Abstract
Background
The Lung Cancer Exercise Training Study (LUNGEVITY) is a randomized trial to investigate the efficacy of different types of exercise training on cardiorespiratory fitness (VO2peak), patient-reported outcomes, and the organ components that govern VO2peak in post-operative non-small cell lung cancer (NSCLC) patients.
Methods/Design
Using a single-center, randomized design, 160 subjects (40 patients/study arm) with histologically confirmed stage I-IIIA NSCLC following curative-intent complete surgical resection at Duke University Medical Center (DUMC) will be potentially eligible for this trial. Following baseline assessments, eligible participants will be randomly assigned to one of four conditions: (1) aerobic training alone, (2) resistance training alone, (3) the combination of aerobic and resistance training, or (4) attention-control (progressive stretching). The ultimate goal for all exercise training groups will be 3 supervised exercise sessions per week an intensity above 70% of the individually determined VO2peak for aerobic training and an intensity between 60 and 80% of one-repetition maximum for resistance training, for 30-45 minutes/session. Progressive stretching will be matched to the exercise groups in terms of program length (i.e., 16 weeks), social interaction (participants will receive one-on-one instruction), and duration (30-45 mins/session). The primary study endpoint is VO2peak. Secondary endpoints include: patient-reported outcomes (PROs) (e.g., quality of life, fatigue, depression, etc.) and organ components of the oxygen cascade (i.e., pulmonary function, cardiac function, skeletal muscle function). All endpoints will be assessed at baseline and postintervention (16 weeks). Substudies will include genetic studies regarding individual responses to an exercise stimulus, theoretical determinants of exercise adherence, examination of the psychological mediators of the exercise - PRO relationship, and exercise-induced changes in gene expression.
Discussion
VO2peak is becoming increasingly recognized as an outcome of major importance in NSCLC. LUNGEVITY will identify the optimal form of exercise training for NSCLC survivors as well as provide insight into the physiological mechanisms underlying this effect. Overall, this study will contribute to the establishment of clinical exercise therapy rehabilitation guidelines for patients across the entire NSCLC continuum.
Trial Registration
NCT00018255
Background
Improvements in surgical techniques together with more effective adjuvant chemotherapeutic regimens has led to significant survival benefit for individuals with non-small cell lung cancer (NSCLC). Approximately 26,000 individuals per year in the United States will survive more than 5 years after initial diagnosis of operable disease. With improving prognosis, acute and long-term disease - and treatment-related morbidity (symptom control) and mortality are now recognized as issues of major clinical importance in the multidisciplinary management of operable NSCLC [1-6]. A parameter of central importance that may mediate acute and late-occurring disease and treatment-related toxicity in lung cancer survivorship is cardiorespiratory fitness. Cardiorespiratory fitness, as measured by an objective exercise tolerance test, reflects the integrative capacity of components in the oxygen (O2) cascade to supply adequate O2 for adenosine triphosphate (ATP) resynthesis. Peak oxygen consumption (VO2peak) provides the gold standard (direct) assessment of cardiorespiratory fitness. Direct or estimated measurement of cardiorespiratory fitness is a well-established independent predictor of mortality in a broad range of non-cancer, adult populations [7,8].
Not surprisingly, operable NSCLC patients have significant and marked reductions in VO2peak. Postsurgical NSCLC patients are subject to the effects of normal ageing, age-related and/or disease-related comorbid conditions, and deconditioning that adversely impact components of the O2 cascade. However, these 'normal' consequences are profoundly accelerated by disease pathophysiology and the use of conventional adjuvant therapy to create a 'perfect deconditioning storm', reducing either the body's ability to deliver and/or utilize O2 and substrate leading to poor VO2peak[9]. Such effects have important implications across the entire NSCLC continuum[10].
First, preoperative VO2peak is a well-established risk stratification tool to determine perioperaitve and postoperative complication risk [11-14]. Second, following resection, VO2peak as well as self-reported exercise behavior (a major determinant of VO2peak), are strong predictors of patient-reported outcomes (PROs) such as overall QOL, fatigue, and other QOL domains[15]. Finally, our group found that pre-operative VO2peak is a strong independent predictor of overall survival in NSCLC surgical candidates even when controlling for performance status, gender, and age[16]. In totality, these data provide strong evidence that VO2peak is an attractive modifiable therapeutic target to improve surgical risk and/or recovery, symptom control and possibly, disease outcome in NSCLC.
Chronic, repeated aerobic training (i.e., continuous activity involving large muscle groups) is widely established as the most effective method to improve VO2peak in healthy humans although a paucity of studies have investigated the role of exercise in NSCLC[10]. Given the preliminary nature of this field, we recently completed two uncontrolled pilot studies investigating the feasibility and preliminary efficacy of supervised aerobic training in the pre-operative and post-operative setting in NSCLC. Results of these pilot studies provided 'proof of principle' that aerobic training is a safe and feasible intervention for NSCLC patients, however, the improvements in VO2peak were modest (<15%), particularly in the post-operative setting (~10%) despite good exercise adherence rates (≥70% of planned sessions) [17,18].
The reasons for the relatively modest improvement in VO2peak in NSCLC relative to other clinical populations (i.e., ~15%-20% improvement in VO2peak following traditional aerobic training recommendations) remain to be elucidated. An obvious potential explanation is a ventilatory limitation or inadequate gas exchange following removal of a substantial portion of lung parenchyma. However, several elegant studies have demonstrated that VO2peak is not limited by ventilation or diffusion capacity [19-22] suggesting that exercise-induced adaptations (or lack thereof) in the other organ components of the O2 cascade are responsible. VO2peak in NSCLC patients is likely principally governed by poor cardiovascular O2 delivery and oxidative capacity as well as unfavorable fiber type distribution and muscle atrophy/weakness similar to the limitations to exercise described in patients with chronic obstructive pulmonary disease (COPD). Major contributors to skeletal muscle dysfunction in NSCLC likely include direct skeletal myopathy (from the use of oral corticosteroids), deconditioning (from physical inactivity), and high levels of systemic inflammation (from underlying disease and therapy)[23].
