Introduction
Normal development and tissue homeostasis in multi-cellular organisms rely on the conserved cell death mechanism of apoptosis (reviewed in [1–4]). There are two principal signaling cascades leading to cell death by apoptosis. They are the extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. The extrinsic pathway is turned on in response to extracellular signals and involves activation of plasma membrane receptors through binding of ligands like Fas/CD95, TNFα and TRAIL [5]. Receptor activation ultimately leads to processes that trigger initiator caspases, like caspase 8 and 10, which in turn activate executioner caspases, like caspase 3 and 7. Executioner caspases orchestrate the appearance of late apoptosis markers such as DNA fragmentation and blebbing of the plasma membrane.
Mitochondria are central players in the intrinsic pathway of apoptosis, which is turned on in response to a diverse set of apoptotic signals, including DNA damage, growth factor withdrawal, and viral infection. Mitochondria release a number of cofactors from their intermembrane space, like cytochrome c, Smac/Diablo, and AIF, which promote and amplify the apoptotic cascade from the formation and activation of the apoptosomes to the final destruction of the cell [1, 3, 6–10]. The Bcl-2 family of proteins is a key regulator of the mitochondrial response to apoptotic signals and contains both pro- and anti-apoptotic members. Many of these proteins localize to mitochondria and finely control apoptosis through regulation of the release of these cofactors from mitochondria (reviewed in [3, 11]). The extrinsic and intrinsic pathways initially appeared to be independent. However, it is now clear that a crosstalk exists between the two pathways that is mediated by the sentinel or ‘BH3 only’ proteins like Bid [12]. For example, caspase 8 is activated in early extrinsic apoptosis and directly turns on the executioner caspase 3. However, caspase 8 also cleaves Bid to form tBid, which facilitates cytochrome c release and enables the cell to mount a more robust apoptotic response. Thus, tBid is generated by the extrinsic pathway and crosses over to activate the intrinsic pathway.
The mechanisms underlying release of pro-apoptotic factors from mitochondria remain a subject of lively debate. This release was attributed to the opening of the permeability transition pore (PTP) in the mitochondrial inner membrane several years ago. Sustained PTP opening causes swelling of the matrix space and bursting of the outer membrane. This rupture of the outer membrane would spill cytochrome c and other intermembrane space proteins into the cytosol. However, this scenario may not normally occur in early apoptosis. Instead, sustained PTP opening is now thought to play a central role in ischemia-reperfusion injury and necrosis [13–15]. The role of PTP in apoptosis may be indirect. Briefly, cyclophilin-D is the target of cyclosporine A and a regulator of the PTP. Surprisingly, cyclophilin-D deficient cells died normally in response to apoptotic stimuli known to activate both the extrinsic and intrinsic pathways. In contrast, these KO cells showed resistance to necrotic cell death induced by either reactive oxygen species or Ca2+ overload [13]. Perhaps the “nail in the coffin” for PTP playing a role in apoptosis was the observation that cytochrome c release occurred in the absence of mitochondrial depolarization and without loss of outer membrane integrity. These observations indicate that, instead of rupturing, a more selective mechanism of permeabilization is operating, like the formation of a pore in the outer membrane [16–21].
Directly patch clamping mitochondria isolated from apoptotic cells enabled identification of the Mitochondrial Apoptosis-induced Channel or MAC. This channel activity is not present in mitochondria of normal cells and is exquisitely regulated by Bcl-2 family proteins. Several observations strongly indicate that MAC provides the aqueous pathway through the mitochondrial outer membrane requisite for the release of pro-apoptotic factors like cytochrome c [17, 20, 21]. Nevertheless, MAC and PTP opening may act alone or in combination, depending on cell type and death stimulus, to remodel the cristae, and facilitate cytochrome c release during amplification of the death signal [19, 22]. The two focuses of this review are the regulation of MAC by Bcl-2 family proteins and how MAC can be a potential therapeutic target in cancer and degenerative diseases.
What is MAC?
MAC is a channel with a giant pore, big enough to allow passage of proteins with diameters >3 nm, like cytochrome c. This channel activity was first detected by directly patch-clamping mitochondria isolated from FL5.12 cells,12 hr after withdrawal of interleukin-3 (IL-3) to induce apoptosis [17]. MAC can also be studied after reconstitution in proteoliposomes formed with mitochondrial outer membranes purified from apoptotic cells. Membrane patches are removed from the proteoliposomes with a micropipette and the ionic current through individual channels is characterized.
MAC is a channel whose conductance is both large and heterogeneous, but is generally between 2.5 and 5 nS [17, 20, 21]. Functional MAC has a peak conductance of >1 nS and displays transitions of up to ~2 nS with multiple sub-conductance levels in 150 mM KCl (Figure 1, Table 1). While flickering between substates is observed, current traces often show MAC occupying a stable fully open state with relatively infrequent transitions [17, 20, 21]. The activity of MAC is significantly different from TOM and VDAC, which are constitutive channels of the mitochondrial outer membrane. The single channel parameters of peak conductance, transition size, selectivity, and voltage dependence for these channels are shown in Table 1.
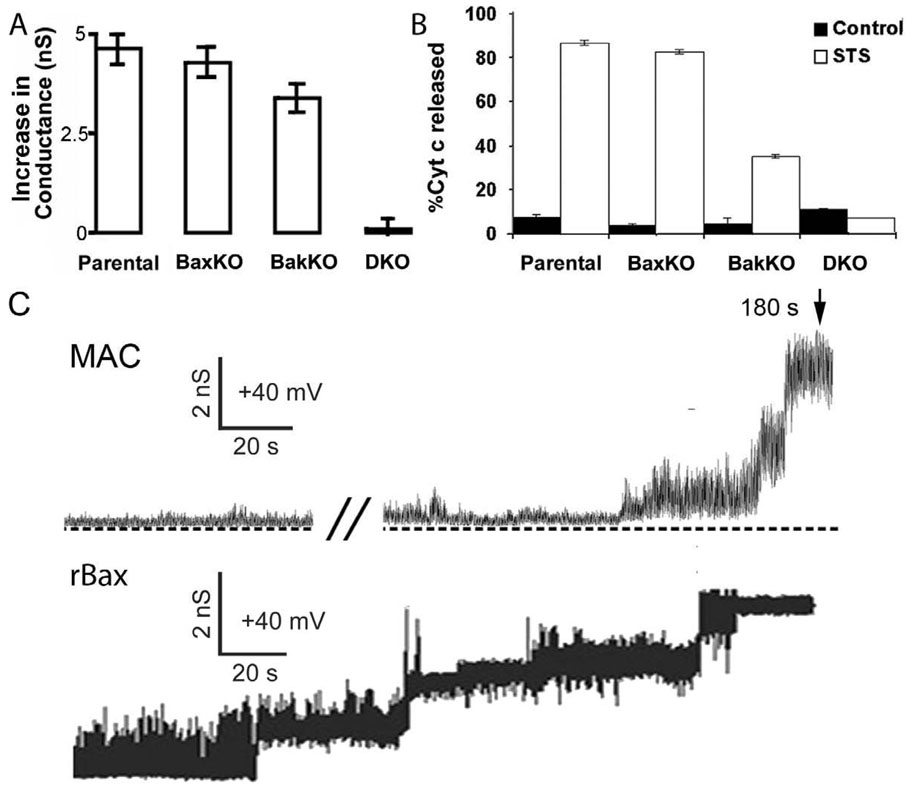
Figure 1. MAC and rBax channel formation.
