Abstract
The olfactory epithelium is remarkable for the persistence of multipotent, neurocompetent progenitor and stem cells throughout life that can replace all of the various cell types of the epithelium following injury. The therapeutic exploitation of the neurocompetent stem cells of the adult olfactory epithelium would be facilitated by the development of a culture system that maintains the in vivo potency of the progenitors while they are expanded and/or manipulated. We have used an air-liquid interface culture protocol, in which a feeder cell layer of 3T3 cells is established on the underside of a culture insert and FACS-isolated or unsorted progenitor cells from the methyl bromide-lesioned adult rodent epithelium are seeded on upper side. Under these conditions, epithelial cells other than HBCs are capable of organizing themselves into complex 3-dimensional, epithelium-lined spheres, which can be passaged. The spheres contain cells with the molecular phenotype of globose basal cells, horizontal basal cells, sustentacular cells and neurons. Spheres derived from mice that express the green fluorescent protein constitutively can be dissociated after 6 days in vitro and directly transplanted into the epithelium of wild type, methyl bromide-lesioned mice via nasal infusion. The resulting clones contain the various cell types observed in aggregate when globose basal cells are transplanted acutely. In contrast, the same cells cultured as two dimensional, submerged cultures undergo fibroblastic transition after transplantation and do not integrate into the epithelium. In conclusion, the culture system described here maintains the potency of progenitors, which can then participate in epitheliopoiesis in vivo.
Keywords: cell surface marker, transplantation, tissue stem cells, progenitors
INTRODUCTION
Data have accumulated demonstrating that the adult olfactory epithelium (OE) retains stem cells that are competent to make neurons, i.e., neural stem cells, and that the population of globose basal cells (GBCs) is likely to include stem cells capable of replacing destroyed neurons and all other epithelial cell types during the recovery of the epithelium from direct or indirect injury. Evidence for the neuropotency of GBCs includes pulse-chase data (Graziadei, 1973; Graziadei and Monti Graziadei, 1979), selective increase in GBC population proliferation after bulbectomy (Schwartz Levey et al., 1991), lineage tracing experiment by infection of GBCs with replication-incompetent, retrovirally derived vectors (RRVV) in normal OE or following selective neuronal depletion (Caggiano et al., 1994; Schwob et al., 1994; Huard et al., 1998), and expression of “neuronal” transcription factors (e.g., Mash1, Ngn1 and NeuroD) (Guillemot et al., 1993; Cau et al., 1997; Guillemot, 1999; Manglapus et al., 2004).
Recently, however, a broader-than-neuronal potency of olfactory progenitor cells, a potency that is close, if not equivalent, to that of the olfactory placodal cells, from which derive the embryonic precursors of the peripheral olfactory system, has been observed. While still subject to investigation, it has been suggested that a set of multipotent olfactory progenitor (MPP) cells resides among the GBC population of the OE and can be activated after lesion when both neurons and non-neuronal cells need to be replaced (i.e., following direct exposure to methylbromide [MeBr] gas). For example, cells that are phenotypically transitional between GBCs and sustentacular cells and between GBCs and HBCs are observed after MeBr lesion (Goldstein and Schwob, 1996; Manglapus et al., 2004; Jang et al., 2007), and truncated Mash1 mRNA persists in both sustentacular cells and the basal cells in Mash-1 knockout mice (Murray et al., 2003). These data are indirect but highly suggestive evidence that the epithelial cells – sustentacular cells, neurons and basal cells (GBCs and HBCs) – share a common origin from GBCs. More direct evidence comes from lineage tracing and transplantation analysis (Goldstein et al., 1998; Huard et al., 1998; Chen et al., 2004). For example, FACS-purified GBCs from normal OE give rise to GBCs, neurons, sustentacular cells, duct/gland cells, columnar respiratory epithelial cells in aggregate in mouse (Chen et al., 2004); HBCs are observed following transplantation of RRVV-labeled GBCs in rats (Goldstein et al., 1998). Moreover, we have data showing that some GBCs retain BrdU label for an extended period after neonatal injection, become activated by MeBr lesion and then re-emerge as label-retaining following injection in the post-lesion period (Chen and Schwob, 2003; Chen et al., 2004). The cycle of apparent mitotic quiescence, activation, and then return to quiescence is characteristic of stem cells in other tissues. Thus, GBCs, by virtue of the capacity for self-renewal, broad multipotency, and mitotic quiescence display many of the characteristics expected of OE stem cells. More recently some lineage data suggested that the HBC population may be a second population with stem-like characteristics (Leung et al., 2007). Thus, the OE apparently contains at least two populations of neurocompetent stem cells that are easily accessible for manipulation and isolation and may be useful as a therapeutic moiety (Murrell et al., 2005). The utility of olfactory stem cells depends on our ability to maintain, expand and differentiate them in culture. To date, most tissue culture protocols have not been successful in maintaining tissue-appropriate differentiation capacity in vitro, nor have they been subject to an assessment of whether the cells maintained in culture can be reintroduced into the epithelium in vivo and participate in its reconstitution. For example, cells from embryonic or early postnatal OE can generate islands and cells that express some of the markers characteristics of olfactory neurons, including NCAM, NST, and, in some cases, OMP, but generally lack the morphology expected of olfactory sensory neurons (OSNs) and fail to maintain production of neurons (Calof and Chikaraishi, 1989; Chuah et al., 1991; Ronnett et al., 1991; Pixley, 1992; Mumm et al., 1996; Cunningham et al., 1999). Of the culture techniques, the one that best mimics the composition and architecture of the in vivo epithelium is the sphere culture observed by Pixley (Pixley et al., 1994). Yet these, too, may not be capable of behaving appropriately when used as replacement cell elements. Cultures derived from a population of cells enriched for (but not solely composed of) HBCs express markers for multiple cell types and rarely show evidence of maintained proliferation, as would be expected of stem cells (Carter et al., 2004).
We report here that MPPs from the MeBr-lesioned OE of adult mice and rats give rise to both neurons and various types of non-neuronal cells when cultured in either submerged monolayer culture or in feeder cell-enhanced air-liquid interface cultures (Gruenert et al., 1995). Only the cells from the three dimensional, air-liquid culture will incorporate successfully after transplantation into the regenerating OE of the host in a manner similar to their growth in culture and to the results of acute isolation and immediate transplantation in vivo. The ability to grow MPPs in vitro would allow us to assay and manipulate them biochemically, pharmacologically and genetically in the future. It would also give a useful way to expand MPPs for transplantation and therapeutic applications.
MATERIALS AND METHODS
Animals
C57BL/6 mice (Charles River Laboratories, Willmington, MA) at the age of 8–12 weeks, or Sprague-Dawley rats (Taconic Farms, Germantown, NY) at 250–300g b.w. were exposed to MeBr gas (200 ppm for 8 hrs for mice and 330ppm for 6 hrs for rats) 40 hrs before sacrifice. For bulbectomy, the right olfactory bulb was exposed via a dorsal craniotomy and removed by suction. The cavity created by the ablation was filled with Oxycel and the skin was sutured. The donor cells for transplantation were harvested from universal and constitutive GFP-expressing transgenic mice of the C57BL6-TgN (ACTbEGFP)1Osb strain created by Dr. Masaru Okabe (Okabe et al., 1997; The Jackson Laboratory, stock number 003291). GFP-expressing transgenic rats of the SD-Tg (ACT-EGFP) CZ-004Osb strain were also used (Sasaki et al., 2001). All animal use protocols were approved by the Institutional Animal Care and Use Committee of Tufts University School of Medicine.
Dissociation of Olfactory Progenitor Cells
Detailed protocols have been reported from our lab (Chen et al., 2004). Briefly, animals were perfused by intracardiac flush with low-Ca2+ Ringer solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM EDTA, 10 mM glucose and 1 mM sodium pyruvate, pH 7.2) after injection of an overdose of a cocktail of ketamine (30 mg/kg), xylazine (6 mg/kg) and acepromazine (1 mg/kg). The olfactory epithelium (OE) was dissected into septum and turbinate scrolls, and then incubated with 0.05 % trypsin/EDTA (Gibco BRL) in low-Ca2+ Ringer solution for 5–10 min at 37°C, followed by dissociation enzyme cocktail (collagenase/hyaluronidase/trypsin inhibitor; 1 mg/ml, 1.5 mg/ml, 0.1 mg/ml, respectively; Worthington Biochemical, Freehold, NJ) in Ringer’s solution (140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose and 1 mM sodium pyruvate, pH 7.2) for 15 min at 37°C with occasional trituration. Dissociated cells were treated with DNase I (Worthington) and subsequently filtered through 35 µm mesh before plating.
Monolayer Cell Culture
Dissociated OE cells were plated in different media (Table 1) in culture flasks, flasks coated with Matrigel (growth factor reduced; Collaborative Biomedical Products, Bedford, MA), or laminin-coated chamber wells (Becton Dickinson, Bedford, MA) at a density of 4–5 × 104/cm2. Cells for immunostaining were plated on 4–8 well chamber slides. Cells on culture flasks were passaged while still in log phase of growth (before confluency).
Table 1.
