Abstract
Considerable evidence supports the idea that cytokines are important mediators of pathophysiologic processes within the central nervous system (CNS). Numerous studies have documented the increased production of various cytokines in the human CNS in a variety of neurological and neuropsychiatric disorders. Deciphering cytokine actions in the intact CNS has important implications for our understanding of the pathogenesis and treatment of these disorders. One approach to address this problem that has been used widely employs transgenic mice with CNS-targeted production of different cytokines. Transgenic production of cytokines in the CNS of mice allows not only for the investigation of complex cellular responses at a localized level in the intact brain, but also more closely recapitulates the expression of these mediators as found in disease states. As discussed in this review, the findings show that these transgenic animals exhibit wide-ranging structural and functional deficits that are linked to the development of distinct neuroinflammatory responses which are relatively specific for each cytokine. These cytokine-induced alterations often recapitulate those found in various human neurological disorders not only underscoring the relevance of these models but also reinforcing the clinicopathogenetic significance of cytokines in diseases of the CNS.
Keywords: central nervous system, cytokine, interferon, interleukin-6, interleukin-12, neurological disease, transgenic mouse, transforming growth factor-beta
Introduction
The central nervous system (CNS) of healthy adult mammals is relatively free of T-lymphocytes and other immunological entities. The presence of the blood-brain barrier (BBB), a paucity of immune accessory molecules (e.g. MHC class I) and professional antigen presenting cells such as dendritic cells as well as the presence of soluble mediators that suppress inflammatory and immune-mediated responses have been taken by some to suggest that the CNS is a site of immune privilege. Nevertheless, it is quite obvious that despite these barriers and regulatory signals, the development of inflammatory and/or autoimmune responses occurs within the CNS compartment and this can contribute to the pathogenesis of some significant neurological diseases such a multiple sclerosis (MS). There is now compelling evidence that cytokines have a key role in such immunoinflammatory processes where they may act both as regulators of immunity as well as mediators of altered neuronal and glial function [1, 2].
Cytokines are a large family of soluble and mostly secreted proteins that are important autocrine or paracrine regulatory factors in the host response to injury or immunological challenge. They coordinate diverse functions necessary for host protection and tissue regeneration after damage. Cytokines are important mediators in bidirectional regulatory circuits between the immune system and the CNS. Furthermore, and similar to the periphery, cytokines are also multifunctional effectors that target a variety of different cell types (“pleiotropic”) within the CNS. Production of cytokines within the CNS may arise from multiple sources including infiltrating leukocytes such as T-cells and macrophages and also from resident neural cells, particularly astrocytes and microglia. The ability of these glial cells to produce a variety of cytokines, including, IL-1α/β, IL-6, IL-10, IL-12, IFN-α, TNF-α and TGF-β [1, 3], highlights the possible existence of a cytokine network within the CNS that is analogous to that of the immune system. Within this network cytokines may influence the production of each other, either in a stimulatory (e.g. IL-1 and TNF-α can induce each other as well as IL-6) or inhibitory fashion (e.g. TGF-β or IL-10 can significantly antagonize the production of the proinflammatory cytokines). Moreover, at a functional level the actions of individual cytokines can often overlap with, as well as be unique from other cytokines, while it is also common for different cytokines to interact with each other being either synergistic or antagonistic.
Not surprisingly given the potential for such a marked biological impact, the cellular production of most cytokine in the CNS, as in other organs, is normally tightly regulated and maintained at very low or negligible levels. However, in many different pathologic states the local production of cytokines is increased significantly in the CNS. These mediators are implicated in the pathogenesis of a variety of neurological disorders (for reviews see: [1, 2, 4]) such as multiple sclerosis (MS), subacute sclerosing panencephalitis (SSPE), HIV-associated dementia (HAD), Alzheimer’s disease (AD), spongiform encephalopathy and meningoencephalitis due to microbial infection and stroke. Due to the complexity of cytokine regulation and actions whether these factors are causative in human disease or associated with beneficial or detrimental outcomes and their mechanisms of action are issues of considerable importance to clarify. The study of the pathogenesis of neurological disease in humans is clearly limited and on the whole has been restricted to postmortem tissue which may not exhibit pivotal processes that were active and contributed to lesion initiation and development. Furthermore, although with this type of approach the knowledge gained has been informative it still suffers from being largely descriptive, telling us little as to whether a particular response or factor is primary or secondary, pathogenic or protective. Therefore, much research in this arena is dependent on reliable animal models for studying specific hypotheses related to human disease processes. The use of genetically-manipulated mouse models in which the expression of specific cytokine genes is targeted to the CNS offers one approach to generating answers to some of the questions posed above. In this review article we discuss these so-called transgenic models in relation to the CNS-directed production of some specific cytokines, highlighting the utility of these models for understanding the functions of cytokines in the CNS and what these models tell us about the potential role of these mediators in human neurological diseases.
1. The multifaceted actions of IL-6 in the CNS
IL-6 belongs to a family of related cytokines that include IL-11, IL-27, leukaemia inhibitory factor (LIF), oncostatin M and ciliary neurotropic factor (CNTF). The receptors for these cytokines have the shared feature of associating with the integral membrane glycoprotein gp130. In general, these cytokines are recognised for their involvement in diverse functions in host defense, bone, muscle and cardiovascular function (reviewed in [5]). In relation to the CNS, members of this cytokine family are known to have important signaling functions in the normal developing and adult brain and in the response to brain injury and disease [6]. IL-6 is the founding member of the gp130 family and was initially discovered as a factor that induced the differentiation of B-cells. However, with time it has become abundantly clear that IL-6 influences a broad range of biological processes in many different tissues (reviewed in [7]).
The functions of IL-6 are mediated by well-defined signal transduction pathways [8, 9]. Binding of IL-6 to the IL6R induces gp130 homodimerization with activation of bound tyrosine kinases of the JAK family that then phosphorylate multiple, highly conserved tyrosine (Y) residues located on the cytoplasmic domains of gp130. This in turn triggers the recruitment of STAT1, STAT3 and SHP2 phosphatase which bind to specific phosphotyrosine sites on gp130. STAT3 and to a lesser extent STAT1, undergo JAK-mediated phosphorylation on a single tyrosine residue, dissociate from the receptor and form homo- and heterodimers that translocate to the nucleus whereupon they bind to specific DNA recognition motifs in target genes regulating transcriptional activity. JAK-mediated phosphorylation of SHP2 results in the activation of the Ras-ERK1/2/ MAPK cascade. The co-ordinate activity of the JAK/STAT and SHP2/MAPK pathways determines the nature of the cellular response to IL-6.
IL-6 likely plays the role of an important mediator of communication between the periphery and the CNS during inflammation [10]. However, IL-6 is also produced locally in the brain and has been implicated in the pathogenesis of a number of progressive neuroinflammatory disorders (reviewed in [11, 12]) including MS, AD, viral and bacterial meningitis, HAD and stroke. Numerous experimental studies point to complex effects of IL-6 in the CNS ranging from increased cellular survival and neuroprotection to the induction of neuroinflammation and subsequent neurodegeneration [11, 12].
IL-6 may well have been the first cytokine gene whose gene expression was targeted specifically to the CNS in mice using a transgenic approach [13]. In this transgenic model, expression of a murine IL-6 cDNA was targeted to astrocytes using a murine GFAP genomic vector. The astrocyte is a relevant cellular target since as stated above these cells are known to produce IL-6 in response to different stimuli. Expression of the transgene-encoded IL-6 gene was localized to astrocytes predominantly in sub-cortical regions such as the thalamus, the cerebellum and the brain stem, with highest expression occurring in the Bergmann glia of the cerebellum. Production of bioactive IL-6 and IL-6 protein also can be found in astrocyte cultures and lysates of cerebellum derived from the GFAP-IL6 mice. Furthermore, the level of transgene-encoded IL-6 mRNA in the CNS of these transgenic mice is similar to that found in EAE and thus falls within a pathophysiological range [14]. Notwithstanding the chronic nature of the production of IL-6 in the brain of these GFAP-IL6 mice, this model has proven to be valuable tool in which to study the actions of IL-6.
Neurobehavioral, neuroendocrine and neurophysiological testing in the GFAP-IL6 animals highlights significant abnormalities in brain function that correlate with the transgene dose and age of these animals. Thus, GFAP-IL6 mice exhibit a progressive age-related decline in avoidance learning performance [15]. In humans, plasma IL-6 levels increase with age and correlate with cognitive decline [16]. Studies in the GFAP-IL6 mice provided the first evidence that local production of IL-6 in the brain might be causative for the age-related decline in learning and cognitive function. Proinflammatory cytokines such as IL-6 also may be key modulators of the hypothalamic-pituitary-adrenal (HPA) axis [17]. Hypothalamic-pituitary adrenal function in the GFAP-IL6 mice is perturbed and while these animals have normal basal plasma corticosterone levels, following restraint stress, levels of this stress hormone increase markedly and well above those in wild type animals [18]. The increased corticosterone levels in the transgenic mice correlate with increased plasma arginine vasopressin (AVP) but not ACTH levels suggesting a role for AVP in the manifestation of this response. A similar mechanism may play a role in the blunted ACTH response and elevated corticosterone levels under pathophysiological conditions observed in humans with high brain levels of IL-6. The HPA axis in humans chronically treated with IL-6 [19] and in patients with various disorders associated with elevated IL-6 levels in the brain and cerebrospinal fluid, including AD, MS, and depression [20, 21], exhibit a blunting of ACTH but not cortisol responses, and the diminished ACTH responses resemble those during chronic stress [22].
As noted above GFAP-IL6 mice with high IL-6 production in the brain exhibit spontaneous seizures. Interestingly, while GFAP-IL6 mice with low production of IL-6 do not have spontaneous seizures these animals develop severe tonic-clonic seizures and die following injection of the glutamatergic excitotoxin kainic acid, at doses that produce little or no behavioral changes in wild type mice [23]. The seizure prone phenotype of the GFAP-IL6 mice is reflected by overt hippocampal excitatory pathophysiology which appears to be due to a loss of inhibitory control [24]. Additionally, in hippocampal slices from GFAP-IL6 mice long-term potentiation in the dentate is significantly reduced in comparison with WT animals [25]. Such disruption of long-term potentiation and the suppression of theta rhythm might be indicative of impaired hippocampal synaptic plasticity and could underlie the altered cognitive function observed in these transgenic mice.
A number of neurodegenerative changes are seen in the brain of the GFAP-IL6 mice that have been linked to the functional impairments noted above. In the hippocampus, dendritic vacuolization, stripping of dendritic spines, decreased synaptic density and significantly decreased numbers of parvalbumin and calbindin immunoreactive neurons is observed [13, 15]. These degenerative neuronal changes likely contribute to the hippocampal pathophysiology and learning deficits seen in the GFAP-IL6 mice. For example, the parvalbumin and calbindin containing neurons are known to be a major source of the inhibitory neurotransmitter GABA [26]. Loss or functional impairment of these cells may result in attenuated inhibitory regulation in the hippocampus and increased excitatory activity as seen in the EEG recordings from the GFAP-IL6 mice. Prominent neurodegenerative disease can also be found in the cerebellum with progressive atrophy and loss of molecular and granular layer neurons (Fig. 1B). In addition, spongiosis and marked axonal dystrophy with consequent secondary demyelination are prevalent throughout the cerebellum [13, 27]. These degenerative changes in the cerebellum likely underlie the motor abnormalities that occur in older GFAP-IL6 mice.
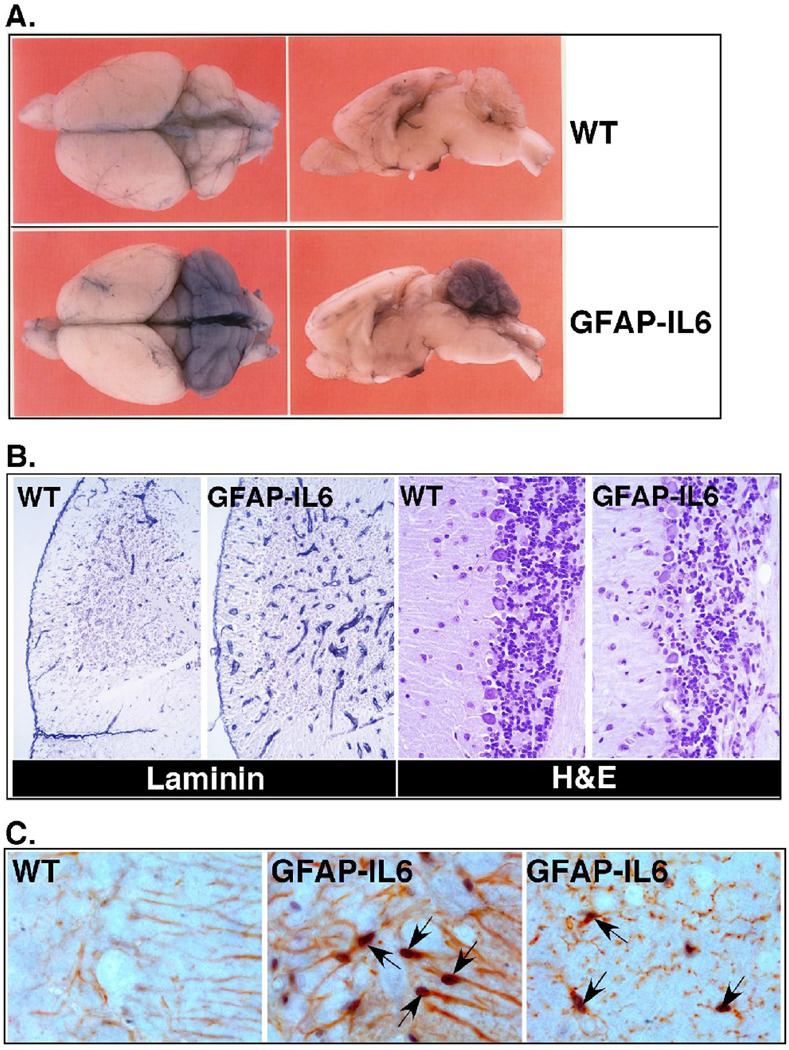
Figure 1. Phenotypic changes in the brain of GFAP-IL6 mice.
