Abstract
The IκB kinase (IKK) complex is a central component in the classic activation of the nuclear factor-κB (NF-κB) pathway. It has been reported to function in physiologic responses, including cell death and inflammation. We have shown that IKK is regulated by oxidative status after transient focal cerebral ischemia (tFCI) in mice. However, the mechanism by which oxidative stress influences IKKs after tFCI is largely unknown. Nuclear accumulation and phosphorylation of IKKα (pIKKα) were observed 1 h after 30 mins of tFCI in mice. In copper/zinc-superoxide dismutase knockout mice, levels of NF-κB-inducing kinase (NIK) (an upstream kinase of IKKα), pIKKα, and phosphorylation of histone H3 (pH3) on Ser10 were increased after tFCI and were higher than in wild-type mice. Immunohistochemistry showed nuclear accumulation and pIKKα in mouse brain endothelial cells after tFCI. Nuclear factor-κB-inducing kinase was increased, and it enhanced pH3 by inducing pIKKα after oxygen–glucose deprivation (OGD) in mouse brain endothelial cells. Both NIK and pH3 interactions with IKKα were confirmed by coimmunoprecipitation. Treatment with IKKα small interfering RNA significantly reduced cell death after OGD. These results suggest that augmentation of NIK, IKKα, and pH3 in response to oxidative stress is involved in cell death after cerebral ischemia (or stroke).
Keywords: focal cerebral ischemia, histone H3 phosphorylation, IκB kinase-α, NF-κB-inducing kinase, oxidative stress, oxygen–glucose deprivation
Introduction
Oxidative stress has been implicated in the progression of cerebral ischemia. Reactive oxygen species (ROS) mediate nuclear factor-κB (NF-κB) activation, which is a principal redox-sensitive transcriptional factor, tightly controlled by IκB kinases (IKKs) (Scheidereit, 1998; Schreck et al, 1991). IκB kinases are composed of at least three subunits, catalytic IKKα and IKKβ, and regulatory IKKγ, which phosphorylate IκB and release the NF-κB protein in the NF-κB signaling cascade (Courtois et al, 2001). Although one of the most specific steps in NF-κB signaling is the activation of IKKs, the mechanism by which they are involved in oxidative stress during transient cerebral ischemia is not fully understood. Cerebral ischemia leads to an increase in IKKβ activity, and interference in IKKβ function in the brain reduces ischemic damage (Han et al, 2003; Herrmann et al, 2005); however, we found decreases in mRNA and IKK protein levels after cerebral ischemia, which were prevented in copper/zinc-superoxide dismutase (SOD1) transgenic mice that had low levels of ROS (Song et al, 2005). This study indicates that IKKs are regulated by redox status. Moreover, although degradation of IKKs was detected after transient focal cerebral ischemia (tFCI) in mice, NF-κB signaling of IKKs was activated downstream. These observations raised the question of whether IKKs may be related to the independent NF-κB pathway. IκB kinase-β has a higher activity for IκBα than does IKKα in the NF-κB pathway. It is a conserved helix–loop–helix ubiquitous kinase that phosphorylates unexpected substrates, such as β-catenin, cyclin D1, and cyclic adenosine monophosphate-responsive element-binding protein (Albanese et al, 2003; Huang et al, 2007; Lamberti et al, 2001). In addition, IKKα has important roles in mammary gland development and keratinocyte differentiation (Descargues et al, 2008; Park et al, 2005). It is also responsible for cytokine-induced phosphorylation of histone H3 in HeLa cells and mouse embryo fibroblast cells (Anest et al, 2003; Yamamoto et al, 2003). However, IKKα is not recruited to the IκBα promoter in cells stimulated with lipopolysaccharide (Saccani et al, 2001). This implies that such recruitment is not a general mechanism. In cerebral ischemia, the cellular function of IKKα in response to oxidative stress is largely unknown. Which molecules interact with IKKα to promote neuronal cell death or neuroprotection? The purpose of this study was to elucidate the effect of oxidative stress in IKKα signaling after cerebral ischemia. We investigated the subcellular changes and the role of IKKα after tFCI using SOD1 knockout (KO) mice that had high levels of ROS, and examined the cellular role of IKKα in oxygen–glucose deprivation (OGD) in mouse brain endothelial cells.
Materials and methods
Focal Cerebral Ischemia
Experiments were conducted in accordance with the National Institutes of Health guidelines and were approved by the Stanford University Administrative Panel on Laboratory Animal Care. CD1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Superoxide dismutase-deficient mice, including SOD1 heterozygous (SOD1−/+) and SOD1 KO mice, designated CD1-SOD1<tm1 Cje>, were produced by Epstein and colleagues (Kondo et al, 1997). No phenotypic differences were observed among wild-type (WT) or SOD1 KO mice. Mouse genotypes were determined by a PCR of DNA obtained from tail biopsies. Adult male mice (weighing 35 to 40 g) were subjected to 30 mins of tFCI and reperfusion. An 11.0-mm 5-0 surgical monofilament nylon suture, blunted at the tip, was introduced into the left internal carotid artery through the external carotid artery stump (Kondo et al, 1997). The mice were anesthetized with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide using a facemask. Rectal temperature was controlled at 37°C using a homeothermic blanket. After 30 mins of middle cerebral artery occlusion, blood flow was restored by withdrawal of the nylon suture. Sham-operated mice underwent exposure of vessels without blood withdrawal.