The composition of media that are used for submerged, monolayer cultures in this study.
| NIC Medium1 (serum-rich) | Neurosphere Medium2 (serum-free) | KGM3 (serum-free) |
|---|---|---|
| OptiMEM | DMEM + F-12 nutrient | KBM |
| Transferrin (5 µ/ml) | Transferrin (100 µg/ml) | |
| Insulin (1 µg/ml) | Insulin (25 µg/ml) | Insulin (50 mg/ml) |
| Sodium selenite (2.5 ng/ml) | Sodium selenite (30 nM) | |
| Glucose (200 mg/ml) | Glucose (6 mg/ml) | |
| Hydrocortisone (10 nM) | Progesterone (20 nM) | Hydrocortisone (1 µg/ml) |
| Thyroxine (40 pg/ml) | Putrescine (60 nM) | |
| Hypoxanthine (4.7 µg/ml) | Glutamine (2 mM) | |
| Folic acid (1 µg/ml) | ||
| Thymidine (0.7µg/ml) | ||
| Magnesium sulfate (0.5 mM) | Sodium bicarbonate (3 mM) | |
| EGF (20 ng/ml) | EGF (200 pg/ml) | |
| BHE (150 µg/ml) | ||
| BPE (50 µg/ml) | BPE (54 µg/ml) | |
Abbreviations: KGM (Keratinocyte Growth Medium), KBM (Keratinocyte Base Medium), BHE (Bovine Hypothalamic Extract), BPE (Bovine Pituitary Extract).
Refer to Goldstein et al. (1997)
Transplantation
The protocol for transplantation into the MeBr-lesioned OE has been described in detail (Chen et al., 2004). In brief, host C57BL/6 mice were exposed to 220 ppm of MeBr gas 18–24 hrs before transplantation. On the day of transplantation, the host mice were anesthetized with intramuscular (IM) injection of ketamine (2 mg/kg) and acepromazine (0.01 mg/kg). The GFP-expressing donor cells were resuspended in DMEM and either injected via a glass micropipette into the dorsal recess of the nasal cavity after dorsal craniotomy in the region of the fronionasal suture, or infused into one naris of trachetomized hosts via PE10 tubing inserted to a depth of 7 mm (Becton Dickinson). Transplant recipients were allowed to survive for 10–14 days before perfusion and fixation with 4% paraformaldehyde for histological and immunocytochemical examination.
Three-Dimensional Air-Liquid Interface Coculture
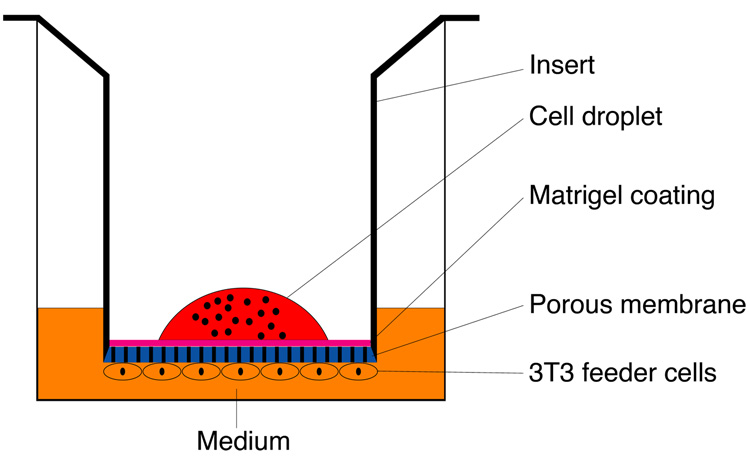
A. Preparing Cell Culture Inserts
One day prior to harvesting olfactory epithelial cells, cell culture inserts were prepared for 3-D air-liquid interface coculture (Fig. 1). The inside surface of Transwell inserts (Corning Incorporated Life Sciences, Acton, MA) were coated for 6 hours with growth-factor-reduced Matrigel Basement Membrane Matrix (BD Biosciences, San Jose, CA). The underside of each insert was then seeded with 100,000 NIH/3T3 mouse fibroblast cells, suspended in Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum. The cells were allowed to adhere to the membrane surface overnight, after which the inserts were inserted and placed in a 24-well tissue culture plate. Culture medium was added to the outside of the insert and the plate was stored at 37°C until seeding of olfactory epithelial cells.
Figure 1.
Schematic diagram illustrating the three-dimensional air-liquid interface culture.
B. Cell Seeding
Following dissociation, OE cells were pelleted and resuspended in a 1:1 mixture of DMEM and F12 media containing 2% fetal bovine serum and N2 supplement (Gibco). To the inside of each insert, 5–10 × 104 cells per well of a 24-well culture plate were seeded in a 40 µL volume. Cultures were incubated at 37°C in a 5%-CO2 environment. Medium was aspirated from the inside of the inserts 18–24 hours after seeding at the time that the OE cells had adhered to the membrane surface. Aspiration was done occasionally as necessary subsequent to plating.
C. Passaging Cells
Cell cultures were passaged every 7–10 days using a protocol similar to that used for dissociation of the primary olfactory cells. Cells were incubated in 0.25% trypsin at 37°C for 15 minutes, then in a mixture of collagenase/hyaluronidase/trypsin inhibitor/papain for 30 minutes, or in dispase (2 U/ml; Worthington) to lift cells from the Matrigel substrate. The reaction was terminated by the addition of Ringer’s solution containing DNase I. The collected cell suspension was then pelleted and resuspended in culture medium. Freshly prepared Transwell inserts were seeded with 1–5 × 104 cells per insert.
D. Cell Sorting
Fluorescence-activated cell sorting (FACS) was performed on a MoFlo cell sorter (Cytomation Inc., Fort Collins, CO). Dissociated cells from 2 day post-MeBr OE of wild type and GFP-transgenic rats (Jang et al., 2007) were prepared as described above, and resuspended in staining solution containing HBSS (Ca2+/Mg2+ free; Invitrogen; Aukland, NJ) with 25 mM HEPES and 1 mM EDTA. To isolate HBCs, the dissociated cells were incubated with a solution containing both the mouse monoclonal antibody (mAb) GBC-3 (Jang et al., 2007) and the biotinylated lectin I from Bandeiraea (Griffonia) simplicifolia (bBS-I; Vector Laboratories, Burlingame, CA) at a concentration of 1:50 each for 30 min. on ice. After washing with the staining solution, R-phycoerythrin (R-PE)-conjugated anti mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) and allophycocyanin (APC)-conjugated streptavidin (eBioscience, San Diego, CA) were applied for 20 min. on ice at concentration of 5 ng/ml and 0.4 ng/ml, respectively, followed by washing with the staining solution. An Innova 90 argon plasma laser (Coherent, Inc., Santa Clara, CA) was used to excite the cells at 488 nm and emission of R-PE was detected at 580nm (FL2), and APC was detected at 660 nm (FL6) using argon/krypton laser with an excitation wavelength of 633 nm. In the case of cells harvested from the GFP rats, the samples were gated in FL1 to include only the GFP positive cells. Additional gating on forward and side scatter and on exclusion of propidium iodide (PI) was used to limit the sort to live cells (Jang et al., 2007). GBC-3(+)/bBS-I(−), bBS-I(+), and double negative cells were collected separately in a solution containing 50% fetal bovine serum (FBS) in the HBSS/HEPES/EDTA solution on ice. After collection, cells were incubated in 37°C water bath for 15 min. and washed twice in the cell seeding media as described above, and seeded at an equal density.
Immunohistochemistry
Table 2 lists the antibodies used in this study. Cells in culture were rinsed in PBS and fixed. For antibodies to CK5/6, CK14, CK18 or BrdU, cells were fixed in cold-methanol at −20°C or 80% ethanol. Cells which were stained with TuJ-1 were fixed with 1% PLP (paraformaldehyde-lysine-periodate) (McLean and Nakane, 1974). After fixation, cells were rinsed and incubated with primary antibodies for 90 min. For BrdU staining, cells were treated with 0.07N NaOH followed by PBS, pH 8.5 before primary antibody incubation. Cultures were washed and incubated with secondary antibodies (either biotinylated, or FITC or Texas Red-conjugated) for 45–60 min. followed by washing. For fluorescent staining, cells are counter stained with either Hoechst dye or DAPI. Biotinylated secondary antibodies were visualized with ABC kit (Vector Laboratories, Burlingame, CA). Primary antibodies used here include: mouse monoclonal anti-neuron-specific tubulin (TuJ-1, Covance, Richmond, CA) 1:100/1:500, mouse monoclonal anti-CK14 (LL002, BioGenex, San Ramon, CA) 1:50/1:300, mouse monoclonal anti-CK18 (RGE 53, ACCU, Westbury, NY) 1:50/1:300, mouse anti-BrdU (Becton Dickinson, San Jose, CA) 1:20/ND, respectively for fluorescent vs. diaminobenzidine (DAB) visualization. The appropriate secondary antibodies were purchased from Jackson ImmunoResearch, West Grove, PA. After transplantation, engrafted donor cells were identified in tissue sections of the host animals by immunostaining with rabbit polyclonal anti-GFP (Abcam, Cambridge, MA) at 1:1200 in combination with TuJ-1, SUS-4 and/or bBS-I (concentrations of 1:50, 1:20 and 1:50, respectively) after steaming in 0.01M citric acid (pH 6.0).
Table 2.
