Abstract
P-selectin and P-selectin glycoprotein ligand 1 (PSGL-1) are vascular adhesion molecules that play an important role in the recruitment of leukocytes to inflamed tissue by establishing leukocyte–endothelial and leukocyte–platelet interaction. P-selectin binds to the amino-terminus of PSGL-1 through recognition of a sialyl Lewisx (SLex) moiety linked to a properly positioned core-2 O-glycan and three tyrosine sulfate residues. We have developed a highly convergent synthesis of the PSGL-1 oligosaccharide linked to threonine based on the use of trichoroacetimidate donors and thioglycosyl acceptors that give products that can immediately be employed in a subsequent glycosylation step without the need for protecting group manipulations. Furthermore, by employing one-pot multi-step glycosylation sequences the number of purification steps could be minimized. The process of oligosaccharide assembly was further streamlined by combining protecting group manipulations and glycosylations as one-pot multi-step synthetic procedure. The resulting PSGL-1 oligosaccharide is properly protected for glycopeptide assembly. It is to be expected that the strategic principles employed for the synthesis of the target compound can be applied for the preparation of other complex oligosaccharides of biological and medical importance.
Introduction
The selectins are a family of three Ca2+ dependent membrane-bound glycoproteins that mediate the adhesion of leukocytes and platelets to vascular surfaces.1,2 Several studies have demonstrated that they play important roles in inflammation, immune responses, hemeostasis and wound repair.3 Selectins also contribute to a broad spectrum of diseases such as arteriosclerosis, thrombosis, organ-transplant rejection, arthritis, sickle cell anemia and tumor metastasis.4-6
Although there are many candidates for selectin ligands, only P-selectin glycoprotein ligand-1 (PSGL-1) has clearly been demonstrated to mediate the adhesion of leukocytes to selectins under flow. P-selectin binds to the amino-terminus of PSGL-1 through recognition of a sialyl Lewisx (SLex) moiety linked to a properly positioned core-2 O-glycan and three tyrosine sulfate residues.7,8
Inhibitors of selectins may possess therapeutic properties for the treatment of a number of diseases.9 In this respect, a recombinant truncated form of a PSGL-1 immunoglobulin fusion protein has already demonstrated effectiveness as such an inhibitor.10-13 This glycoprotein can, however, only be produced in mammalian cells that are co-transfected with fucosyl- and core-2 GlcNAc transferases, making production of even small amounts of glycoprotein difficult. The Davis laboratory is beginning to address these problems by employing a PSGL-1 mimetic by incorporation of azidohomoalanine and cysteine in PSGL-1 using E. coli B834 as a Met-auxotrophic expression system and selectively attachment of sialic acid containing oligosaccharides and a sulfated tyrosine mimics by employing the thiol and azide of the protein as chemical tags.14 Also, it has been shown that conjugation of sialyl Lewisx and sulfates tyrosine to a polyacrylamide gave a polymer with high affinity of L-selectin.15
The N-terminal glycosulfopeptide of PGSL-1 has been obtained by chemo-enzymatic approaches.14,16,17 In these procedures, a glycosulfopeptide that contains an N-acetyl galactosamine linked to a threonine moiety was chemically assembled. Subsequently, glycosyl transferases were employed to assemble the complete oligosaccharide. The problems of this approach include difficulties of preparing sufficient quantities of glycosyltransferases, which often require a eukaryotic cell expression system and the need of expensive sugar nucleotides or the use of a complicated in-situ recycling system. Furthermore, the high selectivity of glycosyltransferases also complicates the preparation of analogs that may exhibit more desirable pharmacological properties.
Recent progress in chemical oligosaccharide synthesis is beginning to provide opportunities for the efficient and large-scale synthesis of complex oligosaccharides18-23 and several laboratories are pursuing the preparation of the oligosaccharide of PSGL-1.24-30 In this respect, Kunz and coworkers have reported the chemical synthesis of a properly protected oligosaccharide of PSGL-1, which was attached to threonine for the preparation of a glycopeptide.26 Although synthetic problems such as anomeric selectivity and the acid and base sensitivity of the PSGL-1 glycopeptide were addressed by employing properly protected saccharide building blocks, the synthetic approach suffered from poor regioselectivity in key glycosylations and a need for replacement of protecting groups at an advanced stage of synthesis. It is to be expected that a highly convergent approach for the synthesis of PSGL-1 will make it possible to prepare a wide range of glycopeptides structural analogs for structure activity relationships. Furthermore, it may offer an opportunity to make mimetics that have improved pharmacokinetic properties.
As part of a program to prepare the PGSL-1 analogs with improved properties, we report here a highly efficient and convergent synthesis of a properly protected oligosaccharide of PSGL-1 linked to threonine (1) that is appropriately protected for solid-phase glycopeptide synthesis. Key features of the approach include an orchestrated use of thioglycosides and trichoroacetimidates31 for oligosaccharide assembly, which minimized protecting group manipulations and made it possible to employ one-pot multi-step glycosylations. The process of oligosaccharide assembly was further streamlined by combining protecting group manipulations and glycosylations as one-pot multi-step synthetic procedure.22,23,32-34 It is to be expected that the strategic principles employed for the synthesis of the target compound can be applied for the preparation of other complex oligosaccharides of biological and medical importance.
Results and Discussion
The synthesis of target compound 1 is complicated by the fact that O-glycosylated peptides are sensitive to acidic and basic conditions. In addition, sufficient quantities of such a complex glycosylated amino acid for glycosulfopeptide assembly can only be obtained by employing a highly convergent synthetic strategy, which uses properly protected monosaccharide building blocks that can be assembled into the target using a minimal number of synthetic steps. In this respect, strategies such as chemoselective, orthogonal, two-directional and one-pot multi-step glycosylations22,23,32-34 have engendered an increased efficiency of oligosaccharide synthesis by minimizing the number of protecting group manipulations on advanced intermediates. Furthermore, combining protecting group manipulations with glycosylations as a one-pot procedure can further expand the scope of these procedures.35-39
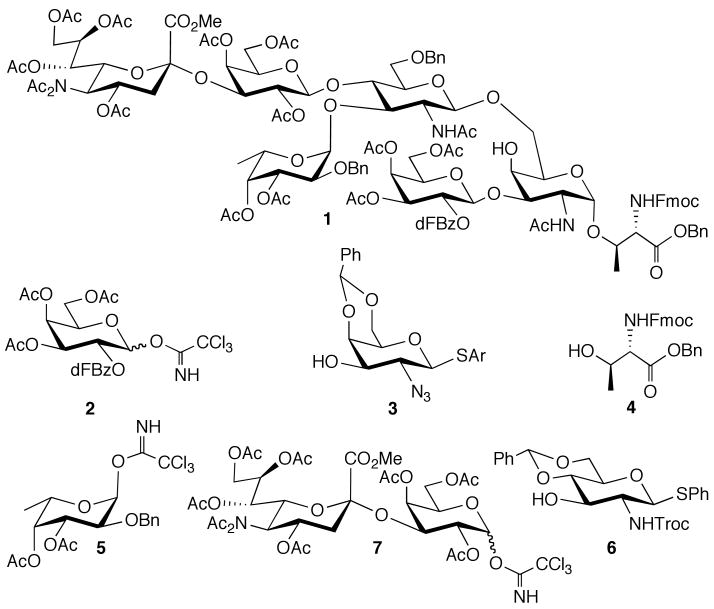
It was envisaged that 1 could be prepared by a combination of chemo- and regioselective glycosylations and one-pot multi-step protocols that combine glycosylations and protecting group manipulations. Thus, the core-2 disaccharide linked to a properly protected threonine (Thr) was prepared by a chemoselective glycosylation of trichloroacetimidate 2 with thioglycoside 3 to give a disaccharide, which immediately can be activated with a thiophilic reagent for coupling with threonine acceptor 440 (Figure 1). Removal of the benzylidene acetal of the resulting compound will give an acceptor for a regioselective coupling with a properly protected SLex derivative. It was envisaged the latter compound could be obtained by chemoselective glycosylations and one-pot reactions using compounds 5,41 642 and 7.
Figure 1.
Target molecule and building blocks.