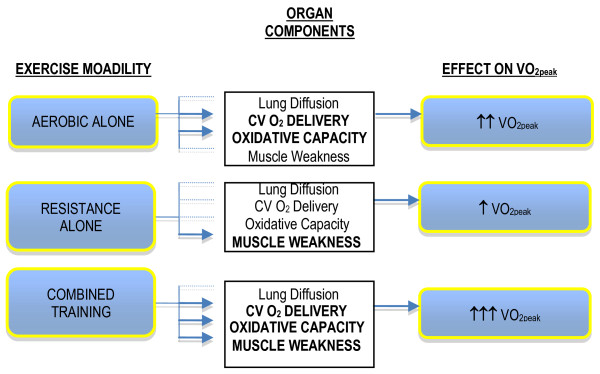
Importantly, aerobic training will cause favorable adaptations in most O2 transport components but will not reverse skeletal muscle atrophy/weakness and will only partially reverse a more glycolytic fiber type distribution. Thus, aerobic training alone may be insufficient to ameliorate skeletal muscle dysfunction likely manifest in NSCLC. Standard resistance training (i.e., activity involving the acute exertion of force) performed according to standard guidelines (i.e., 2-5 times/week, 50%-80% of 1 repetition maximum for 12-24 weeks) is unequivocally acknowledged as the most effective method to improve skeletal muscle function in human subjects [24-28]. Moreover, in severely deconditioned adults, resistance training causes improvements in VO2peak [29-34] although the mechanisms underlying this effect are not clearly understood. In theory, while aerobic and resistance training might independently improve VO2peak in NSCLC, such improvements are likely to be modest (~10%). Instead, the combination of aerobic and resistance training may be the most effective form of exercise training to optimally augment VO2peak. The complementary physiologic adaptations from the combination approach will result in higher cardiovascular O2 delivery, skeletal muscle oxidative phosphoryation, muscle strength and optimal fiber type composition leading to higher muscle endurance, reduced fatiguability, a higher threshold to the metabolic waste products of exercise, and reduced ventilatory requirements during exercise. This approach is hypothesized to maximize physiologic adaptations in the principal factors underlying poor VO2peak in postsurgical NSCLC patients more effectively than either exercise modality alone (Figure 1).
Figure 1.
Hypothesized effects of aerobic training alone, resistance training alone, and aerobic plus resistance training on the components of the oxygen cascade and resultant impact on exercise tolerance (VO2peak).
Against this background, we designed the Lung Cancer Exercise Training Study (LUNGEVITY), a randomized trial to investigate the efficacy of different types of exercise training in post-operative NSCLC patients. The fundamental rationale for this single-center trial is to identify the optimal type of exercise training to improve VO2peak in postoperative NSCLC patients and understand the physiologic mechanisms underlying this effect. The specific aims are: (1) to compare the effects of aerobic training alone, resistance training alone, or both, relative to attention-control, on VO2peak, (2) determine the effects on the mechanisms that govern VO2peak (measurements of the heart-lung-skeletal muscle axis), and (3) to compare the effects on PROs.
Methods/Design
Participants and Setting
In LUNGEVITY, we will recruit and randomize 160 subjects (40 patients/study arm) with histologically confirmed stage I-IIIA NSCLC following curative-intent complete surgical resection at Duke University Medical Center (DUMC). The DUMC institutional review board approved the study and written informed consent will be obtained from all participants prior to initiation of any study procedures. Additional inclusion and exclusion criteria are described in Table 1.
Table 1.
Subject Eligibility Criteria
| Inclusion Criteria | |
| At least 21 years old. | |
| An interval of at least 6 months following surgical resection. | VO2peak has been shown to (spontaneously) recover, to a limited degree, immediately following (~3 months) and stabilize at ~6 months post pulmonary resection. Thus, to accurately determine the effects of exercise training on VO2peak in this setting, we felt it was critically important to initiate study procedures (i.e., recruit and randomize patients) once changes in VO2peak have stabilized to minimize the effects of natural postsurgical recovery on improvements in VO2peak, |
| An interval of no longer than 36 months post-resection | |
| Karnofsky performance status of at least 70% at study entry | |
| Estimated life expectancy of ≥6 months | |
| Ability to read and understand English | |
| Primary attending oncologist approval | |
| Sedentary | Patients not performing regular exercise. Regular exercise is defined as ≥5 days a week, ≥30 minutes each session, at a moderate or vigorous intensity for the past month). |
| Willingness to be randomized | |
| Signed informed consent prior to initiation of study-related procedures | |
| Reside within driving distance of DUMC, as necessitated by the clinic-based assessments and supervised exercise training interventions | |
| Exclusion Criteria | |
| Presence of a concurrent, actively treated other malignancy or history of other malignancy treated within the past 3 years (other than non-melanoma skin cancer) | |
| Presence of metastatic disease | |
| Scheduled to receive any form of adjuvant cancer therapy | |
| Contraindications to maximal exercise testing as recommended by the American Thoracic Society and exercise testing guidelines for cancer patients |
Procedures
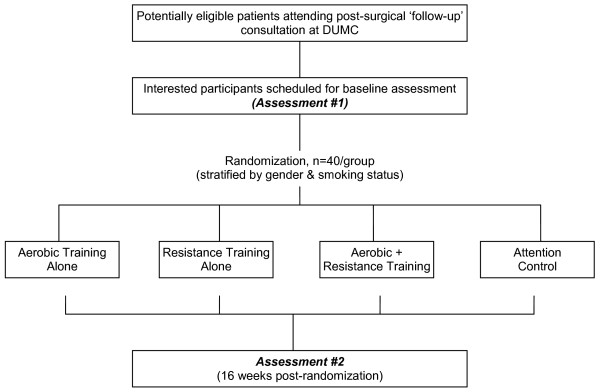
The study will be conducted in accordance with the CONSORT (Consolidated Standards of Reporting Trials) statement for non-pharmacologic interventions[35]. The study flow is presented in Figure 2. Using a 4-arm, randomized design, potential subjects will be identified and screened for eligibility by the study research coordinators via medical record review of NSCLC patients scheduled to attend a 'follow-up' consultation at DUMC as per standard of care. Following the consultation and primary attending oncologist approval, potential eligible subjects will be provided with a thorough review of the study by the study coordinators and asked if they are willing to participate. Interested participants will be provided with the study consent and baseline study questionnaire. Two to five days following the consultation, interested participants will be contacted by telephone by the study coordinators to answer any questions and to schedule the baseline assessment visit. In addition, to broaden recruitment efforts, subjects previously diagnosed with stage I-IIIA NSCLC within 36 months of diagnosis will be identified through the DUMC tumor registry. Permission to contact potential subjects will be obtained from their attending oncologist and letters of study invitation describing the study co-signed by the principal investigator and attending oncologist will be mailed to all potential subjects. Interested participants will be able to call a toll-free telephone number to obtain more information about study participation.
