Summary
Context
The substitution of liothyronine (l-T3) for levothyroxine (l-T4) is commonly employed during thyroid hormone (TH) withdrawal in preparation for diagnostic and therapeutic interventions on thyroid cancer patients. Presently, only limited data are available on the l-T3 for l-T4 therapeutic substitution.
Objective
To characterize the pharmcodynamic equivalence of l-T3 and l-T4.
Design
Randomized, double-blind, cross-over intervention study.
Setting
NIH Clinical Center.
Patients
10 thyroidectomized patients.
Interventions
Study participants were treated with l-T3 or l-T4 with a target TSH ≥0.5≤1.5 mU/l for at least 30 days before undergoing inpatient testing. Following testing, subjects crossed-over according to the same scheme.
Main outcome measures
Area under the serum concentration-time curve of TSH from 0 to 60 minutes (AUC 0-60) and peak TSH serum concentration (Cmax) following thyrotropin-releasing hormone (TRH) stimulation test, total l-T4 and l-T3 dose (mcg/kg), and l-T4/l-T3 ratio.
Results
No difference was observed for time 0 TSH values between l-T3 and l-T4 replacement phases (1.48± 0.77 vs. 1.21± 0.62 mU/l, p=0.293) at average daily doses of 40.3±11.3 mcg lT-3 and 115.2±38.5 mcg lT-4, l-T3: l-T4 ratio 0.36±0.06. TRH stimulation test resulted in similar l-T3 vs.l-T4 TSH responses with AUC 0-60 of 326.1 (95% CI 232.6-457.1) and 247.1 (95% CI 153.8-397.1) mU*min /l (p=0.285); and Cmax of 6.83 (95% CI 4.88-9.55) and 5.23 (95% CI 3.31-8.3) mU/l (p=0.383).
Conclusions
This is the first study addressing the equivalency between l-T3 and l-T4 therapy measured by baseline and TRH-stimulated TSH. The therapeutic substitution of l-T3 for l-T4 was achieved at approximately 1:3 ratio.
Keywords: Hypothyroidism, Levothyroxine, Liothyronine, Deiodinase
Introduction
The circulating pool of thyroid hormones (TH), triiodothyronine (T3) and thyroxine (T4), is the net result of the secretion of these hormones from the thyroid gland, of their clearance, and of the peripheral conversion of the pro-hormone T4 into the hormonally active T3 or into the hormonally inactive reverse T3 by the deiodinases 1. Experimental data 2, 3, 4 and mathematical modeling 1, 4 indicate that the peripheral conversion represents an important physiologic source of circulating T3. However, the actual contribution of each of the deiodinases and of the various organs as sources of circulating T3 has not been empirically demonstrated. Furthermore, very little is known on the relative role of the deiodinases in the maintenance of adequate levels of TH during replacement therapy. Nonetheless, the peripheral conversion of TH allows the use of levothyroxine (l-T4) alone for the TH replacement therapy 5, 6. This treatment modality is simple, safe, and well-tolerated. Conversely, the substitution of liothyronine (l-T3) for l-T4 is commonly employed during the TH withdrawal in preparation for diagnostic and therapeutic nuclear medicine interventions 7. This therapeutic modality substantially reduces the length of symptomatic hypothyroidism, making the TH withdrawal more tolerable. Furthermore, despite the fact that no definite data indicate the superiority of l-T3/l-T4 combination therapy, this remains a common strategy among practitioners for the treatment of hypothyroid patients who complain of hypothyroid symptoms despite adequate serum concentrations of TH. On the other hand, the l-T3 therapy poses a series of challenges stemming from the short half-life of T3 and its relatively low therapeutic index, factors that expose patients to the risk of developing toxicity 8 or under-treatment. Finally, since the use of a simpler once-a-day lT-3 administration regimen does not prevent the onset of severe hypothyroid symptoms 9, a multiple daily dosing scheme would be required to maintain a euthyroid state. Presently, only limited data are available for the assessment of the adequacy of therapeutic substitution with l-T3 for l-T4. In this study we describe the steady-state equivalence of l-T3 substitution for l-T4 in hypothyroid individuals.
