complex subserves the recollection component of item recognition (see also, Section 4.1). We need not assume that such behaviors depend on rat consciousness, as implied by calling these memories “declarative”.
Figure 4.
ROC curves that distinguish familiarity and recollection in a recognition task. From amnesic patients with presumed hippocampal damage and from rats with selective hippocampal lesions. A. Left, ROC curve for amnesic patients (black) and for control subjects (gray). The dashed gray line provides a reference for the symmetry of the ROC curves. Right, estimates for recollection and familiarity components of the ROC curve. Error bars represent ± S.E.M. B. In the format of A, but for rats with selective hippocampal (H) lesions and for controls. A modified from Yonelinas et al. (2002); B modified from Fortin et al. (2004).
We could cite examples of other attributes of conscious memory amenable to similar testing, such as one-trial acquisition, rapid extinction, temporal order information of certain kinds, memory for context, relational knowledge, and so forth, but the example of ROC curves proves the point: we do not require assumptions about animal consciousness to test the attributes of human conscious memory in animals. From this perspective, we can view the attribute-correlation approach two ways. Perhaps certain memories have a given attribute because they are conscious. Alternatively, conscious memories could have the attributes they have, such as a particular shape of the ROC curve, because the hippocampal complex mediates such memories and confers that attribute (along with others) on all of its memories, conscious and subconscious.
6.3. Report-based approach
Obviously, animals cannot provide a verbal report about the contents of their memory. Nevertheless, a relatively recent approach to studying memory in animals requires them to “report” on percepts and memories. Stoerig & Cowey (1997) pioneered this approach in a blindsight task, and Hampton later developed two methods for training monkeys to report a lack of memory. In the direct one (Hampton, 2001), he operantly conditioned monkeys to report the lack of a memory. Monkeys first touched a picture that served as the sample in the short-interval matching task. Next, monkeys could choose one of two pictures, which remained the same across trials: one (picture A) led to delivery of a nonpreferred reward and the other (picture B) led to the short-interval matching test. Successful performance of the test yielded a preferred reward. By touching picture A the monkey could opt out of the memory test and thus “report” either that it had forgotten the sample or that a sample stimulus had not appeared on that trial. For long delay intervals, when the likelihood of forgetting increased, the monkey more often touched picture A, which Hampton interpreted as a report about the loss of the item in short-term memory. In a second, less direct reporting procedure, Hampton, Zivin, & Murray (2004) required monkeys to select among four opaque tubes, one of which contained a food reward. Sometimes the monkeys could see the experimenter bait the tube in advance of its selection, sometimes not. During testing, the monkeys could look down the tube to see if it had food, if they chose to do so. As expected, monkeys looked down the tubes more often when they had not seen the experimenter bait the tube, which suggests that the monkeys knew what they did not know.
Versions of report-based tasks have been used in pigeons, rats, and monkeys in an attempt to use actions to probe the contents of memory (Hampton & Schwartz, 2004; Roberts, Feeney, McMillan, MacPherson, Musolino, & Petter, 2009; Sutton & Shettleworth, 2008). These tests provide a tool to examine behavior that has an important attribute of human conscious memory, the ability to make reports about the content of one’s own memory, without requiring assumptions about animal consciousness. Of course, we cannot rule out the possibility that these “reports” are simply conditioned responses, with the animal’s behavior following a complex pattern of reward probabilities and outcome valuations, especially in relation to cost factors such as prolonged attentiveness. Nevertheless, like the attribute-correlation approach, report-based procedures seek to understand the neural basis of behaviors that resemble aspects of conscious memory in humans. If report-based approaches can be extended to probe long-term memory and examine its neural substrates, then they could yield substantial insight into human amnesia without depending on any assumptions about animal consciousness.
6.4. The granular prefrontal cortex
The ablation-correlation approach relies on the study of homologues, in monkeys, of the structures underlying conscious memory in humans. This approach can extend beyond the hippocampal complex to other brain regions implicated in the encoding or retrieval of conscious memories, such as episodic memories. Neuroimaging reports too numerous to mention (e.g., Spaniol et al., 2009) show that the granular prefrontal cortex plays an important role in episodic memory. But another reason also supports the inclusion of prefrontal cortex in a monkey model of human amnesia: Monkeys, like humans and other primates, have a granular prefrontal cortex, but rodents and other mammals lack these areas. All mammals have agranular frontal areas, which often go by the name prefrontal, but the granular prefrontal cortex is a primate innovation (Preuss, 1995; Wise, 2008). Accordingly, monkey models of amnesia have the considerable advantage that they can include the granular prefrontal cortex. This contention seems strange in the context of current memory research in monkeys, which has focused so intently on the MTL. But all mammals have homologues of the structures composing the MTL: the amygdala, hippocampus, subicular complex, entorhinal cortex, perirhinal cortex, and (with somewhat less confidence) parahippocampal cortex. Only primates have homologues of the granular prefrontal cortex.
Using the methods precluded for humans and apes, the study of monkeys offers additional tools for understanding the functions of the granular prefrontal cortex, as well as studying how prefrontal cortex interacts with the hippocampal complex. Of course, that understanding remains for the future, and we cannot review the vast literature on prefrontal cortex here. But we favor the idea that the granular prefrontal cortex stores knowledge about behavior, including ordered sequences of actions and likely outcomes, along with their contexts (Duncan, 2001; Shallice, 2001; Wise, 2008; Wood, Romero, Makale, & Grafman, 2003).