Figure 2.
Study Flow.
At the baseline study visit, all participants will complete the following assessments in order of presentation: (1) fasting blood draw, (2) pulmonary function test (3) cardiopulmonary exercise test, and (4) echocardiogram at rest and exercise. On the following day, patients will perform the following assessments: (1) one repetition maximum lower extremity strength test, and (2) tissue biopsy of the vastus lateralis. Participants will be asked to adhere to a water-only fast for 8 hours prior to testing on both days. All baseline assessments will be repeated at the end of the intervention (i.e., 16 weeks). To maximize internal validity, study endpoint assessments will be conducted by the same personnel, with the same equipment, in the same order (timing) at baseline and follow-up timepoints.
Group Allocation (Randomization)
Following the successful completion of baseline assessments, participants will be randomly allocated, on an individual basis, to one of the three exercise interventions (i.e., aerobic training, resistance training, the combination of aerobic and resistance training) or an attention-control group. Randomly allocated participants will remain in the same group for the entire duration of the intervention (i.e., no cross-over). To ensure randomized groups are similar at baseline, patient randomization will be stratified based on gender (men vs. female) and smoking status (current vs. non-smoking). For each gender-smoking status participant subgroup, a computer-generated list of random treatment assignments will be created by Dr. Peterson (trial biostatistician) in sequentially numbered sealed envelopes. The random allocation sequences will be concealed from all study personnel (except Dr. Peterson). A permuted block design with allocation weight of 1:1:1:1 will be used to generate the treatment assignments.
Blinding/Masking
Blinding study participants to treatment allocation in clinical trials of exercise training is not possible since participants are aware whether they are exercising or not. Also, it is not possible to blind study personnel monitoring exercise training sessions to the participant group assignment since study personnel are aware whether a participant is performing aerobic or resistance training. Nevertheless, all study personnel conducting the study assessments at baseline and postintervention will be blinded to treatment assignment for the duration of the study. Only the trial statistician and the data monitoring committee will have access to unblinded data, but none will have any contact with study participants or study personnel.
Exercise Training Protocols (General Considerations)
The ultimate goal for all exercise training groups will be 3 supervised exercise sessions per week an intensity above 70% of the individually determined VO2peak for aerobic training and an intensity between 60 and 80% of one-repetition maximum for resistance training, for 30-90 minutes/session. All the exercise interventions are designed such that participants begin exercising at a low intensity (~50%-60% VO2peak) that is subsequently increased to more moderate to vigorous intensity (~70%-80% VO2peak) when appropriate. All interventions will be individually tailored to each patient following the principles of aerobic or resistance training prescription guidelines for adults as recommended by the American College of Sports Medicine (ACSM)[24]. All exercise sessions will also be performed in a supervised setting with one-on-one supervision by an ACSM-certified exercise physiologist. Supervised exercise training sessions are critical to ensure a robust test of exercise training efficacy and safety in this setting.
All exercise sessions will include a 5-min warm-up and 5-min cool down on either a treadmill or cycle ergometer at the beginning and end of each training session, respectively. Heart rate and O2 saturation will be monitored continuously throughout exercise while blood pressure will be assessed prior, during, and immediately following each exercise session. To monitor any exercise performed outside of the supervised sessions, participants will be provided with a heart rate monitor as well as a study exercise log-book to record all sessions.
Study Arm 1: Aerobic Training Alone
Aerobic training will be prescribed according to ASCM guidelines and aimed at increasing VO2peak. The ultimate goal for aerobic training alone is 3 cycle ergometry sessions/week at an intensity above 70% of VO2peak for 30-60 minutes/session. Cycle ergometry was chosen as the mode of aerobic training because exercise prescriptions can be more accurately prescribed and monitored on cycle ergometers than treadmill walking. Also, treadmill walking requires considerable balance; lung cancer patients are typically older, have balance issues, and typically have limited experience with treadmill exercise. Specific details of the aerobic training prescription are provided in Table 2.
Table 2.
Aerobic Training Alone Program
| Supervised Aerobic Training | ||||
|---|---|---|---|---|
| Training Phase | Week | Weekly Sessions | Duration (min/session) | Intensity (% of VO2peak) |
| Introductory | 0-4 | 2-3 | 15-25 | 50-60 |
| Intermediate | 5-8 | 3 | 20-30 | 60-70 |
| Maintenance | 9-16 | 3 | 25-45 | ≥70 |
*Weeks intervals shown are goals and may vary for individual patients; All exercise sessions must be performed in a supervised setting.
Study Arm 2: Resistance Training Alone
Resistance training will be prescribed according the ASCM guidelines and aimed at increasing VO2peak. The ultimate goal for resistance training alone is 3 resistance training sessions/week at an intensity above 60% to 80% of one-repetition maximum (1-RM) for 30-60 minutes/session (Table 3). Resistance training will be performed on stationary weight machines (i.e., no free weights). Patients will be progressively trained to perform three sets of 8 to 12 repetitions of 8 resistance exercise alternating between lower and upper body muscle groups.
Table 3.
Resistance Training Alone Program
| Supervised Resistance Training | |||||
|---|---|---|---|---|---|
| Training Phase | Week | Weekly Sessions | Sets, n | Repetitions, n | Weight, maximum repetitions |
| Introductory | 0-4 | 2-3 | 1-2 | 8-12 | 12 |
| Intermediate | 5-8 | 3 | 2-3 | 8-12 | 12 |
| Maintenance | 9-16 | 3 | 3 | 8-10 | 10 |
*Weeks intervals shown are goals and may vary for individual patients; All exercise sessions must be performed in a supervised setting.
