Abstract
RACK1 (Receptor for Activated C-Kinase 1), an anchoring protein that shuttles activated PKC to cellular membranes, plays an important role in PKC-mediated signal transduction pathways. A significant loss of RACK1 has been found in the brain of aging animals and Alzheimer’s disease (AD) patients, which implicates the potential involvement of RACK1 in altered PKC activation associated with dementia. Our previous studies have demonstrated that GABAergic inhibition in prefrontal cortex, which is important for cognitive processes like “working memory”, is regulated by muscarinic receptors via a PKC-dependent mechanism, and this effect is impaired by β-amyloid peptide (Aβ). In this study, we found that Aβ oligomers decreased RACK1 distribution in the membrane fraction of cortical neurons. Moreover, overexpression of RACK1 rescued the effect of muscarinic receptors on GABAergic transmission in Aβ-treated cortical cultures in vitro and Aβ-injected cortical neurons in vivo. These results suggest that the Aβ-induced loss of RACK1 distribution in the cell membrane may underlie the Aβ impairment of muscarinic regulation of PKC and GABAergic transmission. Thus, RACK1 provides a potential therapeutic target that can restore some of the impaired cellular processes by Aβ.
Keywords: Aβ, RACK1, PKC, muscarinic acetylcholine receptors, IPSC, Sindbis virus, stereotaxic injection
RACK1 is a member of the tryptophan-aspartate (WD) repeat family known for its propeller-like structure (Neer et al., 1994). Like many other WD domain proteins, RACK1 plays different roles upon binding to different partner proteins. RACK1 was first characterized as an intracellular receptor that binds activated PKC and is involved in activation-induced translocation of PKC to the membrane (Mochly-Rosen et al., 1991). RACK1 is present in the particulate fraction when binding activated PKC isozymes (Ron at al., 1994), bringing the signaling enzyme to the appropriate location, in close proximity with its substrate proteins (Jaken and Parker, 2000). In addition to PKC, RACK1 interacts with diverse proteins, including the small subunit of hetero-trimeric G protein Gβ (Dell et al., 2002), IP3 receptors (Patterson et al., 2004), the neuronal transport protein Dynamin 1 (Rodriguez et al., 1999), GABAA receptors (Brandon et al., 1999), and NMDA receptors (Yaka et al., 2002). Thus, RACK1 has been implicated in multiple key neuronal functions, such as intracellular Ca2+ regulation, protein trafficking, synaptic transmission and plasticity (Sklan et al., 2006).
Changes in RACK1 levels have been found in a number of brain pathologies and during aging. For example, several reports show that RACK1 is decreased by ~50% in membrane fractions of aging rat brains (Pascale et al., 1996; Battaini et al., 1997; McCahill et al., 2002). Reports on RACK1 changes in postmortem brains of AD patients are less consistent, with a reduction found in some studies (Battaini et al., 1999), but not others (Shimohama et al., 1998). Moreover, RACK1 levels are significantly decreased in the cortex of Down syndrome patients (Peyrl et al., 2002), all of who develop early onset AD. It suggests that loss of RACK1 may contribute to decreased PKC activity in the aging brain or AD.
The accumulation of β-amyloid (Aβ), a peptide generated from the amyloid precursor protein (APP), is one of the hallmarks of AD (Tanzi and Bertram, 2001; Selkoe and Schenk, 2003). Emerging evidence suggests that Aβ causes “synaptic failure” before the formation of senile plaques and the occurrence of neuron death (Selkoe, 2002). Our previous studies have found that Aβ impairs PKC-dependent regulation of synaptic functions by muscarinic acetylcholine receptors (mAChR) and metabotropic glutamate receptors (mGluR) in cortical neurons (Zhong et al., 2003; Tyszkiewicz and Yan, 2005). However, it is unclear about the mechanism underlying this action of Aβ. In this study, we provide evidence showing that the Aβ-induced impairment of PKC activation and synaptic regulation may be attributed to RACK1 deficit.
MATERIALS AND METHODS
Aβ Oligomer Preparation
Oligomeric Aβ was prepared as what was previously described (Dahlgren et al., 2002; Gu et al., 2009). Briefly, Aβ42 peptide (Sigma) was dissolved in hexafluoroisopropanol (HFIP) to 1 mM. HFIP was then removed under vacuum. The remaining peptide was then dissolved in DMSO to 5 mM and diluted in PBS to 100 μM. The oligermeric Aβ was formed by incubating at 4°C for 24 hr.
Primary Neuronal Culture
All experiments were performed with the approval of the State University of Buffalo Animal Care Committee. Rat cortical cultures were prepared as previously described (Wang et al., 2003). Brief, frontal cortex was dissected from embryonic day 18 embryos, and cells were dissociated using trypsin and triturated through a Pasteur pipette. Neurons were plated on poly-L-lysine coated coverslips in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum at a density of 1×105 cells/cm2. When neurons attached to the coverslip within 24 hr, the medium was changed to Neurobasal medium with B27 supplement (Invitrogen, Carlsbad, CA). Neurons were maintained for 2–3 weeks.
