Abstract
Dithiolethiones are a family of promising cancer chemopreventive agents, and induction of Phase 2 enzymes is key to their chemopreventive activities. Two dithiolethiones have been evaluated in humans for cancer prevention. While some chemopreventive activities were detected in several human studies, potential side effects are a concern. Herein, we report structure-activity relationships of 25 dithiolethiones. Several compounds show exceedingly potent and bladder specific activity in Phase 2 enzyme induction. Structural features responsible for such activity, as well as those inhibiting the activity, are discussed. Moreover, the compounds activate and depend on Nrf2 for their inductive activities. Nrf2 is a major transcriptional stimulator of cytoprotective genes and is critical for cancer prevention. Thus, several new dithiolethiones that are highly promising for bladder cancer prevention have been identified. Because the compounds act specifically in the bladder, the likelihood of potential systemic toxicity may be low.
Introduction
3H-1,2-Dithiole-3-thione and its derivatives (dithiolethiones) have attracted wide interest as potential cancer chemopreventive agents. Indeed, some compounds of this type, 4-methyl-5-pyrazinyl-3H-1,2-dithiole-3-thione (oltipraz) in particular, have been shown to protect against carcinogenesis at various organ sites in animal models.1 Oltipraz and 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione (ADTa) have also been evaluated in humans, but the available data show either moderate chemopreventive activities or an association of such activity with significant toxic effects.1
The cancer chemopreventive activities of dithiolethiones are attributed primarily to their ability to stimulate the transcription of cytoprotective Phase 2 enzymes, such as glutathione S-transferase (GST) and NAD(P)H:quinone oxidoreductase 1 (NQO1), via activation of Nrf2.1 Nrf2 is a transcriptional factor, which binds to a cis-DNA regulatory element, known as the antioxidant response element (ARE), located in the upstream region of Phase 2 genes, and stimulates gene transcription.2 Nrf2 is normally bound to its repressor Keap1 and undergoes proteasomal degradation. Although the mechanism by which dithiolethiones and other compounds activate Nrf2 has not been fully elucidated, it has been demonstrated that these compounds react with the sulfhydryl groups of key cysteine residues of Keap1, specifically Cys273 and Cys288, preventing Keap1-mediated ubiquitination of Nrf2, which leads to inhibition of proteasomal degradation and increased transactivation activity of Nrf2.3
Interestingly, both oltipraz and ADT are relatively weak inducers of Phase 2 enzymes in rodents.1 In previous studies, we have sought dithiolethiones of higher inductive activity, which could possibly increase chemopreventive efficacy and/or reduce toxic side effects. We found that 3H-1,2-dithiole-3-thione itself (1, Table 1) and 5,6-dihydrocyclopenta[c]-1,2-dithiole-3(4H)-thione (16, Table 1) were particularly effective inducers of Phase 2 enzymes.4,5 In rats, these compounds significantly increased enzyme activity in the spleen, liver, kidneys, heart, lungs, urinary bladder and throughout the whole of the gastrointestinal tract. The highest degree of induction was observed, however, in the forestomach, glandular stomach, duodenum, jejunum and urinary bladder. Thus, these compounds show different degrees of activity in different tissues. In a recent dose-response study, 16 was shown to cause significant increases in Phase 2 enzyme activity in the urinary bladder of rats at very low dose-levels, and it was concluded that this substance may be a promising chemopreventative agent in this organ.6
Table 1.