Study Arm 3: Combined Aerobic and Resistance Training
Combined aerobic and resistance training will be prescribed according the ASCM guidelines and aimed at increasing VO2peak. The ultimate goal will be three combined exercise sessions per week at an intensity above 60% VO2peak and above 60% 1-RM for aerobic and resistance training, respectively for 30-60 minutes/session. The general design of the combined intervention is provided in Table 4 and follows similar intensities to the single modality training. In this arm, the duration of aerobic training and resistance training will not be added together but rather the exercise prescription is designed to exploit the complementary properties of aerobic and resistance training to optimally improve VO2peak. The prescription will be balanced to ensure that on days when aerobic training is prescribed at a high-intensity, resistance training (on the same day) will be conducted at a lower (easy) intensity and vice versa. This approach will optimize the desired adaptations without causing excessive fatigue and will help avoid potential interference effects between aerobic and resistance training.
Table 4.
Combined Aerobic and Resistance Training Program
| Aerobic Training | Resistance Training | |||||||
|---|---|---|---|---|---|---|---|---|
| Training Phase | Wk* | Weekly Sessions | Duration (min/session) | Intensity of VO2peak | Duration (min/session) | Sets, n | Repetitions, n | Weight, max repetitions |
| Introductory | 0-4 | 2 | 15-25 | 50-60 | 15-20 | 1 | 8-12 | 12 |
| Intermediate | 5-9 | 3 | 20-30 | >70 | ~25 | 2 | 8-12 | 12 |
| Maintenance | 10-24 | 3 | 30-45 | >70 | ~30 | 2 | 8-10 | 10 |
*Week intervals shown are goals and may vary for individual patients. All exercise sessions must be performed in a supervised setting.
Attention Control Group (Study Arm 4)
Subjects assigned to the attention-control group will perform supervised progressive stretching matched to the exercise interventions in terms of program length (i.e., 16 weeks), social interaction (participants will receive one-on-one instruction), and duration (30-45 mins/session). The progressive stretching program will be prescribed according the ASCM guidelines for older adults and aimed at increasing whole-body flexibility. The ultimate goal for the progressive stretching program is 3 stretching sessions/week for 30-60 minutes/session for 16 weeks (Table 5). Stretching will be performed supine on stretching mats (i.e., no machines). Patients will be progressively trained to perform 8 stretching exercises alternating between lower and upper body muscle groups/joints.
Table 5.
Attention Control Program
| Supervised Aerobic Training | ||||
|---|---|---|---|---|
| Training Phase | Week | Weekly Sessions | Duration (min/session) | Duration of each Stretch (mins) |
| Introductory | 0-4 | 2-3 | 15-25 | 1-2 |
| Intermediate | 5-8 | 3 | 20-30 | 2-5 |
| Maintenance | 9-16 | 3 | 25-45 | ≥5 |
*Weeks intervals shown are goals and may vary for individual patients; All exercise sessions must be performed in a supervised setting.
Adherence Considerations
To maximize adherence, several strategies will be employed including individualized attention at the intervention sessions, telephone calls following missed sessions, individuals meetings to outline goals and providing feedback on study progress. In addition, participants will be asked to perform 3 supervised exercise/stretching sessions per week over a 7-day period, and will be allowed to make-up missed sessions within the 16-week study period. Also, participants will be allowed to schedule supervised exercise training sessions at anytime from 7 am to 7 pm. This degree of scheduling flexibility allows participants to perform exercise training sessions at a convenient time and work around other competing demands such as medical appointments, work, and family commitments. Finally, the study team, consisting of the PI, the exercise physiologist and study coordinator, will also meet on a weekly basis to review each participant's adherence with weekly program goals.
Study Endpoints and Assessments
Table 6 outlines the study assessment schedule while a brief description of study endpoints and endpoint assessments including sub-studies is provided in Table 7.
Table 6.
Study Assessment Schedule
| Baseline | Postintervention (16 weeks) | ||||||
|---|---|---|---|---|---|---|---|
| Assessment | Screening | Day 0 | Day 1 | Day 2 | Day 3-5 | Day 112 | Day 113 |
| Chart Review | x | ||||||
| Patient Approached | x | ||||||
| Informed Consent | x | ||||||
| Day 1 Testing | |||||||
| Blood draw | x | x | |||||
| Body Composition | x | x | |||||
| Pulmonary Function | x | x | |||||
| CPET | x | x | |||||
| Echocardiogram | x | x | |||||
| Day 2 Testing | |||||||
| Strength testing | x | x | |||||
| Muscle biopsy | x | x | |||||
| Day 3 Testing | |||||||
| Repeat CPET | x | ||||||
| Randomization | x | ||||||
| Intervention initiation | x | ||||||
Table 7.
Study Measurements and Sub-Studies
| Physical measurements and tests |
| Height, weight, and body mass index |
| Body composition |
| Resting and exercise heart rate |
| Resting and exercise blood pressure |
| Resting and exercise 12-lead ECG |
| Peak and submaximal oxygen consumption |
| Ventilatory threshold |
| Mechanistic physiological measurements |
| Resting and exercise echocardiogram |
| Skeletal muscle function (fiber type distribution, oxidative capacity, and muscular strength) |
| Pulmonary function |
| Hemoglobin concentration |
| Resting and exercise oxygen saturation |
| Each intervention (exercise or attention control) session: heart rate, blood pressure, oxygen saturation, RPE |
| Patient-reported outcomes (questionnaire-based) |
| Medications |
| Quality of life |
| Fatigue |
| Dyspnea |
| Depression |
| Adverse events (Common Terminology Criteria for Adverse Events; CTCAE v.4.0) |
| Substudies |
| Genetic studies to examine differences to exercise stimulus |
| Predictors of exercise adherence |
| Pilot studies for exercise-induced changes in peripheral gene expression |
Primary Endpoint
VO2peak will be evaluated using a physician-supervised incremental cycle ergometer test with 12-lead ECG monitoring (Mac® 5000, GE Healthcare) will be performed by ACSM-certified exercise physiologists blinded to the patient's randomization group. Expired gases will be analyzed continuously by a metabolic measurement system (ParvoMedics TrueMax, Sandy, UT). Subjects will begin pedaling at 20 Watts for one minute and is increased 5 to 20 Watts every minute until exhaustion or a symptom-limited VO2peak is achieved. This protocol has been previously demonstrated to be appropriate for measuring VO2peak in our prior studies among NSCLC patients[18,36-38]. Exercise will be terminated if any ECG abnormalities are observed.
