Abstract
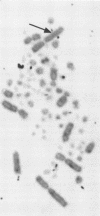
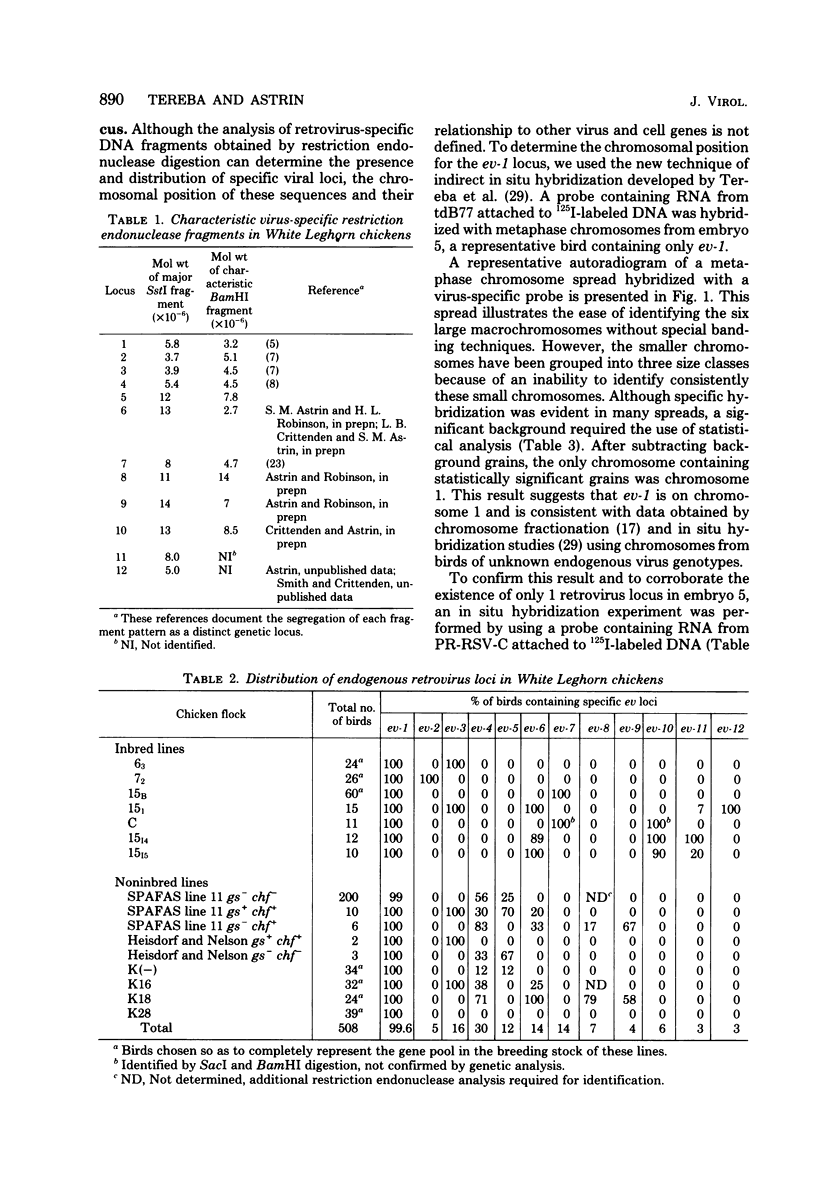
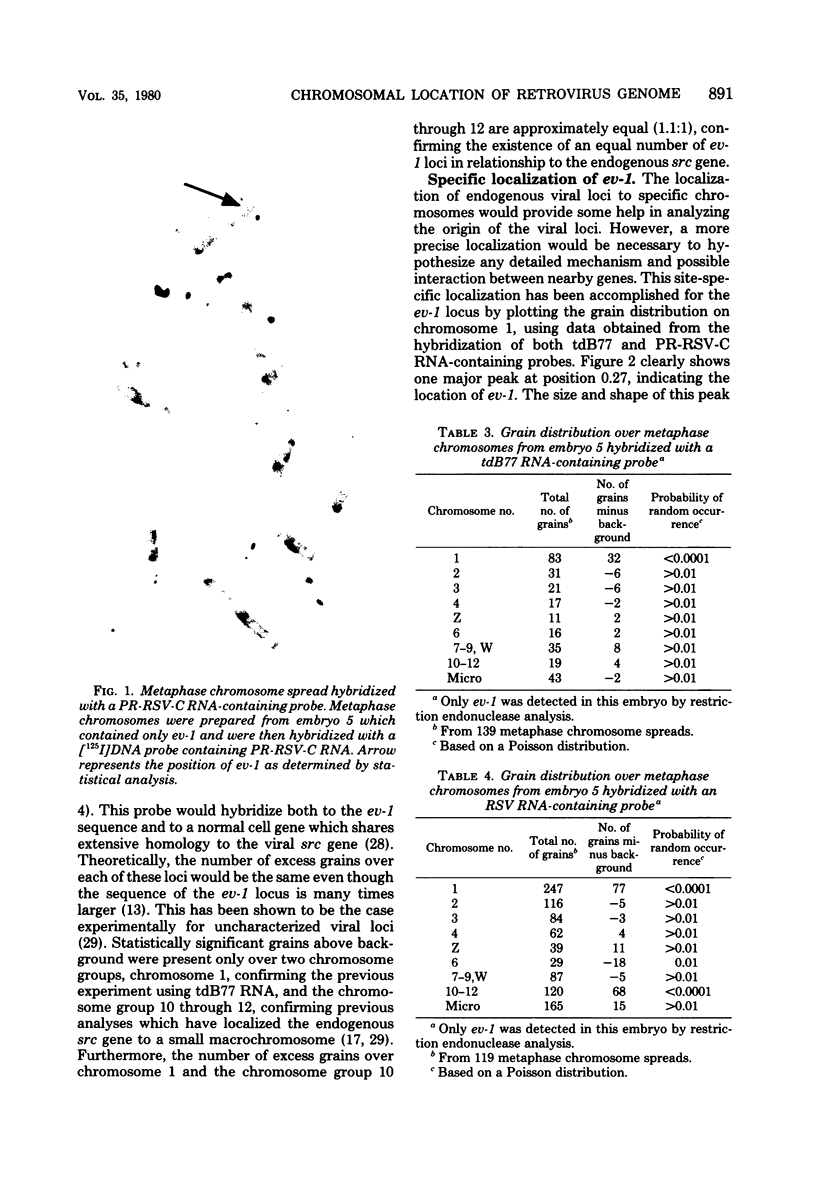
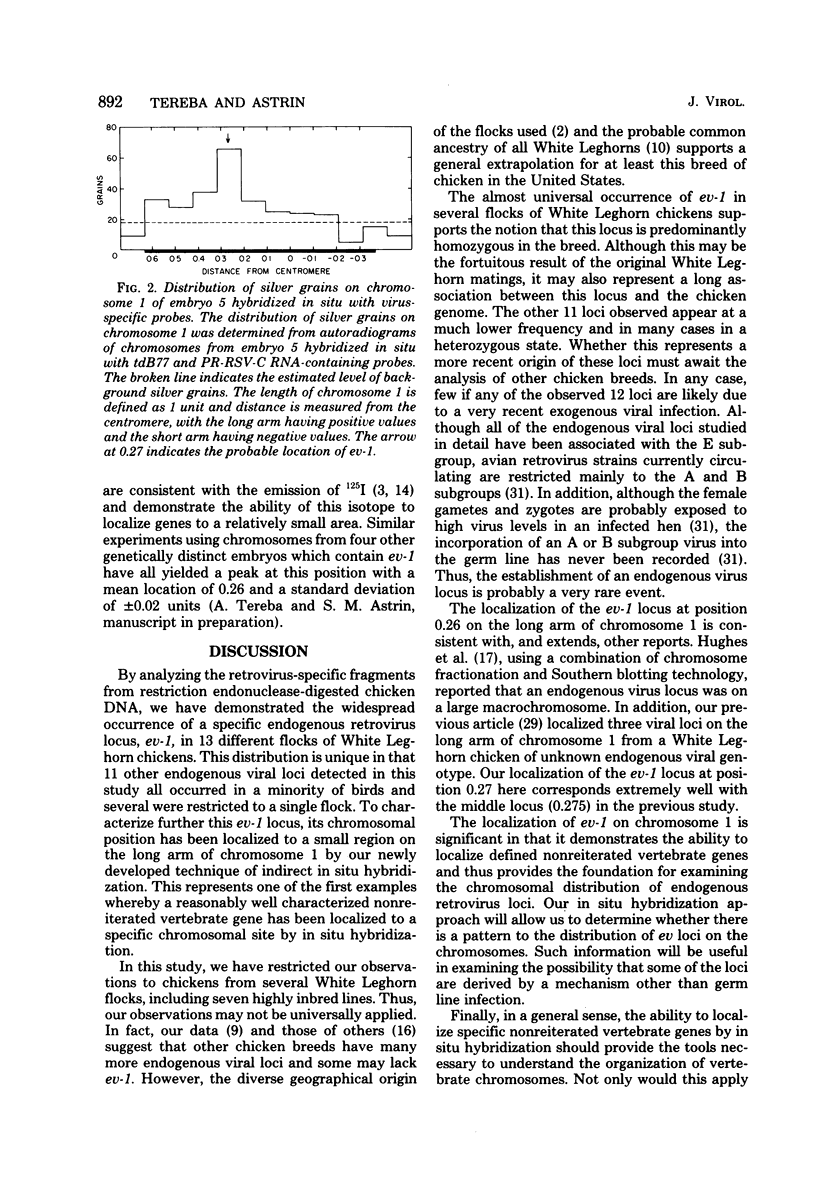
The distribution of 12 endogenous retrovirus loci from 13 White Leghorn flocks of diverse geographical origin was examined, using a total of 508 birds. Only one locus, designated ev-1, was observed to be almost universally present (506 of 508 birds) and thus predominantly homozygous in this breed of chicken. By contrast, 11 other viral loci were observed at much lower frequencies, 3 to 30%, and were occasionally restricted to individual flocks. To further characterize the commonly occurring ev-1 viral locus and to provide a foundation for determining the mechanism by which these 12 endogenous retrovirus loci were generated, a new technique of indirect in situ hybridization was used to localize the chromosomal position of ev-1. By using a combination of specific probes, the position of ev-1 was localized to a small region on the long arm of chromosome 1. This research represents oen of the first instances in which a defined segment of nonreiterated DNA coding for a known set of genes has been localized to a specific region on a vertebrate chromosome by in situ hybridization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Ada G. L., Humphrey J. H., Askonas B. A., McDevitt H. O., Nossal G. J. Correlation of grain counts with radioactivity (125I and tritium) in autoradiography. Exp Cell Res. 1966 Mar;41(3):557–572. doi: 10.1016/s0014-4827(66)80106-4. [DOI] [PubMed] [Google Scholar]