Induction of GST and NQOl by dithiolethiones in rat bladder in vivo
| Relative enzyme activity in the bladder (treated/control) |
||||||
|---|---|---|---|---|---|---|
| Compd | R1 | R2 | X | NQOl | GST | |
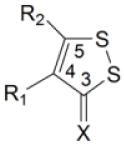 |
1 | H | H | S | 2.78 ± 0.11* | 1.70 ± 0.04* |
| 2 | H | H | O | 1.26 ± 0.09 | 1.01 ± 0.04 | |
| 3 | CH3 | H | S | 1.83 ± 0.12* | 1.23 ± 0.06 | |
| 4 | H | CH3 | S | 1.23 ± 0.06 | 1.08 ± 0.05 | |
| 5 | CH3 | SH | S | 1.29 ± 0.09 | 1.08 ± 0.14 | |
| 6 | C6H5 | SH | S | 1.11 ± 0.07 | 1.03 ± 0.05 | |
| 7 | CO2H | H | S | 3.69 ± 0.30* | 2.11 ± 0.15* | |
| 8 | CO2H | CH3 | S | 1.10 ± 0.04 | 0.99 ± 0.05 | |
| 9 | H | CO2H | S | 1.27 ± 0.10 | 1.11 ± 0.07 | |
| 10 | H | 4-OH-C6H5 | S | 1.02 ± 0.05 | 0.95 ± 0.07 | |
| 11 | CO2CH3 | H | S | 5.05 ± 0.35* | 2.69 ± 0.20* | |
| 12 | CO2C2H5 | H | S | 4.82 ± 0.10* | 2.76 ± 0.11* | |
| 13 | CO2CH3 | CH3 | S | 1.24 ± 0.08 | 1.24 ± 0.08 | |
| 14 | CO2C2H5 | NH2 | S | 2.03 ± 0.23* | 1.25 ± 0.10 | |
| 15 | H | CO2C2H5 | S | 1.13 ± 0.09 | 1.09 ± 0.04 | |
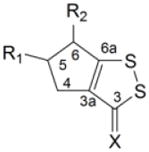 |
16 | H | H | S | 3.14 ± 0.10* | 1.68 ± 0.05* |
| 17 | H | H | O | 1.24 ± 0.08* | 1.11 ± 0.06 | |
| 18 | H | CO2CH3 | S | 4.39 ± 0.31* | 2.31 ± 0.15* | |
| 19 | H | CO2C2H5 | S | 4.47 ± 0.40* | 2.29 ± 0.19* | |
| 20 | CO2C2H5 | H | S | 1.23 ± 0.04* | 1.11 ± 0.07 | |
| 21 | H | CH3 | S | 1.91 ± 0.16* | 1.30 ± 0.06* | |
| 22 | CH3 | H | S | 1.81 ± 0.07* | 1.31 ± 0.05* | |
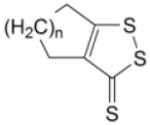 |
23 | n = 2 | 1.46 ± 0.09* | 1.05 ± 0.03 | ||
| 24 | n = 3 | 1.20 ± 0.07 | 1.14 ± 0.05 | |||
| 25 | n = 4 | 1.08 ± 0.06 | 1.11 ± 0.05 | |||
Significantly different from the corresponding control value (P<0.05). Groups of female Sprague-Dawley rats were dosed with vehicle or the test compound by gavage at 7.5 μmol/kg/day for 10 days and sacrificed on the 11th day. Bladders were promptly removed and assayed for enzymatic activities of GST and NQO1. Each value represents the mean ± SEM of results for the 6 animals in each treatment group.
The reason or reasons for the site specificity of Phase 2 induction by certain dithiolethiones is not presently known. Effects in the stomach and upper gastrointestinal tract may simply reflect high concentrations of the dithiolethiones in these tissues after oral administration, prior to absorption. It is also possible that the urinary bladder could similarly be exposed to high concentrations of the dithiolethiones if these substances were excreted in the urine. There is a precedent for this concept in that certain isothiocyanates, which are also potent inducers of Phase 2 enzymes in the urinary bladder, are metabolized to hydrophilic conjugates that are excreted in the urine. In the bladder, these conjugates apparently dissociate back to the parent compounds, so that the bladder epithelium is exposed to high concentrations of the isothiocyanates.7 In experiments with 16, enzyme induction occurred exclusively in the bladder epithelium,6 suggesting that 16 may indeed undergo urinary excretion, leading to high concentrations of itself and/or its active metabolite(s) in urine and thus facilitating uptake by the bladder epithelium. In this context, it is worth noting that very little oltipraz or ADT is excreted in the urine of animals after oral administration,1 and neither oltipraz nor ADT is a good inducer in the bladder.4 However, no definitive information on the urinary excretion of 1 or 16 is available. We considered that urinary excretion could be increased by incorporating hydrophilic substituents into these substances, so that higher inductive activity could be achieved in the bladder. Furthermore, increased urinary excretion could possibly be achieved by replacing the thione moiety of the dithiolethione with a ketone function, since 1,2-dithiole-3-ones are much more hydrophilic than 1,2-dithiole-3-thiones.8 We also considered the potential importance of thiol reactivity of dithiolethiones with regard to their inductive activities, since induction of Phase 2 enzymes by these compounds is believed to depend on their reaction with key thiol groups of Keap1, as mentioned above.
In the experiments described in the present report, 23 derivatives of 1 and 16 have been synthesized, their abilities to induce Phase 2 enzymes in rat tissues determined, important structural features revealed, and the role of Nrf2 in the induction of Phase 2 enzymes by selected compounds assessed. Moreover, several new agents were found to be far more potent than 1 and 16 in inducing Phase 2 enzymes in the bladder in vivo.
Results and Discussion
All dithiolethiones first underwent in vivo evaluation for induction of Phase 2 enzymes in the bladder of female Sprague Dawley rats. We decided to start with in vivo experiments, because in a previous study of 25 isothiocyanates (which are also potent inducers of Phase 2 enzymes in the urinary bladder), we found that cell-based bioassays did not predict the relative activity of these compounds in vivo.9 Groups of 6 rats were treated by gavage with either vehicle or with a dithiolethione at 7.5 μmol/kg/day for 10 days. On the 11th day, the rats were sacrificed and organs of interest were removed for analysis. The dose and treatment duration were based on our recent study6 showing that significant and steady-state enzyme induction in vivo was reached after treatment with 16 at 3.9–7.8 μmol/kg/day for 8–11 days.
