Abstract
The emergence of a highly pathogenic H5N1 influenza virus in Hong Kong in 1997 and the subsequent appearance of other H5N1 strains and their spread to several countries in south-east Asia, Africa, the Middle East, and Europe has evoked fear of a global influenza pandemic. Vaccines offer the best hope to combat the threat of an influenza pandemic. However, the global demand for a pandemic vaccine cannot be fulfilled by the current egg-based vaccine manufacturing strategies, thus creating a need to explore alternative technologies for vaccine production and delivery. Several egg-independent vaccine approaches such as cell culture-derived whole virus or subvirion vaccines, recombinant protein-based vaccines, virus-like particle (VLP) vaccines, DNA vaccines and viral vector-based vaccines are currently being investigated and appear promising both in preclinical and clinical studies. The present review will highlight the various egg-independent alternative vaccine approaches for pandemic influenza.
Keywords: H5N1 influeza, cell-derived vaccine, egg-independent, pandemic influenza, viral vector
INTRODUCTION
Currently circulating H5N1 avian influenza viruses (“avian flu” or “bird flu”) represent a potential pandemic threat, as it is only a matter of time before these viruses acquire the necessary genetic changes enabling efficient human-to-human transmission.1 H5N1 viruses have diverged into antigenically distinct clades and subclades, adding another “unknown” to the nature of the H5N1 pandemic influenza strain.2 The global fatality rate of 75% due to H5N1 infections in humans was alarmingly high in 2008.3 It is estimated that a 1918-like influenza pandemic could result in over 50 million deaths worldwide even though the availability of health care facilities are far better than they were nine decades ago.4
We are presently in the midst of an ongoing 2009 H1N1 influenza A pandemic5 which has spread to more than 100 countries with approximately 134,000 laboratory-confirmed cases and 816 deaths reported to date.6 This pandemic is providing real-time information regarding the dynamics of a modern day pandemic, fortunately without a high fatality rate. The sudden emergence (April 2009) and unprecedented global spread (WHO declared a pandemic in June 2009) of this new H1N1 virus in less than 3 months as compared to approximately a year as seen in the timelines of previous pandemics7 demonstrate the role of human mobility leading to the rapid emergence of an influenza pandemic. Certainly the current pandemic is testing the effectiveness of pandemic preparedness plans (devised for a potential H5N1 pandemic) with regard to early detection and containment of disease outbreaks, school closures, travel restrictions, effective coordination among various agencies, and the availability of effective vaccines for mass immunization. It has also exposed the difficulties in implementing some of the measures for containing the spread of the virus.
New H1N1 influenza vaccine production in eggs (the conventional method) is ongoing, and it is anticipated that over 120 million doses will be available in the U.S. before the regular flu season. However, vaccine manufacturers in their early attempts to grow the virus in eggs have reported issues with virus yields.8 A single dose of the new H1N1 vaccine may not elicit a strong enough protective immune response suggesting the requirement for a two-dose formulation. In a pandemic situation where timely manufacture of vaccines is extremely important, it is very clear that conventional egg-based vaccine production is not the solution for a highly virulent human pandemic strain such as H5N1. The timeline from strain identification to vaccine availability is about 4–6 months, a duration which could prove fatal in a pandemic.
The development of a timely, cost-effective vaccine represents a major challenge in preparing for a possible H5N1 influenza pandemic. With existing manufacturing capacity, 413 million doses of a trivalent seasonal influenza vaccine are being produced every year for the entire world population9. The number of doses of a monovalent pandemic influenza vaccine given at a similar dosage level as the trivalent seasonal influenza vaccine would be 1,239 million doses. In a pandemic situation, the vaccine demand will be at least 5–10-fold that of the current global seasonal influenza vaccine production capability and achieving this target is further challenged by the low yield per egg of H5N1 vaccine strains and the need to have multiple inoculations to elicit protective levels of immunity. Moreover, the supply of embryonated eggs for vaccine production may be adversely affected in an H5N1 pandemic since the virus is lethal to egg-laying poultry. Apart from production issues, delivery of a vaccine to the global population also poses a challenge further compounded by the fact that the majority of vaccine manufacturers are located in developed countries.
Varied strategies for egg-independent vaccine production will greatly expand existing manufacturing capacity and offer flexibility for rapid scale-up in the case of an impending pandemic. Vaccine manufacturers have obtained comparable or higher virus yields in well-characterized certified cell lines compared to those obtained in embryonated eggs.10,11 Furthermore, the development and use of a serum/protein free synthetic media to grow cells has also minimized a potential safety concern regarding the spread of transmissible spongiform encephalopathies or other adventitious agents.12 To increase the global influenza vaccine production capacity, different vaccine approaches should be considered.13,14 The following sections will highlight various egg-independent vaccine approaches for pandemic preparedness.(Fig 1).
Fig. 1.
Egg-independent pandemic influenza vaccine strategies.
CELL-DERIVED VACCINES
The vaccine industry currently possesses the necessary technical expertise for the large scale production of vaccines in certified cell lines, and major vaccine manufacturers are at various stages of developing cell-derived vaccines for seasonal and pandemic influenza.15–21 In 2006, in response to a potential pandemic threat, the U.S. Department of Health and Human Services (DHHS) awarded contracts to GlaxoSmithKline, MedImmune, Novartis Vaccines & Diagnostics, Sanofi Pasteur and DynPort Vaccine to develop cell-based influenza vaccines in the US.22 More recently, DHHS awarded a contract to Novartis to establish the first U.S. facility to manufacture cell-based vaccines for seasonal and pandemic influenza.23 Novartis has already licensed their first cell based seasonal flu vaccine “Optaflu” for the European market.24
Conventional egg-based influenza vaccines involve the annual selection of influenza virus strains that are closely matched antigenically to the circulating epidemic viruses. Reassortant viruses are generated containing the surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA) gene segments from the closely matched circulating strain and the other six gene segments from a high growth master donor strain, A/PR/8/34 (A/Puerto Rico/8/34). High-growth influenza B donor strains do exist (e.g., B/Lee/40), however, the reassortants do not generally grow better than the wild-type viruses. A wild-type strain is therefore used for the influenza B component of the vaccine that necessitates adaptation to eggs for high growth. Adaptation of influenza viruses has been shown to induce amino acid changes in the hemagglutinin protein.25,26,27
Although antigenic variants due to egg adaptation are detected early in vaccine seed virus selection, generating reassortants sometimes poses problems due to poor growth of the original virus in eggs. However, the viruses grown in cell culture usually maintain their antigenicity.28–30 A comparative study between an inactivated influenza vaccine grown in Madin-Darby canine kidney (MDCK) cells and an influenza vaccine grown in eggs suggested that the cell culture-derived vaccine induced a more robust humoral immune response leading to superior protection against challenge in a ferret model compared with egg-grown vaccine.31 However, a clinical trial demonstrated comparable immune responses with an influenza subunit vaccine produced either in MDCK cells or eggs.32 Other significant advantages of cell-derived influenza vaccines are the reduced risk of exogenous contamination in closed scalable bioreactors, and, more importantly, their independence from a continuous egg supply from certified poultry flocks.33
However, potential introduction of adventitious agents or oncogenic elements at any stage of the vaccine manufacturing process would compromise the vaccine safety, and therefore, this concern has to be addressed for all cell-based vaccine production systems. Adventitious agents in influenza vaccines may originate from the original clinical sample or be introduced during growth of the reference strains in eggs. Removal of intact cells and degradation of residual DNA during vaccine processing are some of the required quality control measures to minimize the risk of oncogenicity.34 A risk-assessment model demonstrated that MDCK cell culture-based subunit influenza vaccine manufacturing process is highly efficient in minimizing the risk of adventitious agent to a million-fold below infectious levels.34 Similar to embryonated eggs, MDCK cells also act as an effective filter for a wide range of adventitious agents that might be introduced during vaccine production.34
At present, African green monkey kidney (Vero), MDCK and human PER.C6 cell lines are approved to produce influenza vaccines, and several cell culture-derived influenza vaccines have been tested in pre-clinical and clinical studies.35–37 A cell culture-derived inactivated whole virus vaccine containing clade 1 (A/Vietnam/1203/04) and clade 2 (A/Indonesia/05/05) viruses induced cross-clade protective responses against divergent H5N1 viruses in a mouse model.36 A Vero cell-derived inactivated whole virus (A/Vietnam/1203/2004) vaccine produced in a BSL3 facility was shown to be safe and well tolerated in humans.38 In this study, a two-dose regimen of 7.5 microgram of vaccine formulation without an adjuvant resulted in virus neutralization titers of ≥ 20 in 76% of the subjects by day 42. Furthermore, cross-neutralizing antibody titers (≥ 20) were observed in 76% of subjects against A/Hong Kong/156/97 (clade 0) and in 45% against A/Indonesia/05/05 (clade 2).38 Two phase III trials by Baxter are currently underway to assess the immune responses as well as the safety and tolerability of an H5N1 influenza vaccine (whole virion, Vero cell-derived inactivated influenza vaccine containing H5N1 HA antigen) in adult and elderly populations.39,40
Immune responses for cell culture-derived vaccine appear to be encouraging in both animal and human studies. With the advantages of a reduced risk of contamination in closed scalable bioreactors, they are presently being researched by major pharmaceutical manufacturers. However, great care must be taken to address the issue of possible introduction of adventitious agents or oncogenic elements in the manufacturing process.