Material and Methods
Participants and study design
The study was approved by the NIDDK-NIAMS Institutional Review Board and conducted at the National Institutes of Health Clinical Center in Bethesda Maryland. Written informed consent was obtained from all study participants at the enrollment and before the study procedures. Study volunteers were recruited from the local endocrine clinic and via advertisements in local newspapers and ClinicalTrials.gov (identifier number NCT00106119). Subjects meeting the following criteria were included in the study: age ≥18 years, body mass index (BMI) ≥20≤ 30 kg/m2 and history of primary hypothyroidism on replacement therapy with a daily L-T4 dose ≥ 1.6 mcg/kg. Exclusion criteria consisted of suppressive TH therapy, significant functional thyroid residual as measured by a 24-hour 123I thyroid uptake of greater than 5% (in individuals with thyroid remnant greater than 1 ml as measured by ultrasound) while on replacement therapy; hypertension or cardiovascular disease; pregnancy or use of hormonal contraception; diabetes mellitus; serum cholesterol ≥ 6.2 mmol/l; and/or serum triglycerides ≥ 2.5 mmol/l. The research protocol was designed as a randomized, double blind, cross-over intervention study. After enrollment, study participants were randomized to receive a preprandial three times daily regimen of l-T3 or l-T4 with bi-weekly dosage adjustments in order to achieve a target serum TSH level ≥0.5≤1.5 mU/l. Study volunteers were admitted to the NIH Clinical Center for testing after at least a 30-day period (three consecutive follow-up visits) of target TSH on a steady replacement dose. Following the testing the study volunteers were assigned to the alternative treatment arm with an identical follow-up and target TSH scheme.
Laboratory assessment
Serum TSH (normal, 04-4.0 mU/l), T3 (normal, 1.39-3.31 nmol/l) and free T4 (normal, 10-25 pmol/l) levels were assessed by chemiluminescence immunoassays in the NIH Clinical Center Department of Laboratory Medicine on a Siemens Immulite 2500 analyzer platform. TSH intra- and inter-assay coefficients of variation (CVs) were 4.2 and 3.5 % at mean levels of 0.20 and 15.31 mU/l, respectively, and 5.0 and 3.6 % at mean levels of 0.39 and 30.7 mU/l, respectively. T3 intra- and inter-assay CVs were 11.2 and 5.4 % at mean levels of 1.34 and 5.95 nmol/l, respectively, and 9.9 and 4.5 % at mean levels of 1.26 and 5.56 nmol/l, respectively. Free T4 intra- and inter-assay CVs were 16.43 and 2.58 % at mean levels of 6.0 and 50 pmol/l, respectively, and 18.67 and 5.01 % at mean levels of 5 and 50 pmol/l, respectively.
Serial blood sampling and thyrotropin-releasing hormone (TRH) stimulation test
Serial blood samples for measurement of TSH, T3, and free T4 levels were collected during the second day of hospitalization starting at 06:00 hours. Each TRH stimulation test was performed by intravenous injection of 200 mcg of TRH after a 10-hour fast at 08:00 AM. Samples were collected from an indwelling catheter at times -15, 0, 5, 10, 15, 20, 30, and 60 min.
Study medications and therapeutic adjustments
TH replacement therapy was provided by the NIH Clinical Center Pharmacy Department in identical coded capsules containing l-T4 (5, 10, or 33 mcg), l-T3 (2.5, 10, or 16 mcg) powder, and placebo. Study participants were randomized to either l-T3 or l-T4 treatment according to a computer-generated table, and therapeutic adjustments or changes in placebo were performed bi-weekly by two unblinded physicians (MZ, MCS), and a clinical pharmacist (FP). After randomization the study patients assigned to L-T4 were continued on their latest pre-study total daily dose. Conversely, subjects randomized to L-T3 were initially treated with approximately half of the calculated L-T3 dose to avoid hyperthyroidism due to the presence of residual T4. The L-T3 requirement was estimated as 1/2 of the latest L-T4 dose; subsequently the estimation was adjusted to 1/3. All patients were continued on three-time daily regimen and the total daily dose of study medications was adjusted by changes of ± 5% for TSH levels ≤ 1.0 mU/l from the therapeutic target; conversely the dosage was adjusted by changes of ± 10% for TSH levels > 1.0 mU/l from the therapeutic target. Placebo capsules were used to perform sham adjustments while the subjects were receiving L -T4. Compliance was assessed at each follow-up visit by pill counting and by direct questioning the study volunteers. During each follow-up visit the presence the following symptoms: anxiety, palpitations, tremor, heat intolerance, depression, fatigue, constipation, dry skin, and heat or cold intolerance was assessed and recorded by blinded investigators (FSC, NIB, JDL). In order to avoid unnecessary adjustments, the unblinded team members were made aware by the blinded investigators of the study patients' self-reported compliance to the regimen. Subjects received from one to six capsules per dose depending on total dose requirements. All attempts were made to supply identical amount of study medication in each of three daily doses.
