Abstract
The experimental results reported in this letter suggest that single-walled carbon nanotubes (SWCNTs) have the potential to enhance dielectric contrast between malignant and normal tissue for microwave detection of breast cancer and facilitate selective heating of malignant tissue for microwave hyperthermia treatment of breast cancer. In this study, we constructed tissue-mimicking materials with varying concentrations of SWCNTs and characterized their dielectric properties and heating response. At SWCNT concentrations of less than 0.5% by weight, we observed significant increases in the relative permittivity and effective conductivity. In microwave heating experiments, we observed significantly greater temperature increases in mixtures containing SWCNTs. These temperature increases scaled linearly with the effective conductivity of the mixtures. This work is a first step towards the development of functionalized, tumor-targeting SWCNTs as theranostic (integrated therapeutic and diagnostic) agents for microwave breast cancer detection and treatment.
Index Terms: Breast cancer, carbon nanotubes, contrast agent, dielectric spectroscopy, microwave imaging, microwave hyperthermia, phantoms
I. Introduction
Microwave-frequency dielectric contrast between malignant and normal tissue in the breast serves as the physical basis for emerging microwave methods of detecting and treating breast cancer. Dielectric contrast leads to scattering of an illuminating microwave signal, which is exploited for breast cancer detection, and selective absorption of incident microwave power, which is exploited for localized hyperthermia treatment. The effective dielectric properties of breast tissue are influenced at microwave frequencies by endogenous polar molecules, such as free and bound water, peptides, and proteins. Consequently, the dielectric properties depend on the type and physiological state of the tissue. A recent study conducted by the University of Wisconsin-Madison and the University of Calgary showed that the endogenous dielectric contrast between malignant and normal adipose-dominated tissues in the breast is as large as 10:1, while the contrast between malignant and normal glandular/fibroconnective tissues is no more than about 10% [1].
The effective dielectric properties – both the dielectric constant and effective conductivity – can also be influenced by exogenous molecules introduced as contrast agents. For example, another recent study found that tissue-mimicking (TM) mixtures containing encapsulated microbubbles at clinically relevant concentrations, similar to those used in ultrasound imaging, exhibit significantly lower dielectric properties than pure TM materials [2]. Here, we propose the use of single-walled carbon nanotubes (SWCNTs) as a diagnostic and therapeutic agent – an integrated “theranostic” nanoplatform [3] – for both microwave detection and treatment of breast cancer. The size and unique physiochemical properties of SWCNTs make them ideal candidates for tumor targeting applications [4]. SWCNT-based contrast agents have already shown promise for molecular imaging by MR, PET, nuclear, and photoacoustic imaging modalities [5]–[7].
Our present investigation is motivated by the hypothesis that the accumulation of biocompatible carbon nanotubes in tumors will significantly increase the microwave-frequency dielectric properties of the tumor. Exogenous particles passively accumulate via an enhanced permeability and retention effect in tumor vasculature [8]. Functionalization of these particles with tumor targeting biomarkers may amplify their accumulation properties in tumors [9]. The presence of carbon nanotubes in or near a malignant lesion will result in changes to the dielectric properties of malignant tissue. If these changes are significant, they can be used to enhance the sensitivity of low-power microwave imaging of breast cancer via differential imaging [10], [11] and to enhance the selectivity of higher-power microwave thermal therapy.
In this paper, we report the results of wideband (0.6-20 GHz) dielectric spectroscopy measurements and heating efficiency experiments (at 3 GHz) using TM materials mixed with various concentrations of SWCNTs that have been identified to be non-toxic in mice [12]. Our study addresses the dielectric and thermal effect of SWCNTs over the frequency range that is of interest for microwave imaging and hyperthermia treatment of breast cancer. Frequencies between 0.5 and 3 GHz are commonly employed in microwave imaging via inverse scattering [13] while the ultrawideband range of 3.1 to 10.6 GHz is of interest in tissue-penetrating radar imaging [14]. Microwave-induced thermoacoustic imaging has been explored using 434 MHz [15] and 3 GHz [16]. Microwave hyperthermia treatment of breast cancer has been conducted at 915 MHz [17], although slightly higher frequencies (1.5-4.0 GHz) have been shown recently to be optimal for tightly focusing microwave energy in the breast [18]. We choose 3 GHz for our heating experiments because of its relevance to both microwave-induced thermoacoustic imaging and hyperthermia treatment. Our work complements previous radiofrequency heating studies [19] as well as previous microwave-frequency characterizations of the dielectric properties of mixtures of SWCNTs or multi-walled carbon nanotubes in various host media of interest for electromagnetic shielding and radar absorption [20]–[26].