We focused on the inductive effects of the dithiolethiones on GST and NQO1 in the bladder. Both Phase 2 enzymes may play an important role in bladder cancer prevention in humans. An inverse association in humans between the incidence of bladder cancer and tissue activities of GST and NQO1 has been shown in several studies.10–13
NQO1 was uniformly more inducible than GST by the dithiolethiones. 16 was significantly more effective than 1 in increasing NQO1 activity in the urinary bladder, while GST induction was similar between the two compounds (Table 1). In contrast, 1 was more effective than 16 in inducing NQO1 in the gastrointestinal tract, including the stomach, duodenum, jejunum, ileum and colon, while the two compounds showed similar activity in the kidney, liver and lung (Table 2). As in the bladder, the levels of GST induction by both compounds were lower than that of NQO1 in the gastrointestinal tract and other organs, but 1 appeared to be somewhat more effective than 16 (results not shown). Similar data were also obtained in our previous study.4 Thus, introduction of a cyclopenta ring to 1, to give rise to 16, appears to render the compound a more bladder-specific inducer. Our previous study suggested that the bladder specificity of 16 might arise from selective delivery of this compound and/or its active metabolites to the bladder through urinary excretion.6
Table 2.
Induction of NQOI by dithiolethiones in rat organs in vivo
| Relative NQOI activity (treated/control) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Compd | Stomach† | Duodenum | Jejunum | Ileum | Colon | Liver | Kidney | Lung |
| 1 | 5.31 ± 0.17* | 5.60 ± 0.37* | 2.31 ± 0.10* | 1.76 ± 0.12* | 1.81 ± 0.09* | 1.30 ± 0.13 | 1.82 ± 0.07* | 1.37 ± 0.07* |
| 11 | 2.36 ± 0.18* | 1.17 ± 0.09 | 1.13 ± 0.06 | 0.95 ± 0.07 | 0.89 ± 0.04 | 1.07 ± 0.08 | 1.45 ± 0.07* | 1.07 ± 0.05 |
| 12 | 2.15 ± 0.10* | 1.21 ± 0.13 | 1.04 ± 0.05 | 1.11 ± 0.11 | 0.98 ± 0.02 | 1.04 ± 0.10 | 1.47 ± 0.08* | 1.21 ± 0.04 |
| 16 | 3.55 ± 0.10* | 2.82 ± 0.11* | 1.44 ± 0.05* | 1.36 ± 0.04 | 1.28 ± 0.07 | 1.26 ± 0.04 | 1.87 ± 0.06* | 1.26 ± 0.03 |
| 18 | 1.98 ± 0.13* | 1.14 ± 0.16 | 1.11 ± 0.03 | 1.07 ± 0.07 | 1.07 ± 0.08 | 1.10 ± 0.08 | 1.76 ± 0.12* | 1.08 ± 0.06 |
| 19 | 1.93 ± 0.16* | 1.53 ± 0.20 | 1.38 ± 0.11* | 1.20 ± 0.07 | 1.00 ± 0.03 | 1.17 ± 0.11 | 1.86 ± 0.13* | 1.17 ± 0.08 |
Forestomach.
Significantly different from the corresponding control value (P<0.05). The experimental protocol was the same as that described in the footnote to Table 1.
The inductive effects of the ketone derivatives of both 1 and 16 (Compounds 2 and 17 respectively) on NQO1 and GST were markedly less than those of the respective thiones in the urinary bladder (Table 1), contrary to our initial expectation that 2 and 17 would be more active in the bladder due to potential increase in urinary excretion, as they are more hydrophilic than their parent compounds. Several derivatives of 1 containing other polar groups were also less active than the parent compound itself in the urinary bladder, including the sulfhydryl group (5 vs. 3), the hydroxyphenyl group (10 vs. 1) or the carboxyl group (9 vs. 1) (Table 1). Interestingly, 3-thioxo-3H-1,2-dithiole-4-carboxylic acid (7) induced significantly higher activities of both NQO1 and GST in the urinary bladder than 1. 7 is a strong acid, with a pKa of 2.8,14 and would therefore be extensively ionized at physiological pH. But ionization is unlikely to account for the high activity of this compound, since the isomeric 9, which is an even stronger acid (pKa 1.2),14 was a much weaker inducer than 1 or 7.