A) Intravascular Evans blue dye perfusion showing BBB leakage in the cerebellum of 3 mo old GFAP-IL6 but not WT mice. B) Histological features of the cerebellum of the GFAP-IL6 mice include the presence of increased number and more dilated blood vessels revealed by laminin staining and neurodegeneration affecting the Purkinje cell and granule cell neuron layers of the cerebellum as seen by H&E staining. C) Dual-label immunohistochemical staining for pY-STAT3 (purple) and GFAP (red; left and middle panels) and Iba-1 (red; right panel). Note strong nuclear staining for pY-STAT3 (arrows) in GFAP-IL6 but not WT specimens.
IL-6 is an important mediator in the immune system and is involved in the pathogenesis of autoimmune disease [28]. Not surprisingly the chronic production of low levels of IL-6 in the brain of the GFAP-IL6 mice induces a progressive neuroinflammatory state. Discrete perivascular accumulation of mononuclear cells can be found around some larger vessels in the cerebellar sulci and brain stem [13]. Phenotypically these perivascular mononuclear cells are made up of predominantly B220+ B-cells and/or plasma cells with smaller numbers of CD4+ T-cells. The relative contribution of these immune cells to the degenerative neuropathology is unclear and remains to be determined. In association with the neurodegenerative changes, GFAP-IL6 mice were found to have a diffuse non-proliferative gliosis with marked activation of astrocytes and microglia [13, 29]. Significantly, the level of activation of the microglia associates closely with the degree of neurodegeneration and learning impairment in the GFAP-IL6 mice [15]. How microgliosis might contribute to the development of neurodegeneration in these mice is unknown but conceivably could involve production of an array of inflammatory molecules that, together with IL-6, might mediate neuronal injury. In support of this, evidence has been obtained at the molecular level for cerebral activation of some of these inflammatory functions and includes the upregulation of the genes for the acute-phase response factors α1-antichymotrypsin, complement C3 and metallothionein I+II, expression of other cytokine genes including IL-1α, IL-1β and TNF-α [13, 30, 31]. In addition to the glia, marked changes occur to the cerebrovascular compartment in the GFAP-IL6 mice. Prominent among these is a progressive proliferative angiopathy in the cerebellum and associated breakdown of the blood-brain barrier (BBB) ([13, 32]; see also Fig. 1A and 1B). The failure of the BBB is linked to the endothelial cells not forming typical tight junctions and the maintenance of fenestrated endothelium. It is likely that this is due to the chronic proliferative state of these cells. Surprisingly, despite the gross loss of BBB integrity, the histogenesis of the cerebellum occurs normally [32]. Additional changes reflecting the chronic inflammatory state include dilation of the vessels and associated increases in a variety of vascular adhesion molecules and von Willebrand factor [13, 33]. In all, it is clear from the preceding that in the CNS the glial cells and the vascular endothelium are major responder cells to IL-6. In support of this nuclear accumulation of the key IL6/gp130 signal transduction molecule, phosphotyrosine (pY)-STAT3 occurs predominantly in astrocytes (Fig. 1C), microglia (Fig. 1C) and the vascular endothelial cells in the brain of GFAP-IL6 mice [34]. Changes to the function of these cells induced by IL-6 may therefore be a major mechanism that establishes the proinflammatory milieu of the brain and the resulting neurodegenerative phenotype.
Although the preceding discussion highlights strong, largely localized, proinflammatory and neurodegenerative changes caused by transgene-encoded IL-6 production in the CNS, studies of acute traumatic brain injury (TBI) in the GFAP-IL6 mice support a major neuroprotective function of this cytokine. A number of studies reached the same conclusion that cryolesion-induced injury in the cortex is reduced significantly in the GFAP-IL6 mice [35–37]. The basis for this protection may well be multifactorial and involve the proangiogenic effects of IL-6 resulting in more rapid revascularization of the injury site [37] and decreased oxidative stress and apoptotic cell death associated with induction of protective antioxidant molecules such as metallothionein I+II and the induction of anti-apoptotic genes [35, 36]. Intracerebral levels of IL-6 increase markedly in humans after TBI [38]. While the role of IL-6 in human TBI remains controversial, there are reports of an association between increased IL-6 and improved neurological outcome in TBI patients [39, 40]. The studies in GFAP-IL6 mice potentially offer some insight into the molecular and cellular mechanisms that might underlie any neuroprotective effects exerted by IL-6 in human TBI.
Much evidence indicates that IL-6 has a major role in the pathogenesis of a number of experimental and human autoimmune diseases including MS [41]. IL-6 in combination with TGF-β drives the development of autoreactive CD4+ Th17 T-cells that are major effector cells in various experimental autoimmune disease models [42–44]. However, IL-6 produced locally by the target tissue may also have a significant modulatory role in the development of autoimmunity [45, 46]. The GFAP-IL6 model has been used to examine the induction and evolution of an autoimmune response in a target tissue in which the microenvironment produces and is modified by IL-6. Myelin oligodendrocyte glycoprotein (MOG) immunization-induced EAE in GFAP-IL6 mice in which production of IL-6 is restricted to the cerebellum causes an atypical disease presenting as severe ataxia without the physical signs of spinal cord involvement and ascending paralysis characteristic of classical MOG-EAE [47]. Surprisingly, immune pathology and demyelination are almost completely absent from the spinal cord of MOG-immunized GFAP-IL6 mice while cerebellar immune pathology and tissue damage is markedly increased. These studies show that the local tissue production of IL-6 can target and enhance the inflammatory response directed against an autoantigen. Moreover, in the case of the GFAP-IL6 mice with EAE, the IL-6 primed cerebellum seems to generate a leukocyte “sink” that diverts trafficking and inflammation away from the normally dominant antigenic site of the spinal cord. The mechanisms underlying this process remain to be determined but likely include the preexisting production of specific cytokines and chemokines as well as the increased vascular activation and loss of integrity of the BBB in the cerebellum.
2. Tumor necrosis factor is a mediator of meningoencephalomyelitis and demyelinating disease
Like IL-6, tumor necrosis factor (TNF) is a plurifunctional cytokine involved in many facets of immune system development and function. TNF belongs to a large family of cytokines that in addition to TNF, includes lymphotoxin (LT)-α, LT-β and CD40 (reviewed in [48]). Studies of these cytokines in the CNS and their role in neuroinflammation have been limited somewhat to TNF. TNF is produced by a variety of cell types, and is synthesized in large amounts by activated macrophage/microglia. There are two biologically active forms of TNF, a 26 kDa transmembrane precursor, which is a major player in local cellular interactions and responses [49] and a 17 kDa secreted form, which is derived from the former by proteolytic enzyme cleavage-this soluble TNF plays an important role both locally and systematically [50]. TNF binds to two high affinity receptors termed TNFRp55 and TNFRp75 that are co-expressed in most tissues including the CNS [51]. Many studies have demonstrated that TNF is important in a variety of inflammatory disorders including those of the CNS as exemplified by bacterial meningitis, HAD, cerebral malaria, cerebral ischemia and MS (reviewed in [51]).
The relative significance of TNF in the pathogenesis of inflammatory CNS disease has been extensively evaluated using transgenic modelling with a variety of different transgene constructs that result in production of different forms of TNF in different cell types within the brain. These included a murine TNF transgene controlled by its own promoter [52] that was apparently expressed in glial progenitor cells [53], a human wild type or transmembrane TNF expressed in astrocytes or neurons [54] and a murine TNF gene targeted to astrocytes using the GFAP-promoter [55]. In all cases, with the exception of human transmembrane TNF produced by neurons, these different transgenic mice develop severe, progressive motor impairment, paralysis and cachexia leading to premature death. This clinical phenotype is accompanied by extensive meningoencephalomyelitis with widespread accumulation of leukocytes in the brain. In GFAP-MuTNF mice, large numbers of B-cells, CD4+ and CD8+ T-cells accumulate at predominantly perivascular sites while parenchymal lesions contain mostly CD45+ high, MHC class II+ and Mac-1+ cells of the macrophage lineage with lower numbers of neutrophils and few CD4+ and CD8+ T-cells [55]. In these latter GFAP-MuTNF mice, the development of primary and secondary demyelination and neurodegeneration follows the onset of the immunoinflammatory response in the brain. These findings suggest that the cellular injury and degeneration in this model are due primarily to TNF-induced inflammation rather than direct toxic actions of the cytokine. On the otherhand, in transgenic mice expressing murine TNF in glial progenitor cells, myelin vacuolation and oligodendrocyte apoptosis are present prior to BBB breakdown and immune cell infiltration in the CNS [53]. Thus, it is likely that some pathogenic effects of TNF in the CNS depend upon the context of its expression with both the cellular location and time of production being key factors. This latter idea is further reinforced by the observation that mice producing human transmembrane TNF in astrocytes develop severe immunoinflammatory disease of the CNS while mice producing the same transmembrane form of TNF in neurons do not [54].
The primary effect of TNF when expressed as a transgene product in the brain appears to be to stimulate the recruitment and trafficking of immunoinflammatory cells into the CNS. This process is likely facilitated by the pronounced activation of the cerebrovascular endothelium that occurs prior to immune cell infiltration [55]. However, unlike GFAP-IL12 mice (see below), the majority of lymphocytes found in the CNS of GFAP-MuTNF mice are in a relatively non-activated state and do not produce IFN-γ. Therefore, although TNF production in these transgenic mice promotes the robust recruitment of T- and B-cells to the CNS, these cells do not appear to be pathogenic. In fact, in GFAP-MuTNF mice crossed with SCID mice, which lack T- and B-cells, neurological disease is exacerbated with earlier onset of motor impairment and more extensive inflammatory lesions [55]. The lesions in the CNS of these immunodeficient GFAP-MuTNF mice are dominated by highly activated macrophage/microglia. Thus, there is a central role of macrophage/microglia in mediating destructive inflammatory disease in the CNS in response to TNF. A similar conclusion was reached from studies of EAE in a transgenic mouse model with oligodendrocyte-targeted production of TNF [56]. These mice do not develop spontaneous neurological disease, however, following induction of EAE, a more persistent disease with markedly increased macrophage/microglial reactivity is seen.
The TNF transgenic mice offer a useful tool to dissect out the relative functions of the TNFRp55 and TNRp75 receptors in mediating the actions of TNF in the CNS. A crucial role for TNFRp55 signaling in both inflammation and demyelination is demonstrated by the finding that disease is completely abrogated in transgenic mice producing soluble MuTNF in glial progenitor cells [53]. Conversely, in this same model, inflammatory demyelinating disease is unaltered by the absence of TNFRp75 indicating that this receptor does not contribute to pathogenic signaling by TNF in these mice. However, TNFRp75 signaling does appear to contribute to immune pathology in the CNS when human transmembrane TNF is targeted to astrocytes in transgenic mice in which the human TNFRp75 is overexpressed in the absence of MuTNFRp55 [57]. In this complex model system, transmembrane TNF/TNFRp75 interactions cause endothelial cell activation and the upregulation of adhesion molecules, with the development of chronic inflammation but no oligodendrocyte apoptosis or demyelination. The severity of this disease process is increased and its onset occurs earlier when TNFRp55 is introduced back into the CNS, indicating there is co-operation between the two receptors in mediating the effects of the transmembrane TNF. In all, the results of these studies highlight a proinflammatory role for TNFRp75 when signaling alone in the CNS and point to an exclusive role for TNFRp55 in TNF-mediated oligodendrocyte apoptosis and primary demyelination.
3. Interleukin-12 (IL-12) stimulates type I, cell-mediated immunity in the CNS
Interleukin-12 is a heterodimeric cytokine consisting of a p35 and a p40 subunit that has a pivotal role in innate and adaptive immunity (reviewed in [58]). IL-12 is produced by macrophages, dendritic cells and B-cells and has the primary function of promoting type I cellular immune responses. The IL-12 receptor is a heterodimer consisting of an alpha and a beta subunit that following binding of IL-12 initiates signal transduction via the JAK/STAT pathway resulting in activation of STAT4. Consistent with its function as a key mediator of cellular immunity, IL-12 stimulates naive and activated CD4- and CD8- T-cells as well as NK-cells. This stimulation leads to increased proliferation, activity and – most importantly – induces the production of IFN-γ in T- and NK-cells which is a key signature of a T helper 1 (Th1) response. The immune response of mice and men against intracellular parasites such as Leishmania and Toxoplasma gondii, bacteria, viruses, tumors, transplanted tissue and autoimmune disorders rely heavily on the actions of IL-12.