6.5. Prefrontal–hippocampal interactions
A monkey model of human amnesia also permits an analysis of prefrontal–hippocampal interactions. These two sets of structures have both direct (Barbas & Blatt, 1995; Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000; Goldman-Rakic, Selemon, & Schwartz, 1984; Insausti & Muñoz, 2001) and indirect connections via entorhinal, perirhinal, and parahippocampal cortex (Insausti, Amaral, & Cowan, 1987; Muñoz & Insausti, 2005; Suzuki & Amaral, 1994). Yet little, if anything, is known about the functions of these interactions. The current model emphasizes the role of the entorhinal cortex as the sensory gateway from neocortex (e.g., perirhinal and parahippocampal cortex) to the hippocampus and back. If the focus of an improved model shifts to the hippocampal complex, then entorhinal cortex takes on increased importance as an indirect route connecting the hippocampus to the granular prefrontal cortex, along with the direct projections between the hippocampal complex and granular prefrontal cortex. On this view, the entorhinal cortex subserves a “relay function” in a broad framework that places perirhinal, parahippocampal, granular prefrontal, and other neocortical areas on an even footing.
One anatomical clue about prefrontal–hippocampal interactions concerns the distinction between the temporal parts of the hippocampal complex (called anterior in primates and ventral in rodents) and the septal parts (called posterior in primates and dorsal in rodents). The anterior region has the bulk of direct interconnections with the prefrontal cortex (for example, see Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000), and there is ample evidence for differential roles of the anterior and the posterior parts of the hippocampal complex. The posterior region subserves accurate spatial navigation and the anterior region seems to be important in other functions (Bannerman, Grubb, Deacon, Yee, Feldon, & Rawlins, 2003; Kjelstrup, Tuvnes, Steffenach, Murison, Moser, & Moser, 2002). Among their differences, the two regions differ in the size of their place fields, the parts of a rodent’s environment for which a neuron in the hippocampal complex increases its discharge rate. The posterior hippocampus has smaller place fields (<1 m) and the anterior hippocampus has larger ones (~10 m) (Kjelstrup, Solstad, Brun, Hafting, Leutgeb, Witter, Moser, & Moser, 2008). The large place fields in the anterior hippocampus may aid in generalizing spatial contexts, and therefore mediating navigation in a more general sense than usually understood, whereas the posterior hippocampus may mediate navigation in the more usual sense.
So far, we have discussed how an improved monkey model of human amnesia might focus on the hippocampal complex and its interaction with the prefrontal cortex. In the next section, we put forward the idea that conscious memory in humans depends on these two structures and their interactions.
7. What history can tell us about human amnesia
7.1. What have we got that they ain’t got?
What makes the Hottentot so hot?
What puts the “ape” in apricot?
What have they got that I ain’t got?
—The Cowardly Lion, from The Wizard of Oz (1939)
The Cowardly Lion posed some probing questions. The Hottentots have something hot alright: human consciousness. But instead of pointing to that, The Lion agreed to a different answer: “Courage!” That was wrong; The Cowardly Lion had plenty of bravery. In terms of prowess, he was more of a lion than a “mowess [sic]”. Yet he was not a lion at all: just another construction of human fiction. The creation of The Cowardly Lion epitomizes the human ability to imagine animals acting like people, which requires analogical and metaphorical thinking across two cognitive domains: social knowledge and biological (natural history) knowledge (Mithen, 1996). We return to this theme shortly.
We discussed above how an improved monkey model of human amnesia might focus on the hippocampal complex and its interaction with the prefrontal cortex. Could conscious memory depend on these interactions? We think that it might. In order to place this idea in a concrete conceptual framework, we relate a story about the evolution of human consciousness, drawn primarily from two sources (Klein & Edgar, 2002; Mithen, 1996). For readers uncomfortable with untestable evolutionary stories, or simply uninterested in them, we suggest skipping to Section 7.2. The ideas presented there and elsewhere in this article do not depend on our evolutionary story. As one of our sources says: “the crux here is logic and parsimony, not evidence” (Klein & Edgar, 2002, p. 273). We present these speculations in order to make our idea about prefrontal–hippocampal interactions more concrete, with no claim of originality or adequate scholarship. But it seems that every neuroscientist over the age of 55 eventually gets the urge to write an article on consciousness, and ours follows. In it, we refrain from parsing the distinctive meanings of declarative memory, explicit knowledge, sentience, consciousness (in general), access consciousness, phenomenal consciousness, awareness, and so forth. Searle’s famous Chinese Room was, until this point, unmentioned, and we will not mention it again. If these issues could be decided by dueling definitions or thought experiments, that would have happened a long time ago. But when we address the question, What can monkeys tell us about human amnesia?, it would be dishonest to assume that they view the world with a consciousness that very much resembles ours. The fact is that we do not know, and we doubt very much that anyone else knows either, whether monkeys or other animals have a human-like consciousness. In the remainder of this article, however, we assume that nonhuman animals lack any cognitive capacity that closely resembles the conscious awareness of modern humans. The assumption implies that our kind of consciousness arose some time during human evolution, after the divergence of our ancestors from the lineages that gave rise to other apes.
As we mentioned in Section 1, monkeys diverged from the human lineage about 30 million years ago. In those 30 million years, humans developed language, extensive tool use, and a culture capable of transmitting innovations to succeeding generations. Monkeys display both cultures (or ‘traditions’) and tool use (Visalberghi, Addessi, Truppa, Spagnoletti, Ottoni, Izar, & Fragaszy, 2009), but with meager capacities compared to ours, and the same goes for language (Hauser, Chomsky, & Fitch, 2002). We do not deny that glimpses of these abilities exists in other animals, and we do not need to deny such glimpses in order to recognize the huge gulf between human and animal cognition. As the poet quoted in the introduction made clear: we humans seem of two minds when it comes to other animals; we either deny any similarity or deny any difference. In collections of papers like the current special issue or academic anthologies, one can read an article by one author agonizing about whether chimpanzees, for example, have anything remotely resembling human consciousness, alongside an article from a different author assuming without reservation that rats have a consciousness just like ours. We believe that the differences between human and animal cognition amount to a cognitive discontinuity between them and us (Penn, Holyoak, & Povinelli, 2008), without denying any of the cognitive similarities documented by primatologists, for example, intent on disproving every claim of unique human capacities.