Statistical analysis
Unless stated otherwise, data are presented as a mean ± standard deviation (SD). The area under the curve (AUC 0-60) for TRH-stimulated TSH response following l-T3 and l-T4 study phases was calculated by the trapezoidal rule using WinNonLin® Professional version 3.2 from Pharsight (Mountain View, CA). The maximum serum TSH concentration (Cmax) following the TRH administration was obtained by direct inspection of the serum concentration-time profiles. Results for both TSH AUC 0-60 and Cmax are reported as geometric means and their associated 95% confidence intervals. Paired t-tests were used for group comparisons, and the results were confirmed by Wilcoxon signed-rank tests. A mixed model was used for comparing paired serial thyroid function tests data. Analyses were performed using SAS® and JMP® statistical software packages (SAS Institute Cary NC, USA). Two-tailed P values of less than 0.05 are considered statistically significant.
Results
Patient recruiting and characteristics
During the period June 2005 - November 2007 14 eligible individuals were enrolled in the study. Of them, 10 post-surgical hypothyroid patients (9 females, 1 male, age 51.3±3.4 years) completed both phases of the study. The characteristics of the study patients are reported on Table I. The overall recruiting, screening, drop out, and completion figures are reported in Figure 1. Four subjects withdrew from the study; three secondary to inability of following the medication regimen, and one due to relocation. No serious adverse event was recorded. None of the patients complained of hyperthyroid (anxiety, palpitations, tremor, heat intolerance) or hypothyroid (depression, fatigue, constipation, dry skin) symptoms at the time of the admissions. Systolic blood pressure on l-T3 therapy was 117.6±12.5 mmHg vs. 111.1±6.9 mmHg while on l-T4 (n.s.); diastolic blood pressure on l-T3 therapy was 73.11±11.3 mmHg vs. 69.2±8.2 mmHg while on l-T4 (n.s.). Heart rate on l-T3 therapy was 66.0±5.4 beats/min vs. 64.9±6.9 beats/min while on l-T4 (n.s.). At the end of the study three patients expressed overall preference for the l-T3 therapy, four for the l-T4 therapy, and three had no preference.
Table 1.
Baseline characteristics of the study population (n=10).
| Parameters | ||
|---|---|---|
| Female/Male | 9/1 | |
| Age (yr) | 51.3±3.4 | |
| Ethnicity | 7 Caucasian, 2 Asian, 1 African American | |
| Height (cm) | 162.9±5.3 | |
| Weight (kg) | 69.01±11.4 | |
| Body mass index (kg/m2) | 25.9±3.7 | |
| Etiology of hypothyroidism (all post-thyroidectomy) | 4 multinodular goiter 2 adenoma 3 papillary thyroid cancer 1 medullary thyroid cancer |
|
| Duration of hypothyroidism (years) | 3.8±2.7 | |
| L-T4 replacement dose (mcg/day) | 123.7±35.9 TSH (mU/l) |
1.03±1.40 |
| Thyroid function tests at baseline | FreeT4 (pmol/l) | 21±6 |
| T3 (nmol/l) | 1.51±0.36 | |
Figure 1.
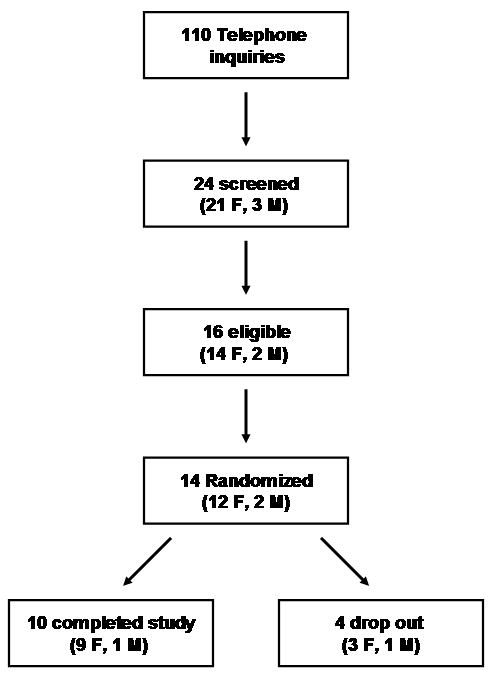
CONSORT flow-chart: research protocol recruiting, screening, drop out, and completion rate.