In the brain, IL-12 has been observed in the course of endotoxemia in mice and in MS-lesions of humans [59–61]. The cellular source of IL-12 in the CNS include microglia [59, 60, 62] and astrocytes [60, 63]. Studies of EAE [64] and a recent clinical trial in MS [65] found that genetic loss and neutralization of IL-12 respectively, does not alter the development or progression of disease suggesting that IL-12 does not play a crucial role in the pathogenesis of these autoimmune diseases. The strong induction of IL-12 in the brain mediated by LPS suggests that a key role of this cytokine may be in anti-microbial defence [59, 60]. In support of this, endogenous IL-12 is required for the long-term maintenance of IFN-γ-dependent resistance against intracellular pathogens such as Toxoplasma gondii and that absence of IL-12 results in a lethal encephalitis [66].
In order to further evaluate the functions of IL-12 in the CNS, transgenic mice that produce IL-12 under the transcriptional control of the GFAP promotor were established [67]. This was accomplished by the simultaneous microinjection into the germline of a GFAP-IL12p40 and a GFAP-IL12p35 transgene. At an age of 4–6 months, low expressor GFAP-IL12 transgenic mice spontaneously develop adverse clinical signs characterized by progressive ataxia, diminished body weight and muscle atrophy. In contrast to the GFAP-IL12 mice, transgenic mice that produce only the p40 subunit of IL-12 in the brain do not develop neurological disease. Histologically, severe immunoinflammatory lesions localized predominantly to areas of highest IL-12 production in the cerebellum and the overlying meninges occur in the brain of diseased GFAP-IL12 mice (Fig. 2). These lesions contain large numbers of CD4+ and CD8+ T- and NK-cells as well as numerous calcium deposits. The infiltrating T-cells present in the lesions have an activated phenotype and produce high amounts of IFN-γ, IL-1 and TNF indicating a type 1 (cell-mediated) immune response in the CNS. The meningioencephalitis findings resemble the human disorders MS and viral or chronic bacterial encephalitis. The calcium deposits on the other hand are not a usual finding in adults with these neurological disorders.
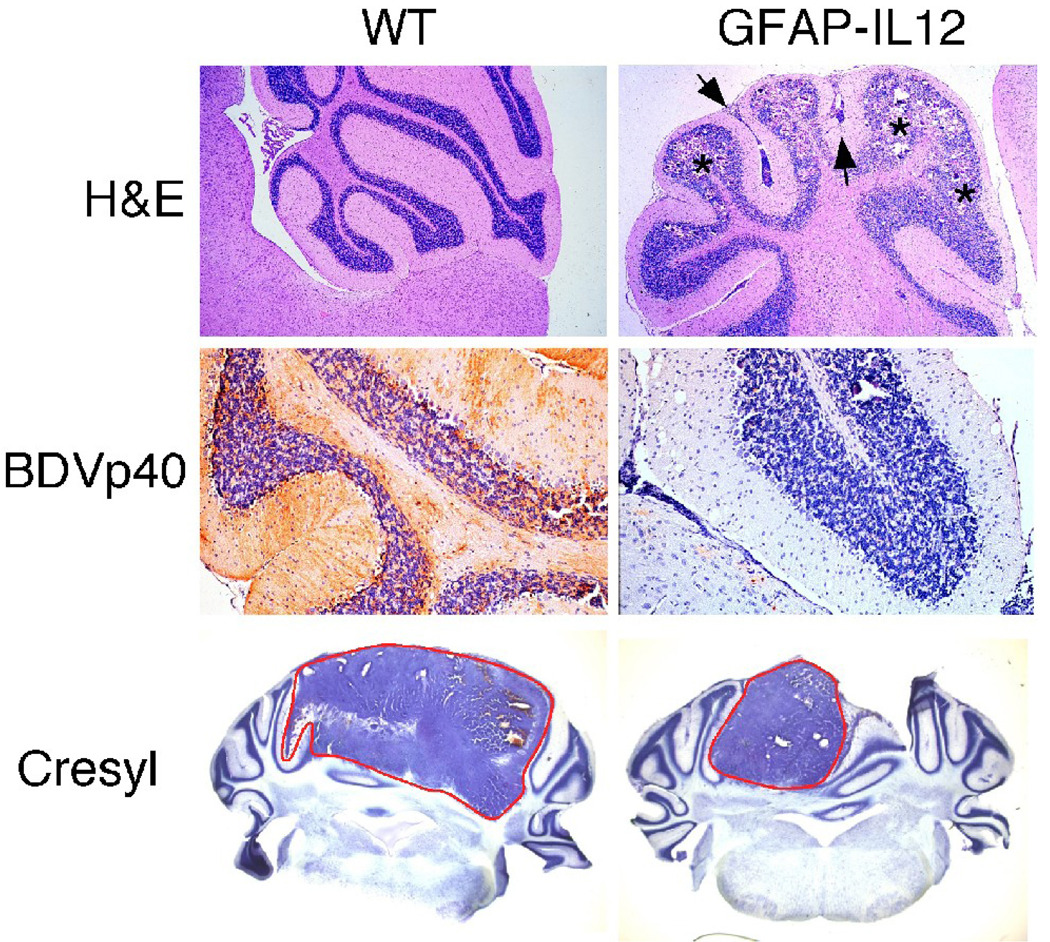
Figure 2. Histological features as well as antiviral and antitumor properties of the brain in GFAP-IL12 mice.
Top row) Routine stain shows the normal histology of a wild type (WT) cerebellum in contrast to calcifications in the granule cell layer (asterisks) and lymphocyte infiltrations in the meninges (arrows) and the parenchyma of GFAP-IL12 mice. Middle row) Immunohistochemistry for the nucleoprotein of Borna disease virus (BDVp40) revealed strong staining in all regions of the WT cerebellum while the cerebellum of GFAP-IL12 mice had only minor foci of immunoreactivity. Bottom row) In WT mice GL261 glioma cells formed large tumor masses in the cerebellum which contrasted with the findings in GFAP-IL12 mice that had smaller or no tumors.
The development of disease in the GFAP-IL12 mice depends on the presence of lymphocytes and IFN-γ, respectively. Thus, GFAP-IL12 mice with a homozygous disruption of either the RAG2 gene or the IFN-γ gene do not develop neurological disease [68]. These findings demonstrate that lymphocyte entry into the CNS is the pivotal event in the pathogenesis of neurological disease in the GFAP-IL12 mice. Lymphocytes that enter the brain in transgenic mice are stimulated by IL-12 and are induced to produce IFN-γ. The latter in turn leads to the induction of the plethora of interferon-induced genes in CNS resident as well as in immune cells that have a multitude of effects such as upregulation of transgene expression via reactive astrocytosis, upregulation of adhesion molecules on endothelial cells that leads to the enhanced recruitment of lymphocytes to the brain. Thus, a vicious cycle occurs of IL-12 production, lymphocyte entry into the CNS and IFN-γ production. CNS resident cells are not the major targets of IL-12 since the brain is unaffected in GFAP-IL12 mice that lack lymphocytes and/or IFN-γ. It is likely that CNS resident cells lack surface expression of either or both IL-12 receptor subunits and are therefore unresponsive to IL-12.
Immunization of young GFAP-IL12 mice with complete Freund’s adjuvant without a specific CNS antigen induces earlier onset and more severe disease [69]. The changes in the CFA-stimulated GFAP-IL12 mice reproduces the spontaneous disease that develops in this line thereby underlining the notion that the spontaneous disease might be triggered by inter-current peripheral immune stimuli that need not be specific for the CNS. The trafficking of lymphocytes into the brain is modulated by the immune status of the host and is increased during peripheral immune challenge [70, 71]. In addition, expression of the IL-12 receptor is known to accompany the transition of T-cells from a naïve to primed state [72, 73]. Peripheral immunization of the GFAP-IL12 mice most likely leads to an increase in the number of IL-12 receptor bearing T-cells that enter the brain and can respond to the presence of the transgene - encoded IL-12. A proposed model for the genesis of cell-mediated immunity in the CNS of the GFAP-IL12 mice is given in Fig. 3. These findings may be highly relevant for human disease since it is well known that MS bouts often occur or are exacerbated after viral or bacterial infections that would increase systemic immune activation [74–76].
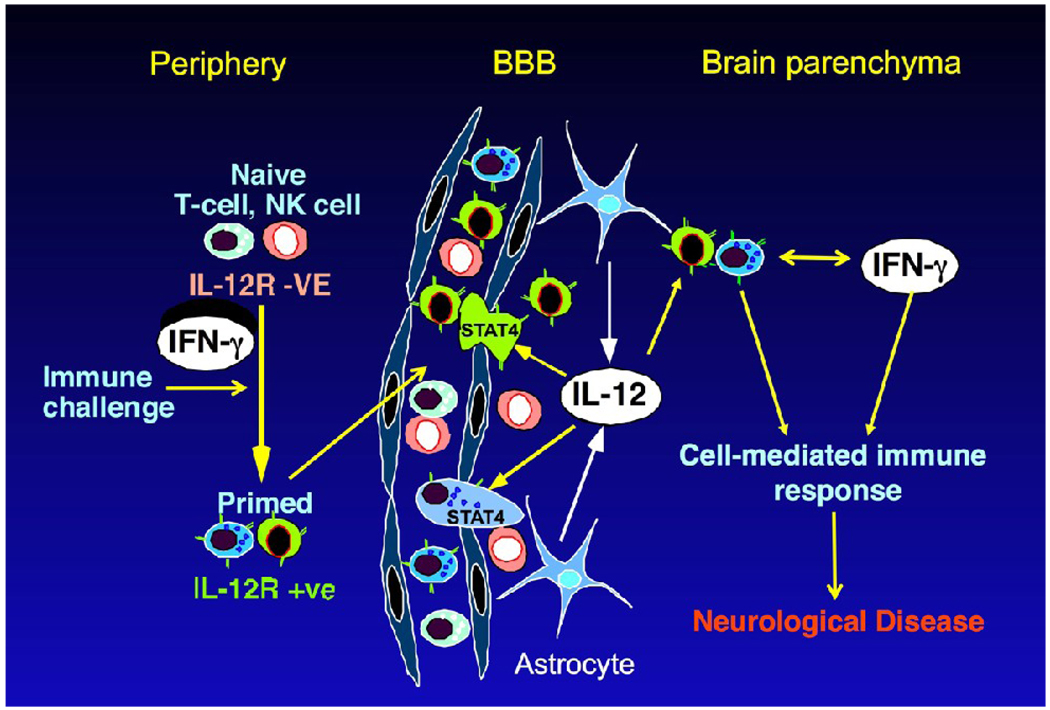
Figure 3. Model for cell-mediated immunity in CNS of GFAP-IL12 mice.
Naive T-cells in the periphery that are exposed to stimulation from immune challenge acquire surface expression of the functional IL-12 receptor (IL-12R). These cells have increased propensity for immunosurveillance of the CNS. The exposure of these immune cells to transgene produced IL-12 from the astrocytes in the CNS stimulates their differentiation and the STAT4-dependent induction of IFN-γ producing T-cells and NK cells. Continued stimulation by IL-12, localized effects of IFN-γ as well as further recruitment of immune cells fuels the cell-mediated immune response with destruction of neural tissues giving rise to neurological disease.
As indicated above the binding of IL-12 to its receptor results in the activation of STAT4, a key transcriptional inducer of the IFN-γ gene [77]. While not detectable in the brain from WT or healthy GFAP-IL12 mice, STAT4 is present in the brain of diseased GFAP-IL12 mice and localizes to the infiltrating T-cells [78]. This restricted localization of STAT4 further underscores the point that the effects of IL-12 are primarily directed at the immune cells that enter the CNS rather than neural cells. STAT1 is another member of the STAT family whose expression is increased dramatically in the brain of diseased GFAP-IL12 mice. However, in contrast to STAT4, STAT1 is localized predominantly to various neural cells including astrocytes, neurons and oligodendrocytes and at much lower levels in the infiltrating immune cells [78]. As discussed further below, STAT1 is a crucial transcriptional mediator in IFN-γ-regulated gene expression. The changes in STAT1 that occur in the brain of the GFAP-IL12 mice therefore reflect the presence and actions of IFN-γ in the brain of these animals. The strength and duration of the cellular response to IFN-γ is regulated negatively by the physiological feedback inhibitors suppressor of cytokine signaling (SOCS) 1 and SOCS3 [79, 80]. The action of these SOCS is to reduce the levels of activated STAT1 thus dampening down IFN-γ signaling and reducing the cellular response to this cytokine. The expression of both SOCS1 and SOCS3 is induced in the brain of diseased GFAP-IL12 mice and like STAT4, localized predominantly to the infiltrating mononuclear cells [78]. The restricted localization of these SOCS in the brain during the cellular immune response in the GFAP-IL12 mice helps to explain the observation that the levels of STAT1 are much lower in the infiltrating mononuclear cell population versus the neural cells. These findings also suggest that the response of the neural cells to IFN-γ may be exaggerated due to less robust negative feedback control mediated by SOCS1 and SOCS3. Similar temporal and spatial regulation of the expression of these different STAT and SOCS genes is found in the brain of mice with MOG-EAE [78]. This indicates that common regulatory processes likely modulate the cellular response in the CNS in the GFAP-IL12 mice and in MOG-EAE. The interaction between the positive (STAT) and negative (SOCS) signaling circuits and their distinct cellular locations no doubt play a defining role in coordinating the actions of IL-12 and IFN-γ during the pathogenesis of cell-mediated immune responses in the CNS.