A, B. Mitochondria were isolated from Parental, Bax KO, Bak KO and DKO (Double Knockout) MEF cell lines as indicated 16 hours after apoptosis was induced by 1 µM staurosporine (STS). A. The peak conductances of outer membranes were measured by directly patch clamping mitochondria isolated from control and STS-treated MEF cells. Histograms show the increase in conductance corresponding to the difference between the mean conductance of STS-treated and control patches. These permeability increases were consistent with the presence of MAC in mitochondria of apoptotic Parental, Bax KO and Bak KO cells, but not in the STS-treated DKO cells. Data were mean of 20–23 independent patches, error bars indicate SE. B. Histograms show the cytochrome c release to the cytosol monitored by ELISA and expressed relative to the positive control alamethicin (80 µg/106 cells). Data were mean ± SE of at least 3 different preparations. C. Current traces are shown of the formation of MAC and recombinant Bax (rBax) channels. A mitochondrion isolated from healthy parental MEF cells was patch clamped with a micropipette containing 20 nM tBid. Time indicated was since seal formation. Current trace labeled rBax was from a liposome patch that showed an increase in conductance with time when 380 ng/µl of monomeric BaxΔC and 35 ng/µl tBid were included in the micropipette. The conductance increased in multiple steps of ~300 pS until it reached about 1200 pS. Modified from ref. [32].
Table I.
Electrophysiological properties of MAC containing Bax and/or Bak, VDAC and TOM channelsa
| Parental | Bax KO | Bak KO | rBax | VDAC | TOM | |
|---|---|---|---|---|---|---|
| Peak conductance (nS) | 3.5±1.8 (n=7) | 3.1±2.0 (n=8) | 3.0±1.9 (n=8) | 5.0±3.0 | 0.7±0.1 | 0.7±0.1 |
| Transition size (nS) | Up to 2 | Up to 2 | Up to 2 | Up to 2 | 0.35 | 0.35 |
| Ion selectivity PK+/PCl− | 3.1±1.4 (n=5) | 3.7 (n=1) |
3.4±1.2 (n=4) | 6.8±1.0 | 0.5 | 3.6±0.8 |
|
Voltage dependent (±50 mV) |
No | No | No | No | Yes | Yes |
| Predicted diameter (nm) | 6 | 5.6 | 5.5 | 5.1±1.7 | 2.0±0.2 | 2.0±0.1 |
|
Predicted # helices forming Pore |
18 | 17 | 17 | 22 | n. a. | n. a. |
|
[tBid] EC50 (nM) cyt. c release |
7 | 17 | 247 | n. a. | n. a. | n. a. |
Measurements were made in 150 mM KCl containing buffer. n. a. stands for not applicable. n = number of determinations. rBax, recombinant oligomeric BaxΔC20 channels in artificial membranes[17, 21]. Diameters of MAC and number of helices were calculated from conductances as previously described [17, 32]. VDAC selectivity was from ref. [78].
MAC is typically a voltage-independent channel [17, 20, 21, 23], although it has also been reported to occupy less than peak conductances at higher potentials [24]. The channel is slightly cation-selective, which is consistent with MAC’s putative role in releasing the cationic protein cytochrome c. The polymer exclusion method was used to estimate the pore size of MAC [20], as has been done with other channels [25–30]. These studies indicate that MAC with a conductance of 4 nS is permeable to 10 and 17 kDa, but not 45 and 71 kDa Dextran. The polymer exclusion experiments suggest that 1.5 to 5 nS MAC have pore diameters in the range of 2.9 to 7.6 nm.
MAC is the cytochrome c release channel
MAC provides the pathway through the outer membrane for release of cytochrome c early in apoptosis and this notion is supported by a variety of observations. In patch clamp experiments, cytochrome c modifies the behavior of MAC in a manner consistent with its entrance into the pore, which is an essential step of translocation [20, 21]. Physiological concentrations (0.1–1 mM) of cytochrome c reduced MAC conductance with increased channel noise in a voltage dependent manner [20, 31]. The pore size of MAC estimated as mentioned above is sufficient to allow the passage of 12.5 kDa cytochrome c. In addition, the onset of MAC activity coincides with cytochrome c release in several systems [17, 20, 21, 23, 32, 33]. The temporal association of MAC formation and cytochrome c release is consistent with biochemical findings. Specifically, MAC conductance is found in mitochondria isolated from apoptotic cells at the same time that Bax is translocated to the outer membrane and cytochrome c was released [21]. Also, proteoliposomes made from apoptotic membranes express MAC activity and fail to retain cytochrome c compared with those prepared from control cells [17]. Hence, cytochrome c permeability increases early in apoptosis when MAC activity is present. Finally, the multitude of effects of Bcl-2 family proteins on this activity described below also strongly support MAC’s identification as the cytochrome c release channel.
Balance within the Bcl-2 family of proteins
The Bcl-2 family of proteins is the fundamental regulator of mitochondrial apoptosis. As shown in Table 2, proteins in this family contain between one and four Bcl-2 homology (BH) domains. These α-helical domains allow the Bcl-2 family proteins to form heterodimers by interacting with each other, a process that is central to the mechanism by which they regulate apoptosis. There are three main functional groups of proteins in this family. The anti-apoptotic proteins contain four BH domains (BH1 to BH4). The BH1, BH2 and BH3 domains form a hydrophobic cleft that binds the hydrophobic face of BH3 domains of pro-apoptotic proteins. The multi-domain pro-apoptotic proteins have the BH1, BH2, and BH3 domains, but lack a BH4 domain. The pro-apoptotic sentinels, or BH3-only proteins, share only a BH3 domain with the rest of the Bcl-2 family. BH3-only proteins are also subdivided into direct activators and sensitizers and whether PUMA is a sensitizer or direct activator remains a subject of debate (Table 2).
