Abstract
Background and aims
There is little epidemiological evidence regarding the association of impaired glucose metabolism with recurrent cardiovascular events. We therefore examined potential sex differences in the effect of impaired fasting glucose (IFG) on recurrent cardiovascular disease (CVD) in a community-based study of survivors of a first acute myocardial infarction (MI).
Methods and results
This report focuses on 1,226 incident MI cases (28.4% women) discharged alive from area hospitals in the Western New York Acute MI Study (1996–2004). Deaths and underlying cause of death were determined via query of the National Death Index (Plus) Retrieval Program with follow-up through December 31, 2004. Outcomes reported included fatal or nonfatal coronary heart disease (CHD) or coronary revascularization surgery and total stroke. Traditional CHD risk factors and other explanatory variables were determined by clinical examination after the first acute event. Impaired fasting glucose was defined as fasting blood glucose between 100 and 125 mg/dl. During a mean follow-up of 4.5 years, there were 91 recurrent events (26.1%) in women and 173 recurrent events (19.7%) in men. After multivariable adjustment, the hazard ratios for recurrent cardiovascular events were 1.96 (95% CI: 1.15–3.16) and 2.59 (1.56–4.30) in women with IFG and with diabetes, respectively, compared to normoglycemic women. Among men, neither IFG nor diabetes was independently related to risk of recurrence.
Conclusions
In this study, IFG was a strong risk factor for recurrent cardiovascular events only among women. These results suggest that increased cardiovascular risk in MI survivors begins at lower glucose levels in women than men.
Keywords: glucose, gender, recurrent CVD, population, epidemiology, follow-up
Introduction
Over the past thirty years the mortality rate from coronary heart disease has fallen dramatically in the United States. This decline has been more pronounced in men than women 1. However, the population with type 2 diabetes has failed to share in this decline 2 . While both primary prevention as well as secondary preventive efforts no doubt lies behind much of the decline in the non-diabetic population, the lack of improvement among the diabetic population is puzzling. Extant data suggest that women with diabetes are more likely to have recurrent CVD than men 3. Few data are available that have examined potential sex differences in the effects of impaired fasting glucose (IFG) (100–125 mg/dl) on recurrent cardiovascular events, though it is known that diabetes is an especially important risk factor among women 4, and that risk of coronary heart disease (CHD) in women may be elevated at lower glucose levels than for men 5–6.
To examine the role of IFG in terms of secondary prevention, we hypothesized that IFG among those discharged alive after suffering an initial acute MI (AMI) would be at higher risk for recurrent cardiovascular disease (CVD) than their normoglycemic counterparts, with potential sex differences.
The Western New York Acute MI study is an on-going study of residents of Erie and Niagara Counties who were hospitalized at area hospitals with confirmed AMI. This population-based study affords a unique examination of IFG and post-discharge survival and the use of various treatment modalities in a community-wide setting on any sex differences post-discharge.
Methods
Study Population and Design
Measurements and Definitions
Enrolled cases completed a series of health and lifestyle questionnaires and underwent a comprehensive interview and physical examination in our Center for Preventive Medicine an average of 4.4 months (± 1.3 months) after the index MI. This window of time was selected in order to minimize the acute effects of the MI on other variables of interest 10. Participants were asked to bring to the clinic with them all prescription and over-the-counter medications they had taken over the last thirty days including aspirin. Hypertension was defined as a systolic BP ≥ 140 mmHG , or a diastolic BP ≥ 90 mmHG or taking any medications known to have blood pressure lowering effects. Data collection also included information on hospitalizations since discharge, socio-demographic characteristics, anthropometric variables including: BMI (metric weight / metric height squared), as well as personal and family medical history, alcohol use, smoking, and physical activity. Anyone who reported a 24-hour stay at the hospital or anyone who reported a surgical procedure for CVD (ie, angioplasty, CABG) was not included in this report. Diabetes was defined by 1) a positive answer to “has a physician ever told you that you have diabetes?” and concurrent use of insulin or oral medications, or 2) fasting blood glucose above 125 mg/dl for those without such a history. While in the clinic, fasting blood (at least 12 hours) was drawn into a fluoridated tube for the glucose assessment. Impaired fasting glucose was defined as fasting blood glucose between 100 and 125 mg/dl which is consistent with guidelines issued by the American Diabetes Association for epidemiologic studies11. A complete lipoprotein profile was not available; hyperlipidemia was defined as a total cholesterol concentration of 200 mg/dl or greater or on lipid-lowering medication. Data on nutritional intake before or after the MI were not available. Physical activity was assessed but was so minimal that it made no impact on the findings.