II. Materials and methods
A. Construction of samples
The TM materials were constructed from oil-in-gelatin dispersions following the procedure outlined in [27]. The dielectric properties of these materials can be customized to mimic the properties of a variety of human soft tissues by controlling the concentrations of gelatin, safflower oil, kerosene, and preservatives. The results reported in [1] show that a 10%-oil mixture adequately replicates the microwave properties of malignant breast tissue over our frequency range of interest (0.6 GHz to 20 GHz). Furthermore, these materials are relatively inexpensive to fabricate and possess long term stability.
For this study we constructed 10%-oil TM materials with various concentrations of SWCNTs. The SWCNTs were synthesized by a commercial vendor (Cheap Tubes Inc., Brattleboro, VT) using a chemical vapor deposition technique and acid treated for purification. According to the manufacturer's specifications, the nanotubes were 1-2 nm in diameter and 5-30 μm in length, and were composed of mainly SWCNTs (>90% by weight) with trace amounts of multi-walled carbon nanotubes and metal catalyst (e.g. cobalt, 1% by weight). We mixed various concentrations (1 mg/mL, 2 mg/mL, and 3 mg/mL) of SWCNTs into a 1% by weight mixture of Pluronic (F127) and deionized water using a probe sonicator for 25 minutes at a 120 W power level. Then we substituted these mixtures for the pure deionized water in the TM recipe described in [27]. The resulting percent-by-weight concentrations of SWCNTs in the samples were 0.07%, 0.15%, and 0.22%, respectively. For reference, we also constructed a pure TM mixture with the same amount of Pluronic as the other samples, but with no SWCNTs.
B. Characterization of the dielectric properties
The dielectric properties of the TM samples were characterized using a well established open-ended coaxial probe technique described in [28]. The measurement uncertainty of this technique is no more than approximately 10% [28]. We poured the liquid oil-gelatin mixtures, described in Section II-A, into small covered glass jars and allowed the mixture to gel unperturbed. We positioned the tip of the precision coaxial probe against the surface of the sample [27]. We conducted these measurements at room temperature (approximately 22°C) on three TM samples of each SWCNT concentration.
C. Characterization of the heating response
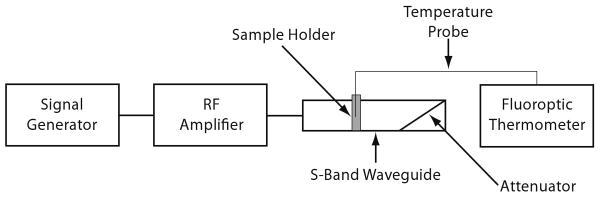
The configuration for the heating experiment is shown in Figure 1. We prepared the samples for the heating experiments by pouring the liquid TM mixture, as described in Section II-A, into a 1.1-mm-inner-diameter glass capillary tube. The mixture was allowed to gel around a fiber-optic temperature probe connected to a calibrated fluoroptic thermometer (Luxtron, 3100). The capillary tube was inserted through a small hole drilled into the center of the broad wall of an S-band (WR-284) rectangular waveguide with cross-sectional dimensions of 72 mm × 34 mm. The vertical extent of the sample in capillary tube spanned the entire height of the waveguide. A microwave synthesizer (Agilent, 83623B) and amplifier (Mini-Circuits, ZHL-42W) generated 1 W of continuous microwave power at 3 GHz that was delivered to the sample via the waveguide. The waveguide was terminated with a matched load to suppress standing waves and ensure single-pass electromagnetic wave illumination. Network analyzer measurements verified that the small hole drilled in the waveguide and the presence of the sample did not significantly perturb the fields inside the waveguide; namely, these waveguide alterations increased |S11| by approximately 0.6 dB. The fluoroptic thermometer recorded the time-varying temperature of each sample as it was heated. We heated each sample for three minutes, then turned off the microwave source and allowed it to cool for five minutes.