RECOMBINANT PROTEIN-BASED VACCINES
The technology of baculovirus expressed recombinant proteins produced in cultured insect cells is a viable means to generate large amounts of recombinant immunogenic proteins.41 In the case of influenza, this approach can result in large amounts of purified recombinant HA protein devoid of egg-derived allergens in a short duration (6–8 weeks) following identification of the target influenza strain.42 Baculoviruses have a narrow host range, and therefore, do not replicate in mammalian cells, but are capable of efficiently transducing them.43 In addition, the protein expression is very efficient with one of the strongest promoters (polyhedrin) found in the nature.44 Baculovirus recombinant proteins expressed in insect cells are correctly folded since these cells also support many of the posttranslational modifications that occur in mammalian cells.45 However, insect cell-based production of recombinant glycoproteins suffer a major limitation due to the lack of complex-type N-glycans containing terminal sialic acid residues.45 This may affect the immunogenicity of insect cell-derived influenza antigens. Nevertheless, several approaches are being pursued to address this issue.46,47
Baculovirus expressed recombinant HA has also been shown to be well tolerated and immunogenic in a number of clinical trials.42,48–50 A seasonal trivalent recombinant HA vaccine from Protein Science was safe and immunogenic in a healthy adult population, although at a three-fold higher dose rate than that of an egg-derived seasonal flu vaccine. Nonetheless, it demonstrated the potential feasibility of this approach to be used for the development of a pandemic flu vaccine.51 In another study, a two dose regimen (90 microgram) of a purified recombinant HA derived from a clade 0 H5N1 virus (A/Hong Kong/156/97) was well tolerated, and 52% of the naive subjects generated potentially protective titers of 1:80 by day 42.52 In a follow up study after 8 years involving the same subjects (now primed), a single dose of an egg-derived inactivated subvirion clade 1 vaccine (RG A/Vietnam/1203/04) generated seroconversion (≥1:40) in 68% of the primed subjects compared to 43% of unprimed subjects receiving two doses of 90 micrograms of the vaccine, suggesting the importance of priming the population during a pre-pandemic period.53
Clinical trials have shown the potential of recombinant protein-based vaccines as a viable potential pandemic influenza vaccine. To enhance the immunogenicity and dose sparing of recombinant HA protein-based vaccine formulations, the use and development of novel and improved adjuvants with an enhanced safety profile will be important. The addition of conserved internal protein/s in the vaccine formulation containing recombinant HA may further broaden the vaccine efficacy.
VIRUS-LIKE PARTICLES (VLP)-BASED VACCINES
Virus-like particles (VLP) are composed of highly organized particles that self-assemble from virus-derived structural proteins and are devoid of the viral nucleic acid. Currently, this approach is getting much attention for a pandemic influenza vaccine owing to its safety and immunogenic attributes. A VLP vaccine mimics live virus with regard to its interactions at the cellular level but is completely non-infectious. VLP have been shown to possess excellent adjuvant properties capable of enhancing humoral and cellular immune responses.54 They can be produced in established protein expression host systems such as yeast, insect or mammalian cells. The baculovirus expression system and insect cells form the most promising VLP technology for viral vaccines.55,56
The availability of a VLP-based vaccine for human papillomavirus (Gardasil) has demonstrated the safety of this approach for human use. A H5N1 influenza VLP vaccine expressing HA, NA & M1 of clade 1 (A/VN/1203/2004) or clade 2 (A/Indonesia/05/2005) was used to immunize mice with two doses (0.6 or 3 microgram), and it protected mice against lethal challenge with homologous and heterologous reassortant H5N1 viruses.57 Interestingly, a single immunization with either of the two vaccine amounts was also protective even in the absence of detectable neutralizing antibodies. Further, the immune responses generated by the clade 1 VLP vaccine (A/Vietnam/1203/04) was shown to be long lasting (30 weeks) and protected mice against homologous H5N1 challenge.58
A candidate clade 2 influenza VLP vaccine induced hemagglutination inhibition (HI) antibodies against the homologous H5N1 clade 2.1 strain, as well as heterologous strains from H5N1 clades 1, 2.2, and 2.3 in a dose-dependent manner in ferrets.59 The vaccine was protective against homologous or heterologous virus challenge even at a low vaccine dose (0.6 microgram of HA) given twice. The vaccine also reduced the virus shedding in the vaccinated animals.59 More recently, mucosal administration of a VLP vaccine containing HA, NA and M1 of the 1918 H1N1 virus not only protected against lethal challenge with the reconstructed 1918 H1N1 virus but also offered heterosubtypic cross-protection against a lethal H5N1 virus challenge in mice and ferrets.60
A Phase I/IIa clinical trial for evaluation of safety and immunogenicity of a clade 2 H5N1 (A/Indonesia/05/2005) VLP vaccine showed that it was well tolerated and immunogenic.10 The study evaluated individuals from 18 to 40 years of age who received two injections of 15, 45, or 90 micrograms of the vaccine or a placebo. There were dose-dependent increases in HI titers. In the 90 microgram group, 63% of subjects achieved a four-fold or greater (≥1:40) rise in HI titers compared to the baseline.10 A multivalent VLP incorporating HA from different subtypes broadened the protection coverage.61
Despite some encouraging pre-clinical and clinical results, VLP strategy warrants further stringent clinical evaluation to assess neutralizing and cross-neutralizing immune responses.. Importantly, clinical studies should also be done to evaluate the role of adjuvants in further enhancing the immunogenicity and dose-sparing to make this strategy feasible.
DNA VACCINES
A DNA vaccine involves administration of plasmid DNA carrying one or more genes representing antigenic proteins. The immunogenicity of a DNA vaccine is dependent, to a great extent, on the delivery method used. The most efficacious approach for DNA immunization is the bombardment with particles coated with DNA (gene-gun) or electroporation into the epidermal layer of skin.62,63 Unlike conventional vaccines, a DNA plasmid can be easily manipulated to incorporate gene inserts representing single/multiple antigens and immunostimulatory molecules to enhance the magnitude and type of immune responses. DNA vaccines have demonstrated both an excellent safety profile64 and the ability to induce humoral and cellular immune responses. DNA vaccination usually elicits a strong Th 1 response.65
The first demonstration of protective efficacy of a DNA vaccine in an animal model was reported for influenza in 1993.66 Despite all its advantages, clinical development of DNA vaccines has been hampered by the inability to induce consistent, high-level immune responses in humans.67 The development of new delivery systems that enhance the immunogenicity of DNA vaccines would be critical for their clinical use.68 For pandemic influenza vaccine development, this strategy can be rapidly adapted to emerging influenza variants. The ease of introducing multiple antigens and immunostimulants offer flexibility in designing improved vaccine formulations.64
A DNA vaccine based on the consensus sequence of HA generated neutralizing antibodies against clade 1 and clade 2 H5N1 viruses and offered complete protection in mice against both clades.69 A similar study using an HA consensus sequence-based DNA vaccine induced protective neutralizing antibody titers against clade 1 and clade 2 viruses in ferrets and showed significant reductions in virus shedding after challenge.70 This vaccine also induced cross-clade neutralizing antibody titers in a primate model. DNA vaccines based on the conserved nucleoprotein and matrix protein have been shown to induce a cross-protective cell-mediated immune response thereby reducing the morbidity associated with the disease.71,72 Electroporation of synthetic DNA resulted in robust induction of cross-reactive cellular and humoral immune responses capable of providing protection from influenza infection in rhesus macaques.73 Vaxfectin (a cationic lipid delivery system74,75)-formulated DNA vaccine encoding the HA-derived from A/Vietnam/1203/04, was protective in mice and ferrets in a two dose regimen.76,77 However, only a single dose of a Vaxfectin-formulated multivalent (HA + NP + M2) DNA vaccine was equally protective in ferrets.