Cell Culture
An immortalized mouse brain endothelial cell line, bEnd.3 (Montesano et al, 1990), was purchased from the American Type Culture Collection (Manassas, VA, USA), and the cells were grown according to the supplier's instructions in Dulbecco's modified Eagle's medium with 4.5 g/L glucose, 3.7 g/L sodium bicarbonate, 4 mmol/L glutamine, 10% fetal bovine serum, 100 Units/mL penicillin, and 100 μg/mL streptomycin. Cells were maintained in a humidified cell culture incubator at 37°C and 5% CO2/95% room air as instructed by the manufacturer. For all experiments, cells were trypsinized and seeded at a density of 0.5 to 1.0 × 104 cells per cm2 onto tissue culture-treated plastic ware.
Oxygen–Glucose Deprivation
Cultures were transferred to an anaerobic chamber (Plas Labs, Lansing, MI, USA) with an atmosphere of 5% H2 and 90% N2. The culture medium was replaced three times with deoxygenated, glucose-free balanced salt solution (BSS0), pH 7.4, containing phenol red (10 mg/L), and the following (in mmol/L): NaCl 116, CaCl2 1.8, MgSO4 0.8, KCl 5.4, NaH2PO4 1, NaHCO3 14.7, and HEPES 10. BSS5.5 that contained 5.5 mmol/L glucose. Cultures were placed in a humidified 37°C incubator within the anaerobic chamber. Oxygen tension was monitored using an oxygen electrode meter and was kept under 0.02%. Oxygen–glucose deprivation was ended by adding glucose to the culture medium to yield a final concentration of 5.5 mmol/L and returning the cultures to the normoxic incubator for the indicated time period before sampling.
Transfection of Small Interfering RNA
Both IKKα small interfering RNA (siRNA) (product name: Mm_Chuk_1 HP siRNA) and negative control siRNA were purchased from Qiagen (Valencia, CA, USA). bEnd.3 cells (3 × 105), seeded onto 60-mm plates and incubated overnight, were 50% to 60% confluent. HiPerFect transfection reagent (Qiagen) and IKKα siRNA (Qiagen) or nonfunctional negative control siRNA (Qiagen) were dissolved separately in Optimem I (Invitrogen, Carlsbad, CA, USA). After equilibration at room temperature, each siRNA solution was combined with the respective volume of the HiPerFect transfection reagent, mixed gently, and allowed to form siRNA liposome for a further 10 mins at room temperature. The transfection mixture was added to the antibiotic-free cell culture medium to a final concentration of 50 nmol/L IKKα siRNA and 12 μL HiPerFect transfection reagent. After incubating for 4 h under normal cell culture conditions, Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum was added to reach the final volume of the well for 48 h before treatment.
Immunohistochemistry
The effect of cerebral ischemia on the expression level and phosphorylation of IKKα (pIKKα) was examined by immunocytochemistry. Animals were transcardially perfused with 10 Units/mL heparin and subsequently with 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline, pH 7.4, at 1 h of reperfusion. The brains were quickly removed, postfixed for 12 h, and sectioned at 30 μm on a cryotome. To avoid nonspecific binding, the tissue was treated with 20% normal goat serum, and the sections were incubated overnight at 4°C with rabbit anti-IKKα polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-phospho-IKK (pIKK)α/β polyclonal antibody (Cell Signaling Technology, Beverly, MA, USA), and rabbit anti-pIKKα polyclonal antibody (Abcam, Cambridge, MA, USA). The sections were incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG) antibody (Vector Laboratories, Burlingame, CA, USA) for 60 mins at room temperature and were subsequently incubated with avidin–biotin–peroxidase complex (ABC-Elite Kit; Vector Laboratories) for 30 mins and then developed using 3,3-diaminobenzidine (DAB substrate kit; Vector Laboratories) as a color substrate. Methyl green was used for counterstaining. Negative control samples were run in parallel using adjacent sections incubated without a primary or a secondary antibody.
Western Blotting
Animals were decapitated after reperfusion under deep anesthesia with isoflurane (n=4). Samples of 1-mm thickness (coronal levels 3, 4, 5, 6, and 7) were obtained from the middle cerebral artery territory brain tissue on the ischemic side, including the striatum and cortex, and were quickly frozen in powdered dry ice and kept at −80°C until use. Cytosolic and nuclear fractions were prepared from the ischemic brains using ProteoExtract (EMD Chemicals, Gibbstown, NJ, USA). A lysate was run on a sodium dodecyl sulfate gel, subsequently transferred to a polyvinylidene difluoride membrane, and incubated with primary antibodies for 24 h at 4°C, and then with secondary antibodies. We used primary antibodies to IKKα (Millipore, Billerica, MA, USA), pIKKα (Abcam), pIKKα/β (Cell Signaling Technology), NF-κB-inducing kinase (NIK) (Cell Signaling Technology), phosphorylation of histone H3 (pH3) (Cell Signaling Technology), HNE (4-hydroxy-2-nonenal) (Oxis International, Beverly Hills, CA, USA), nitrotyrosine (Millipore), carbonylated protein (Millipore), transcription factor II D (TFIID) (Santa Cruz Biotechnology), and α-tubulin (Sigma-Aldrich, St Louis, MO, USA). After washing, the membrane was incubated with horseradish peroxidase-conjugated anti-mouse IgG (GE Healthcare, Piscataway, NJ, USA) or horseradish peroxidase-conjugated anti-rabbit IgG at a 1:5,000 dilution for 60 mins. The signal was then detected using a chemiluminescent kit (Thermo Scientific, Rockford, IL, USA). Signals were normalized with α-tubulin or TFIID to standardize equal protein loading. Multi-Analyst 1.0.2 software (Bio-Rad Laboratories, Hercules, CA, USA) was used for data analysis.