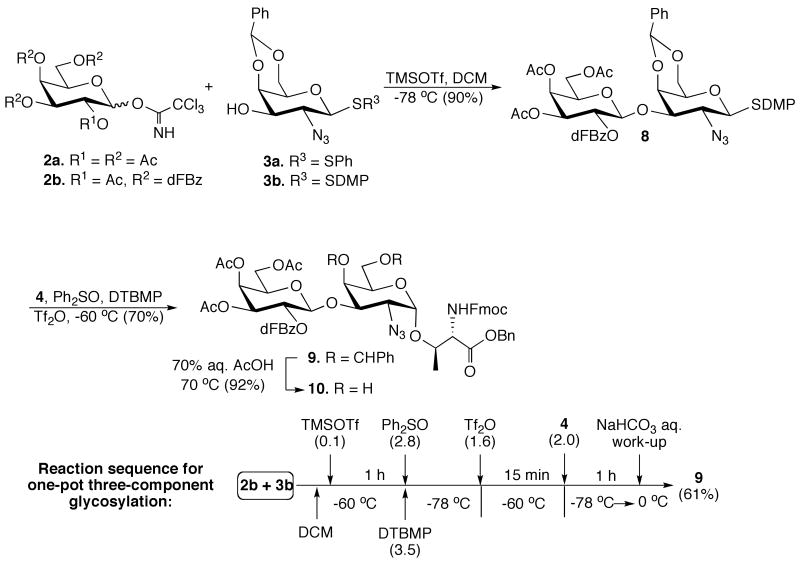
Unfortunately, coupling of galactosyl trichloroacetimidate 2a43 with thioglycosyl acceptor 3a44 in the presence of TMSOTf led to a complex mixture of products that included the required disaccharide, the corresponding ortho-ester45 and a thiophenyl galactoside derived from aglycon-transfer46 (Scheme 1). It is well known that the use of C-2 benzoyl esters will suppress ortho-ester formation, however, this protecting group is not compatible with glycopeptide synthesis because the rather strong basic conditions required for its removal47 will result in β-elimination of the O-glycopeptide linkage. It has been shown that a 2,5-di-fluoro-benzoyl esters (dFBz) is an efficient neighboring group participant that suppresses ortho-ester formation.48,49 This protecting group has, however, as an advantage that it can be removed under mild basic conditions without affecting threonine and serine glycosides. Thus, dFBz-protected glycosyl donor 2b,48,49 having a 2,5-di-fluoro-benzoyl (dFBz) ester at C-2 and acetyl esters at C-3, C-4 and C-6, was coupled with thioglycosyl acceptor 3a50 using TMSOTf as the catalyst.51 Although orthoester formation was suppressed, the aglycon-transfer byproduct was still formed. Recently, it was reported that aglycon transfer of thioglycosyl acceptors can be avoided by employing a 2,6-dimethylthiophenyl glycoside.52 The rationale of this observation is that the bulky 2,6-dimethylthiophenyl hinders reaction with an activated glycosyl donor, thereby reducing aglycon transfer. Indeed, trimethylsilyl triflate (TMSOTf) promoted glycosylation of 2b with 3b52 gave the corresponding disaccharide 8 in an excellent yield of 90% as only the β-anomer. Next, the core-2 O-glycan 9 was obtained in high yield with exclusively α-selectivity by a diphenylsulfoxide/triflic anhydride mediated glycosylation53 of thioglycoside 8 with threonine derivative 4.
Scheme 1.
One-pot three component reaction; SDMP = 2,6-dimethylthiophenyl, dFBz = 2,5-difluorobenzyl.
Having established efficient reaction conditions for the synthesis of 9, attention was focused on its preparation by a one-pot procedure. Thus, coupling of galactosyl trichloroacetimidate 2b with the galactosyl acceptor 3b in presence of TMSOTf followed by activation of the resulting thio-disaccharide 8 by addition of diphenyl sulfoxide and triflic anhydride in presence of DTBMP53 and coupling with threonine 4 gave oligosaccharide 9 in an overall yield of 61%. Finally, glycosyl acceptor 10 was obtained by the removal of the benzylidene acetal of 9 using aqueous acetic acid at 70°C.
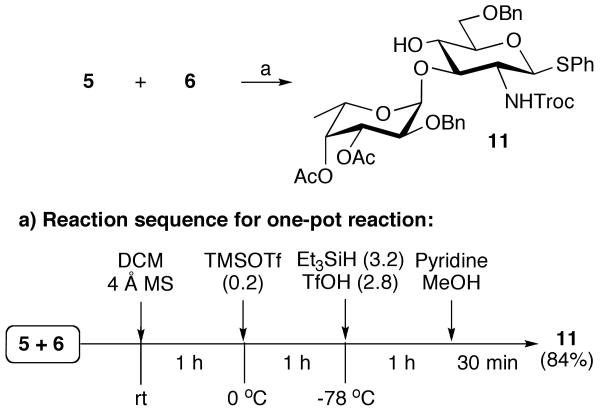
The next stage of the synthesis entailed the preparation of properly protected SLex glycosyl donor 15 for coupling with glycosyl acceptor 10 to give protected PSGL-1 16. Compound 15 was prepared from the readily available saccharide building blocks 5, 6, and 7 (Schemes 2 and 3). Thus, fucosyl trichloroacetimidate 5 was coupled with phenyl thioglycosyl acceptor 6 using TMSOTf as the promoter to give the corresponding disaccharide, exclusively as the α-anomer, which was then treated with triethylsilane and trifluoromethanesulfonic acid (TfOH) for regioselective opening of the benzylidene acetal39,54 to provide glycosyl acceptor 11 in an overall yield of 84% with excellent stereo- and regio-selectivity. The regioselectivity of the latter reaction was confirmed by acetylation of compound 11 and the 1H-NMR of the resulting derivative showed a significant down field shift for H-4 (4.94 ppm).
Scheme 2.
One-pot glycosylation followed by reductive opening of the benzylidene acetal.
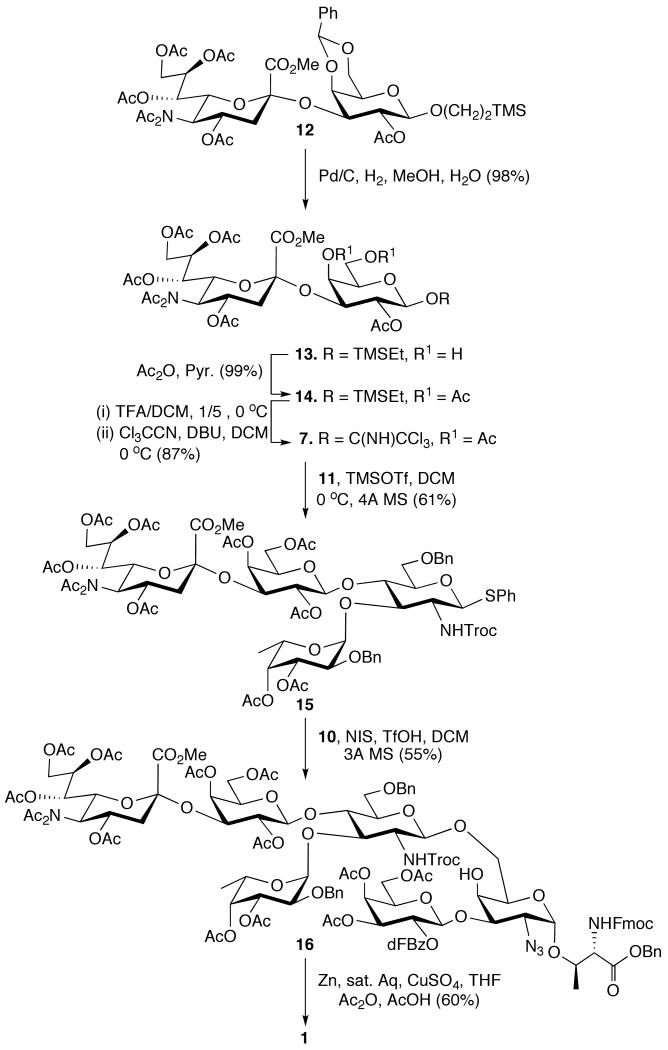
Scheme 3.
Synthesis of target molecule.
Glycosyl donor 7 could be obtained in facile manner from the known disaccharide 1255 by a four-step reaction sequence. Thus, hydrogenation of 12 over Pd/C to remove the benzylidene acetal was followed by acetylation of the hydroxyls of the resulting compound 13 to give 14 in a quantitative overall yield. Next, the anomeric trimethylsilylethyl moiety of 14 was cleaved by treatment with trifluoroacetic acid in dichloromethane and the resulting lactol was converted into trichloroacetimidate 7 by reaction with trichloroacetonitrile and DBU in dichloromethane.