Thyroid hormone replacement therapy
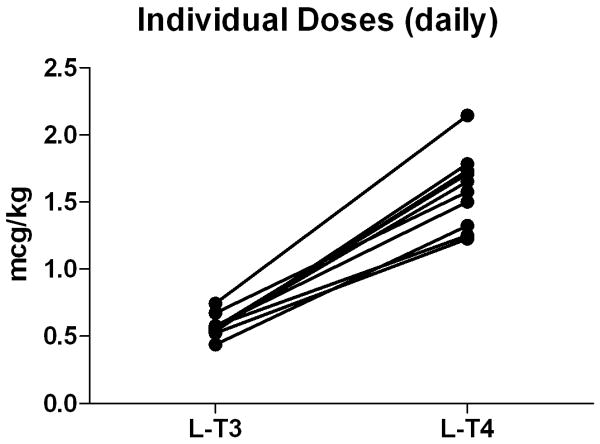
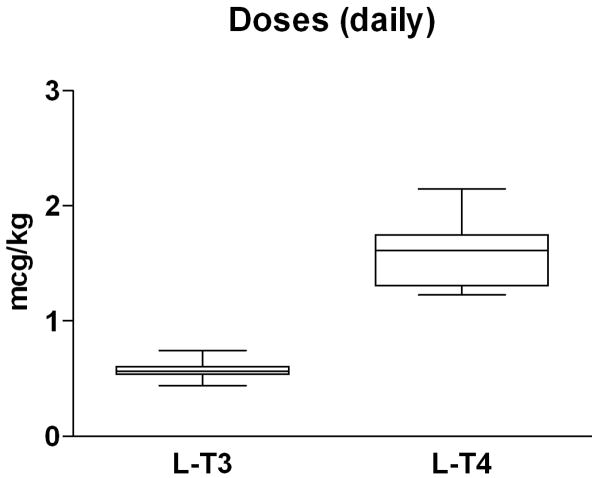
Both treatment formulations required an extensive amount of time to reach the therapeutic target TSH (≥0.5 and ≤1.5 mU/L), 228±142 days (range 117-559) on l-T3 vs. 223±75 days (range 119-290) on l-T4. The average replacement dose of l-T3 was 40.3±11.3 mcg/day (0.57±0.08 mcg/kg/day) vs. 115.2±38.5 mcg/day (1.59±0.28 mcg/kg/day) of l-T4 with a l-T3:l-T4 mcg/mcg ratio of 0.36±0.06 (Fig. 2). Individual l-T3:l-T4 ratios ranged from 0.29 to 0.46.
Figure 2.
Equivalency for thyroid hormone replacement therapy. A: individual l-T3 l-T4 equivalency at steady state. B: box-plot indicating median and distribution of the daily doses of l-T3 and l-T4 replacement therapy. Data are expressed as mcg/kg daily dose on a thrice daily administration scheme.
Thyroid hormone levels
As expected, at the time of admission after a minimum of thirty days at a stable replacement dose and a TSH level on target, the free and total T4 levels fell below the levels of detection in the group treated with l-T3 (Table II). On the other hand, the mean free T4 levels (21±4 pmol/l) in the group treated with l-T4 were within the normal range (10-25 pmol/l). Conversely, although in both the treatment modalities the total T3 levels were within normal range, they were significantly higher in the l-T3 treated group, 2.84±1.54 nmol/l L-T3 vs. 1.49±0.29 nmol/l l-T4, p=0.017. No difference was observed for TSH values between the two replacement phases, 1.48± 0.78 mU/l on l-T3 vs. 1.21± 0.62 mU/l on l-T4, p=0.293. The serum levels of TSH and total and free thyroid hormones are reported in Table II. In order to assess the changes in the circulating TH levels, serial blood samples were obtained for one day. The mean total T3 levels were within normal range throughout the 24-h period of observation on l-T3 treatment, but the concentrations appeared to be more variable when compared to those observed in the l-T4 treated group (Fig. 3a). In agreement with the previous observation, the 24-hour serial T3 serum concentrations were higher during the l-T3 replacement therapy than during l-T4 replacement therapy (respective means: 2.74 nmol/l vs. 1.39 nmol/l, p<0.0001); these differences were similar at all sampling time points. Conversely, the 24-hour serum TSH profiles (Fig 3b) were similar between the two treatment arms (respective means 1.16 mU/l l-T3 and 1.20 mU/l l-T4, p=0.81).
Table 2.
Comparison of thyroid function tests following thyroid hormone replacement with lT-3 and lT-4.
| Parameter | Reference Interval | l-T4 Replacement | l-T3 Replacement | P valuea |
|---|---|---|---|---|
| TSH (mU/l) | 0.40 - 4.00 | 1.21 ± 0.62 | 1.48 ± 0.78 | 0.293 |
| Free T4 (pmol/l) | 10-25 | 21 ± 4 | Below detection | <0.001 |
| Total T4 (nmol/l) | 58-161 | 106 ± 22 | Below detection | <0.001 |
| Free T3 (pmol/l) | 2.8 - 6.5 | 4.0 ± 0.4 | 5.9 ± 2.6 | 0.044 |
| Total T3 (nmol/l) | 1.39-3.31 | 1.49 ± 0.29 | 2.84 ± 1.54 | 0.017 |
Data are presented as mean±SD.