Whole-cell Recordings
Standard voltage-clamp techniques (Liu et al., 2006) were used for whole-cell recordings of spontaneous IPSCs in cultured neurons. The external solution contained (mM): 127 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 12 glucose, pH 7.3–7.4, 300–305 mOsm. The internal solution contained (in mM): 100 CsCl, 30 N-methyl-D-glucamine, 10 HEPES, 1 MgCl2, 4 NaCl,5 EGTA, 0.1 QX314, 12 phosphocreatine, 2 MgATP, 0.2 Na3GTP, 0.1 leupeptin, pH = 7.2–7.3, 265–270 mOsm. The AMPA/KA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) and NMDA receptor antagonist D-aminophosphonovalerate (APV, 25 μM) were present in the external solution throughout the recording. Cells were held at −70 mV.
Whole-cell voltage-clamp techniques were used for recordings of slices from young adult (3–4 weeks postnatal) Sprague-Dawley rats (Zhong et al., 2003). The brain slice (300 μm) was submerged in oxygenated artificial cerebrospinal fluid (ACSF) containing CNQX (10 μM) and APV (25 μM). The internal solution was the same as that used for culture recordings. Cells were visualized with a water-immersion lens and illuminated with near-IR light. Tight seals (2–10 GΩ) from visualized pyramidal neurons were obtained by applying negative pressure. The membrane was disrupted with additional suction and the whole-cell configuration was obtained. The access resistances ranged from 13 to 18 MΩ and were compensated 50–70%. The recording of spontaneous IPSCs were performed on neurons (held at −70 mV) using a Multiclamp 700A amplifier (Axon Instruments).
Subcellular Fractionation of Proteins and Western Blot Analysis
Cultured cortical neurons (DIV 11–16) were treated with Aβ oligomer (1 μM) for 48–72 hrs. After treatment, cultured neurons were homogenized on ice with the lysis buffer (0.3 M sucrose, 0.15 M NaCl, 20 mM Tris-HCl (pH 7.4), 2 mM EDTA, 0.3 mM PMSF, and 10 μg/ml leupeptin). Homogenates were centrifuged at 1,000× g for 10 min at 4°C, and supernatant fractions were collected for ultracentrifugation. Cytosol and membrane fractions were separated by ultracentrifugation at 100,000× g for 90 min at 4°C. The supernatant constituted the cytosol fraction, and the pellet was resuspended and homogenized in the above lysis buffer with 0.2% Triton X-100 added. This resuspended fraction represented the membrane fraction.
Samples were boiled in 2× SDS loading buffer for 5 min, loaded at 30 μg of cytosol fractions and membrane fractions, separated on 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The blots were incubated with primary antibodies, including RACK1 (Santa Cruz), PKC (Santa Cruz), or actin (Santa Cruz). For detecting activated PKC, a phospho-PKC (pan) antibody (Cell Signal) that recognizes PKCα, βI, βII, ε, η, and δ isoforms only when phosphorylated at a carboxy-terminal residue homologous to Ser660 of PKCβII was used in the Western blot analysis. The Aβ antibody (Chemicon, 6E10, 1:500) was used for detecting oligomeric Aβ. After incubation with secondary antibodies conjugated with horseradish peroxidase (Amersham Biosciences), quantification was obtained from densitometric measurements of immunoreactive bands on films.
siRNA Transfection
To knockdown RACK1, the target-specific 19–25 nt siRNA designed against rat RACK1 (Santa Cruz) was transfected into cultured cortical neurons (DIV 11–16) using Lipofectamine 2000 reagent as we described before (Gu et al., 2009). RACK1 siRNA (20 nM) was co-transfected with EGFP (0.5 μg/ml). Cultures were recorded 2–3 days after transfection.
Construction of Sindbis Viruses and in vivo Infection
The cDNAs encoding GFP and GFP-RACK1 were subcloned to pSinRep5 vector (Invitrogen) according to the manufacturer’s protocol. Recombinant GFP-pSinRep5 and GFP-RACK1-pSinRep5 were linearized with NotI. The DH26S plasmid was linearized using XhoI. The linearized templates were transcribed in vitro using mMessage Machine SP6 kit (Ambion) and the RNAs were electroporated into baby hamster kidney (BHK) cells. The extracellular medium containing the recombinant viruses was harvested after 24–48 hr. The medium was concentrated on a discontinuous sucrose gradient (55% and 20% sucrose) using ultracentrifugation (160,000× g, 90 min at 4°C) (Hu et al., 2006).
Cultured cortical neurons (11–16 DIV) were infected by adding the viral suspension directly to the medium (10 μl added to 100 μl culture medium, 1 hr later add 900 μl new medium). After 24 hrs of infection, more than 80% of the neurons appeared GFP positive. Recordings were performed 2–3 days after infection. The virus did not cause apparent toxicity to cultured neurons at least 4 days after infection.
For viral expression in vivo (McCormack et al., 2006; Kopec et al., 2007), rats (3–4 weeks old) were anesthetized by an i.p. injection of pentobarbital (50 mg/kg), and placed on a stereotaxic instrument. The viral suspension (0.5 μl) and Aβ oligomer (100 μM, 0.5 μl) were mixed and injected with a Hamilton syringe (needle gauge 31) at a speed of ~0.2 μl/min, and the needle was kept in place for an additional 5 min. The virus and Aβ were delivered bilaterally to prefrontal cortex using the following coordinates (mm): AP 2.5, ML 0.75, and DV 3.5. After 48–72 hr of injection, the slices from the infected brains were used for recording.