Preliminary results of an ongoing clinical trial using a trivalent Vaxfectin-formulated DNA vaccine encoding the HA of A/Vietnam/1203/04 and the consensus sequences of two highly conserved influenza proteins, NP and M2, delivered intramuscularly or with a Biojector 2000 needle-free injection system showed promising safety and immunogenicity results.78 At least 67% of the subjects developed protective levels of antibody response (HI titers ranged from 40 to 640) by Day 56 in higher dose cohorts receiving 0.5 mg or 1 mg vaccine doses given twice.78 Similarly, another clinical trial involving a three dose regimen of a DNA plasmid encoding HA from a more recent strain of H5N1 (A/Indonesia/05/05) given intramuscularly via Biojector is currently underway.79
DNA vaccines have a good safety profile in clinical trials, but while testing has shown significant development of immune responses, this was accomplished only with several doses. In the event of a pandemic, however, a three dose regimen may not be feasible.
VIRAL VECTOR-BASED VACCINES
The viral vector-based vaccine approach involves the insertion of the genetic material encoding the important antigen/s into the genome of a harmless virus resulting in the generation of a recombinant virus. These modified viruses serve as gene delivery systems to efficiently carry the gene/s of interest into the host cells for expression of immunogenic antigen/s. Thus, viral vector-based vaccines function as live vaccines, but do not involve the complete pathogen. The antigens are expressed in infected cells similar to a natural infection, thereby inducing both humoral and cell-mediated immune responses. In addition, the inherent ability of certain viral vectors to enhance innate immune responses may boost the efficacy of such vaccines. Viral vectors serve as a natural nanoparticle-based gene delivery system. These vectors can be grown to high titers in certified cell lines in a short period of time and without the safety challenges associated with the production of highly pathogenic viruses. Furthermore, this approach can also be used to deliver multiple antigens and/or genetic adjuvants at the same time to broaden the protection coverage. As a tool for producing large amounts of vaccine quickly in an influenza pandemic, viral vector-based vaccines hold considerable potential.
Adenovirus vectors
Adenovirus (Ad)-based vectors have several features that are desirable in a vaccine vector. The virus is nonpathogenic, infects both dividing and non-dividing cells, grows to high titers in cell culture, enables high levels of transgene expression and lacks the ability to integrate into the host genome.80 Ad vectors have been shown to exert an adjuvant effect by stimulating the innate immune system by both Toll-like receptor-dependent and independent pathways.81[Sharma and Mittal unpublished data]. Interaction of Ad capsids with cellular receptors induces expression of pro-inflammatory cytokines/chemokines, which results in recruitment of effector cells of the innate and adaptive immune system to the site of vector delivery.82 These cytokines also activate functions of antigen-presenting cells (APCs). Several preclinical and clinical studies using Ad vector-based vaccines for measles, severe acute respiratory syndrome (SARS), human immunodeficiency virus, hepatitis B and Ebola have highlighted the versatility of this approach.83–87
With regard to influenza virus, our studies have demonstrated that a human adenovirus (HAd)-based H5N1 vaccine expressing HA gene of HK/156/97 elicited significantly high levels of virus-neutralizing antibody titers in a mouse model.88 The vaccine also elicited a significantly high frequency of HA-518 (a conserved CD8 T cell epitope in all human H5N1 viruses isolated since 1997) epitope-specific interferon-gamma secreting CD8+ T cells and offered complete protection against lethal challenge with homologous as well as antigenically distinct H5N1 influenza viruses.88 The generation of robust cell-mediated immune responses by the vaccine is particularly important as it has been shown to play a role in viral clearance and shortening the duration of illness.89
A similar study conducted in mice and chickens also demonstrated the efficacy of HAd-based H5N1 vacccine.90 The HAd-H5HA vaccine induced durable serological responses, long-term persistence of epitope-specific CD8+ T-cell responses and conferred long-term protective immunity for at least 12 months post-immunization in mice.91 Interestingly, two doses of vaccine as little as 1 × 106 plaque forming unit (p.f.u.) was completely protective.91 The protective efficacy of HAd-based H5N1 vaccine can be further broadened by including HA from clade 1 and clade 2 viruses, as well as conserved NP, in the vaccine formulation, thereby offering complete protection in mice against challenge with either clade 1 or clade 2 viruses.92
In a similar study, a replication incompetent complex Ad vector (CAdVax) devoid of E1, E3, and most of E4 regions of the Ad genome and carrying multiple genes (HA, NA, and M1) from clade 1 virus (CAdVax-FluAv) was shown to be 100% protective in mice against challenge with both clade 1 and clade 2 viruses.93 Ad5 vector-based delivery system for influenza vaccine has been shown to be safe and immunogenic in humans.94,95 In a clinical trial involving 24 healthy human subjects, intranasal administration of a two dose regimen of 5 × 108 virus particles expressing HA from A/PR/8/34 resulted in a fourfold increase in HI titers in 83% of the subjects. A topical application of 10–1000-fold higher viral particles resulted only in 33–67% seroconversion.95,94
One of the potential problems with Ad vectors is the role of vector immunity in blunting the immune response generated against the antigen delivered by HAd5 vectors. Several pre-clinical studies have addressed vector immunity,80,96 but to date this has not been effectively established in clinical studies. Moreover, the impact of Ad vector immunity on an HAd-based HIV vaccine in a phase I/II clinical trial was shown to be overcome by increasing the vaccine dose.97 However, the much publicized failure of Merck’s replication–defective HAd5 HIV-1 vaccine trial (STEP trial) has raised some concerns regarding the use of HAd5 vector as vaccine delivery system.98,99 The trial showed the lack of vaccine efficacy. Surprisingly there was a higher incidence rate (2.3%) of HIV-1 acquisition in vaccinees with baseline HAd5-specific neutralizing antibodies compared with controls.98,99 However, the scientific community is still debating the potential explanations100,101,102 of the observed results of the STEP trial. Whether the findings were unique to HIV pathogenesis is yet to be determined conclusively. Moreover, the implications of the STEP trial’s findings on the use of other rare human or nonhuman Ad serotypes should be favorable since the basic principle behind their use is a low or no seroprevalence in the human population.
Nonetheless, several alternative approaches are being explored to circumvent the limitations of human Ad vectors, such as the use of nonhuman Ad vector based vaccines80,103,104 or the use of DNA priming and an Ad vector boost.105,106 Our studies have shown that a bovine Ad subtype 3 (BAd) vector-based H5N1 vaccine offered complete protection in a mouse model in the presence of high levels of preexisting HAd vector immunity.104 In addition, the prime-boost strategy using HAd5/BAd vectors resulted in significant higher levels of immune responses compared to responses generated with either vector alone, suggesting the importance of two vector systems in inducing better immune responses.104
Advantages of Ad-based vaccines are numerous, and results of both animal studies and clinical trials have shown an Ad-vector-based delivery system for influenza vaccine to be safe and immunogenic in humans.94,95 However, the safety and efficacy of the HAd5-based H5N1 vaccine needs to be thoroughly tested in clinical trials involving individuals of varied age groups and health status.