Next, a TMSOTf mediated coupling of trichloroactimidate 7 with 11 gave the properly protected SLex tetrasaccharide 15 in good yield. Up to this stage of the synthesis, the thiophenyl moiety of 15 has functioned as an effective anomeric-protecting group. However, in the next step it was activated with the thiophilic promoter NIS/TfOH for coupling with 10 to give the hexasaccharide 16 in a yield of 55%. As expected, no glycosylation of the less reactive C-4 hydroxyl of 11 was observed, which was confirmed by a range of two-dimensional NMR experiments. Thus, Heteronuclear Multiple Bond Correlation NMR Spectroscopy (HMBC) of 16 showed a cross peak between H-1 of β-GluNTroc (4.62 ppm) and C-6 of α-GalN3 (69.3 ppm), confirming that the glycosylation had occurred at the C-6 hydroxyl of 10. The latter was also supported by Nuclear Overhauser Enhancement Spectroscopy (NOESY), which revealed cross peaks between the H-1 of β-GluNTroc and H-6a and H-6b of the α-GalN3 moiety. Furthermore, due to effective neighboring group participation of the N-Troc group of 15 only the β-glycoside was formed, which was confirmed by a large coupling constant between H-1 and H-2 (10.0 Hz).
Finally, the Troc and the azido moiety of 16 were converted into acetamido functions by reduction with Zn/CuSO4 in a mixture of THF, acetic acid and acetic anhydride56 to give the target compound 1. It is envisaged that the benzyl ester of compound 1 can be removed by performing hydrogenolysis over palladium in a mixture of isopropanol and pyridine.57 The benzyl ethers in the glycan will be removed after glycopeptide assembly using the previously described “low TfOH” method.58,59
In conclusion, a properly protected PSGL-1 oligosaccharide linked to threonine has been described that is appropriately protected for glycosulfopeptide assembly. A highly convergent strategy utilizing six strategically protected building blocks, combined with one-pot, chemoselective and regioselective glycosylations was employed to minimize the number of protecting group manipulations and purifications during oligosaccharide assembly. The longest linear sequence entailed only seven chemical steps and gave the target compounds in an excellent overall yield of 17%. Previous attempts to chemically synthesize the PSGL-1 oligosaccharide suffered from extensive replacement of protecting groups at advanced stages of the synthesis and poor regioselectivities in crucial glycosylation steps compromising the poor overall yield of target compound.26 It is to be expected that the strategic principles employed for the synthesis of 1 will be relevant for the synthesis of many other complex oligosaccharides of biological and medical importance.
Experimental Section
2,6-Dimethylphenyl [2-Azido-4,6-O-benzylidene-2-deoxy-3-O-(3,4,6-tri-O-acetyl-2-O-(2,5-difluorobenzoyl)-β-D-galactopyranosyl)]-1-thio-α-d-galactopyranoside (8)
A mixture of galactosyl acceptor 3b (93 mg, 0.22 mmol), galactosyl trichloroacetaimidate donor 2b (200 mg, 0.34 mmol), and 4Å MS in CH2Cl2 (3 ml) was placed under an atmosphere of argon and stirred at room temperature for 1 h. The reaction mixture was then cooled to -78 °C. TMSOTf (0.023 mmol, 0.23m solution in CH2Cl2) was added and the temperature was raised to -15 °C with stirring over a period of 2 h. The progress of the reaction was monitored by TLC and MALDI-ToF MS. The reaction mixture was diluted with CH2Cl2 (20 ml), filtered, and washed with sat. aq. NaHCO3 solution (10 ml), water (10 ml), and brine (10 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 2:1, v:v) to afford compound 8 (170 mg, 90%) as a white foam. Analytical data for 8: Rf = 0.35 (Hexanes:EtOAc, 2:1, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.52-6.97 (m, 6H, aromatic), 5.49 (dd, 1H, J1′,2′ = 8.2 Hz, J2′,3′ = 10.2 Hz, H-2′), 5.43 (s, 1H, CHPh), 5.38 (bd, 1H, J = 3.2 Hz, H-4′), 5.12 (dd, 1H, J3′,4′ = 3.1 Hz, J2′,3′ = 10.5 Hz, H-3′), 4.96 (d, 1H, J1′,2′ = 7.9 Hz, H-1′), 4.18 (m, 2H, H-1, H-4), 4.15-4.05 (m, 3H, H-6a, H-6a′, H-6b′), 3.91-3.85 (m, 2H, H-5′, H-6b), 3.72 (t, 1H, J1,2 = J2,3 = 9.9 Hz, H-2), 3.46 (dd, 1H, J3,4 = 3.1 Hz, J2,3 = 9.9 Hz, H-3), 3.17 (bs, 1H, H-5), 2.51 (s, 6H, 2 × CH3, SDMP), 2.11 (s, 3H, COCH3), 1.98 (s, 3H, COCH3), 1.87 (s, 3H, COCH3) ppm. 13C from HSQC (125.7 MHz, CDCl3) : δ = 102.3 (C-1′), 101.1 (CHPh), 89.4 (C-1), 80.9 (C-3), 75.3 (C-4), 71.4 (C-5′), 71.2 (C-3′), 70.3 (C-2′), 70.0 (C-5), 69.6 (C-6), 67.3 (C-4′), 62.4 (C-2), 61.8 (C-6′), 22.9 (CH3-SDMP), 21.0, 20.9, 20.8 (3 × OAc); HR-MALDI-ToF/MS: m/z: calc. for C40H41F2N3O13S [M+Na]+: 864.2226; found 864.2231.
N-(9-Fluorenylmethyloxycarbonyl)-O-[2-Azido-4,6-O-benzylidene-2-deoxy-3-O-(3,4,6-tri-O-acetyl-2-O-(2,5-difluorobenzoyl)-β-D-galactopyranosyl)-α-D-galactopyranosyl]-l-threonine benzylester (9)
Method A
A mixture of disaccharide donor 8 (170 mg, 0.20 mmol), Ph2SO (114 mg, 0.56 mmol), and 4Å MS in CH2Cl2 (5 ml) was placed under an atmosphere of argon and stirred at room temperature for 1 h. The reaction mixture was then cooled to -60 °C after the addition of 2,6-di-tert-butyl-4-methylpyridine (124 mg, 0.60 mmol). Stirring was continued for 10 min. at the same temperature followed by the addition of Tf2O (47 μL, 0.28 mmol). Stirring was continued for another 15 min at the same temperature followed by the addition of a solution of threonine acceptor 4 (173 mg, 0.40 mmol) in CH2Cl2 (2 ml). The temperature of the reaction mixture was raised to 0 °C over a period of 1 h. The progress of reaction was monitored by TLC and MALDI-ToF MS. The reaction mixture was diluted with CH2Cl2 (30 ml), filtered, and washed with sat. aq. NaHCO3 solution (15 ml), water (15 ml), and brine (15 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 2:1, v:v) to afford compound 9 (156 mg, 70%) as a white amorphous solid.