Statistics
Spontaneous synaptic events were analyzed with Mini Analysis Program (Synaptosoft, Leonia, NJ). Statistical comparisons of the amplitude and frequency of mEPSC were made using the Kolmogorov-Smirnov (K-S) test. ANOVA tests were performed to compare the differential degrees of current modulation between groups subjected to different treatment.
RESULTS
Aβ treatment decreased RACK1, PKC and p-PKC in the membrane fraction of cortical cultures
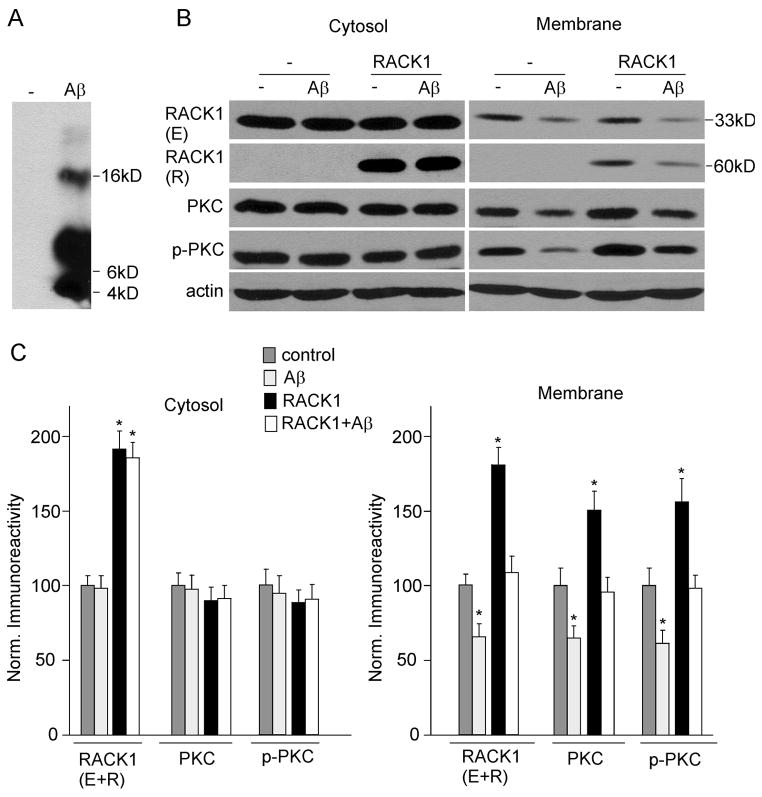
Given the involvement of RACK1 in the activation-induced translocation of PKC to the membrane (Mochly-Rosen et al., 1991), we speculate that RACK1 is potentially involved in Aβ-induced impairment of PKC activation. To test this, we measured the effect of Aβ on the distribution of RACK1 by treating cultured cortical neurons with oligomeric Aβ (1 μM). As shown in the Western blot of oligomeric Aβ (Fig. 1A), there was a smear band of signals between 4–22 kDa, suggesting the presence of Aβ monomers, dimers, trimers and tetramer.
FIG. 1.
Aβ42 treatment decreases RACK1, total and activated PKC in membrane fractions of cultured cortical neurons. A, Western blots showing the oligomeric Aβ B, Western blots showing endogenous (E) and recombinant (R) RACK1, PKC, p-PKC and actin in cytosolic and membrane fractions from cortical cultures (uninfected or infected with GFP-RACK1 virus) treated without or with Aβ oligomer (1 μM, 48 hrs). C, Quantifications by densitometric analysis of RACK1 (E+R), PKC, p-PKC in cytosolic and membrane fractions from non-treated or Aβ-treated cortical cultures without or with RACK1 overexpression. Protein levels (normalized to actin) were expressed as the percent of controls. *: p < 0.01, ANOVA, compared to untreated and uninfected neurons (control).
After ~48 hr of treatment with Aβ oligomer, neurons were homogenized, and the cytosol and membrane fraction were separated using ultracentrifugation. As shown in Fig. 1B, Aβ treatment did not alter endogenous RACK1 (33 KDa) in the cytosol fraction, but induced a marked reduction of RACK1 in the membrane fraction.
Since RACK1 is an anchoring protein that translocates activated PKC from the cytosol fraction to the membrane fraction (Mochly-Rosen et al., 1991; Ron et al., 1994), we examined whether the Aβ-induced reduction of RACK1 distribution at cellular membranes also affected translocation and activation of PKC. Because the catalytic competence of many PKC isozymes depends on autophosphorylation at the carboxyl terminus on a conserved residue (Behn-Krappa and Newton, 1999), a phosphospecific pan PKC antibody that detects PKC isoforms only when phosphorylated at this residue was used to detect activated PKC. As shown in Fig. 1B, Aβ treatment also decreased the distribution of PKC and p-PKC (activated) in the membrane fraction, but not in the cytosol fraction. Quantification data (Fig. 1C) show that RACK1 in the membrane fraction was decreased to 68.7 ± 4.1% of control (n = 6, p < 0.01, ANOVA) by A treatment. Similarly, PKC and p-PKC in the membrane fraction were reduced to 66.8 ± 3.5% and 70.5 ± 5.7% of control (n = 6, p < 0.01, ANOVA), respectively, by Aβ treatment. No significant difference was found on cytosolic RACK1, PKC and p-PKC between control and Aβ-treated neurons. These data indicate that oligomeric Aβ treatment reduces the level of RACK1, PKC and p-PKC at cellular membranes of cortical cultures.
