Abstract
Bilateral subthalamic (STN) deep brain stimulation (DBS) provides significant symptom relief for the majority of well-screened patients suffering with Parkinson’s disease (PD). Implantation of stimulating electrodes bilaterally in a single session has become standard in most operating theaters worldwide. There is, however, limited evidence-based support for this approach. Although bilateral surgical procedures have been shown, using standardized clinical ratings, to provide greater motor benefits compared to unilateral procedures, bilateral procedures are more likely to be associated with increased acute and long- term complications including post-operative confusion, speech difficulties and cognitive dysfunction. Unilateral stimulation has been shown to provide significant benefits for appendicular and axial symptoms. The relative benefit of implanting one versus two sides and whether the degree of benefit associated with the second side is worth the potential risk of doing so have not been examined systematically. The relative magnitude of benefit associated with unilateral versus bilateral procedures is likely to vary from patient to patient, particularly in those patients with asymmetric symptomatology. As such, there are likely subsets of patients who do not require and therefore should not be exposed to the potential complications associated with bilateral simultaneous implantation. This review and commentary will outline our current understanding of the benefits associated with unilateral and bilateral STN DBS and discuss the role of unilateral or staged unilateral procedures as an alternative surgical approach for patients with advanced PD.
Introduction
The ability to adjust stimulation parameters to optimize function and minimize side effects in patients with Parkinson’s disease (PD) and other neurological disorders is a unique advantage of deep brain stimulation (DBS) over ablative surgical procedures. Although no large scale head-to-head studies with lesion therapy have been performed, the flexibility and reversibility associated with DBS has led to the general “clinical acceptance” of DBS as the preferred surgical treatment for select candidates with advanced PD. The current operative approach taken by the vast majority of centers performing DBS surgeries for PD is to simultaneously implant the STN on both sides of the brain in a single surgical session 1, 2. Following bilateral STN DBS, clinical ratings typically improve 30–60 percent with notable improvements in levodopa responsive symptoms (the degree of response to surgery depending heavily on the pre-operative response to levodopa). Typically, STN DBS results in reductions in antiparkinsonian medication, duration of “OFF” time and episodes of motor fluctuations 1–7.
The recent paper from Tabbal and colleagues8, our previous work 9, 10, and data from others 11, 12 offer experimental evidence that provides a rationale for unilateral STN DBS as a viable alternative approach to bilateral simultaneous implantations for the treatment of advanced PD. These studies have demonstrated that unilateral DBS produces significant contralateral and ipsilateral benefits as well as an improvement in axial symptoms. Taken together, these recent unilateral studies provide compelling evidence for a re-evaluation of the current practice of routinely placing DBS leads on both sides of the brain (in a single surgical session) for all patients undergoing DBS surgery for PD. The goal of this commentary is to open a dialogue based on the studies by Tabbal and others to address what is known about the bilateral effects of unilateral stimulation, whether bilateral STN DBS may be associated with previously unappreciated side effects in cognitive and motor tasks and to provide the rationale for consideration of unilateral or staged STN DBS as an alternative approach to bilateral simultaneous implantation for the treatment of patients with advanced PD.
Why has there been a propagation of simultaneous bilateral STN DBS for the treatment of advanced PD?
It is difficult to know exactly why the field marginated so quickly toward simultaneous bilateral implantations of DBS leads in a single surgical session. Early arguments centered on the perceived greater efficiency and overall benefit of the surgical procedure. Specifically, it was argued that implanting the second side could be accomplished more quickly by mirroring the targeting of the first site, once electrophysiological mapping had uncovered the optimal location for the first lead. This approach would require less mapping, thus decreasing the amount of time necessary to implant the second side. There are however, no studies to our knowledge that support this argument. Indeed, our own preliminary data suggest that just the opposite may be true (Vitek et al unpublished observations). Another rationale for simultaneous implantation has been that advanced PD patients have bilateral symptoms and axial symptoms are likely to improve only with bilateral lead placement. This belief has stemmed, at least in part, from previous experiences with unilateral pallidotomy in which the majority of studies reported only transient benefit in gait and balance13–15. Another argument is that PD, although asymmetrical, is a bilateral progressive disease that will eventually require the placement of electrodes bilaterally. Finally, it is believed that reducing antiparkinsonian medication, to alleviate dyskinesia, would not be possible with unilateral STN stimulation as symptoms ipsilateral to the stimulator would still require the same dose of levodopa for optimal management.