Lactate Dehydrogenase Assay
Cell viability after OGD was estimated by quantification of lactate dehydrogenase (LDH) release. A medium of bEnd.3 cells was sampled at 24 h of reoxygenation and was detected using a cytotoxicity detection kit (LDH Kit; Roche Diagnostics, Indianapolis, IN, USA). The percentage of death (percentage of LDH release) was calculated by dividing the experimental time points by the full-kill values × 100.
Viability Testing
Viability was tested after 4 h of OGD in mouse brain endothelial cells (bEnd.3) treated with the vehicle, scrambled siRNA, or IKKα siRNA. Cell cultures were stained with 2 μmol/L of calcein AM and 4 μmol/L of ethidium homodimer (EthD-1) using a Live/Dead Kit (Invitrogen). Calcein AM and EthD-1 fluorescence were observed through a fluorescence microscope (excitation 470 to 490 nm, dichroic mirror 505 nm, emission 470 to 490 and 590 nm). As calcein AM is converted to green fluorescence by intracellular esterase, green staining indicated metabolically active cells. The ethidium homodimer is an indicator of membrane damage and shows dead cells. The fluorescence image was obtained by × 100 magnification.
Immunoprecipitation
For immunoprecipitation, cells were lysed in a mild lysis buffer (50 mmol/L Tris-Cl pH 8.0, 150 mmol/L NaCl, 1% NP-40, 0.1 mmol/L phenylmethylsufonyl fluoride, 5 μg/mL aprotinin, and 5 μg/mL leupeptin). A total of 500 μg of the protein sample was incubated overnight at 4°C with an anti-IKKα antibody (Santa Cruz Biotechnology) and with protein G-Sepharose (GE Healthcare). The 1,000 × g pellets were washed three times and used as samples bound to each antibody. After adding the same volume of Tris-glycine sodium dodecyl sulfate sample buffer to the samples, we boiled these samples to remove the Sepharose beads. After centrifugation at 1,000 × g for 1 min, the supernatant was immunoblotted with anti-NF-κB-inducing kinase/pIKKα/pH3 (Ser10) antibody as described in the Western blot method.
Quantification and Statistical Analysis
Data are expressed as mean±s.d. Comparisons among multiple groups were performed by one-way analysis of variance with appropriate Bonferroni or Dunnet tests (Prism; GraphPad, San Diego, CA, USA). P-values <0.05 were considered statistically significant.
Results
Oxidative Stress by Cerebral Ischemia Induced Nuclear Accumulation of IκB Kinase-α After Transient Focal Cerebral Ischemia in Mice
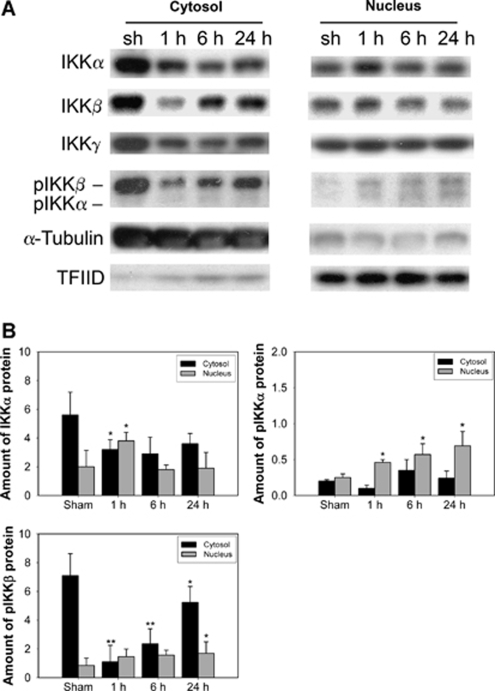
Diverse signals such as oxidative stress, cytokines, and pathogens are conducted through distinct signaling pathways that converge on the activation of IKKs. IκB kinase-β-directed phosphorylation initiates IκB phosphorylation and ubiquitination of phosphorylated IκB, resulting in rapid nuclear accumulation of NF-κB subunits. IκB kinase-α, but not IKKβ, was found to constitutively shuttle between the cytoplasm and the nucleus, implying a nuclear role for IKKα (Birbach et al, 2004). To confirm whether the subcellular localization of IKKα changes in oxidative cerebral ischemia, nuclear and cytoplasmic extracts obtained from the brain infarct after tFCI were immunoblotted with antibodies against the IKKα, IKKβ, and IKKγ subunits. Oxidative stress after 30 mins of tFCI resulted in significant nuclear accumulation of IKKα at 1 h (Figure 1A) in the mouse brains. Although both IKKβ and IKKγ were present in the nucleus and cytoplasm, they showed no significant changes in the nucleus after tFCI. Phosphorylated IKKα/β increased in the nucleus until 24 h after tFCI (Figure 1A). The purity of the nuclear and cytoplasmic fractions was confirmed with TFIID and α-tubulin antibodies, respectively (Figure 1A). The graph in Figure 1B shows the significant nuclear accumulation of IKKα as early as 1 h after tFCI, and nuclear increases in phosphorylated IKKα/β after 30 mins of tFCI. It also shows that IKKα and phosphorylated IKKα/β were distributed from the cytoplasm to the nucleus in response to oxidative stress after tFCI.