Fig. 1.
Experimental setup for characterizing the heating response of a TM sample.
III. Results and discussion
A. Dielectric properties
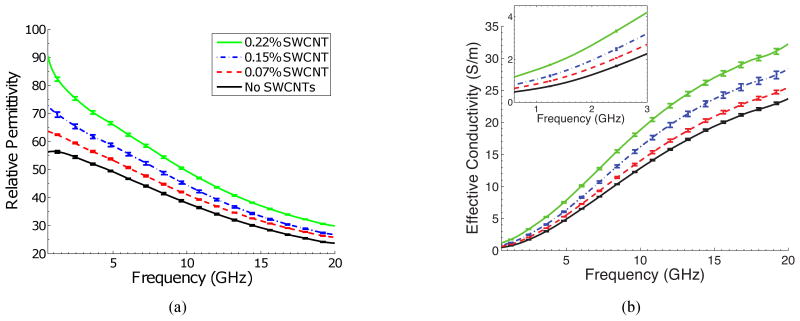
Figures 2(a) and 2(b) show the relative permittivity and effective conductivity of the TM materials with various concentrations of SWCNTs over a frequency range of 0.6-20 GHz. Each wideband curve represents the average properties (relative permittivity or effective conductivity) of the three samples with the same SWCNT concentration. The vertical bars span the maximum and minimum values at specific frequencies. Table I summarizes the dielectric properties of the different TM mixtures at 3 GHz. This particular frequency is of interest for microwave inverse scattering [10], [11], microwave-induced thermoacoustic imaging [16], and microwave hyperthermia [18]. Both Figure 2 and Table I show that the permittivity and conductivity increase with small increases in the concentration of SWCNTs.
Fig. 2.
(a) Relative permittivity and (b) effective conductivity of TM materials with varying concentrations of SWCNTs, measured over the frequency range from 0.6 to 20 GHz. Each curve represents the average properties of three different samples of the same concentration. The bars span the maximum and minimum values at specific frequencies. Both the permittivity and effective conductivity of the TM materials increase with increasing concentration of SWCNTs.
TABLE I. Summary of data presented in Figure 2 at 3 GHz.
| Percent by weight concentration of SWCNTs | Average εr (3 GHz) | Average σ (3 GHz) | Average percent change εr (3 GHz) | Average percent change σ (3 GHz) |
|---|---|---|---|---|
| None | 53.3 | 2.3 S/m | - | - |
| 0.07 % | 57.9 | 2.7 S/m | 9% | 16 % |
| 0.15 % | 63.7 | 3.2 S/m | 19 % | 38 % |
| 0.22 % | 73.0 | 4.2 S/m | 37 % | 81 % |
These substantial changes in the dielectric properties caused by the carbon nanotubes will have a significant impact on microwave scattering and absorption. The results reported here were used in a microwave imaging computational test bed in order to determine the impact of these changes in microwave breast cancer detection [10], [11]. Briefly, microwave inverse scattering techniques were used to reconstruct images of anatomically realistic numerical breast phantoms with a compact malignant lesion. Two images were reconstructed: an image of a phantom with the endogenous dielectric properties of the tumor and another with elevated dielectric properties values due to the carbon nanotubes. A differential image was formed by subtracting the two images obtaining from pre- and post-contrast measurements. Using this differential imaging technique, Shea et al. ([10], [11]) reported detection of previously undetectable tumors in four different classes of numerical phantoms that ranged from “mostly fatty” to “extremely dense” in radiographic density. These results suggest that the changes in dielectric properties shown in Figure 2 are significant enough to dramatically improve the sensitivity of microwave imaging.