Borna disease virus (BDV) is a neurotropic, non-cytolytic negative strand RNA virus that leads to an encephalitis in horses, sheep and other animals (reviewed in [81]). Whether BDV induces disease in humans has been the cause of hot debate. A mouse model for Borna disease has been established [82]. Neonatal mice on a H2Kk genetic background are susceptible to BDV while mice on a H2Kb background (e.g. C57BL/6 mice) are largely resistant. The induction of disease in mice depends on the recognition of the TELEISSI epitope of the BDVp40 nucleoprotein [83]. Infection of neonatal GFAP-IL12 mice that are on a H2Kb/s genetic background causes an earlier onset and a much higher incidence of the disorder that develops spontaneously in these mice, while WT mice on this background do not exhibit adverse effects [84]. In the CNS of BDV-infected GFAP-IL12 mice, there is strong upregulation of chemokine genes such as CXCL10, CCL 5 and CCL6 as well as IFN-γ, TNF and NOS-2 genes. Most importantly, transgene expressing mice show a significant, IL-12 dose-dependent reduction of BDV in the brain (Fig. 2) [84]. This effect is reproduced in vitro in cerebellar slice cultures by IFN-γ but not by IL-12 [85]. In all, these studies show that IL-12 can drive an otherwise ineffective anti-viral T-cell response in the CNS and from which IFN-γ directly inhibits viral replication and/or spread. Furthermore, in GFAP-IL12 mice bearing the H2Kk haplotype, BDVp40-specific T-cells are found almost exclusively in the areas of IL-12 expression although other CNS areas contain larger amounts of virus [68]. Thus, transgenic expression of IL-12 in one part of the CNS seems to act as kind of a lymphocyte sink to prevent other areas of the CNS that lack IL-12 from being infiltrated with anti-viral T-cells.
The specific interaction between BDV-infection and transgenic production of IL-12 in the CNS was further scrutinized in GFAP-IL12 mice deficient for either IFN-γ or RAG2 [68]. While BDV-infection does not lead to clinical disease in RAG2−/− GFAP-IL12 mice, all IFN-γ−/− GFAP-IL12 animals develop disease symptoms - although at a later time point as compared with GFAP-IL12 mice with functional IFN-γ genes. GFAP-IL12 mice lacking IFN-γ show no reduction of BDVp40 RNA and no induction of NOS-2 RNA in the cerebellum thereby confirming the crucial role of IFN-γ in inhibiting BDV replication. Upon BDV-infection, additional proinflammatory factors such as chemokines (e.g. CXCL10) are upregulated that under the influence of IL-12 may induce immune pathology in the absence of IFN-γ. Thus, while IL-12 induces inflammatory CNS-disease predominantly via IFN-γ induction, in the absence of this factor different pathways are possible. One possibility here is that BDV-infection in the GFAP-IL12 mice induces type I IFN production in the brain. This would then explain the observed increase in the chemokine CXCL10 which is known to be induced by type I IFNs.
The antitumor activity of cerebral IL-12 was examined using glioma cells of the GL261 line implanted in the cerebellum of GFAP-IL12 mice. Cerebral production of IL-12 mediates strong rejection of GL261 cells with transgenic mice exhibiting smaller tumors (Fig. 2) and better health [86]. The rejection of tumor cells in GFAP-IL12 mice is mediated predominantly by CD8+ T-cells. Surprisingly, GFAP-IL12 mice lacking IFN-γ show a similar capability for tumor rejection [86]. This finding is unexpected since a number of reports demonstrate that IFN-γ is an important mediator of IL-12 induced anti-tumor effects [87–90]. IFN-γ acts against neoplasms on several levels including anti-angiogenic effects and induction of NOS-2 that has cytotoxic effects on glioma cells in vitro [89]. However, IFN-γ can have opposite effects as well, since it can upregulate the immunosuppressive factor B7-H1 on glioma cells [91]. The actions of IFN-γ in the context of tumor immunity are not unilateral and more experiments are needed to clarify this issue.
4. Interferons - antiviral cytokines that also induce neurological disease
Interferons (IFNs) are a heterogeneous group of cytokines that have multiple functions both in the innate and adaptive immune systems (reviewed in [92, 93]). Based on biological properties, the IFNs are grouped into three families. The type I IFN family contains multiple members, including several IFN-α isoforms and a single IFN-β that are produced by various cell types. Deficiencies in type I IFN function render humans [94, 95] and mice [96, 97] highly susceptible to viral infection highlighting their integral role in host defence. In contrast, IFN-γ is the only member of the type II IFN family. IFN-γ production occurs mainly in activated T- and NK cells and is important in type 1 cell-mediated immunity (for reviews see: [98, 99]. The type III IFN family has been recently classified (reviews in [100]) and contains IFN-λ1, - λ2 and λ3 (interleukin (IL)-29, -28A and 28B, respectively). Though not as well characterized as the type I and type II IFNs, the biological activities of type III IFNs appear to be similar to the type I IFNs.
All members of the type I IFN family bind to a common receptor, termed IFNAR, whereas IFN-γ and the type III IFNs bind to their own unique receptors, termed IFNGR and IL28RA, respectively [92]. Binding of type I IFNs to the IFNAR activates receptor-associated tyrosine kinases that belong to the Janus-kinase family (Tyk2 and Jak1) [92, 101, 102]. These JAKs phosphorylate tyrosine residues in the cytoplasmic domain of the IFNAR as well as tyrosine residues of the latent cytoplasmic transcription factors signal transducer and activator of transcription (STAT) 1 and STAT2. Activated STAT1 and STAT2 form heterodimers that translocate to the nucleus and interact with a third molecule, interferon regulatory factor 9 (IRF9), forming a heterotrimeric complex. This complex, called interferon stimulated gene factor 3 (ISGF3), binds to interferon-stimulated response elements (ISREs) found in the promoter region of type I IFN regulated genes. In contrast, IFN-γ binding to the IFNGR activates JAK1 and JAK2 which phosphorylate STAT1. Phosphorylated STAT1 forms homodimers which translocate to the nucleus and bind to gamma activated sequences (GAS) found in the promoter region of type II IFN-regulated genes [98]. Similar to the type I IFNs, activation of the IL28RA induces the formation of the ISGF3 complex [100].
4.1. IFN-α
Detection of microorganisms by host pathogen-associated molecular pattern recognition receptors such as the TLRs or RIG-I induces the production of the type I IFNs IFN-α and IFN-β [103]. In the CNS, resident cells such as astrocytes and microglia can secrete considerable amounts of type I IFNs [104, 105]. Activation of IFNAR-coupled signaling pathways regulates the transcription of several hundred type I IFN-regulated genes resulting in a plethora of biological effects [106]. Beside being antiviral, type I IFNs influence metabolism, cell growth and differentiation, immune function and tumor development [92, 107]. On the other hand, type I IFNs are also implicated in the pathogenesis of several neurological diseases such as HAD and the genetic disorders Aicardi-Goutirères syndrome (AGS) and Cree encephalitis [108–110]. Intrathecal production of type I IFNs is linked to neuropsychiatric symptoms in patients with chronic viral infections or systemic lupus erythematosus [111–114]. While being a very effective drug in the therapy of some chronic viral infections and malignant tumors [115], treatment with type I IFNs often has severe neurological side-effects and may result in death [116, 117]. Therefore, a thorough understanding of the biological actions of this cytokine and of the signal pathways responsible for these actions is of considerable importance.
The impact of type I IFN production in the CNS has been studied in transgenic mice that produce IFN-α1 under the control of the GFAP-promoter (GIFN mice) [118, 119]. The GIFN mice express the transgene predominantly in the medial-ventral forebrain and the cerebellum causing a dose-dependent neurological disorder. One GIFN transgenic line with moderate levels of IFN-α develops progressive disease with seizures, weight loss and premature death. A second GIFN line, which produces much lower levels of IFN-α in the brain, exhibits behavioral changes and learning deficits [119]. Aged mice of this line develop milder signs of a neurological disease. The morphological correlates of this disease are foci of necrosis with prominent calcification in the medial-ventral forebrain and cerebellum that lead to a loss of neurons. Surrounding the necrotic foci, activation of blood vessels, reactive astrogliosis and infiltrating leukocytes are conspicuous. These cellular changes are accompanied by the markedly increased expression of characteristic type I IFN-regulated genes such as 2’5’ oligoadenylate synthetase (2’5’OAS), protein kinase R (PKR) and MHC class I, indicating that the observed effects are linked to IFN-α [118]. Importantly, the characteristic calcifications in the deep white matter and basal ganglia of GIFN mice bear a striking resemblance to the changes seen in the brains of children with AGS, congenital HIV infection or CVE [108, 120–122]. The hippocampus is particularly vulnerable to HIV [123] and infection of the CNS causes neurodegenerative changes in the hippocampus that likely contribute to the symptoms of progressive cognitive impairment and cerebral seizures [124]. Similar symptoms are also observed as side-effects of IFN-α therapy [117] or in patients with AGS [125] indicating a potential link between chronic viral infection and increased production of this cytokine in the brain. Electrophysiological studies in GIFN mice show there is neuronal hyperexcitability and dysfunction in the hippocampus which further supports this idea [119]. The morphological and functional changes may also form the basis for the seizures seen in GIFN mice with higher levels of transgene-encoded IFN-α. In summary, the chronic production of IFN-α in the CNS of GIFN mice leads to pathological changes and clinical symptoms that resemble findings from human diseases that coincide with increased cerebral production of IFN-α such as viral infections or certain genetic and autoimmune disorders such as AGS and SLE encephalopathy, respectively.
Although IFN-α has potent antiviral properties its clinical use in humans as a drug for viral encephalitis has been limited to a few cases of West Nile virus (WNV) encephalitis [126] and HIV-associated progressive multifocal leukoencephalopathy (PML) [127, 128]. In these cases IFN-α therapy was associated with increased recovery of neurologic function and prolonged survival. Concordantly, several studies show that GIFN mice are protected from infection with a variety of different classes of virus including lymphocytic choriomeningitis virus (LCMV), Herpes simplex virus (HSV) and BDV [118, 129, 130]. In all cases protection is associated with a significant decrease in viral replication and spread throughout the CNS and reduced immune pathology. These results demonstrate the important role of IFN-α in the anti-viral host defense in the brain. In addition, the studies in the GIFN mice highlight the role of IFN-α as a “double-edged sword”. While protecting against detrimental viral infections, chronic expression of IFN-α in the CNS also causes inflammation, calcification and a destructive neurological disease.
As outlined above, formation of the ISGF3 transcription factor complex is central in the cellular responses to type I IFNs. But it has been shown that other, so-called non-canonical pathways and transcription factors, are activated as well [131]. To elucidate the role of the various pathways in the context of type I IFN actions in the CNS, GIFN mice have been interbred with mice deficient for the canonical signaling molecules STAT1 [132] or STAT2 [133]. Surprisingly, GIFN mice with low IFN-α production in the brain lacking either of these canonical signaling molecules develop severe neurological disease with an early-onset age. These findings clearly demonstrate that non-canonical type I IFN signaling occurs in the CNS. Moreover a balance between ISGF3-dependent and ISGF3-independent signaling is critical to minimize the adverse effects of type I IFNs. Lack of either STAT1 or STAT2 in the GIFN mice has distinct clinical and pathophysiological effects pointing to the existence of multiple non-canonical signaling pathways.
In STAT1-deficient GIFN mice extensive tissue destruction and inflammatory lesions and higher expression of pro-inflammatory cytokines (e.g. TNF, IL-1α, IL-β, IFN-γ) are seen in the CNS. Expectedly, expression of characteristic type I IFN- regulated genes (e.g. 2’5’OAS, PKR, MHC I) was diminished in STAT1-deficient GIFN mice. This shows that non-canonical signaling pathways activated by type I IFNs can contribute to disease highlighting the fact that an equilibrium of signaling pathways is critical in programming the biological actions of the type I IFNs. Dysregulation in these signaling pathways may occur for example, following viral infection [134] and could enhance the pathophysiological actions of the type I IFNs.
In contrast to GIFN mice lacking STAT1, STAT2-deficient GIFN mice develop primitive neuroectodermal tumors in the cerebellum and die around one month of age [133]. The tumors are reminiscent of medulloblastomas which are found mainly in children where they are the most common of the malignant pediatric brain tumors [135]. Dysregulation of the Sonic Hedgehog (Shh) signaling pathway is implicated in the pathogenesis of a subgroup of medulloblastomas in children [136, 137]. Both Shh and the downstream transcriptional factor Gli1 are aberrantly expressed in the cerebellar granule neurons and tumor cells in STAT2-deficient GIFN mice. It is likely that chronic activation of this developmental pathway in progenitor granule neurons accounts for the abnormal proliferation and eventual neoplastic transformation of these cells seen in the brain of the STAT2-deficient GIFN mice [133]. In these mice, concomitant with tumor development there is a pronounced cell-mediated immune response that results in the localized production of IFN-γ. Interestingly, IFN-γ, but not IFN-α, induces the STAT1-dependent expression of the sonic hedgehog (Shh) gene in cultured cerebellar granule neurons [133]. Studies in transgenic mice with inducible expression of IFN-γ in the CNS (see below) confirm the important role of IFN-γ as a mediator of Shh gene expression. Thus, the immune system via IFN-γ is capable of markedly altering the function of a key developmental pathway in the brain resulting in tumorigenesis.