Method B
A mixture of galactosyl acceptor 3b (65 mg, 0.16 mmol), galactosyl trichloroacetaimidate donor 2b (120 mg, 0.20 mmol), and 4Å MS in CH2Cl2 (2 ml) was placed under an atmosphere of argon and stirred at room temperature for 1 h. The reaction mixture was then cooled to -60 °C. TMSOTf (0.016 mmol, 0.16M solution in CH2Cl2) was added and stirring was continued for 1 h at the same temperature. The reaction mixture was then cooled to -78 °C followed by addition of Ph2SO (88 mg, 0.44 mmol) and 2,6-di-tert-butyl-4-methylpyridine (112 mg, 0.55 mmol). After stirring for 10 min. at the same temperature, Tf2O (37 μL, 0.22 mmol) was added followed by increasing the temperature to -60 °C over a period of 15 min. The reaction mixture was again cooled to -78 °C followed by addition of a solution of threonine acceptor 4 (100 mg, 0.23 mmol) in CH2Cl2 (1 ml). The temperature of the reaction mixture was raised to 0 °C over a period of 1 h. The progress of reaction was monitored by TLC and MALDI-ToF MS. The reaction mixture was diluted with CH2Cl2 (20 ml), filtered, and washed with sat. aq. NaHCO3 solution (10 ml), water (10 ml), and brine (10 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 2:1, v:v) to afford compound 9 (106 mg, 61%) as a white amorphous solid. Analytical data for 9: Rf = 0.25 (Hexanes:EtOAc, 2:1, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.72-6.87 (m, 21H, aromatic), 5.66 (d, 1H, J = 9.4, NHFmoc), 5.51-5.45 (m, 2H, CHPh, H-2′), 5.40 (bd, 1H, H-4′), 5.15 (dd, 1H, J3′,4′ = 3.2 Hz, J2′,3′ = 10.2 Hz, H-3′), 5.10 (bt, 2H, CH2, Bn), 4.88 (d, 1H, J1′,2′ = 7.9 Hz, H-1′), 4.83 (d, 1H, J1,2 = 3.5, H-1), 4.46-4.25 (m, 5H, OCHCH3 threonine, CH2CH-Fmoc, CHCOOBn threonine, H-4,), 4.21-4.08 (m, 4H, H-6a′, CH2CH-Fmoc, H-6b′, H-6a), 3.96-3.92 (m, 3H, H-6b, H-3, H-5′), 3.69 (dd, 1H, J1,2 = 3.5 Hz, J2,3 = 10.8 Hz, H-2), 3.56 (bs, 1H, H-5), 2.11 (s, 3H, OCH3), 1.97 (s, 3H, COCH3), 1.87 (s, 3H, COCH3), 1.23 (d, 3H, OCHCH3 threonine) ppm. 13C from HSQC (125.7 MHz, CDCl3) : δ = 102.3 (C-1′), 100.8 (CHPh), 99.4 (C-1), 76.2 (OCHCH3 threonine), 75.9 (C-4), 75.8 (C-3), 71.2 (C-5′), 71.1 (C-3′), 70.3 (C-2′), 69.3 (C-6), 68.0 (CH2Ph), 67.6 (CH2CH-Fmoc), 67.3 (C-4′), 63.7 (C-5), 61.5 (C-6′), 59.5 (C-2), 58.9 (CHCOOBn threonine), 47.4 (CH2CH-Fmoc), 21.0, 20.9, 20.7 (3 × OAc), 19.0 (OCHCH3 threonine); HR-MALDI-ToF/MS: m/z: calc. for C58H56F2N4O18 [M+Na]+: 1157.3455; found 1157.3460 [M+Na]+.
N-(9-Fluorenylmethyloxycarbonyl)-O-[2-azido-2-deoxy-3-O-(3,4,6-tri-O-acetyl-2-O-(2,5-difluorobenzoyl)-β-d-galactopyranosyl)-α-d-galactopyranosyl]-l-threonine benzyl ester (10)
A solution of compound 9 (80 mg, 0.072 mmol) in 5 ml of 70% aq. acetic acid was heated at 70 °C for 3 h. The progress of the reaction was monitored by TLC and MALDI-ToF MS. The reaction was cooled to rt and concentrated by co-evaporation with toluene in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 1:2, v:v) to afford compound 10 (59 mg, 92%) as a white amorphous solid. Analytical data for 10: Rf = 0.25 (Hexanes:EtOAc, 1:2, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.72-6.94 (m, 16H, aromatic), 5.60 (d, 1H, J = 9.5 Hz, NHFmoc), 5.50-5.46 (q, 1H, J1′,2′ = 8.6 Hz, J3′,2′ = 10.3 Hz, H-2′), 5.40 (bd, 1H, H-4′), 5.16 (q, 1H, J3′,4′ = 2.9 Hz, J2′,3′ = 10.3 Hz, H-3′), 5.12-5.01 (dd, 2H, CH2Ph), 4.81 (d, 1H, J1′,2′ = 7.8 Hz, H-1′), 4.76 (d, 1H, J1,2 = 3.4 Hz, H-1), 4.41-4.35 (m, 3H, OCHCH3 threonine, CHCOOBn threonine, CHH-Fmoc), 4.25-4.21 (m, 1H, CHH-Fmoc), 4.16-4.05 (m, 4H, H-6a′, H-6b′, H-4, CH2CH-Fmoc), 3.96 (m, 1H, H-5′), 3.91-3.88 (dd, 1H, J3,4 = 2.7 Hz, J2,3 = 10.7 Hz, H-3), 3.83-3.70 (m, 3H, H-5, H-6a, H-6b), 3.46-3.44 (dd, 1H, J1,2 = 3.7 Hz, J2,3 = 10.5 Hz, H-2), 2.75 (bs, 1H, C4-OH), 2.32 (bs, 1H, C6-OH), 2.13 (s, 3H, OCH3), 2.00 (s, 3H, COCH3), 1.90 (s, 3H, COCH3), 1.24 (d, 3H, OCHCH3) ppm. 13C from HSQC (125.7 MHz, CDCl3) : δ = 101.8 (C-1′), 99.2 (C-1), 78.1 (C-3), 76.2 (OCHCH3 threonine), 71.7 (C-5′), 70.7 (C-3′), 69.8 (C-2′), 69.7 (C-5), 69.3 (C-4), 67.8 (CH2Ph), 67.4 (CH2CH-Fmoc), 67.2 (C-4′), 62.7 (C-6), 61.6 (C-6′), 59.0 (C-2), 58.7 (CHCOOBn threonine), 47.4 (CH2CH-Fmoc), 20.8, 20.7, 20.6 (3 × OAc), 18.6 (OCHCH3 threonine); HR-MALDI-ToF/MS: m/z: calc. for C51H52F2N4O18 [M+Na]+: 1069.3142; found 1069.3140.
Phenyl 3,4-di-O-acetyl-2-O-benzyl-6-deoxy-5-methyl-α-l-fucopyranosyl-(1→3)-2-(2,2,2-trichloroethoxy)carbonyl amino-6-O-benzyl-2-deoxy-1-thio-β-d-glucopyranoside (11)
A mixture of glycosyl acceptor 6 (50 mg, 0.09 mmol), trichloroacetimidate donor 5 (63 mg, 0.13 mmol), and 4Å MS in CH2Cl2 (1 ml) was placed under an atmosphere of argon and stirred at room temperature for 1 h. The reaction mixture was then cooled to 0 °C. TMSOTf (0.018 mmol, 0.18m solution in CH2Cl2) was added and stirring was continued for 30 min at the same temperature. The reaction mixture was then cooled to -78 °C followed by addition of TfOH (23 μL, 0.26 mmol) and triethylsilane (48 μL, 0.30 mmol). The reaction mixture was then stirred at -78 °C for 30 min. The progress of the reaction was monitored by TLC and MALDI-ToF MS. The reaction was quenched by the addition of pyridine (25 μL) and MeOH (0.2 ml), diluted with CH2Cl2 (20 ml), and washed with sat. aq. NaHCO3 solution (10 ml), water (10 ml), and brine (10 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 2:1, v:v) to afford compound 11 (67 mg, 84%) as a white amorphous solid. Analytical data for 11: Rf = 0.40 (Hexanes:EtOAc, 1:1, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.50-7.17 (m, 15H, aromatic), 5.46 (d, 1H, J = 6.6 Hz, NHTroc), 5.30-5.27 (m, 2H, H-3′, H-4′), 5.09 (m, 2H, H-1, H-1′), 4.70-4.57 (m, 6H, 2 × CH2, Bn, OCH2CCl3), 4.42 (m, 1H, H-5′), 3.89-3.83 (m, 3H, H-2′, H-3, H-6a), 3.78-3.75 (dd, 1H, H-6b), 3.68 (bs, 1H, C4-OH), 3.58-3.55 (m, 2H, H-4, H-5), 3.31 (m, 1H, H-2), 2.13 (s, 3H, COCH3), 1.98 (s, 3H, COCH3), 1.12 (d, 3H, J = 6.6 Hz, CH3 fucose) ppm. 13C from HSQC (125.7 MHz, CDCl3) : δ = 98.6 (C-1′), 85.9 (C-1), 83.8 (C-3), 78.7 (C-5), 74.1-73.6 (2 × CH2Ph, CH2Troc), 73.8 (C-2′), 71.2 (C-3′), 71.0 (C-4), 70.3 (C-4′), 70.2 (C-6), 66.0 (C-5′), 55.7 (C-3), 21.0, 20.8 (2 × OAc), 16.4 (C-6′); HR-MALDI-ToF/MS: m/z: calc. for C39H44Cl3NO12S [M+Na]+: 878.1547; found 878.1543.