Table 2.
The Bcl-2 family of proteins is divided into three functional groups based on their composition of BH domainsa
| Class | Subclass | Members | Domains | Normal function |
Role in MAC regulation |
|---|---|---|---|---|---|
| Anti-apoptotic Effectors (AE) |
Multi- domain |
Bcl-2, Bcl-xL, Mcl-1, Bcl-w, A1 (Bfl-1) |
BH1–4 | Inhibit PEs & sentinels at MOM through direct interaction [3, 10] |
-Bcl-2 inhibits MAC formation [17] - Bcl-xL inhibits MAC-like activity in yeast expressing Bax [17] |
| Pro-apoptotic Effectors (PE) |
Multi- domain |
Bax, Bak, Bok |
BH1–3 | Permeabilize MOM during apoptosis [3, 10] |
-Bax is a MAC component [21] -Bax and Bak are redundant regarding MAC formation [32] |
| Pro-apoptotic Sentinels (BH3-only) |
Direct Activators |
Bid, Bim, p53, PUMA? |
BH3 | Relay pro- apoptotic signal by interacting with PEs [3, 10] |
-tBid induces MAC formation in either Bax−/− or Bak−/− mitochondria [21, 32] |
| Sensitizers | Bad, NOXA, Hrk, PUMA? |
BH3 | Relay pro- apoptotic signal by interacting with AEs [3, 10] |
-n. d. |
BH: Bcl-2 Homology domains; n. d. stands for not determined.
Many Bcl-2 family proteins eventually localize to mitochondria and finely control the process of releasing apoptosis mediators into the cytosol (recently reviewed in [1, 3, 8, 10]). Some translocate upon apoptotic signaling like Bcl-xL and Bax, while others, like Bcl-2 and Bak, are resident proteins in the outer membrane and contact sites [34]. The pro-apoptotic multi-domain proteins like Bax are essential to the machinery underlying permeabilization of the outer membrane and, as described below, are structural components of MAC. On the other hand, anti-apoptotic members such as Bcl-2, inhibit this process by directly binding to and sequestering the pro-apoptotic effector proteins [11]. Finally, the sentinels are pro-apoptotic proteins that relay the apoptotic signal to mitochondria by interacting with pro- (direct activators) or anti- (sensitizers) apoptotic Bcl-2 family proteins [1, 3, 11]. Members of each of these classes have effects on MAC formation and activity as shown in Table 2. Importantly, the combined signaling through protein-protein interactions of the various types of Bcl-2 family proteins controls the immediate fate of the cell, i.e., the decision to or not to induce permeabilization of the outer membrane when challenged with apoptotic stimuli [3]. Finally, mounting evidence indicates that this permeabilization phenomenon occurs through formation of the cytochrome c release channel MAC (see [8, 10, 35] for reviews).
MAC activity is tightly linked to Bax and Bak
Previous studies using single and double knock out cell lines for Bax and Bak found these two proteins are functionally redundant with respect to their role in apoptosis (see [36–39] for reviews). Patch-clamp studies of mitochondria isolated from cells deficient in one or both Bax and Bak show that at least one of the proteins must be present for formation of MAC [32]. Figure 1 shows cytochrome c release and an increase in mitochondrial permeability corresponding to MAC assembly, is recorded in mitochondria containing Bax and/or Bak, but not in those of the double knockout after apoptosis is induced. As indicated in Table 1, although some differences were detected, the single channel behaviors of MACs containing exclusively Bax or Bak are similar [32]. Considering Bax was shown to be a component of MAC [21], these data support the notion that Bak may replace Bax as a structural component of MAC in cells deficient in Bax. That is, Bax and Bak are functionally redundant with respect to MAC.
Other studies determined that it is oligomeric, not monomeric, Bax that contributes to MAC activity. Bax is a component of MAC of staurosporine-treated HeLa cells because MAC activity is depleted after immunoprecipitation of oligomeric Bax. In this system, MAC activity is present in total mitochondrial lysates and fractions containing oligomeric, but not monomeric, Bax [21]. This is expected as Bax oligomers form in the outer membrane following Bax translocation to mitochondria at the time that cytochrome c is released [19, 21, 40, 41]. Importantly, MAC activity is depleted from solubilized mitochondrial fractions by Bax antibodies raised against an N-terminal epitope of the protein [21]. This epitope is inaccessible in monomeric Bax but becomes exposed following Bax activation. Thus, these antibodies selectively immunoprecipitate oligomeric Bax [42, 43]. The concomitant loss of MAC activity and oligomeric Bax by immunoprecipitation provides strong evidence that activated/oligomeric Bax is a component of MAC.
The role of Bax in MAC structure and function are reflected in a variety of data. While Bax monomers form channels, the conductance corresponds to a pore that is likely too small to allow passage of cytochrome c [44, 45]. However, non-selective, voltage-independent channels that show a gradual increase in conductance up to 5.4 nS form if Bax is oligomerized prior to insertion into planar bilayers [46]. The diameter estimated from the peak conductance of 5.4 nS for rBax channels is >5 nm, which should easily allow the passage of ~3 nm cytochrome c [47]. This discovery was confirmed in a study in which the channel forming activity of oligomeric Bax was monitored by patch-clamp techniques (Figure 1, Table 1) [21]. Cytochrome c and RNAse A have the same effects on MAC and recombinant BaxΔC20 channels, while hemoglobin has no effect. Similarly, heterologous expression of Bax induces cell death and cytochrome c release in yeast [48, 49]. Using this system, we found expression of c-myc tagged human Bax (hBax) induced a MAC-like activity in yeast mitochondria. Patch-clamping yeast mitochondria expressing hBax in a VDAC-less strain detected a novel channel activity, which again, was similar to MAC activity found in apoptotic mammalian mitochondria [17, 21]. Furthermore, MAC activity appears when Bax levels increase in mitochondria of apoptotic FL5.12 cells [17]. MAC is also detected as an increase in outer membrane permeability by patch-clamp techniques in staurosporine-treated HeLa cells at the time Bax-GFP forms clusters in mitochondria and cytochrome c is released [21]. While correlative, this evidence supports the mechanistic link between Bax translocation and oligomerization, MAC formation, and cytochrome c release that was directly tested by the molecular and immunological studies described above.