Although these arguments provide an initial rationale and at least partially explain the large-scale movement favoring bilateral simultaneous implantations, there is little evidence-based data to fully support this position. An important lesson was learned with bilateral pallidotomy and thalamotomy in that large, bilaterally placed lesions which infringed on non-motor areas resulted in pseudobulbar and/or cognitive problems16–18. These observations led us to essentially discontinue bilateral ablative procedures. The lesion literature also demonstrated that unilateral lesions, restricted to the motor area, produced significant ipsilateral motor benefit without the cognitive problems associated with bilateral procedures and that axial symptoms could be significantly improved and maintained at two years post-pallidotomy when larger lesions were made within the sensorimotor territory of the GPi 19. Yet, in spite of these historical lessons we in the medical community seem to have lost sight of the fact that unilateral DBS may also provide meaningful ipsilateral benefits and that bilateral procedures may be associated with more side effects.
While there are important lessons gained from our past experiences that provide a rationale for choosing either unilateral or bilateral surgical procedures, large-scale comparisons of unilateral versus bilateral DBS are lacking in the literature. The recent paper from Tabbal and colleagues (2008) along with our previous work (Alberts et al., 2004; Alberts et al., 2008) provide evidence to suggest we need to systematically evaluate the effects of unilateral relative to bilateral STN DBS. While these recent studies serve as a starting point, the unique advantages and disadvantages of each procedure relative to one another remain ill-defined. A vital question that begs an answer is “How much better is bilateral compared to unilateral STN DBS?” this question can only be answered by first understanding the effects of unilateral stimulation.
What are the effects of unilateral DBS on motor performance?
Although limited in number, those studies that have examined the effect of unilateral DBS on motor performance report significant improvements in UPDRS III ratings as well as ipsilateral improvement in motor function and gait. Improvements in UPDRS III scores range from 26–37 percent 9–12, 20, and ipsilateral benefits range between 15–20 percent. Decreases in the daily dose of levodopa ranging between 15–36 percent were also reported together with marked improvement in bimanual functional dexterity (Alberts et al., 2008; Alberts et al., 2004) and gait (see next section) [Bastian et al., 2003].
The results from our bimanual dexterity study suggest that unilateral DBS improves force control in each limb. The strength of this finding lies in the notion that the experimental bimanual task utilized is a better assessment of how patients may function in daily activities as it replicates the bimanual function required in these activities. Thus, the finding that unilateral STN DBS significantly improved performance of both limbs during this task suggests this surgical strategy can enhance functional performance. Additionally, we have recently evaluated the effects of unilateral STN DBS on force control in the ipsilateral and contralateral limb separately. Twelve patients, all at least 12 months post DBS surgery, performed a sine-wave force tracking paradigm. The target sine wave ranged from 5% to 25% of the maximum voluntary contraction (MVC) produced while the patient was OFF stimulation. Patients were instructed to track, as accurately as possible, the displayed sine wave. Representative force-tracking data for each limb relative to the target sine wave are provided in the upper plots of Figure 1 while “on” and “off” unilateral DBS. The coefficient of coordination (Kc) was used to quantify performance. The middle plots of Figure 1 depict the rate of force production as a function of the actual force produced by the patient. If the patient tracks the target force perfectly, then the relationship between the rate of force production and actual force is a circle and the Kc measure is equals 1; departures from the circle represent variability in motor performance. As illustrated in the middle row of plots in Figure 1, when the patient was OFF stimulation force tracking performance was highly variable (e.g. deviations from ideal circle were great). Values for Kc reflected this variability at 0.28 and 0.20 for contralateral and ipsilateral limbs respectively. Unilateral stimulation resulted in significant improvements in force tracking (lower plots) for both limbs as the Kc values improved to 0.81 and 0.65 for the contralateral and ipsilateral limbs respectively. These data indicate the ipsilateral and contralateral improvements in force control associated with unilateral STN are relatively long term. The interconnectedness of the basal ganglia with motor cortical circuits may provide insight into why unilateral DBS improves ipsilateral and contralateral motor function.