Antibodies and markers used in the study
| Antibody | Source (catalog/clone number) | Antigen | OE Cell Types Labeled |
|---|---|---|---|
| Mouse antiBrdU | Becton Dickinson (347580/B44) | BrdU | Mitotically active cell types |
| Biotinylated BS-I (bBS-I) | Vector Laboratories | Carbohydrate | HBCs, some duct/gland cells |
| Mouse anti-CK 14 | Novocastra (NCLLL002) | 50 kD cytokeratin protein (cytokeratin 14) | HBCs |
| Mouse anti-CK 18 | ACCU-SPEC (YM3026/RGE53) | 45 kD cytokeratin protein (cytokeratin 18) | Sustentacular cells |
| Mouse GBC-3 | Jang et al. (2007) | 40 kD nonintegrin laminin receptor precursor protein | GBCs, some immature sensory neurons |
| Rabbit anti-GFP | Abcam (ab6556) | 27 kD green fluorescent protein (GFP) | Engrafted donor cells |
| Mouse SUS-4 | Goldstein and Schwob (1996) | Rat OE | Sustentacular cells, duct/gland cells |
| Mouse TuJ-1 | Covance (MMS-435P/TUJ1) | 50kD Neuron-specific tubulin (III) | Immature sensory neurons |
RESULTS
The experiments reported here contrast the behavior of olfactory epithelial progenitor cells in submerged monolayer culture vs. air-liquid insert culture, both in vitro and in vivo.
Submerged Monolayer Cultures of the Dissociated Adult Olfactory Epithelium
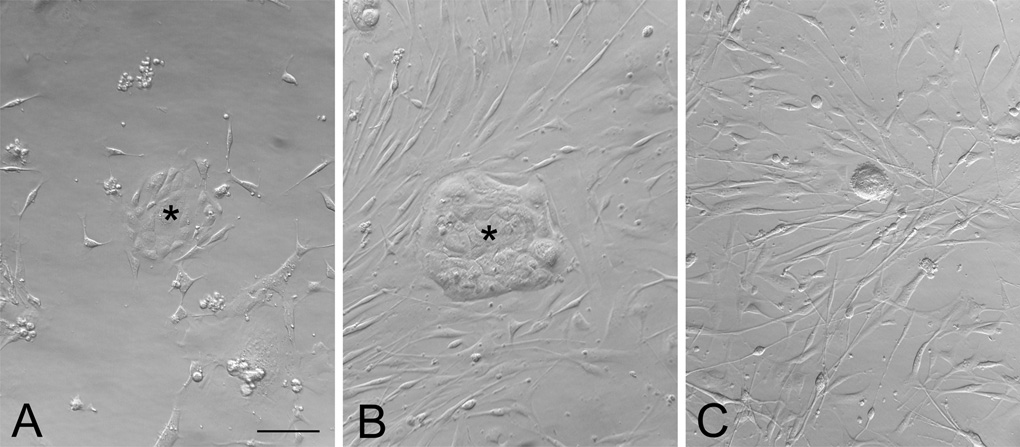
Submerged cultures were prepared exclusively on Matrigel- or laminin-coated culture plates. When cultured in serum-rich medium, the dissociated olfactory epithelial cells from normal vs. MeBr-lesioned vs. bulbectomized mice showed different growth patterns (Fig. 2). Cultures derived from normal and MeBr-exposed OE contained two kinds of constituents at 3 days in vitro (3 DIV): tightly adherent cells forming epithelioid islands, and dispersed, elongated, spindled cells surrounding the islands (Fig. 2A and B). The epithelioid islands appear to be very similar to the ones reported by other investigators from neonatal (Mumm et al., 1996) or postnatal mice (Carter et al., 2004). In OE cultures from bulbectomized mice, the epithelioid islands form only rarely; instead, the spindled cells are much more the predominant cell type (Fig. 2C).
Figure 2.
Epithelial islands develop from adult olfactory epithelium in submerged cultures. Bright field photomicrographs showing the two-dimensional submerged cultures of dissociated cells from the olfactory epithelium (OE) from normal (A), MeBr-exposed (B; at 2 days postlesion), and bulbectomized (C; at 5 days postlesion) mice grown on matrigel in serum-rich NIC medium. In normal and MeBr-exposed OE cultures, epithelioid islands are seen (asterisks in A and B) as well as scattered, elongated, spindle-shaped cells around the islands. In bulbectomized OE culture, the spindled cells are much more prevalent (C). Scale bar in A is 50 µm, and applies to B and C.
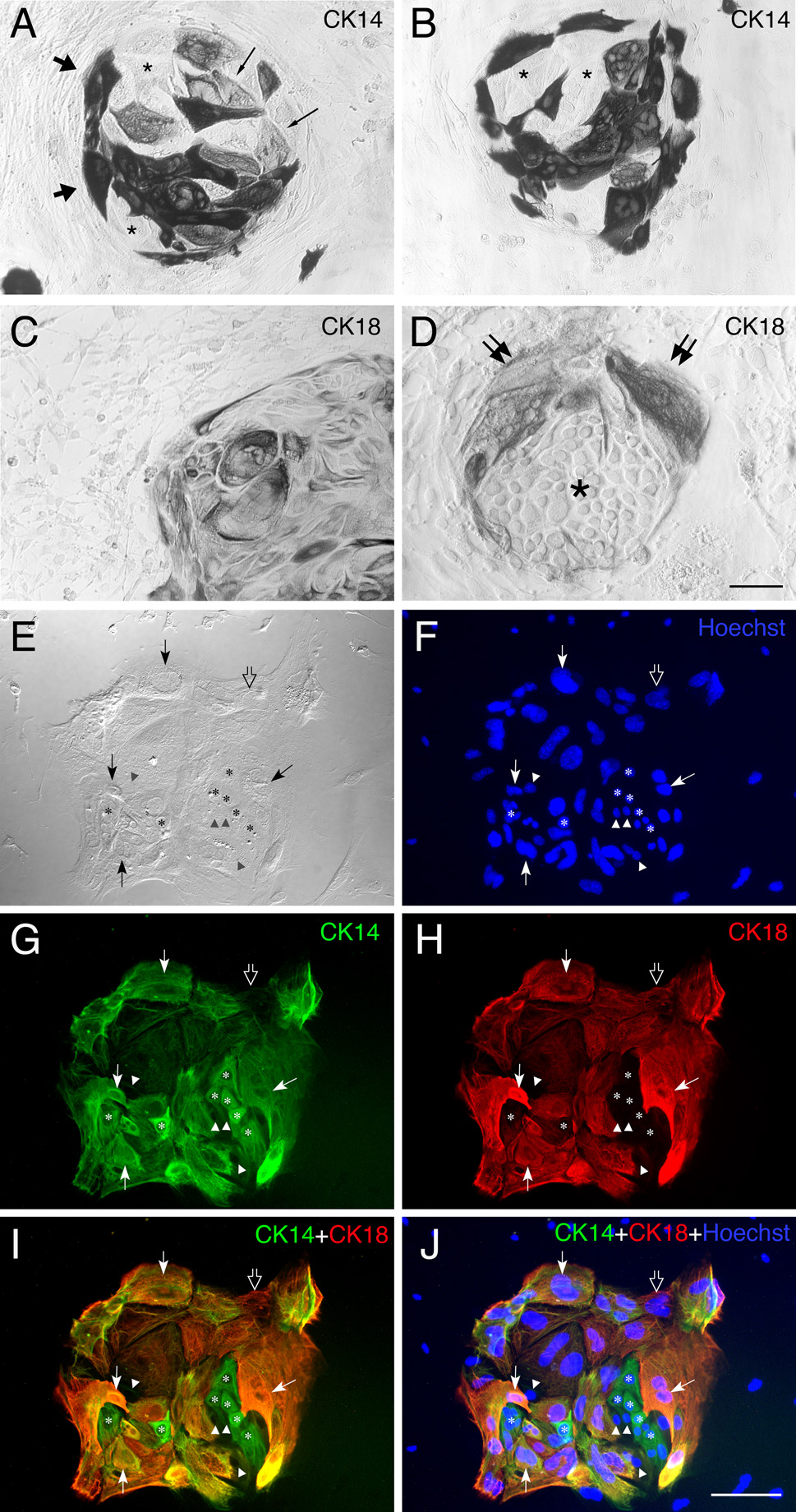
We analyzed marker expression by cells in the epithelioid islands and the dispersed spindle cells. As expected, the epithelioid islands are composed of cytokeratin (CK)-expressing cells, while the dispersed cells around the islands are devoid of cytokeratin expression (Fig. 3). Regardless of growth substrate (Matrigel or laminin) or culture medium composition (serum-rich or serum-free medium), the epithelioid islands are labeled by anti-CK14 (a marker for HBCs in vivo; Fig. 3A, B) and anti-CK18 (a marker for sustentacular cells in vivo; Fig. 3C, D). Cells within the epithelioid islands are heterogeneous with respect of their expression of cytokeratins. With regard to CK14, some cells are heavily labeled, some are lightly labeled and some remain unstained (Fig. 3A, B). This is true of cultures grown in either serum-rich or serum-free medium (cf. Figs. 3A and B). Cytokeratin18 (CK18)-expressing cells are also present in the vast majority of islands. With regard to CK18, the density of labeled cells differ across islands; in some islands most of the cells are CK18(+), while others are composed of mixture of CK18(+) and CK18 (−) cells (cf. Fig. 3C and D). Double staining with both anti-CK18 and CK14 demonstrates the surprising finding that a majority of cells in the islands stain with both, although CK18(+)-only and CK14(+)-only cells are also seen (Fig. 3E–J). Our results indicate that the epithelioid islands developing in culture from the MeBr-lesioned OE show the phenotypic characteristics of HBCs, of sustentacular cells, and of an intermediate phenotype.
Figure 3.
