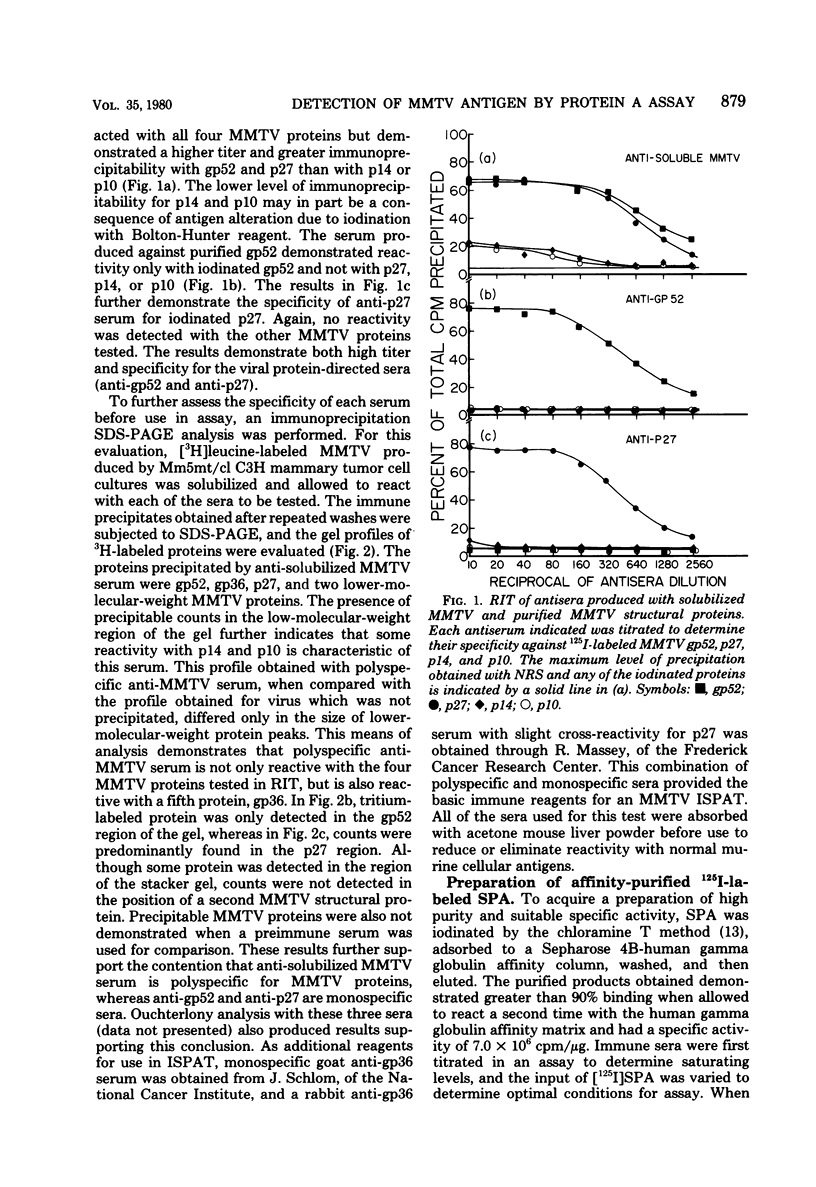
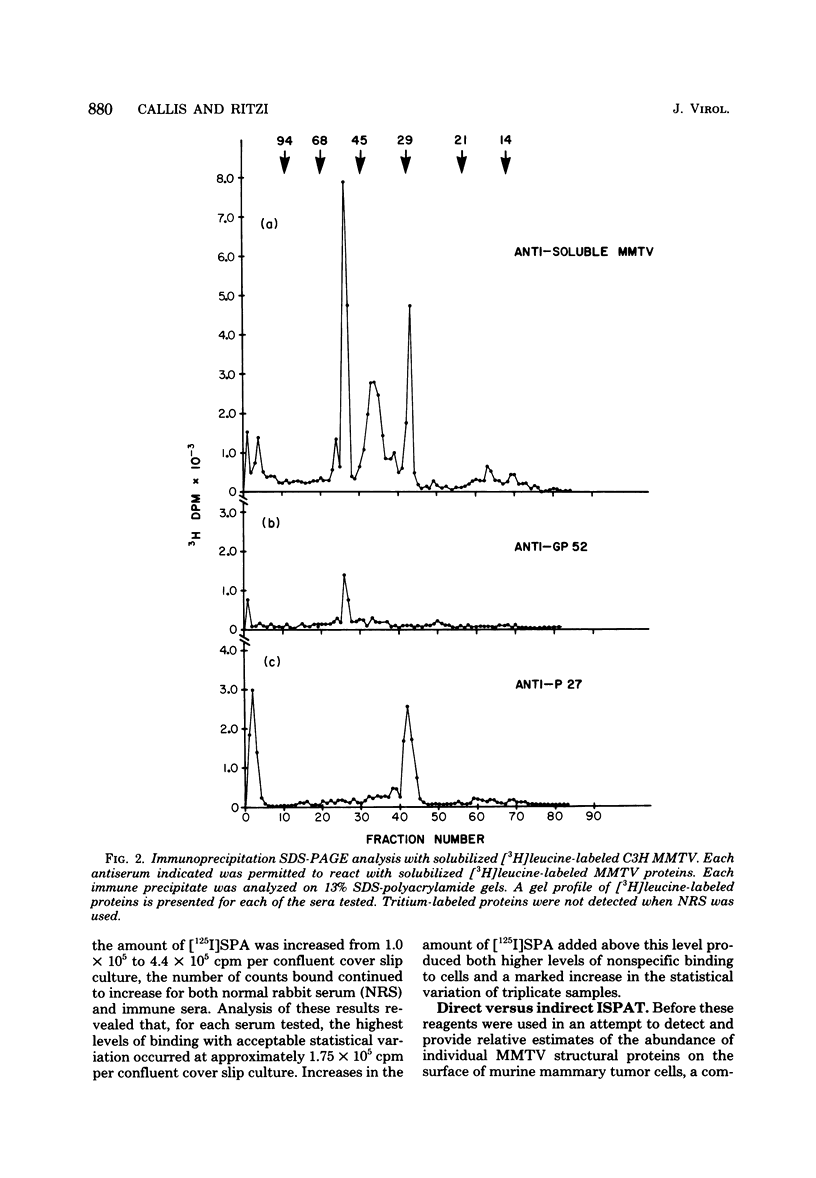
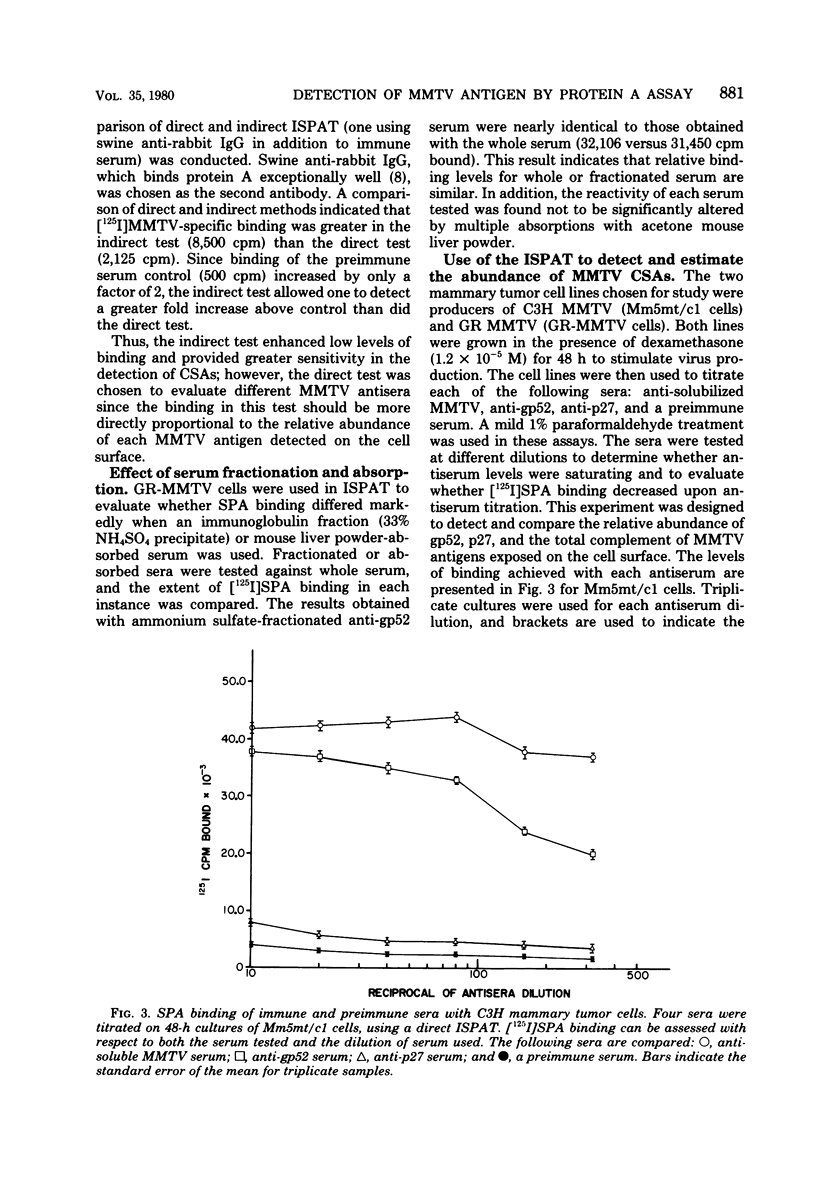
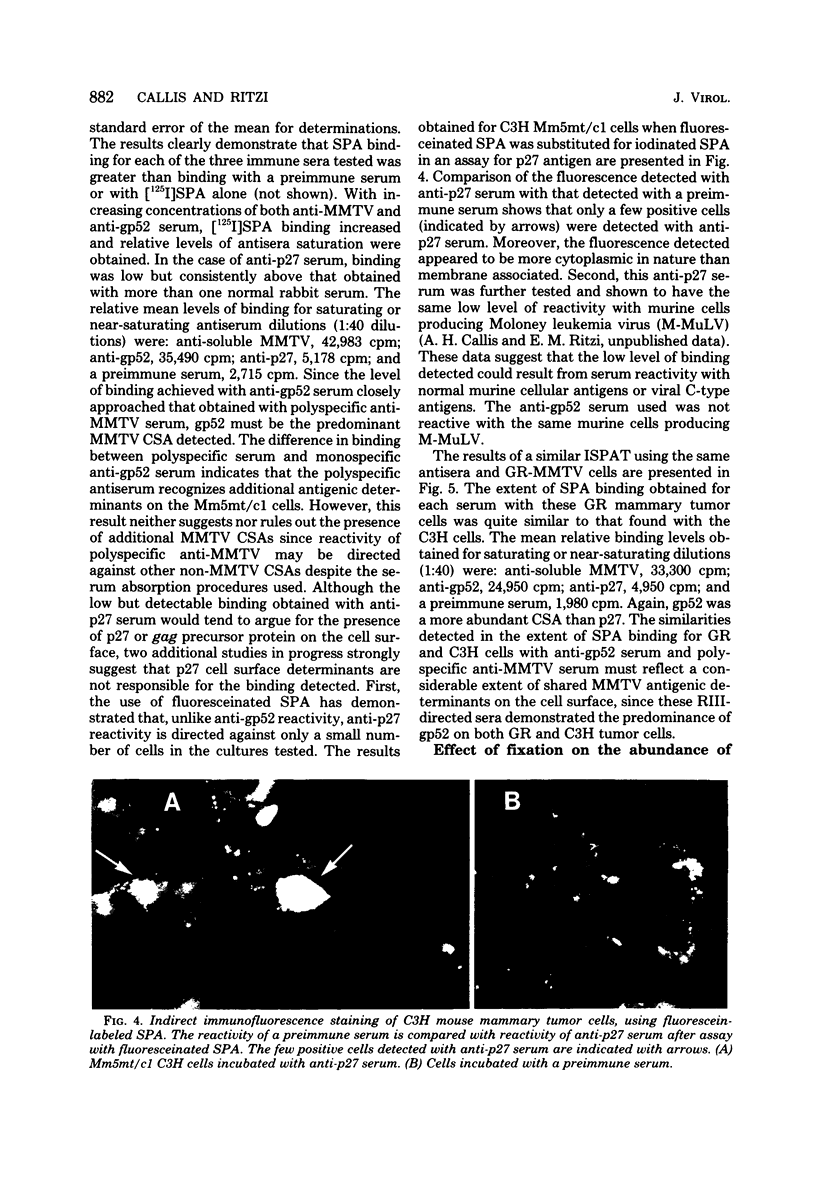
Abstract
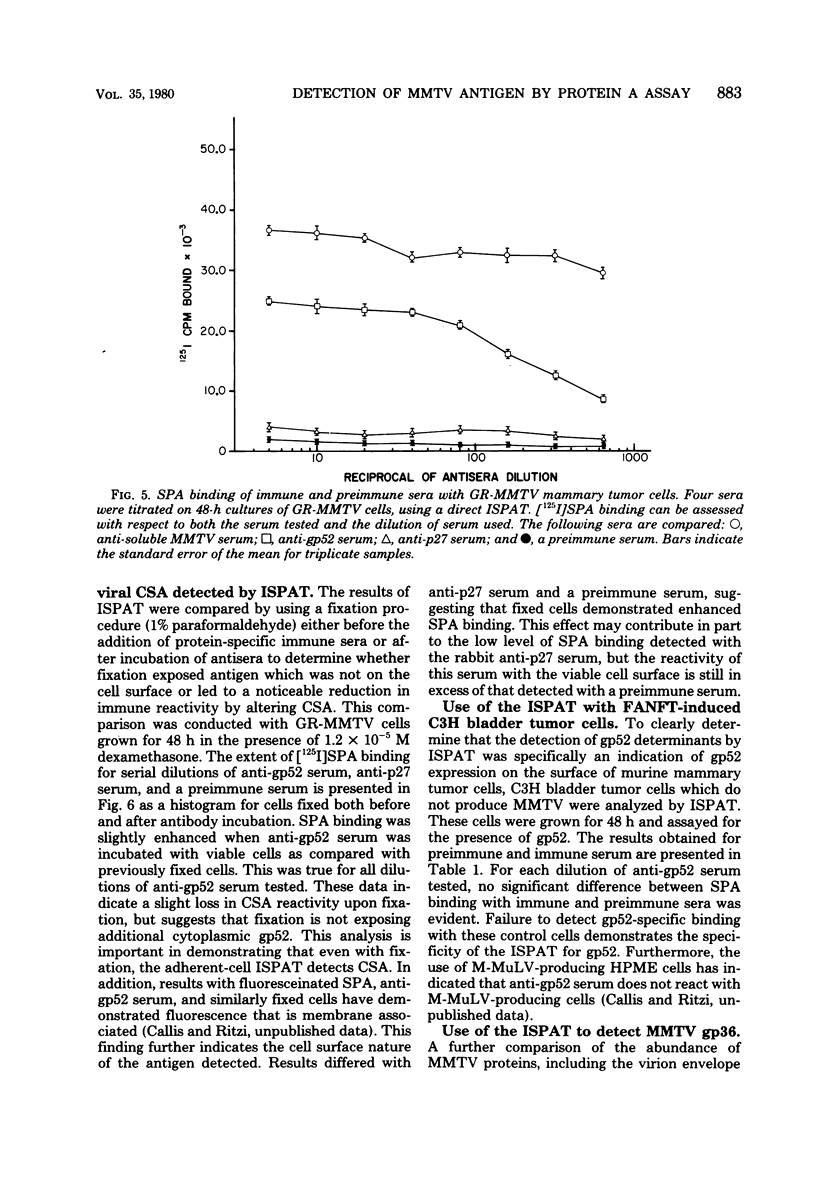
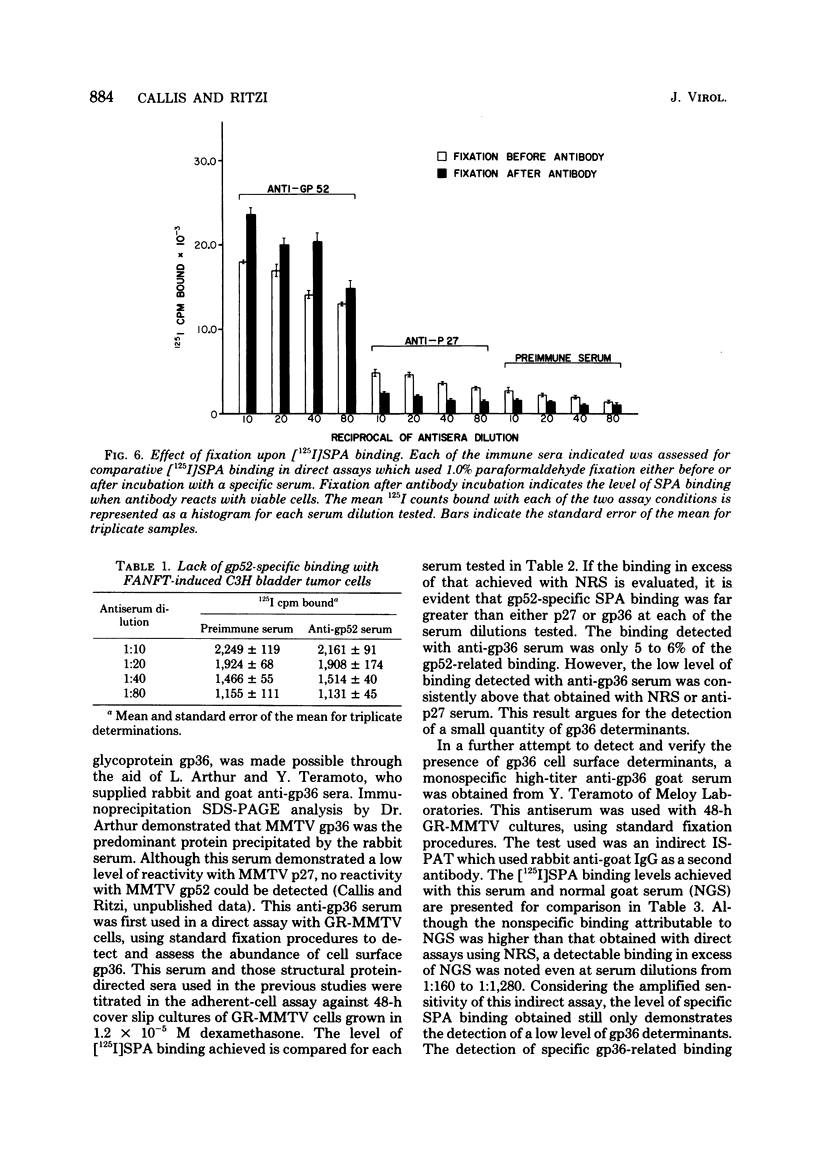
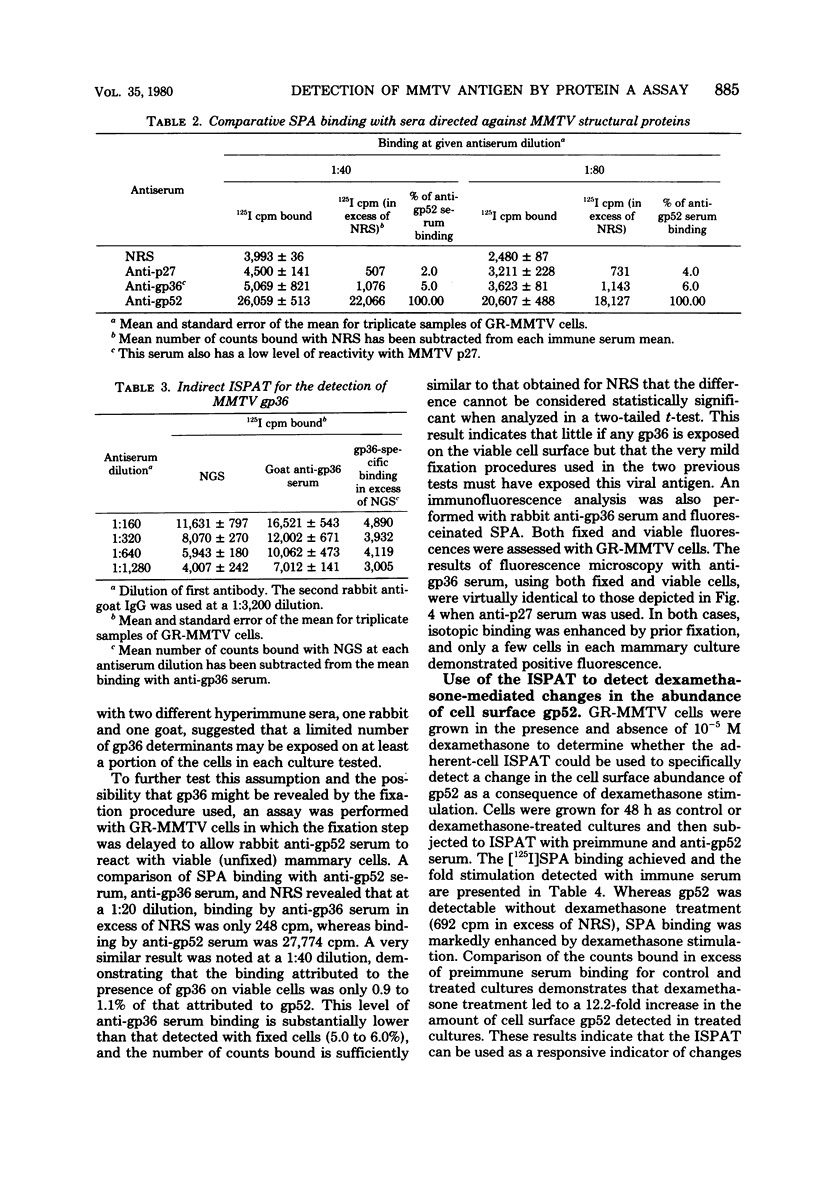
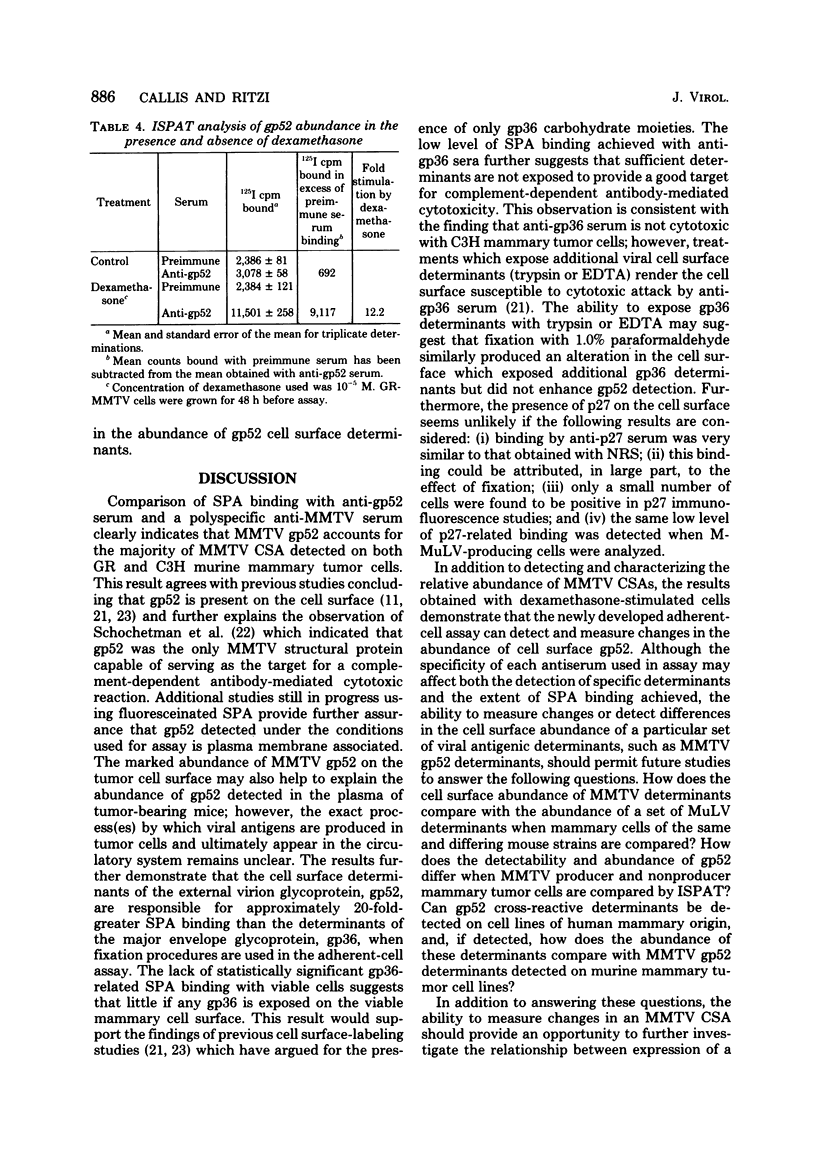
Antisera against the following mouse mammary tumor virus (MMTV) structural proteins were used to detect MMTV cell surface antigens: (i) the 27,000-dalton nucleoid protein, p27; (ii) the 36,000-dalton envelope glycoprotein, gp36; and (iii) the 52,000-dalton exterior envelope glycoprotein, gp52. We report here the development of an adherent-cell isotopic staphylococcal protein A (SPA) test (ISPAT) for MMTV structural proteins which allows for the detection of an MMTV membrane-associated antigen as well as an estimate of its relative abundance on the cell surface. This test demonstrated that the gp52 was the predominant MMTV cell surface antigen