In summary, IFN-receptor signaling is clearly complex, involving the coexistence of multiple JAK/STAT as well as alternative pathways. The balance in the activity of these pathways ultimately determines the repertoire of CNS responses regulated by IFN-α. Primary signaling occurs via the ISGF3 pathway leading to the induction of ISRE-dependent genes that favor protective processes in the CNS (Fig. 4). However, a reduction or loss of signaling via the primary ISGF3 pathway results in a shift to favor the activation of non-canonical signaling pathways. The altered signaling resulting from this shift accentuates more destructive processes and increased severity of neurological disease mediated by IFN-α.
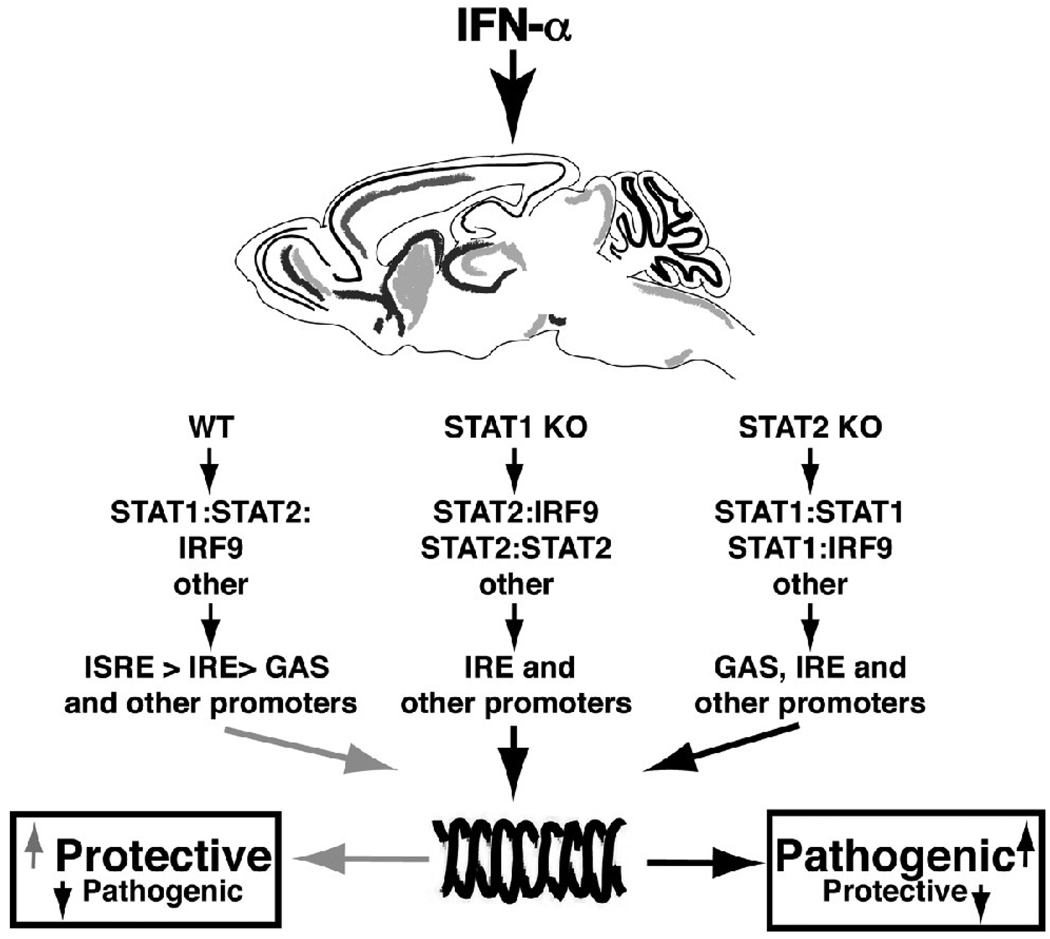
Figure 4. Model for canonical and non-canonical type I IFN signaling in glial cells.
In WT cells, IFN-α induces the formation of the ISGF3 complex that consists of STAT1:STAT2:IRF9 trimers. This results in increased expression of predominantly ISRE-regulated genes that are more protective. In the absence of STAT1 or STAT2 alternative signaling complexes are activated resulting in increased expression from GAS-, IRE- and possibly other response element- regulated genes that lead to more pathogenic effects of type I IFNs in the murine CNS.
4.2. IFN-γ
Similar to the type I IFNs, IFN-γ is a plurifunctional cytokine. Originally described as a potent antiviral cytokine like the type I IFNs, IFN-γ exerts a wide range of effects within and outside the immune system (reviewed in [98]). In the mature CNS, consistent with its restricted production to activated T- and NK-cells, IFN-γ is not present at detectable levels normally but is found in a variety of immunoinflammatory states such as following infection or in autoimmune diseases such as MS. Within the CNS there is considerable evidence that IFN-γ has a wide range of actions that affects all the major neural cells (reviewed in: [138, 139]). Such actions include alterations in the growth and differentiation of neurons and oligodendrocytes as well as modulation of the function of microglia and astrocytes.
Several groups independently developed IFN-γ-transgenic mice to study the impact of this cytokine on the CNS. In these studies the mice produced IFN-γ constitutively either under the control of the MBP-promoter in oligodendrocytes [140, 141] or under the control of the GFAP-promoter in astrocytes [142]. Progressive ataxia, shivering and tremors resulting in premature death are characteristic features of most IFN-γ transgenic mice independent of the promoter used. The disease is accompanied by extensive hypo- and demyelination in the CNS [140–142]. In addition, foamy macrophages containing myelin fragments suggestive of active demyelination are present in the CNS in some IFN-γ transgenic mice [141]. However, in general these different IFN-γ transgenic mice exhibit little or only minor leukocyte accumulation in the CNS. This suggests that developing myelin-producing oligodendrocytes are highly sensitive to IFN-γ. In support of this is data showing that overexpression of SOCS1 in IFN-γ transgenic mice ameliorates the loss of oligodendrocytes and hypomyelination [143]. This highlights the functional significance of negative feedback signaling in down regulating IFN-γ actions. In the case of oligodendrocytes, SOCS1 levels seem to be insufficient under normal circumstances to prevent the harmful effects of IFN-γ.
The mechanism by which IFN-γ can cause damage to oligodendrocytes in these transgenic mice may involve endoplasmic reticulum (ER)-stress and apoptosis [143–145]. Phosphorylation of eIF-2α by the pancreatic ER kinase (PERK), a process termed integrated stress response (ISR), is an important protective mechanism from ER-stress and has been shown to occur in a number of disorders of myelinating cells [146]. However, severe or prolonged ER stress leads to the activation of apoptosis and the eventual death of the cell. Decreased activity of the PERK-eIF2a pathway via PERK inactivation exacerbates IFN-γ– induced oligodendrocyte apoptosis and hypomyelination [145]. In addition to the harmful effects of IFN-γ on developing oligodendrocytes and axonal myelination, IFN-γ also inhibits remyelination in mice with EAE or following cuprizone-induced demyelination [147]. Similar to the observations in developing oligodendrocytes, evidence indicates that this was associated with increased ER-stress.
Another line of transgenic mice with IFN-γ production by oligodendrocytes have increased demyelination after induction of EAE [148]. These mice have no spontaneous phenotype but develop a chronic progressive form of EAE with extensive demyelination whereas non-transgenic mice had an acute self-limiting form of the disease. However, the findings of this study contrast with those from two studies that found IFN-γ protects oligodendrocytes from immune-induced damage and the CNS from subsequent demyelination in EAE [149, 150]. Surprisingly, the protective effect of IFN-γ in EAE was attributed to modest ER stress induced by IFN-γ in mature oligodendrocytes conferring resistance to EAE-induced demyelination, axonal damage, and oligodendrocyte loss [150]. The results of these EAE studies in the IFN-γ transgenic mice seemingly contradict previous findings by this group discussed above showing that ER stress induced by IFN-γ in myelinating or remyelinating oligodendrocytes is detrimental causing oligodendrocyte apoptosis and myelin abnormalities. To resolve these differences the authors propose that the outcomes of ER stress induced by IFN-γ in oligodendrocytes are determined by the developmental status of the cells. Actively myelinating oligodendrocytes respond with cell death to ER stress, while in mature oligodendrocytes this reaction is protective. The exact molecular mechanisms underlying this differential outcome to ER stress are not known. However, in contrast to mature cells developing oligodendrocytes that are in the process of (re-)myelinating axons require an immense amount of newly synthesized proteins. It is conceivable that even a short-term disruption of this process has severe consequences on various critical cellular processes resulting in apoptosis and cell death.
A developmental disorder has also been described in transgenic mice with IFN-γ production by either oligodendrocytes [140] or astrocytes [142] consisting of cerebellar dysplasia with the persistence and gross hyperplasia of the external granule layer. More recently, conditional induction of perinatal IFN-γ production by astrocytes in the CNS recapitulated these developmental abnormalities and promoted the development of medulloblastoma [151]. A direct causal relationship between IFN-γ production and the activation of the Shh signaling pathway in granular neuron cells was established [151, 152]. As discussed above, IFN-γ-mediated activation of the Shh signaling pathway in granular neuron cells leads to the autocrine stimulation of these cells, inappropriate EGL hyperplasia and formation of medulloblastoma.
5. TGF-β is a major player in the development of hydrocephalus and cerebral amyloidosis
The cytokine TGF-β was originally described as a growth factor for fibroblasts. Since then numerous studies have demonstrated a wide range of additional functions indicating that TGF-β is produced by most cell types and is involved in a multitude of biological processes including embryogenesis, carcinogenesis, cell growth, cell differentiation and activation and immunoregulation (reviewed in: [153–155]). Not surprisingly, TGF-β deficiency in mice is either embryonically or perinatally lethal. In mammals three isoforms exist (TGF-β1, -β2 and -β3) that are produced as pro-peptides and activated involving a multi-step cascade. The pro-peptide is cleaved into mature TGF-β and the latency-associated protein (LAP) that form non-covalently linked complexes. Following secretion complexed TGF-β must be liberated by a factor termed TGF-β activator (TA) in order to be able to bind to its receptor. TGF-β and TA can originate from different cell types allowing for a sophisticated regulation of TGF-β activity. Activated TGF-β is recognized by three different TGF-β receptors (TGF-βR) that cooperate in the regulation of TGF-β signaling [155]. The type III TGF-βR exists as a soluble and membrane-bound form. The soluble form acts as a decoy receptor that intercepts TGF-β before it can bind to a membrane bound receptor while the membrane-bound type III TGF-βR binds active TGF-β and passes it to the type II TGF-βR. Complexes consisting of the type II and type I TGF-βR (also called ALK-5) form an active receptor complex that functions as a serine-threonine kinase. In the primary TGF-β signaling pathway phosphorylated receptor-associated Smad2 and Smad3 form transcriptionally active complexes with Smad4 that bind to Smad DNA-binding elements (SBEs) to modulate transcription of TGF-β regulated genes. TGF-β has also been shown to stimulate the type I receptor ALK1 activating Smad1. In addition, several Smad-independent signaling pathways including the MAPK- or the PI3K kinase pathways contribute to the biological effects of TGF-β [156, 157].
The effects of TGF-β on the CNS are multifaceted and poorly understood (reviewed in [158, 159]). Most neural cell types can produce TGF-β and low levels are detectable in the CSF of healthy humans. Expression of TGF-β2 and TGF-β3 is predominantly controlled by hormonal and developmental signals indicating a more important role in physiological processes such as CNS development. In contrast, TGF-β1 expression is increased in response to various danger signals such as trauma, infection, inflammation or neurodegeneration. Following traumatic injury, increased production of TGF-β1 is observed predominantly in astrocytes and microglia. Elevated levels of TGF-β1 in the CNS or CSF are also observed in HAD, AD and in MS.
To study the effects of TGF-β on the CNS in health and disease two groups independently reported the generation of transgenic mice with astrocyte-directed production of TGF-β1 under the control of the GFAP promoter [160, 161]. Both studies report that transgenic mice with higher TGF-β1 production develop a severe spontaneous neurological disease with a communicating non-obstructive hydrocephalus. Furthermore, while transgenic mice with low levels of TGF-β1 do not develop a spontaneous disease, traumatic CNS injury also induces the development of hydrocephalus in these mice [161]. Intraventricular injection of TGF-β1 can induce hydrocephalus in mice [162] and high TGF- β1 levels in the CSF of patients with intracranial bleeding are associated with increased risk to develop a hydrocephalus [163]. Taken together, these findings clearly indicate that excessive TGF-β1 production in the CNS is involved in the development of hydrocephalus. In addition to hydrocephalus, Galbreath and colleagues [160] describe a hypoplasia of the cerebellum with a reduced number of cerebellar foliae in the spontaneously diseased TGF-β1 transgenic mice. These findings indicate that TGF-β1 might act negatively on neural and/or neuronal cell proliferation. In line with this, in vitro studies show that TGF-β1 inhibits the proliferation of neuronal progenitor cells and aged TGF-β1 transgenic mice show reduced adult neurogenesis in the hippocampus [164]. Interestingly, no differences in the total number of neurons in the hippocampus of adult wild type and TGF-β1 transgenic mice are seen, suggesting that TGF-β1 may also act as a survival factor for mature neurons. This possibility is strengthened by the observations that transgenic mice with low-levels of CNS TGF-β1 [165] or mice with adenoviral induced production of TGF-β1 in the CNS [166] are more resistant to kainic acid induced neuronal excitotoxicity. Likewise, hypoxia induced damage and infarction size is significantly reduced in mice with adenoviral expression of TGF-β1 in the CNS [167]. Conversely, three-week-old TFG-β1 deficient mice display reduced synaptogenesis in the cortex and hippocampus and increased neuronal loss in response to kainic acid [165]. Thus, TGF-β1 seemingly has both negative as well as positive roles in neuronal differentiation and neuroprotection. While the molecular mechanisms of TGF-β1-mediated neuroprotection are unclear, several studies have demonstrated that TGF-β1 decreases pro-apoptotic pathways and induces production of anti-apoptotic proteins [168, 169]. Furthermore, TGF-β has been shown to enhance neuronal survival mediated by neurotrophins and nerve growth factor [170] and to positively regulate expression of laminin which promotes neurite outgrowth [161, 165].