Regulation of MAC by the sentinel or BH3-only proteins
The BH3-only proteins are the sentinels that translate the survival and apoptotic signals emanating from throughout the cell. There are two functional classes of sentinels; they are direct activators or sensitizers (Table 2). Bid, Bim, p53, and perhaps PUMA directly interact with the multidomain pro-apoptotic Bax and Bak to cause a conformational change, which presumably triggers their oligomerization leading to MAC formation [3, 50]. Bad, NOXA, and perhaps PUMA are sensitizers which bind the hydrophobic pocket of the anti-apoptotic proteins like Bcl-2 to displace the normally sequestered direct activators, like Bim, or even possibly the pro-apoptotic effectors Bax and Bak [3, 50]. The elevation in liberated pro-apoptotic proteins shifts the balance within the family towards apoptosis. Nevertheless, Bax and Bak presumably remain inert until a direct activator sentinel induces the conformational change that allows their oligomerization. Yet another layer of regulation exists within the BH3-only group. For example, Bad must be dephosphorylated while Bid needs to be cleaved in order to assume their sentinel status as sensitizers and direct activators, respectively [51, 52].
During apoptosis, inactive Bid is cleaved to form a C-terminal truncated form referred to as tBid, which functions as a direct activator. The fragment tBid triggers oligomerization of both Bax and Bak in the mitochondrial outer membrane presumably to form MAC, which causes cytochrome c release (Figure 2 left) [53, 54]. Furthermore, tBid can trigger oligomerization of recombinant monomeric Bax in artificial membranes [21, 46]. The oligomerization results in formation of voltage independent and slightly cationic channels with conductances of up to several nS, which are detected by patch-clamp techniques (Figure 1, Table 1) [21]. Moreover, cytochrome c is transported through these tBid induced Bax channels, which again makes them very similar to MAC [21].
Figure 2. MAC formation in mitochondria from normal and Bcl-2-overexpressing cells.
Left: Upon apoptotic stimulus, the sentinel Bim ( ) translocates to mitochondrial outer membrane (mom) where it catalyzes Bax (
) translocates to mitochondrial outer membrane (mom) where it catalyzes Bax ( ) activation and conversion of MAC precursors into functional MAC. MAC releases cytochrome c (
) activation and conversion of MAC precursors into functional MAC. MAC releases cytochrome c ( , cyt c) from the intermembrane space (ims) in the commitment step to death. As yet unidentified interacting proteins (
, cyt c) from the intermembrane space (ims) in the commitment step to death. As yet unidentified interacting proteins ( ) are also shown. iMACs block the release of cytochrome c and death by inhibiting MAC. Center: In cells overexpressing Bcl-2 (
) are also shown. iMACs block the release of cytochrome c and death by inhibiting MAC. Center: In cells overexpressing Bcl-2 ( ), an apoptotic signal induces accumulation of Bim/Bcl-2 complexes, but MAC is still in its precursor form and cytochrome c is not released. Accumulation of Bim/Bcl2-containing complexes represents the putative mechanism by which Bcl-2 overexpression primes cells to die. Right: Addition of ABT-737 disrupts Bcl-2 complexes with Bax and Bim to allow MAC formation and cytochrome c release. Bax, Bim, and Bcl-2 represent a pro-apoptotic effector, a sentinel and an anti-apoptotic effector protein, respectively.
), an apoptotic signal induces accumulation of Bim/Bcl-2 complexes, but MAC is still in its precursor form and cytochrome c is not released. Accumulation of Bim/Bcl2-containing complexes represents the putative mechanism by which Bcl-2 overexpression primes cells to die. Right: Addition of ABT-737 disrupts Bcl-2 complexes with Bax and Bim to allow MAC formation and cytochrome c release. Bax, Bim, and Bcl-2 represent a pro-apoptotic effector, a sentinel and an anti-apoptotic effector protein, respectively.
We used tBid to induce MAC activity in mitochondria in order to visualize formation of the pore of MAC in real time [32]. As shown in Figure 1, a large conductance developed with time when control mitochondria were patch-clamped with micropipettes containing tBid [32]. Nanomolar levels of tBid catalyzed MAC formation and cytochrome c release with a time course of minutes. Interestingly, the amount of tBid needed to form MAC and release cytochrome c in mitochondria lacking Bax and/or Bak was different. MAC formed from Bak with an EC50 of about 20 nM tBid but MAC needed >200 nM tBid to form from Bax [32]. It is not known if this was due to differences in available Bax or Bak in these mitochondria. As expected, MAC did not form in mitochondria lacking both Bax and Bak.
Mathematical analysis of the step-wise changes in conductance associated with tBid and MAC formation was consistent with pore assembly by a barrel-stave model. Assuming the staves are two transmembrane α-helices in Bax and Bak, mature MAC pores would typically contain ~9 monomers and have diameters of 5.5–6 nm. The role of other sentinel proteins like Bad and Bim on MAC formation and activity is still under investigation. However, there are reports that lipids may also play a central role in cytochrome c release. For example, the lipid ceramide forms channels in mitochondrial outer membranes that are in part regulated by Bcl-2 family proteins and the channels formed from Bax and peptides mimicking regions of Bax may indeed form lipidic pores [55–57]. Hence, the assembly mechanism of MAC is not yet resolved.
Regulation of MAC by the anti-apoptotic proteins
Bcl-2 is one of the best studied anti-apoptotic proteins in the Bcl-2 family (Table 2) [36–39, 55]. However, several other members of this subclass co-exist with Bcl-2 within the cell as shown in Table 2. Some differences exist in their abilities to bind other members to form heterodimers. Bcl-2 and Bcl-xL seem to bind almost all the pro-apoptotic members. A hydrophobic cleft is formed by the BH1, BH2 and BH3 domains of anti-apoptotic proteins that provides a binding site for the hydrophobic face of BH3 domains of pro-apoptotic proteins. Presumably, it is at this site that Bcl-2 and Bcl-xL inhibit apoptosis by sequestering Bax, Bak and direct activator sentinels like Bim. Interestingly, Mcl-1 is the only member that binds the sentinel NOXA and Bcl-xL is the only one that binds Hrk [7, 58]. As described below (Figure 2), the inhibitors of anti-apoptotic action, like ABT-737, target this cleft to release pro-apoptotic proteins [50]. Importantly, and unlike most anti-apoptotic proteins, the action of Mcl-1 is not inhibited by ABT-737.