Alphavirus vectors
Alphaviruses are positive strand RNA viruses, and the vectors derived from them are also known as “replicons” due to the self-amplification of the vector RNA in the cytoplasm of infected cells. Alphavirus vectors have been primarily developed using the Venezuelan equine encephalitis, Sindbis, and Semliki Forest viruses.107 The attractive features of these vaccine vectors are the high levels of antigen expression leading to development of robust cellular, humoral and mucosal immune responses, the induction of innate immune responses through the double-stranded RNA intermediates, and the absence of preexisting immunity in humans against these vectors.108,109 Alphavirus vector-based vaccines have shown protection in numerous models for infectious diseases including influenza, human papilloma, and Ebola. 110,111,112
In one study, alphavirus replicon particles containing the HA gene from an H5N1 isolate (A/HK/156/97) was protective in chickens at a dose of 107 infectious units.111 Alphavirus-like replicon particles expressing the HA of A/Wyoming/03/2003 (H3N2) was immunogenic in preclinical studies in mice, rabbits and macaques.113 A phase 1 clinical trial involving 216 healthy adults immunized with one or two doses of a low or high concentration of the same H3N2 vaccine induced protective HI titers in 77 and 80% of subjects receiving a single low or high antigen dose of vaccine, respectively.114 A second immunization in these individuals enhanced seroprotective responses to 86% for both dosage levels and extended the duration of T cell response as compared to the single immunization. No significant differences in immune responses were observed between subcutaneous and intramuscular routes of immunization.114
The advantages inherent in alphavirus replicon particles such as a lack of preexisting immunity in humans indicates that this vaccine strategy should be further investigated. Since an initial clinical study showed promising results, H5N1 vaccine candidates developed using this technology should be tested extensively for safety and efficacy in clinical trials.
Newcastle disease virus (NDV) vectors
With the advent of the reverse genetics system, the manipulation of NDV (a negative sense RNA virus) has become straightforward (similar to other DNA and positive-sense RNA viruses) and has helped to develop NDV as a safe and efficacious vaccine vector.115 NDV naturally infects via the mucosal surfaces of respiratory and alimentary tracts thus making it well suited for the control of respiratory virus infections such as influenza. The needle-free intranasal route of inoculation also makes it suitable for mass immunization. NDV is highly attenuated for replication in primates and is restricted to the respiratory tract, precluding concerns associated with potential spread to distal sites. The high level of host range restriction in primates offers a potential advantage to being developed as a vaccine vector for humans. In addition, NDV-based vectors grow to high titers in certified Vero cells.116
Several preclinical studies in mouse and chicken models have demonstrated the immunogenicity and protective efficacy of a NDV-vectored influenza vaccine against highly pathogenic avian influenza.117,118 Recently, DiNapoli et al have demonstrated the utility of an NDV vectored vaccine for human use in a nonhuman primate model.119 They engineered a live attenuated NDV-based vaccine (NDV-HA) expressing HA of highly pathogenic H5N1 virus (A/Vietnam/1203/2004) and showed that the vaccine was highly attenuated in nonhuman primates. A single inoculation by both the intranasal and intratracheal routes with 107 p.f.u. of NDV-HA per site induced substantial serum IgG and mucosal IgA responses.119
Although the safety of the vector has been demonstrated in the mouse, chicken and, importantly, in nonhuman primate models, there are concerns regarding the safety associated with the recombination between a vaccine vector and its circulating wild-type counterpart. Additionally, there are issues regarding the foreign gene insertion site in the NDV genome which affects the immunogenicity and vaccine efficacy.120
Vesicular stomatitis virus (VSV) vectors
Several naturally occurring or recombinant strains of VSV have been developed as potential therapeutic and vaccine vectors for various diseases.121–124 The low seroprevalance of VSV in humans makes it suitable as a vector for human use. Its immunogenicity and safety profile has been evaluated in several preclinical trials.125–127 VSV has a broad host range due to its utilization of phosphatidylserine, an universal component of cell-surface membranes, for virus entry.128 Using mice, Schwartz et al.129 showed that a recombinant VSV vector expressing HA of an H5N1 virus isolated in 1997 induced robust neutralizing antibody titers against the homologous and more recent antigenically different H5N1 viruses. This vaccine provided protection up to 7 months against lethal challenge with the homologous H5N1 virus even in animals receiving a single dose of the vaccine.129 Efficacy of a VSV-based vaccine expressing HA of A/FPV/Rostock/34 (H7N1) in place of the VSV G gene was also evaluated in chickens.130 The vaccinated birds were protected against challenge with a homologous H7N1 virus but not against an H5N2 virus.130
A VSV-vector vaccine has the potential to protect against different subtypes of avian influenza viruses in poultry. However, while low seroprevalance in humans is a definite advantage to using VSV as a potential pandemic influenza vaccine, its value in designing a pandemic influenza vaccine for humans requires more preclinical studies before clinical trials on humans would be feasible.
Poxvirus vectors
Vaccinia virus, a member of the family Poxviridae was successfully used to eradicate small pox. Poxviruses are the largest viruses known, and vectors derived from them can accommodate large/multiple gene inserts (>25kb).131 Modified vaccinia Ankara (MVA) vectors are highly attenuated with comparable immunogenicity and recombinant gene expression in mammalian cells indicating their safety and usefulness as vaccine vectors.132–134 Immunogenicity and safety of MVA vectors has been demonstrated in several preclinical and clinical trials for HIV, malaria and small pox.135–138
MVA recombinants expressing influenza virus antigens have been evaluated in several preclinical trials.139–141 Immunization with two doses (108 p.f.u.) of a MVA expressing A/Vietnam/1194/04 HA, given at 4 weeks part, induced strong antibody responses in mice.142 Furthermore, the mice were protected from infection when challenged with the homologous clade 1 (A/VN/1194/04) or clade 2 (A/Indonesia/05/05) virus.142 The same vaccine (MVA-HA-VN/04) also induced protective immune responses in nonhuman primates (Cynomolgus macaques) against clade 1 and clade 2 viruses.143 Another vaccinia virus-based multivalent H5N1 influenza vaccine expressing HA, NA & NP from A/Vietnam/1203/04 and M1 & M2 from A/CK/Indonesia/PA/2003 and adjuvanted with IL-15 elicited protective neutralizing antibody titers (1:80) against both clade 1 and clade 2.2 viruses in mice.144 The vaccine induced influenza-specific antibody response was detectable in appreciable amounts for 14 months post-immunization.144 Similarly, a single dose of a replication defective vaccinia H5N1 vaccine based on A/Vietnam/1203/2004 induced substantial cell-mediated immune (CMI) responses and provided cross-clade protection in mice.145
The pre-clinical data on studies using poxviruses as vectors is encouraging. Given the large size of poxviruses able to accommodate multiple gene inserts and the proven record as a vaccine against small pox, the strategy of using poxviruses as vectors warrants further clinical evaluation as an H5N1 pandemic vaccine.
CONCLUSION
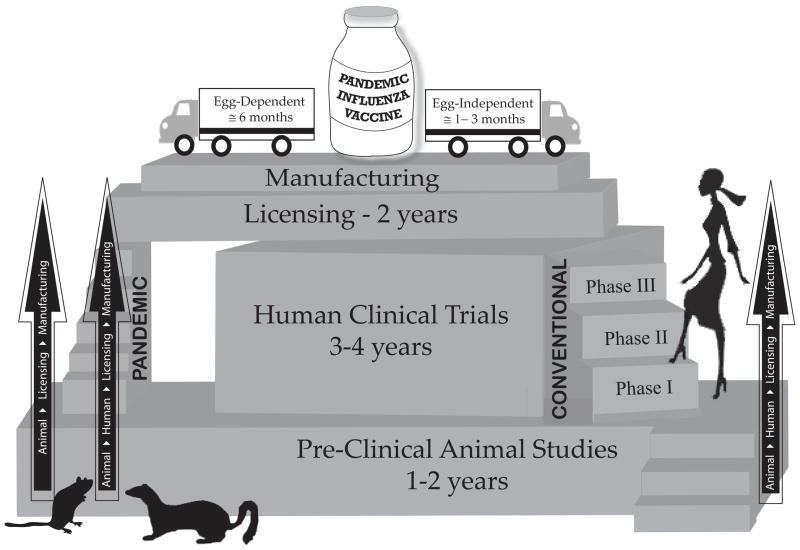
Developing effective vaccines in a timely manner remains our best option for influenza pandemic preparedness. Egg-dependent influenza vaccine approaches may not be able to meet the huge global demand in a pandemic scenario, due to lack of manufacturing capacity as well as potential problems with the availability of embryonated chicken eggs. Therefore, an egg-independent vaccine manufacturing technology/infrastructure will not only augment the existing vaccine production capability but also offer shorter production timelines than egg-based technologies (Fig. 2). The ongoing 2009 H1N1 influenza pandemic can be seen struggling with the challenge to meet the global vaccine demand with primarily egg-based strategies.