B. Heating response
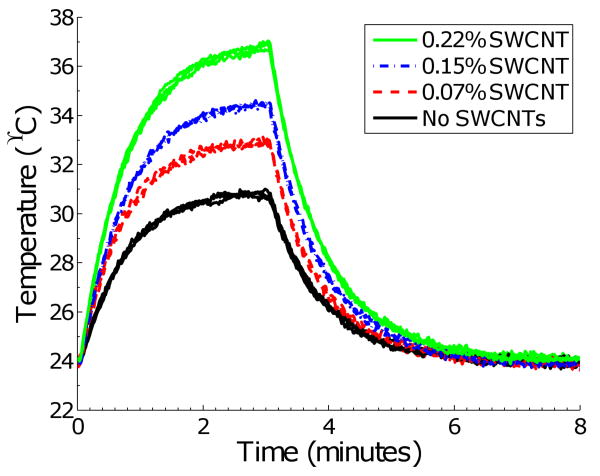
Figure 3 shows the time-dependent temperature rise of the various TM mixtures under continuous microwave illumination. The individual temperature value on each curve was a rolling average of five different temperature measurements of a single sample that was recorded every 0.25 seconds. For each TM mixture concentration, measurements were performed on three different samples of each TM mixture. Figure 3 demonstrates the measurement repeatability for each concentration, as well as the statistical significance of the differences observed across concentrations. Table II summarizes the principal characteristics of the results shown in Figure 3. These data show that as the concentration of SWCNTs in the TM material increases, the maximum temperature of that sample also increases. This increase in temperature for each TM mixture scales roughly linearly with the increase in conductivity reported in Table I, as expected for a configuration in which various samples are exposed to approximately the same electric field intensity. In other experiments, we have observed that microwave-heated capillary tubes of liquids and SWCNT-containing liquids similarly exhibit a temperature rise proportional to their effective conductivity. Therefore, this heating response scaling appears to be a general trend, not restricted to just TM material mixtures.
Fig. 3.
Microwave heating response of TM materials with various concentrations of SWCNTs. Each curve shows the temperature profile of a sample that was heated via 3-GHz microwave illumination for 3 minutes and allowed to cool for 5 minutes. The maximum temperature of the TM mixtures increases with increasing concentration of SWCNTs.
TABLE II. Summary of data presented in Figure 3.
| Percent by weight concentration of SWCNTs | Average steady-state temperature | Average temperature rise | ΔT increase compared to SWCNT-free sample |
|---|---|---|---|
| None | 30.9°C | 6.9°C | - |
| 0.07 % | 33.0°C | 9.0°C | 2.1°C |
| 0.15 % | 34.5°C | 10.5°C | 3.6°C |
| 0.22 % | 36.9°C | 12.9°C | 6.0°C |
The enhanced heating shown in Figure 3 due to the carbon nanotubes is of interest to microwave-induced thermoacoustic imaging and microwave hyperthermia of breast cancer. Our results suggest that the increase in heating response will lead to a larger thermoacoustic response for a malignant tumor that is infused with carbon nanotubes in comparison to a tumor in a pre-contrast stage. In addition, the results of Figure 3 suggest that malignant tissue that has preferentially accumulated carbon nanotubes will heat more efficiently; this selective heating is a desired property for microwave hyperthermia of breast cancer. As seen in Figure 2(b), various samples exhibit a large spread in effective conductivity over the range of 0.6 to 20 GHz. Therefore, we expect a similar heating response to those seen in Figure 3 for these samples over the 0.6 to 20 GHz frequency range.
IV. Conclusion
We characterized the dielectric properties of TM materials with several different concentrations of SWCNTs. Our results indicate that low concentrations of SWCNTs significantly impact the dielectric properties and heating response of the TM materials. For example, at 3 GHz, SWCNTs concentrations as small as 0.22% by weight increased the relative permittivity of the TM material by 37% and the effective conductivity by 81%. In our heating experiments, this concentration of SWCNTs led to an average steady-state temperature rise that was 6°C higher than the rise observed in the TM material without SWCNTs. These results suggest that SWCNTs may enhance contrast for microwave imaging and facilitate selective microwave heating for treatment of breast cancer.