Figure 1.
Nuclear accumulation of endogenous IKKα after 30 mins of tFCI in mice. (A) IKKα, β, and γ are constitutively present in the nucleus and cytoplasm of sham-operated brains (sh). Cerebral ischemia resulted in marked nuclear accumulation of IKKα at 1 h, and pIKKα/β increased until 24 h. IKKγ exhibited no appreciable changes in the nucleus after tFCI and showed constant high levels. (B) The graphs illustrate the relative changes in the amount of IKKα, pIKKα, and pIKKβ in the cytosol and nucleus 1, 6, and 24 h, respectively, after tFCI. All data are mean±s.d. from three independent experiments (*P<0.05 compared with sham-operated mice, **P<0.01). α-Tubulin and TFIID were used as internal controls.
Higher Oxidative Stress in Copper/Zinc-Superoxide Dismutase Knockout Mice Enhanced the Phosphorylation of IκB Kinase-α and Histone H3 by NF-κB-Inducing Kinase After Transient Focal Cerebral Ischemia
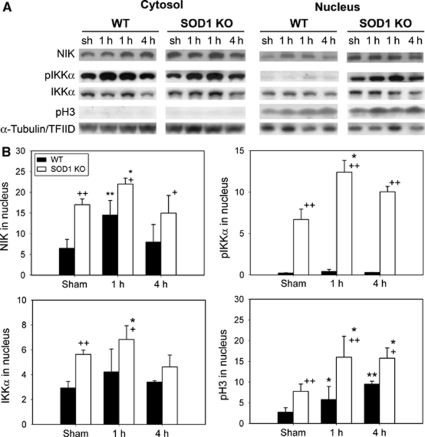
Oxidative stress after tFCI leads to a marked nuclear accumulation of IKKα. We reported on the degradation of IKKs by oxidative stress after tFCI (Song et al, 2005). Our study implies that modulation of IKKs is redox sensitive. However, how ROS relate to IKKs remains unclear. To determine the effect of ROS on IKKα after tFCI, we investigated the changes in IKKα in WT and SOD1 KO mice that had high ROS levels and increased intracellular ROS. The SOD1 KO mice showed higher levels of oxidative stress-damaged proteins, including the carbonyl group, HNE-conjugated proteins, and nitrotyrosine, compared with the WT mice 1, 6, and 24 h and 7 days, respectively, after tFCI (data not shown). Cerebral ischemia resulted in increases in pIKKα and induced nuclear accumulation of IKKα in the cytosol and nucleus of the WT mice 1 h after tFCI (Figure 2A). In the SOD1 KO mice, high levels of pIKKα were observed in the nucleus after tFCI (Figure 2A). This suggests that IKKα may have a predominant role in the nucleus. The figure shows that oxidative stress increased accumulation and phosphorylation of IKKα. Upstream regulation of IKKα by oxidative stress is not well documented in cerebral ischemia. Nuclear factor-κB-inducing kinase, an upstream kinase in the NF-κB activation pathway, preferentially phosphorylates IKKα, leading to the activation of IKKα. The physiologic role of NIK in cerebral ischemia remains unclear. Oxidative stress caused by cerebral ischemia increased the levels of NIK in the cytosol and nucleus, which were higher in SOD1 KO mice than in WT mice after tFCI. Increased histone H3 phosphorylation was also detected in accordance with increased pIKKα in the nucleus after tFCI in SOD1 KO mice (Figure 2A). Quantification confirmed that nuclear levels of NIK, pIKKα, IKKα, and pH3 were significantly higher in SOD1 KO mice than in WT mice after tFCI (Figure 2B). These results show that NIK was rapidly increased after high oxidative stress and that phosphorylated IKKα and histone H3 were in the nucleus of SOD1 KO mice after tFCI.
Figure 2.
High oxidative stress increased phosphorylation and nuclear accumulation of IKKα after 30 mins of tFCI in SOD1 KO mice. (A) In the Western blot analysis, IKKα was shown as a single band of molecular mass of 85 kDa in the cytosol and nucleus. Three strong bands that had immunoreactivity with a pIKKα antibody were identified in the nucleus. A monomer of pIKKα at 85 kDa was detected in the nucleus of WT and SOD1 KO mice after tFCI. In SOD1 KO mice, high levels of NIK, pIKKα, and pH3 were seen in the nucleus after tFCI. (B) The graphs illustrate the relative changes in the amounts of NIK, pIKKα, IKKα, and pH3 after tFCI. All data are mean±s.d. from three independent experiments (*P<0.05, **P<0.01 compared with sham-operated mice (sh), +P<0.05, ++P<0.01 compared with WT mice). α-Tubulin and TFIID were used as internal controls.