Epithelial islands contain cells expressing mixed phenotypic markers, i.e., CK14, a maker of HBCs in vivo, and CK18, a marker of sustentacular cells in vivo. (A, B) CK14(+) cells are prominent in cultures derived from MeBr-lesioned OE grown on laminin in serum-rich NIC medium. Some are densely positive (thick arrows), others are weakly positive (thin arrows) and still others are completely unstained (asterisks) within a single island. Elongated cells found outside of the islands are CK14(−). (C, D) CK-18(+) cells are also present in cultures of MeBr-lesioned OE maintained under identical conditions. Note the heterogeneity of size and shape as well as intensity of CK18 immunoreactivity. Furthermore, the proportion of CK18 cells also varies such that the island in C is dominated by CK18(+) cells, while the island in D is sharply divided with CK18(+) wedge-shaped cells at the surface (double arrows) and CK18(−) round cells in the interior (asterisks). Spindle-shaped cells outside of islands are CK18-negative. (E–J) Doubling labeling with CK14 (green) and CK18 (red) demonstrates that the island consists of CK14-positive presumptive HBCs (asterisks), CK18-positive presumptive sustentacular cells (open arrow), double positive cells (thin arrows) and unstained cells (triangles). The brightfield image and Hoechst staining of the same island are shown in E and F, respectively. Scale bar in D is 50 µm for A and B, 100 µm for C, and 25 µm for D. Scale bar in J is 50 µm and also applies to E–I.
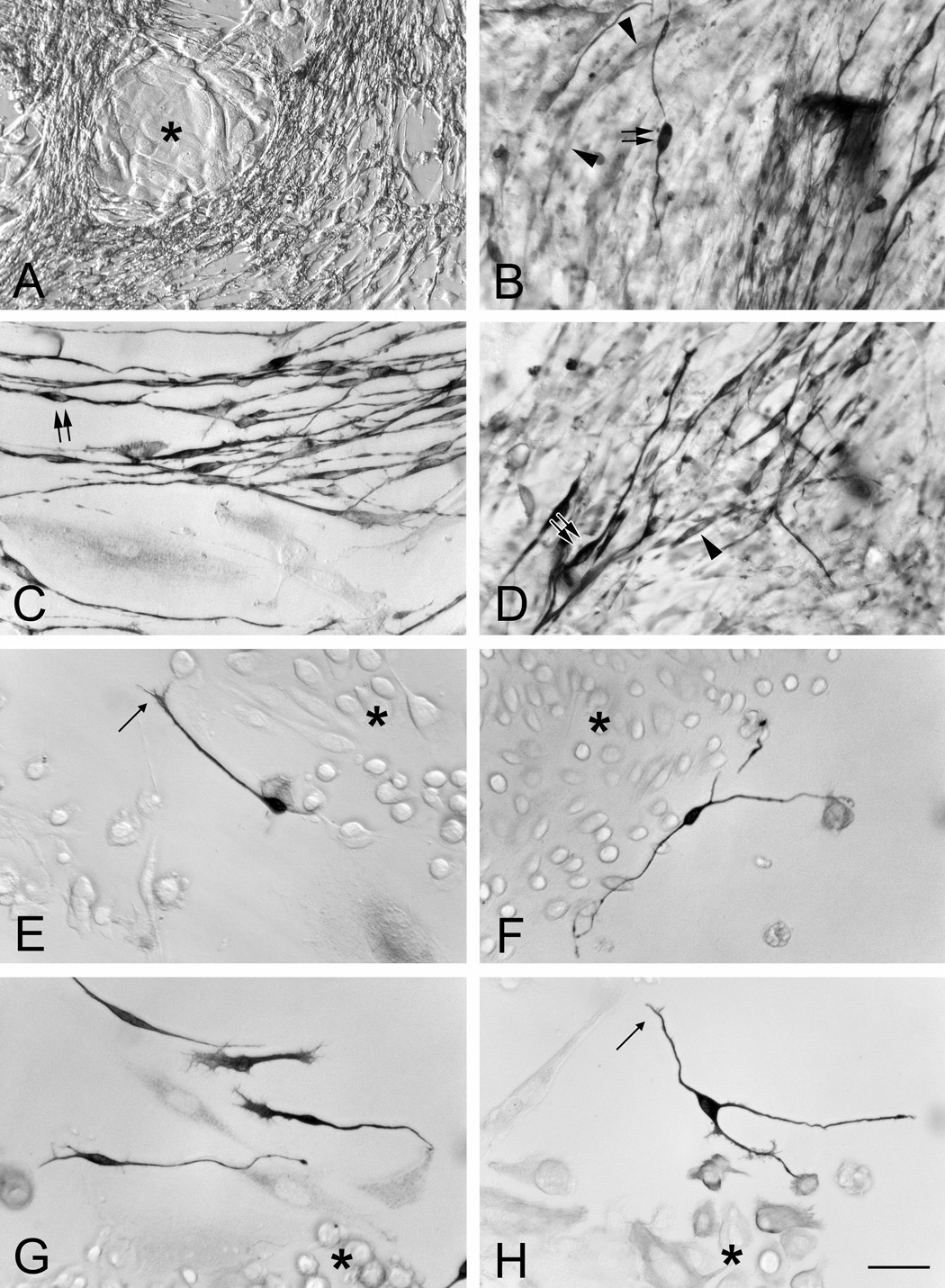
There is one noticeable difference between cultures grown in serum-rich vs. serum-free medium. In serum-rich medium, the spindle cells surrounding the islands are relatively abundant and form a dense mat (Fig. 4A–D). In contrast, in serum-free medium, the spindle cells are less numerous (Fig. 3E–H). Some, but not all, of these spindle cells label with TuJ-1 antibody and presumably express the neuronal marker, βIII (neuron-specific tubulin). Heavily labeled TuJ-1(+) cells (Fig. 4B–H) are bipolar in shape. Some TuJ-1(+) cells show growth cone-like structures (Fig. 4E and H).
Figure 4.
TuJ-1(+) cells are present in the monolayer cultures of MeBr-lesioned OE grown on laminin in serum-rich NIC medium (A–D) and serum-free KGM (E–H). The TuJ-1(+) cells are elongated cells present surrounding epithelioid islands (asterisks in A and E–H). Note the difference in abundance of TuJ-1(+) cells in the two media conditions. In serum-rich condition, some TuJ-1(+) cells are heavily stained and clearly bipolar (double arrows in B, C and D), suggesting neuronal differentiation, while others are some weakly stained cells, and are usually spindle-shaped (arrowheads in B and D). In serum-free condition, such heavy labeled cells are scarce, but clearly neuronal-like in shape. Indeed, the leading process of some TuJ-1(+) cells closely resembles growth cones (thin arrow in E and H), particularly when found around the edges of the islands (asterisks). Note that not all scattered cells are TuJ-1(+). Scale bar in H is 50 µm for A and 25 µm for B–H.
Since monolayer cultures give rise to both neuronal-like and non-neuronal-like cells that show some phenotypic similarities with epithelial cell types in vivo, we attempted to re-introduce the cultured cells by transplantation in vivo. Transplantation into the MeBr-lesioned epithelium of both mice and rats has been used successfully by our lab in the past to engraft cells – both unsorted and sorted as to type – immediately after isolation from normal, bulbectomized, or MeBr-lesioned animals (i.e., without an intervening period in culture) (Goldstein et al., 1998; Chen et al., 2004). In this case, cells from GFP-expressing mice were maintained in monolayer cultures for 6 DIV (without passaging) or 15 DIV (passaged twice) in serum-rich medium before transplantation into the host C57BL/6 mice (n = 4 for the 6 DIV group and n = 2 for the 15 DIV group) 1 day after MeBr lesion (Fig. 5). At perfusion 11 days after transplantation, only a few small clusters of GFP-expressing, donor-derived cells were found (less than 10 across all animals). The transplant-derived cells were located either in fibrous scar tissue that projects above the surrounding OE into in the nasal cavity and is often observed at a few spots in MeBr lesioned-recovered mice (Fig. 5A–D), or below the basal lamina dispersed within the lamina propria, in a manner and appearance reminiscent of fibroblasts or fibroblast-like mesenchymal cells (Fig. 5E–H). In none of the six cases of transplanted monolayer cultures were donor-derived cells observed to integrate into the regenerating epithelium, and all are rather found in abnormal locations. Some donor-derived cells are weakly TuJ-1(+), which is similar to the phenotype observed in monolayer cultures (see Fig. 4). The results obtained with the cells from monolayer culture stand in sharp contrast to the successful integration of epithelial cells that are transplanted immediately after dissociation or when maintained in three-dimensional air-liquid interface cultures (see next section).
Figure 5.
Cells harvested from the MeBr-lesioned OE and maintained in submerged monolayer cultures do not integrate into the epithelium. The donor cells from the 1.5 days MeBr-lesioned OE of GFP-expressing mice were cultured as monolayers in serum-rich media for 6 days (A–D) or 15 days (E–H). In host wild type mice (see Materials and Methods), the donor-derived GFP-expressing cells (green) are found either in scar tissue (asterisks) (A–D), or below the basal lamina (arrowheads) (E–H) 11 days post-transplantation. Note that some engrafted cells are weakly TuJ-1(+) (arrows in A–D). (A, E) TuJ-1 only; (B, F) transplanted GFP cells; (C, G) merging of TuJ-1 and GFP; (D, H) merging of TuJ-1, GFP and Hoechst (blue). Scale bar in D is 25 µm and applies to A–C, and scale bar in H is 50 µm and applies to E–F.
In summary, the MeBr-lesioned OE from adult mice can be effectively expanded in monolayer cultures, which contain both neuronal and non-neuronal cells, somewhat like the epithelium in vivo. Serum-rich culture condition favors the robust production of both neuron-like cells and epithelioid islands, in contrast to serum-free media, which heavily favors the production of epithelioid islands over neurons. However, cells cultured as a monolayer failed to incorporate into the regenerating epithelium, indicating some loss of differentiation capacity of cells as compared to in vivo.