Figure 1.
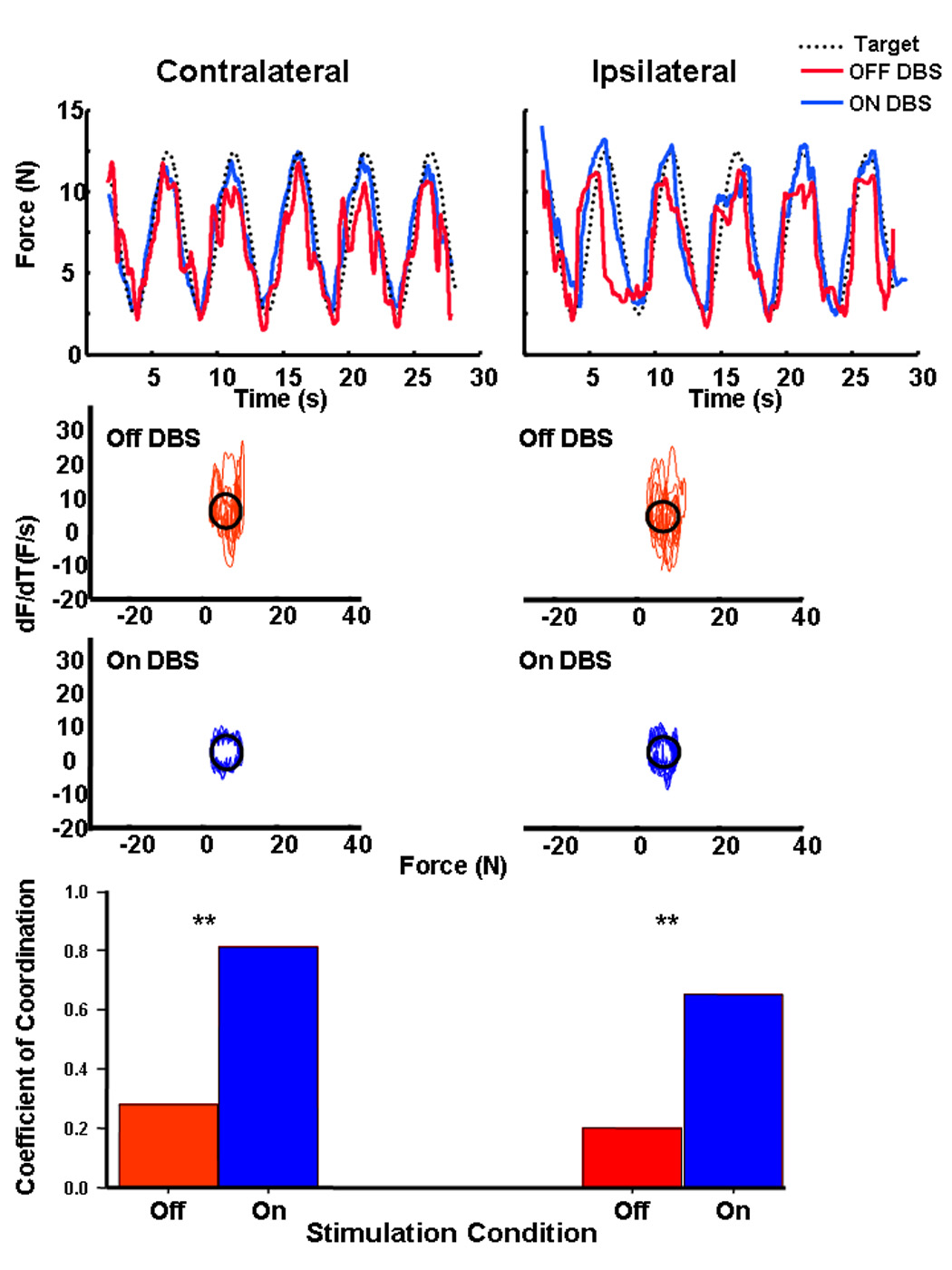
Representative force-tracking performance for one patient for the limb contralateral and ipsilateral to the DBS electrode while Off (red) and On(blue) DBS. The middle plots depict the rate of force produced as a function of actual force to assess force variability during tracking for data provided above. Perfect performance would yield a circle. Perfect tracking of the sine wave would result in a circle. The lower plots represent the group average coefficient of coordination for each limb while Off (red bar) and On (blue bar) stimulation.