To detect whether overexpression of RACK1 restores Aβ-induced loss of PKC and p-PKC in the membrane fraction, we infected cortical cultures with GFP-RACK1 Sindbis virus. After 2 days of Aβ-treatment, neurons were subjected to Western blot assays. As shown in Fig. 1B, 1C, the overexpression of recombinant GFP-RACK1 (60 KDa) was confirmed in RACK1-infected neurons. The level of PKC and p-PKC in the membrane fraction was elevated by RACK1 overexpression. After Aβ treatment, the membrane level of PKC and p-PKC in RACK1-infected neurons was similar to that in untreated neurons without infection (control), suggesting that the loss of PKC and p-PKC by Aβ is restored by RACK1 overexpression.
Overexpression of RACK1 rescued the muscarinic regulation of GABAergic transmission in Aβ-treated cortical cultures
Our previous study has shown that mAChR activation increases the amplitude of spontaneous inhibitory postsynaptic current (sIPSC) in cortical slices via a PKC-dependent mechanism, which was impaired by Aβ25–35 treatment or in APP transgenic mice, probably due to the Aβ-mediated interference of mAChR activation of PKC (Zhong et al., 2003). Since Aβ treatment reduces the level of RACK1 and activated PKC at the membrane fraction of cortical cultures, we would like to know whether overexpression of RACK1 could rescue the effect of mAChRs on GABAergic transmission in Aβ-treated neurons.
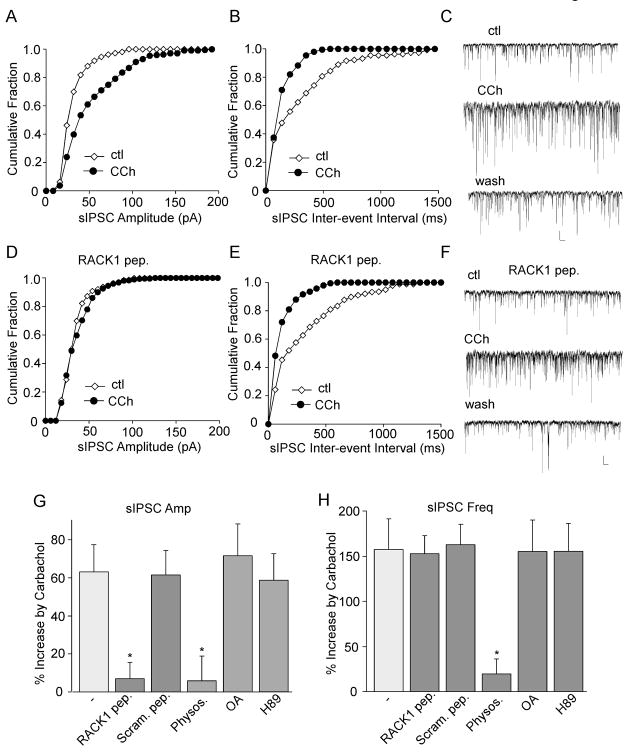
First, we examined the involvement of RACK1 in muscarinic regulation of GABAergic transmission. As shown in Fig. 2A–C, bath application of the mAChR agonist carbachol (20 μM) caused a significant (p < 0.01, ANOVA) increase in the amplitude and frequency of sIPSC (amplitude: 63.2 ± 14.3%, frequency: 157.6 ± 33.3%, n = 20; Fig. 2G, H), consistent with our previous finding (Zhong et al., 2003). To test the involvement of RACK1 in muscarinic regulation of sIPSC, we dialyzed neurons with a peptide derived from the sixth WD40 repeats of RACK1 (DGGDIINALCFSPNR) to inhibit PKC binding to RACK1 (Ron et al., 1994; Feng et al., 2001). As shown in Fig. 2D–H, dialysis with the RACK1 peptide (40 μM) significantly diminished the enhancing effect of carbachol on sIPSC amplitude (6.9 ± 8.8%, n = 17), while did not alter the effect of carbachol on sIPSCs frequency (152.8 ± 19.5%, n = 17). On the other hand, dialysis with a scrambled control peptide (FDSRGIGPDINCANL) did not block the enhancing effect of carbachol on sIPSC amplitude or frequency (Fig. 2G, H). It suggests that muscarinic modulation of sIPSC amplitudes requires RACK1 to anchor activated PKC to the membrane of inhibitory synapses.
FIG. 2.
RACK1 is involved in muscarinic regulation of sIPSC amplitude. A–C, Cumulative plots of the distribution of sIPSC amplitudes (A) and inter-event interval (B), and representative sIPSC traces (C) in a cultured cortical neuron before (ctl) and after carbachol (CCh, 20 μM) application. D–F, Cumulative plots of the distribution of sIPSC amplitudes (D) and inter-event interval (E), and representative sIPSC traces (F) showing the effect of carbachol in a cultured cortical neuron dialyzed with a RACK1 peptide (40 μM). Scale bars: 30 pA, 1s. G,H, Cumulative data (mean ± SEM) showing the percent increase of sIPSC amplitude (G) or frequency (H) by carbachol in the absence or presence of RACK1 peptide, a scrambled control peptide (40 μM), acetylcholinesterase (AChE) inhibitor physostigmine (40 μM), protein phosphatase inhibitor okadaic acid (OA, 1 μM), or PKA inhibitor H89 (10 μM). *: p < 0.01, ANOVA, compared to the effect of carbachol in the control condition (−).