Follow-up and Outcomes
Following the baseline study interview, non-fatal events were tracked prospectively via mailed surveys (1998, 1999, 2003, 2004) to participants who identified the occurrence of all hospitalizations lasting 24 hours or more, emergency room visits, and out-patient procedural visits. Participants not responding to mailed surveys were contacted by telephone and asked to return the survey by mail or complete the survey at the telephone contact. At least one survey was completed by 93.5% of enrolled study participants and nearly 75% of the participants completed all surveys. All events reported by study participants were adjudicated through review of medical records by an Outcomes Committee that was blinded to the glycemic status of the participants. When members disagreed, the majority opinion was recorded. Deaths and underlying cause of death were determined via query of the National Death Index (Plus) Retrieval Program with follow-up through the end of 2004. Outcomes considered in this report included fatal or nonfatal CHD, or coronary revascularization surgery or stroke (ICD9 410–414 and 427, 430–438). Only the first recurrence of an event was counted. Through comparison with the New York Department of Heath records, we estimate we identified 95% of the acute MI’s in Eire and Niagara Counties.
Statistical Analysis
The primary endpoint of this analysis was time to recurrent event. Survival time was defined as the period from discharge to the date of a recurrent MI, stroke, cardiac intervention, or death due to a cardiac cause. Participants known not to have had an event were censored on December 31, 2004; the most recent date mortality information could be identified via the National Death Index. The association between continuous variables was examined with general linear models and chi-square tests were used for categorical variables. Sex-specific Kaplan-Meier estimates were created to obtain the survival curves for each glycemic group. Differences between survival curves were tested with the log-rank test. Two multivariable models were examined. In model 1 we adjusted for age (years), current smoker (yes, no), ever use of alchol (yes,no), current aspirin use (yes, no), ethnicity (white vs otherwise), and body mass index(kg/m2). Model 2 included the above variables plus hypercholesterolemia and hypertension as previously defined.. The adjusted hazard ratios and 95% CI were estimated with normoglycemic persons serving as the reference group. Interactions were tested using likelihood ratio statistics and compared with chi-square tests. All tests were two-sided and an alpha level of < 0.05 was considered statistically significant. Only those with complete data were included in the multivariable analyses. There was no evidence to show that the hazards were not proportional over the follow-up period. SPSS version 16.0 (SPSS Inc., Chicago, IL) was used for all analyses.
Results
Of the 2060 eligible, 59.5% attended our clinic (n=1226). We randomly telephoned over 500 of the nonresponders in order to characterize them to some degree. The only significant differences between the two were the following: the nonrespondants were significantly less likely to be male (66.7% vs 71.0%), more likely to report “ever smoker” (80.4% vs 74.8%), more likely to be nonwhite (93.8% vs 87.4 %) and had less formal education (12.7 years vs 13.3 years; all p < 0.05).
There were 91 events recorded in 348 women at risk for a recurrent event (crude case fatality rate) was 26.5 %. Non-fatal MI comprised the majority of the events; with 65 non-fatal CHD events and 8 fatal MI’s. There were only 9 events attributable to angioplasty or coronary artery bypass graft (CABG) and an equal number of non-fatal strokes (Table 1). Among men, there were 173 events and 705 men without an event (crude CFR of 19.7%; P = 0.005 vs. women). There was no significant sex difference in the distribution of events between the two sexes. The median time to follow-up among men was 3.73 years and among women was 3.49 years.
Table 1.
Distribution (n and %) of Recurrent Events according to Gender
| Gender | |||||
|---|---|---|---|---|---|
| Female | Male | ||||
| Count | Col % | Count | Col % | ||
| Recurrent CVD | no | 257 | 73.9% | 705 | 80.3% |
| yes | 91 | 26.1% | 173 | 19.7% | |
| Total | 348 | 100.0% | 878 | 100.0% | |
| Gender | |||||
|---|---|---|---|---|---|
| Female | Male | ||||
| Count | Col % | Count | Col % | ||
| Type of Recurrent | no event | ||||
| CVD | 257 | 73.9% | 705 | 80.3% | |
| non-fatal CHD | 65 | 18.7% | 126 | 14.4% | |
| fatal CHD | 8 | 2.3% | 28 | 3.2% | |
| 9 | 2.6% | 13 | 1.5% | ||
| PTCA/angioplasty | |||||
| non-fatal stroke | 9 | 2.6% | 6 | .7% | |
| Total | 348 | 100.0% | 878 | 100.0% | |
Chi-square = 0.013
Chi –Square = 0.007
CHD and stroke (ICD 410–414,427,430–438) PTCA or CABG
Diabetes defined as fasting glucose >125 or physician diagnosed and taking DM medication.
Impaired fasting glucose defined as fasting glucose 100–125mg/dl.