2-tail paired Student's t test; all significant p values ≤0.002 with the Wilcoxon signed-rank test.
Figure 3.
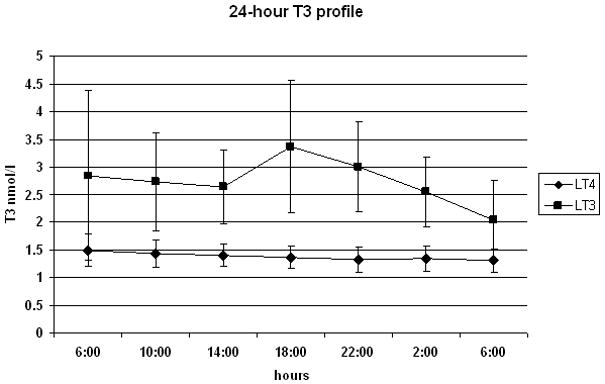
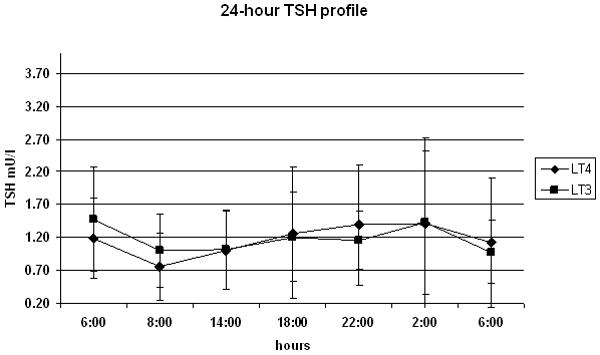
24-hour profile of total T3 levels (panel A) and TSH (panel B) on ◆ lT-3 and (●) l-T4 thyroid hormone replacement therapy. Samples were obtained at 4-hour intervals, except for the second TSH time-point where the time 0 TRH stimulation test (08:00) was substituted for the 10:00 time-point to avoid the artifact of the TRH-stimulated rise in TSH.
TRH stimulation test
In order to study the pituitary end-organ response to the two different treatments, eight study volunteers underwent a 200 mcg TRH stimulation test. Following pharmacological stimulation, the TSH Cmax of 6.83 (95% CI 4.88-9.55) mU/l on l-T3 vs. 5.23 (95% CI 3.31-8.3) mU/l on l-T4, p=0.383; and AUC 0-60 326.1 (95% CI 232.6-457.1) mU*min/l on l-T3 vs. 247.1 (95% CI 153.8-397.1) mU*min/l on l-T4 (p=0.285), were similar, indicative of pharmacodynamic equivalency (Fig. 4). TSH Cmax and AUC 0-60 results were also similar using a Wilcoxon signed-rank test (data not shown).
Figure 4.
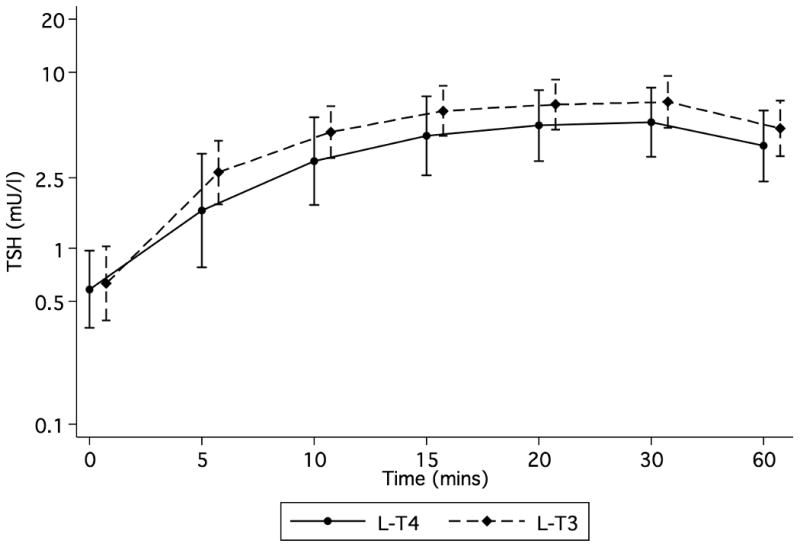
Results of the TRH stimulation test. Study volunteers (n=8) received a 200 mcg intravenous injection of TRH while on ◆ lT-3 and ● l-T4 thyroid hormone replacement therapy at 08:00. Time 0 indicates the average of times -15 and 0 min. Data expressed as geometric means and associated 95% confidence limits.