The extensive interhemispheric connections between the SMA21 and ipsilateral projections from the SMA to striatum22 provide anatomical support for the notion that improved SMA functioning leads to improvements in motor function in the ipsilateral and contralateral limbs. The importance of the SMA in the control and planning of bimanual activities in particular23 may explain why ipsilateral motor function of PD patients during DBS is enhanced during bimanual activities. Pallidotomy has been shown to increase in movement related cortical activity in the SMA24; similar findings have been reported in patients with GPi DBS25. Additionally two PET studies have shown that STN DBS leads to increased activation centered around the SMA (ipsilateral to stimulation and contralateral to movement) during internally generated movements 26, 27. Improved SMA function in the hemisphere ipsilateral to the stimulation site likely contributes to the bilateral motor improvements associated with unilateral stimulation.
Collectively, these studies indicate that unilateral DBS reliably improves ipsilateral and contralateral motor function and allows for a reduction in antiparkinsonian medication. These ipsilateral benefits translate to improved bimanual dexterity and these improvements persisted at least one year following DBS surgery9, 10. Overall, these findings argue against several of the premises that have provided the rationale for performing bilateral simultaneous procedures (no ipsilateral benefit, unable to reduce medication, unknown functional benefit and waning effectiveness).
How much better is bilateral compared to unilateral STN DBS?
To our knowledge, only three studies have directly compared the effects of unilateral and bilateral STN DBS on motor function in the same cohort of PD patients. Collectively, the results from these three studies indicate that unilateral and bilateral STN DBS both produce significant improvements in PD motor function 8, 28, 29. A formal comparison of the differential effects of unilateral and bilateral stimulation on UPDRS III scores was not reported by Tabbal (2008). Kumar (1999) and Bastian (2003) indicated bilateral DBS did improve total UPDRS III scores approximately 20–30 percent more than unilateral stimulation. Unfortunately there are some limitations with these studies that may cloud the results when comparing unilateral to bilateral procedures. These include: the small number of patients assessed, six in one study and 10 in another; the short time period between stimulation conditions in the Kumar study; both studies’ grouping results from individual patients together, thus not allowing a comparison across patients; and in the Kumar study collapsing the data across side for unilateral stimulation (indeed where results from individual patients were reported in the Bastian study, in three of six patients adding the second stimulator only gained then less than half again the benefit that was gained with one stimulator). In the Kumar study only 15 minutes was allowed between stimulation conditions and the results for all unilateral conditions were grouped together, which does not allow one to compare whether one side may have provided greater benefit than another. This is critically important as many patients have asymmetry to their disease and when performing unilateral procedures the more affected side would be treated. Averaging stimulation benefits across both sides when doing unilateral stimulation would wash out this difference and decrease the relative difference between unilateral stimulation of the more affected or dominant side compared to bilateral stimulation.
Interestingly, however, Kumar and colleagues (1999) did report significant ipsilateral improvements in reaction time and timed tapping with unilateral stimulation. The ipsilateral effects of unilateral stimulation on other PD symptoms assessed by the UPDRS III were not reported. Biomechanical data characterizing upper extremity reaching from Bastian and colleagues (2003) indicated that unilateral and bilateral stimulation improved upper extremity reaching speed compared to no stimulation. The ipsilateral effects of unilateral stimulation on reaching speed, however, were not reported29. Similar to our previous observations (Alberts, et al., 2004, Alberts, et al., 2008) the data from Tabbal (2008) clearly indicate ipsilateral improvements in rigidity and bradykinesia with unilateral stimulation when objective biomechanical measures are utilized.