To confirm the specificity of PKC involvement, we also examined the potential role of several other molecules in muscarinic regulation of GABAergic transmission. Application of the acetylcholinesterase (AChE) inhibitor physostigmine (40 μM) increased sIPSC (amplitude: 51.1 ± 9.3%, frequency: 148.5 ± 39.3%, n = 10), mimicking the effect of carbachol. Furthermore, the enhancing effect of carbachol on sIPSC was occluded by physostigmine (Fig. 2G, H, amplitude: 5.91 ± 2.9%, frequency: 19.6 ± 16.8%, n = 10), suggesting its dependence on acetylcholine. On the other hand, the effect of carbachol was not significantly altered by the protein phosphatase inhibitor okadaic acid (OA, 1 μM, amplitude: 71.6 ± 16.8%, frequency: 155.3 ± 34.5%, n = 10) or the PKA inhibitor H89 (10 μM, amplitude: 58.7 ± 13.8%; frequency: 155.6 ± 30.6%, n = 10, Fig. 2G, H), which ruled out the involvement of these signaling molecules.
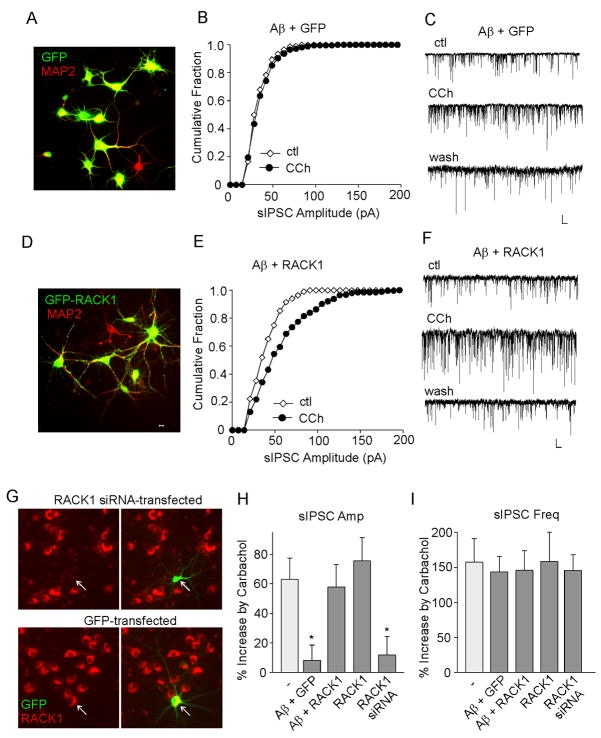
We further examined whether overexpression of RACK1 could restore the impaired muscarinic regulation of GABAergic transmission in Aβ-treated neurons. We infected cortical cultures (DIV 11–16) with GFP or GFP-tagged RACK1 Sindbis viruses. Sindbis virus vector can infect neurons with high efficiency and has been widely applied in neuroscience research (Schlesinger et al., 1999; Maletic-Savatic et al., 1999; Ehrengruber et al 1999). After 2–3 days of infection, we stained neurons with MAP2, a dendritic marker, to examine their viability and morphological features. As shown in Fig. 3A and 3D, most MAP2-labeled neurons were also GFP positive, and they looked healthy, confirming the high infection efficiency and low neuronal toxicity. The expression pattern of GFP and GFP-RACK1 was different, with GFP showing even distribution throughout the neurons, while GFP-RACK1 displaying more punctuate pattern, especially in the dendrites.
FIG. 3.
Overexpression of RACK1 restores the Aβ-induced loss of muscarinic regulation of sIPSC amplitudes in cortical cultures. A,D, Immunocytochemical images of MAP2(red)-stained cortical cultures infected with GFP (A) or GFP-RACK1 (D) Sindbis viruses. B,E, Cumulative plots of the distribution of sIPSC amplitudes showing the effect of carbachol (CCh, 20 μM) in A (1 μM, 2–3 days)-treated cortical cultures infected with GFP (B) or GFP-RACK1 (E) Sindbis viruses. C,F, Representative sIPSC traces from the neurons used to construct B and E. Scale bars: 30 pA, 1s. G, Immunostaining of RACK1 (red) in cultured cortical neurons transfected with RACK1 siRNA (co-transfected with GFP) or GFP alone. Arrowheads point to GFP+ neurons. H, I, Cumulative data (mean ± SEM) showing the percent increase of sIPSC amplitude (H) or frequency (I) by carbachol in cortical neurons under different conditions. *: p < 0.01, ANOVA, compared to the effect of carbachol in the control condition (−).
During infection, cortical neurons were also treated with oligomeric A (1 μM). After 2–3 days of treatment, GFP+ neurons were subjected to recording. As show in Fig. 3B and 3C, Aβ diminished the effect of carbachol on sIPSC amplitude in neurons overexpressing GFP (8.0 ± 10.6%, n = 15, Fig. 3H). In contrast, overexpression of GFP-RACK1 rescued the enhancing effect of carbachol on sIPSC amplitude (Fig. 3E, F, 57.8 ± 15.3%, n = 15, Fig. 3H). Overexpression of RACK1 alone did not significantly alter the effect of carbachol on sIPSC amplitude (75.7 ± 15.7%, n = 8, Fig. 3H). The effect of carbachol on sIPSC frequency, which is PKC-independent (Zhong et al., 2003), was not altered by Aβ in neurons infected with GFP or GFP-RACK1 (Fig. 3I). These data suggest that overexpressing RACK1 in vitro restores the Aβ-induced loss of muscarinic effects on sIPSC amplitudes in cortical cultures.