Table 2 presents descriptive characteristics of the study participants by sex and recurrent MI status. Among women, who had a recurrent event had higher concentrations of total cholesterol and fasting glucose than those who did not experience a recurrence. Thirty-one percent of women who had suffered a recurrent MI, reported use of insulin or hypoglycemic medication compared to 13.9% in women who did not.. Use of calcium channel blockers were also more frequent among women who had a recurrent event (P = 0.02). Use of beta-blockers was relatively low, about 67%, as was use of aspirin and statin use. Frequency of medication use in women ranged from 61.5% for lipid-lowering medication to approximately 85 % for hypertension. Among men, there were fewer significant differences in therapy except for diuretic use which was more frequent in those who suffered another CVD event (P= 0.001). Men who had a recurrence were more likely to have continued to smoke (19.3% vs. 12.9%; P = 0.03).
Table 2.
Age-adjusted risk factors by sex and recurrent event
| Women | Men | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent event | Recurrent event | |||||||||
| No | Yes | No | Yes | |||||||
| Mean | (SD) | Mean | (SD) | p | Mean | (SD) | Mean | (SD) | p | |
| Age (years) | 57.0 | (8.3) | 55.1 | (8.9) | .070 | 54.5 | (8.7) | 54.8 | (8.7) | .610 |
| Fasting total cholesterol (mg/dl) | 193.4 | (40.2) | 209.2 | (46.2) | .003 | 180.5 | (42.9) | 182.4 | (41.4) | .598 |
| Serum creatinine (mg/l) | 0.9 | (0.8) | 0.9 | (0.4) | .534 | 1.1 | (0.9) | 1.0 | (0.3) | .846 |
| Fasting glucose (mg/dl) | 111.1 | (43.6) | 124.1 | (54.6) | .028 | 107.6 | (30.4) | 107.0 | (30.2) | .834 |
| BMI (wt in kg/ht in m**2) | 29.8 | (6.1) | 30.6 | (5.9) | .321 | 29.0 | (4.6) | 29.2 | (5.0) | .595 |
| SBP (mmHg) | 117.2 | (16.4) | 115.2 | (12.8) | .301 | 115.5 | (14.1) | 113.7 | (15.4) | .130 |
| DBP (mmHG) | 69.8 | (9.3) | 68.6 | (8.0) | .269 | 71.6 | (9.1) | 70.7 | (9.6) | .282 |
| Number of types of HTN meds used past 30 days | 1.5 | (1.0) | 1.7 | (0.9) | .118 | 1.4 | (0.9) | 1.5 | (0.9) | .256 |
| % | % | p | % | % | p | |||||
| Hypertension>140/90 or reported tx | 54.5 | 61.1 | .277 | 43.0 | 46.5 | .403 | ||||
| High blood cholesterol>=200 or med use | 85.2 | 93.8 | .043 | 81.7 | 76.9 | .149 | ||||
| CHD in mother, father, sister, brother | 39.9 | 30.8 | .140 | 35.3 | 40.5 | .228 | ||||
| Diabetes in mother, father, sister, brother | 43.4 | 46.5 | .620 | 35.3 | 38.1 | .512 | ||||
| Lipid lowering drugs use past 30 days | 60.3 | 64.9 | .440 | 63.7 | 58.9 | .247 | ||||
| Statin use past 30 days | 58.3 | 60.6 | .708 | 60.0 | 55.4 | .276 | ||||
| HTN medication use past 30 days | 82.2 | 92.0 | .030 | 88.2 | 91.2 | .261 | ||||
| Diuretic use past 30 days | 22.0 | 23.7 | .730 | 9.1 | 18.5 | .001 | ||||
| Alpha blocker use past 30 days | 0.4 | 0.0 | .997 | 3.7 | 1.6 | .164 | ||||
| Beta blocker use past 30 days | 66.0 | 69.7 | .521 | 75.8 | 73.9 | .597 | ||||
| Calcium channel blocker use past 30 days | 21.5 | 33.8 | .021 | 19.5 | 20.9 | .669 | ||||
| Ace inhibitor use past 30 days | 31.0 | 32.2 | .833 | 32.2 | 35.3 | .439 | ||||
| Angiotensin receptor blockers use past 30 days | 5.9 | 5.4 | .862 | 2.6 | 2.3 | .844 | ||||
| Other type HTN med use past 30 days | 1.6 | 2.0 | .851 | 1.0 | 0.0 | .996 | ||||
| DM medication use past 30 days, eg insulin or Oral hypoglyemic agents | 13.9 | 30.1 | .001 | 12.4 | 13.2 | .778 | ||||
| Daily aspirin past 30 days | 78.5 | 81.6 | .536 | 85.1 | 84.3 | .794 | ||||
| Caucasian race | 82.3 | 89.5 | .111 | 91.5 | 96.5 | .031 | ||||
| Current smoker | 23.0 | 19.7 | .484 | 12.9 | 19.3 | .029 | ||||
| Ever drink alcohol | 84.6 | 89.6 | .242 | 95.0 | 96.6 | .382 | ||||
Recurrent event includes CHD and stroke (ICD 410–414,427,430–438), PTCA or CABG
Some variables have missing data.