As expected, an excess of anti-apoptotic proteins blocks apoptosis by preventing outer membrane permeabilization. MAC has never been detected in IL-3 deprived FL5.12 cells that overexpress Bcl-2. Furthermore, overexpression of Bcl-xL eliminated the MAC-like activity of mitochondria from yeast expressing hBax [17]. These results suggest that these anti-apoptotic proteins inhibit MAC formation. However, the molecular mechanisms of this inhibition are, as yet, poorly defined. For example, does Bcl-2 block Bax translocation, activation, or just oligomerization?
Recombinant Bcl-2 can form channels in planar bilayers [45, 59]. In contrast, no new channel activities are detected when Bcl-2 is overexpressed in FL5.12 or MDA-231 cells, suggesting this protein does not form channels in native mitochondrial membranes [17, 60]. However, channels whose conductances are between 0.75–1 nS are detected in isolated mitochondria after addition of caspase cleaved recombinant Bcl-xL (ΔN-Bcl-xL) [61]. These channels have conductances and other properties similar to mitochondrial channels detected in squid giant synapses during early stages of hypoxia-mediated apoptosis, when Bcl-xL is cleaved by caspases [62, 63]. In particular, conductances of up to 3.8 nS were detected in media containing 570 mM KCl in the micropipette. For purposes of comparison, this peak conductance should correspond to about 1 nS in symmetrical 150 mM KCl. Hence, the pore size may be too small to allow for cytochrome c transport and therefore these channels are unlikely to have the same role as MAC during early apoptosis.
Importantly, the anti-apoptotic proteins like Bcl-2 cause an accumulation of sentinel proteins, like Bim, in mitochondria [64]. While overexpression of Bcl-2 suppresses apoptosis, the excess of sentinel proteins may be an Achilles’ heel that might be exploited to selectively target cancer cells to die.
Antagonists and Agonists of MAC or the pharmacology of cell death
MAC is a potential therapeutic target because of its role in the commitment step of apoptosis, i.e., cytochrome c release. Hence, we have striven to expand the pharmacological profile of MAC so that this channel can be turned on or turned off depending on the pathological condition being treated.
It has been previously shown that trifluoperazine and propranolol prevent apoptosis in some cell lines [65, 66], and trifluoperazine and dibucaine also block mitochondrial depolarization induced by glutamate in neurons [67]. Dibucaine, trifluoperazine and propranolol also block cytochrome c release from mitochondria induced by recombinant Bax and BH3-only proteins like tBid [68]. We found using patch-clamping that dibucaine, propranolol and trifluoperazine inhibited MAC in a dose-dependent manner. The IC50 are 39 µM, 52 µM and 1 µM for dibucaine, propranolol and trifluoperazine, respectively [23]. In contrast, lidocaine, a structural homolog of dibucaine, and cyclosporine A, a well known PTP blocker [69–71], had no effect on MAC activity [23]. Dibucaine, trifluoperazine and propranolol also block cytochrome c release from mitochondria induced by recombinant Bax and BH3-only proteins like tBid [68]. Hence, these studies provide yet another link between Bax and MAC. However, each of these agents has low potency and pleiotropic effects.
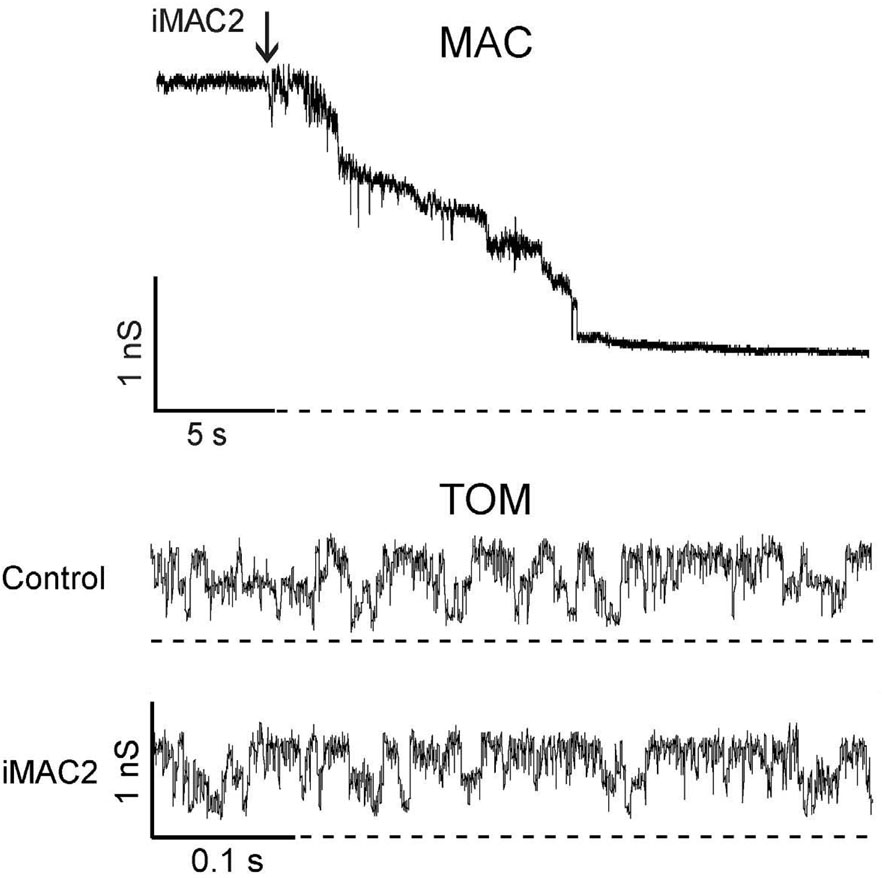
Previously, Antonsson and colleagues identified two novel agents that block the channel activity of recombinant Bax in planar bilayers and inhibit release of cytochrome c induced by tBid [72]. Hence, these two agents were named Bax channel inhibitors or Bci1 and Bci2. Furthermore, Bci1 and Bci2 block apoptosis of neurons in an animal model of global brain ischemia. Antonsson’s group also identified 3,6 dibromocarbazole piperazine derivatives of 2-propanol that blocked cytochrome c release induced by tBid in isolated mitochondria; some have IC50 values in the nM range [73]. As described below, we later named these compound inhibitors of MAC (iMAC). We tested Bci1, Bci2, and several potential iMACs derived from 2-propanolol for their ability to block MAC, and began an assessment of their effects on apoptosis. Several of these agents were effective in closing MAC in patch clamp experiments at nM concentrations (Table 3) [74]. As shown in Figure 3, 25 nM iMAC2 rapidly closed MAC. The effects of iMAC2 showed specificity for MAC as 500 nM iMAC2 had no effect on the activity of TOM, a constitutive channel of the outer membrane. In all, five iMACs and the two Bci compounds were tested and their IC50 values in patch clamp experiments are listed in Table 3 [74]. The toxicity of these compounds was also determined. Importantly, the ratios of LD50 over IC50 values for Bci1, iMAC1, iMAC2 and iMAC3 were over 200, which also suggest that these pharmacological agents have wide therapeutic windows.