To further confirm the role of RACK1 in carbachol regulation of GABAergic transmission, we performed cellular knockdown experiments by transfecting RACK1 siRNA to cortical cultures. The specific suppression of RACK1 expression in siRNA-transfected (GFP+) neurons is illustrated in Fig. 3G. Knockdown of RACK1 abolished the enhancing effect of carbachol on sIPSC amplitude (11.8 ± 12.5%, n = 8, Fig. 3H), but not on sIPSC frequency (145.52 ± 2.7%, n = 8, Fig. 3I).
Injection of Aβ into animals impaired muscarinic regulation of GABAergic transmission, which was rescued by RACK1 expresssion in vivo
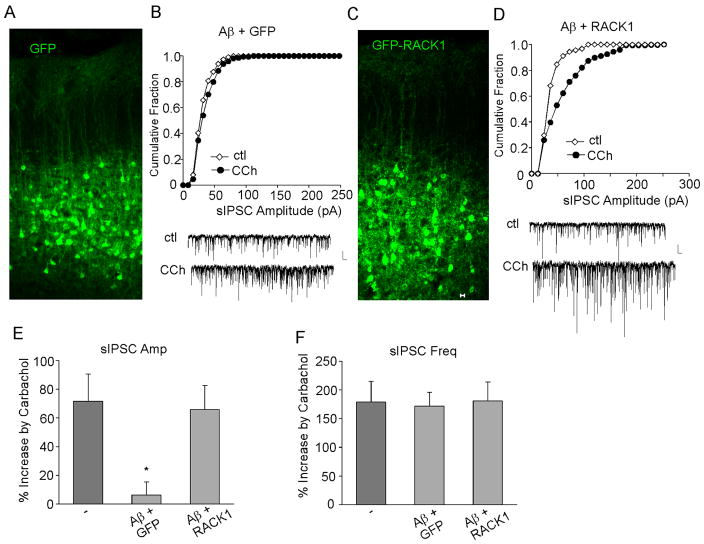
To confirm the in vitro results, we examined the impact of Aβ and RACK1 in vivo. Sindbis virus vector-based gene delivering enables rapid and efficient heterologous protein expression in neurons (McCormack et al., 2006; Kopec et al., 2007), so we used it for transient RACK1 expression in vivo. Oligomeric Aβ (100 μM) was delivered to rat frontal cortex via a stereotaxic injection. GFP or GFP-RACK1 Sindbis viruses were also injected simultaneously. As shown in Fig. 4A and 4C, after 2–3 days of infection, these viruses were efficiently expressed in neurons at the proximity of injected sites, and the GFP+ neurons showed normal morphology. In cortical pyramidal neurons without Aβ injection, application of carbachol (20 μM) increased sIPSC amplitude by 71.7 ± 18.9% (n = 26, Fig. 4E), consistent with our previous study (Zhong et al., 2003). However, in GFP-infected cortical neurons from Aβ-injected animals, the enhancing effect of carbachol on sIPSC amplitudes was largely abolished (Fig. 4B, 6.3 ± 9.2%, n = 15, Fig. 4E). Interestingly, in RACK1-infected cortical neurons from Aβ-injected animals, the enhancing effect of carbachol on sIPSC amplitudes was restored (Fig. 4D, 65.9 ± 16.7%, n = 13, Fig. 4E). Again, the effect of carbachol on sIPSC frequency was not altered by Aβ injection (Fig. 4F). These data indicate that oligomeric Aβ blocks the enhancement of carbachol on sIPSC amplitudes in rat cortical neurons in vivo, which is rescued by RACK1 expression.
FIG. 4.
RACK1 expression restores the Aβ-induced loss of muscarinic regulation of sIPSC amplitudes in vivo. A,C, Immunocytochemical images of cortical slices from rats stereotaxically injected with GFP (A) or GFP-RACK1 (C) Sindbis viruses. B,D, Cumulative plots of the distribution of sIPSC amplitudes showing the effect of carbachol (CCh, 20 μM) in cortical neurons from A (1 μM)-injected rats that were infected with GFP (B) or GFP-RACK1 (D) Sindbis viruses. Inset: representative sIPSC traces. Scale bars: 30 pA, 1s. E,F, Cumulative data (mean ± SEM) showing the percent increase of sIPSC amplitude (E) or frequency (F) by carbachol in cortical neurons (from Aβ-injected rats) overexpressing GFP or GFP-RACK1. *: p < 0.01, ANOVA, compared to the effect of carbachol in the control condition (−).
Discussion
Cholinergic system is involved in many cognitive functions, such as attention (Voytko et al., 1994), learning (Fine et al., 1997) and memory (Hasselmo et al., 1992). Selective degeneration of cholinergic neurons in basal forebrain is a major feature of AD (Whitehouse et al., 1982; Selkoe, 2001). The action of acetylcholine is mediated by muscarinic and nicotinic receptors. It has been reported that nicotinic receptors are significantly lost in cortical and hippocampal areas of AD brains (Perry et al., 1995), whereas m1 muscarinic receptors are preserved in most AD patients (Araujo et al., 1988). However, several studies indicate that the interaction between m1 muscarinic receptors and G-proteins is affected in AD brains (Flynn et al., 1991; Cowburn et al., 1996). So it is important to elucidate the downstream signaling pathway of muscarinic receptors in the normal condition and how it goes awry in the AD state.