Fig 2.
Pandemic influenza vaccine development: From pre-clinical testing to vaccine distribution.
In a pandemic scenario, there will be a need for improvising regulatory requirements for vaccine approval and facilitating a fast-track approval of vaccines made using current or evolving technologies. Over the years, regulatory agencies in the US and Europe have come up with strategies and guidelines for accelerated approval of pandemic vaccines.146–148 Strategies with a “mock-up” or “pandemic-like” vaccine have been outlined wherein vaccine manufacturers go through a special advance licensing process, producing a “core dossier” of safety and immunogenicity data on their vaccines and manufacturing processes prior to a pandemic. Then, if warranted, they may apply for a “pandemic variation” to switch to the subtype covered by the vaccine to the pandemic virus. Nonetheless, harmonization of the diverse regulatory pathways in different countries for the licensure of a pandemic influenza vaccine would further simplify the regulatory requirements for a vaccine manufactured using new technologies or with new adjuvant formulations. One of the major concerns in the endorsement of new vaccine technologies is the lack of official calibrated reagents and assays, thus contributing to significant delays in the approval process.
Cell-derived whole virus and subunit influenza vaccines are in advanced phases of clinical trials149 (Table 1) and have demonstrated safety and efficacy. They have also been approved for use in several countries. Other approaches such as VLP, DNA vaccines and viral vector-based vaccines appear very attractive as these carrier systems favorably impact the innate immune system and generate strong humoral and cell-mediated immune responses. VLP-based vaccines for influenza are fast gaining acceptance and are likely to be approved in Europe and the US within 2–3 years.150
Table 1.
Egg-independent influenza vaccine strategies that are at various stages of clinical trial.
| Approach | Subtype | Virus strain and/or gene/s | Substrate | Company | Clinical phases | References |
|---|---|---|---|---|---|---|
| Inactivated whole virus | H5N1 | A/Vietnam/1203/2004 | Vero cells | Baxter, Austria | I/II, III | 38, 39, 40 |
| Inactivated whole virus | H5N1 | A/Vietnam/1194/2004 | Primary Monkey Kidney cells | Vabiotech, Vietnam | I | 149 |
| Inactivated split virus | H7N1 | A/chicken/Italy H7N1xPR8(RD-3) | PER. C6 cells | Sanofi Pasteur, France | I | 20 |
| Inactivated subunit | H5N1 | A/Vietnam/1194/2004 | MDCK cells | Solvay Pharmaceuticals, Netherlands | I | 19 |
| Live-attenuated (NS1 deleted virus) | H5N1 | A/Vietnam/1203/2004 | Vero cells | Green Hill Biotechnology, Austria | I | 149 |
| Recombinant M2 | Consensus | Consensus M2/M2e sequence/motif | E.coli | Cytos Biotechnology, Switzerland | I | 19 |
| Recombinant HA | H5N1 | (i)A/Hong Kong/156/97 and A/Hong Kong/483/97 (ii)A/Vietnam/1203/2004 | SF9 Insect cells | Protein Sciences, USA; UMN Pharma, Japan | I/II; I | 52, 149 |
| VLP | H5N1 | HA, NA & M1 from A/Indonesia/05/2005 | SF9 Insect cells | Novavax, USA | I/II | 10 |
| DNA vaccine | H5N1 | (i) HA, NP, M2 from A/Vietnam/1203/2004 (ii) HA of A/Indonesia/05/2005 | E.coli | Vical, USA; NIH, USA | I; I/II | 78;79 |
| Adenovirus vector | H1N1 | HA from A/PR/8/34 | 293 cells | Vaxin Inc, USA | I | 95 |
| Alphavirus vector | H3N2 | HA from A/Wyoming/03/2003 | Vero Cells | Alphavax, USA | I | 114 |
The development of a Pandemic Influenza Preparedness Plan includes more factors than the development and stockpiling of vaccines. In addition to vaccine programs, plans will need to ensure the availability of antiviral and antibiotics drugs and to utilize passive immune therapy with human polyclonal antibodies against common epitopes to treat infected individuals in order to curtail the spread of a pandemic. Other non-pharmaceutical interventions such as regulating travel, social distancing and personal hygiene will also help to mitigate the severity of a pandemic. Furthermore, additional strategies need to be developed to ensure that infants less than six months old, immunocompromised individuals and the elderly are especially protected against a pandemic virus, since they comprise the most vulnerable populations and do not respond adequately to vaccines. Existing vaccine capacity can be further increased by dose-sparing and by developing novel adjuvants that will not only reduce the amount of antigen required for protection but also will help achieve the desirable objective of a single shot pandemic vaccine, most likely in a primed population. New vaccine technologies, however, are one of the most important tools in pandemic preparedness with their potential to meet the global demand for vaccine manufacturing and stockpiling.
Acknowledgments
This work was supported by Public Health Service grant AI059374 from the National Institute of Allergy and Infectious Diseases to SKM and an NVPO grant to SS. We are thankful to Jane Kovach for her secretarial assistance.
References
- 1.Peiris JS, de J, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. WHO; 2009. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_03_02/en/index.html. [Google Scholar]
- 4.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–2218. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 5.World now at the start of 2009 influenza pandemic. 2009 http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html.
- 6.Pandemic (H1N1) 2009 - update 59. 2009 http://www.who.int/csr/don/2009_07_27/en/index.html.
- 7.Pandemic (H1N1) 2009 briefing note 3 (revised) 2009 http://www.who.int/csr/disease/swineflu/notes/h1n1_surveillance_20090710/en/index.html.
- 8.Swine flu vaccine production hits a snag: yield so far is ‘less than optimal’. 2009 http://www.google.com/hostednews/canadianpress/article/ALeqM5hJMZ2o0rf1lyVv_1ZIxwdlZOqJuQ .
- 9.Influenza Vaccine Strategies for Broad Global Access. 2009 doi: 10.1007/978-3-540-92165-3_23. http://www.path.org/publications/details.php?i=1525. [DOI] [PubMed]
- 10.Novavax Presents Favorable Results From Phase I/IIa Pandemic Influenza Vaccine Program; World Health Organization Conference; 2009. http://www.medicalnewstoday.com/articles/139352.php. [Google Scholar]
- 11.Audsley JM, Tannock GA. Cell-based influenza vaccines: progress to date. Drugs. 2008;68:1483–1491. doi: 10.2165/00003495-200868110-00002. [DOI] [PubMed] [Google Scholar]
- 12.Grillberger L, Kreil TR, Nasr S, Reiter M. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol J. 2009;4:186–201. doi: 10.1002/biot.200800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev. 2006;19:614–636. doi: 10.1128/CMR.00005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horimoto T, Kawaoka Y. Strategies for developing vaccines against H5N1 influenza A viruses. Trends Mol Med. 2006;12:506–514. doi: 10.1016/j.molmed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Baxter Receives EMEA Positive Opinion for CELVAPAN, the First Cell Culture-based Pandemic Flu Vaccine. 2009 http://www.baxter.com/about_baxter/news_room/news_releases/2008/12_18_08_celvapan.html.