While there are clearly substantive data supporting improvement in ipsilateral bradykinesia and rigidity, the ability of unilateral DBS to improve gait has been questioned and most centers subscribe to the widely held notion that unilateral procedures do not improve gait and postural stability. In reviewing the literature this notion may not be supported and in fact it would appear that unilateral stimulation may indeed improve gait and posture. Piper and colleagues 30 examined gait in patients who had undergone staged STN DBS. Unilateral stimulation significantly improved gait parameters (speed, cadence, step length and joint kinematics) particularly in the Off medication state. Unilateral DBS restored the ability to walk in 5/9 of the patients who could not walk in the off medication state prior to surgery. Of note, the authors did not identify any differences between unilateral and bilateral stimulation. However, “due to the long time lag between unilateral and bilateral testing sessions” (pg. 231); they declined to make a formal comparison between unilateral and bilateral DBS. Bastian and colleagues (2003) studied the relative effect of unilateral to bilateral stimulation and reported that four of the six patients exhibited improvements in walking speed and stride length with unilateral stimulation. Only two of the six patients required bilateral stimulation to improve gait. Gait asymmetry, assessed through cadence, was not different between unilateral and bilaterally stimulated patients. Therefore, unilateral stimulation did not induce asymmetric gait. Similarly, Kumar and colleagues (1999) and Tabbal and colleagues (2008) indicated that unilateral stimulation led to significant improvements in the time to walk a specified distance. Bilateral stimulation led to a further reduction in time, approximately eight percent, compared to unilateral stimulation. While improvements in timed gait performance may have clinical importance, it only reflects changes in quantity of movement and not changes in underlying control processes. Further, timed gait tasks do not provide insight into how gait / balance function is maintained during tasks of daily living especially those requiring concurrent performance of locomotor and cognitive tasks. Indeed, effective human locomotion is characterized by the ability to perform multiple tasks simultaneously. For example, serving a meal includes dual tasks such as carrying food in a dish while walking towards the table and the ability to talk while walking is known to be related to fall risk. Thus, the “true” effects of DBS on locomotor function needs to tested in research paradigms that better reflect the context in which daily activities are performed.
Does bilateral STN DBS adversely affect cognitive function under “real life” conditions?
A similar argument to that for locomotion can be made for assessing the effect of DBS on cognition. Although the vast majority of studies observe deficits in verbal fluency with bilateral STN DBS, declines in attention, working memory, general cognition and learning appear equivocal. Overall, the body of literature concerning cognitive impairment and DBS is complex, difficult to interpret and may be misleading due to the following methodological issues: 1) small sample sizes with inadequate statistical power, 2) lack of baseline data prior to DBS, 3) variable testing conditions (e.g. On or Off medications; stimulation parameters; On/Off DBS stimulation; cognitive battery), 4) variability in the timing of post-operative evaluations, 5) variation in patient age, 6) little information regarding lead location, 7) ignoring practice effects, 8) failure to examine the relative effect of stimulation on the dominant versus nondominant hemisphere and 9) the use of neuropsychological evaluations that may not sufficiently challenge the patient or provide a reliable estimate of cognitive function when the patients are performing activities of daily living
Recently, several long-term bilateral STN DBS studies (follow up 3–5 years after surgery) have reported varying levels of decline in overall cognitive functioning, including verbal fluency 31, 32 and working memory 5, 6. Rodriguez-Oroz reported that 24 percent of the patients with STN DBS experienced cognitive declines 3–4 years after surgery 5. Schupbach described significant worsening of the UPDRS I score, Mattis scale and frontal scores in patients 60 months after surgery6. While Funkieweiz and colleagues 31 noted a significant decrease in verbal fluency immediately after surgery, no further deterioration over time (up to 3 years following surgery) was observed. Although some of these long term results may be due to natural progression of PD, these studies provide data that suggest that bilateral STN DBS could adversely affect various aspects of cognitive function. These potential adverse side-effects have not been reported in patients with unilateral STN DBS33, 34. It is acknowledged that the relatively few patients implanted unilaterally and subsequently studied may contribute to the decreased reports of cognitive side effects, thus providing further rationale for comparative studies.