Prefrontal cortex (PFC), which has long been implicated in cognitive processes (Dalley et al., 2004), such as “working memory” (Goldman-Rakic 1995), is a major target area of the basal forebrain cholinergic system. The cholinergic innervation of PFC is critically involved in “working memory” (Dunnett et al., 1990; Broerse et al., 1995), while the underlying mechanism is unclear. It has been suggested that “working memory” relies on GABAergic inhibition in PFC, which controls the timing of neuronal activity during cognitive operations (Constantinidis et al., 2002). Our previous studies show that muscarinic receptors regulate PFC GABAergic transmission via a PKC-dependent mechanism (Ma et al., 2003), and this effect is impaired in APP transgenic mice and Aβ-treated slices (Zhong et al., 2003). In this study, we demonstrate that the impairment is likely due to Aβ-induced decrease of RACK1 distribution in the neuronal membrane.
RACK1 is a scaffold protein with complex functions because of its promiscuous and dynamic capacity to interact with multiple partners (Sklan et al., 2006). PKC is the first identified RACK1 partner protein. PKC typically translocates to the membrane upon activation, however its residence on membranes is transient if not stabilized by interacting with RACK1 (Mochly-Rosen et al., 1991; Ron et al., 1995; McCahill et al., 2002). PKC isozymes located at different subcellular compartments are differentially involved in distinct organ functions, such as the regulation of ion channels, neurotransmission, synaptic plasticity, learning and memory (Tanaka and Nishizuka, 1994). The disparate functional effects of specific PKC isozymes can be determined by binding to RACKs (Sklan et al., 2006).
AD-related changes in the expression and activity of PKC isoforms have been investigated, with several reports indicating that PKC activities are reduced in AD brains (Cole et al., 1988, Wang et al., 1994; Matsushima et al., 1996). There is also evidence indicating that the level of RACK1 is decreased in the membrane fraction of aging rat cortex (Battaini et al., 1997; Pascale et al., 1996) and aging rabbit hippocampus (Van der Zee et al., 2004), as well as in both soluble and membrane fractions of AD brains (Battaini et al., 1999). Accompanying to the reduced RACK1 is the impaired translocation of PKC from the cytosol to the plasma membrane (Battaini et al., 1997; Pascale et al., 1996; Van der Zee et al., 2004). Consistent with these, we found that Aβ treatment reduces RACK1 levels in the membrane fraction of PFC neurons, with a concomitant decrease of total PKC and activated PKC levels in the membrane.
The Aβ-induced loss of RACK1 membrane distribution could underlie the Aβ-induced loss of m1/PKC regulation of GABA transmission in PFC. The best supporting evidence is the rescue experiments. We have shown that overexpression of RACK1 restores the muscarinic effect on GABA transmission in Aβ-treated PFC neurons. This in vitro result is confirmed by using viral-based gene delivering in vivo. It suggests that RACK1 is a potential therapeutic target that can rescue some of the impaired cellular processes by Aβ.
Acknowledgments
This work was supported by National Institutes of Health (AG21923, MH84233) to Z.Y. and National Natural Science Foundation of China to F.D. (NSFC 30600099) and Z.Y. (NSFC 30528009). We would like to thank Xiaoqing Chen and Dr. Yong Ren for their technical support.
Footnotes
DISCLOSURE STATEMENT
None of the authors has actual or potential conflicts of interest. None of the authors’ institution has contracts relating to this research. There is no other agreement of authors or their institutions that could be seen as involving a financial interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer’s disease. J Neurochem. 1988;50(6):1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the ageing rat brain. Trends Neurosci. 1997;20:410–415. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- Battaini F, Pascale A, Lucchi L, Pasinetti GM, Govoni S. Protein kinase C anchoring deficit in postmortem brains of Alzheimer’s disease patients. Exp Neurol. 1999;159:559–564. doi: 10.1006/exnr.1999.7151. [DOI] [PubMed] [Google Scholar]
- Behn-Krappa A, Newton AC. The hydrophobic phosphorylation motif of conventional protein kinase C is regulated by autophosphorylation. Curr Biol. 1999;9:728–37. doi: 10.1016/s0960-9822(99)80332-7. [DOI] [PubMed] [Google Scholar]
- Broersen LM, Heinsbroek RPW, de Bruin JPC, Uylings HBM, Oliver B. The role of the medial prefrontal cortex of rats in short term memory functioning: further support for involvement of cholinergic, rather than dopaminergic mechanisms. Brain Res. 1995;674:221–229. doi: 10.1016/0006-8993(95)00025-l. [DOI] [PubMed] [Google Scholar]
- Cole G, Dobkins KR, Hansen LA, Terry RD, Saitoh T. Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 1988;452:165–174. doi: 10.1016/0006-8993(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat Neurosci. 2002;5(2):175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, Fowler CJ, O’Neill C. Neurotransmitter Receptor/G-protein Mediated Signal Transduction in Alzheimer’s Disease Brain. Neurodegeneration. 1996;5:483–488. doi: 10.1006/neur.1996.0067. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dell EJ, Connor J, Chen S, Stebbins EG, Skiba NP, Mochly-Rosen D, Hamm HE. The betagamma subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem. 2002;277(51):49888–49895. doi: 10.1074/jbc.M202755200. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Wareham AT, Torres EM. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1:61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Lundstrom K, Schweitzer C, Heuss C, Schlesinger S, Gähwiler BH. Recombinant Semliki Forest virus and Sindbis virus efficiently infect neurons in hippocampal slice cultures. Proc Natl Acad Sci U S A. 1999;96(12):7041–7046. doi: 10.1073/pnas.96.12.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Cai X, Zhao JH, Yan Z. Serotonin receptors modulate GABAA receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM. Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neuroscience. 1997;81:331–343. doi: 10.1016/s0306-4522(97)00208-x. [DOI] [PubMed] [Google Scholar]