Table 3.
Potency and toxicity of agents that block MAC
| Compound | IC50 (nM) | LD50 (nM) |
|---|---|---|
| iMAC1 | 300 | 50,000 |
| iMAC2 | 28 | 15,000 |
| iMAC3 | 19 | 15,000 |
| iMAC4 | 170 | 5,000 |
| iMAC5 | 450 | 7,500 |
| Bci1 | 84 | 20,000 |
| Bci2 | 966 | 30,000 |
Figure 3. Effect of iMAC2 on MAC and TOM channels.
MAC and TOM activities were recorded in patches excised from reconstituted mitochondrial outer membranes of apoptotic FL5.12 cells. Representative current traces at +20 mV of MAC and TOM channels upon perfusion with buffer containing 25 nM and 500 nM iMAC2, respectively. The current trace of TOM in the presence of iMAC2 was recorded ~1 min after perfusion. Dashed lines indicate 0 current levels. Modified from ref. [74].
Imbalances in the interaction network of Bcl-2 family proteins, e.g. through the variation of the expression level of various members, likely underlies some degenerative diseases and is known to cause cancer. But, these imbalances may also reveal selective therapeutic targets. For example, genetic events leading to overexpression of the Bcl-2 proto-oncogene suppresses apoptosis and are associated with tumor formation and, more particularly, B-Cell non-Hodgkin’s lymphoma [75–77]. Prolymphocytic cell lines overexpressing Bcl-2 exhibit a resistance to mitochondrial apoptosis and induce lymphoma upon injection in mice [17, 33, 75, 78]. Hence, overexpression of Bcl-2 is associated with cancer. While these cells are more resistant to death, apoptotic stimuli cause the sequestration of sentinel proteins such as Bim in mitochondria [64, 79]. This accumulation has been coined sensitization or “primed to die” and is illustrated in Figure 2. Small molecules that mimic the important BH3 domain can function as competitive inhibitors and bind to the hydrophobic cleft in Bcl-2. In doing so, these BH3 mimetics release sequestered pro-apoptotic proteins and the primed cells now die. Like most apoptotic stimuli, BH3-mimetics alone often do not kill these cells. However, the BH3-mimetics may act synergistically with chemotherapeutic agents (which provide the apoptotic signal) to trigger an increase in MAC formation and selectively kill cancer cells overexpressing anti-apoptotic proteins like Bcl-2. That is, BH3 mimetics, represented by ABT-737 in Figure 2, are able to selectively kill these cancer cells by inhibiting the interactions e.g., between Bcl-2 and Bim [50, 58, 64] and triggering an increase of MAC formation (Figure 2), a property potentially optimized by the activity of direct MAC activators which are yet to be found. It is important to note that ABT-737 inhibits all the known anti-apoptotic proteins except Mcl-1. Naturally, this “priming for death” approach will only work on cancer cells whose survival relies on overexpression of anti-apoptotic proteins and accumulation of pro-apoptotic proteins in mitochondria. Hence, development of personalized chemotherapy using BH3 mimetics will likely require pre-diagnosis of such an anti-apoptotic addiction through BH3 profiling [50, 58, 64].
Future perspectives
MAC is central to the commitment step of apoptosis and a potential therapeutic target. Turning MAC on should induce cell death and turning MAC off should suppress cell death. Recently identified pharmacological agents that modulate MAC are of special interest [23, 32, 74]. Also, our recent findings that MAC opening is linked to a bystander effect is intriguing but is still a relatively unexplored area of potential therapeutics [80]. Important questions remain about the function and interplay amongst the Bcl-2 family of proteins and the use of pharmacological compounds can be useful in elucidating their answers. For example, in the recently described “priming for death” mechanism, BH3 mimetics, like ABT-737, interfere with the interaction of Bcl-2 with BH3-only or pro-apoptotic effector proteins in presence of an apoptotic stimulus (Fig. 2). Thus, the combined action of apoptosis inducers and BH3 mimetics would lead to MAC formation, followed by cytochrome c release and cell death. In this scenario, what is the mechanism of MAC formation? Do BH3-only proteins directly activate Bax upon action by BH3 mimetics? Do BH3 mimetics also displace Bcl-2 from Bax? Also of intense interest is the definition of the molecular identity of MAC, which undoubtedly will require a proteomic approach to be fulfilled. In summary, a great deal of information establishes MAC as a pivotal player in a deadly plot with Bcl-2 members, which also provides clues for selective therapeutic strategies for the treatment of cancer and degenerative diseases.