Recent reviews and meta-analyses have attempted to clarify the conflicting body of literature related to the effects of bilateral DBS on various aspects of cognitive functioning34–37. According to Woods, bilateral STN DBS resulted in significant declines in verbal fluency, learning and memory compared to pre-surgery or OFF DBS scores. In a meta-analysis that included data from 1398 patients with bilateral STN DBS, cognitive problems were present in 41 percent of patients37. Cognitive problems varied from a moderate deterioration in verbal memory to significant declines in executive functioning. More recently, Parsons and colleagues (2006) reviewed 28 studies and noted that declines in executive and verbal learning, memory domains and verbal fluency were significant following bilateral STN DBS.
Although most earlier studies documented some minimal degree of change in various aspects of cognitive function associated with bilateral STN DBS, the lack of more significant declines in these studies could be due to the variation in difficulty of cognitive tests across studies38. Hershey and colleagues38 found that bilateral STN stimulation decreased working memory under testing conditions that were more cognitively demanding than those used in most previous studies where minimal declines in cognitive function were reported. These findings38 are consistent with our preliminary data that indicate bilateral STN DBS may compromise cognitive and motor performance to a greater degree than unilateral STN DBS as task difficulty is increased during a dual cognitive-motor task (Alberts et al., In review).
Activities of daily living are typically performed under modestly complex conditions and require the simultaneous performance of cognitive and motor tasks39. Therefore, even slight or subtle declines in cognitive function for PD patients may impact performance of routine activities such as dressing, grooming, cooking, walking and driving40. Determining the effects of unilateral and bilateral stimulation on dual task performance may provide a more accurate indicator of a patient’s performance in daily life. While the UPDRS Part II utilizes self-report to assess patients’ performance of daily activities, these items fail to objectively measure the most relevant aspects of motor function under different degrees of cognitive loading that may provide a more accurate representation of the performance of these tasks in our lives.
Recommendation: Data over Dogma
The fundamental goal of DBS is to modulate and to alter pathological neural activity within the basal ganglia thalamocortical network to optimally improve motor function while minimizing side effects. Although the DBS field has gravitated quickly toward bilateral DBS, there is little data to support this exclusive approach for all PD patients. In light of recent evidence documenting the bilateral benefits and improvement in axial symptoms following unilateral DBS together with the observation that bilateral STN DBS may be associated with a higher incidence of cognitive side effects and impairment in cognitive-motor function under dual task conditions, there is reason to pause and reassess the fundamental rationale for simultaneous lead implantations for PD patients. There are likely subsets of patients, particularly those with pronounced asymmetry, where unilateral DBS would be a reasonable and perhaps a more rationale approach for initiating DBS therapy.