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Astrin S. M., Crittenden L. B., Buss E. G. Ev 2, a genetic locus containing structural genes for endogenous virus, codes for Rous-associated virus type 0 produced by line 72 chickens. J Virol. 1980 Jan;33(1):250–255. doi: 10.1128/jvi.33.1.250-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M. Endogenous viral genes of the White Leghorn chicken: common site of residence and sites associated with specific phenotypes of viral gene expression. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5941–5945. doi: 10.1073/pnas.75.12.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L. Gs, an allele of chickens for endogenous avian leukosis viral antigens, segregates with ev 3, a genetic locus that contains structural genes for virus. J Virol. 1979 Aug;31(2):420–425. doi: 10.1128/jvi.31.2.420-425.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A. Widespread presence, in chickens, of DNA complementary to the RNA genome of avian leukosis viruses. Proc Natl Acad Sci U S A. 1972 Mar;69(3):576–580. doi: 10.1073/pnas.69.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano H. S., Dougherty R. M. Mechanisms for congenital transmission of avian leukosis virus. J Natl Cancer Inst. 1966 Dec;37(6):869–883. [PubMed] [Google Scholar]
- Duesberg P., Vogt P. K., Beemon K., Lai M. Avian RNA tumor viruses: mechanism of recombination and complexity of the genome. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):847–857. doi: 10.1101/sqb.1974.039.01.099. [DOI] [PubMed] [Google Scholar]
- Ertl H. H., Feinendegen L. E., Heiniger H. J. Iodine-125, a tracer in cell biology: physical properties and biological aspects. Phys Med Biol. 1970 Jul;15(3):447–456. doi: 10.1088/0031-9155/15/3/005. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Payvar F., Spector D., Schimke R. T., Robinson H. L., Payne G. S., Bishop J. M., Varmus H. E. Heterogeneity of genetic loci in chickens: analysis of endogenous viral and nonviral genes by cleavage of DNA with restriction endonucleases. Cell. 1979 Oct;18(2):347–359. doi: 10.1016/0092-8674(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Stubblefield E., Payvar F., Engel J. D., Dodgson J. B., Spector D., Cordell B., Schimke R. T., Varmus H. E. Gene localization by chromosome fractionation: globin genes are on at least two chromosomes and three estrogen-inducible genes are on three chromosomes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1348–1352. doi: 10.1073/pnas.76.3.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess R. E., Hayward W. S., Hanafusa H. Cellular information in the genome of recovered avian sarcoma virus directs the synthesis of transforming protein. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3154–3158. doi: 10.1073/pnas.76.7.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W., Hanafusa H., Tilghman S., Skalka A. Structural studies on oncornavirus-related sequences in chicken genomic DNA: two-step analyses of EcoRI and Bgl I restriction digests and tentative mapping of a ubiquitous endogenous provirus digests and tentative mapping of a ubiquitous endogenous provirus. Proc Natl Acad Sci U S A. 1979 May;76(5):2165–2169. doi: 10.1073/pnas.76.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- RUBIN H. Interactions between Newcastle disease virus (NDV), antibody and cell. Virology. 1957 Dec;4(3):533–562. doi: 10.1016/0042-6822(57)90085-5. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Astrin S. M., Salazar F. H. V-15B, an allele of chickens for the production of a noninfectious avian leukosis virus. Virology. 