Addition of a methyl group at the 4- or 5-position of 1 (Compounds 3 and 4), or at the 5- or 6-position of 16 (Compounds 22 and 21) greatly diminished inductive activity (Table 1). Likewise, addition of a methyl group at the 5-position of 7 or 11, to give rise to 8 or 13 respectively, also greatly diminished inductive activity, as did addition of an amino group at the 5-position (Compound 14). Whether this effect reflects the electron-donating activity of the alkyl or amino substituent, or the steric effect of the substitution is not presently known.
The methyl ester of 7 (Compound 11) was much more active than 7, as was the ethyl ester (Compound 12), and both these compounds were significantly more effective than 1 in the urinary bladder (Table 1). There was no significant difference between the methyl and ethyl esters. However, esterification of 9, which itself was a poor inducer, yielding Compound 15, did not significantly increase inductive activity. Modification of 16 by addition of a carboxylic ester function at the 6-position (Compounds 18 and 19) significantly increased the inductive effect in the urinary bladder. In contrast, carboxylic ester substitution at the 5-position of 16 (Compound 20) almost completely abolished the inductive activity.
Homologs of 16 containing 6, 7, or 8-membered rings (Compounds 23, 24 and 25) proved to be poor inducers, and in the case of NQO1 induction, it can be clearly seen that the larger the ring size, the lower the inductive activity (Table 1).
The inductive activities of the four most potent compounds, 11, 12, 18 and 19, together with 1 and 16, were compared in other rat organs as well. The specimens were obtained from the same rats from which bladders were removed and analyzed as described above. We focused on NQO1 induction, since it was more responsive than GST. While both 1 and 16 exhibited significant inductive activities in most of the organs examined, including colon, duodenum, ileum, jejunum, kidney, lung and stomach, 11, 12, 18 and 19 were either much weaker inducers than the parent compounds or totally inactive in these organs, except for the kidney (Table 2). In the kidney, the inductive activities of 11 and 12 were only slightly lower than that of 1, and the inductive activities of 18 and 19 closely resembled that of 16. Thus, carboxylic ester substitution at the 4-position of 1 or at the 6-position of 16 significantly increased the inductive activity in the bladder, decreased the inductive activity in other organs, and retained the inductive activity in the kidney. Such a change in the inductive activity of these compounds supports the notion that the increased inductive activities of the derivatives result from increased urinary excretion of them or their active metabolites.
11 and 19 were further investigated for induction of Phase 2 enzymes by Western blot analysis and the role of Nrf2 in such induction. Treatment of rat bladder NBT-II cells with either 11 or 19 at 25 or 50 μM for 24 h led to a significant and dose-dependent increase in both GST and NQO1 expression (Figure 1). In the case of GST, each compound induced both GST-α and GST-μ, but not GST-π (result not shown). Previous epidemiological studies have shown that GST-α and GST-μ may play an important role in bladder cancer prevention in humans.9,10 Moreover, both compounds significantly induced glutamate cysteine ligase (GCL), a Phase 2 enzyme which plays a key role in glutathione biosynthesis. Glutathione is a cofactor of several important cytoprotective enzymes. 19 seems to be a more effective inducer of GCL and GST-μ than 11, but the two compounds showed similar efficacy in inducing GST-α and NQO1. Increased expression of GCL, GST-α, GST-μ and NQO1, but not GST-π, were also detected in the bladder tissues of rats after treatment with 11 or 19 at 7.5 μmol/kg/day for 10 days, although, not surprisingly, there was some degree of individual variation in the induction of each enzyme within each group (Figure 2). These results not only show that the bladder cells in culture predict the inductive activity of each agent in the bladder in vivo, but also extend those obtained through measurement of enzymatic activities shown in Table 1. In the bladder tissues of rats treated with each agent, increased NQO1 protein level closely correlates with increase in its enzymatic activity, and the increase in GST enzymatic activity may result mainly from induction of both GST-α and GST-μ. Induction of GCL by both 11 and 19 in bladder cells and tissues is also consistent with the well-known fact that inducers of Phase 2 enzymes frequently cause simultaneous induction of many such enzymes through the Nrf2 signalling pathway.
Figure 1.
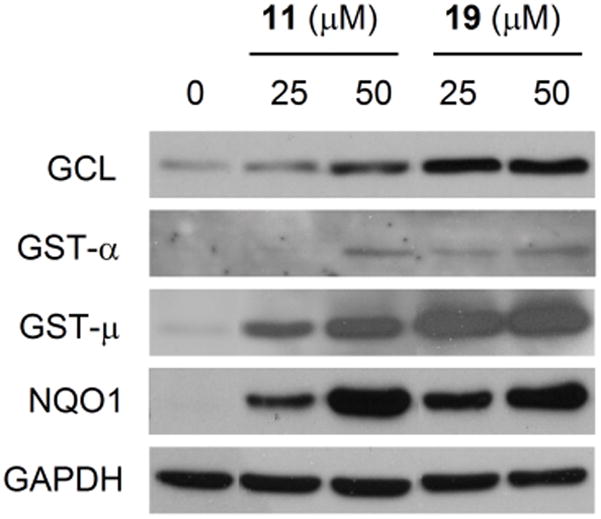
Induction of Phase 2 enzymes by dithiolethiones in NBT-II cells. Cells were treated with compounds 11 or 19 at the indicated concentrations for 24 h and then harvested for Western blot analysis. GAPDH was used as a loading control. The results are representative of at least two experiments.