detected on both C3H and GR mouse mammary tumor cells. In a comparative study with anti-gp52 and anti-gp36 sera, SPA-specific binding with anti-gp36 serum was found to be only 5 to 6% of that obtained for the external virion glycoprotein, gp52. Both direct and indirect ISPAT indicated the presence of a low but detectable number of gp36 determinants on GR-MMTV cells; however, these gp36 determinants, unlike gp52 determinants, appeared to be exposed by the fixation procedure used. Only 0.9 to 1.1% of the gp52-specific binding was detected when anti-gp36 serum was allowed to react with viable cells. The binding of [125I]SPA achieved with anti-p27 serum was even less than that detected with gp36-directed reagents, indicating that p27 is not a cell surface antigen. The use of fluoresceinated SPA further demonstrated that p27 and gp36 reactivity was only associated with a small number of cells in each of the mammary cultures tested. When N-[4-(5-nitro-2-furyl)-2-thiazoly]-formamide-induced C3H bladder tumor cells were subjected to a gp52-directed ISPAT, the failure to detect gp52-specific binding demonstrated the specificity of this assay for MMTV gp52-expressing cells. In addition to detecting and characterizing MMTV cell surface antigens, the newly developed adherent cell assay could measure changes in the abundance of cell surface gp52. When dexamethasone-treated and untreated GR cells were compared, measurements of gp52-specific SPA binding indicated that dexamethasone stimulation leads to a 12.2-fold increase in the amount of cell surface gp52 detected.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bauer R. F., Orme L. S., Fine D. L. Coexistence of the mouse mammary tumor virus (MMTV) major glycoprotein and natural antibodies to MMTV in sera of mammary tumor-bearing mice. Virology. 1978 Jun 15;87(2):266–275. doi: 10.1016/0042-6822(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Buijs F., Hageman P. C., Links J., Hilgers J., Hekman A. Distribution