2-(Trimethylsilyl)ethyl [methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2-non-ulopyranosylonate]-(2→3)-O-β-d-galactopyranoside (13)
To a solution of 12 (250 mg, 0.283 mmol) in CH2Cl2:MeOH (30:1, v:v; 15 mL) under an argon atmosphere was added Pd, 10 wt. % on activated carbon, (150 mg) and the mixture stirred for 20 min. at room temperature. The argon was replaced with H2(g), and the reaction stirred for 8 h. The solution was diluted with CH2Cl2 (50 mL) and filtered through celite. The solvent was removed by evaporation under reduced pressure and the residue purified by silica gel column chromatography (Toluene:Acetone, 5:2, v:v) to afford compound 13 (220 mg, 98%) as a white amorphous solid. Analytical data for 13: Rf = 0.48 (Toluene:Acetone, 1:1, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 5.52 (ddd, 1H, H-4′), 5.34 (dd, 1H, H-8′), 5.15 (dd, 1H, J = 8.2 Hz, H-7′), 4.94 (d, 1H, J = 10.3 Hz, H-6′), 4.42 (d, 1H, J = 7.6 Hz, H-1), 4.33 (dd, 1H, H-9a′), 4.21 (t, 1H, J = 10.2 Hz, H-5′), 4.12 (dd, 1H, J2,3 = 9.4 Hz, J3,4 = 3.1 Hz, H-3), 4.08 (dd, 1H, H-9b′), 4.02 (m, 1H, OCHH), 3.91 (dd, 1H, H-6), 3.92-3.82 (m, 5H, H-6a, H-6b,COOCH3), 3.70-3.60 (m, 3H, H-4, H-2, OCHH), 3.55 (t, 1H, H-5), 2.86 (dd, 1H, H-3′eq), 2.35, 2.28 (2 × s, 6H, N(COCH3)2), 2.10, 2.09, 2.00, 1.97 (4 × s, 12H, 4 × COCH3), 1.93 (dd, 1H, H-3′ax), 1.13-0.85 (m, 2H, CH2SiMe3), 0.01 (s, 9H, Si(CH3)3); 13C from HSQC (125.7 MHz, CDCl3) : δ = 102.7 (C-1), 77.2 (C-3), 73.8 (C-5), 70.4 (C-6′), 69.5 (C-2), 68.9 (C-4), 68.7 (C-8′), 67.3 (CH2CH2SiMe3), 66.9 (C-7′), 66.8 (C-4′), 62.6 (C-6), 62.2 (C-9′), 56.9 (C-5′), 53.7 (COOCH3), 38.6 (C-3′), 28.3, 26.3 (NAc2), 21.4, 21.1, 21.0 (3 × OAc), 18.3 (CH2SiMe3); HR-MALDI-ToF/MS: m/z: calc. for C33H53NO19Si [M+Na]+: 818.2879; found 818.2880.
2-(Trimethylsilyl)ethyl [methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-glycero-α-d-galacto-2-non-ulopyranosylonate]-(2→3)-O-(2,4,6-tri-O-acetyl-β-galactopyranoside) (14)
Compound 13 (215 mg, 0.270 mmol) was dissolved in pyridine (10 mL) and acetic anhydride (5 mL) and the reaction stirred for 14 h at room temperature. The solvent was removed by co-evaporation with toluene (3 × 50 mL). Silica gel column chromatography (Hexanes:EtOAc, 1:1, v:v) of the residue afforded compound 14 (246 mg, 99%) as a white solid. Analytical data for 14: Rf = 0.55 (Hexanes:EtOAc, 1:3, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 5.53-5.47 (m, 2H, H-4′, H-8′), 5.14 (dd, 1H, H-7′, J = 9.3 Hz, 2.4 Hz), 4.98-4.94 (m, 2H, H-3, H-4), 4.58-4.53 (m, 3H, H-2, H-6′, H-1), 4.28-4.25 (m, 2H, H-5′, H-9a′), 4.08-3.98 (m, 3H, H-6a, H-6b, H-9b′), 3.97-3.92 (dt, 1H, OCHHCH2Si(CH3)3), 3.85 (s, 3H, COOCH3), 3.83 (t, 1H, H-5), 3.59-3.54 (dt, 1H, OCHHCH2Si(CH3)3), 2.63 (dd, 1H, J = 5.4 Hz, 12.7 Hz, H-3′eq), 2.32, 2.25 (2 × s, 6H, N(COCH3)2), 2.17, 2.15, 2.04, 2.01, 2.00, 1.99, 1.91 (7 × s, 21H, 7 × COCH3), 1.60 (t, 1H, J = 12.2 Hz, H-3′ax), 1.01-0.86 (m, 2H, CH2Si(CH3)3), 0.00 (s, 9H, Si(CH3)3); 13C from HSQC (125.7 MHz, CDCl3) : δ = 100.8 (C-1), 71.8 (C-2), 70.6 (C-5), 70.4 (C-3), 69.6 (C-6′), 67.9 (C-4), 67.8 (C-4′), 67.6 (CH2CH2SiMe3), 67.4 (C-7′), 67.3 (C-8′), 62.7 (C-6), 62.4 (C-9′), 56.5 (C-5′), 53.3 (COOCH3), 38.7 (C-3′), 28.4, 27.0 (NAc2), 22.0-20.6 (7 × OAc), 18.4 (CH2SiMe3), 1.3 (SiMe3); HR HR-MALDI-ToF/MS: m/z: calc. for C39H59NO22Si [M+Na]+: 944.3196; found 944.3194.
Methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2-non-ulopyranosylonate-(2→3)-O-(2,4,6-tri-O-acetyl-β-d-galactopyranosyl) trichloroacetimidate (7)
TFA (2 mL) was added to a solution of compound 14 (240 mg, 0.260 mmol) in CH2Cl2 (10 mL) at 0 °C and the reaction stirred for 4 h at the same temperature. The solvent was removed by co-evaporation with toluene (5 × 20 mL). The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 2:5, v:v). Trichloroacetonitrile (130 μL, 1.26 mmol) and 1,8-diazabicyclo[5.4.0]-undec-7-ene (14 μL, 94.8 μmol) were added to a solution of methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2- non-ulopyranosylonate-(2→3)-O-(2,4,6-tri-O-acetyl-β-d-galactopyranoside) (207 mg, 0.252 mmol), in CH2Cl2 (5 mL). The reaction mixture was stirred for 1 h and then concentrated in vacuo. Silica gel column chromatography (Hexanes:EtOAc, 1:2, v:v) of the syrup afforded compound 7 (220 mg, 87% over two steps, 3:2 α:β) as a white foam. Analytical data for 7: Rf = 0.30 (Hexanes:EtOAc, 1:2, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 8.65 (s, 1H, NH), 8.61 (s, 1H, NH), 6.48 (d, 1H, J1,2 = 3.8 Hz, H-1α), 5.93 (d, 1H, J1,2 = 8.2 Hz, H-1β), 5.56-5.50 (m, 2H, H-4′α, H-8′α), 5.37-5.35 (m, 2H, H-4′β, H-8′β), 5.27-5.24 (m, 2H, H-2α, H-2β), 5.15-5.12 (m, 2H, H-7′α, H-7′β), 5.04 (bd, 1H, H-4β), 4.99 (dd, 1H, J = 3.4 Hz, 10.5 Hz, H-3α), 4.77 (dd, 1H, J = 3.4 Hz, 10.0 Hz, H-3β), 4.62 (m, 2H, H-6′α, H-6′β), 4.32-4.28 (m, 2H, H-5′α, H-6α), 4.23-4.06 (m, 4H, H-5′β, H-6b, H-9′aα, H-9′bα), 4.02-3.95 (m, 2H, H-9′aβ, H-9′bβ), 3.88 (s, 3H, COOCH3α), 3.85 (s, 3H, COOCH3β) 2.70 (dt, 1H, H-3′eq), 2.35, 2.33 (2 × s, 6H, NCOCH3α), 2.28, 2.27 (2 × s, 6H, NCOCH3β), 2.16-1.93 (7 × s, 21H, 7 × COCH3), 1.68 (dd, 1H, H-3′ax); 13C from HSQC (125.7 MHz, CDCl3) : δ = 96.3 (C-1β), 94.2 C-1α), 72.0 (C-5), 71.2 (C-3β), 69.8 (C-6′), 68.4 (C-2), 68.3 (C-3α), 67.9 (C-4′α), 67.8 (C-8′α), 67.5 (C-4β), 67.3 (C-4′β), 67.2 (C-8′β), 67.1 (C-7′), 62.5 (C-6), 62.3 (C-9′β), 61.8 (C-9′α), 56.6 (C-5′α), 56.1 (C-5′β), 53.3 (COOCH3), 38.9 (C-3′), 28.4, 28.3 (NAc2α), 26.9, 26.8 (NAc2β), 23.0-21.8 (7 × OAc α/β);