1979 Nov;99(1):10–20. doi: 10.1016/0042-6822(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Robinson H. L., Robinson W. S., Hanafusa T., Hanafusa H. DNA in uninfected and virus-infected cells complementary to avian tumor virus RNA. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2336–2340. doi: 10.1073/pnas.68.10.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tereba A., Lai M. M., Murti K. G. Chromosome 1 contains the endogenous RAV-0 retrovirus sequences in chicken cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6486–6490. doi: 10.1073/pnas.76.12.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereba A., Skoog L., Vogt P. K. RNA tumor virus specific sequences in nuclear DNA of several avian species. Virology. 1975 Jun;65(2):524–534. doi: 10.1016/0042-6822(75)90057-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Vogt P. K. Appearance of virus-specific DNA in mammalian cells following transformation by Rous sarcoma virus. J Mol Biol. 1973 Mar 15;74(4):613–626. doi: 10.1016/0022-2836(73)90052-1. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Weiss R. A., Friis R. R., Levinson W., Bishop J. M. Detection of avian tumor virus-specific nucleotide sequences in avian cell DNAs (reassociation kinetics-RNA tumor viruses-gas antigen-Rous sarcoma virus, chick cells). Proc Natl Acad Sci U S A. 1972 Jan;69(1):20–24. doi: 10.1073/pnas.69.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigne R., Breitman M. L., Moscovici C., Vogt P. K. Restitution of fibroblast-transforming ability in src deletion mutants of avian sarcoma virus during animal passage. Virology. 1979 Mar;93(2):413–426. doi: 10.1016/0042-6822(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Halpern C. C., Nadel M., Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]