IκB Kinase was Localized in Endothelial Cells of the Ischemic Brain After Transient Focal Cerebral Ischemia
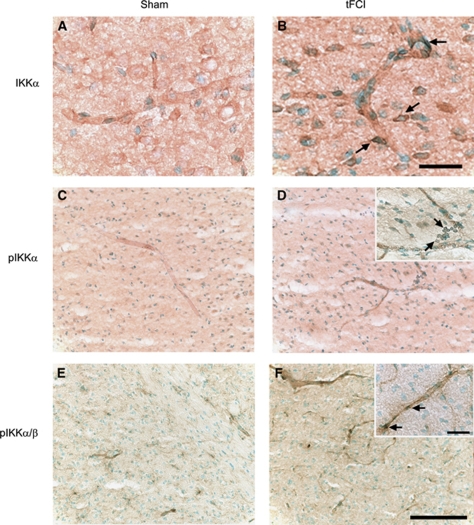
To clarify the spatial distribution of IKKα, we investigated the level of IKKα expression in mouse brains after tFCI using immunocytochemistry. The sham-manipulated animals showed weak IKKα that was predominantly cytoplasmic, with low but detectable nuclear levels in the endothelial cells (Figure 3A). Animals subjected to 30 mins of tFCI exhibited IKKα-positive immunostaining in the endothelial cells of the ischemic brains at 1 h. Levels of IKKα in the nucleus were higher (Figure 3B). Phosphorylation of both IKKα and IKKα/β was low in the unstimulated brains, but there were detectable levels in the cytosol (Figures 3C and 3E), whereas the ischemic brains showed higher levels of phosphorylation 1 h after tFCI (Figures 3D and 3F). Nuclear accumulation of phosphorylated IKKα and IKKα/β was increased 1 h after tFCI. Immunostaining with an anti-IKKα antibody and anti-pIKKα antibody showed distribution of IKKα in brain endothelial cells and confirmed the Western blot analysis.
Figure 3.
Immunohistochemistry of IKKα, pIKKα, and pIKKα/β 1 h after 30 mins of tFCI in mice. (A) Weak IKKα immunopositivity was observed in sham-operated mice. (B) Cerebral ischemia induced an increase in expression and nuclear accumulation of IKKα in endothelial cells of the infarcted brains. (C, E) Phosphorylation of IKKα and IKKα/β was detected at low levels in sham-operated mice. (D, F) After tFCI, pIKKα and pIKKα/β significantly increased in endothelial cells 1 h after 30 mins of tFCI. Scale bar=25 μm (panel B), scale bar=100 μm (panel F), and scale bar=25 μm (panel F, inset).
Nuclear IκB Kinase-α Phosphorylated Histone H3 (Ser10) After Oxygen–Glucose Deprivation in Mouse Brain Endothelial Cells
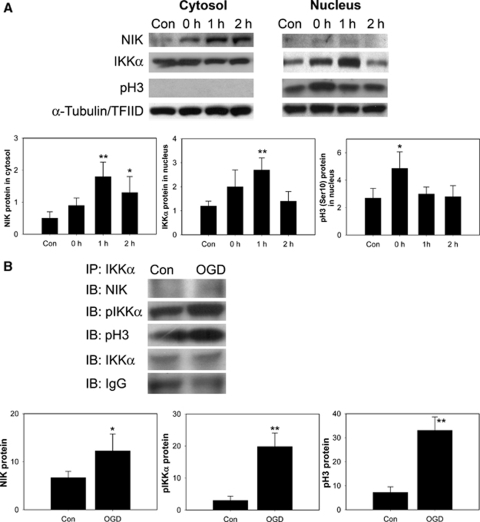
We have shown that IKKα was distributed in mouse brain endothelial cells and was increased in the nucleus of these cells after 30 mins of tFCI. To confirm the nuclear role of IKKα in response to oxidative stress, OGD was used on bEnd.3 cells. It has been reported that IKKα had histone H3 kinase activity after cytokine exposure to mouse embryo fibroblasts (Yamamoto et al, 2003). These authors suggested that IKKα was responsible for tumor necrosis factor-α-induced phosphorylation and for the subsequent acetylation in histone H3. We investigated whether IKKα might be associated with phosphorylation of histone H3 through NIK after OGD in bEnd.3 cells. Nuclear factor-κB-inducing kinase was rapidly increased in accordance with nuclear accumulation of IKKα. Phosphorylation of histone H3 (Ser10) was increased in bEnd.3 cells compared with controls (Figure 4A). This implies that IKKα, increased by OGD, and which may interact with other proteins, has a role in the IKK found in mouse cerebral endothelial cells. To confirm whether IKKα interacted with NIK and histone H3 in these bEnd.3 cells, we treated cells with 4 h of OGD and captured endogenous IKKα by immunoprecipitation with an anti-IKKα antibody. Western blot analysis showed an increase in NIK, pIKKα, and pH3 (Figure 4B). Equal amounts of IKKα and IgG were confirmed in each lane (bottom two rows). These results verify that IKKα has a role as a kinase for histone H3 through interaction with NIK in response to oxidative stress in mouse brain endothelial cells.