Figure 2.
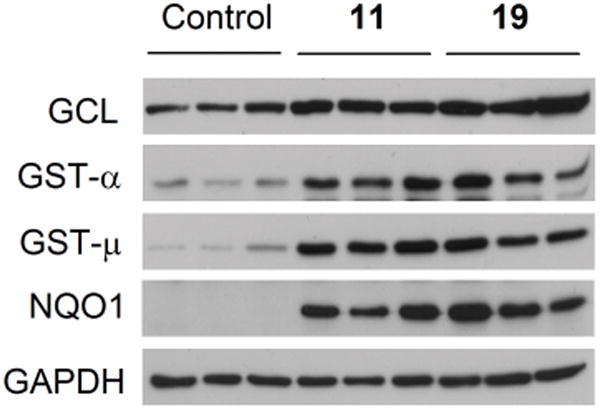
Induction of Phase 2 enzymes by dithiolethiones in the bladder in vivo. Female Sprague-Dawley rats were dosed by gavage with compounds 11 or 19 at 7.5 μmol/kg or with vehicle once daily for 10 days. On the 11th day, the rats were sacrificed and the bladders were collected for analysis of Phase 2 enzymes by Western blot analysis. Three animals were used in each group. GAPDH was used as a loading control.
In order to determine the role of Nrf2 in Phase 2 enzyme induction, Nrf2 in NBT-II cells was silenced by Nrf2 siRNA. The Nrf2 level in NBT-II cells decreased significantly after treatment with Nrf2 siRNA for 48 h, compared with cells treated with control siRNA for the same time period (Figure 3). The cells were then treated with 11 or 19 at 25 μM for 24 h. In control cells, both compounds elevated Nrf2, but 19 was markedly more effective than 11 (Figure 3). Elevation of Nrf2 level by these compounds is consistent with the notion that inducers of Phase 2 enzymes activate Nrf2 by inhibiting its proteasomal degradation. It also suggests that these compounds may increase the expression of other Nrf2-regulated cytoprotective genes. The stimulating effect of both compounds on Nrf2 in cells treated with Nrf2 siRNA was either not detectable (11) or markedly attenuated (19). More importantly, while both compounds significantly induced GCL, GST-α, GST-μ and NQO1 in the control cells (treated with control RNAi) as expected, none of these enzymes was induced by either compound in cells treated with Nrf2 RNAi (Figure 3). Thus, the inductive effects of these compounds appear to depend completely on Nrf2. It is also noteworthy that our recent study showed that Phase 2 enzyme induction by 16 also depended on Nrf2.6
Figure 3.
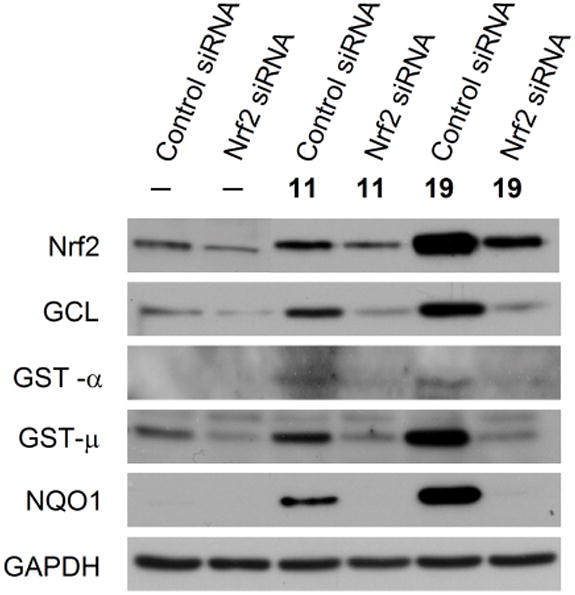
The role of Nrf2 in Phase 2 enzyme induction by dithiolethiones. NBT-II cells were treated with control RNAi or Nrf2 RNAi for 48 h, followed by treatment with vehicle or compounds 11 or 19 at 25 μM for 24 h. The cells were then harvested for Western blot analysis of Nrf2 and Phase 2 enzymes. GAPDH was used as a loading control.