Phenyl [O-methyl 5-(A-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2-non-ulopyranosylonate]-(2→3)-O-(2,4,6-tri-O-acetyl-β-d-galactopyranosyl)-(1→4)-O-[(3,4-di-O-acetyl-2-O-benzyl-α-l-fucopyranosyl)-(1→3)]-O-[6-O-benzyl-2-deoxy-1-thio-2-(2,2,2-trichloroethoxycarbonylamino)-β-d-glucopyranoside] (15)
A mixture of disaccharide acceptor 11 (30 mg, 0.035 mmol) and trichloroacetimidate donor 7 (50 mg, 0.052 mmol) in CH2Cl2 (4 ml) was placed under an atmosphere of argon and stirred at room temperature with 4Å MS for 1 h. The reaction mixture was then cooled to 0 °C. TMSOTf (3.0 μmol, 0.035M solution in CH2Cl2) was added and stirring was continued for 1 h at the same temperature. The progress of reaction was monitored by TLC and MALDI-ToF MS. The reaction was quenched by the addition of pyridine (25 μL), diluted with CH2Cl2 (10 ml), filtered, and washed with sat. aq. NaHCO3 solution (10 ml), water (10 ml), and brine (10 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (CHCl3:EtOAc, 1:1, v:v) to afford compound 15 (35 mg, 61%) as a white amorphous solid. Analytical data for 15: Rf = 0.25 (Acetone:Toluene, 1:3, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.43-7.13 (m, 15H, aromatic), 5.56-5.51 (m, 2H, H-8‴, H-4‴), 5.32 (d, 1H, J = 7.3 Hz, NHTroc), 5.27 (bs, 1H, H-4″), 5.21-5.14 (m, 3H, H-1, H-1″, H-3″), 4.99 (d, 1H, J = 1.0 Hz, H-4′), 4.91-4.86 (m, 2H, H-5″, H-2′), 4.83 (d, 1H, J1′,2′ = 8.2 Hz, H-1′), 4.79 (d, 1H, CHHPh), 4.68-4.90 (m, 7H, CH2Ph, CHHPh, CH2CCl3, H-3′, H-6″), 4.29 (t, J4′,5′ = J5′,6′ = 10.1 Hz, H-5′), 4.23-4.14 (m, 4H, H-6a′, H-6b′, H-9a‴, H-3), 4.03 (m, 1H, H-9b‴), 3.96 (t, 1H, J3,4 = J5,4 = 9.0 Hz, H-4), 3.87-3.80 (m, 5H, COOCH3, H-6a, H-6b), 3.78 (m, 1H, H-5′), 3.52(bd, 1H, H-5), 3.10 (bm, 1H, H-2), 2.62 (m, 1H, H-3a′), 2.33, 2.26 (2s, 6H, 2× NCOCH3), 2.15-1.92 (9s, 27H, 9× COCH3), 1.62 (m, 1H, H-3b‴), 1.17 (d, 3H, J = 6.6 Hz, CH3 fucose) ppm; 13C from HSQC (125.7 MHz, CDCl3) : δ = 99.6 (C-1′), 97.7 (C-1″), 84.5 (C-1), 79.7 (C-5), 75.7 (C-3), 74.6 (CH2Ph), 74.4 (C-2″), 73.9 (C-4), 73.2 (CH2Ph), 72.3 (C-4″), 71.8 (C-3′), 71.2 (C-5′), 70.8 (C-5″), 70.6 (C-3″), 69.7 (C-6′), 68.7 (C-6), 67.8 (C-4′), 67.5 (C-8′), 67.4 (C-7′), 67.3 (C-4‴), 64.8 (C-2′), 62.4 (C-9′), 62.0 (C-6′), 57.9 (C-2), 56.2 (C-5′), 53.4 (COOCH3), 38.7 (C-3‴), 28.6, 27.2 (NAc2), 21.6-20.5 (9 × OAc), 16.3 (C-6″); HR-MALDI-ToF/MS: m/z: calc. for C73H89Cl3N2O33S [M+Na]+: 1681.4032; found 1681.4029.
N-(9-Fluorenylmethyloxycarbonyl)-O-[2-azido-2-deoxy-3-O-(3,4,6-tri-O-acetyl-2-O-(2,5-difluorobenzoyl)-β-d-galactopyranosyl)-6-O-(O-methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2-non-ulopyranosylonate)-(2→3)-O-(2,4,6-tri-O-acetyl-β-d-galactopyranosyl)-(1→4)-O-[(3,4-di-O-acetyl-2-O-benzyl-α-l-fucopyranosyl)-(1→3)]-O-(6-O-benzyl-2-deoxy-2-(2,2,2-trichloroethoxycarbonylamino)-β-d-glucopyranosyl)-α-d-galactopyranosyl]-l-threonine benzyl ester (16)
A mixture of disaccharide acceptor 10 (22 mg, 0.025 mmol) and tetrasaccharide donor 15 (32 mg, 0.019 mmol) in CH2Cl2 (2 ml) was placed under an atmosphere of argon and stirred at room temperature with 4Å MS for 1 h. The reaction mixture was then cooled to 0 °C. N-iodosuccinimide (22 mg, 0.096 mmol) and TfOH (0.019 mmol, 0.20m solution in CH2Cl2) were added sequentially and stirring was continued for 1 h at the same temperature. The progress of reaction was monitored by TLC and MALDI-ToF MS. The reaction was diluted with CH2Cl2 (10 ml), filtered, and washed with sat. aq. NaHCO3 solution (10 ml), water (10 ml), and brine (10 ml). The organic layer was dried (MgSO4), filtered, and the filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography (Hexanes:EtOAc, 1:2, v:v) to afford compound 16 (29 mg, 55%) as a white amorphous solid. Analytical data for 16: Rf = 0.30 (Hexanes:EtOAc, 1:2, v:v); 1H-NMR (500 MHz, CDCl3) : δ = 7.77-6.42 (m, 26H, 3 × Bn, Fmoc, dFBz), 5.92-5.88 (m, 2H, H-4‴″, H-8‴″), 5.84 (t, 1H, H-2′), 5.74 (m, 2H, H-3‴, H-4‴), 5.61 (d, 1H, J = 3 Hz, H-1‴), 5.47-5.41 (m, 4H, H-2‴′, H-7‴″, H-4′, H-4‴), 5.34 (m, 1H, H-5‴), 5.27 (m, 1H, H-3′), 5.19 (d, 1H, J = 8.1 Hz, H-1‴&prime), 5.04-4.99 (m, 3H, H-6‴″, H-3‴′, COOCHHPh), 4.95 (d, 1H, OCHHPh), 4.86-4.77 (m, 3H, COOCHHPh, COOCHHCCl3, OCHHPh), 4.73-4.49 (m, 9H, COOCHHCCl3, H-6a‴′, OCHHPh, H-1″, H-1, H-5‴″, OCHCH3 threonine, OCHHPh, H-6b‴′), 4.44-4.18 (m, 9H, CH2Fmoc, H-9a‴″, H-9b‴″, H-5‴′, CHCOOBn threonine, H-1′, H-3‴, H-4′), 4.15-3.79 (m, 11H, H-6a, H-6b, H-2‴, H-6a′, H-6b′, CH2CHFmoc, H-5, H-4, H-6a′, H-6b′, H-3), 3.77 (s, 3H, COOCH3), 3.46 (m, 4H, H-2″, H-2, H-5′, H-5″), 2.85 (dd, 1H, H-3‴″), 2.26, 2.22 (2s, 6H, 2× NCOCH3), 1.87 (m, 1H, H-3‴″), 1.88-1.65 (11s, 33H, 11× COCH3), 1.60 (d, 1.623H, J = 6.5 Hz, CH3 fucose), 1.57 (s, 3H, COCH3), 1.34 (m, 3H, CH3 threonine) ppm; 13C from HSQC (125.7 MHz, CDCl3) : δ = 101.6 (C-1′), 100.7 (C-1″), 100.5 (C-1‴′), 99.9 (C-1), 97.4 (C-1‴), 78.4 (C-3), 76.5 (OCHCH3 threonine), 75.6 (C-5″), 74.9 (C-2‴), 74.8 (C-3″), 74.7 (C-4″), 74.5 (CH2Ph), 73.6(CH2Ph), 73.1 (CH2Troc), 72.6 (C-4‴), 72.4 (C-3‴′), 71.7 (C-5′), 71.5 (C-5‴′), 71.1 (C-3′), 71.0 (C-2‴′), 70.8 (C-7‴″), 70.6 (C-3‴), 70.5 (C-6‴″), 70.3 (C-2′), 69.9 (C-5), 69.3 (C-6), 69.1 (C-6″), 68.1 (C-4), 67.9 C-4‴′), 67.7 (CH2Fmoc), 67.6 (COOCH2Ph threonine), 67.5 (C-4′), 67.4 (C-4‴″), 67.3 (C-8‴″), 65.2 (C-5‴), 62.5 (C-9‴″), 62.1 (C-6‴′), 61.6 (C-6′), 59.5 (OCHCH3 threonine), 59.3 (C-2), 58.9 (C-2″), 56.3 (C-5‴″), 52.9 (COOCH3), 47.6 (CH2CHFmoc), 39.0 (C-3‴″), 21.0, 20.9 (NAc2), 20.8-20.1 (12 × OAc), 18.9 (CH3 threonine), 16.5 (C-6‴); HR-MALDI-ToF/MS: m/z: calc. for C118H135Cl3F2N6O51 [M+Na]+: 2617.7086; found 2617.7091.