Figure 4.
Changes in NIK, IKKα, and pH3 (Ser10) after 4 h of OGD in mouse cerebral endothelial (bEnd.3) cells. (A) NIK, an upstream kinase of IKKα, and pH3 (Ser10) were increased compared with the controls (Con) in accordance with nuclear accumulation of IKK in bEnd.3 cells. α-Tubulin and TFIID were used as internal controls. Quantitative analysis showed the relative changes in the amount of NIK, IKKα, and pH3 (Ser10) (n=4, *P<0.05, **P<0.01 compared with controls). (B) Endogenous IKKα was captured by immunoprecipitation (IP) with an anti-IKKα antibody in the total fraction of bEnd.3 cells. Results were detected by immunoblotting (IB) using antibodies to NIK, pIKKα, and pH3. Coimmunoprecipitation analysis for pIKKα/NIK/pH3 in bEnd.3 cells showed significant increases in OGD in bEnd.3 cells compared with control cells. Input lysates showed no differences. Grouped quantitative data are presented as mean±s.d. (n=3, *P<0.05, **P<0.01).
The Role of IκB Kinase-α After Oxygen–Glucose Deprivation in Mouse Brain Endothelial Cells
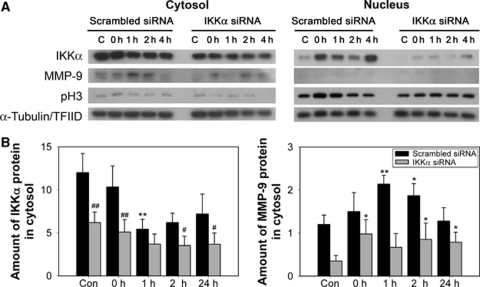
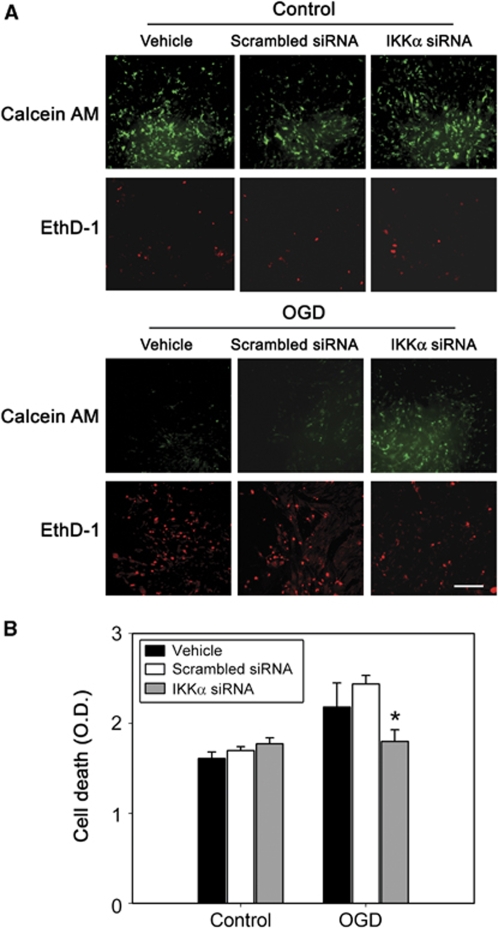
As oxidative stress such as cerebral ischemia and OGD resulted in IKKα activity for histone H3 through NIK, we investigated the physiologic role of IKKα. To determine whether inhibition of IKKα affects OGD-induced cell death, bEnd.3 cells were transfected with either a scrambled siRNA or siRNA targeting IKKα. Oxygen–glucose deprivation was terminated by adding oxygen and glucose to the medium (reperfusion). After 4 h of OGD, expression of matrix metalloproteinase-9 (MMP-9) and pH3 (Ser10) was increased compared with controls, in accordance with nuclear accumulation of IKKα in bEnd.3 cells (Figure 5A). IκB kinase-α siRNA was effective in attenuating the expression of MMP-9 and IKKα, as well as the pH3 levels after OGD in mouse cerebral endothelial cells. A quantitative analysis showed that the absence of IKKα significantly reduced the levels of proinflammatory MMP-9 after hypoxia (Figure 5B). Viability of the cells was assessed using calcein AM (green) and EthD-1 (red). Living cells were stained green, and dead cells were stained red. Most of the cells treated with scrambled siRNA or IKKα siRNA were viable before the injury (Figure 6A). After 4 h of OGD, dead cells were confirmed by EthD-1 staining in the vehicle- or scrambled siRNA-treated cells after 4 h of OGD. Cell death was reduced in IKKα siRNA-treated bEnd.3 cells after OGD. The increase in cell viability was confirmed by the increase in calcein AM cells in IKKα siRNA-treated bEnd.3 cells. The medium was then sampled for LDH release as a measure of cell injury. Four hours of OGD increased cell death in vehicle- or scrambled siRNA-treated cells (Figure 6B). Treatment with IKKα siRNA showed less cell death than did treatment with scrambled siRNA after OGD. This suggests that activation of IKKα by oxidative stress is related to cell death after OGD.
Figure 5.