Transgenic mice with low levels of TGF-β1 in the CNS do not develop spontaneous hydrocephalus but show signs of a progressive cerebral angiopathy. Electron microscopy reveals that these TGF-β1 transgenic mice have a thickening of the vascular basal lamina and morphologic changes to endothelial cells [171]. These changes result in a focally reduced blood perfusion rate [172]. Importantly, these alterations precede the deposition of amyloid in the vessel walls [171]. The deposition of amyloid in vessel walls is of special interest as it is also a characteristic feature of cerebral amyloid angiopathy (CAA) in humans [173]. CAA is a common cause of spontaneous cerebral hemorrhage in aged humans and it is associated with AD [174]. Patients with AD show elevated levels of TGF-β in the affected brain areas [175]. Furthermore, TGF-β1 is more strongly expressed around cerebral vessels in AD patients with CAA than in AD patients without CAA, further supporting a role for TGF-β in CAA [176]. Interestingly, levels for the type II TGF-βR are reduced in the CNS of patients with AD [177] suggesting there may be reduced responsiveness to this cytokine. Thus, it might well be that the increased TGF-β levels in AD are in part, the consequence of reduced signaling due to decrease of the receptor. In contrast to the CNS of patients with AD, TGF-β1 transgenic mice do not develop parenchymatous amyloid plaque formation in the brain [171, 176]. Interestingly, while hAPP transgenic mice develop extensive amyloid plaque pathology, plaques are absent in hAPP-TGF-β1 transgenic mice. However, the double-transgenic mice show an accelerated and enhanced vascular amyloid deposition compared with TGF-β1 single-transgenic mice [176, 178]. These findings suggest TGF-β1 mediates amyloid clearance from the brain but conversely, promotes amyloid deposition in vessel walls thus, contributing to the development of CAA. The mechanism responsible for the clearance of the amyloid plaques from hAPP-TGF-β1 transgenic mice appears to involve an innate immune response with activation of plaque associated microglia and complement-mediated phagocytosis of amyloid [178, 179].
Increased numbers of T-cells occur in the parenchyma of TGF-β1 and hAPP-TGF-β1 double-transgenic mice following immunization with amyloid-beta peptide [180] showing that local cerebral expression of TGF-β1 increases the sensitivity of the CNS towards inflammatory insults. Supporting such a process is the finding that TGF-β1 transgenic mice develop more severe EAE with more extensive leukocyte infiltrates in the spinal cord and brain [181, 182]. Furthermore, TGF-β is expressed in the CNS of wild type mice several days before onset of EAE-symptoms or infiltration by lymphocytes and inhibition of cerebral TGF-β signaling ameliorates EAE symptoms and reduces lymphocyte infiltration into the CNS [181]. Importantly this has no effect on T-cell populations in the spleen or CNS including Th17 and Th1 CD4+ T-cells. These results indicate that cerebral production of TGF-β1 in the mouse CNS induces a state that makes the CNS more susceptible to peripheral immune responses. Localized expression of TGF-β occurs in plaque lesions of MS patients suggesting that TGF-β may have a similar role in MS [183].
Importantly, the dual nature of TGF-β1 action is also illustrated by the EAE disease model where peripheral administration of TGF-β inhibits the development of EAE [184–186] indicating that the anatomical location of TGF-β release is crucial for the outcome of its biological effects. As noted above, TGF-β in combination with IL-6 plays an important role in the differentiation of pro-inflammatory, autoreactive Th17 CD4+ T-cells that are crucial in EAE development. Mice with T-cells that are unresponsive to TGF-β do not produce Th17 T-cells and are resistant to EAE induction [44] whereas mice with increased production of TGF-β1 in T-cells develop more severe EAE [42]. These findings clearly distinguish the systemic from the localized CNS effects of TGF-β and have important implications for any attempts at therapeutic modulation of this cytokine in the treatment of diseases such as MS.
6. Concluding Comments
Targeting the expression of individual genes to the CNS in transgenic mice has provided a powerful approach to the investigation of complex cellular responses at a localized level. The results of studies in these transgenic mice have greatly increased knowledge of the fundamental mechanisms in the brain that are linked to a variety of neurological disorders that involve cytokines (summarized in Table 1). Importantly, the cerebral production of the various cytokines discussed above, induce distinct clinical, behavioral, physiological, cellular and molecular phenotypes and while there are some overlapping features, the differences are more prominent reflecting the unique actions of each cytokine. In relation to the relevance of these models to our understanding of the role of cytokines in human disease, it should be borne in mind that like all animal models, the CNS-cytokine transgenic mice discussed in this review have limitations. In this regard, the nature of the transgene-encoded cytokine production itself being consititutive and present throughout the life of the animal does not replicate the more episodic production of cytokines found in various neurological disease states in humans. Nevertheless, as highlighted here, there are remarkable overlaps between the structural and functional changes observed in the different CNS-cytokine transgenic mice and those found in various human neurodegenerative and demyelinating diseases. Such overlap reinforces the importance of the transgenic models for studying cytokine-mediated CNS inflammation and disease and solidifies the concept that locally produced cytokines have a primary role as direct effectors in the pathogenesis of CNS disease.
Table 1.
Comparative features of some CNS-cytokine transgenic mouse modelsa
| Promoter / target cell | Clinical features | Cellular changes | Infiltrating leukocytes | Human disease correlates | |
|---|---|---|---|---|---|
| IL-6 | GFAP / astrocyte | Cognitive decline, ataxia, hippocampal electrophysiological hyperexcitability, impaired HPA function | Proliferative angiopathy, BBB leakage, gliosis, inflammation, reduced neurogenesis neurodegeneration | Mild. B-cell > CD4+ T-cell > macrophage | Stroke, HIV-associated dementia, Alzheimer’s disease, traumatic injury |
| TNF | TNF / oligodendrocyte progenitor GFAP / astrocyte | Progressive motor deterioration, paralysis, wasting, premature death | Encephalomyelitis, demyelination, gliosis neurodegeneration | Florid, macrophage > CD4+ T cell> B-cell > CD8+ T cell | Multiple sclerosis, stroke, bacterial meningoencephalitis |
| IL-12 | GFAP / astrocyte | Progressive weight loss, ataxia, premature death | Meningoencephalitis, calcification, gliosis, neurodegeneration | Florid. CD4+ T-cell > CD8+ T-cell > NK-cell | Multiple Sclerosis, viral encephalitis |
| IFN-α | GFAP / astrocyte | Learning deficits, reduced activity, weight loss, seizures, premature death | Encephalitis, gliosis, multifocal calcifying necrosis with neuronal loss | Mild. CD4+ >> CD8+ T-cell > B cell > macrophage | Aicardi-Goutières Syndrome, Cree encephalitis, congenital viral encephalopathy, HIV-associated dementia |
| IFN-γ | MBP / oligodendrocyte and GFAP / astrocyte | Progressive ataxia, shivering and tremors, premature death | Hypomyelination, demyelination, macrophagocytosis, gliosis, cerebellar hyperplasia and tumor | Mild. Macrophage > T cell | Multiple sclerosis (MS) Medulloblastoma |
| TGF-β1 | GFAP / astrocyte | Moribund, premature death in high TGF-β expressing mice | Hydrocephalus (high expession), amyloid angiopathy, blood vessel fibrosis, gliosis, neuroprotection, reduced neurogenesis | Mild. T-cell | Cerebral amyloid angiopathy, Alzheimer’s disease |
Citations not included, refer to text for details.
Acknowledgements
The authors wish to acknowledge grant funding support from the National Institutes of Health (MH50426; MH62231 & NS036979), the National Health and Medical Research Council of Australia (512407) and the New South Wales Government (Spinal Cord and Related Neurological Disorders Project Grant) to ILC. MJH was supported by the Deutsche Forschungsgemeinschaft through a postdoctoral fellowship (DFG HO3298/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell NJ, Hopkins SJ. Cytokines and the nervous system II: actions and mechanisms of action. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins SJ, Rothwell NJ. Cytokines and the nervous system 1:expression and recognition. Trends Neurosci. 1995;18:83–88. [PubMed] [Google Scholar]
- 4.Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118:3557–3563. doi: 10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 6.Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- 7.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol. Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 8.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20–27. doi: 10.1111/j.1749-6632.2002.tb04198.x. [DOI] [PubMed] [Google Scholar]
- 11.Gadient RA, Otten UH. Interleukin-6 (IL-6)-A molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997;52:379–390. doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 12.Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- 13.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MBA, Mucke L. Neurologic disease induced in transgenic mice by the cerebral overexpression of interleukin 6. Proc. Natl. Acad. Sci. USA. 1993;90:10061–10065. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell IL. Structural and functional impact of the transgenic expression of cytokines in the CNS. Ann. N.Y. Acad. Sci. 1998;840:83–96. doi: 10.1111/j.1749-6632.1998.tb09552.x. [DOI] [PubMed] [Google Scholar]
- 15.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA. 1997;94:1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weaver J, Seeman T. Interleukin-6 as a predictor of cognitive function and cognitive decline. J. Am. Geriatr. Soc. 2000;48:S2–S8. [Google Scholar]
- 17.Sternberg EM. Neural-immune interactions in health and disease. J. Clin. Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raber J, O'Shea RD, Bloom FE, Campbell IL. Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J. Neurosci. 1997;17:9473–9480. doi: 10.1523/JNEUROSCI.17-24-09473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993;77:1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 20.Gold PW, Licinio J, Wong ML, Chrousos GP. Corticotropin releasing hormone in the pathophysiology of melancholic and atypical depression and in the mechanism of action of antidepressant drugs. Ann N Y Acad Sci. 1995;771:716–729. doi: 10.1111/j.1749-6632.1995.tb44723.x. [DOI] [PubMed] [Google Scholar]
- 21.Hatzinger M, Z'Brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, Heuser IJ. Hypothalamic-pituitary-adrenal system function in patients with Alzheimer's disease. Neurobiol Aging. 1995;16:205–209. doi: 10.1016/0197-4580(94)00159-6. [DOI] [PubMed] [Google Scholar]
- 22.Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- 23.Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, Campbell IL. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73:176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- 24.Steffensen SC, Campbell IL, Henriksen SJ. Site-specific hippocampal pathophysiology due to cerebral overexpression of interleukin-6 in transgenic mice. Brain Res. 1994;652:149–153. doi: 10.1016/0006-8993(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 25.Bellinger FP, Madamba SG, Campbell IL, Siggins G. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neurosci. Letts. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- 26.Celio MR. Parvalbumin in most g-aminobutyric acid-containing neurons of the rat cerebral cortex. Science. 1986;231:995–997. doi: 10.1126/science.3945815. [DOI] [PubMed] [Google Scholar]
- 27.Powell HC, Campbell IL. Ultrastructural abnormalities of glia and the blood brain barrier in a transgenic mouse overexpressing interleukin-6. Brain Pathol. 1994;4:278. [Google Scholar]
- 28.Nishimoto N. Cytokine signal regulation and autoimmune disorders. Autoimmunity. 2005;38:359–367. doi: 10.1080/08916930500124106. [DOI] [PubMed] [Google Scholar]
- 29.Chiang C-S, Stalder A, Samimi A, Campbell IL. Reactive gliosis as a consequence of interleukin-6 expression in the brain. Studies in transgenic mice. Dev. Neurosci. 1994;16:212–221. doi: 10.1159/000112109. [DOI] [PubMed] [Google Scholar]
- 30.Barnum SR, Jones JL, Muller-Ladner U, Samimi A, Campbell IL. Chronic complement C3 gene expression in the CNS of transgenic mice with astrocyte-targeted interleukin-6 expression. Glia. 1996;18:107–117. doi: 10.1002/(SICI)1098-1136(199610)18:2<107::AID-GLIA3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Carrasco J, Hernandez J, Campbell IL, Hidalgo J. Localization of metallothionein-I and-III expression in the CNS of transgenic mice with astrocyte-targeted expression of interleukin-6. Exp. Neurol. 1998;153:184–194. doi: 10.1006/exnr.1998.6861. [DOI] [PubMed] [Google Scholar]
- 32.Brett FM, Mizisin AP, Powell HC, Campbell IL. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J. Neuropathol. Exp. Neurol. 1995;54:766–775. doi: 10.1097/00005072-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Milner R, Campbell IL. Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci. 2006;33:429–440. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanz E, Hofer MJ, Unzeta M, Campbell IL. Minimal role for STAT1 in interleukin-6 signaling and actions in the murine brain. Glia. 2007;56:190–199. doi: 10.1002/glia.20602. [DOI] [PubMed] [Google Scholar]