Discussion
THs play an important regulatory role in the development and function of virtually all organ systems, and their homeostasis is maintained by a highly regulated, multi-step redundant system. The peripheral conversion of the THs, by regulating the availability of T3 at the target tissue level, represents an important pre-receptor modulation of the hormonal action 10. The TH replacement therapy indeed is based on the assumption that the peripheral conversion of exogenously administered T4 (as l-T4) into T3 provides adequate replacement to the various target tissues. Nonetheless, clinical and in vitro observations 11, 12 indicate that the peripheral conversion of T4 could play an important role in the variable clinical presentation of thyroid disease among patients. Further, it is a common observation that, despite normal circulating levels of TSH and THs, a sizable number of individuals treated with l-T4 alone complain of hypothyroid symptoms 13. These observations have led to the human experimentation of combination therapy with both l-T4 and l-T3 in the treatment of hypothyroidism 14. Some studies indeed suggested marginal improvement of hypothyroid symptoms 14-18, but the vast majority of the large trials do not support the need for 19, or the superiority of the combination therapy 20-24. Nonetheless, this modality remains a common practice among endocrinologists and general practitioners 25. It is worth noting that most of the studies did not provide a physiological TH replacement, since the combination therapy was administered on a once-a-day regimen 14, 16-18, 21, 23, 24 and/or with a fixed l-T3 dose 14-16, 18, 20, 21, 24. On the other hand, therapeutic substitution of l-T3 on a twice-a-day administration regimen for l-T4 during the TH withdrawal in thyroid cancer patients in preparation for nuclear medicine studies and radioiodine therapy has become the standard of care 7. This modality is generally well-tolerated and is used to reduce the duration of hypothyroid symptoms 26. However, the narrow therapeutic index and the relatively short half-life of l-T3 represent substantial impediments to achieving a sustained TH replacement with l-T3 alone. Surprisingly, no formal comparison of the pharmacodynamic equivalency of l-T3 vs. l-T4 using a narrow TSH range as a therapeutic target is currently available. The only detailed evaluation of l-T3 as a single agent replacement therapy for hypothyroidism was performed in a study population with TSH ranging from subclinical to severely hypothyroid levels 27. At the present, therapeutic substitution of l-T3 for l-T4 is mostly performed on a fixed-dose of 50% of the l-T4 dose requirements, with minimal empirical evidence for the adequacy of replacement.
In this study we chose to substitute l-T3 for l-T4 on a three times daily administration regimen to maintain reasonably steady levels of T3 throughout the day, avoiding the potential risk of supratherapeutic levels of T3 28. Our findings demonstrate that a steady-state pharmacodynamic equivalence can be achieved by substituting l-T3 for l-T4 using a thrice daily regimen at an approximate ratio of 1:3. It is worth noting that the l-T4 dosage required to achieve the target TSH on a thrice daily regimen is comparable to the baseline once daily regimen (Table I). The characteristics of our study design make the results particularly robust. By implementing a randomized, cross-over, treat-to-target, prolonged intervention, we have virtually avoided most of the biases associated with pharmacodynamic equivalence studies. Consequently, we do not expect lead-time biases or any carry-over effects from the first to the second treatment phase. Further, the use of a cross-over design and careful selection of the study volunteers, who were otherwise healthy and completely devoid of endogenous TH secretion, have allowed a rather precise evaluation of the required dose. Finally we have tested the l-T3 vs. l-T4 equivalency not simply on a single time point at baseline, but also throughout a 24-hour period, and after a dynamic pituitary stimulation with TRH. Despite the fact that TRH stimulation test may not add any substantial diagnostic advantage over the assessment of baseline TSH 29 in primary hypothyroidism, we chose to perform this additional dynamic test to uncover subtle differences in the pituitary response to the two drug formulations. Although the two therapies were not significantly different, the 95% confidence interval for the true AUC ratio could be within 0.75 to 2.33 because of the variability in TSH AUC values among patients under each therapeutic cycle. However, a pharmacodyamic equivalency between the two treatments was further supported by finding non-significant differences between baseline TSH serum concentrations and TSH Cmax determinations following TRH stimulation tests, as well as similar diurnal TSH profiles. Furthermore, the results were comparable with normative data 30, indicating a state of euthyroidism. Nonetheless it is possible that some very subtle differences in the pituitary response to the TRH stimulation might not have been demonstrated because of type-II error.
Compared to individuals treated with l-T4, the circulating levels of T3 were higher in the l-T3 treated group. Consistent with previous observations 6, the circulating levels of T3 in the individuals treated with l-T4 were comparable to the ones observed in healthy volunteers (data not shown). It is important to note that albeit elevated, the mean circulating T3 levels remained within the normal range throughout the 24 hours, indicating that the three times daily l-T3 substitution regimen at a 1:3 ratio represents a more physiologic and possibly safer therapeutic modality than the use of less frequent l-T3 dosing intervals.