So if something important happened to animal consciousness after the divergence of humans from our closest primate relatives, how did this come about? The line of thought we outline here comes from anthropologists and archeologists who study the artifacts of human prehistory (Klein & Edgar, 2002; Mithen, 1996). Somewhere around 6 million years ago, give or take a few million years, an ancestral species split into the lineage that evolved into the chimpanzees and bonobos of modern times and a lineage from which the first anatomically modern humans descended. Later, these anatomically modern humans became “behaviorally modern” as well. Although their interpretations remain somewhat controversial, these archaeologists base their conclusion on the observation that tool kits, art, and other artifacts left behind by these people changed dramatically about 50,000 years ago, although their brain (and postcranial) anatomy remained fairly constant. At 25 years per generation, these were our grandparents2000, or something like that. As Klein and Edgar (2002, p. 271) put it:
the relationship between anatomical and behavioral change shifted abruptly about 50,000 years ago. Before this time, anatomy and behavior appear to have evolved more or less in tandem, very slowly, but after this time anatomy remained relatively stable while behavioral (cultural) change accelerated rapidly. What could explain this better than a neural change that promoted the extraordinary modern human ability to innovate?
Often called the creative explosion, these innovations came long after the increases in brain size that occurred in our ancestors ~2.5 million years ago and again about 600,000 years ago. As summarized by Klein and Edgar (2002, p. 235), the innovations of these people included “solidly built houses, tailored clothing, more efficient fire places, and new hunting technology that not only allowed … [them] to displace their predecessors but also to colonize the harshest, more continental parts of Eurasia where no one had lived before.” The production of cave art exemplifies their many departures from the behavior of previous people. It appears that these people first evolved in east Africa and later took over the world, displacing people who had done much the same thing earlier in human history. According to this view of human prehistory, within the past 50,000 years or so our ancestors evolved from a benign if clever mammal into one capable of creating The Cowardly Lion.
Not only did the tool kit and other artifacts become much more diverse 50,000 years ago, but certain kinds of artifacts appeared that people had never (or only very rarely) made before. Among the items that caught the attention of the archaeologist Stephen Mithen (1996), behaviorally modern humans fashioned parts of animals into tools and other things. This observation, among others, led him to propose that a major change in human cognition occurred at the time these new people emerged. The cognitive characteristics of these humans allowed them to represent and model their physical and social worlds, and their place in it. Specifically, Mithen proposed that before about 50,000 years ago our ancestors had several specialized cognitive modules, one for social knowledge, another for knowledge of animals and other aspects of the ecosystem and environment, and yet another for technical knowledge. According to his view, before about 50,000 years ago language was not very important outside the social domain within which it evolved (see also Burling, 2005; MacNeilage, 2008). After a few fortuitous mutations, the barriers between these domains of knowledge broke down, resulting in a creative explosion of cross-domain analogies, the ability to apply language to technical and biological knowledge, improved and innovative tool manufacture and use, and more efficient (as well as safer) hunting.
Mithen has used a cathedral metaphor to explain his idea. Imagine a cathedral with a number of chapels walled off from each other. These represent the domains of knowledge before the creative explosion. Knowledge in one domain could not contribute to any other or to anything that might be construed as general knowledge. The creative explosion followed the penetration of those walls by conceptual passage ways allowing humans, for example, to conceive of using animal material as tools. Before the creative explosion, according to Mithen, tool making and knowledge about animals had been “walled off” from each other. Afterward, interaction between the two domains of knowledge allowed the linkage of these knowledge sets. In a sense, these metaphorical passage ways enabled our ancestors to “see” a tool inside an animal bone, much as their specialized technical knowledge had previously allowed them and their ancestors to “see” a tool within a rock. The interaction of knowledge domains underlies higher-order analogical reasoning, the ability to attribute events to unseen causes, and a well developed ability to attribute mental states to others, all now identified as likely areas of discontinuity between human and nonhuman animals (Penn et al., 2008).
As Penn et al. put it (2008, p. 123):
The crux of the matter, then, is to identify the specific changes to the hominid architecture that enabled … [behaviorally modern humans] to reason about higher order relations in a structurally systematic and inferentially productive fashion, and ultimately resulted in the evolution of our unique linguistic, mentalistic, logical, and causal reasoning abilities.
7.2. Prefrontal products and the hippocampal complex
We suggest that conscious memory evolved in our human ancestors after changes in their (our) granular prefrontal cortex made it possible for different domains of knowledge to interact with each other and with a representation of self. The fruits of these interactions, when exchanged with the hippocampal complex, could mediate human consciousness. We note that the appearance of the granular prefrontal cortex predates the creative explosion by tens of millions of years; it appeared before the divergence of monkeys from the human-ape lineage (Preuss, 1995, 2007a, b; Wise, 2008). Thus, the evolution of a granular prefrontal cortex did not lead directly to the creative explosion, but a change in its organization might have done so. We have previously noted, in Section 4.1, that the frontal pole cortex expanded in humans to become the largest part of the granular prefrontal cortex. Taken as a group, the granular prefrontal areas bring into physical proximity neural representations from virtually every sensory, emotional, and cognitive domain. Accordingly, the neural interconnections among these domains are more direct and presumably stronger than cross-domain interconnections among more posterior parts of neocortex. Such close proximity could promote mappings or associations among different domains of knowledge. In terms of Mithen’s cathedral metaphor, the proximity of distinct cognitive domains in the prefrontal cortex, along with some organizational or connectional change, could have promoted the perforation of walls that separate each chapel from the others.