- 16.Sanofi Pasteur Cell Culture-Based Seasonal Influenza Vaccine Enters First Clinical Trial. 2009 http://www.medicalnewstoday.com/articles/52770.php.
- 17.Novartis launches first seasonal flu vaccine to use cell culture technology in UK. 2009 http://www.manufacturingchemist.com/story.asp?storycode=52934.
- 18.Cox MM. Vaccines in development against avian influenza. Minerva Med. 2007;98:145–153. [PubMed] [Google Scholar]
- 19.Tables on the Clinical trials of pandemic influenza prototype vaccines. 2009 http://www.who.int/vaccine_research/immunogenicity/immunogenicity_table.xls.
- 20.Sanofi Pasteur Broadens Pandemic Preparedness With First Clinical Trial of Novel Cell-Based H7N1 Vaccine. 2009 http://www.redorbit.com/news/health/661903/sanofi_pasteur_broadens_pandemic_preparedness_with_first_clinical_trial_of/index.html.
- 21.Solvay Pharmaceuticals builds new factory for cell-cultured influenza vaccine INFLUVAC®TC. 2009 http://www.solvayhealthcare.co.uk/news/newsitems/0,,19781-2-0,00.htm.
- 22.US awards $1 billion for cell-based flu vaccines. 2009 http://www.cidrap.umn.edu/cidrap/content/influenza/panflu/news/may0406vaccines.html.
- 23.HHS Awards $487 Million Contract to Build First U.S. Manufacturing Facility for Cell-Based Influenza Vaccine. 2009 http://www.hhs.gov/news/press/2009pres/01/20090115b.html.
- 24.Novartis gains European approval for its innovative flu vaccine Optaflu®. 2009 http://www.novartisvaccines.com/press-room/news/20070613_Optaflu_approved.shtml.
- 25.Robertson JS, Naeve CW, Webster RG, Bootman JS, Newman R, Schild GC. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985;143:166–174. doi: 10.1016/0042-6822(85)90105-9. [DOI] [PubMed] [Google Scholar]
- 26.Schild GC, Oxford JS, de Jong JC, Webster RG. Evidence for host-cell selection of influenza virus antigenic variants. Nature. 1983;303:706–709. doi: 10.1038/303706a0. [DOI] [PubMed] [Google Scholar]
- 27.Hardy CT, Young SA, Webster RG, Naeve CW, Owens RJ. Egg fluids and cells of the chorioallantoic membrane of embryonated chicken eggs can select different variants of influenza A (H3N2) viruses. Virology. 1995;211:302–306. doi: 10.1006/viro.1995.1405. [DOI] [PubMed] [Google Scholar]
- 28.Robertson JS, Nicolson C, Major D, Robertson EW, Wood JM. The role of amniotic passage in the egg-adaptation of human influenza virus is revealed by haemagglutinin sequence analyses. J Gen Virol. 1993;74 (Pt 10):2047–2051. doi: 10.1099/0022-1317-74-10-2047. [DOI] [PubMed] [Google Scholar]
- 29.Robertson JS, Cook P, Attwell AM, Williams SP. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine. 1995;13:1583–1588. doi: 10.1016/0264-410x(95)00085-f. [DOI] [PubMed] [Google Scholar]
- 30.Robertson JS. An overview of host cell selection. Dev Biol Stand. 1999;98:7–11. [PubMed] [Google Scholar]
- 31.Katz JM, Webster RG. Efficacy of inactivated influenza A virus (H3N2) vaccines grown in mammalian cells or embryonated eggs. J Infect Dis. 1989;160:191–198. doi: 10.1093/infdis/160.2.191. [DOI] [PubMed] [Google Scholar]
- 32.Palache AM, Brands R, van Scharrenburg GJ. Immunogenicity and reactogenicity of influenza subunit vaccines produced in MDCK cells or fertilized chicken eggs. J Infect Dis. 1997;176 (Suppl 1):S20–S23. doi: 10.1086/514169. [DOI] [PubMed] [Google Scholar]
- 33.Use of Cell Lines for the Production of Influenza Virus Vaccines: An Appraisal of Technical, Manufacturing, and Regulatory Considerations. 2009 http://www.who.int/vaccine_research/diseases/influenza/WHO_Flu_Cell_Substrate_Version3.pdf.
- 34.Gregersen JP. A quantitative risk assessment of exposure to adventitious agents in a cell culture-derived subunit influenza vaccine. Vaccine. 2008;26:3332–3340. doi: 10.1016/j.vaccine.2008.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard MK, Kistner O, Barrett PN. Pre-clinical development of cell culture (Vero)-derived H5N1 pandemic vaccines. Biol Chem. 2008;389:569–577. doi: 10.1515/bc.2008.060. [DOI] [PubMed] [Google Scholar]
- 36.Kistner O, Howard MK, Spruth M, Wodal W, Bruhl P, Gerencer M, et al. Cell culture (Vero) derived whole virus (H5N1) vaccine based on wild-type virus strain induces cross-protective immune responses. Vaccine. 2007;25:6028–6036. doi: 10.1016/j.vaccine.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palker T, Kiseleva I, Johnston K, Su Q, Toner T, Szymkowiak C, et al. Protective efficacy of intranasal cold-adapted influenza A/New Caledonia/20/99 (H1N1) vaccines comprised of egg- or cell culture-derived reassortants. Virus Res. 2004;105:183–194. doi: 10.1016/j.virusres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich HJ, Muller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med. 2008;358:2573–2584. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 39.Phase 3 Immunogenicity and Safety Study of an Inactivated H5N1 Influenza Vaccine (Whole Virion, Vero Cell Derived) 2009 http://clinicaltrial.gov/ct2/show/NCT00462215?term=avian+influenza&rank=16.
- 40.Phase III Study of a H5N1 Vaccine in Adults, Elderly and Specified Risk Groups. 2009 http://clinicaltrial.gov/ct2/show/NCT00711295?term=avian+influenza&rank=34.
- 41.Ikonomou L, Schneider YJ, Agathos SN. Insect cell culture for industrial production of recombinant proteins. Appl Microbiol Biotechnol. 2003;62:1–20. doi: 10.1007/s00253-003-1223-9. [DOI] [PubMed] [Google Scholar]
- 42.Insect-cell-based flu vaccine looks good in clinical trial. 2009 http://www.cidrap.umn.edu/cidrap/content/influenza/panflu/news/apr1207cell.html; file://C:\ProgramFiles\Reference Manager 11\review.rmd. http://www.cidrapumnedu/cidrap/content/influenza/panflu/news/apr1207cellhtml.