N-(9-Fluorenylmethyloxycarbonyl)-O-[2-(N-acetamido)-2-deoxy-3-O-(3,4,6-tri-O-acetyl-2-O-(2,5-difluorobenzoyl)-β-d-galactopyranosyl)-6-O-(O-methyl 5-(N-acetylacetamido)-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-d-glycero-α-d-galacto-2-non-ulopyranosylonate)-(2→3)-O-(2,4,6-tri-O-acetyl-β-d-galactopyranosyl)-(1→4)-O-[(3,4-di-O-acetyl-2-O-benzyl-α-l-fucopyranosyl)-(1→3)]-O-(6-O-benzyl-2-deoxy-2-(N-acetamido)-β-d-glucopyranosyl)-α-d-galactopyranosyl]-l-threonine benzyl ester (1)
Zn dust (400 mg, 6.12 mmol) and saturated aq. CuSO4 (25 μL) were added to a solution of 16 (20 mg, 7.70 μmol) in THF (3 mL), Ac2O (2 mL), and AcOH (1 mL) and the reaction stirred at rt for 3 h. The reaction mixture was filtered and co-evaporated with toluene (3 × 5 mL). The residue was purified by silica gel column chromatography (CHCl3:Acetone, 2/1, v/v) to afford compound 1 (11.5 mg, 60%) as a white amorphous solid. Analytical data for 1: Rf = 0.30 (CHCl3:Acetone, 2/1, v/v); 1H-NMR (600 MHz, acetone-D6) : δ = 7.75-7.08 (m, 26H, 3 × Bn, Fmoc, dFBz), 6.99 (d, 1H, NH, GlcNAc), 6.51 (d, 1H, NH, GalNAc), 6.42 (d, 1H, NHFmoc, threonine), 5.5 (m, 1H, H-8‴″), 5.45 (m, 1H, H-4‴″), 5.35 (d, 1H, J = 3.7 Hz, H-1‴), 5.29 (d, 1H, J = 3.5 Hz, H-4′), 5.25 (dd, 1H, J = 8.1 Hz, J = 10.5 Hz, H-2′), 5.19 (bd, 1H, J = 2.6 Hz, H-4‴), 5.12-5.08 (m, 3H, H-3‴, H-7‴″, H-3′), 4.99 (bd, 1H, J = 3.7 Hz, H-4‴′), 4.97-4.93 (dd, 2H, COOCH2Ph, threonine), 4.88 (q, 1H, H-5‴), 4.84-4.81 (m, 2H, H-2‴′, H-1‴′), 4.79 (d, 1H, J = 8.1 Hz, H-1″), 4.67 (d, 2H, 2 × CHHPh), 4.60 (m, 1H, H-3‴′), 4.57 (d, 1H, J = 2.8 Hz, H-1), 4.55 (dd, 1H, H-6‴″), 4.49 (d, 1H, J = 7.5 Hz, H-1″), 4.45 (d, 1H, CHHPh), 4.38 (d, 1H, CHHPh), 4.35 (m, CHHFmoc), 4.27-4.22 (m, 2H, CHHFmoc, H-2), 4.20 (m, 1H, H-5‴″), 4.15-4.05 (m, 8H, OCHCH3 threonine, H-6a‴′, CHCOOBn threonine, CHFmoc, H-6b‴′, H-9a‴″, H-5′ H-6a′), 4.03-3.91(m, 5H, H-4″, H-6b′, H-9b‴″, H-4, H-3″), 3.87-3.81 (m, 3H, H-6a″, H-2″, H-5), 3.76-3.73 (m, 6H, H-5‴′, COOCH3, H-6b″, H-3), 3.70 (dd, 1H, H-2‴), 3.54 (m, 2H, H-6a, H-6b), 3.47 (m, 2H, C4-OH, H-5″), 2.48 (dd, 1H, H-3‴″), 2.24, 2.20 (2 × s, 6H, NAc2), 2.14-1.74 (14 × s, 42H, 12 × OAc, 2 × NHAc), 1.43 (t, 1H, H-3‴″), 1.16 (d, 3H, CH3 threonine), 1.03 (d, 3H, J = 6.6 Hz, CH3 fucose) ppm; 13C from HSQC (150.9 MHz, CDCl3) : δ = 104.3 (C-1′), 104.1 (C-1″), 102.2 (C-1‴′), 101.9 (C-1), 98.6 (C-1‴), 81.0 C-3), 77.6 (C-5″), 77.4 (OCHCH3 threonine), 77.3 (C-3″), 76.3 (C-2‴), 76.1 (C-4‴), 75.1 (CH2Ph), 74.6 (C-4‴), 74.2 (C-3‴′), 73.8 (CH2Ph), 73.6 (C-5‴′), 73.5 (C-5′), 73.4 (C-3′), 73.1 (C-2‴′), 72.8 (C-2′), 72.5 (C-3‴), 72.3 (C-6‴″), 72.2 (C-6), 72.1 (C-5), 71.4 (C-4), 71.2 (C-6″), 70.4 (C-8‴″), 70.2 (C-4‴′), 70.0 (C-4′), 69.8 (C-7‴″), 69.5 (COOCH2Ph), 69.2 (C-4‴″), 69.1 (CH2CHFmoc), 66.4 (C-5‴), 64.7 (C-9‴″), 64.1 (C-6‴′), 63.9 (C-6′), 61.8 (OCHCH3 threonine), 58.6 (C-2″), 58.5 (C-5‴″), 55.2 (COOCH3), 50.5 (C-2), 49.8 (CH2CHFmoc), 41.1 (C-3‴″), 29.8, 28.4 (NAc2), 25.5-22.4 (12 5 OAc, 2 × NHAc), 21.4 (CH3 threonine), 18.3 (C-6‴); HR-MALDI-ToF/MS: m/z: calc. for C119H140F2N4O51 [M+Na]+: 2501.8350; found 2501.8353.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Christian Heiss for help with elucidating NMR spectra. This work was supported by NIH NIGM grant No. R01-GM065248 and R01-GM61761.