The strict requirement for Nrf2 in the induction of Phase 2 enzymes by the dithiolethiones strongly suggests that chemical modification (alkylation or oxidation) of key cysteine thiols of Keap1 is critical to their inducer activity. As described before, activation of Nrf2 by inducers of Phase 2 enzymes primarily involves chemical reaction of the inducer with key cysteine thiols of Keap1, thereby preventing Keap1-mediated ubiquitination of Nrf2 and its proteasomal degradation. However, at the present time, we are unable to determine if the inducer activity of a dithiolethione correlates with its reactivity with Keap1 thiols, since little is known about the potential metabolism of these compounds in vivo. Oltipraz was previously shown to be extensively metabolized; a total of 13 metabolites were detected in the plasma and urine of animals and humans.15 The lack of information on the potential metabolism of dithiolethiones certainly contributes to the difficulty in understanding why certain structural features rendered the compounds more potent inducers and others rendered the compounds weaker inducers. However, from the results of the present study it appears that hydrophilicity is not a prerequisite for inductive activity, and substitution in the 5-position of 1 or 16 with electron-donating groups decreases activity. It may be suggested that derivatives of 1 with electron-withdrawing groups in the 4 position but unsubstituted in the 5-position, and derivatives of 16 with electron-withdrawing groups in the 6 position would be worthy of further study.
In summary, several dithiolethiones that are exceedingly potent and bladder-specific inducers of Phase 2 enzymes have been identified. Such organ specificity suggests that these compounds may be useful for bladder cancer prevention with potential low systemic toxicity. Structural features responsible for such activities have been identified. Structural features that inhibit the inductive activity of dithiolethiones have also been revealed. The dithiolethiones activate Nrf2, a key stimulator of cytoprotective defence against carcinogens and oxidants, and Nrf2 in turn is essential for induction of Phase 2 enzymes by these compounds. Bladder cancer is the fourth commonest cancer in men and the eighth commonest in women, and its incidence continues to rise. In the United States alone, there were an estimated 70980 new cases in 2009.16 To the best of our knowledge, no chemopreventive agents are currently available for prevention of primary bladder cancer. Aromatic amines from tobacco smoke and from environmental and occupational exposure are the main causes of bladder cancer.17 It would be of interest to determine if the dithiolethiones are able to protect against these carcinogens.
Experimental Section
Chemistry
Starting materials for syntheses were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Acros Organics (Geel, Belgium) and were used without further purification. NMR spectra were obtained from solutions in chloroform-d (CDCl3) (99.96 atom % D; Sigma Aldrich) using a Bruker Avance DRX 400 MHz spectrometer fitted with a 5 mm dual inverse-probe. NMR data were acquired and processed using Topspin V1.3 software. Chemical shifts were determined at 30 C and spectra calibrated relative to 7.24 ppm (1H NMR) and 77.23 ppm (13C NMR). Assignments were obtained from examination of 1H, 13C, DEPT-135, COSY, TOCSY, g-HSQC and g-HMBC NMR spectra. 13C NMR signal multiplicities (s, d, t or q) were determined using the DEPT-135 sequence. Two-dimensional COSY and inverse-mode g-HMBC were obtained in absolute value mode. TOCSY and g-HSQC spectra were obtained in phase-sensitive mode. HRMS experiments were performed on a Bruker Daltonics MicrOTOF spectrophotometer. Merck silica gel (40 – 60 mesh) was used for column chromatography. Analytical TLC employed Merck TLC 60 F254 silica gel plates and preparative TLC Merck PLC 60 F254 silica gel plates.
3H-1,2-Dithiole-3-thione (1),18 3H-1,2-dithiole-3-one (2),19 4-methyl-3H-1,2-dithiole-3-thione (3),20 5-methyl-3H-1,2-dithiole-3-thione (4),20 5-mercapto-4-methyl-3H-1,2-dithiole-3-thione (5),21 5-mercapto-4-phenyl-3H-1,2-dithiole-3-thione (6),21 3-thioxo-3H-1,2-dithiole-4-carboxylic acid (7),22 5-methyl-3-thioxo-3H-1,2-dithiole-4-carboxylic acid (8),22 3-thioxo-3H-1,2-dithiole-5-carboxylic acid (9),23 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (10),24 methyl 3-thioxo-3H-1,2-dithiole-4-carboxylate (11),25 ethyl 3-thioxo-3H-1,2-dithiole-4-carboxylate (12),25 methyl 5-methyl-3H-1,2-dithiole-3-thione-4-carboxylate (13),22 ethyl 5-amino-3H-1,2-dithiole-3-thione-4-carboxylate (14),26 ethyl 3-thioxo-3H-1,2-dithiole-5-carboxylate (15),25 5,6-dihydrocyclopenta[c][1,2]dithiole-3(4H)-thione (16),27 5,6-dihydrocyclopenta[c][1,2]dithiol-3(4H)-one (17),28 4,5,6,7-tetrahydrobenzo[c][1,2]dithiole-3-thione (23),20 5,6,7,8-tetrahydrocyclohepta[c][1,2]dithiole-3-thione (24),27 4,5,6,7,8,9-hexahydrocycloocta[c][1,2]dithiole-3-thione (25),29 were prepared following described procedures.