- 43.Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safdar A, Cox MM. Baculovirus-expressed influenza vaccine. A novel technology for safe and expeditious vaccine production for human use. Expert Opin Investig Drugs. 2007;16:927–934. doi: 10.1517/13543784.16.7.927. [DOI] [PubMed] [Google Scholar]
- 45.Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 46.Hang GD, Chen CJ, Lin CY, Chen HC, Chen H. Improvement of glycosylation in insect cells with mammalian glycosyltransferases. J Biotechnol. 2003;102:61–71. doi: 10.1016/s0168-1656(02)00364-4. [DOI] [PubMed] [Google Scholar]
- 47.Jarvis DL, Finn EE. Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat Biotechnol. 1996;14:1288–1292. doi: 10.1038/nbt1096-1288. [DOI] [PubMed] [Google Scholar]
- 48.Lakey DL, Treanor JJ, Betts RF, Smith GE, Thompson J, Sannella E, et al. Recombinant baculovirus influenza A hemagglutinin vaccines are well tolerated and immunogenic in healthy adults. J Infect Dis. 1996;174:838–841. doi: 10.1093/infdis/174.4.838. [DOI] [PubMed] [Google Scholar]
- 49.Powers DC, McElhaney JE, Florendo OA, Jr, Manning MC, Upshaw CM, Bentley DW, et al. Humoral and cellular immune responses following vaccination with purified recombinant hemagglutinin from influenza A (H3N2) virus. J Infect Dis. 1997;175:342–351. doi: 10.1093/infdis/175.2.342. [DOI] [PubMed] [Google Scholar]
- 50.Treanor JJ, Betts RF, Smith GE, Anderson EL, Hackett CS, Wilkinson BE, et al. Evaluation of a recombinant hemagglutinin expressed in insect cells as an influenza vaccine in young and elderly adults. J Infect Dis. 1996;173:1467–1470. doi: 10.1093/infdis/173.6.1467. [DOI] [PubMed] [Google Scholar]
- 51.Treanor JJ, Schiff GM, Hayden FG, Brady RC, Hay CM, Meyer AL, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297:1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 52.Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O’Brien D, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 53.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198:635–641. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 54.Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig C, Wagner R. Virus-like particles-universal molecular toolboxes. Curr Opin Biotechnol. 2007;18:537–545. doi: 10.1016/j.copbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roy P, Noad R. Virus-like particles as a vaccine delivery system: myths and facts. Hum Vaccin. 2008;4:5–12. doi: 10.4161/hv.4.1.5559. [DOI] [PubMed] [Google Scholar]
- 57.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3:e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang SM, Yoo DG, Lipatov AS, Song JM, Davis CT, Quan FS, et al. Induction of long-term protective immune responses by influenza H5N1 virus-like particles. PLoS ONE. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mahmood K, Bright RA, Mytle N, Carter DM, Crevar CJ, Achenbach JE, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 60.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009 doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–3361. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharpe M, Lynch D, Topham S, Major D, Wood J, Loudon P. Protection of mice from H5N1 influenza challenge by prophylactic DNA vaccination using particle mediated epidermal delivery. Vaccine. 2007;25:6392–6398. doi: 10.1016/j.vaccine.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Wang S, Zhang C, Zhang L, Li J, Huang Z, Lu S. The relative immunogenicity of DNA vaccines delivered by the intramuscular needle injection, electroporation and gene gun methods. Vaccine. 2008;26:2100–2110. doi: 10.1016/j.vaccine.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster RG, Robinson HL. DNA vaccines: a review of developments. BioDrugs. 1997;8:273–292. doi: 10.2165/00063030-199708040-00004. [DOI] [PubMed] [Google Scholar]
- 65.Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 66.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 67.Luxembourg A, Evans CF, Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–1664. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- 68.Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Chen MW, Cheng TJ, Huang Y, Jan JT, Ma SH, Yu AL, et al. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc Natl Acad Sci U S A. 2008;105:13538–13543. doi: 10.1073/pnas.0806901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laddy DJ, Yan J, Kutzler M, Kobasa D, Kobinger GP, Khan AS, et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS ONE. 2008;3:e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, Subbarao K, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, et al. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine. 2005;23:5404–5410. doi: 10.1016/j.vaccine.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 73.Laddy DJ, Yan J, Khan AS, Anderson H, Cohn A, Greenhouse J, et al. Electroporation of Synthetic DNA Antigens Offers Protection in Non-Human Primates Challenged with Highly Pathogenic Avian Influenza. J Virol. 2009 doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hartikka J, Bozoukova V, Ferrari M, Sukhu L, Enas J, Sawdey M, et al. Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine. 2001;19:1911–1923. doi: 10.1016/s0264-410x(00)00445-x. [DOI] [PubMed] [Google Scholar]
- 75.Reyes L, Hartikka J, Bozoukova V, Sukhu L, Nishioka W, Singh G, et al. Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine. 2001;19:3778–3786. doi: 10.1016/s0264-410x(01)00090-1. [DOI] [PubMed] [Google Scholar]
- 76.Lalor PA, Webby RJ, Morrow J, Rusalov D, Kaslow DC, Rolland A, et al. Plasmid DNA-based vaccines protect mice and ferrets against lethal challenge with A/Vietnam/1203/04 (H5N1) influenza virus. J Infect Dis. 2008;197:1643–1652. doi: 10.1086/588431. [DOI] [PubMed] [Google Scholar]
- 77.Jimenez GS, Planchon R, Wei Q, Rusalov D, Geall A, Enas J, et al. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum Vaccin. 2007;3:157–164. doi: 10.4161/hv.3.5.4175. [DOI] [PubMed] [Google Scholar]
- 78.Vical Announces Breakthrough For Pandemic Influenza DNA Vaccines With Preliminary Human Data. 2009 http://www.medicalnewstoday.com/articles/115420.php.
- 79.NIAID DNA Vaccine For H5N1 Avian Influenza Enters Human Trial. 2009 http://www.medicalnewstoday.com/articles/60091.php.
- 80.Bangari DS, Mittal SK. Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 2006;24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muruve DA. The innate immune response to adenovirus vectors. Hum Gene Ther. 2004;15:1157–1166. doi: 10.1089/hum.2004.15.1157. [DOI] [PubMed] [Google Scholar]
- 83.Fooks AR, Schadeck E, Liebert UG, Dowsett AB, Rima BK, Steward M, et al. High-level expression of the measles virus nucleocapsid protein by using a replication-deficient adenovirus vector: induction of an MHC-1-restricted CTL response and protection in a murine model. Virology. 1995;210:456–465. doi: 10.1006/viro.1995.1362. [DOI] [PubMed] [Google Scholar]
- 84.Gao W, Tamin A, Soloff A, D’Aiuto L, Nwanegbo E, Robbins PD, et al. Effects of a SARS-associated coronavirus vaccine in monkeys. Lancet. 2003;362:1895–1896. doi: 10.1016/S0140-6736(03)14962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez-Roman VR. HIV/AIDS prevention programs in developing countries are deficient without an appropriate scientific research infrastructure. AIDS. 2003;17:1114–1116. doi: 10.1097/00002030-200305020-00034. [DOI] [PubMed] [Google Scholar]
- 86.Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K, et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A. 1989;86:6763–6767. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bender BS, Small PA., Jr Influenza: pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- 90.Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoelscher MA, Jayashankar L, Garg S, Veguilla V, Lu X, Singh N, et al. New pre-pandemic influenza vaccines: an egg- and adjuvant-independent human adenoviral vector strategy induces long-lasting protective immune responses in mice. Clin Pharmacol Ther. 2007;82:665–671. doi: 10.1038/sj.clpt.6100418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoelscher MA, Singh N, Garg S, Jayashankar L, Veguilla V, Pandey A, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis. 2008;197:1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, et al. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26:2627–2639. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 94.Hoelscher M, Gangappa S, Zhong W, Jayashankar L, Sambhara S. Vaccines against epidemic and pandemic influenza. Expert Opin Drug Deliv. 2008;5:1139–1157. doi: 10.1517/17425247.5.10.1139. [DOI] [PubMed] [Google Scholar]
- 95.Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 96.Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- 97.Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hudson CP. HIV-1 step study. Lancet. 2009;373:805–806. doi: 10.1016/S0140-6736(09)60471-2. [DOI] [PubMed] [Google Scholar]
- 101.O’Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009 doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Robb ML. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet. 2008;372:1857–1858. doi: 10.1016/S0140-6736(08)61593-7. [DOI] [PubMed] [Google Scholar]
- 103.Roy S, Kobinger GP, Lin J, Figueredo J, Calcedo R, Kobasa D, et al. Partial protection against H5N1 influenza in mice with a single dose of a chimpanzee adenovirus vector expressing nucleoprotein. Vaccine. 2007;25:6845–6851. doi: 10.1016/j.vaccine.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh N, Pandey A, Jayashankar L, Mittal SK. Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus. Mol Ther. 2008;16:965–971. doi: 10.1038/mt.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA, et al. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J Virol. 2003;77:8729–8735. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lundstrom K. Alphavirus vectors for vaccine production and gene therapy. Expert Rev Vaccines. 2003;2:447–459. doi: 10.1586/14760584.2.3.445. [DOI] [PubMed] [Google Scholar]
- 108.Perri S, Driver DA, Gardner JP, Sherrill S, Belli BA, Dubensky TW, Jr, et al. Replicon vectors derived from Sindbis virus and Semliki forest virus that establish persistent replication in host cells. J Virol. 2000;74:9802–9807. doi: 10.1128/jvi.74.20.9802-9807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Polo JM, Gardner JP, Ji Y, Belli BA, Driver DA, Sherrill S, et al. Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev Biol (Basel) 2000;104:181–185. [PubMed] [Google Scholar]
- 110.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75:11677–11685. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, Johnston R, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278:55–59. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]
- 112.Velders MP, McElhiney S, Cassetti MC, Eiben GL, Higgins T, Kovacs GR, et al. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer Res. 2001;61:7861–7867. [PubMed] [Google Scholar]
- 113.Hubby B, Talarico T, Maughan M, Reap EA, Berglund P, Kamrud KI, et al. Development and preclinical evaluation of an alphavirus replicon vaccine for influenza. Vaccine. 2007;25:8180–8189. doi: 10.1016/j.vaccine.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alphavax announces results from phase I influenza vaccine clinical trial. 2009 http://www.alphavax.com/docs/pr/release_43.pdf.