Take, for example, our introspective views about the consequences of our own actions in pocket billiards. We believe that the cue ball hit the 8-ball because of our intention to send it there with our cue stick. So when we see circles bounce off each other on a video display, we attribute intention to them, as well. According to the idea espoused here, the attribution of intent to circles comes from a mapping between representations of intention in one part of prefrontal cortex and the representation of inanimate objects such as circles, also in prefrontal cortex. Except for prefrontal cortex, these representations are remote from each other and weakly interconnected. In prefrontal cortex, they are closer and more directly connected.
According to Mithen, the key cognitive domains specific to humans include knowledge about other people (social knowledge, including moral and language knowledge), knowledge about tools and objects (technical knowledge), and knowledge about plants and animals (natural-history knowledge). Technical knowledge means more than just the ability to use and modify sticks; for example, it entails the ability to imagine the tool within an otherwise much-less-useful rock. Cross-domain analogies and metaphors depend on associative mappings between representations of these different domains of knowledge. When we connect social knowledge to natural-history knowledge, it creates intuitions about human nature; when we connect technical knowledge to social knowledge, it creates animism, the belief that objects such as tools and rocks have feelings and intentions; when we connect natural-history knowledge to social knowledge, we generate anthropomorphism, antivivisectionism, many of Gary Larson’s cartoons, and The Cowardly Lion. Cross-domain interactions could enable analogical and metaphorical relations that not only provide the contents of consciousness, but when combined with self-reference also establish consciousness per se.
Gaffan (2002) has argued that the monkey and human prefrontal cortex function in a domain-general manner, without functional specializations of any kind. His subsequent neuropsychological studies and those of others (Baxter, Gaffan, Kyriazis, & Mitchell, 2009; Buckley et al., 2009; Dias, Robbins, & Roberts, 1996), along with decades of neuroanatomical and neurophysiological research, shows that his idea was overstated in the form expressed at that time. Viewed more generally, however, the granular prefrontal cortex might well function in the development of cross-domain knowledge of the sort required for domain-general information processing, and this idea accords well with existing theories of prefrontal cortex function (Duncan, 2001; Miller & Cohen, 2001; Shallice, 2001). Duncan, especially, has pointed to the granular prefrontal cortex as the substrate for fluid intelligence, the same term that Mithen uses for uniquely human consciousness. This idea also resembles the conception of consciousness as a global workspace (Baars, Ramsoy, & Laureys, 2003) subserved, at least in part, by the granular prefrontal cortex.
If the granular prefrontal cortex changed in a way that perforated the walls in Mithen’s cognitive cathedral, it would generate novel cognitive representations. This creative information could then interact with the hippocampal complex in the way that prefrontal interactions always do, whatever that way is. This idea incorporates elements of the global workspace, but still recognizes the specializations within the granular prefrontal cortex. According to this idea, human consciousness arises from an interaction between the hippocampal complex and knowledge representations in the prefrontal cortex, but becomes stored in both areas, and perhaps in other areas, as well. A model of this kind could explain why neither prefrontal lobotomy (Al-Hai, 2005) nor hippocampectomy obliterates consciousness.
These ideas could also account for deficits in the acquisition of semantic knowledge. If prefrontal–hippocampal interactions subserve conscious memory in humans, they must be essential not only for the acquisition of episodic memories, as already discussed, but also for the acquisition of facts available to conscious awareness. H. M. could not create either kind of memory. We propose that item information, such as that processed and stored by the inferior temporal and perirhinal cortex (Davies, Halliday, Xuereb, Kril, & Hodges, 2009; Levy, Bayley, & Squire, 2004; Schmolck, Kensinger, Corkin, & Squire, 2002), is conveyed to the granular prefrontal cortex, which, in turn, interacts with the hippocampal complex through both direct (prefrontal–hippocampal) pathways as well as indirect ones (via entorhinal cortex). This idea contrasts with the current orthodoxy, which views the perirhinal and parahippocampal cortex as playing the key role, if not the sole role, in funneling information into the hippocampus (e.g., Mishkin et al., 1997). Thus, in considering a monkey model of human amnesia, we favor an approach that focuses as much on prefrontal-hippocampal interactions as on inputs to hippocampus relayed by entorhinal cortex, in part because we do not ascribe special status to neocortical areas included in a “thing” called the MTL (Murray & Wise, 2004). Prefrontal–hippocampal interactions could complement inputs to the hippocampus relayed via perirhinal, parahippocampal and entorhinal cortex to underlie the acquisition of explicit semantic knowledge.
Finally, the ideas put forward here also lead directly to the proposed autonoetic functions of the prefrontal cortex (Wheeler et al., 1997), perhaps in association with the hippocampal complex. The phenomenological experience of remembering is thought to rely on a self-knowing (i.e., autonoetic) consciousness (Tulving, 2001, 2002), a concept related to prospection (Buckner & Carroll, 2007). Suddendorf & Corballis (2007a) have proposed that the ability to place oneself in the past or future, termed mental time travel (MTT), enables reconstruction of the particulars of past events. Recall what H. M. said about his daily life experience: “it is like waking from a dream … every day is alone in itself ….” (Section 2.1). Hurford (2007, p. 79) put this idea in the context of episodic memory when he hypothesized “that an instance of human uniqueness is the inability of non-humans explicitly to recall episodes before the last period of sleep, related to a qualitative difference between MTT, which perhaps only humans can do.” Hurford (2007) also noted the connection between episodic memory and a sense of self: episodic memory appears later in human development than does semantic memory, with neonatal amnesia giving way to lifelong conscious memories at about the same age as episodic memory first appears. Recall also the distinction drawn by Maguire and her colleagues between two aspects of episodic memory: scene memory and embedding oneself in events (Section 4.1). The latter involves the connection between episodic memory and a sense of self. As Hurford (2007, p. 68) put it: “My episodic memories are memories of what I experienced—I was there.” Self-referential analysis, combined within an ability to navigate through remembered and imaginary places and times, could be a key link between the novel knowledge generated by prefrontal cortex and the ancestral function of the hippocampal complex in navigation. Together, cross-domain mappings in the granular prefrontal cortex, self-reference, and prefrontal-hippocampal interactions could have lead to a creative explosion.