Next we compared risk factor variables across levels of glycemia (Table 3). Again in women only, BMI, , systolic blood pressure, and the number of different types of antihypertensive agents were all positively associated with increased level of glycemia. The mean concentration of glucose was 90.0 mg/dl in the normoglycemic women, 108.5 mg/dl in those with impaired fasting glucose, 92.0 mg/dl in normoglycemic men and 175.0 mg/dl among those with type 2 diabetes (p < 0.001). BMI increased with increasing glycemic level in both sexes. A positive family history of type 2 diabetes was more frequent with increased glycemic level. Reported use of anti-hypertensive agents was somewhat more common in women (p=0.06) while no difference in beta-blocker therapy was evident with increasing level of glycemia.
Table 3.
Age-adjusted risk factors by sex and diabetes status
| Women | Men | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NGT (n=176) |
Impaired fasting glucose n=(83) |
Diabetic (n= 89) |
NGT (n=420) |
Impaired fasting glucose (n=307) |
Diabetic (n=151) |
|||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | p | Mean | (SD) | Mean | (SD) | Mean | (SD) | p | |
| Age (years) | 56.3 | (8.6) | 56.6 | (8.6) | 56.6 | (8.2) | .920 | 53.4 | (9.0) | 55.0 | (8.5) | 56.8 | (7.6) | <.001 |
| Fasting total cholesterol (mg/dl) | 196.8 | (44.2) | 194.0 | (40.6) | 202.1 | (39. | .462 | 177.9 | (40. | 185.3 | (40.6) | 180.4 | (51.5) | .068 |
| Serum creatinine (mg/l) | .9 | (.8) | .8 | (.2) | 1.0 | (0.7) | .277 | 1.0 | (.6) | 1.1 | (1.2) | 1.0 | (0.3) | .293 |
| Fasting glucose (mg/dl) | 90.0 | (6.7) | 108.5 | (6.4) | 175.0 | 65.3 | <.001 | 92.0 | (5.2) | 107.9 | (6.5) | 153.1 | 52.4 | <.001 |
| BMI (wt in kg/ht in m**2) | 28.2 | (5.2) | 31.2 | (6.2) | 32.7 | (6.2) | <.001 | 27.9 | (4.2) | 29.3 | (4.1) | 31.7 | (5.8) | <.001 |
| SBP (mmHG) | 115.0 | (14.9) | 114.6 | (15.8) | 121.7 | (15. | .002 | 114.7 | (14. | 114.0 | (13.9) | 118.6 | (14.9) | .004 |
| DBP (mmHG) | 69.6 | (8.9) | 68.8 | (10.4) | 69.9 | (7.7) | .718 | 71.1 | (9.5) | 71.6 | (9.0) | 71.9 | (9.0) | .642 |
| Number of types of HTN meds used past 30 days | 1.4 | (1.0) | 1.5 | (0.9) | 1.8 | (0.9) | .005 | 1.3 | (0.8) | 1.6 | (0.9) | 1.7 | (1.0) | <.001 |
| % | % | % | p | % | % | % | p | |||||||
| Hypertension>140/90 or reported tx | 54.8 | 45.7 | 69.3 | .007 | 38.8 | 44.0 | 56.7 | .001 | ||||||
| High blood cholesterol>=200 or brought meds | 86.4 | 85.3 | 91.7 | .392 | 80.2 | 80.7 | 82.7 | .819 | ||||||
| CHD in mother, father, sister, brother | 35.8 | 41.4 | 37.0 | .716 | 37.0 | 37.8 | 31.5 | .444 | ||||||
| Diabetes in mother, father, sister, brother | 30.4 | 60.3 | 56.8 | <.001 | 28.6 | 34.7 | 58.9 | <.001 | ||||||
| Lipid lowering drugs use past 30 days | 64.8 | 59.0 | 57.3 | .434 | 65.9 | 57.8 | 64.1 | .075 | ||||||
| Statin use past 30 days | 61.9 | 59.0 | 52.8 | .360 | 63.5 | 53.5 | 58.0 | .024 | ||||||