While the limited number of participants might be considered a weakness of our study, its main limitation is the tight follow-up schedule with the requirement of multiple daily TH replacement doses, and the narrow therapeutic target adopted in this research protocol, which cannot be realistically replicated for a sustained period of time in the general endocrine practice. The time-to-target was similarly prolonged for both treatment arms. This is somewhat surprising since one would think that it should be easier to achieve the TSH goal with l-T4 than with l-T3. This finding is probably explained by the fact that the thrice daily regimen almost invariably introduces an element of non-compliance. Furthermore, while adjustments in the l-T3 regimen can be effectively made every two weeks, this is not possible on LT-4 because of its longer half-life.
Nonetheless, should the fractional dosage of l-T3 become available, l-T3 for l-T4 substitution on a thrice daily regimen at an approximate ratio of 1:3 could be implemented for a limited period of time during the l-T4 withdrawal for 131I scan and treatment in thyroid cancer patients. This would avoid the risks of supra-therapeutic T3 levels or substantial under-dosing, thus minimizing the duration of TSH stimulation and hypothyroid symptoms. Furthermore, the data reported in this manuscript could constitute the proof-of-concept for therapeutic substitution of l-T3 for l-T4 or combination TH therapy when a sustained-release l-T3 formulation becomes commercially available 19, 31.
Acknowledgments
The authors gratefully acknowledge the help and professionalism of the nursing, laboratory, pharmacy (especially George Grimes Judy Starling), and ancillary personnel of the NIH Clinical Center. This research could have not been accomplished without the selflessness participation of the study participants.
This study was supported by the Intramural Research Program of the NIDDK-NIH, program Z01-DK047057-02 and the Intramural Research Program of the NIH Clinical Center. ClinicalTrials.gov identifier: NCT00106119
Footnotes
Competing interests/financial disclosure: The authors have nothing to disclose
References
- 1.Eisenberg M, Samuels M, DiStefano JJ., 3rd L-T4 bioequivalence and hormone replacement studies via feedback control simulations. Thyroid. 2006;16:1279–1292. doi: 10.1089/thy.2006.0144. [DOI] [PubMed] [Google Scholar]
- 2.Saberi M, Sterling FH, Utiger RD. Reduction in extrathyroidal triiodothyronine production by propylthiouracil in man. Journal of Clinical Investigation. 1975;55:218–223. doi: 10.1172/JCI107924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. Journal of Clinical Investigation. 2005;115:2524–2533. doi: 10.1172/JCI25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen TT, DiStefano JJ, 3rd, Yamada H, Yen YM. Steady state organ distribution and metabolism of thyroxine and 3,5,3′-triiodothyronine in intestines, liver, kidneys, blood, and residual carcass of the rat in vivo. Endocrinology. 1993;133:2973–2983. doi: 10.1210/endo.133.6.8243325. [DOI] [PubMed] [Google Scholar]
- 5.Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. New England Journal of Medicine. 1987;316:764–770. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 6.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. Jama. 2008;299:769–777. doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JM, Line BR, Aamodt RL, Robbins J. Influence of triiodothyronine withdrawal time on 131I uptake postthyroidectomy for thyroid cancer. Journal of Clinical Endocrinology and Metabolism. 1980;50:734–739. doi: 10.1210/jcem-50-4-734. [DOI] [PubMed] [Google Scholar]
- 8.Dahlberg PA, Karlsson FA, Wide L. Triiodothyronine intoxication. Lancet. 1979;2:700. doi: 10.1016/s0140-6736(79)92105-6. [DOI] [PubMed] [Google Scholar]
- 9.Leboeuf R, Perron P, Carpentier AC, Verreault J, Langlois MF. L-T3 preparation for whole-body scintigraphy: a randomized-controlled trial. Clinical Endocrinology. 2007;67:839–844. doi: 10.1111/j.1365-2265.2007.02972.x. [DOI] [PubMed] [Google Scholar]
- 10.Gereben B, Zeold A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cellular and Molecular Life Sciences. 2008;65:570–590. doi: 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escobar-Morreale HF, del Rey FE, Obregon MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502. doi: 10.1210/endo.137.6.8641203. [DOI] [PubMed] [Google Scholar]
- 12.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. Journal of Clinical Investigation. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clinical Endocrinology. 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [DOI] [PubMed] [Google Scholar]