8. Conclusion and summary
Neuropsychological research on animals remains an essential tool for gaining a deep understanding of memory mechanisms and their failures. Notwithstanding its well known limitations, impairments in behavior caused by selective lesions or inactivations can tell us something with a durable logic and a straightforward interpretation that other methods lack. We know that many neuroscientists believe that functional neuroimaging, neurophysiological research, or others methods can provide the same insight. Every neuroscientist has an opinion, of course, but these attitudes reflect a deep misunderstanding of the relevant research methods and their interpretational limitations. As important as they are, measures of local blood-flow rates, neuronal spike density, and electrical potentials of dubious provenance cannot substitute for the interpretational logic of experimental neuropsychology, perhaps better understood by its traditional name: comparative and physiological psychology.
Despite the promise offered by a monkey model of human amnesia, its goals have yet to be attained. Nevertheless, the setbacks and false starts related in Section 3 point to problems in practice, not deficiencies in principle. As the poet in our opening quotation says, humans are born to err in “reas’ning”, and the self-corrective aspect of science works reasonably well over the long term. With improved experimental designs and interpretations, proper attention to control procedures, adherence to the principles of comparative and evolutionary biology, and a forthright approach to the issue of animal consciousness, research on monkeys can achieve its goal of developing an improved and more useful model of human amnesia.
In conclusion, we consider the baseball adage that you can’t replace somebody with nobody. That doesn’t mean a whole lot in the world of memory research, and, in baseball, it doesn’t mean a whole lot more. But it does remind us to state an alternative to the orthodox model of human amnesia in equally attractive, parsimonious, and easy-to-remember terms. Section 4.1 cites precedents for some parts of our idea, which, despite many uncertainties, goes like this: The hippocampal complex, through interactions with the cross-domain knowledge generated by the granular prefrontal cortex, creates conscious memories. It does so, in part, by extending its ancestral function in spatial navigation to other forms of navigation, which guide an individual’s representation of self through space and time, ordered sequences of events, and the memories of objects, people, plants, and animals, not to mention cowardly lions.
Footnotes
For a special issue of Neuropsychologia on “Animal models of amnesia and the organization of memory”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network in left temporal lobe epilepsy. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, Halpin S, Wiles CM, Kamel H, Brennan P, Carton S, Gaffan D. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. 2000;123:800–815. doi: 10.1093/brain/123.4.800. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Nicol RM, Huston AE, Fairbairn AF. The performance of amnesic subjects on tests of experimental amnesia in animals: delayed matching-to-sample and concurrent learning. Neuropsychologia. 1988;26:265–272. doi: 10.1016/0028-3932(88)90079-6. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research. Philos Trans R Soc Lond B Biol Sci. 2001;356:1467–1482. doi: 10.1098/rstb.2001.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton JP, Shaw C. Amnesia and recognition memory: a re-analysis of psychometric data. Neuropsychologia. 1996;34:51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Al-Hai J. The Lobotomist: A Maverick Medical Genius and His Tragic Quest to Rid the World of Mental Illness. Hoboken: John Wiley & Sons; 2005. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Angeli SJ, Murray EA, Mishkin M. Hippocampectomized monkeys can remember one place but not two. Neuropsychologia. 1993;31:1021–1030. doi: 10.1016/0028-3932(93)90030-4. [DOI] [PubMed] [Google Scholar]
- Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Parkinson JK, Mishkin M. Visual recognition in monkeys: effects of separate vs. combined transection of fornix and amygdalofugal pathways. Exp Brain Res. 1985;57:554–561. doi: 10.1007/BF00237842. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Warrington EK. Amnesia and the distinction between long- and short-term memory. J Verb Learn Verb Behav. 1970;9:176–189. [Google Scholar]
- Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Banta Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: not all space is created equal. Hippocampus. 2009;19:8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee ACH, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Gaffan D, Kyriazis DA, Mitchell AS. Ventrolateral prefrontal cortex is required for performance of a strategy implementation task but not reinforcer devaluation effects in rhesus monkeys. Eur J Neurosci. 2009;29:2049–2059. doi: 10.1111/j.1460-9568.2009.06740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Kohler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci USA. 2007;104:16382–16387. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Role of the hippocampal system in associative learning beyond the spatial domain. Brain. 2003;126:1202–1223. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Conditional motor learning in the nonspatial domain: effects of errorless learning and the contribution of the fornix to one-trial learning. Behav Neurosci. 2005;119:662–676. doi: 10.1037/0735-7044.119.3.662. [DOI] [PubMed] [Google Scholar]
- Brooks DN, Baddeley A. What can amnesic patients learn? Neuropsychologia. 1976;14:111–122. doi: 10.1016/0028-3932(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Browning PG, Gaffan D. Prefrontal cortex function in the representation of temporally complex events. J Neurosci. 2008a;28:3934–3940. doi: 10.1523/JNEUROSCI.0633-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning PGF, Gaffan D. Global retrograde amnesia but selective anterograde amnesia after frontal-temporal disconnection in monkeys. Neuropsychologia. 2008b;46:2494–2502. doi: 10.1016/j.neuropsychologia.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Mansouri FA, Hoda H, Mahboubi M, Browning PG, Kwok SC, Phillips A, Tanaka K. Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325:52–58. doi: 10.1126/science.1172377. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Wilson CR, Gaffan D. Fornix transection impairs visuospatial memory acquisition more than retrieval. Behav Neurosci. 2008;122:44–53. doi: 10.1037/0735-7044.122.1.44. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Stefanacci L, Squire L, Zola SM. A reexamination of the concurrent discrimination learning task: the importance of anterior inferotemporal cortex, area TE. Behav Neurosci. 1998;112:3–14. doi: 10.1037//0735-7044.112.1.3. [DOI] [PubMed] [Google Scholar]