Secondary Endpoints
Patient Reported Outcomes will include QOL, fatigue, dyspnea, and depression. QOL will be assessed using the Functional Assessment of Cancer Therapy - Lung (FACT-L) scale developed for the assessment of patient symptoms and QOL in lung cancer patients [39]. The FACT-L contains four subscales for physical (7-items), functional (7-items), emotional (6-items), social/family (7-items) well-being plus a lung cancer specific subscale (15-items) which will be summed to obtain the FACT-L score (all 42 items). Fatigue will be assessed using the 13-item FACT-fatigue scale for the assessment of fatigue in cancer patients[40]. Dyspnea will be assessed using the Cancer Dyspnea Scale (CDS)[41]. The CDS is a 12-item scale comprised of three factors (sense of sense/sense of anxiety/sense of discomfort). This instrument has been demonstrated to be a brief, valid, and feasible scale for assessing cancer-related dyspnea among inoperable NSCLC patients[42,43]. Finally, depression will be assessed using the Center for Epidemiologic Studies Depression scale (CES-D)[44]. We have found these instruments to be reliable, valid, responsive, brief, and easy to administer in our on-going study and prior reports in lung cancer patients.
Physiologic Mechanisms of VO2peak will include pulmonary function, cardiovascular O2 delivery, and skeletal muscle function. Pulmonary Function will be determined using standard spirometry to assess FEV1. All measures will be performed in a sitting position according to the American Thoracic Society guidelines [45]. Cardiovascular O2Delivery will be comprised of: (1) Cardiac Output: Left ventricular volumes will be performed with a commercially available ultrasound system (GE Vivid 7 or Philips i33). Apical two- and four- chamber views will be assessed at rest and exercise to determine left ventricular end-diastolic volume and end-systolic volume by modified Simpson's rule [46,47]. Stroke volume will be calculated as end-diastolic volume minus end-systolic volume. Cardiac output will be calculated by stroke volume multiplied by heart rate, (2) Hemoglobin (Hb) Concentration (O2 carrying capacity of blood) will be assessed via a venous blood draw according to standard guidelines. Normal range for hemoglobin is ~13 - 18 grams per deciliter for men and 12 - 16 for women. Mean cell Hb (amount of Hb/red blood cell) and platelet count (number of platelets in blood volume) will also be calculated, and (3) Arterial O2 Saturation will be assessed at rest and continuously during exercise using pulse oximetry (Biox 3700, Ohmeda Medical, Boulder, CO), which provides the most accurate noninvasive assessment of blood arterial O2 saturation levels. Pulse oximetry works on the principle of the red and infrared light absorption characteristics of oxygenated and deoxygenated Hb. Normal range is 96% to 100% at sea level for healthy humans.
Skeletal Muscle Function will be determined by: (1) Fiber Type Distribution will be determined by tissue biopsy of the vastumus laterialis using a modified Bergstrom needle technique [48]. Biopsy sites will be first anesthetized with a 2% lidocaine solution. Next, a 0.5 cm incision will be made through the skin and fascia lata. All samples will be prepared immediately by weighing and then dividing the samples for subsequent analysis as previously described[48]. A portion will be mounted in cross-section in optimal temperature compound (OCT) media immediately prior to being frozen in isopentane cooled by liquid nitrogen for fiber type determination. Specifically, myosin heavy-chain (MHC) isoforms I, IIa, and IIx will be identified by order of migration as described by Duscha et al [48]. Gels will be scanned electronically, and relative proportions of MHC isoform will be measured using NIH image 1.60 for Macintosh and Jandel PeakFit for Windows, (2) Oxidative Capacity (Enzymology) will be assessed via maximal activities of several oxidative pathways representative of different energy pathways using frozen tissue samples as previously described [48]. Phosphofructokinase and succinic dehydrogenase activities will be performed on fresh homogenates while the enzymes malate dehydrogenase and 3-hydoxyl-Co-A dehydrogenase will be performed on the frozen homogenates stored at -80°C, and (3) Lower Extremity Maximal Muscular Strength will be assessed as a voluntary one-repetition maximum (1-RM) using the following exercises: (1) leg press, (2) leg extension, and (3) leg curl. These tests will be repeated twice and the heaviest weight lifted while adhering to strict technique and form will be used as the score.
Tracking and Monitoring of Adverse Events
Tracking and monitoring of adverse events will be assessed using the following methods: (1) during intervention sessions, all patients will receive one-on-one supervision and all adverse events (e.g., knee pain, back pain) will be recorded on the patient case report form (CRF). In addition, heart rate, blood pressure, and O2 saturation will be recorded prior to, during, and following every intervention session, (2) at the beginning of each week, the exercise physiologist will spend the first 10 minutes of every session discussing any potential negative side-effects of the intervention assignment and any injuries that may have occurred. All events will be recorded in the patient CRF, (3) every six months a meeting of all investigators will be scheduled to review and discuss all reported non-serious and serious adverse events for early identification of negative issues and development of solutions. All serious adverse events will be immediately reported to Duke IRB and immediately circulated to all study investigators for appropriate discussion, and (4) early stopping rules in response to a differential higher frequency of adverse events in a particular study group.