| HTN medication use past 30 days | 80.8 | 85.5 | 92.0 | .061 | 85.1 | 92.1 | 92.4 | .005 | ||||||
| Diuretic use past 30 days | 20.5 | 16.8 | 31.4 | .052 | 8.0 | 12.3 | 16.6 | .013 | ||||||
| Alpha blocker use past 30 days | 0.6 | 0.0 | 0.0 | .995 | 2.3 | 4.4 | 3.9 | .149 | ||||||
| Beta blocker use past 30 days | 63.7 | 67.4 | 73.0 | .316 | 73.1 | 79.8 | 72.9 | .091 | ||||||
| Calcium channel blocker use past 30 days | 22.1 | 20.5 | 33.8 | .072 | 13.5 | 23.6 | 29.6 | <.001 | ||||||
| Ace inhibitor use past 30 days | 27.9 | 37.3 | 32.5 | .301 | 31.1 | 32.5 | 38.1 | .295 | ||||||
| Angiotensin receptor blockers use past 30 days | 4.5 | 7.2 | 6.8 | .613 | 1.7 | 1.6 | 6.6 | .007 | ||||||
| Other type HTN med use past 30 days | 1.1 | 1.2 | 3.4 | .382 | 0.2 | 1.3 | 1.4 | .237 | ||||||
| DM medication use past 30 days-insulin or OHG | 0.0 | 0.0 | 70.8 | 0.0 | 0.0 | 73.2 | ||||||||
| Daily aspirin past 30 days | 82.4 | 83.1 | 69.6 | .035 | 86.3 | 85.2 | 80.4 | .229 | ||||||
| Caucasian race | 84.2 | 87.9 | 80.8 | .446 | 92.8 | 93.4 | 89.6 | .314 | ||||||
| Current smoker | 21.3 | 25.6 | 20.6 | .649 | 15.3 | 12.7 | 14.0 | .627 | ||||||
| Ever drink alcohol | 85.7 | 84.4 | 87.5 | .839 | 95.4 | 95.2 | 95.2 | .984 | ||||||
| Had recurrent event | 17.5 | 30.2 | 39.4 | .001 | 19.1 | 19.2 | 22.4 | .668 | ||||||
Diabetes defined as fasting glucose >125 or physician diagnosed and taking DM medication. Impaired fasting glucose defined as fasting glucose 100–125 mg/dl.
Recurrent event includes CHD and stroke (ICD 410–414,427,430–438), PTCA or angioplasty.
Some variables have missing data. P - values from GLM or logistic regression.
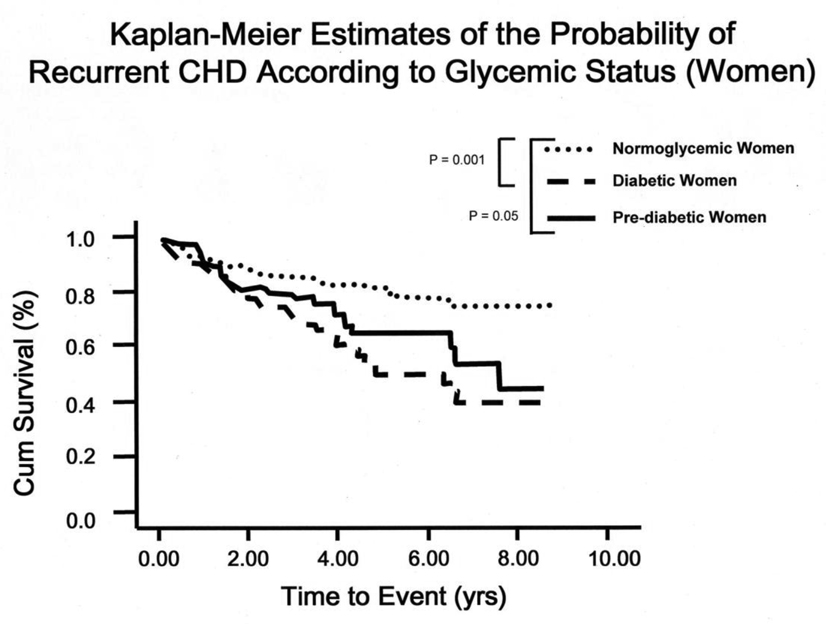
Figure 1 presents the Kaplan-Meier Survival curves in women according to glycemic status. Women with impaired fasting glucose at baseline fared significantly worse than those who were normoglycemic (p = 0.02), and the diabetic women had the lowest survival rate (p = 0.001) (log-rank test = 0.001). The log-rank test was not statistically significant for men (P = 0.56) (data not shown).
Figure 1.
Kaplan-Meier Estimates of the Probability of Recurrent CHD According to Glycemic Status: Log-rank ≤ 0.01
Table 4 presents age-adjusted and multivariate adjusted hazard ratios (95% CI) for recurrent CVD in both sexes. Adjusted only for age, there was a clear monotonic trend in women with increasing degree of glycemia (P = 0.001). After multivariable adjustment, the hazard ratios were 1.96 (95% CI: 1.15, 3.16) and 2.59 (95% CI: 1.56, 4.30) among women with IFG and diabetic women, respectively, compared to normoglycemic women. Consideration of other potential mediators (e.g., beta blocker use, diuretic use, ACE inhibitor use, daily aspirin use) had no material effect on these results. Among men, neither IFG nor diabetes was statistically significant.
Table 4.