- Burling R. The Talking Ape. Oxford: Oxford University Press; 2005. [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2001;115:971–982. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci. 2004;24:2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Emery NJ, Dickinson A. Prometheus to Proust: The case for behavioural criteria for ‘mental time travel’. Trends Cogn Sci. 2003;7:436–437. doi: 10.1016/j.tics.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Memory for the content of caches by scrub jays (Aphelocoma coerulescens) J Exp Psychol Anim Behav Process. 1999;25:82–91. [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–200. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41:334–340. doi: 10.1016/s0028-3932(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. J Neurosci. 1984;2:1214–1229. [Google Scholar]
- Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H M’s medial temporal lobe lesion: findings from magnetic resonance imaging. J Neurosci. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll RE, Scoville WB. Effects of medial temporal lesions on visual discrimination performance. J Comp Physiol Psychol. 1965a;60:175–181. doi: 10.1037/h0022290. [DOI] [PubMed] [Google Scholar]
- Correll RE, Scoville WB. Performance on delayed match following lesions of medial temporal lobe structures. J Comp Physiol Psychol. 1965b;60:360–367. doi: 10.1037/h0022549. [DOI] [PubMed] [Google Scholar]
- Correll RE, Scoville WB. Significance of delay in the performance of monkeys with medial temporal lobe resections. Exp Brain Res. 1967;4:85–96. doi: 10.1007/BF00235220. [DOI] [PubMed] [Google Scholar]
- Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR. The neural basis of semantic memory: evidence from semantic dementia. Neurobiol Aging. 2009;30:2043–2052. doi: 10.1016/j.neurobiolaging.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Day M, Langston R, Morris RGM. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424:205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Easton A. Episodic memory in animals: remembering which occasion. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.11.002. this issue. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition for small sets, and impaired identification for large sets, following rhinal cortex ablation in monkeys. Eur J Neurosci. 1994;6:1466–1478. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Zinkivskay A, Eacott MJ. Recollection is impaired, but familiarity remains intact in rats with lesions of the fornix. Hippocampus. 2009;19:837–843. doi: 10.1002/hipo.20567. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Bunsey M. On the binding of associations in memory: clues from studies on the role of the hippocampal region in paired-associate learning. Curr Dir Psychol Sci. 1995;4:19–23. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Social cognition in humans. Curr Biol. 2007;17:R724–R732. doi: 10.1016/j.cub.2007.05.068. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 1988;7:157–177. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Recognition impaired and association intact in the memory of monkeys after transection of the fornix. J Comp Physiol Psychol. 1974;86:1100–1109. doi: 10.1037/h0037649. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Idiothetic input into object-place configuration as the contribution to memory of the monkey and human hippocampus: a review. Exp Brain Res. 1998;123:201–209. doi: 10.1007/s002210050562. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Against memory systems. Philos Trans R Soc Lond B Biol Sci. 2002;357:1111–1121. doi: 10.1098/rstb.2002.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertrial intervals and fail at matching to sample despite double sample presentations. Behav Neurosci. 1992;106:30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Parker A, Easton A. Dense amnesia in the monkey after transection of fornix, amygdala and anterior temporal stem. Neuropsychologia. 2001;39:51–70. doi: 10.1016/s0028-3932(00)00097-x. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Brandt KR, Baddeley AD, Vargha-Khadem F, Mishkin M. Charting the acquisition of semantic knowledge in a case of developmental amnesia. Neuropsychologia. 2008;46:2865–2868. doi: 10.1016/j.neuropsychologia.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. Functional specialization within rostral prefrontal cortex (Area 10): a meta-analysis. J Cogn Neurosci. 2006;18:932–948. doi: 10.1162/jocn.2006.18.6.932. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Goulet S, Dore FY, Murray EA. Aspiration lesions of the amygdala disrupt the rhinal corticothalamic projection system in rhesus monkeys. Exp Brain Res. 1998;119:131–140. doi: 10.1007/s002210050326. [DOI] [PubMed] [Google Scholar]
- Hampton RR. Rhesus monkeys know when they remember. Proc Natl Acad Sci USA. 2001;98:5359–5362. doi: 10.1073/pnas.071600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14:808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Schwartz BL. Episodic memory in nonhumans: what, and where, is when? Curr Opin Neurobiol. 2004;14:192–197. doi: 10.1016/j.conb.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Zivin A, Murray EA. Rhesus monkeys (Macaca mulatta) discriminate between knowing and not knowing and collect information as needed before acting. Anim Cogn. 2004;7:239–246. doi: 10.1007/s10071-004-0215-1. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. The construction system of the brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1263–1271. doi: 10.1098/rstb.2008.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MD. Moral Minds. New York: HarperCollins; 2006. [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT. The faculty of language: what is it, who has it, and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Isaac CL, Gong Q, Roberts N. Differential involvement of the hippocampus and temporal lobe cortices in rapid and slow learning of new semantic information. Neuropsychologia. 2002;40:748–768. doi: 10.1016/s0028-3932(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Hurford JR. The Origins of Meaning. Oxford: Oxford University Press; 2007. [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Insausti R, Muñoz M. Cortical projections of the non-entorhinal hippocampal formation in the cynomolgus monkey (Macaca fascicularis) Eur J Neurosci. 2001;14:435–451. doi: 10.1046/j.0953-816x.2001.01662.x. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kapur N. Remembering Norman Schwartzkopf: evidence for two distinct long-term fact learning mechansisms. Cogn Neuropsychol. 1994;11:661–670. [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser MB. Finite scale of spatial representation in the hippocampus. Science. 2008;321:140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RG, Edgar B. The Dawn of Human Culture. New York: John Wiley & Sons; 2002. [Google Scholar]