General procedure for the synthesis of Compounds 18–22
Methyl 2-oxocyclopentanecarboxylate, ethyl 2-oxocyclopentanecarboxylate, 3-methylcyclopentanone, 2-methylcyclopentanone or 3-oxo-1-cyclopentane carboxylic acid (0.04 mole) was refluxed with piperidine (0.06 mole) in benzene (15 ml) under azeotropic conditions. After water loss was complete (1–7 hr), the reaction mixtures were evaporated to dryness under vacuum. The crude enamines (0.02 mole) were dissolved in 5 ml THF and added dropwise at RT to carbon disulfide (0.06 mole), and sulfur (0.19 gram-atom) in 15 ml of THF.27 Heat was evolved, and the solution became orange or red in colour. After all the enamine had been added, stirring was continued at RT for 1 hr, when the reaction mix was poured into ice water (75 ml) and extracted with dichloromethane. The organic layer was washed four times with water and dried over sodium sulfate. Evaporation of the solvent gave crude samples of 18, 19, 21, and 22 and of 3,4,5,6-tetrahydro-3-thioxocyclopenta[c][1,2]dithiole-5-carboxylic acid. The latter was esterified by refluxing with ethanol in benzene with addition of 100 μl of concentrated sulfuric acid under azeotropic conditions. After 1 hr, the reaction mix was poured into water. The benzene layer was separated, washed with water, saturated sodium bicarbonate solution and again with water. Evaporation gave crude 20, which was purified by preparative TLC using benzene-dichloromethane (9:1) as eluent. The crude samples of 18, 19, 21 and 22 were purified by column chromatography on silica gel with benzene as eluent. The materials so obtained were recrystallized from methanol. The purity of the compounds, as determined by GCMS, was between 96 and 99%. For NMR assignments, the ring numbering is shown in Table 1.
Methyl 3,4,5,6-tetrahydro-3-thioxocyclopenta[c][1,2]dithiole-6-carboxylate (18)
Pale orange crystals, yield 34%. 1H NMR δ 2.77 (m, 4H, CH2 at positions 4 and 5), 3.81 (s, 3H, ester CH3), 4.14 (t, 1H, CH at position 6). 13C NMR δ 28.4 (C-5), 32.8 (C-4), 51.7 (C-6), 53.2 (ester methyl), 155.6 (C-3a), 169.4 (C-6a), 170.1 (ester carbonyl), 208.6 (C-3). HRMS (ESI) calculated for C8H7O2S3 230.9608 [M – H]−, found 230.9614.
Ethyl 3,4,5,6-tetrahydro-3-thioxocyclopenta[c][1,2]dithiole-6-carboxylate (19)
Pale yellow crystals, yield 28%. 1H NMR δ 1.30 (t, 3H, ester methyl), 2.72 (m, 2H, CH2 at position 4), 2.81 (m, 2H, CH2 at position 5), 4.10 (s, 1H, CH at position 6), 4.25 (m, 2H, ester methylene). 13C NMR δ 14.3 (ester methyl), 28.3 (C-4), 32.5 (C-5), 51.9 (C-6), 62.4 (ester methylene), 155.5 (C-3a), 169.5 (ester carbonyl*), 169.7 (C-6a*), 208.5 (C-3). HRMS (ESI) calculated for C9H9O2S3 244.9765 [M – H]−, found 244.9770.
Ethyl 3,4,5,6-tetrahydro-3-thioxocyclopenta[c][1,2]dithiole-5-carboxylate (20)
Orange crystals, yield 18%. 1H NMR δ 1.25 (t, 3H, ester methyl), 2.90 –3.00 (m, 2H, CH2 at position 6*), 3.15 – 3.32 (m, 2H, CH2 at position 4*), 3.80, (m, 1H, CH at position 5), 4.15 – 4.18 (q, 2H, ester methylene). 13C NMR δ 14.3 (ester methyl), 32.9 (C-6), 36.5 (C-4), 48.2 (C-5), 61.5 (ester methylene), 152.9 (C-3a), 171.9 (C-6a), 173.3 (ester carbonyl), 208.0 (C-3). HRMS (ESI) calculated for C9H9O2S3 244.9765 [M – H]−, found 244.9770.
5,6-Dihydro-6-methylcyclopenta[c][1,2]dithiole-3(4H)-thione (21)
Pale orange crystals, yield 41%. 1H NMR δ 1.32 (s, 3H, methyl), 2.11 and 2.70 (m, 2H, CH2 at position 5), 2.61 (m, 2H, CH2 at position 4), 3.34 (s, 1H, CH at position 6). 13C NMR δ 20.1 (methyl), 28.3 (C-4), 38.8 (C-5), 42.1 (C-6), 154.4 (C-3a), 180.8 (C-6a), 208.8 (C-3). HRMS (ESI) calculated for C7H7S3 186.9710 [M – H]−, found 186.9715.