Footnotes
Supporting Information Available: 1H-NMR spectra and HSQC of all synthesized compounds are being furnished. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D, Blanks JE. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 3.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, Xia L, Kazazian HH, Schaub RG, McEver RP, Wagner DD. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 4.McNicol A, Israels SJ. Cardiovasc Hematol Disord: Drug Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- 5.Geng JG, Chen M, Chou KC. Curr Med Chem. 2004;11:2153–2160. doi: 10.2174/0929867043364720. [DOI] [PubMed] [Google Scholar]
- 6.Platt OS. J Clin Invest. 2000;106:337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings RD. Braz J Med Biol Res. 1999;32:519–28. doi: 10.1590/s0100-879x1999000500004. [DOI] [PubMed] [Google Scholar]
- 8.Moore KL, Stults NL, Diaz S, Smith DF, Cummings RD, Varki A, Mcever RP. J Cell Biol. 1992;118:445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantin G. Drug News & Perspectives. 2004;17:579–586. doi: 10.1358/dnp.2004.17.9.872571. [DOI] [PubMed] [Google Scholar]
- 10.Hicks AER, Nolan SL, Ridger VC, Hellewell PG, Norman KE. Blood. 2003;101:3249–3256. doi: 10.1182/blood-2002-07-2329. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakides C, Favuzza J, Wang Y, Austen WG, Moore FD, Hechtman HB. British J Surg. 2001;88:825–830. doi: 10.1046/j.0007-1323.2001.01795.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayward R, Campbell B, Shin YK, Scalia R, Lefer AM. Cardiovasc Res. 1999;41:65–76. doi: 10.1016/s0008-6363(98)00266-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang K, Zhou XR, Zhou ZM, Tarakji K, Qin JX, Sitges M, Shiota T, Forudi F, Schaub RG, Kumar A, Penn MS, Topol EJ, Lincoff AM. Thromb Haemostasis. 2002;88:149–154. [PubMed] [Google Scholar]
- 14.van Kasteren SI, Kramer HB, Jensen HH, Campbell SJ, Kirkpatrick J, Oldham NJ, Anthony DC, Davis BG. Nature. 2007;446:1105–1109. doi: 10.1038/nature05757. [DOI] [PubMed] [Google Scholar]
- 15.Enders S, Bernhard G, Zakrzewicz A, Tauber R. Biochim Biophys Acta Gen Subj. 2007;1770:1441–1449. doi: 10.1016/j.bbagen.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Zeng S, Gutierrez Gallego R, Dinter A, Malissard M, Kamerling JP, Vliegenthart JFG, Berger EG. Glycoconj J. 1999;16:487–497. doi: 10.1023/a:1007065803554. [DOI] [PubMed] [Google Scholar]
- 17.Leppanen A, Mehta P, Ouyang YB, Ju TZ, Helin J, Moore KL, van Die I, Canfield WM, McEver RP, Cummings RD. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- 18.Davis BG. J Chem Soc, Perkin Trans 1. 2000:2137–2160. [Google Scholar]
- 19.Sears P, Wong CH. Science. 2001;291:2344–2350. doi: 10.1126/science.1058899. [DOI] [PubMed] [Google Scholar]
- 20.Boons GJ. Contemp Org Synth. 1996;3:173–200. [Google Scholar]
- 21.Seeberger PH, Werz DB. Nature. 2007;446:1046–1051. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 22.Codee JDC, Litjens REJN, van den Bos LJ, Overkleeft HS, van der Marel GA. Chem Soc Rev. 2005;34:769–782. doi: 10.1039/b417138c. [DOI] [PubMed] [Google Scholar]
- 23.Douglas NL, Ley SV, Lucking U, Warriner SL. J Chem Soc, Perkin Trans 1. 1998:51–65. [Google Scholar]
- 24.Koeller KM, Smith MEB, Huang RF, Wong CH. J Am Chem Soc. 2000;122:4241–4242. [Google Scholar]
- 25.Pratt MR, Bertozzi CR. Org Lett. 2004;6:2345–2348. doi: 10.1021/ol0493195. [DOI] [PubMed] [Google Scholar]
- 26.Baumann K, Kowalczyk D, Kunz H. Angew Chem Int Ed. 2008;47:3445–3449. doi: 10.1002/anie.200705762. [DOI] [PubMed] [Google Scholar]
- 27.Hanashima S, Castagner B, Esposito D, Nokami T, Seeberger PH. Org Lett. 2007;9:1777–1779. doi: 10.1021/ol0704946. [DOI] [PubMed] [Google Scholar]
- 28.Otsubo N, Ishida H, Kiso M. Tetrahedron Lett. 2000;41:3879–3882. [Google Scholar]
- 29.Xia J, Alderfer JL, Locke RD, Piskorz CF, Matta KL. J Org Chem. 2003;68:2752–2759. doi: 10.1021/jo020698u. [DOI] [PubMed] [Google Scholar]
- 30.Huang KT, Wu BC, Lin CC, Luo SC, Chen CP, Wong CH, Lin CC. Carbohydr Res. 2006;341:2151–2155. doi: 10.1016/j.carres.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 31.Yu B, Yu H, Hui YZ, Han XW. Tetrahedron Lett. 1999;40:8591–8594. [Google Scholar]
- 32.Boons GJ. Tetrahedron. 1996;52:1095–1121. [Google Scholar]
- 33.Wang YH, Ye XS, Zhang LH. Org Biomol Chem. 2007;5:2189–2200. doi: 10.1039/b704586g. [DOI] [PubMed] [Google Scholar]
- 34.Wang YH, Zhang LH, Ye XS. Comb Chem High Throughput Screening. 2006;9:63–75. doi: 10.2174/138620706775213912. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan S, Kahne D. J Am Chem Soc. 1993;115:1580–1581. [Google Scholar]
- 36.Demchenko A, Boons GJ. Tetrahedron Lett. 1997;38:1629–1632. [Google Scholar]
- 37.Zhu T, Boons GJ. Angew Chem Int Ed. 1998;37:1898–1900. [Google Scholar]
- 38.Wang CC, Lee JC, Luo SY, Fan HF, Pai CL, Yang WC, Lu LD, Hung SC. Angew Chem Int Ed. 2002;41:2360–2362. doi: 10.1002/1521-3773(20020703)41:13<2360::AID-ANIE2360>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Vohra Y, Vasan M, Venot A, Boons GJ. Org Lett. 2008;10:3247–3250. doi: 10.1021/ol801076w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen XT, Sames D, Danishefsky SJ. J Am Chem Soc. 1998;120:7760–7769. [Google Scholar]
- 41.Rencurosi A, Lay L, Russo G, Caneva E, Poletti L. J Org Chem. 2005;70:7765–7768. doi: 10.1021/jo050704x. [DOI] [PubMed] [Google Scholar]
- 42.Benakli K, Zha CX, Kerns RJ. J Am Chem Soc. 2001;123:9461–9462. doi: 10.1021/ja0162109. [DOI] [PubMed] [Google Scholar]
- 43.Cheng H, Cao XH, Xian M, Fang LY, Cai TB, Ji JJ, Tunac JB, Sun DX, Wang PG. J Med Chem. 2005;48:645–652. doi: 10.1021/jm049693a. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka H, Adachi M, Takahashi T. Tetrahedron Lett. 2004;45:1433–1436. [Google Scholar]
- 45.Veeneman GH. In: Carbohydrate Chemistry. Boons GJ, editor. Blackie Academic & professional; London: 1998. pp. 98–167. [Google Scholar]
- 46.Zhu T, Boons GJ. Carbohydr Res. 2000;329:709–715. doi: 10.1016/s0008-6215(00)00252-4. [DOI] [PubMed] [Google Scholar]
- 47.Berces A, Whitfield DM, Nukada T, do Santos I, Obuchowska A, Krepinsky JJ. Can J Chem. 2004;82:1157–1171. [Google Scholar]
- 48.Sjolin P, Kihlberg J. J Org Chem. 2001;66:2957–65. doi: 10.1021/jo001584q. [DOI] [PubMed] [Google Scholar]
- 49.Cato D, Buskas T, Boons GJ. J Carbohydr Chem. 2005;24:503–516. [Google Scholar]
- 50.Cheshev PE, Kononov LO, Tsvetkov YE, Shashkov AS, Nifantiev NE. Russian J Bioorg Chem. 2002;28:419–429. [Google Scholar]
- 51.Schmidt RR, Kinzy W. Adv Carb Chem Biochem. 1994;50:21–123. doi: 10.1016/s0065-2318(08)60150-x. [DOI] [PubMed] [Google Scholar]
- 52.Li Z, Gildersleeve JC. J Am Chem Soc. 2006;128:11612–11619. doi: 10.1021/ja063247q. [DOI] [PubMed] [Google Scholar]
- 53.Codee JDC, van den Bos LJ, Litjens REJN, Overkleeft HS, van Boeckel CAA, van Boom JH, van der Marel GA. Tetrahedron. 2004;60:1057–1064. [Google Scholar]
- 54.Sakagami M, Hamana H. Tetrahedron Lett. 2000;41:5547–5551. [Google Scholar]
- 55.Demchenko AV, Boons GJ. J Org Chem. 2001;66:2547–2554. doi: 10.1021/jo001477w. [DOI] [PubMed] [Google Scholar]
- 56.Winans KA, King DS, Rao VR, Bertozzi CR. Biochemistry. 1999;38:11700–11710. doi: 10.1021/bi991247f. [DOI] [PubMed] [Google Scholar]
- 57.Venot A, Swayze EE, Griffey RH, Boons GJ. Chembiochem. 2004;5:1228–1236. doi: 10.1002/cbic.200400105. [DOI] [PubMed] [Google Scholar]
- 58.Tam JP, Heath WF, Merrifield RB. J Am Chem Soc. 1986;108:5242–5251. [Google Scholar]
- 59.Takano Y, Habiro M, Someya M, JHojo H, Nakahara Y. Tetrahedron Lett. 2002;43:8395–8399. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.