Three-dimensional Culture (Air-Liquid Interface) of the Dissociated Adult Olfactory Epithelium
Although the monolayer culture system described above phenotypically mimics in vivo cellular events of reconstitution of the OE, its nature (i.e. monolayer) does not favor interactions between different cell types, which may be necessary for cells remain able to participate in the reconstitution of the OE in vivo. In order to mimic the architecture of the in vivo condition, we developed a three-dimensional (3D) culture system, with the key component of an air-liquid interface and a feeder layer of 3T3 cells on the underside of the insert (Fig. 1).
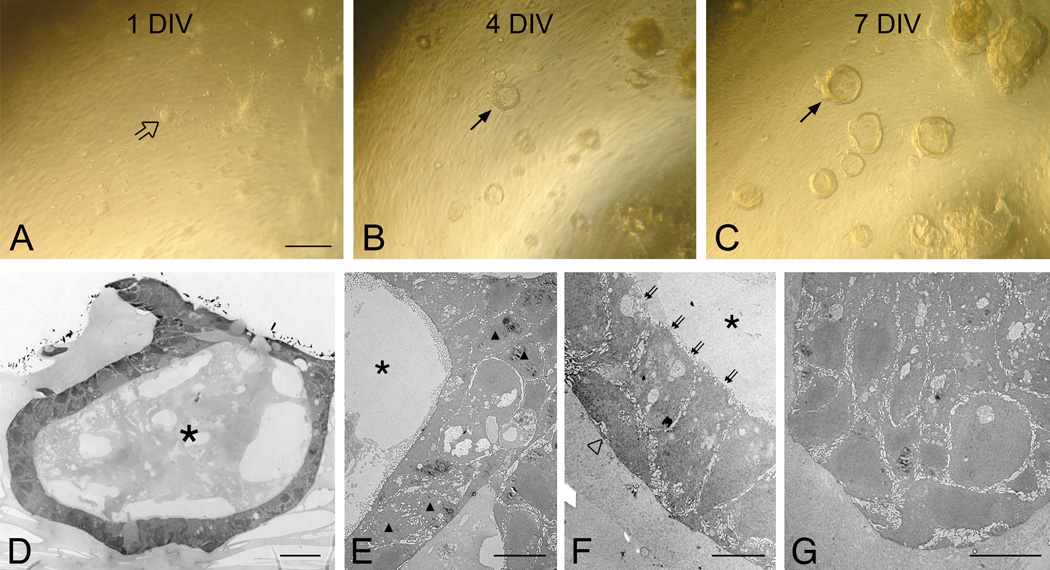
When the dissociated cells from the MeBr-lesioned OE of adult mice were seeded onto the Matrigel-coated insert, they formed aggregates initially (1 DIV; Fig. 6A), but soon began to form “spheres” that were elevated above the insert membrane (4 DIV and 7 DIV; Fig. 6B–C). These spheres continuously grow in size and some adjacent spheres merge to form even bigger spheres at later times in culture. Examination of semi-thin sections through spheres on the insert membrane reveals that the spheres form as either a hollow or compact structure (Fig. 6D–G). Electron microscopic examination shows that the hollow spheres contain 1) round-shaped, 2) flattened and 3) columnar-shaped cells (Fig. 6E and F). Compact, solid spheres contain many round cells (Fig. 6G), which are otherwise relatively non descript (Fig. 6E–F). The cells in spheres do not resemble the more differentiated cells found in OE in normal animals or at an advanced stage after MeBr lesion, but are consistent with the ones found in the epithelium at short survivals after MeBr exposure and in the early embryonic OE (Cuschieri and Bannister, 1975; Schwob et al., 1995; Jang et al., 2003). Sphere formation is absent when we culture cells harvested from either normal or bulbectomized adult OE (data not shown), indicating that this phenomenon depends on the specific capacity of cells found in the MeBr-lesioned OE. We also observed that the presence of the feeder layer was necessary for the sphere formation; in the absence of the feeder layer cells assort into epithelial islands or remain as dispersed, spindle-shaped cells.
Figure 6.
Dissociated OE cells from MeBr-lesioned mice form complex spheres when cultured at the air-liquid interface with a 3T3 feeder cell layer on the underside of the insert. Cultures were photographed at (A) 1 day in vitro (1 DIV); (B) 4 DIV; and (C) 7 DIV. The dissociated cells aggregate within 24 hrs in culture (open arrow in A) and progressively form dome-like spheres (arrows in B and C). (D) Semithin sections of spheres examined with a light microscope. Electron microscopies (EM) of representative spheres (E–G). (D–F) The sphere shown here is hollow (asterisks in D–F) and consists of rounded (triangles in D), flattened (open triangle in F) and columnar (double arrows in F) cells. (G) Another sphere forms a solid ball-like structure composed of mostly rounded cells. Scale bar in A is 100 µm and applies to B and C. Scale bars in D–G are 20, 10, 5 ad 10 µm, respectively.
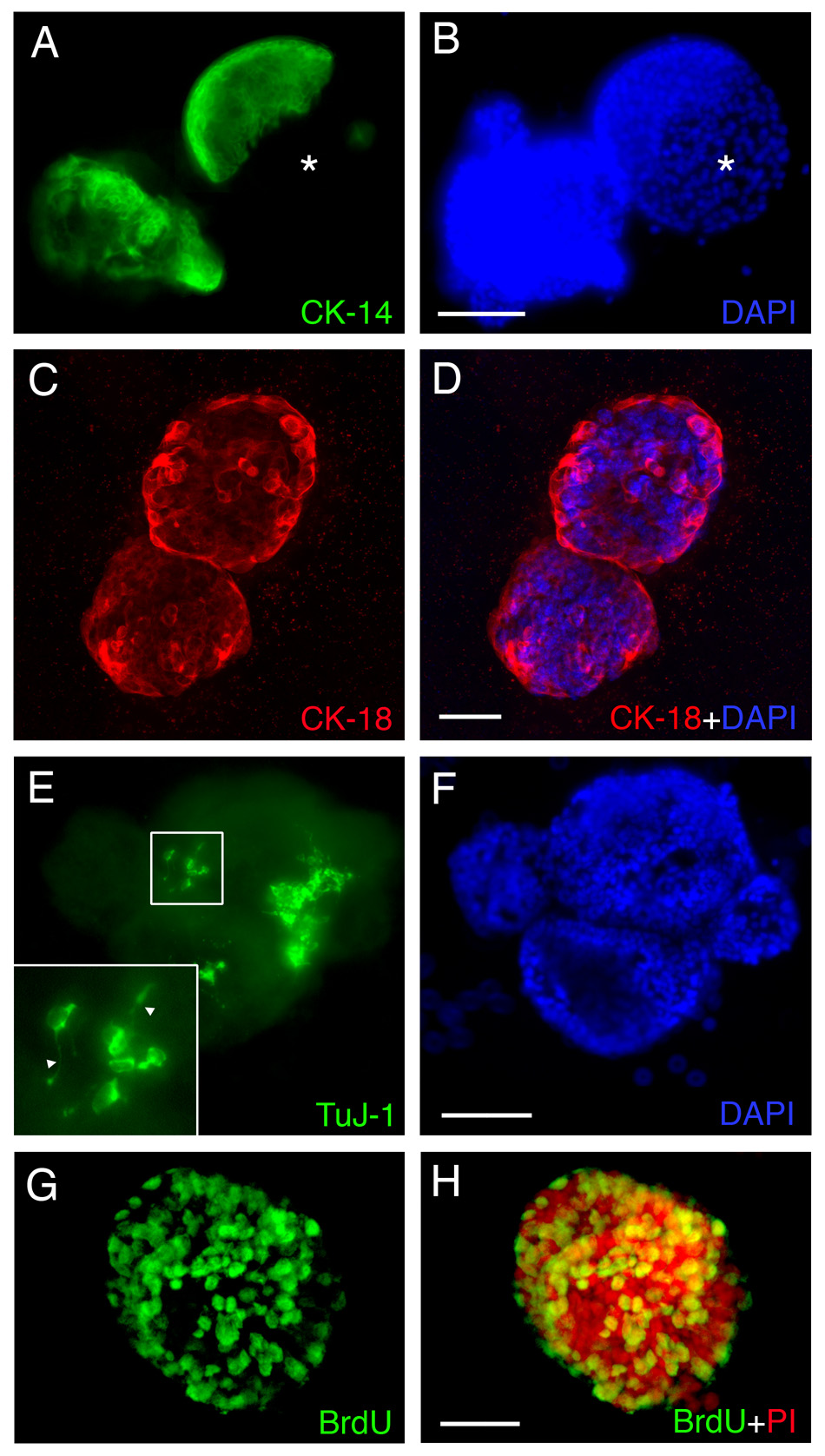
When we performed immunohistochemical analysis on spheres, we found a similar molecular profile as in the monolayer cultures described above. Cytokeratin-expressing cells are prominent (Fig. 7A–D). While many of the cells in the spheres are CK14 (or CK5/6: data not shown)(+) cells (Fig. 7A and B), others are CK18(+) (Fig. 7C and D). On occasion, the spheres are sharply divided into segregated territories containing CK14(+) cells vs. CK14(−) cells, suggesting different origin for these distinct regions. In many cases, the CK18(+) cells line the periphery of the spheres. Rare Tuj-1 positive cells emerge within the spheres (Fig. 7E and F). In contrast to the monolayer culture, the Tuj-1(+) cells are integrated within the sphere. A substantial number of cells in the sphere label acutely with BrdU and must, therefore, remain in the mitotic cycle (Fig. 7G and H, 6 DIV); thus, the growth of the spheres in size is due in part to cellular proliferation, although the cell clumping and aggregation noted earlier probably also contribute to sphere growth. Furthermore, the cells in this culture setup were able to maintain the capacity to form spheres up to at least five passages (the limit of our attempt at passaging, data not shown). The results indicate that the air-liquid interface culture system mimics the regenerating OE in vivo with marker-defined epithelial cell types present.