Results of Cox Proportional Hazards Modeling on Risk of Recurrent CVD
| Women | Men | ||||
|---|---|---|---|---|---|
| Hazard Ratio |
95% CI | Hazard Ratio |
95% CI | ||
| Normoglycemia | 1.00 | ---- | 1.00 | ---- | |
| Model 1 | Impaired fasting glucose |
2.14 | 1.15, 3.96 | 0.84 | 0.57, 1.22 |
| Diabetes | 3.23 | 1.81, 5.76 | 1.11 | 0.70, 1.77 | |
| P trend = <0.001 | P trend = 0.567 | ||||
| Normoglycemia | 1.00 | ---- | 1.00 | ---- | |
| Model 2 | Impaired fasting glucose |
2.24 | 1.20, 4.18 | 0.83 | 0.57, 1.21 |
| Diabetes | 2.96 | 1.65, 5.30 | 1.09 | 0.68, 1.75 | |
| P trend = <0.001 | P trend = 0.836 | ||||
Adjusted for age, current smoker (yes vs. otherwise), Ever use alcohol (yes vs. otherwise), hypertension (yes vs. otherwise), hypercholesterolemia (yes vs. otherwise), aspirin use (yes vs. otherwise), BMI (continuous)
Recurrent event defined as CHD and stroke (ICD 410–414,427,430–439), PTCA or angioplasty.
Discussion
The present study highlighted sex differences in the role of impaired fasting glucose in recurrent cardiovascular events in a community-based cohort of survivors of a first acute myocardial infarction. Specifically, the principle finding of this report is that impaired fasting glucose, defined as fasting plasma glucose between 100 mg/dl and 125 mg/dl, was a predictor of recurrent CVD only among women, imparting a multivariate hazard ratio of 2.24. Neither IFG nor diabetes was independently related to risk of recurrence in men.
The Worcester Heart Attack Study has followed survivors of AMI for up to ten years and reported no sex difference for total mortality 12. The SPRINT study suggested that among those who took insulin for their diabetes, women had poorer survival than men; an observation not seen among users of oral hypoglycemic agents 13.
In the primary CVD prevention setting, a few studies point to potential sex differences in the association of impaired fasting glucose with cardiovascular risk. For example, recent findings from the Framingham Offspring cohort study showed that both the 1997 and 2003 IFG definitions were predictive of CHD in women but not men 5. In agreement with this, a cross-sectional analysis of NHANES 1999–2004 showed that compared to normoglycemic subjects, women with IFG (100–125 mg/dl) had a significant 83% increased 10-year risk of developing CHD whereas no significant risk was observed in men6.
Several mechanisms may be involved to help explain these results. For example, women with impaired fasting glucose may have a greater degree of insulin resistance and/or have greater endothelial dysfunction14, 15. Unfortunately, we did not have the necessary clinical information to examine these possibilities. Diabetic women may also have experienced greater left ventricular dysfunction than their male counterparts 20 although there were few differences in cardiac enzyme levels post MI (data not shown). Furthermore, in the present study men with IFG were more healthy and lean than women with IFG. This could explain, at least in part, the observed sex differences in the effect of IFG on recurrent CVD.
In the past, treatment guidelines have tended to focus on optimal glycemic control in the diabetic patients. Recent findings from three clinical trials have shown, however that glycemic control is not strongly related to risk of first MI 16–18 . The prevention of CVD disease may be more strongly related to treatment of conventional CVD risk factors as well as nonglycemic factors, and influenced not only by the management of glycemic control, but also aggressive management of the conventional coronary risk factors including enhancing insulin sensitivity 19. In this observational study, pharmacologic therapy was quite common making it very difficult to tease out a unique contribution of a specific drug to risk of recurrent cardiovascular disease.
We have shown in previous work that women who progressed from normoglycemia to impaired fasting glucose had evidence of greater endothelial dysfunction than their male counterparts 15 and had a higher index of insulin resistance, consistent with findings from the WISE study 20. Whether this also extends into the post MI period deserves further epidemiologic scrutiny. We also did not have data concerning in-hospital MI characteristics such as new Q wave, anterior vs. posterior MI, ST vs. non-ST elevation, heart failure or cardiogenic shock. These variables likely reflect the degree of damage to left ventricular function and risk for ventricular arrhythmia and it is unclear how they would have affected our findings.
Some limitations of this investigation deserve comment. We limited our study population to those aged 35 to 69 years who were discharged alive from the hospital. Thus those of both genders at highest risk for AMI and diabetes were not included. Our results therefore, may not be applicable to this group of patients. We did not collect data on in-hospital mortality as the focus of our research was on secondary prevention post-discharge. We also had largely a white, male, and somewhat more highly educated group of participants than nonparticipants which would limit the external but not internal validity of our findings. The Finnish contribution to the MONICA study 21 showed that diabetic men had a higher out-of-hospital mortality rate than did women, but that diabetic women who survived to admission had a higher in-hospital and one year case-fatality rate than their male counterparts. Many studies have shown a greater case-fatality rate in women with AMI than men that persists during the first year of discharge 22. However, restricting our study to patients who survived at least 28 days post-discharge could involve an underestimation of effects of diabetes in men who died early. Thus, we cannot totally dismiss the possibility that men with IFG died earlier than women with IFG. Our finding of a sex difference related to impaired fasting glucose over a mean follow-up of 4.5 years is unique and suggests that risk of recurrent CVD in women post-MI extends below the diabetic range. However, a longer follow-up period is needed to firmly establish this finding. We had no information on time-dependent covariates as we conducted a single clinical exam. It is difficult to speculate with any reasonable degree of certainty on how, for instance, change in medication or dose might have affected our results.