- 35.Penkowa M, Giralt M, Lago N, Camats J, Carrasco J, Hernandez J, Molinero A, Campbell IL, Hidalgo J. Astrocyte-targeted expression of IL-6 protects the CNS against a focal brain injury. Exp Neurol. 2003;181:130–148. doi: 10.1016/s0014-4886(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 36.Quintana A, Molinero A, Borup R, Nielsen FC, Campbell IL, Penkowa M, Hidalgo J. Effect of astrocyte-targeted production of IL-6 on traumatic brain injury and its impact on the cortical transcriptome. Dev Neurobiol. 2008;68:195–208. doi: 10.1002/dneu.20584. [DOI] [PubMed] [Google Scholar]
- 37.Swartz KR, Liu F, Sewell D, Schochet T, Campbell IL, Sandor M, Fabry Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86–95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- 38.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T. Modulation of immune response by head injury. Injury. 2007;38:1392–1400. doi: 10.1016/j.injury.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Chiaretti A, Antonelli A, Mastrangelo A, Pezzotti P, Tortorolo L, Tosi F, Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25:225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- 40.Winter CD, Pringle AK, Clough GF, Church MK. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127:315–320. doi: 10.1093/brain/awh039. [DOI] [PubMed] [Google Scholar]
- 41.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendel I, Katz A, Kozak N, Ben-Nun A, Revel M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 1998;28:1727–1737. doi: 10.1002/(SICI)1521-4141(199805)28:05<1727::AID-IMMU1727>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 47.Quintana A, Muller M, Frausto RF, Ramos R, Getts DR, Sanz E, Hofer MJ, Krauthausen M, King NJ, Hidalgo J, Campbell IL. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J Immunol. 2009;183:2079–2088. doi: 10.4049/jimmunol.0900242. [DOI] [PubMed] [Google Scholar]
- 48.Ware CF. The TNF Superfamily-2008. Cytokine Growth Factor Rev. 2008;19:183–186. doi: 10.1016/j.cytogfr.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 50.Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251–258. doi: 10.1016/0092-8674(90)90158-b. [DOI] [PubMed] [Google Scholar]
- 51.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system-specific expression of tumor necrosis factorα. Proc. Natl. Acad. Sci. USA. 1995;92:11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassman H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice. Am. J. Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akassoglou K, Probert L, Kontogeorgos G, Kollias G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 1997;158:438–445. [PubMed] [Google Scholar]
- 55.Stalder AK, Carson MJ, Pagenstecher A, Asensio VC, Kincaid C, Benedict M, Powell HC, Masliah E, Campbell IL. Late-onset chronic inflammatory encephalopathy in immune-competent and severe combined immune-deficient (SCID) mice with astrocyte-targeted expression of tumor necrosis factor. Am. J. Pathol. 1998;153:767–783. doi: 10.1016/S0002-9440(10)65620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taupin V, Renno T, Bourbonniere L, Peterson AC, Rodriguez M, Owens T. Increased severity of experimental autoimmune encephalomyelitis, chronic macrophage/microglial reactivity, demyelination in transgenic mice producing tumor necrosis factor-α in the central nervous system. Eur. J. Immunol. 1997;27:905–913. doi: 10.1002/eji.1830270416. [DOI] [PubMed] [Google Scholar]
- 57.Akassoglou K, Douni E, Bauer J, Lassmann H, Kollias G, Probert L. Exclusive tumor necrosis factor (TNF) signaling by the p75TNF receptor triggers inflammatory ischemia in the CNS of transgenic mice. Proc Natl Acad Sci U S A. 2003;100:709–714. doi: 10.1073/pnas.0236046100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 59.Park J-H, Shin S-H. Induction of IL-12 gene expression in the brain in septic shock. Biochemical And Biophysical Research. 1996;224:391–396. doi: 10.1006/bbrc.1996.1038. [DOI] [PubMed] [Google Scholar]
- 60.Stalder A, Pagenstecher A, Yu NC, Kincaid C, Chiang C-S, Hobbs MV, Bloom FE, Campbell IL. Lipopolysaccharide induced interleukin-12 expression in the CNS and cultured astrocytes and microglia. J. Immunol. 1997;159:1344–1351. [PubMed] [Google Scholar]
- 61.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 1995;182:1986–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aloisi F, Penna G, Cerase J, Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- 63.Constantinescu CS, Frei K, Wysocka M, Trinchieri G, Malipiero U, Rostami A, Fontana A. Astrocytes and microglia produce interleukin-12 p40. Ann. N.Y. Acad. Sci. 1996;795:328–333. doi: 10.1111/j.1749-6632.1996.tb52684.x. [DOI] [PubMed] [Google Scholar]
- 64.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 66.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 67.Pagenstecher A, Lassmann S, Carson MJ, Kincaid CL, Stalder AK, Campbell IL. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J. Immunol. 2000;164:4481–4492. doi: 10.4049/jimmunol.164.9.4481. [DOI] [PubMed] [Google Scholar]
- 68.Hofer M, Hausmann J, Staeheli P, Pagenstecher A. Cerebral expression of interleukin-12 induces neurological disease via differential pathways and recruits antigen-specific T cells in virus-infected mice. Am J Pathol. 2004;165:949–958. doi: 10.1016/S0002-9440(10)63356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lassmann S, Kincaid C, Asensio VC, Campbell IL. Induction of type I immune pathology in the brain following immunization without central nervous system autoantigen in transgenic mice with astrocyte-targeted expression of IL-12. J. Immunol. 2001;167:5485–5493. doi: 10.4049/jimmunol.167.9.5485. [DOI] [PubMed] [Google Scholar]
- 70.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 71.Williams KC, Hickey WF. Traffic of hematogenous cells through the central nervous system. Curr. Topics Microbiol. Immunol. 1995;202:221–245. doi: 10.1007/978-3-642-79657-9_15. [DOI] [PubMed] [Google Scholar]
- 72.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann. Neurol. 1994;36:S25–S28. doi: 10.1002/ana.410360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsunoda I, Tolley ND, Theil DJ, Whitton JL, Kobayashi H, Fujinami RS. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 1999;9:481–493. doi: 10.1111/j.1750-3639.1999.tb00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy KM, Murphy TL, Szabo SJ, Jacobson NG, Guler ML, Gorham JD, Gubler U. Regulation of IL-12 receptor expression in early T-helper responses implies two phases of Th1 differentiation: capacitance and development. Chem Immunol. 1997;68:54–69. doi: 10.1159/000058694. [DOI] [PubMed] [Google Scholar]
- 78.Maier J, Kincaid C, Pagenstecher A, Campbell IL. Regulation of signal transducer and activator of transcription and suppressor of cytokine signaling gene expression in the brain of mice with astrocyte-targeted production of IL-12 and experimental autoimmune encephalomyelitis. Am. J. Pathol. 2002;160:271–288. doi: 10.1016/S0002-9440(10)64371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hilton DJ, Nicholson SE. The SOCS proteins: a new family of negative regulators of signal transduction. J. Leukoc. Biol. 1998;63:665–668. doi: 10.1002/jlb.63.6.665. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, CampbelI IL. Cytokine signaling in the brain: putting a SOCS in it? J. Neurosci. Res. 2002;67:423–427. doi: 10.1002/jnr.10145. [DOI] [PubMed] [Google Scholar]
- 81.de la Torre JC. Bornavirus and the brain. J Infect Dis. 2002;186 Suppl 2:S241–S247. doi: 10.1086/344936. [DOI] [PubMed] [Google Scholar]
- 82.Hallensleben W, Schwemmle M, Hausmann J, Stitz L, Volk B, Pagenstecher A, Staeheli P. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J Virol. 1998;72:4379–4386. doi: 10.1128/jvi.72.5.4379-4386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schamel K, Staeheli P, Hausmann J. Identification of the immunodominant H-2K(k)-restricted cytotoxic T-cell epitope in the Borna disease virus nucleoprotein. J Virol. 2001;75:8579–8588. doi: 10.1128/JVI.75.18.8579-8588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freude S, Hausmann J, Hofer M, Pham-Mitchell N, Campbell IL, Staeheli P, Pagenstecher A. Borna disease virus accelerates inflammation and disease associated with transgenic expression of interleukin-12 in the central nervous system. J Virol. 2002;76:12223–12232. doi: 10.1128/JVI.76.23.12223-12232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedl G, Hofer M, Auber B, Sauder C, Hausmann J, Staeheli P, Pagenstecher A. Borna disease virus multiplication in mouse organotypic slice cultures is site-specifically inhibited by gamma interferon but not by interleukin-12. J Virol. 2004;78:1212–1218. doi: 10.1128/JVI.78.3.1212-1218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vetter M, Hofer M, Roth E, Pircher H, Pagenstecher A. Intracerebral Interleukin 12 Induces Glioma Rejection in the Brain Predominantly by CD8+ T Cells and Independently of Interferon-γ. J Neuropath Exp Neurol. 2009;68:525–534. doi: 10.1097/NEN.0b013e3181a2afa0. [DOI] [PubMed] [Google Scholar]
- 87.Badn W, Visse E, Darabi A, Smith KE, Salford LG, Siesjo P. Postimmunization with IFN-gamma-secreting glioma cells combined with the inducible nitric oxide synthase inhibitor mercaptoethylguanidine prolongs survival of rats with intracerebral tumors. J Immunol. 2007;179:4231–4238. doi: 10.4049/jimmunol.179.6.4231. [DOI] [PubMed] [Google Scholar]
- 88.Fathallah-Shaykh HM, Zhao LJ, Kafrouni AI, Smith GM, Forman J. Gene transfer of IFN-gamma into established brain tumors represses growth by antiangiogenesis. J Immunol. 2000;164:217–222. doi: 10.4049/jimmunol.164.1.217. [DOI] [PubMed] [Google Scholar]
- 89.Kito T, Kuroda E, Yokota A, Yamashita U. Cytotoxicity in glioma cells due to interleukin-12 and interleukin-18-stimulated macrophages mediated by interferon-gamma-regulated nitric oxide. J Neurosurg. 2003;98:385–392. doi: 10.3171/jns.2003.98.2.0385. [DOI] [PubMed] [Google Scholar]
- 90.Yu C-R, Lin J-X, Fink DW, Akira S, Bloom ET, Yamauchi A. Differential utilization of janus kinase-signal transducer and activator of transcription signaling pathways in the stimulation of human natural killer cells by IL-2, IL-12, and IFN-α. J. Immunol. 1996;157:126–137. [PubMed] [Google Scholar]
- 91.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 92.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 93.Sen GC, Ransohoff RM. Interferon-induced antiviral actions and their regulation. Adv. Virus Res. 1993;42:57–102. doi: 10.1016/s0065-3527(08)60083-4. [DOI] [PubMed] [Google Scholar]
- 94.Jouanguy E, Zhang SY, Chapgier A, Sancho-Shimizu V, Puel A, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Human primary immunodeficiencies of type I interferons. Biochimie. 2007;89:878–883. doi: 10.1016/j.biochi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Levin S, Hahn T. Interferon deficiency syndrome. Clinical And Experimental Immunology. 1985;60:267–273. [PMC free article] [PubMed] [Google Scholar]
- 96.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 97.van den Broek MF, Muller U, Huang S, Aguet M, ZRM Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J. Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boehm U, KT, Groot M, Howard JC. Cellular responses to interferon-gamma. Ann. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 99.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 100.Ank N, West H, Paludan SR. IFN-lambda: novel antiviral cytokines. J Interferon Cytokine Res. 2006;26:373–379. doi: 10.1089/jir.2006.26.373. [DOI] [PubMed] [Google Scholar]
- 101.Schindler C, Strehlow I. Cytokines and STAT signaling. Adv. Pharmacol. 2000;47:113–174. doi: 10.1016/s1054-3589(08)60111-8. [DOI] [PubMed] [Google Scholar]
- 102.Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Ann. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 103.Alsharifi M, Mullbacher A, Regner M. Interferon type I responses in primary and secondary infections. Immunol Cell Biol. 2008;86:239–245. doi: 10.1038/sj.icb.7100159. [DOI] [PubMed] [Google Scholar]
- 104.Akiyama H, Ikeda K, Katoh M, McGeer EG, McGeer PL. Expression of MRP14, 27E10, interferon-α and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J. Neuroimmunol. 1994;50:195–201. doi: 10.1016/0165-5728(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 105.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams RG. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 107.Belardelli F, Gresser I. The neglected role of type 1 interferon in the T-cell response: implications for its clinical use. Immunol. Today. 1996;17:369–372. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 108.Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109. [DOI] [PubMed] [Google Scholar]
- 109.Black DN, Watters GV, Andermann E, Dumont C, Kabay ME, Kaplan P, Meagher-Villemure K, Michaud J, O'Gorman G, Reece E, Tsoukas C, Wainberg MA. Encephalitis among Cree children in Northern Quebec. Ann. Neurol. 1988;24:483–489. doi: 10.1002/ana.410240402. [DOI] [PubMed] [Google Scholar]
- 110.Rho MB, Wesselingh S, Glass JD, McArthur JC, Choi S, Griffin J, Tyor WR. A potential role for interferon-α in the pathogenesis of HIV-associated dementia. Brain Behav. Immun. 1995;9:366–377. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- 111.Dussaix E, Lebon P, Ponsot G, Huault G, Tardieu M. Intrathecal sythesis of different alpha-interferons in patients with various neurological diseases. Acta Neurol. Scand. 1985;7:504–509. doi: 10.1111/j.1600-0404.1985.tb03235.x. [DOI] [PubMed] [Google Scholar]