The Tabbal study (2008) provides an excellent example of the importance of using precise and objective methods of motor assessment to determine the effects of unilateral and bilateral stimulation on motor function. Their data and that of others indicate that unilateral DBS is associated with improvements in ipsilateral appendicular as well as axial motor symptoms. We and others have shown that medication can be reduced with unilateral stimulation. Although preliminary, the collective results from these studies should prompt us to reconsider unilateral and/or staged procedures as an alternative approach to bilateral simultaneous implantation. Greater consideration should be given to tailoring surgical therapies for PD patients and for developing guidelines, based on objective measures and class I data. These guidelines should help to select between unilateral, bilateral, or staged DBS operations. Finally, the benefits and side effects of right versus left DBS (lateralization) should be weighed carefully with respect to both motor and non-motor features such as mood and cognition. We would argue that as we expand our understanding of the issues, we should focus the interpretation of our investigations in a direction that is derived from published studies and results should be used to tailor therapy to individual patients. Decisions over which approach should be taken should be based on the existing corpus of medical information in addition to individual patient needs. We conclude that in select cases and when considering all factors two leads may not always be better than one. The challenge now will be to develop rational and objective patient selection and evaluation criterion to determine when one or two leads should be used. In the interim, we need to keep a critical eye on current methodology, be willing to challenge ourselves and to move the field forward by designing thoughtful experiments, and relying on data, not dogma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Deep Brain Stimulation Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345(13):956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Chabardes S, Seigneuret E. Deep-brain stimulation in Parkinson's disease: long-term efficacy and safety - What happened this year? Curr Opin Neurol. 2005;18(6):623–630. doi: 10.1097/01.wco.0000186839.53807.93. [DOI] [PubMed] [Google Scholar]
- 3.Israel Z, Hassin-Baer S. Subthalamic stimulation for Parkinson's disease. Isr Med Assoc J. 2005;7(7):458–463. [PubMed] [Google Scholar]
- 4.Rodriguez-Oroz MC, Gorospe A, Guridi J, et al. Bilateral deep brain stimulation of the subthalamic nucleus in Parkinson's disease. Neurology. 2000;55 12 Suppl 6:S45–S51. [PubMed] [Google Scholar]
- 5.Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 6.Schupbach WM, Chastan N, Welter ML, et al. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5 year follow up. J Neurol Neurosurg Psychiatry. 2005;76(12):1640–1644. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 2003;349(20):1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- 8.Tabbal SD, Ushe M, Mink JW, et al. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in parkinson disease. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberts JL, Okun MS, Vitek JL. The persistent effects of unilateral pallidal and subthalamic deep brain stimulation on force control in advanced Parkinson's patients. Parkinsonism Relat Disord. 2008 doi: 10.1016/j.parkreldis.2007.11.014. In Press, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberts JL, Elder CM, Okun MS, Vitek JL. Comparison of pallidal and subthalamic stimulation on force control in patient's with Parkinson's disease. Motor Control. 2004;8(4):484–499. doi: 10.1123/mcj.8.4.484. [DOI] [PubMed] [Google Scholar]
- 11.Slowinski JL, Putzke JD, Uitti RJ, et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106(4):626–632. doi: 10.3171/jns.2007.106.4.626. [DOI] [PubMed] [Google Scholar]
- 12.Chung SJ, Jeon SR, Kim SR, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced parkinson's disease. Eur Neurol. 2006;56:127–132. doi: 10.1159/000095704. [DOI] [PubMed] [Google Scholar]
- 13.Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. N Engl J Med. 1997;337(15):1036–1042. doi: 10.1056/NEJM199710093371503. [DOI] [PubMed] [Google Scholar]
- 14.Roberts-Warrior D, Overby A, Jankovic J, et al. Postural control in Parkinson's disease after unilateral posteroventral pallidotomy. Brain. 2000;123(Pt 10):2141–2149. doi: 10.1093/brain/123.10.2141. [DOI] [PubMed] [Google Scholar]