Statistical Considerations
Sample Size Calculation
This randomized phase II trial will accrue 160 subjects with postsurgical NSCLC over an accrual period of ~48 months. For each of the primary and secondary endpoints, three separate t-tests will be used to compare each experimental arm to the control arm in mean change across time of the endpoint. For each endpoint, the overall alpha level will be controlled at a two-sided 0.05 by using Holm's procedure[49]. That is, Holm's procedure first ranks the three p-values from lowest to highest. The first (lowest) p-value has to be less than 0.05/3 (0.0167) to be significant and permit continuation to the other t-tests. The Holm's procedure continues sequentially in this fashion using alpha levels of 0.05/2 (0.025) and 0.05/1 (0.05) for the remaining two t-tests, respectively. Power for this study is defined as the probability that at least one of the three t-tests of the arm effect on VO2peak is significant; in other words, power is the probability that the first of the 3 ordered t-tests are significant. We assume that change in VO2peak will have a standard deviation of 3.0 mL.kg.-1min-1 as observed in our pilot work. Statistical power depends upon the configuration of mean change in VO2peak across the 4 arms. Thus, for example, 80% power is obtained when the mean change in VO2peak across Arms 1, 2, 3, and 4 is 0.60, 0.60, 2.10, and 0.0 (mL.kg.-1min-1), respectively.
Analytic Plan
The principal analysis of the study endpoints will employ the intention-to-treat (ITT) approach. The ITT analysis will include all randomized participants in their randomly assigned allocation. The intervention group assignment will not be altered based on the participant's adherence to the randomly allocated study arm. Patients who are lost-to-follow-up will be included in all primary and secondary analyses by assuming zero change across time. For the primary analysis, a multiple regression model will be used to regress change in VO2peak on study group, the baseline value of the endpoint, and other pertinent baseline variables that may influence change in the study endpoints (e.g., co-morbid conditions/medications, self-reported exercise history, age).
Discussion
Methodological Considerations
Several issues were considered when designing LUNGEVITY. First, was the decision to investigate exercise in patients with operable (early) or inoperable (advanced) NSCLC. The majority of patients (~75%) diagnosed with NSCLC present with inoperable (advanced) disease. From a population health perspective, exercise trials in inoperable disease are likely to greater impact in comparison to those in patients with operable disease. Our decision to target early-stage patients was based on several important factors, primarily, exercise training safety and patient eligibility (recruitment). Inoperable NSCLC patients have poor prognosis and commonly present with significant smoking-related comorbid disease, advanced disease and symptoms, and are heavily treated with aggressive chemotherapy and concurrent radiotherapy [37]. As a result, the majority present with KPS scores <70% which may preclude participation in a moderate-intensity exercise training program and increase the risk of an exercise-related adverse [37]. Accordingly, it is prudent to focus on patients with operable disease; these patients have, in general, better performance status (and prognosis) and issues of NSCLC survivorship are becoming increasingly important aspect of multidisciplinary care. A second important consideration was the decision to investigate the efficacy of exercise following completion of, as opposed to, during adjuvant therapy. In our pilot work, aerobic training was associated with a 2% and 11% improvement in VO2peak among patients receiving or not receiving chemotherapy, respectively for postoperative NSCLC[17]. Clearly, without a non-intervention control group, it is not known whether maintenance of VO2peak during chemotherapy is important. Nevertheless, given the lack of benefit of exercise training during chemotherapy, subjects in LUNGEVITY will be recruited ≥6 months following the completion of adjuvant therapy, if appropriate.
Ancillary Studies
A number of ancillary studies are planned for LUNGEVITY. First, as part of the informed consent process, a sample of white cells (buffy coat) will be collected from each subject in order to conduct genetic studies. A question of great interest that has not yet been addressed in the cancer populations is assessing genetic contributions to inter-individual differences in response to exercise training. Second, we will conduct ancillary study to examine predictors of adherence to the interventions as well as extent of exercise 'drop-in' in the attention control group across different exercise protocols in NSCLC. To address this question, we will adopt the guiding principles of the theory of planned behavior (TPB)[50]. This data will be used to inform the design and implementation of future trials. Third, in addition to assessing the physiological mediators of the exercise - VO2peak relationship, LUNGEVITY also provides a unique opportunity to examine which psychological variables that may mediate the exercise - PROs relationship. To this end, we will assess whether self-efficacy, affect, and social support mediate the effect of the exercise interventions and attention-control on anticipated improvements in PROs. Finally, we will include pilot studies to investigate the effects of exercise training on changes in peripheral gene expression (from analysis of whole blood mRNA) using high-density mRNA microarrays. Such studies will provide insight into the molecular mechanistic properties of exercise in NSCLC.
Summary
The past decade has witnessed a dramatic increase in clinical and research interest of the application of exercise following a cancer diagnosis [51-58]. Recent systematic reviews conclude that exercise is a safe and feasible supportive intervention to improve symptom control and cardiorespiratory fitness in cancer patients with early-stage disease either during or following the completion of adjuvant therapy [59-63]. Moreover, recent observational studies provided the first evidence that regular exercise (i.e., 30 minutes of brisk walking at least 5 days/week) may be associated with substantial reductions in cancer-specific mortality and all-cause mortality among patients with early breast and colorectal cancer following the completion of adjuvant therapy [64-68]. Despite this growing evidence, investigation of the role of exercise training following a diagnosis of NSCLC remains limited.
The pathophysiology of NSCLC together with conventional therapeutic management is associated with unique and varying degrees of adverse physiological impairments that dramatically reduce patient's ability to tolerate exercise. Poor VO2peak likely predisposes to increased susceptibility to other common age-related diseases, greater symptoms, poor QOL, and even premature death. In recent years, a limited number of pilot studies have emerged that provide 'proof-of-principle' that supervised aerobic training is a safe and feasible supportive intervention associated with improvements in cardiopulmonary function and select patient-reported outcomes in postsurgical NSCLC. In addition, VO2peak may be a strong, independent predictor of long-term all-cause mortality in this population. Against this background, LUNGEVITY was designed to identify the most effective type of exercise training to improve VO2peak and to elucidate the physiologic mechanisms underlying this improvement. To our knowledge, LUNGEVITY will be the first trial to compare different types of exercise training protocols in NSCLC as well as the first to study the effects of exercise on changes in the organ components that govern exercise tolerance in any cancer population. In totality, LUNGEVITY will address many critical unanswered questions regarding the role and mechanistic properties of exercise in NSCLC and will set the stage for more definite trials. In the long-term, we hope that this research will contribute to the establishment of clinical exercise therapy rehabilitation guidelines for patients across the entire NSCLC continuum.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LWJ: conception and design, drafting of manuscript, and final approval for publication. NDE: conception and design and final approval for publication. WEK: conception and design and final approval for publication. AP: conception and design and final approval for publication. JC: conception and design and final approval for publication. JAB: conception and design and final approval for publication. BLP: drafting of manuscript and final approval for publication. PSD: conception and design, drafting of manuscript, and final approval for publication.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Lee W Jones, Email: jones442@mc.duke.edu.