Inhibition of IKKα reduced expression of proinflammatory MMP-9 after OGD in mouse cerebral endothelial cells. (A) The protein levels of IKKα, MMP-9, and pH3 in bEnd.3 cells were determined by Western blot with anti-IKKα, MMP-9, and pH3 (Ser10). Treatment of IKKα siRNA reduced the level of MMP-9 and phosphorylation of histone H3 after OGD in mouse cerebral endothelial (bEnd.3) cells. α-Tubulin and TFIID were used as internal controls. (B) Quantitative analysis showed that the absence of IKKα significantly decreased the levels of MMP-9 after OGD in bEnd.3 cells (*P<0.05, **P<0.01 compared with control, #P<0.05, ##P<0.01 compared with scrambled siRNA treatment).
Figure 6.
Viability of IKKα siRNA-treated bEnd.3 cells after OGD. (A) Viability of the cells was assessed using calcein AM (green) and EthD-1 (red). Living cells were stained green and dead cells were stained red. Upper panels: Most of the cells treated with the vehicle, scrambled siRNA, or IKKα siRNA were viable before the injury. Lower panels: After 4 h of OGD, dead cells were confirmed by EthD-1 staining in vehicle- or scrambled siRNA-treated cells after 4 h of OGD. Cell death was reduced in the IKKα siRNA-treated bEnd.3 cells after OGD. The increase in cell viability was confirmed by the increase in calcein AM cells in IKKα siRNA-treated bEnd.3 cells. Scale bar=50 μm. (B) bEnd.3 cells were transfected with IKKα siRNA and were then exposed to 4 h of OGD, followed by reperfusion until 24 h. Cell death was assessed by LDH release. Values are expressed as mean±s.d. of three different experiments (*P<0.05 compared with scrambled siRNA).
Discussion
IκB kinase-α is one of the catalytic kinases in the classic IKK complex. Unlike the effect of the other catalytic kinase (IKKβ) on IκB degradation and NF-κB in the canonical pathway, IKKα has a crucial function to facilitate NF-κB-dependent gene transcription (Ghosh and Karin, 2002; Lee and Hung, 2008; Li et al, 2002). IκB kinase activation does more than merely induce IκB degradation and NF-κB release. The IκB-independent targets of IKKα and IKKβ are in the process of being identified, revealing that activation of IKKs could result in more widespread effects on cell signaling than realized previously (Perkins, 2007). It has also been reported that IKKα requires nuclear translocation for it to function and that it is independent of NF-κB signaling (Anest et al, 2003). We showed that tFCI resulted in nuclear translocation and phosphorylation of IKKα. In cerebral ischemia, how ROS regulate IKKα is still largely unknown. Although downstream mechanisms of IKKs in oxidative stress are well documented, an understanding of their upstream molecular events remains to be elucidated. In the results of our study, higher oxidative stress in SOD1 KO mice dramatically increased nuclear translocation and phosphorylation of IKKα in accordance with increases in NIK after cerebral ischemia. In response to treatment with an endotoxin, NIK accumulated in the nuclear compartment where it had a role in the activation of IKKα (Park et al, 2006). Nuclear factor-κB-inducing kinase had both functional nuclear import and nuclear export signals, resulting in continuous shuttling between the cytoplasm and the nucleus with relatively rapid kinetics (Birbach et al, 2004), causing pIKKα and subsequent phosphorylation of histone H3 in macrophage Raw 264.7 cells (Park et al, 2006). Our results showed that NIK was increased in the nuclei of the ischemic brains and in endothelial cells after oxidative stress, even though the distribution of NIK was larger in the cytosol than in the nucleus. We suggest that oxidative stress increased the nuclear translocation of NIK from the cytosol and interacted with histone H3 and IKKα in the nucleus.
What factors convey IKKα to the nucleus? Nuclear entry depended on a nuclear localization sequence within the IKKα domain, the disruption of which prevents the induction of keratinocyte differentiation (Sil et al, 2004). Our results showed that oxidative stress increased NIK expression and stimulated nuclear translocation and phosphorylation of IKKα in cerebral ischemia. Although many new IKKα functions have recently been discovered, there is no report on how oxidative stress affects IKKα in the brain. We found that IKKα accumulated in the nuclei of mouse brain cells after cerebral ischemia. The expression and phosphorylation of IKKα, especially, were increased in brain endothelial cells after tFCI, and this confirmed the nuclear accumulation of IKKα. To clarify the nuclear role of IKKα in oxidative stress, we investigated IKKα activity for histone H3 using OGD in bEnd.3 cells. Histone H3 modification is one of the key regulatory steps essential for transcriptional activation. Stimulation of both the extracellular signal-regulated kinase pathway and the stress-activated p38 pathway also resulted in an increase in phosphorylation of histone H3 (Ser10) during activation and expression of immediate early genes, such as c-myc and c-fos (Chadee et al, 1999). Cytokine-induced phosphorylation of Ser10 by IKKα in histone H3 is important for the subsequent acetylation of Lys14 by cyclic adenosine monophosphate-response element-binding protein for NF-κB-directed gene expression (Yamamoto et al, 2003). The nuclear role of IKKα associated with oxidative stress after cerebral ischemia is not known. We showed that phosphorylation of histone H3 (Ser10) is increased in accordance with nuclear accumulation of IKKα after tFCI in mice and after OGD in mouse brain endothelial cells. It has been reported that pIKKα was detected with pH3, which showed the strong histone H3 kinase activity of phosphorylated IKKα (Park et al, 