Acknowledgments
This work was supported by the Department of Defense Breast Cancer Research Program under grant W81XWH-07-1-0629, the National Institutes of Health under grant R01CA112398 awarded by the National Cancer Institute, and the Carol M. Baldwin Breast Cancer Research Fund.
Contributor Information
Alireza Mashal, Email: amashal@wisc.edu, Department of Electrical and Computer Engineering, University of Wisconsin, Madison, WI, 53706 USA.
Balaji Sitharaman, Department of Biomedical Engineering, Stony Brook University, Stony Brook, NY, 11794.
Xu Li, Department of Biomedical Engineering, Northwestern University, Evanston, IL, 60208.
Pramod Avti, Department of Biomedical Engineering, Stony Brook University, Stony Brook, NY, 11794.
Alan V. Sahakian, Department of Biomedical Engineering, Northwestern University, Evanston, IL, 60208.
John H. Booske, Department of Electrical and Computer Engineering, University of Wisconsin, Madison, WI, 53706 USA.
Susan C. Hagness, Email: hagness@engr.wisc.edu, Department of Electrical and Computer Engineering, University of Wisconsin, Madison, WI, 53706 USA.
References
- 1.Lazebnik M, Popovic D, McCartney L, Watkins CB, Lindstrom MJ, Harter J, Sewall S, Ogilvie T, Magliocco A, Breslin TM, Temple W, Mew D, Booske JH, Okoniewski M, Hagness SC. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys Med Biol. 2007;52:6093–6115. doi: 10.1088/0031-9155/52/20/002. [DOI] [PubMed] [Google Scholar]
- 2.Mashal A, Booske JH, Hagness SC. Toward contrast-enhanced microwave-induced thermoacoustic imaging of breast cancer: An experimental study of the effects of microbubbles on simple thermoacoustic targets. Phys Med Biol. 2009;54:641–650. doi: 10.1088/0031-9155/54/3/011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumer B, Gao J. Theranostic nanomedicine for cancer. Nanomedicine. 2008;3(2):137–140. doi: 10.2217/17435889.3.2.137. [DOI] [PubMed] [Google Scholar]
- 4.McDevitt M, Chattopadhyay D, Kappel B, Jaggi J, Schiffman S, Antczak C, Njardarson J, Brentjens R, Scheinberg D. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48(7):1180. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 5.Sitharaman B, Wilson L. Gadofullerenes and gadonanotubes: A new paradigm for high-performance magnetic resonance imaging contrast agent probes. Journal of Biomedical Nanotechnology. 2007;3(4):342–352. [Google Scholar]
- 6.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 7.De La Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi1 J, Smith1 BR, Ma TJ, Oralkan O, Cheng Z, Chen X, Dai H, Khuri-Yakub BT, Gambhir SS. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nat Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeda H, Wua J, Sawaa T, Matsumurab Y, Horic K. Tumor vascular permeability and the epr effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 9.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Delivery Rev. 2002;54 doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 10.Shea JD, Kosmas P, Hagness SC, Van Veen BD. Contrast-enhanced microwave breast imaging. 13th International Symposium on Antenna Technology and Applied Electromagnetics (ANTEM) and the Canadian Radio Science Meeting (URSI/CNC); Banff, Canada. 2009. [Google Scholar]
- 11.Shea JD, Kosmas P, Hagness SC, Van Veen BD. Contrast-enhanced microwave imaging of breast tumors. Inverse Prob. doi: 10.1088/0266-5611/26/7/074009. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by raman spectroscopy. PNAS. 2008;105(5):1410. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubæk T, Meaney PM, Meincke P, Paulsen KD. Nonlinear microwave imaging for breast-cancer screening using Gauss–Newton's Method and the cgls inversion algorithm. IEEE Trans Antennas Propag. 2007;55(8):2320–2331. [Google Scholar]