- 115.Huang Z, Elankumaran S, Panda A, Samal SK. Recombinant Newcastle disease virus as a vaccine vector. Poult Sci. 2003;82:899–906. doi: 10.1093/ps/82.6.899. [DOI] [PubMed] [Google Scholar]
- 116.Bukreyev A, Collins PL. Newcastle disease virus as a vaccine vector for humans. Curr Opin Mol Ther. 2008;10:46–55. [PubMed] [Google Scholar]
- 117.Veits J, Wiesner D, Fuchs W, Hoffmann B, Granzow H, Starick E, et al. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc Natl Acad Sci U S A. 2006;103:8197–8202. doi: 10.1073/pnas.0602461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ge J, Deng G, Wen Z, Tian G, Wang Y, Shi J, et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–158. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.DiNapoli JM, Yang L, Suguitan A, Jr, Elankumaran S, Dorward DW, Murphy BR, et al. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J Virol. 2007;81:11560–11568. doi: 10.1128/JVI.00713-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han GZ, Liu XP, Li SS. Caution about Newcastle disease virus-based live attenuated vaccine. J Virol. 2008;82:6782. doi: 10.1128/JVI.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geisbert TW, ddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–6900. doi: 10.1016/j.vaccine.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iyer AV, Pahar B, Boudreaux MJ, Wakamatsu N, Roy AF, Chouljenko VN, et al. Recombinant vesicular stomatitis virus-based west Nile vaccine elicits strong humoral and cellular immune responses and protects mice against lethal challenge with the virulent west Nile virus strain LSU-AR01. Vaccine. 2009;27:893–903. doi: 10.1016/j.vaccine.2008.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kapadia SU, Simon ID, Rose JK. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376:165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roediger EK, Kugathasan K, Zhang X, Lichty BD, Xing Z. Heterologous boosting of recombinant adenoviral prime immunization with a novel vesicular stomatitis virus-vectored tuberculosis vaccine. Mol Ther. 2008;16:1161–1169. doi: 10.1038/mt.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zinkernagel RM, Adler B, Holland JJ. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46:53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]
- 126.Geisbert TW, ddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4:e1000225. doi: 10.1371/journal.ppat.1000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, et al. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwartz JA, Buonocore L, Roberts A, Suguitan A, Jr, Kobasa D, Kobinger G, et al. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology. 2007;366:166–173. doi: 10.1016/j.virol.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalhoro NH, Veits J, Rautenschlein S, Zimmer G. A recombinant vesicular stomatitis virus replicon vaccine protects chickens from highly pathogenic avian influenza virus (H7N1) Vaccine. 2009;27:1174–1183. doi: 10.1016/j.vaccine.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 131.Smith GL, Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983;25:21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- 132.McCurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin Infect Dis. 2004;38:1749–1753. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 133.Moss B, Carroll MW, Wyatt LS, Bennink JR, Hirsch VM, Goldstein S, et al. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992;89:10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dorrell L, Williams P, Suttill A, Brown D, Roberts J, Conlon C, et al. Safety and tolerability of recombinant modified vaccinia virus Ankara expressing an HIV-1 gag/multiepitope immunogen (MVA. HIVA) in HIV-1-infected persons receiving combination antiretroviral therapy. Vaccine. 2007;25:3277–3283. doi: 10.1016/j.vaccine.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 136.Im EJ, Hanke T. MVA as a vector for vaccines against HIV-1. Expert Rev Vaccines. 2004;3:S89–S97. doi: 10.1586/14760584.3.4.s89. [DOI] [PubMed] [Google Scholar]
- 137.Moorthy VS, Pinder M, Reece WH, Watkins K, Atabani S, Hannan C, et al. Safety and immunogenicity of DNA/modified vaccinia virus ankara malaria vaccination in African adults. J Infect Dis. 2003;188:1239–1244. doi: 10.1086/378515. [DOI] [PubMed] [Google Scholar]
- 138.Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24:2065–2070. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 139.Altstein AD, Gitelman AK, Smirnov YA, Piskareva LM, Zakharova LG, Pashvykina GV, et al. Immunization with influenza A NP-expressing vaccinia virus recombinant protects mice against experimental infection with human and avian influenza viruses. Arch Virol. 2006;151:921–931. doi: 10.1007/s00705-005-0676-9. [DOI] [PubMed] [Google Scholar]
- 140.Jakeman KJ, Smith H, Sweet C. Mechanism of immunity to influenza: maternal and passive neonatal protection following immunization of adult ferrets with a live vaccinia-influenza virus haemagglutinin recombinant but not with recombinants containing other influenza virus proteins. J Gen Virol. 1989;70 (Pt 6):1523–1531. doi: 10.1099/0022-1317-70-6-1523. [DOI] [PubMed] [Google Scholar]
- 141.Sutter G, Wyatt LS, Foley PL, Bennink JR, Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 142.Kreijtz JH, Suezer Y, van AG, de MG, Schnierle BS, Wood JM, et al. Recombinant modified vaccinia virus Ankara-based vaccine induces protective immunity in mice against infection with influenza virus H5N1. J Infect Dis. 2007;195:1598–1606. doi: 10.1086/517614. [DOI] [PubMed] [Google Scholar]
- 143.Kreijtz JH, Suezer Y, de MG, van den Brand JM, van AG, Schnierle BS, et al. Recombinant modified vaccinia virus Ankara expressing the hemagglutinin gene confers protection against homologous and heterologous H5N1 influenza virus infections in macaques. J Infect Dis. 2009;199:405–413. doi: 10.1086/595984. [DOI] [PubMed] [Google Scholar]
- 144.Poon LL, Leung YH, Nicholls JM, Perera PY, Lichy JH, Yamamoto M, et al. Vaccinia virus-based multivalent H5N1 avian influenza vaccines adjuvanted with IL-15 confer sterile cross-clade protection in mice. J Immunol. 2009;182:3063–3071. doi: 10.4049/jimmunol.0803467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mayrhofer J, Coulibaly S, Hessel A, Holzer G, Schwendinger M, Bruhl P, et al. Non-replicating vaccinia vectors expressing the H5 influenza hemagglutinin produced in modified Vero cells induce robust protection. J Virol. 2009 doi: 10.1128/JVI.02081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. 2009 http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm.
- 147.GUIDELINE ON DOSSIER STRUCTURE AND CONTENT FOR PANDEMIC INFLUENZA VACCINE MARKETING AUTHORISATION APPLICATION. 2009 http://www.emea.europa.eu/pdfs/human/vwp/471703enfin.pdf.
- 148.Hampson AW. Vaccines for pandemic influenza. The history of our current vaccines, their limitations and the requirements to deal with a pandemic threat. Ann Acad Med Singapore. 2008;37:510–517. [PubMed] [Google Scholar]
- 149.Hayden FG, Howard WA, Palkonyay L, Kieny MP. Report of the 5th meeting on the evaluation of pandemic influenza prototype vaccines in clinical trials: World Health Organization, Geneva, Switzerland, 12–13 February 2009. Vaccine. 2009;27:4079–4089. doi: 10.1016/j.vaccine.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 150.Novavax in multi-million Spanish deal on vaccines. 2009 http://news.moneycentral.msn.com/provider/providerarticle.aspx?feed=AP&date=20090630&id=10102279.