Figure 7.
Cells in spheres derived from MeBr-lesioned rat OE express a variety of differentiated cell markers when maintained in air-liquid interface culture as in the regenerating OE, including CK14 (A, B), CK18 (C, D), TuJ-1 (E, F). (G–H) Some of the cells are labeled by their incorporation of BrdU added to the culture, and thus, are proliferating in vitro. (A, B) 10 DIV. CK14 positive cells are found in the sphere, and in some cases, the sphere is sharply divided into CK14 positive (green) and CK14 negative (asterisks) area. (C, D) 7 DIV. CK18 positive cells (red) are found in the spheres, and often times are found at the periphery of the spheres. (E, F) 10 DIV. TuJ-1 positive cells (green) are found clustered in the sphere, and often bear axon-like processes (triangles in higher magnification inset). (G, H) 6 DIV. A majority of cells in the sphere incorporate BrdU (anti-BrdU, green), indicating that spheres can grow via proliferation. Propidium iodide (PI, red) was used to stain the nuclei (H). Scale bars in B and F are 100 µm and apply to A and E. Scale bars in D and H are 50 µm and apply to C and G.
The air-liquid interface culture of the dissociated cells from postnatal rats (PN 1) shows some similar features to the culture of the dissociated cells from adults (Fig. 8), but is distinct in many ways. In addition to the fact that the cells assemble more rapidly into spheres than in the adult cultures, they often times sprout dense rays of spindle cells (Fig. 8B). Tuj-1(+) neurons are much more frequent in the cultures established with neonatal OE cells (Fig. 8C, D; Tuj-1). Similar to the culture of the adult OE cells, CK14 and CK18(+) cells are found in a single sphere (Fig. 8E–H)
Figure 8.
Cells harvested from rat OE on postnatal day 1 form spheres when maintained in air-liquid interface culture. (A) At 5 DIV, spheres appear and grow rapidly, indicating rapid proliferation. (B) Spheres appear either hollow with an empty cavity (asterisk) or clustered into a pile. Spheres are surrounded by a radiating array of elongated, spindle-shaped cells (arrowheads), which are rarely seen in the cultures derived from MeBr-lesioned OE. (C, D) Neurons are found with a greater frequency than in the cultures with adult OE cells. Confocal microscopy illustrates two areas with neuronal populations that are interconnected via axons (arrows). (E–H) Both CK14(+) (E) and CK18(+) (F) cells are found in a sphere and some cells are double positive with anti-CK14 and 18 (G). Scale bars in A and B are 200 µm and 100 µm, respectively. Scale bar in D is 50 µm and applies to C, and scale bar in H is 100 µm and applies to E, F and G.
Are GBCs or HBCs or Duct/gland cells Responsible for the Formation of the Sphere? – Cultures from Sorted Cells
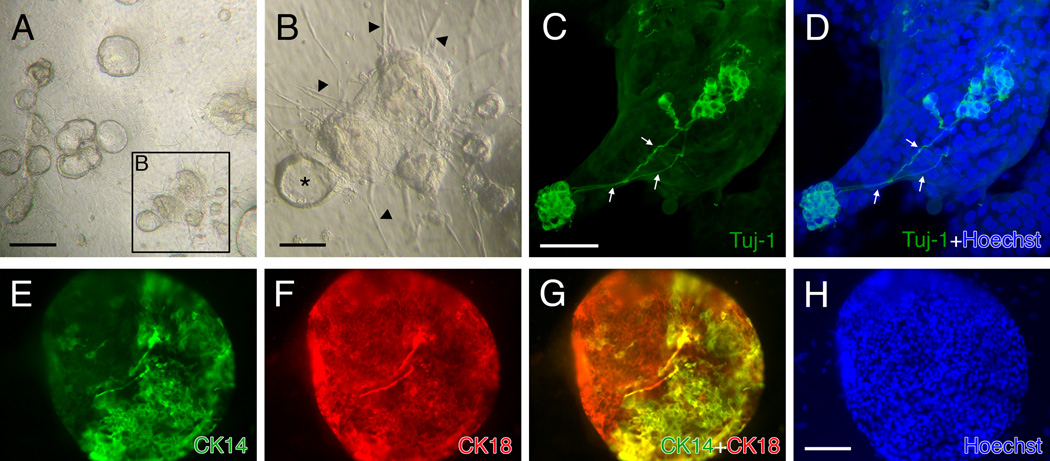
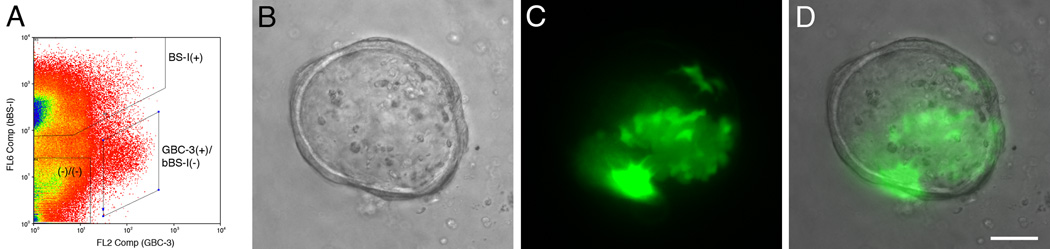
It is likely that several types of epithelial cells exhibit capacity as multipotent progenitors and possibly stem cells, which identification is additionally based on persistent mitotic quiescence, among other criteria (Chen and Schwob, 2003; Chen et al., 2004; Murdoch and Roskams, 2007). After exposure to MeBr gas, the remaining epithelial cells are basal cells (GBCs and HBCs) and gland/duct cells (Schwob et al., 1995; Jang et al., 2003). To assess which cell types contribute to, or are responsible for, the formation of spheres, we harvested OE cells from the 1.5 day MeBr-lesioned rat OE and sorted them on the basis of marker expression. FACS-sorting after surface labeling with the biotinylated lectin BS-I (bBS-I) isolates a population of CK-positive-confirmed HBCs (Chen et al., 2004). When sorted bBS-I(+) and bBS-I(−) cells were seeded in the air-liquid interface culture, only bBS-I(−) cells generated spheres, which appeared similar to spheres formed from non-sorted, post-MeBr OE cells (Fig. 9). Cells labeled with bBS-I gave rise to spheres only rarely. This result suggests that the population of HBCs does not compel to the formation of the spheres at least under these conditions. Since the bBS-I(−) population contain both GBCs and duct/gland cells, we attempted to sort GBCs for culture on the basis of surface expression of immature laminin receptor precursor (iLRP) with monoclonal antibody GBC-3 (Jang et al., 2007). Of the total population of bBS-I(−) cells, both the bBS-I(−)/GBC-3(−) and the bBS-I(−)/GBC-3(+) subpopulations reliably gave rise to spheres, although the latter subpopulation of putative GBCs gave rise to fewer spheres than bBS-I(−)/GBC-3(−) cells. When a small number of bBS-I(−)/GBC-3(+) cells from GFP-expressing rats were added to bBS-I(−)/GBC-3(−) cells from wildtype rats at the time of seeding, spheres that form contained GFP-positive cells, suggesting that GBC-3(+) GBCs incorporated and participated in sphere formation (Fig. 9).
Figure 9.
FACS-sorted GBCs will participate in the formation of spheres in the air-liquid interface cultures. (A) FACS-profile of cells from 1.5 days post-MeBr lesioned rat OE sorted with GBC-3 (a marker selective for GBCs) and BS-I (an HBC marker). The GBC population corresponds to the GBC-3(+)/bBS-I(−) population. (B–D) Addition of FACS-sorted GBCs (green) to the non-GBC/HBC cells (double negative fraction, (−)/(−), shown in A) at a 1:99 ratio. The GFP-expressing cells are incorporated into the sphere (C, D), indicating that GBCs contribute to sphere formation. The sphere was photographed at 3 DIV and shown in brightfield (B), green channel epifluorescence (C) and merged (D). Scale bar in D is 50 µm and applies to A and B.