- 14.Li X, Bond EJ, Veen BDV, Hagness SC. An overview of ultrawideband microwave imaging via space-time beamforming for early-stage breast cancer detection. IEEE Antennas Propag Mag. 2005;47:19–34. [Google Scholar]
- 15.Kruger R, Miller K, Reynolds H, Kiser W, Reinecke D, Kruger G. Breast cancer in vivo: Contrast enhancement with thermoacoustic ct at 434 MHz-feasibility study. Radiology. 2000;216(1):279–283. doi: 10.1148/radiology.216.1.r00jl30279. [DOI] [PubMed] [Google Scholar]
- 16.Xu M, Ku G, Jin X, Wang LV, Fornage BD, Hunt KK. Breast cancer imaging by microwave-induced thermoacoustic tomography. Proc SPIE. 2005;5697:45–48. [Google Scholar]
- 17.Vargas HI, Dooley WC, Gardner RA, Gonzalez KD, Venegas R, Heywang-Kobrunner SH, Fenn AJ. Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol. 2004;11(2):139–146. doi: 10.1245/aso.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 18.Zastrow E, Hagness SC, Van Veen BD. 3D computational feasibility study of non-invasive patient-specific microwave hyperthermia treatment of breast cancer. Phys Med Biol. doi: 10.1088/0031-9155/55/13/003. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannon C, Cherukuri P, Yakobson B, Cognet L, Kanzius J, Kittrell C, Weisman R, Pasquali M, Schmidt H, Smalley R, Curley S. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110(12):2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 20.Roberts J, Imholt T, Ye Z, Dyke C, Price D, JR, Tour J. Electromagnetic wave properties of polymer blends of single wall carbon nanotubes using a resonant microwave cavity as a probe. J Appl Phys. 2004;95:4352. [Google Scholar]
- 21.Xiang C, Pan Y, Liu X, Sun X, Shi X, Guo J. Microwave attenuation of multiwalled carbon nanotube-fused silica composites. Appl Phys Lett. 2005;87:123103. [Google Scholar]
- 22.Fan Z, Luo G, Zhang Z, Zhou L, Wei F. Electromagnetic and microwave absorbing properties of multi-walled carbon nanotubes/polymer composites. Mater Sci Eng, B. 2006;132:85–89. [Google Scholar]
- 23.Li YH, Lue JT. Dielectric constants of single-wall carbon nanotubes at various frequencies. J Nanosci Nanotechnol. 2007;7:3185–3188. doi: 10.1166/jnn.2007.658. [DOI] [PubMed] [Google Scholar]
- 24.Moayed NNA, Khan U, Oboll M, Gupta S, Afsar MN. Characterization of single- and multi-walled carbon nanotubes at microwave frequencies. IEEE Instrum Meas Tech Conf. 2007 [Google Scholar]
- 25.Wang L, Zhou R, Xin H. Microwave (8-50 GHz) characterization of multiwalled carbon nanotube papers using rectangular waveguides. IEEE Trans Microwave Theory Tech. 2008;56(2):499–506. [Google Scholar]
- 26.Challa RK, Kajfez D, Demir V, Gladden JR, Elsherbeni AZ. Characterization of multiwalled carbon nanotube (mwcnt) composites in a waveguide of square cross section. IEEE Microwave Wireless Compon Lett. 2008;18:161–163. [Google Scholar]
- 27.Lazebnik M, Madsen EL, Frank GR, Hagness SC. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys Med Biol. 2005;50:4245–4258. doi: 10.1088/0031-9155/50/18/001. [DOI] [PubMed] [Google Scholar]
- 28.Popovic D, McCartney L, Beasley C, Lazebnik M, Okoniewski M, Hagness SC, Booske JH. Precision open-ended coaxial probes for in vivo and ex vivo dielectric spectroscopy of biological tissues at microwave frequencies. IEEE Trans Microwave Theory Tech. 2005;53(5):1713–1722. [Google Scholar]