Neil D Eves, Email: neves@ukin.ucalgary.ca.
William E Kraus, Email: william.kraus@duke.edu.
Anil Potti, Email: anil.potti@duke.edu.
Jeffrey Crawford, Email: jeffrey.crawford@duke.edu.
James A Blumenthal, Email: james.blumenthal@duke.edu.
Bercedis L Peterson, Email: bercedis.peterson@duke.edu.
Pamela S Douglas, Email: pamela.douglas@duke.edu.
Acknowledgements
This study was supported by the National Institutes of Health (R01 CA138624).
References
- Woodward RM, Brown ML, Stewart ST, Cronin KA, Cutler DM. The value of medical interventions for lung cancer in the elderly: results from SEER-CMHSF. Cancer. 2007;110(11):2511–2518. doi: 10.1002/cncr.23058. [DOI] [PubMed] [Google Scholar]
- Kenny PM, King MT, Viney RC, Boyer MJ, Pollicino CA, McLean JM, Fulham MJ, McCaughan BC. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol. 2008;26(2):233–241. doi: 10.1200/JCO.2006.07.7230. [DOI] [PubMed] [Google Scholar]
- Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol. 2007;14(5):173–179. doi: 10.3747/co.2007.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull DE, Thomas ML, Meade GE, Updyke GM, Arocho MA, Chin HW, Adebonojo SA, Little AG. Determinants of quality of life in patients following pulmonary resection for lung cancer. Am J Surg. 2006;192(5):565–571. doi: 10.1016/j.amjsurg.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Rumble ME, Keefe FJ, Edinger JD, Porter LS, Garst JL. A pilot study investigating the utility of the cognitive-behavioral model of insomnia in early-stage lung cancer patients. J Pain Symptom Manage. 2005;30(2):160–169. doi: 10.1016/j.jpainsymman.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Li WW, Lee TW, Yim AP. Quality of life after lung cancer resection. Thorac Surg Clin. 2004;14(3):353–365. doi: 10.1016/S1547-4127(04)00023-4. [DOI] [PubMed] [Google Scholar]
- Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106(6):666–671. doi: 10.1161/01.CIR.0000024413.15949.ED. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Waner E, Joy AA. Exercise therapy across the lung cancer continuum. Curr Oncol Rep. 2009;11(4):255–262. doi: 10.1007/s11912-009-0036-0. [DOI] [PubMed] [Google Scholar]
- Win T, Jackson A, Groves AM, Sharples LD, Charman SC, Laroche CM. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax. 2006;61(1):57–60. doi: 10.1136/thx.2005.043547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckles MA, Spiro SG, Colice GL, Rudd RM. The physiologic evaluation of patients with lung cancer being considered for resectional surgery. Chest. 2003;123(1 Suppl):105S–114S. doi: 10.1378/chest.123.1_suppl.105S. [DOI] [PubMed] [Google Scholar]
- Beckles MA, Spiro SG, Colice GL, Rudd RM. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123(1 Suppl):97S–104S. doi: 10.1378/chest.123.1_suppl.97S. [DOI] [PubMed] [Google Scholar]
- Dales RE, Dionne G, Leech JA, Lunau M, Schweitzer I. Preoperative prediction of pulmonary complications following thoracic surgery. Chest. 1993;104(1):155–159. doi: 10.1378/chest.104.1.155. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, Mabe S, Harpole D, Kraus WE, Douglas PS. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical non-small cell lung cancer patients: A pilot study. Cancer. 2008;113(12):3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- Kohman L, Watson D, Herndon JE, Eves ND, Haithcock B, Loewen G, Jones LW. CALGB 140803 - Association between cardiorespiratory fitness and overall survival in operable lung cancer patients: ancillary analysis of protocol 9238. J Clin Oncol. 2009;27(15s) [Google Scholar]
- Jones LW, Eves ND, Peterson BL, Garst J, Crawford J, West MJ, Mabe S, Harpole D, Kraus WE, Douglas PS. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: a pilot study. Cancer. 2008;113(12):3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- Jones LW, Peddle CJ, Eves ND, Haykowsky MJ, Courneya KS, Mackey JR, Joy AA, Kumar V, Winton TW, Reiman T. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007;110(3):590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- Degraff AC Jr, Taylor HF, Ord JW, Chuang TH, Johnson RL Jr. Exercise Limitation Following Extensive Pulmonary Resection. J Clin Invest. 1965;44:1514–1522. doi: 10.1172/JCI105258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia CC, Carlin JI, Ramanathan M, Cassidy SS, Johnson RL Jr. Estimation of diffusion limitation after pneumonectomy from carbon monoxide diffusing capacity. Respir Physiol. 1991;83(1):11–21. doi: 10.1016/0034-5687(91)90089-2. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Herazo LF, Ramanathan M, Johnson RL Jr. Cardiopulmonary adaptations to pneumonectomy in dogs. IV. Membrane diffusing capacity and capillary blood volume. J Appl Physiol. 1994;77(2):998–1005. doi: 10.1152/jappl.1994.77.2.998. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Dane DM, Estrera AS, Wagner HE, Wagner PD, Johnson RL Jr. Shifting sources of functional limitation following extensive (70%) lung resection. J Appl Physiol. 2008;104(4):1069–1079. doi: 10.1152/japplphysiol.01198.2007. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11(6):681–686. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101(7):828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- Fodor JG, Whitmore B, Leenen F, Larochelle P. Lifestyle modifications to prevent and control hypertension. 5. Recommendations on dietary salt. Canadian Hypertension Society, Canadian Coalition for High Blood Pressure Prevention and Control, Laboratory Centre for Disease Control at Health Canada, Heart and Stroke Foundation of Canada. Cmaj. 1999;160(9 Suppl):S29–34. [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Kraemer WJ. Performance and physiologic adaptations to resistance training. Am J Phys Med Rehabil. 2002;81(11 Suppl):S3–16. doi: 10.1097/00002060-200211001-00003. [DOI] [PubMed] [Google Scholar]
- King L. The effects of resistance exercise on skeletal muscle abnormalities in patients with advanced heart failure. Prog Cardiovasc Nurs. 2001;16(4):142–151. doi: 10.1111/j.0889-7204.2001.00616.x. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol. 1997;82(4):1305–1310. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Thomas SG, Goodman JM, Plyley MJ. Quantitating the capillary supply and the response to resistance training in older men. Pflugers Arch. 1997;433(3):238–244. doi: 10.1007/s004240050273. [DOI] [PubMed] [Google Scholar]