Notable strengths of this study include the community-based nature of the investigation, the recency of the past few years, results which may reflect the current profiles of patients with AMI in this catchment area, the careful assessment of risk factors, the collection of pharmaceutical medications, the standardization of the endpoints, the number of events and the completeness of follow-up. Our response rate of 60% is consistent with other large epidemiologic studies including the Atherosclerosis in Community Study (ARIC) 23 and the Cardiovascular Health Study (CHS) 24 where response rates varied from 48% to 60%. The response rate would be unlikely to affect the internal validity of this study in any event.
A strong survivorship bias would seem an unlikely explanation to completely explain the observed sex-differences in the current investigation. For example, if women with diabetes were more likely to die during hospitalization than non-diabetic women, this would tend to bias the hazard rate towards the null. Indeed, the median survivorship was 3.49 years in women and 3.73 years in men. There are no comparable data that we know of for women with impaired fasting glucose, but a similar direction of attenuation seems plausible. Longer term data are necessary to better address this question. We had information from one baseline examination which was not repeated during the follow-up time. Thus, we could not directly examine the proportion of impaired fasting glucose women who may have progressed to develop type 2 diabetes. Some of the study participants classified as IFG case patients at the follow-up examination may have had undetected type 2 diabetes. Such misclassification of diabetes would tend to increase the hazard ratio. However, fasting glucose measures are more highly correlated over time than the 2-h post-challenge glucose level, and, upon repeat testing, many with newly detected type 2 diabetes are found to “revert” to either impaired glucose tolerance (IGT) or normal 25–28. The Hoorn Study 25 has reported that the risk for conversion to diabetes over 6.5 years is nearly identical among those with impaired fasting glucose (51.4/1,000 person-years) and those with impaired glucose tolerance (57.9/1,000 person-years). Thus, we would expect relatively few women to progress to type 2 diabetes over our follow-up time especially because they are under care from their physician.
There is abundant evidence that indicates that women patients with AMI do not receive the same standard of care as men. In patients admitted to 37 Minnesota hospitals, women were less likely than men to receive beta-blockers, aspirin and thrombolytic therapy 29. A similar pattern was noted in the Cooperative Cardiovascular Project 30. Although we noted different disparities, consideration of these variables in multivariate analyses did not materially alter our results (data not shown).
In summary, impaired fasting glucose and type 2 diabetes were found to be significant predictors of recurrent CVD in women MI survivors after consideration of other risk factors and medical management. These results suggest that this increased CVD risk in these AMI survivors begins at lower levels of fasting glucose than are currently used to define type 2 diabetes. Recent findings from the ACCORD, VADT, and ADVANCE16–18 randomized clinical trials clearly show that tight glycemic control does not eliminate the risk of macro-vascular complications in the diabetic population. Targeted screening of conventional CVD risk factors and secondary interventions proven to enhance insulin sensitivity should be more heavily emphasized and managed, particularly among women.