2006). Histone H3 phosphorylation was proposed to recruit and activate histone acetyl transferase in the cyclic adenosine monophosphate-response element-binding protein. Modification of specific residue in the N-terminal tails of histones served as a signal for the binding of specific coactivators, resulting in increased gene expression of hippocampal neurons (Crosio et al, 2003). Our IKKα siRNA experiments confirmed that treatment with IKKα siRNA decreased the level MMP-9 and levels of phosphorylated Ser10 histone H3. Although the immortalized mouse brain endothelial cell line bEnd.3 could not exactly reflect the status of mouse brain endothelial cells, our data show that IKKα was activated in both endothelial cells of mouse brains after tFCI and in bEnd.3 cells after OGD. For a further understanding of the molecular mechanism regulating cerebral ischemia, a study of primary cultures of brain microvessel endothelial cells would be valuable. Our data indicate that nuclear IKKα has a role in phosphorylation of Ser10 in histone H3 in brain endothelial cells after oxidative stress, resulting in cell death, which was reduced by siRNA targeting of IKKα. Targeting IKKs is common for the development of novel therapeutic interventions for many human diseases (Lee and Hung, 2008). A selective inhibitor of IKKβ has been suggested for stroke therapy, because IKKβ has a major role in neurotoxicity during cerebral ischemia (Herrmann et al, 2005). We found that nuclear translocation and phosphorylation of IKKα through NIK in response to oxidative stress resulted in the phosphorylation of histone H3 and cell death. Modulation of IKKα is a new potential therapeutic target for clinical application in stroke.
Acknowledgments
We thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance, and Elizabeth Hoyte for assistance with figures.
The authors declare no conflict of interest.
References
- Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, Hulit J, Sakamaki T, Fu M, Ben-Ze'ev A, Bromberg JF, Lamberti C, Verma U, Gaynor RB, Byers SW, Pestell RG. IKKα regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IκB kinase-α in NF-κB-dependent gene expression [Letter] Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- Birbach A, Bailey ST, Ghosh S, Schmid JA. Cytosolic, nuclear and nucleolar localization signals determine subcellular distribution and activity of the NF-κB inducing kinase NIK. J Cell Sci. 2004;117:3615–3624. doi: 10.1242/jcs.01224. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, Davie JR. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J Biol Chem. 1999;274:24914–24920. doi: 10.1074/jbc.274.35.24914. [DOI] [PubMed] [Google Scholar]
- Courtois G, Smahi A, Israël A. NEMO/IKKγ: linking NF-κB to human disease. Trends Mol Med. 2001;7:427–430. doi: 10.1016/s1471-4914(01)02154-2. [DOI] [PubMed] [Google Scholar]
- Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang X-J, Karin M. IKKα is a critical coregulator of a Smad4-independent TGFβ-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci USA. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-κB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Köhrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M. IKK mediates ischemia-induced neuronal death. Nat Med. 2005;11:1322–1329. doi: 10.1038/nm1323. [DOI] [PubMed] [Google Scholar]
- Huang W-C, Ju T-K, Hung M-C, Chen C-C. Phosphorylation of CBP by IKKα promotes cell growth by switching the binding preference of CBP from p53 to NF-κB. Mol Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Reaume AG, Huang T-T, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti C, Lin K-M, Yamamoto Y, Verma U, Verma IM, Byers S, Gaynor RB. Regulation of β-catenin function by the IκB kinases. J Biol Chem. 2001;276:42276–42286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- Lee D-F, Hung M-C. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14:5656–5662. doi: 10.1158/1078-0432.CCR-08-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Massa PE, Hanidu A, Peet GW, Aro P, Savitt A, Mische S, Li J, Marcu KB. IKKα, IKKβ, and NEMO/IKKγ are each required for the NF-κB-mediated inflammatory response program. J Biol Chem. 2002;277:45129–45140. doi: 10.1074/jbc.M205165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Pepper MS, Möhle-Steinlein U, Risau W, Wagner EF, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- Park GY, Wang X, Hu N, Pedchenko TV, Blackwell TS, Christman JW. NIK is involved in nucleosomal regulation by enhancing histone H3 phosphorylation by IKKα. J Biol Chem. 2006;281:18684–18690. doi: 10.1074/jbc.M600733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-J, Krishnan V, O'Malley BW, Yamamoto Y, Gaynor RB. Formation of an IKKα-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell. 2005;18:71–82. doi: 10.1016/j.molcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. Two waves of nuclear factor κB recruitment to target promoters. J Exp Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C. Docking IκB kinases. Nature. 1998;395:225–226. doi: 10.1038/26121. [DOI] [PubMed] [Google Scholar]
- Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IκB kinase-α acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Song YS, Lee Y-S, Chan PH. Oxidative stress transiently decreases the IKK complex (IKKα, β, and γ), an upstream component of NF-κB signaling, after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:1301–1311. doi: 10.1038/sj.jcbfm.9600123. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Verma UN, Prajapati S, Kwak Y-T, Gaynor RB. Histone H3 phosphorylation by IKK-α is critical for cytokine-induced gene expression [Letter] Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]