- Koch C, Crick F. The zombie within. Nature. 2001;411:893. doi: 10.1038/35082161. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The human hippocampus: cognitive maps or relational memory? J Neurosci. 2005;25:7254–7259. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005a;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee AC, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS. Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic’ view. Neuropsychologia. 2005b;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proc Natl Acad Sci USA. 2004;101:6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF. The Origin of Speech. Oxford: Oxford University Press; 2008. [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahut H, Moss M. The monkey and the sea horse. In: Isaacson RL, Pribram KH, editors. The Hippocampus. New York: Plenum Press; 1986. pp. 241–279. [Google Scholar]
- Mahut H, Zola-Morgan S, Moss M. Hippocampal resections impair associative learning and recognition memory in the monkey. J Neurosci. 1982;2:1214–1229. doi: 10.1523/JNEUROSCI.02-09-01214.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamut BL, Saunders RC, Mishkin M. Monkeys with combined amygdalo-hippocampal lesions succeed in object discrimination learning despite 24-hour intertrial intervals. Behav Neurosci. 1984;98:759–769. doi: 10.1037//0735-7044.98.5.759. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38:127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton B, O’Reilly R. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. The memory defect in bilateral hippocampal lesions. Psychiatr Res Rep Am Psychiatr Assoc. 1959;11:43–58. [PubMed] [Google Scholar]
- Milner B. Physiologie de l Hippocampe. Paris: Centre National de la Recherche Scientifique; 1962. Les troubles de la memoire accompagnant des lesions hippocampiques bilaterales; pp. 257–272. [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Milner B. The medial temporal-lobe amnesic syndrome. Psychiatr Clin North Am. 2005;28:599–611. 609. doi: 10.1016/j.psc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of hippocampal amnesic syndrome: 14-year follow-up study of H.M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- Milner D, Karnath HO, Desmurget M. The cognitive and neural bases of visually guided action. Exp Brain Res. 2003;153:133. [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978;273:297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 1982;298:83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithen S. The Prehistory of the Mind. London: Thames and Hudson; 1996. [Google Scholar]
- Morris RGM. Is the distinction between procedural and declarative memory useful with respect to animal models of amnesia? In: Lynch G, McGaugh JL, Weinberger N, editors. Neurobiology of Learning and Memory. New York: Guilford; 1984. pp. 119–124. [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: in defense of the multiple-trace model. Curr Opin Neurobiol. 1998;8:297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) Eur J Neurosci. 2005;22:1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- Muñoz M, Mishkin M, Saunders RC. Resection of the medial temporal lobe disconnects the rostral superior temporal gyrus from some of its projection targets in the frontal lobe and thalamus. Cereb Cortex. 2009;19:2114–2130. doi: 10.1093/cercor/bhn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. What have ablation studies told us about the neural substrates of stimulus memory? Seminars Neurosci. 1996;8:13–22. [Google Scholar]
- Murray EA, Baxter MG, Gaffan D. Monkeys with rhinal cortex damage or neurotoxic hippocampal lesions are impaired on spatial scene learning and object reversals. Behav Neurosci. 1998;112:1291–1303. doi: 10.1037//0735-7044.112.6.1291. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Severe tactual as well as visual memory deficits follow combined removal of the amygdala and hippocampus in monkeys. J Neurosci. 1984;4:2565–2580. doi: 10.1523/JNEUROSCI.04-10-02565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Visual recognition in monkeys following rhinal cortical ablations combined with either amygdalectomy or hippocampectomy. J Neurosci. 1986;6:1991–2003. doi: 10.1523/JNEUROSCI.06-07-01991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Wise SP. Role of the hippocampus plus subjacent cortex but not amygdala in visuomotor conditional learning in rhesus monkeys. Behav Neurosci. 1996;110:1261–1270. doi: 10.1037//0735-7044.110.6.1261. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. What, if anything, is the medial temporal lobe, and how can the amygdala be part of it if there is no such thing? Neurobiol Learn Mem. 2004;82:178–198. doi: 10.1016/j.nlm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nadel L. Multiple memory system: what and why? J Cogn Neurosci. 1992;4:179–188. doi: 10.1162/jocn.1992.4.3.179. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Orbach J, Milner B, Rasmussen T. Learning and retention in monkeys after amygdala-hippocampus resection. Arch Neurol. 1960;3:230–251. doi: 10.1001/archneur.1960.00450030008002. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Mamillary body lesions in monkeys impair object-in-place memory: functional unity of the fornix-mamillary system. J Cogn Neurosci. 1997a;9:512–521. doi: 10.1162/jocn.1997.9.4.512. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. The effect of anterior thalamic and cingulate cortex lesions on object-in-place memory in monkeys. Neuropsychologia. 1997b;35:1093–1102. doi: 10.1016/s0028-3932(97)00042-0. [DOI] [PubMed] [Google Scholar]
- Parkinson JK, Murray EA, Mishkin M. A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J Neurosci. 1988;8:4159–4167. doi: 10.1523/JNEUROSCI.08-11-04159.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE. The Frontal Lobes and Voluntary Action. Oxford: Oxford University Press; 1993. [Google Scholar]
- Passingham RE. How good is the macaque monkey model of the human brain? Curr Opin Neurobiol. 2009;19:6–11. doi: 10.1016/j.conb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Mathieson G. Memory: autopsy findings and comments on the role of hippocampus in experiential recall. Arch Neurol. 1974;31:145–154. doi: 10.1001/archneur.1974.00490390027001. [DOI] [PubMed] [Google Scholar]