of virus particles and mammary tumor virus antigens in mouse mammary tumors, transformed BALB-c mouse kidney cells, and GR ascites leukemia cells. J Natl Cancer Inst. 1974 Oct;53(4):977–992. doi: 10.1093/jnci/53.4.977. [DOI] [PubMed] [Google Scholar]
- Daams J. H., Calafat J., Lasfargues E. Y., Kramarsky B., Bentvelzen P. Mammary tumor virus associated antigens on the membrane of infected mouse spleen cells. Virology. 1970 May;41(1):184–186. doi: 10.1016/0042-6822(70)90068-1. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Arthur L. O., PLOWMAN J. K., Hillman E. A., Klein F. In vitro system for production of mouse mammary tumor virus. Appl Microbiol. 1974 Dec;28(6):1040–1046. doi: 10.1128/am.28.6.1040-1046.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette R. W., Robertson S., Brown R., Blackman K. E. Expression of mammary tumor virus antigen on the membranes of lymphoid cells. J Natl Cancer Inst. 1974 Aug;53(2):499–505. doi: 10.1093/jnci/53.2.499. [DOI] [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hilgers J. H., Theuns G. J., van Nie R. Mammary tumor virus (MTV) antigens in normal and mammary tumor-bearing mice. Int J Cancer. 1973 Nov 15;12(3):568–576. doi: 10.1002/ijc.2910120305. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Williams W. C., Myers B., Dmochowski L. Detection of antigens of the mouse mammary tumor (MTV) and murine leukemia virus (MuLV) in cells of cultures derived from mammary tumors of mice of several strains. Virology. 1971 Aug;45(2):470–483. doi: 10.1016/0042-6822(71)90347-3. [DOI] [PubMed] [Google Scholar]
- Holder W. D., Jr, Peer G. W., Bolognesi D. P., Wells S. A., Jr Detection of the major glycoproteins of Friend leukemia virus (gp71) and the murine mammary tumor virus (gp52) on the surface of mouse cells. Cancer Res. 1976 Sep;36(9 PT1):3217–3224. [PubMed] [Google Scholar]