Acknowledgements
This research was supported by NIH grant GM57249 to KWK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. DOI: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. DOI: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 3.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. DOI: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. DOI: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz I, Kirchhoff S, Krammer PH. Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol. 2000;32:1123–1136. doi: 10.1016/s1357-2725(00)00048-0. DOI: 10.1016/S1357-2725(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 6.Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27 Suppl 1:S93–S104. doi: 10.1038/onc.2009.47. DOI: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 7.Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27 Suppl 1:S149–S157. doi: 10.1038/onc.2009.52. DOI: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dejean LM, Martinez-Caballero S, Kinnally KW. Is MAC the knife that cuts cytochrome c from mitochondria during apoptosis? Cell Death Differ. 2006;13:1387–1395. doi: 10.1038/sj.cdd.4401949. DOI: 10.1038/sj.cdd.4401949. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi P, Forte M. The mitochondrial permeability transition pore. Novartis Found Symp. 2007;287:157–164. discussion 164-159. [PubMed] [Google Scholar]
- 10.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. DOI: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 11.Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. DOI: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. DOI: 10.1016/S0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. DOI: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 14.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. DOI:10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 15.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. DOI: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 17.Pavlov EV, Priault M, Pietkiewicz D, Cheng EH, Antonsson B, Manon S, Korsmeyer SJ, Mannella CA, Kinnally KW. A novel, high conductance channel of mitochondria linked to apoptosis in mammalian cells and Bax expression in yeast. J Cell Biol. 2001;155:725–731. doi: 10.1083/jcb.200107057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. DOI: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, Hanson GT, Remington SJ, Youle RJ, Ichas F. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. Faseb J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. DOI: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Pietkiewicz D, Pavlov EV, Grigoriev SM, Kasianowicz JJ, Dejean LM, Korsmeyer SJ, Antonsson B, Kinnally KW. Effects of cytochrome c on the mitochondrial apoptosis-induced channel MAC. Am J Physiol Cell Physiol. 2004;286:C1109–C1117. doi: 10.1152/ajpcell.00183.2003. DOI: 10.1152/ajpcell.00183.2003. [DOI] [PubMed] [Google Scholar]
- 21.Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, Kinnally KW. Oligomeric Bax Is a Component of the Putative Cytochrome c Release Channel MAC, Mitochondrial Apoptosis-induced Channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. DOI: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. DOI: 10.1016/S0006-291X(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Caballero S, Dejean LM, Kinnally KW. Some amphiphilic cations block the mitochondrial apoptosis-induced channel, MAC. FEBS Lett. 2004;568:35–38. doi: 10.1016/j.febslet.2004.05.006. DOI: 10.1016/j.febslet.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Guihard G, Bellot G, Moreau C, Pradal G, Ferry N, Thomy R, Fichet P, Meflah K, Vallette FM. The mitochondrial apoptosis-induced channel (MAC) corresponds to a late apoptotic event. J Biol Chem. 2004;279:46542–46550. doi: 10.1074/jbc.M405153200. [DOI] [PubMed] [Google Scholar]
- 25.Truscott KN, Kovermann P, Geissler A, Merlin A, Meijer M, Driessen AJ, Rassow J, Pfanner N, Wagner R. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat Struct Biol. 2001;8:1074–1082. doi: 10.1038/nsb726. DOI: 10.1038/nsb726. [DOI] [PubMed] [Google Scholar]
- 26.Krasilnikov OV, Sabirov RZ, Ternovsky VI, Merzliak PG, Muratkhodjaev JN. A simple method for the determination of the pore radius of ion channels in planar lipid bilayer membranes. FEMS Microbiol Immunol. 1992;5:93–100. doi: 10.1111/j.1574-6968.1992.tb05891.x. [DOI] [PubMed] [Google Scholar]
- 27.Krasilnikov OV, Da Cruz JB, Yuldasheva LN, Varanda WA, Nogueira RA. A novel approach to study the geometry of the water lumen of ion channels: colicin Ia channels in planar lipid bilayers. J Membr Biol. 1998;161:83–92. doi: 10.1007/s002329900316. DOI: 10.1007/s002329900316. [DOI] [PubMed] [Google Scholar]
- 28.Grigoriev SM, Muro C, Dejean LM, Campo ML, Martinez-Caballero S, Kinnally KW. Electrophysiological approaches to the study of protein translocation in mitochondria. Int Rev Cytol. 2004;238:227–274. doi: 10.1016/S0074-7696(04)38005-8. DOI: 10.1016/S0074-7696(04)38005-8. [DOI] [PubMed] [Google Scholar]
- 29.Bezrukov SM, Kasianowicz JJ. The charge state of an ion channel controls neutral polymer entry into its pore. Eur Biophys J. 1997;26:471–476. doi: 10.1007/s002490050101. DOI: 10.1007/s002490050101. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Caballero S, Grigoriev SM, Herrmann JM, Campo ML, Kinnally KW. Tim17p regulates the twin pore structure and voltage gating of the mitochondrial protein import complex TIM23. J Biol Chem. 2007;282:3584–3593. doi: 10.1074/jbc.M607551200. DOI: 10.1074/jbc.M607551200. [DOI] [PubMed] [Google Scholar]
- 31.Gupte SS, Hackenbrock CR. The role of cytochrome c diffusion in mitochondrial electron transport. J Biol Chem. 1988;263:5248–5253. [PubMed] [Google Scholar]
- 32.Martinez-Caballero S, Dejean LM, Kinnally MS, Oh KJ, Mannella CA, Kinnally KW. Assembly of the mitochondrial apoptosis-induced channel, MAC. J Biol Chem. 2009;284:12235–12245. doi: 10.1074/jbc.M806610200. DOI: 10.1074/jbc.M806610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. Embo J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. DOI: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marzo I, Brenner C, Zamzami N, Susin SA, Beutner G, Brdiczka D, Remy R, Xie ZH, Reed JC, Kroemer G. The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med. 1998;187:1261–1271. doi: 10.1084/jem.187.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Caballero S, Dejean LM, Jonas EA, Kinnally KW. The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J Bioenerg Biomembr. 2005;37:155–164. doi: 10.1007/s10863-005-6570-z. DOI: 10.1007/s10863-005-6570-z. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. DOI: 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol Cell Biochem. 2004;256–257:141–155. doi: 10.1023/b:mcbi.0000009865.70898.36. DOI: 10.1023/B:MCBI.0000009865.70898.36. [DOI] [PubMed] [Google Scholar]
- 38.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. DOI:10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 39.Green DR, Kroemer G. The Pathophysiology of Mitochondrial Cell Death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. DOI: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 40.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 41.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. DOI: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 42.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. Embo J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. DOI: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. DOI: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 45.Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roucou X, Montessuit S, Antonsson B, Martinou JC. Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein. Biochem J. 2002;368:915–921. doi: 10.1042/BJ20020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan SK, Tulloss I, Margoliash E. Primary structure of the cytochrome c from the snapping turtle, Chelydra serpentina. Biochemistry. 1966;5:2586–2597. doi: 10.1021/bi00872a016. [DOI] [PubMed] [Google Scholar]