- 112.Lebon P, Badoual J, Ponsot G, Goutieres F, Hemeury-Cukier F, Aicardi J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J. Neurol. Sci. 1988;84:201–208. doi: 10.1016/0022-510x(88)90125-6. [DOI] [PubMed] [Google Scholar]
- 113.Shiozawa S, Kuroki Y, Kim M, Hirohata S, Ogino T. Interferon-α in lupus psychosis. Arthritis Rheum. 1992;35:417–422. doi: 10.1002/art.1780350410. [DOI] [PubMed] [Google Scholar]
- 114.Winfield JB, Shaw M, Silverman LM, Eisenberg RA, Wilson HA, 3rd, Koffler D. Intrathecal IgG synthesis and blood-brain barrier impairment in patients with systemic lupus erythematosus and central nervous system dysfunction. Am J Med. 1983;74:837–844. doi: 10.1016/0002-9343(83)91075-6. [DOI] [PubMed] [Google Scholar]
- 115.Gutterman JU. Cytokine therapeutics: Lessons from interferon α. Proc. Natl. Acad. Sci. USA. 1994;91:1198–1205. doi: 10.1073/pnas.91.4.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bocci V. Central nervous system toxicity of interferons and other cytokines. J. Biol. Regulators Homeostat. Agents. 1988;2:107–118. [PubMed] [Google Scholar]
- 117.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 118.Akwa Y, Hassett DE, Eloranta ML, Sandberg K, Masliah E, Powell H, Whitton JL, Bloom FE, Campbell IL. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 1998;161:5016–5026. [PubMed] [Google Scholar]
- 119.Campbell IL, Krucker T, Steffensen S, Akwa Y, Powell HC, Lane T, Carr D, Gold LH, Henriksen SJ, Siggins GR. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res. 1999;835:46–61. doi: 10.1016/s0006-8993(99)01328-1. [DOI] [PubMed] [Google Scholar]
- 120.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 121.Price DB, Inglese CM, Jacobs J, Haller JO, Kramer J, Hotson GC, Loh JP, Schlusselberg D, Menez-Bautista R, Rose AL, et al. Pediatric AIDS. Neuroradiologic and neurodevelopmental findings. Pediatr Radiol. 1988;18:445–448. doi: 10.1007/BF00974074. [DOI] [PubMed] [Google Scholar]
- 122.Shaw DW, Cohen WA. Viral infections of the CNS in children: imaging features. AJR Am J Roentgenol. 1993;160:125–133. doi: 10.2214/ajr.160.1.8416608. [DOI] [PubMed] [Google Scholar]
- 123.Brenneman DE, McCune SK, Mervis RF, Hill JM. gp120 as an etiologic agent for NeuroAIDS: neurotoxicity and model systems. Advances in neuroimmunology. 1994;4:157–165. doi: 10.1016/s0960-5428(06)80252-4. [DOI] [PubMed] [Google Scholar]
- 124.Belman AL, Ultmann MH, Horoupian D, Novick B, Spiro AJ, Rubinstein A, Kurtzberg D, Cone-Wesson B. Neurological complications in infants and children with acquired immune deficiency syndrome. Annals Of Neurology. 1985;18:560–566. doi: 10.1002/ana.410180509. [DOI] [PubMed] [Google Scholar]
- 125.Aicardi J. Aicardi-Goutieres syndrome: special type early-onset encephalopathy. Eur. J. Paediatr. Neurol. 2002;6 Suppl A:A1–A7. doi: 10.1053/ejpn.2002.0567. [DOI] [PubMed] [Google Scholar]
- 126.Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, Fayad P, Freifeld AG. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin Infect Dis. 2005;40:764–766. doi: 10.1086/427945. [DOI] [PubMed] [Google Scholar]
- 127.Huang SS, Skolasky RL, Dal Pan GJ, Royal W, 3rd, McArthur JC. Survival prolongation in HIV-associated progressive multifocal leukoencephalopathy treated with alpha-interferon: an observational study. J Neurovirol. 1998;4:324–332. doi: 10.3109/13550289809114533. [DOI] [PubMed] [Google Scholar]
- 128.Steiger MJ, Tarnesby G, Gabe S, McLaughlin J, Schapira AH. Successful outcome of progressive multifocal leukoencephalopathy with cytarabine and interferon. Annals Of Neurology. 1993;33:407–411. doi: 10.1002/ana.410330415. [DOI] [PubMed] [Google Scholar]
- 129.Carr DJ, Veress LA, Noisakran S, Campbell IL. Astrocyte-targeted expression of IFN-alpha1 protects mice from acute ocular herpes simplex virus type 1 infection. J. Immunol. 1998;161:4859–4865. [PubMed] [Google Scholar]
- 130.Staeheli P, Sentandreu M, Pagenstecher A, Hausmann J. Alpha/beta interferon promotes transcription and inhibits replication of borna disease virus in persistently infected cells. J. Virol. 2001;75:8216–8223. doi: 10.1128/JVI.75.17.8216-8223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 132.Wang J, Schreiber RD, Campbell IL. STAT1 deficiency unexpectedly and markedly exacerbates the pathophysiological actions of IFN-α in the central nervous system. Proc. Natl. Acad. Sci. USA. 2002;99:16209–16214. doi: 10.1073/pnas.252454799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang J, Pham-Mitchell N, Schindler C, Campbell IL. Dysregulated sonic hedgehog signaling and medulloblastoma consequent to IFN-α-stimulated STAT2-independent production of IFN-γ in the brain. J. Clin. Invest. 2003;112:535–543. doi: 10.1172/JCI18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 135.Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 136.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature Rev. Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 137.Wechsler-Reya R, Scott MP. The developmental biology of brain tumors. Ann. Rev. Neurosci. 2001;24:385–428. doi: 10.1146/annurev.neuro.24.1.385. [DOI] [PubMed] [Google Scholar]
- 138.Chesler DA, Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13:441–454. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 139.Popko B, Corbin JG, Baerwald KD, Dupree J, Garcia AM. The effects of interferon-gamma on the central nervous system. Mol. Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, Popko B. Targeted CNS expression of interferon-γ in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol. Cell. Neurosci. 1996;7:354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- 141.Horwitz MS, Evans CF, McGavern DB, Rodriguez M, Oldstone MBA. Primary demyelination in transgenic mice expressing IFN-γ. Nature Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-targeted expression of interferon-γ. J. Mol. Neurosci. 2000;15:45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- 143.Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci. 2006;26:5143–5152. doi: 10.1523/JNEUROSCI.0737-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baerwald KD, Popko B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J Neurosci Res. 1998;52:230–239. doi: 10.1002/(SICI)1097-4547(19980415)52:2<230::AID-JNR11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 145.Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin W, Popko B. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 2009;12:379–385. doi: 10.1038/nn.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 148.Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, Rodriguez M, Peterson A, Owens T. Interferon-γ in progression to chronic demyelination and neurological deficit following acute EAE. Mol. Cell Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- 149.Balabanov R, Strand K, Goswami R, McMahon E, Begolka W, Miller SD, Popko B. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:2013–2024. doi: 10.1523/JNEUROSCI.4689-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lin W, Kemper A, McCarthy KD, Pytel P, Wang J, Campbell IL, Utset MF, Popko B. Interferon-γ-induced medulloblastoma in the developing cerebellum. J. Neurosci. 2004;10:10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang J, Lin W, Popko B, CampbelI IL. Inducible production of interferon-γ in the developing brain causes cerebellar dysplasia with the activation of the sonic hedgehog pathway. Mol. Cell. Neurosci. 2004;27:489–496. doi: 10.1016/j.mcn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 153.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 154.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 156.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 157.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Finch CE, Laping NJ, Morgan TE, Nichols NR, Pasinetti GM. TGF-beta 1 is an organizer of responses to neurodegeneration. J. Cell. Biochem. 1993;53:314–322. doi: 10.1002/jcb.240530408. [DOI] [PubMed] [Google Scholar]
- 159.Pratt BM, McPherson JM. TGF-beta in the central nervous system: potential roles in ischemic injury and neurodegenerative diseases. Cytokine Growth Factor Rev. 1997;8:267–292. doi: 10.1016/s1359-6101(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 160.Galbreath E, Kim S-J, Park K, Brenner M, Messing A. Overexpression of TGF-β1 in the central nervous system of transgenic mice results in hydrocephalus. J. Neuropathol. Exp. Neurol. 1995;54:339–349. doi: 10.1097/00005072-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 161.Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-β1. Am. J. Pathol. 1995;147:53–67. [PMC free article] [PubMed] [Google Scholar]
- 162.Tada T, Kanaji M, Kobayashi S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. J Neuroimmunol. 1994;50:153–158. doi: 10.1016/0165-5728(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 163.Kitazawa K, Tada T. Elevation of transforming growth factor-beta 1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke. 1994;25:1400–1404. doi: 10.1161/01.str.25.7.1400. [DOI] [PubMed] [Google Scholar]
- 164.Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169:154–164. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40:1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 166.Boche D, Cunningham C, Gauldie J, Perry VH. Transforming growth factor-beta 1-mediated neuroprotection against excitotoxic injury in vivo. J Cereb Blood Flow Metab. 2003;23:1174–1182. doi: 10.1097/01.WCB.0000090080.64176.44. [DOI] [PubMed] [Google Scholar]
- 167.Pang L, Ye W, Che XM, Roessler BJ, Betz AL, Yang GY. Reduction of inflammatory response in the mouse brain with adenoviral-mediated transforming growth factor-ss1 expression. Stroke. 2001;32:544–552. doi: 10.1161/01.str.32.2.544. [DOI] [PubMed] [Google Scholar]
- 168.Prehn JH, Bindokas VP, Marcuccilli CJ, Krajewski S, Reed JC, Miller RJ. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type beta confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci U S A. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J. Transforming growth factor-beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci. 2002;22:3839–3909. doi: 10.1523/JNEUROSCI.22-10-03898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krieglstein K, Farkas L, Unsicker K. TGF-beta regulates the survival of ciliary ganglionic neurons synergistically with ciliary neurotrophic factor and neurotrophins. J Neurobiol. 1998;37:563–572. [PubMed] [Google Scholar]
- 171.Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gaertner RF, Wyss-Coray T, Von Euw D, Lesne S, Vivien D, Lacombe P. Reduced brain tissue perfusion in TGF-beta 1 transgenic mice showing Alzheimer's disease-like cerebrovascular abnormalities. Neurobiol Dis. 2005;19:38–46. doi: 10.1016/j.nbd.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 173.Love S, Miners S, Palmer J, Chalmers K, Kehoe P. Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Front Biosci. 2009;14:4778–4792. doi: 10.2741/3567. [DOI] [PubMed] [Google Scholar]
- 174.Smith EE, Greenberg SM. Beta-amyloid, blood vessels, and brain function. Stroke. 2009;40:2601–2606. doi: 10.1161/STROKEAHA.108.536839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Masliah E, Ho G, Wyss-Coray T. Functional role of TGF beta in Alzheimer's disease microvascular injury: lessons from transgenic mice. Neurochem Int. 2001;39:393–400. doi: 10.1016/s0197-0186(01)00046-8. [DOI] [PubMed] [Google Scholar]
- 176.Wyss-Coray T, Masliah E, Mallory M, McConologue L, Johnson-Wood K, Lin C, Mucke L. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer's disease. Nature. 1997;389:603–606. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 177.Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, Mucke L, Masliah E, Wyss-Coray T. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006;116:3060–3069. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wyss-Coray T, Lin C, Yan F, Yu G-Q, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 179.Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci U S A. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buckwalter MS, Coleman BS, Buttini M, Barbour R, Schenk D, Games D, Seubert P, Wyss-Coray T. Increased T cell recruitment to the CNS after amyloid beta 1–42 immunization in Alzheimer's mice overproducing transforming growth factor-beta 1. J Neurosci. 2006;26:11437–11441. doi: 10.1523/JNEUROSCI.2436-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Luo J, Ho PP, Buckwalter MS, Hsu T, Lee LY, Zhang H, Kim DK, Kim SJ, Gambhir SS, Steinman L, Wyss-Coray T. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-β1 enhances inflammatory central nervous system disease in transgenic mice. J. Neuroimmunol. 1997;77:45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 183.De Groot CJ, Montagne L, Barten AD, Sminia P, Van Der Valk P. Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol. 1999;58:174–187. doi: 10.1097/00005072-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 184.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino MA, Thorbecke GJ. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J. Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 186.Santambrogio L, Hochwald GM, Saxena B, Leu CH, Martz JE, Carlino JA, Ruddle NH, Palladino MA, Gold LI, Thorbecke GJ. Studies on the mechanisms by which transforming growth factor-beta (TGF-beta) protects against allergic encephalomyelitis. Antagonism between TGF-beta and tumor necrosis factor. J Immunol. 1993;151:1116–1127. [PubMed] [Google Scholar]