Acknowledgements
This study was supported by the National Institute of Alcohol and Addictions, National Institutes of Health (2 P50 AA09802-06 and HL 08561) and had no involvement in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Access and Responsibility: Dr Donahue had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281(14):1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147(3):149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 3.Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260(23):3456–3460. [PubMed] [Google Scholar]
- 4.Donahue RP, Orchard TJ. Diabetes mellitus and macrovascular complications. An epidemiological perspective. Diabetes Care. 1992;15(9):1141–1155. doi: 10.2337/diacare.15.9.1141. [DOI] [PubMed] [Google Scholar]
- 5.Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol. 2008;51(3):264–270. doi: 10.1016/j.jacc.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Koro CE, Bowlin SJ, Rabatin V, Fedder DO. Cardiovascular disease risk among subjects with impaired fasting glucose in the United States: Results from NHANES 1999–2004. Diabetes and Metabolic Syndrome: Clinical Research and Reviews. 2008;2(4):239–244. [Google Scholar]
- 7.Donahue RP, Stranges S, Rejman K, Rafalson LB, Dmochowski J, Trevisan M. Elevated cystatin-C concentration is associated with progression to prediabetes: the Western New York Study. Diabetes Care. 2007;30(7):1724–1729. doi: 10.2337/dc07-0040. [DOI] [PubMed] [Google Scholar]
- 8.Stranges S, Dorn JM, Donahue RP, Browne RW, Freudenheim JL, Hovey KM, Trevisan M. Oxidation, type 2 diabetes and coronary heart disease, a complex interaction. Findings from a population-based study. Diabetes Care. 2008;31(9):1864–1866. doi: 10.2337/dc08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaglehole R, Stewart AW, Butler M. Comparability of old and new World Health Organization criteria for definite myocardial infarction. Int J Epidemiol. 1987;16(3):373–376. doi: 10.1093/ije/16.3.373. [DOI] [PubMed] [Google Scholar]
- 10.Hamsten A, Walldius G, Dahlen G, Johansson B, De Faire U. Serum lipoproteins and apolipoproteins in young male survivors of myocardial infarction. Atherosclerosis. 1986;59(2):223–235. doi: 10.1016/0021-9150(86)90051-1. [DOI] [PubMed] [Google Scholar]
- 11.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 12.Donahue RP, Goldberg RJ, Chen Z, Gore JM, Alpert JS. The influence of sex and diabetes mellitus on survival following acute myocardial infarction: a community-wide perspective. J Clin Epidemiol. 1993;46(3):245–252. doi: 10.1016/0895-4356(93)90072-9. [DOI] [PubMed] [Google Scholar]
- 13.Behar S, Boyko V, Reicher-Reiss H, Goldbourt U. Ten-year survival after acute myocardial infarction: comparison of patients with and without diabetes. SPRINT Study Group. Secondary Prevention Reinfarction Israeli Nifedipine Trial. Am Heart J. 1997;133(3):290–296. doi: 10.1016/s0002-8703(97)70222-9. [DOI] [PubMed] [Google Scholar]
- 14.Donahue RP, Bean JA, Donahue RD, Goldberg RB, Prineas RJ. Does insulin resistance unite the separate components of the insulin resistance syndrome? Evidence from the Miami Community Health Study. Arterioscler Thromb Vasc Biol. 1997;17(11):2413–2417. doi: 10.1161/01.atv.17.11.2413. [DOI] [PubMed] [Google Scholar]
- 15.Donahue RP, Rejman K, Rafalson LB, Dmochowski J, Stranges S, Trevisan M. Sex differences in endothelial function markers before conversion to pre-diabetes: does the clock start ticking earlier among women? The Western New York Study. Diabetes Care. 2007;30(2):354–359. doi: 10.2337/dc06-1772. [DOI] [PubMed] [Google Scholar]
- 16.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 18.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 19.Goodarzi MO, Psaty BM. Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA. 2008;300(17):2051–2053. doi: 10.1001/jama.2008.510. [DOI] [PubMed] [Google Scholar]
- 20.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21(1):69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Sprafka JM, Burke GL, Folsom AR, McGovern PG, Hahn LP. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care. 1991;14(7):537–543. doi: 10.2337/diacare.14.7.537. [DOI] [PubMed] [Google Scholar]
- 23.Shahar E, Folsom AR, Jackson R. The effect of nonresponse on prevalence estimates for a referent population: insights from a population-based cohort study. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Ann Epidemiol. 1996;6(6):498–506. doi: 10.1016/s1047-2797(96)00104-4. [DOI] [PubMed] [Google Scholar]
- 24.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 25.de Vegt F, Dekker JM, Stehouwer CD, Nijpel G, Bouter LM, Heine RJ. The 1997 American Diabetes Association criteria versus the 1985 World Health Organization criteria for the diagnosis of abnormal glucose tolerance: poor agreement in the Hoorn Study. Diabetes Care. 1998;21(10):1686–1690. doi: 10.2337/diacare.21.10.1686. [DOI] [PubMed] [Google Scholar]
- 26.Feskens EJ, Bowles CH, Kromhout D. Intra- and interindividual variability of glucose tolerance in an elderly population. J Clin Epidemiol. 1991;44(9):947–953. doi: 10.1016/0895-4356(91)90058-h. [DOI] [PubMed] [Google Scholar]
- 27.McDonald GW, Fisher GF, Burnham C. Reproducibility of the Oral Glucose Tolerance Test. Diabetes. 1965;14:473–480. doi: 10.2337/diab.14.8.473. [DOI] [PubMed] [Google Scholar]
- 28.Olefsky JM, Reaven GM. Insulin and glucose responses to identical oral glucose tolerance tests performed forty-eight hours apart. Diabetes. 1974;23(5):449–453. doi: 10.2337/diab.23.5.449. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin TJ, Soumerai SB, Willison DJ, Gurwitz JH, Borbas C, Guadagnoli E, McLaughlin B, Morris N, Cheng SC, Hauptman PJ, Antman E, Casey L, Asinger R, Gobel F. Adherence to national guidelines for drug treatment of suspected acute myocardial infarction: evidence for undertreatment in women and the elderly. Arch Intern Med. 1996;156(7):799–805. [PubMed] [Google Scholar]
- 30.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Engl J Med. 1998;339(8):489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]