- 14.Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ., Jr Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. New England Journal of Medicine. 1999;340:424–429. doi: 10.1056/NEJM199902113400603. [DOI] [PubMed] [Google Scholar]
- 15.Appelhof BC, Fliers E, Wekking EM, Schene AH, Huyser J, Tijssen JG, Endert E, van Weert HC, Wiersinga WM. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. Journal of Clinical Endocrinology and Metabolism. 2005;90:2666–2674. doi: 10.1210/jc.2004-2111. [DOI] [PubMed] [Google Scholar]
- 16.Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. Journal of Clinical Endocrinology and Metabolism. 2005;90:805–812. doi: 10.1210/jc.2004-1672. [DOI] [PubMed] [Google Scholar]
- 17.Slawik M, Klawitter B, Meiser E, Schories M, Zwermann O, Borm K, Peper M, Lubrich B, Hug MJ, Nauck M, Olschewski M, Beuschlein F, Reincke M. Thyroid hormone replacement for central hypothyroidism: a randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. Journal of Clinical Endocrinology and Metabolism. 2007;92:4115–4122. doi: 10.1210/jc.2007-0297. [DOI] [PubMed] [Google Scholar]
- 18.Bunevicius R, Jakubonien N, Jurkevicius R, Cernicat J, Lasas L, Prange AJ., Jr Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves' disease. Endocrine. 2002;18:129–133. doi: 10.1385/ENDO:18:2:129. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DS. Combined T4 and T3 therapy--back to the drawing board. Jama. 2003;290:3002–3004. doi: 10.1001/jama.290.22.3002. [DOI] [PubMed] [Google Scholar]
- 20.Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. Jama. 2003;290:2952–2958. doi: 10.1001/jama.290.22.2952. [DOI] [PubMed] [Google Scholar]
- 21.Escobar-Morreale HF, Botella-Carretero JI, Gomez-Bueno M, Galan JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Annals of Internal Medicine. 2005;142:412–424. doi: 10.7326/0003-4819-142-6-200503150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. Journal of Clinical Endocrinology and Metabolism. 2003;88:4551–4555. doi: 10.1210/jc.2003-030139. [DOI] [PubMed] [Google Scholar]
- 23.Siegmund W, Spieker K, Weike AI, Giessmann T, Modess C, Dabers T, Kirsch G, Sanger E, Engel G, Hamm AO, Nauck M, Meng W. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14 : 1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clinical Endocrinology. 2004;60:750–757. doi: 10.1111/j.1365-2265.2004.02050.x. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JP, Shiels L, Lim EM, Bhagat CI, Ward LC, Stuckey BG, Dhaliwal SS, Chew GT, Bhagat MC, Cussons AJ. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. Journal of Clinical Endocrinology and Metabolism. 2003;88:4543–4550. doi: 10.1210/jc.2003-030249. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard KR. Dosage recommendations for combination regimen of thyroxine and 3,5,3′-triiodothyronine. Journal of Clinical Endocrinology and Metabolism. 2004;89:1486–1487. doi: 10.1210/jc.2003-031971. author reply 1487-1488. [DOI] [PubMed] [Google Scholar]
- 26.Schlumberger MJ, Filetti S, Hay ID. Nontoxic diffuse and nodular goiter and thyroid neoplasia. In: Kronenberg HM, editor. In Williams Textbook of Endocrinology. 11th. Saunders; Philadelphia: 2008. pp. 411–445. [Google Scholar]
- 27.Saberi M, Utiger RD. Serum thyroid hormone and thyrotropin concentrations during thyroxine and triiodothyronine therapy. Journal of Clinical Endocrinology and Metabolism. 1974;39:923–927. doi: 10.1210/jcem-39-5-923. [DOI] [PubMed] [Google Scholar]
- 28.Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, Free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Experimental and Clinical Endocrinology & Diabetes. 2007;115:261–267. doi: 10.1055/s-2007-973071. [DOI] [PubMed] [Google Scholar]
- 29.Spencer CA, Schwarzbein D, Guttler RB, LoPresti JS, Nicoloff JT. Thyrotropin (TSH)-releasing hormone stimulation test responses employing third and fourth generation TSH assays. Journal of Clinical Endocrinology and Metabolism. 1993;76:494–498. doi: 10.1210/jcem.76.2.8432796. [DOI] [PubMed] [Google Scholar]
- 30.Sawin CT, Hershman JM. The TSH response to thyrotropin-releasing hormone (TRH) in young adult men: intra-individual variation and relation to basal serum TSH and thyroid hormones. Journal of Clinical Endocrinology and Metabolism. 1976;42:809–816. doi: 10.1210/jcem-42-5-809. [DOI] [PubMed] [Google Scholar]
- 31.Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid. 2004;14:271–275. doi: 10.1089/105072504323030924. [DOI] [PubMed] [Google Scholar]