- Penfield W, Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch Neurol Psychiatry. 1958;79:475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behav Brain Sci. 2008;31:109–130. doi: 10.1017/S0140525X08003543. [DOI] [PubMed] [Google Scholar]
- Phillips RR, Malamut BL, Bachevalier J, Mishkin M. Dissociation of the effects of inferior temporal and limbic lesions on object discrimination learning with 24-h intertrial intervals. Behav Brain Res. 1988;27:99–107. doi: 10.1016/0166-4328(88)90035-6. [DOI] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci. 2000;3:729–736. doi: 10.1038/76694. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Desmurget M, Grea H. Neural control of on-line guidance of hand reaching movements. Prog Brain Res. 2003;142:155–70. 155–170. doi: 10.1016/S0079-6123(03)42012-8. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagasto M, editors. Primate Origins: Adaptations and Evolution. New York: Springer; 2007a. pp. 625–675. [Google Scholar]
- Preuss TM. Primate brain evolution in phylogenetic context. In: Kaas JH, Preuss TM, editors. Evolution of Nervous Systems. Oxford: Elsevier; 2007b. pp. 2–34. [Google Scholar]
- Rempel-Clower N, Zola S, Squire LR, Amaral D. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA, Feeney MC, McMillan N, MacPherson K, Musolino E, Petter M. Do pigeons (Columba livia) study for a test? J Exp Psychol Anim Behav Process. 2009;35:129–142. doi: 10.1037/a0013722. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Lopez JC, Vargas JP, Broglio C, Gomez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: Insights from reptiles and teleost fish. Brain Res Bull. 2002;57:499–503. doi: 10.1016/s0361-9230(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Murray EA, Mishkin M. Further evidence that amygdala and hippocampus contribute equally to recognition memory. Neuropsychologia. 1984;22:785–796. doi: 10.1016/0028-3932(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Schmolck H, Kensinger EA, Corkin S, Squire LR. Semantic knowledge in patient H. M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus. 2002;12:520–533. doi: 10.1002/hipo.10039. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW. Prefrontal cortex in humans and apes: a comparative study of area 10. Am J Phys Anthropol. 2001;114:224–241. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time dependent motor memory processes in amnesia. J Neurophysiol. 1998;80:1590–1597. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shallice T. ‘Theory of mind’ and the prefrontal cortex. Brain. 2001;124:247–248. doi: 10.1093/brain/124.2.247. [DOI] [PubMed] [Google Scholar]
- Sidman M, Stoddard LT, Mohr JP. Some additional observations of immediate memory in a patient with bilateral hippocampal lesions. Neuropsychologia. 1968;6:245–254. [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behav Neurosci. 1988;102:210–221. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia after damage to the medial temporal lobe: a neuroanatomical and neuropsychological profile of patient E.P. J Neurosci. 2000;20:7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoerig P, Cowey A. Blindsight in man and monkey. Brain. 1997;120:535–559. doi: 10.1093/brain/120.3.535. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. Mental time travel across the disciplines: the future looks bright. Behav Brain Sci. 2007a;30:335–345. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: what is mental time travel, and is it unique to humans? Behav Brain Sci. 2007b;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Sutton JE, Shettleworth SJ. Memory without awareness: pigeons do not show metamemory in delayed matching to sample. J Exp Psychol Anim Behav Process. 2008;34:266–282. doi: 10.1037/0097-7403.34.2.266. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the medial temporal lobe: evaluating the current evidence. Neuron. 2009;61:657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Baxter MG. Memory, perception, and the medial temporal lobe: a synthesis of opinions. Neuron. 2009;61:678–679. doi: 10.1016/j.neuron.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nat Neurosci. 2009 doi: 10.1038/nn.2453. published online 6 December 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and common sense: how far apart? Phil Trans Roy Soc Lond B Biol Sci. 2001;356:1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, Montaldi D, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci USA. 2009;106:5442–5447. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Mishkin M. Dissociations in cognitive memory: the syndrome of developmental amnesia. Philos Trans R Soc Lond B Biol Sci. 2001;356:1435–1440. doi: 10.1098/rstb.2001.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Addessi E, Truppa V, Spagnoletti N, Ottoni E, Izar P, Fragaszy D. Selection of effective stone tools by wild bearded capuchin monkeys. Curr Biol. 2009;19:213–217. doi: 10.1016/j.cub.2008.11.064. [DOI] [PubMed] [Google Scholar]
- Waxler M, Rosvold HE. Delayed alternation in monkeys after removal of the hippocampus. Neuropsychologia. 1970;8:137–146. doi: 10.1016/0028-3932(70)90001-1. [DOI] [PubMed] [Google Scholar]
- Wegner DM. The Illusion of Conscious Will. Cambridge: MIT Press; 2002. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Role of the hippocampal system in conditional motor learning: mapping antecedents to action. Hippocampus. 1999;9:101–117. doi: 10.1002/(SICI)1098-1063(1999)9:2<101::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wise SP, Murray EA. Arbitrary associations among antecedents and actions. Trends Neurosci. 2000;23:271–276. doi: 10.1016/s0166-2236(00)01570-8. [DOI] [PubMed] [Google Scholar]
- Wood JN, Romero SG, Makale M, Grafman J. Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci. 2003;15:236–248. doi: 10.1162/089892903321208178. [DOI] [PubMed] [Google Scholar]
- Yanike M, Wirth S, Suzuki WA. Representation of well-learned information in the monkey hippocampus. Neuron. 2004;42:477–487. doi: 10.1016/s0896-6273(04)00193-x. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20:451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Preserved learning in monkeys with medial temporal lesions: sparing of motor and cognitive skills. J Neurosci. 1984;4:1072–1085. doi: 10.1523/JNEUROSCI.04-04-01072.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99:22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Mishkin M. The neuroanatomy of amnesia: amygdala-hippocampus versus temporal stem. Science. 1982;218:1337–1339. doi: 10.1126/science.6890713. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4:483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]