- Flynn DD, Weinstein DA, Mash DC. Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer’s disease: implications for the failure of cholinergic replacement therapies. Ann Neurol. 1991;29:256–262. doi: 10.1002/ana.410290305. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gu Z, Liu W, Yan Z. {beta}-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284(16):10639–49. doi: 10.1074/jbc.M806508200. Epub 2009 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. J Neurophysiol. 1992;67:1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- Hu XD, Huang Q, Roadcap DW, Shenolikar SS, Xia H. Actin-associated neurabin-protein phosphatase-1 complex regulates hippocampal plasticity. J Neurochem. 2006;98:1841–1851. doi: 10.1111/j.1471-4159.2006.04070.x. [DOI] [PubMed] [Google Scholar]
- Jaken S, Parker PJ. Protein kinase C binding partners. Bioessays. 2000;22(3):245–254. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yuen EY, Allen PB, Feng J, Greengard P, Yan Z. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc Natl Acad Sci USA. 2006;103:18338–18343. doi: 10.1073/pnas.0604560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XH, Zhong P, Gu Z, Feng J, Yan Z. Muscarinic potentiation of GABA(A) receptor currents is gated by insulin signaling in the prefrontal cortex. J Neurosci. 2003;23(4):1159–1168. doi: 10.1523/JNEUROSCI.23-04-01159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- Matsushima H, Shimohama S, Chachin M, Taniguchi T, Kimura J. Ca2+-dependent and Ca2+-independent protein kinase C changes in the brain of patients with Alzheimer’s disease. J Neurochem. 1996;67:317–323. doi: 10.1046/j.1471-4159.1996.67010317.x. [DOI] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–1273. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- McCormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Khaner H, Lopez J. Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci U S A. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Pascale A, Fortino I, Govoni S, Trabucchi M, Wetsel WC, Battaini F. Functional impairment in protein kinase C by RACK1 (receptor for activated C kinase 1) deficiency in aged rat brain cortex. J Neurochem. 1996;67:2471–2477. doi: 10.1046/j.1471-4159.1996.67062471.x. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Nat Acad Sci U S A. 2004;101:2328–2332. doi: 10.1073/pnas.0308567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, Irving D, Brown A, Perry RH. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: Possible index of early neuropathology. Neuroscience. 1995;64:385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- Peyrl A, Weitzdoerfer R, Gulesserian T, Fountoulakis M, Lubec G. Aberrant expression of signaling-related proteins 14-3-3 gamma and RACK1 in fetal Down syndrome brain (trisomy 21) Electrophoresis. 2002;23(1):152–157. doi: 10.1002/1522-2683(200201)23:1<152::AID-ELPS152>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S, Dubensky TW. Alphavirus vectors for gene expression and vaccines. Curr Opin Biotechnol. 1999;10:434–439. doi: 10.1016/s0958-1669(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Kamiya S, Taniguchi T, Kimura J. Intracellular receptors for activated C-kinase in the postmortem human brain: no alteration in Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12(4):384–386. doi: 10.1097/00002093-199812000-00022. [DOI] [PubMed] [Google Scholar]
- Sklan EH, Podoly E, Soreq H. RACK1 has the nerve to act: Structure meets function in the nervous system. Prog Neurobiol. 2006;78:117–134. doi: 10.1016/j.pneurobio.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. New frontiers in Alzheimer’s disease genetics. Neuron. 2001;32(2):181–184. doi: 10.1016/s0896-6273(01)00476-7. [DOI] [PubMed] [Google Scholar]
- Tyszkiewicz JP, Yan Z. beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J Neurophysiol. 2005;93(6):3102–3111. doi: 10.1152/jn.00939.2004. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Palm IF, O’Connor M, Maizels ET, Hunzicker-Dunn M, Disterhoft JF. Aging-related alterations in the distribution of Ca2+-dependent PKC isoforms in rabbit hippocampus. Hippocampus. 2004;14:849–860. doi: 10.1002/hipo.20000. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Pisano MR, Friedman E. Attenuated protein kinase C activity and translocation in Alzheimer’s disease brain. Neurobiol of Aging. 1994;15:293–298. doi: 10.1016/0197-4580(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–9861. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99(8):5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Gu Z, Wang X, Jiang H, Feng J, Yan Z. Impaired modulation of GABAergic transmission by muscarinic receptors in a mouse transgenic model of Alzheimer’s disease. J Biol Chem. 2003;278:26888–26896. doi: 10.1074/jbc.M302789200. [DOI] [PubMed] [Google Scholar]