- Hagerman FC, Walsh SJ, Staron RS, Hikida RS, Gilders RM, Murray TF, Toma K, Ragg KE. Effects of high-intensity resistance training on untrained older men. I. Strength, cardiovascular, and metabolic responses. J Gerontol A Biol Sci Med Sci. 2000;55(7):B336–346. doi: 10.1093/gerona/55.7.b336. [DOI] [PubMed] [Google Scholar]
- Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS. Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol. 2002;88(1-2):50–60. doi: 10.1007/s00421-002-0681-6. [DOI] [PubMed] [Google Scholar]
- Haykowsky M, Muhll I Vonder, Ezekowitz J, Armstrong P. Supervised exercise training improves aerobic capacity and muscle strength in older women with heart failure. Can J Cardiol. 2005;21(14):1277–1280. [PubMed] [Google Scholar]
- Lawrenson L, Hoff J, Richardson RS. Aging attenuates vascular and metabolic plasticity but does not limit improvement in muscle VO(2) max. Am J Physiol Heart Circ Physiol. 2004;286(4):H1565–1572. doi: 10.1152/ajpheart.01070.2003. [DOI] [PubMed] [Google Scholar]
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Hayowsky M, Joy AA, Douglas PS. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9(8):757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Mackey JR, Peddle CJ, Haykowsky M, Joy AA, Courneya KS, Tankel K, Spratlin J, Reiman T. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. 2007;55(2):225–232. doi: 10.1016/j.lungcan.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Jones LW, Garst J, Eves ND, West M, Mabe S, Crawford J. Effect of aerobic exericse training on cardiorespiratory fitness and quality of life in postsurgical non-small cell lung cancer patients. Journal of Clinical Oncology: 2008 ASCO Annual Meeting Proceedings (abstract #7577) 2008.
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-F. [DOI] [PubMed] [Google Scholar]
- Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19. [PubMed] [Google Scholar]
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82(4):800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario A, Ferri L, Galetta D, Pasqua F, Bonassi S, Clini E, Biscione G, Cardaci V, di Toro S, Zarzana A. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57(1):118–9. doi: 10.1016/j.lungcan.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C, A'Hern R. Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. Bmj. 1999;318(7188):901–904. doi: 10.1136/bmj.318.7188.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128(6):1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- Kreider ME, Grippi MA. Impact of the new ATS/ERS pulmonary function test interpretation guidelines. Respir Med. 2007;101(11):2336–2342. doi: 10.1016/j.rmed.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Parisi AF, Moynihan PF, Feldman CL, Folland ED. Approaches to determination of left ventricular volume and ejection fraction by real-time two-dimensional echocardiography. Clin Cardiol. 1979;2(4):257–263. doi: 10.1002/clc.4960020404. [DOI] [PubMed] [Google Scholar]
- Schiller NB, Acquatella H, Ports TA, Drew D, Goerke J, Ringertz H, Silverman NH, Brundage B, Botvinick EH, Boswell R. Left ventricular volume from paired biplane two-dimensional echocardiography. Circulation. 1979;60(3):547–555. doi: 10.1161/01.cir.60.3.547. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39(7):1170–1174. doi: 10.1016/S0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Ajzen I. Nature and operation of attitudes. Annu Rev Psychol. 2001;52:27–58. doi: 10.1146/annurev.psych.52.1.27. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, Dipietro L, Mayne ST, Yu H. Randomized Controlled Trial of Aerobic Exercise on Insulin and Insulin-like Growth Factors in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15(1):45–51. doi: 10.1111/j.1524-4741.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- Reigle BS, Wonders K. Breast cancer and the role of exercise in women. Methods Mol Biol. 2009;472:169–189. doi: 10.1007/978-1-60327-492-0_7. full_text. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Partridge A, Giobbie-Hurder A, Golshan M, Emmons K, Winer EP. Physical Activity Behaviors in Women with Newly Diagnosed Ductal Carcinoma-In-Situ. Ann Surg Oncol. 2008;20(8):1523–8. doi: 10.1245/s10434-008-0174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, Meeske KA, Bernstein L, Baumgartner KB, Ballard-Barbash R. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: a prospective analysis. J Cancer Surviv. 2007;1(2):116–128. doi: 10.1007/s11764-007-0014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CM, Day JM, Katz ML, Herndon JE, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology. 2009;18(2):128–133. doi: 10.1002/pon.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, Snyder DC, Giguere JK, Shaw E. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8(1):70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, Adloff K, Keshaviah A, Winer EP. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- Ballard-Barbash R, McTiernan A. Is the whole larger than the sum of the parts? The promise of combining physical activity and diet to improve cancer outcomes. J Clin Oncol. 2007;25(17):2335–2337. doi: 10.1200/JCO.2007.10.7326. [DOI] [PubMed] [Google Scholar]
- Ingram C, Courneya KS, Kingston D. The effects of exercise on body weight and composition in breast cancer survivors: an integrative systematic review. Oncol Nurs Forum. 2006;33(5):937–947. doi: 10.1188/06.ONF.937-950. quiz 948-950. [DOI] [PubMed] [Google Scholar]
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol. 2006;7(12):1017–1026. doi: 10.1016/S1470-2045(06)70976-7. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Weltzien E, Quesenberry CP Jr, Castillo AL, Kwan M, Slattery ML, Caan BJ. Physical Activity and Risk of Recurrence and Mortality in Breast Cancer Survivors: Findings from the LACE Study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, Baron JA, Egan KM, Willett WC. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, Al-Delaimy WK, Thomson CA, Kealey S, Hajek R. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]