- 48.Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure-function analysis of Bcl-2 protein. Identification of conserved domains important for homodimerization with Bcl-2 and heterodimerization with Bax. J Biol Chem. 1995;270:11962–11969. doi: 10.1074/jbc.270.20.11962. [DOI] [PubMed] [Google Scholar]
- 49.Manon S, Chaudhuri B, Guerin M. Release of cytochrome c and decrease of cytochrome c oxidase in Bax-expressing yeast cells, and prevention of these effects by coexpression of Bcl-xL. FEBS Lett. 1997;415:29–32. doi: 10.1016/s0014-5793(97)01087-9. DOI: 10.1016/S0014-5793(97)01087-9. [DOI] [PubMed] [Google Scholar]
- 50.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. DOI: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Youle RJ. The Role of Mitochondria in Apoptosis. Annu Rev Genet. 2009 doi: 10.1146/annurev-genet-102108-134850. in press. DOI: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. DOI: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 53.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. DOI: 10.1101/gad.14.16.2060. [PMC free article] [PubMed] [Google Scholar]
- 54.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Qian S, Wang W, Yang L, Huang HW. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc Natl Acad Sci U S A. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. 0807764105 [pii] 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M. Anti-apoptotic Bcl-2 Family Proteins Disassemble Ceramide Channels. J Biol Chem. 2008;283:6622–6630. doi: 10.1074/jbc.M706115200. M706115200 [pii] 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. DOI: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 59.Schendel SL, Azimov R, Pawlowski K, Godzik A, Kagan BL, Reed JC. Ion channel activity of the BH3 only Bcl-2 family member, BID. J Biol Chem. 1999;274:21932–21936. doi: 10.1074/jbc.274.31.21932. DOI: 10.1074/jbc.274.31.21932. [DOI] [PubMed] [Google Scholar]
- 60.Murphy RC, Schneider E, Kinnally KW. Overexpression of Bcl-2 suppresses the calcium activation of a mitochondrial megachannel. FEBS Letters. 2001;497:73–76. doi: 10.1016/s0014-5793(01)02440-1. DOI: S0014-5793(01)02440-1. [DOI] [PubMed] [Google Scholar]
- 61.Jonas EA, Hickman JA, Chachar M, Polster BM, Brandt TA, Fannjiang Y, Ivanovska I, Basanez G, Kinnally KW, Zimmerberg J, Hardwick JM, Kaczmarek LK. Proapoptotic N-truncated BCL-xL protein activates endogenous mitochondrial channels in living synaptic terminals. Proc Natl Acad Sci U S A. 2004;101:13590–13595. doi: 10.1073/pnas.0401372101. DOI: 10.1073/pnas.0401372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jonas EA, Hickman JA, Hardwick JM, Kaczmarek LK. Exposure to hypoxia rapidly induces mitochondrial channel activity within a living synapse. J Biol Chem. 2005;280:4491–4497. doi: 10.1074/jbc.M410661200. DOI: 10.1074/jbc.M410661200. [DOI] [PubMed] [Google Scholar]
- 63.Jonas EA. Molecular participants in mitochondrial cell death channel formation during neuronal ischemia. Exp Neurol. 2009;218:203–212. doi: 10.1016/j.expneurol.2009.03.025. DOI: 10.1016/j.expneurol.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. DOI: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 65.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J. 1995;307(Pt 1):99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freedman AM, Kramer JH, Mak IT, Cassidy MM, Weglicki WB. Propranolol preserves ultrastructure in adult cardiocytes exposed to anoxia/reoxygenation: a morphometric analysis. Free Radic Biol Med. 1991;11:197–206. doi: 10.1016/0891-5849(91)90172-y. [DOI] [PubMed] [Google Scholar]
- 67.Hoyt KR, Sharma TA, Reynolds IJ. Trifluoperazine and dibucaine-induced inhibition of glutamate-induced mitochondrial depolarization in rat cultured forebrain neurones. Br J Pharmacol. 1997;122:803–808. doi: 10.1038/sj.bjp.0701442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polster BM, Basanez G, Young M, Suzuki M, Fiskum G. Inhibition of Bax-induced cytochrome c release from neural cell and brain mitochondria by dibucaine and propranolol. J Neurosci. 2003;23:2735–2743. doi: 10.1523/JNEUROSCI.23-07-02735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lenartowicz E, Bernardi P, Azzone GF. Phenylarsine oxide induces the cyclosporin A-sensitive membrane permeability transition in rat liver mitochondria. J Bioenerg Biomembr. 1991;23:679–688. doi: 10.1007/BF00785817. [DOI] [PubMed] [Google Scholar]
- 70.Szabo I, Bernardi P, Zoratti M. Modulation of the mitochondrial megachannel by divalent cations and protons. J Biol Chem. 1992;267:2940–2946. [PubMed] [Google Scholar]
- 71.Broekemeier KM, Carpenter-Deyo L, Reed DJ, Pfeiffer DR. Cyclosporin A protects hepatocytes subjected to high Ca2+ and oxidative stress. FEBS Lett. 1992;304:192–194. doi: 10.1016/0014-5793(92)80616-o. [DOI] [PubMed] [Google Scholar]
- 72.Hetz C, Vitte PA, Bombrun A, Rostovtseva TK, Montessuit S, Hiver A, Schwarz MK, Church DJ, Korsmeyer SJ, Martinou JC, Antonsson B. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. DOI: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- 73.Bombrun A, Gerber P, Casi G, Terradillos O, Antonsson B, Halazy S. 3,6-dibromocarbazole piperazine derivatives of 2-propanol as first inhibitors of cytochrome c release via Bax channel modulation. J Med Chem. 2003;46:4365–4368. doi: 10.1021/jm034107j. DOI: 10.1021/jm034107j. [DOI] [PubMed] [Google Scholar]
- 74.Peixoto PM, Ryu SY, Bombrun A, Antonsson B, Kinnally KW. MAC inhibitors suppress mitochondrial apoptosis. Biochem J. 2009;423:381–387. doi: 10.1042/BJ20090664. DOI: 10.1042/BJ20090664. [DOI] [PubMed] [Google Scholar]
- 75.Meijerink JP, Van Lieshout EM, Beverloo HB, Van Drunen E, Mensink EJ, Macville M, Pieters R. Novel murine B-cell lymphoma/leukemia model to study BCL2-driven oncogenesis. Int J Cancer. 2005;114:917–925. doi: 10.1002/ijc.20822. DOI: 10.1002/ijc.20822. [DOI] [PubMed] [Google Scholar]
- 76.Egle A, Harris AW, Bath ML, O'Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004;103:2276–2283. doi: 10.1182/blood-2003-07-2469. DOI: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- 77.Swanson PJ, Kuslak SL, Fang W, Tze L, Gaffney P, Selby S, Hippen KL, Nunez G, Sidman CL, Behrens TW. Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol. 2004;172:6684–6691. doi: 10.4049/jimmunol.172.11.6684. [DOI] [PubMed] [Google Scholar]
- 78.Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Del Gaizo Moore V, Letai A. Rational design of therapeutics targeting the BCL-2 family: are some cancer cells primed for death but waiting for a final push? Adv Exp Med Biol. 2008;615:159–175. doi: 10.1007/978-1-4020-6554-5_8. [DOI] [PubMed] [Google Scholar]
- 80.Peixoto PM, Ryu SY, Pruzansky DP, Kuriakose M, Gilmore A, Kinnally KW. Mitochondrial apoptosis is amplified through gap junctions. Biochem Biophys Res Commun. 2009;390:38–43. doi: 10.1016/j.bbrc.2009.09.054. DOI: 10.1016/j.bbrc.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colombini M. Voltage gating in the mitochondrial channel, VDAC. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]