Transplantation of Dissociated Cells from 3-D Culture
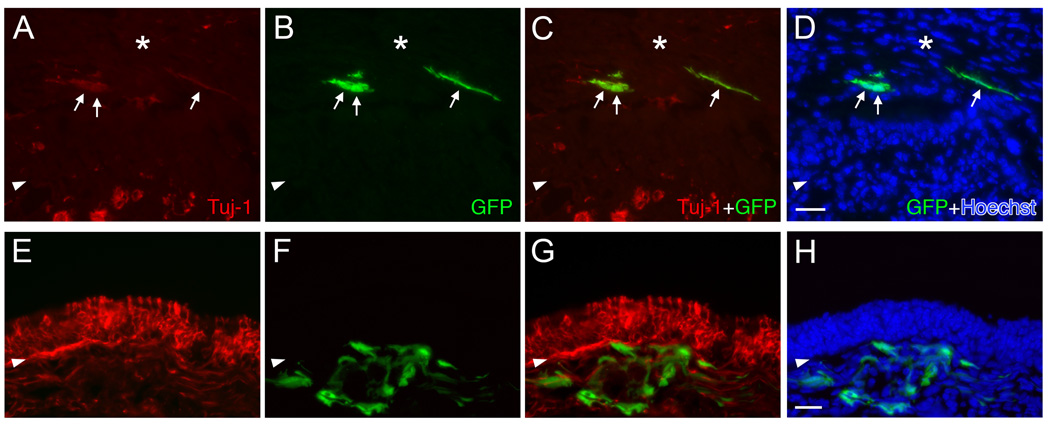
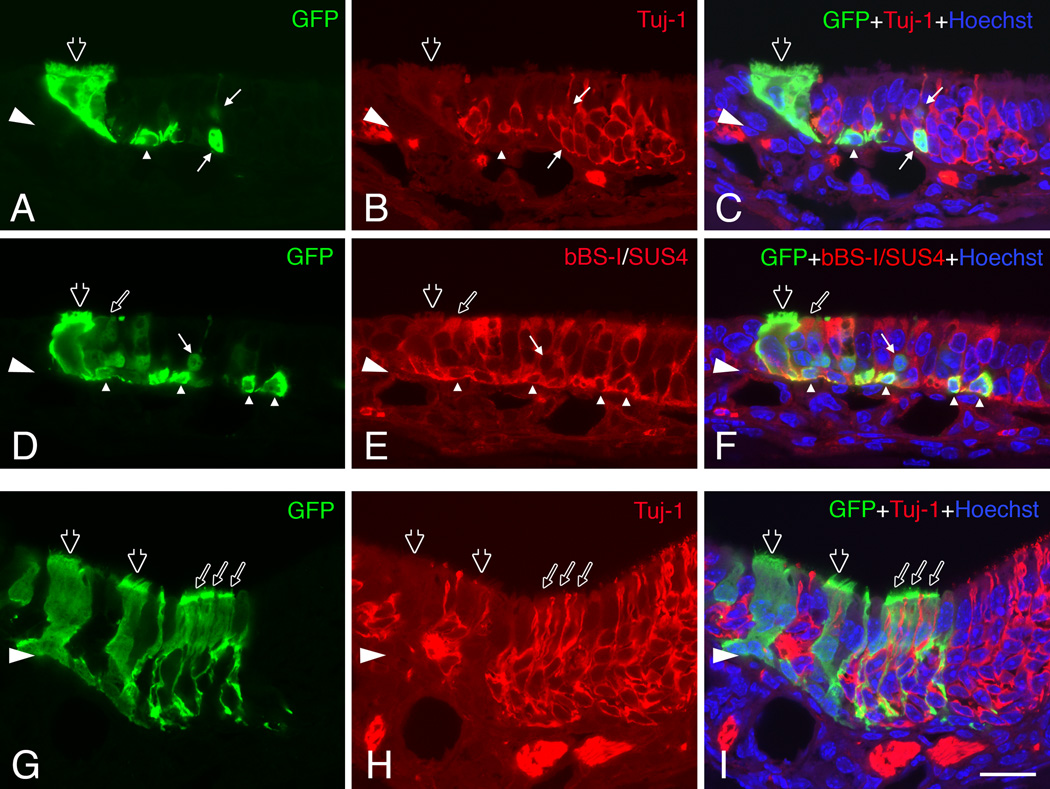
To test whether the cells from our 3-D culture system are able to incorporate into the reconstituting OE in vivo, we transplanted cells from spheres that had been maintained in culture for 6 days into the MeBr-lesioned, regenerating epithelium via nasal infusion. Spheres formed by the dissociated cells from MeBr-lesioned OE from GFP-expressing mice were selectively lifted from the membrane by condition of limited trypsin dissociation. Examination of the cultures after this step indicates that only the spheres have been lifted from the membrane, with little or no disruption of the spindle cell mat that surrounded them. That impression was confirmed by direct microscopic inspection of the released spheres before further dissociation. Cells were released from the spheres by incubation with enzyme cocktail used to dissociate cells from the epithelium and trituration. After filtering, the single cell suspension was infused into the naris of wild-type host mice, which were exposed to MeBr 1 day before transplantation. A total of 6 hosts were infused with 10,000–100,000 cells per animal. At 11 days after transplantation, inspection of the septum and turbinates of the host animals reveals numerous colonies composed of GFP-expressing cells; 10 or more colonies were observed in three of the six host mice. In two other host mice, four to six colonies were found in a whole-mount examination. At 11 days post-transplantation, the regenerating OE contains donor-derived GFP expressing cells identified as GBCs, HBCs, sustentacular cells, and neurons as well as some columnar ciliated respiratory epithelial cells (Fig. 10). Many of the donor-derived cells are either HBCs or sustentacular cells. This cellular composition is similar to that observed in the culture setting (see Fig. 7 A–D). Our results indicate that the cultured cells grown in our 3-D air-liquid model can integrate into the reconstituting epithelium and give rise to cell types that are typical of the host OE or of cells that arise following the acute transplantation of GBCs harvested from the normal (Chen et al., 2004) or lesioned OE (Goldstein et al., 1998).
Figure 10.
Cells harvested from spheres that formed in air-liquid interface cultures incorporate into the regenerating OE of the host after transplantation. (A–C) (D–F) Adjacent sections through a single graft-derived cluster 11 days post-transplantation. This cluster contains donor-derived (green) neurons (thin arrows) double labeled by Tuj-1 staining (red in B–C), sustentacular cells (thin open arrows) and HBCs (triangles) double labeled by bBS-I and SUS-4 staining (red in E–F) and ciliated respiratory epithelial cells (thick open arrows in A–F). (G–I) Another clone consists mainly of sustentacular cells (thin open arrows) positioned above the layer of neurons and marked by thin foot processes extending toward the basal lamina. Ciliated respiratory epithelial cells (thick open arrows in G–I) are also seen in this clone. The images were obtained with a confocal microscope. Scale bars in I is 20 µm and apply to A–H.
DISCUSSION
The results presented here demonstrate that the conditions under which progenitor cells and stem cells of the adult OE are maintained in tissue culture have a profound effect on the kind of cellular differentiation observed in vivo and in vitro. Thus, 2-D, submerged cultures of OE harvested under a variety of conditions (from normal, bulbectomized, or MeBr-lesioned rats and mice) show some evidence of cell-type specific differentiation, as monitored by expression of cell-type specific markers, but do not engraft in an appropriate manner after transplantation in vivo. By comparison, air-liquid interface cultures maintained with a feeder layer of fibroblast develop into spheres architecture that can either be solid or hollow, with cell-specific differentiation AND the capacity to engraft and participate in the reconstitution of the OE after transplantation.
The work represents an advance on previous means of culturing the OE in several respects. First, it uses adult OE, which, of necessity, will be the most likely tissue source for therapeutic use of these neurocompetent stem cells. The majority of previous reports utilize embryonic or neonatal cells (Calof and Chikaraishi, 1989; Chuah et al., 1991; Ronnett et al., 1991; Pixley, 1992; Mumm et al., 1996; Cunningham et al., 1999; McEntire and Pixley, 2000; Carter et al., 2004). Exceptions are found in a few reports describing a culture system of the cells harvested from adult OE (Grill and Pixley, 1997; Liu et al., 1998; Feron et al., 1999; Newman, 2000).
Second, it harvests cells from MeBr-lesioned OE less than 40 hours after exposure to the toxin, at which time the multipotency of residual basal cells and their activation during wholesale epithelial reconstitution has been demonstrated by several investigators and approaches (Goldstein et al., 1998; Huard et al., 1998; Chen et al., 2004; Leung et al., 2007). Certainly the robust generation of cells with marker-defined phenotypes (CK14[+], CK18[+], TuJ-1[+] cells, an HBC, sustentacular cell and neuronal marker, respectively) is consistent with that mutipotency, which is further suggested by the cell types produced after infusion and engraftment of the cells maintained in air-liquid, feeder cell-enriched cultures.
Despite differences from previous culture protocols – with respect to starting material, media, substrate and other variables – some similar results were obtained in both the submerged and air-liquid cultures. For example, epithelial islands were a prominent feature of the submerged cultures shown here and elsewhere (Calof and Chikaraishi, 1989; Pixley, 1992; Mumm et al., 1996; Liu et al., 1998; Carter et al., 2004), although ours is the first analysis to show the specific cytokeratin profile of cells in the islands. The occurrence of cells that express both type-specific cytokeratins (i.e., CK14 and CK18) suggests a common origin. HBCs in vivo can apparently give rise to sustentacular cells based on transitional cell types defined morphologically and on lineage tracing grounds (Schwob et al., 1995; Huard et al., 1998; Leung et al., 2007). In like manner, islands develop in the air-liquid culture in the absence of a feeder layer, with similar characteristics as in submerged cultures. Finally, in the feeder cell-supported spheres, both CK14(+) and CK18(+) cells are observed.
Previously unappreciated aspect of these islands is the presence of intermediate phenotypes, marked by co-expression of the HBC and sustentacular cell-types of cytokeratins, in addition to the more “differentiated” cells expressing one, but not the other cytokeratin protein. That result, too, is indication of a kind of multipotency in the culture setting.
Without a doubt, the most novel aspect of the work is the formation of the spheres, which emerge when the cells from the MeBr-lesioned OE are cultured in air-liquid cultures with 3T3 cell feeder layer on the underside of the insert membrane. Elaboration of spheres was also strictly dependent on the presence of the feeder cells; conditioned media was markedly less effective. Furthermore, addition of identified growth factors (TGFα, EGF, among others) has been ineffective to date in stimulating and maintaining the formation of the spheres (Jang et al., unpublished data).