- 15.Baron MS, Vitek JL, Bakay RA, et al. Treatment of advanced Parkinson's disease by unilateral posterior GPi pallidotomy: 4-year results of a pilot study. Mov Disord. 2000;15(2):230–237. doi: 10.1002/1531-8257(200003)15:2<230::aid-mds1005>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Merello M, Starkstein S, Nouzeilles MI, Kuzis G, Leiguarda R. Bilateral pallidotomy for treatment of Parkinson's disease induced corticobulbar syndrome and psychic akinesia avoidable by globus pallidus lesion combined with contralateral stimulation. J Neurol Neurosurg Psychiatry. 2001;71(5):611–614. doi: 10.1136/jnnp.71.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.York MK, Lai EC, Jankovic J, et al. Short and long-term motor and cognitive outcome of staged bilateral pallidotomy: a retrospective analysis. Acta Neurochir (Wien) 2007;149(9):857–866. doi: 10.1007/s00701-007-1242-x. discussion 866. [DOI] [PubMed] [Google Scholar]
- 18.Gross RE, Lombardi WJ, Lang AE, et al. Relationship of lesion location to clinical outcome following microelectrode-guided pallidotomy for Parkinson's disease. Brain. 1999;122(Pt 3):405–416. doi: 10.1093/brain/122.3.405. [DOI] [PubMed] [Google Scholar]
- 19.Vitek JL, Bakay RA, Freeman A, et al. Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol. 2003;53(5):558–569. doi: 10.1002/ana.10517. [DOI] [PubMed] [Google Scholar]
- 20.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101(1):36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 21.Wiesendanger M, Rouiller EM, Kazennikov O, Perrig S. Is the supplementary motor area a bilaterally organized system? Adv Neurol. 1996;70:85–93. [PubMed] [Google Scholar]
- 22.Lehericy S, Ducros M, Krainik A, et al. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004;14(12):1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- 23.Cunnington R, Bradshaw JL, Iansek R. The Role of the Supplementary motor area in the control of voluntary movement. Human Movement Science. 1996;15:627–647. [Google Scholar]
- 24.Grafton ST, Waters C, Sutton J, Lew MF, Couldwell W. Pallidotomy increases activity of motor association cortex in Parkinson's disease: a positron emission tomographic study. AnnNeurol. 1995;37(6):776–783. doi: 10.1002/ana.410370611. [DOI] [PubMed] [Google Scholar]
- 25.Davis KD, Taub E, Houle S, et al. Globus pallidus stimulation activates the cortical motor system during alleviation of parkinsonian symptoms. Nat Med. 1997;3(6):671–674. doi: 10.1038/nm0697-671. [DOI] [PubMed] [Google Scholar]
- 26.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson's disease. Ann Neurol. 1997;42(3):283–291. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 27.Ceballos-Baumann AO, Boecker H, Bartenstein P, et al. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol. 1999;56(8):997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- 28.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;53(3):561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 29.Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW. Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson's disease. Mov Disord. 2003;18(9):1000–1007. doi: 10.1002/mds.10493. [DOI] [PubMed] [Google Scholar]
- 30.Piper M, Abrams GM, Marks WJ., Jr Deep brain stimulation for the treatment of Parkinson's disease: overview and impact on gait and mobility. NeuroRehabilitation. 2005;20(3):223–232. [PubMed] [Google Scholar]
- 31.Funkiewiez A, Ardouin C, Caputo E, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(6):834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contarino MF, Daniele A, Sibilia AH, et al. Cognitive outcome 5 years after bilateral chronic stimulation of subthalamic nucleus in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78(3):248–252. doi: 10.1136/jnnp.2005.086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahanshahi M, Ardouin CM, Brown RG, et al. The impact of deep brain stimulation on executive function in Parkinson's disease. Brain. 2000;123(Pt 6):1142–1154. doi: 10.1093/brain/123.6.1142. [DOI] [PubMed] [Google Scholar]
- 34.Woods SP, Fields JA, Troster AI. Neuropsychological sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a critical review. Neuropsychol Rev. 2002;12(2):111–126. doi: 10.1023/a:1016806711705. [DOI] [PubMed] [Google Scholar]
- 35.Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol. 2006;5(7):578–588. doi: 10.1016/S1474-4422(06)70475-6. [DOI] [PubMed] [Google Scholar]
- 36.Vitek JL. Deep Brain Stimulation for Parkinson's Disease: A Critical Re-Evaluation of STN versus GPi DBS. Stereotact Funct Neurosurg. 2002;78:119–131. doi: 10.1159/000068959. [DOI] [PubMed] [Google Scholar]
- 37.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: A systematic review. Parkinsonism Relat Disord. 2006 doi: 10.1016/j.parkreldis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Hershey T, Revilla FJ, Wernle A, Gibson PS, Dowling JL, Perlmutter JS. Stimulation of STN impairs aspects of cognitive control in PD. Neurology. 2004;62(7):1110–1114. doi: 10.1212/01.wnl.0000118202.19098.10. [DOI] [PubMed] [Google Scholar]
- 39.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9(3):187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 40.Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson's disease. Brain. 2007;130(Pt 9):2433–2440. doi: 10.1093/brain/awm178. [DOI] [PubMed] [Google Scholar]