- Hoshino M., Dmochowski L. Electron microscope study of antigens in cells of mouse mammary tumor cell lines by peroxidase-labeled antibodies in sera of mammary tumor-bearing mice and of patients with breast cancer. Cancer Res. 1973 Nov;33(11):2551–2561. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Baldi A., Spiegelman S. The purification of a gs antigen of the murine mammary tumor virus and its quantitation by radioimmunoassay. Virology. 1976 Nov;75(1):188–197. doi: 10.1016/0042-6822(76)90017-9. [DOI] [PubMed] [Google Scholar]
- Ritzi E., Martin D. S., Stolfi R. L., Spiegelman S. Plasma levels of a viral protein as a diagnostic signal for the presence of mammary tumor: the effect of tumor removal. J Exp Med. 1977 Apr 1;145(4):999–1013. doi: 10.1084/jem.145.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzi E., Martin D. S., Stolfi R. L., Spiegelman S. Plasma levels of a viral protein as a diagnostic signal for the presence of tumor : the murine mammary tumor model. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4190–4194. doi: 10.1073/pnas.73.11.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Arthur L. O., Long C. W., Massey R. J. Mice with spontaneous mammary tumors develop type-specific neutralizing and cytotoxic antibodies against the mouse mammary tumor virus envelope protein gp52. J Virol. 1979 Oct;32(1):131–139. doi: 10.1128/jvi.32.1.131-139.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Fine D. L., Massey R. J. Mouse mammary tumor virus and murine leukemia virus cell surface antigens on virus producer and nonproducer mammary epithelial tumor cells. Virology. 1978 Jul 15;88(2):379–383. doi: 10.1016/0042-6822(78)90294-5. [DOI] [PubMed] [Google Scholar]
- Shigematsu T., Dmochowski L., Williams W. C. Studies on mouse mammary tumor virus (MTV) and mouse leukemia virus (MuLV) by immunoelectron microscopy. Cancer Res. 1971 Dec;31(12):2085–2097. [PubMed] [Google Scholar]
- Tanaka H., Moore D. H. Electron microscopic localization of viral antigens in mouse mammary tumors by ferritin-labeled antibody. I. The homologous systems. Virology. 1967 Oct;33(2):197–214. doi: 10.1016/0042-6822(67)90138-9. [DOI] [PubMed] [Google Scholar]
- Yang J., Tang R., Nandi S. Identification of the mammary tumor virus envelope glycoprotein (gp52) on mouse mammary epithelial cell surface. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1044–1050. doi: 10.1016/0006-291x(77)90961-5. [DOI] [PubMed] [Google Scholar]
- Zangerie P. F., Calberg-Bacq C. M., Colin C., Franchimont P., François C., Gosselin L., Kozma S., Osterrieth P. M. Radioimmunoassay for glycoprotein gp47 of murine mammary tumor virus in organs and serum of mice and search for related antigens in human sera. Cancer Res. 1977 Dec;37(12):4326–4331. [PubMed] [Google Scholar]
- van Pelt F. G., Houweling A., Aarssen A. M., Bentvelzen P. Lack of interference in antigen production in vitro between B-type and C-type oncornaviruses as determined by double stain immunofluorescence. Eur J Cancer. 1976 Apr;12(4):321–323. doi: 10.1016/0014-2964(76)90113-4. [DOI] [PubMed] [Google Scholar]