Spheres are best formed by a complex mixture of cells: FACS-purified HBCs are ineffective at forming spheres on feeder layers; purified GBCs can form spheres, but are less effective as a starting material than the heterogeneous mix of cells harvested by release from the 1.5 days post MeBr-lesioned OE. As a consequence we cannot yet, nor may it be sensible, to talk about “the” cell type that can give rise to the spheres in culture.
Air-interface cultures have been used for other epithelial tissues, including trachea and bronchial epithelium and epidermis, and found to provide a better degree of differentiation and tissue organization in vitro than submerged culture (Prunieras et al., 1983; Asselineau et al., 1985; Whitcutt et al., 1988; Mak et al., 1991; Yamaya et al., 1992; You et al., 2002). Of course, one of the features that is remarkable here is the formation of neurons. To date only limited success has been reported when using these cultures to define the specific progenitor cell type, which fits with our experience here. An analytical approach in which such heterogeneous cultures are seeded with specific marker-identified cell types may be a more successful strategy for analyzing differentiation capacity, as our preliminary results with FACS-isolated GBCs suggest.
The relative lack of sphere formation in air-liquid interface cultures from epithelium that is solely neurogenic – i.e. from normal or bulbectomized animals – suggests that the active generation of multiple cell types by the cells remaining after MeBr lesion is a critical aspect of sphere formation. It appears as if all three of the spared cell types in vivo have some capacity for multipotency – GBCs, HBCs and duct/gland cells. With respect to the first two, essentially all cell types of the OE are generated following MeBr lesion, whereas the duct/gland cells are capable of making sustentacular cells as well as themselves.
Sphere formation from the dissociated olfactory epithelial cells is not completely without precedent. The closest example to our sphere cultures is the one from the work of Pixley’s group (Pixley et al., 1994) wherein they found neuron-containing and neuron-free spheres with central cavities when starting from dissociated newborn rat nasal cells, termed “micro-nose”. The formation of spheres and their complex composition were found only when the cultures were maintained in supension with a coculture of CNS astrocytes, and were not observed in submerged monolayer culture. The noticeable difference between Pixley’s micro-noses and our spheres is the frequency of neurons found in the cultures. This is likely due to the fact that Pixley’s micro-noses were formed from the OE cells of newborn rats at a time when neurogenesis is robust. Interestingly, when we grew the dissociated OE cells from perinatal rats (PN 1) in our air-liquid culture condition, we also find a large population of neurons (Fig. 8), and our spheres closely resemble the Pixley’s. More recently, adult mouse OE cells have been cultured as 3-D neurospheres in suspension, which then could be differentiated into a hair cell-like phenotype in vitro (Doyle et al., 2007).
The most significant aspect of the sphere-forming culture protocol is the capacity of sphere-derived cells to engraft into the regenerating OE and participate in its reconstitution. In that regard, cells that have been maintained in 3-D, sphere cultures retain a potency that is apparently lost in 2-D, submerged cultures. The cells in the sphere tend to lack the differentiated characteristics typically seen in the intact, unlesioned OE and resemble the morphology of the relatively non-descript cells seen in the OE in vivo at short survivals after MeBr-exposure (Schwob et al., 1995; Jang et al., 2003). Thus, some as yet unidentified feature of the sphere-culture paradigm permits the cells to retain the morphological and functional features characteristics of the cells in vivo.
In summary, we report that dissociated cells from adult OE that are cultured in three-dimensional air-liquid interface maintain the cellular phenotypes and architecture of the OE in vivo, and, furthermore, can be used as source for transplantation to participate in the reconstitution of the OE. Refining our culture system to achieve greater insight into manipulating (e.g., expanding and directing the cell fate of) the cultured cells is ongoing in our lab. The establishment of a culture system enhance the proliferative capacity of the OE stem/progenitor cells as well as to maintain the full capacity for cellular and molecular differentiation will likely provide better understanding of epitheliopoiesis and future therapeutic use of OE-derived stem and progenitor cell.
Supplementary Material
Acknowledgments
The work presented here was supported by grants from the NIH (R01 DC002167 and R21 DC006517) from the Ellison Foundation, and a core grant to the Tufts Center for Neuroscience Research (P30 NS047243).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Asselineau D, Bernhard B, Bailly C, Darmon M. Epidermal morphogenesis and induction of the 67 kD keratin polypeptide by culture of human keratinocytes at the liquid-air interface. Exp Cell Res. 1985;159:536–539. doi: 10.1016/s0014-4827(85)80027-6. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Chen X, Schwob JE. Quiescent globose basal cells are present in the olfactory epithelium. Chem Senses. 2003;28:A5. [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Chuah MI, David S, Blaschuk O. Differentiation and survival of rat olfactory epithelial neurons in dissociated cell culture. Brain Res Dev Brain Res. 1991;60:123–132. doi: 10.1016/0165-3806(91)90040-p. [DOI] [PubMed] [Google Scholar]
- Cunningham AM, Manis PB, Reed RR, Ronnett GV. Olfactory receptor neurons exist as distinct subclasses of immature and mature cells in primary culture. Neuroscience. 1999;93:1301–1312. doi: 10.1016/s0306-4522(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Cuschieri A, Bannister LH. The development of the olfactory mucosa in the mouse: electron microscopy. Journal of Anatomy. 1975;119:471–498. [PMC free article] [PubMed] [Google Scholar]
- Doyle KL, Kazda A, Hort Y, McKay SM, Oleskevich S. Differentiation of adult mouse olfactory precursor cells into hair cells in vitro. Stem Cells. 2007;25:621–627. doi: 10.1634/stemcells.2006-0390. [DOI] [PubMed] [Google Scholar]
- Feron F, Mackay-Sim A, Andrieu JL, Matthaei KI, Holley A, Sicard G. Stress induces neurogenesis in non-neuronal cell cultures of adult olfactory epithelium. Neuroscience. 1999;88:571–583. doi: 10.1016/s0306-4522(98)00233-4. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16:4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Wolozin BL, Schwob JE. FGF2 suppresses neuronogenesis of a cell line derived from rat olfactory epithelium. J Neurobiol. 1997;33:411–428. [PubMed] [Google Scholar]
- Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9:1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- Graziadei PP. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5:113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Grill RJ, Pixley SK. In vitro generation of adult rat olfactory sensory neurons and regulation of maturation by coculture with CNS tissues. J Neurosci. 1997;17:3120–3127. doi: 10.1523/JNEUROSCI.17-09-03120.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Vertebrate bHLH genes and the determination of neuronal fates. Exp Cell Res. 1999;253:357–364. doi: 10.1006/excr.1999.4717. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–140. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim KP, Schwob JE. Nonintegrin laminin receptor precursor protein is expressed on olfactory stem and progenitor cells. J Comp Neurol. 2007;502:367–381. doi: 10.1002/cne.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Liu N, Shields CB, Roisen FJ. Primary culture of adult mouse olfactory receptor neurons. Exp Neurol. 1998;151:173–183. doi: 10.1006/exnr.1998.6810. [DOI] [PubMed] [Google Scholar]
- Mak VH, Cumpstone MB, Kennedy AH, Harmon CS, Guy RH, Potts RO. Barrier function of human keratinocyte cultures grown at the air-liquid interface. J Invest Dermatol. 1991;96:323–327. doi: 10.1111/1523-1747.ep12465212. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- McEntire JK, Pixley SK. Olfactory receptor neurons in partially purified epithelial cell cultures: comparison of techniques for partial purification and identification of insulin as an important survival factor. Chem Senses. 2000;25:93–101. doi: 10.1093/chemse/25.1.93. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Shou J, Calof AL. Colony-forming progenitors from mouse olfactory epithelium: evidence for feedback regulation of neuron production. Proc Natl Acad Sci U S A. 1996;93:11167–11172. doi: 10.1073/pnas.93.20.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B, Roskams AJ. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Histol. 2007 doi: 10.1007/s10735-007-9141-2. [DOI] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, Lander AD, Calof AL. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell W, Feron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G, Mackay-Sim A. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- Newman MP, Feron F, Mackay-Sim A. Growth factor regulation of neurogenesis in adult olfactory epithelium. Neurosci. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. 'Green mice' as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Pixley SK. Purified cultures of keratin-positive olfactory epithelial cells: identification of a subset as neuronal supporting (sustentacular) cells. J Neurosci Res. 1992;31:693–707. doi: 10.1002/jnr.490310413. [DOI] [PubMed] [Google Scholar]
- Pixley SK, Bage M, Miller D, Miller ML, Shi M, Hastings L. Olfactory neurons in vitro show phenotypic orientation in epithelial spheres. Neuroreport. 1994;5:543–548. doi: 10.1097/00001756-199401000-00003. [DOI] [PubMed] [Google Scholar]
- Prunieras M, Regnier M, Woodley D. Methods for cultivation of keratinocytes with an air-liquid interface. J Invest Dermatol. 1983;81:28s–33s. doi: 10.1111/1523-1747.ep12540324. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Hester LD, Snyder SH. Primary culture of neonatal rat olfactory neurons. J Neurosci. 1991;11:1243–1255. doi: 10.1523/JNEUROSCI.11-05-01243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Akiyama Y, Uede T, Hashi K, Kocsis JD. Transplantation of an acutely isolated bone marrow fraction repairs demyelinated adult rat spinal cord axons. Glia. 2001;35:26–34. doi: 10.1002/glia.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Huard JM, Luskin MB, Youngentob SL. Retroviral lineage studies of the rat olfactory epithelium. Chem Senses. 1994;19:671–682. doi: 10.1093/chemse/19.6.671. [DOI] [PubMed] [Google Scholar]
- Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.