5,6-Dihydro-5-methylcyclopenta[c][1,2]dithiole-3(4H)-thione (22)
Orange crystals, yield 15%. 1H NMR δ 1.20 (s, 3H, methyl), 2.25 – 2.83 (m, 2H, CH2 at position 4*), 2.54 – 3.08 (m, 2H, CH2 at position 6*), 3.11 (m, 1H, CH at position 5). 13C NMR δ 21.7 (methyl), 37.1 (C-4), 40.4 (C-5), 42.0 (C-6), 154.7 (C-3a), 174.2 (C-6a), 208.4 (C-3). HRMS (ESI) calculated for C7H7S3 186.9710 [M – H]−, found 186.9715.
Rat treatment with test agents and measurement of Phase 2 enzyme activity
Female Sprague-Dawley rats, 11–12 weeks old, were bred at the AgResearch laboratory. Animals were randomly assigned to treatment groups, and housed in solid-bottomed cages containing Sani-Chips bedding (P. J. Murphy Forest Products, Montville, NJ, USA). They were allowed free access to food (Rat and Mouse Cubes, Specialty Feeds Ltd., Glen Forrest, Western Australia) and tap water. The room temperature was maintained at 21 – 23°C with a 12-hour light-dark cycle. All experimental protocols were approved by the Institutional Animal Ethics Committee.
Groups of rats were dosed with freshly-prepared solutions of the test compounds in soya oil at 7.5 μmoles/kg/day each day for 10 days. Control rats received soya oil alone. On the eleventh day, the rats were killed and the urinary bladders and other organs of each animal were dissected out. The contents of the gastrointestinal tract were removed under running cold water and the tissues blotted dry on absorbent paper. All tissues were then stored at −80°C until analysis.
For measurement of GST and NQO1 activities in rat organs, the tissues were homogenized in ice-cold 0.2% Triton X-100 using a Polytron tissue homogenizer, The homogenates were cleared by centrifugation and activities of NQO1 and GST in the supernatants were measured by the methods of Ernster et al.30 and Habig et al.31 respectively.
Cell culture, treatment with test agents, and Nrf2 knockdown
NBT-II rat bladder carcinoma cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and were grown in Eagle’s Minimum Essential Medium (Mediatech, Manassas, VA), supplemented with 10% (v/v) fetal bovine serum (Life Technologies, Grand Island, NY). Approximately 1.5–2 million cells were plated with 10 ml medium in a 10-cm dish overnight and then incubated with each test compound at the desired concentrations for 24 h. Each test compound was dissolved in DMSO, and the final concentration of DMSO in the media was 0.1% (v/v). Cells were harvested by trypsin treatment and low speed centrifugation, rinsed once with ice-cold PBS, and processed for measurement of Nrf2 and Phase 2 proteins by Western blot analysis.
To knock down Nrf2, NBT-II cells were treated with Nrf2 stealth RNAi (Catalog # RSS343557) or negative universal control stealth RNAi (Catalog # 12935300) (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Briefly, 1.5 million cells were grown in each 10-cm dish with 10 ml medium overnight and incubated with RNAi in fresh medium for 48 h. RNAi (0.584 nmol) was diluted in Opti-MEM I reduced serum medium (Invitrogen), mixed with lipofectamine 2000, and the mixture added to the medium in each dish. At the end of RNAi treatment, cells were either harvested to check the level of Nrf2 or treated with each test compound for 24 h.
Western blot analysis of bladder cells and tissues
Cells harvested from each dish were suspended in cell lysis buffer (Cell Signaling Technology, Beverly, MA), supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma, St Louis, MO) and sonicated using a Branson’s Model 450 sonifier. Rat bladder tissues were homogenized in 10 mM Tri-HCl, pH 7.4 (10 μl/mg tissue), containing 0.25 M sucrose, 1 mM PMSF and 1 μg/ml leupeptin (Roche, Indianapolis, IN). Protein levels were determined using Pierce’s BCA assay kit. Each sample (25–40 μg protein) was resolved by SDS-PAGE (6–12%) and transferred to a polyvinylidine difluoride membrane. The membranes were blocked at room temperature for 1 h with either 5% milk or 1x Detector Block (KPL, Gaithersburg, MD), and then probed with an antibody overnight at 4°C. Immunoreactivity was detected by incubation with an HRP-linked secondary antibody (either mouse or rabbit, Amersham, Piscataway, NJ) at room temperature for 1 h, and the band of interest was visualized using either ECL (Pierce) or ECL+ (Amersham) chemiluminescence systems. The primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), including anti-GCS (Catalog # sc-22755), anti-Nrf2 (Catalog # sc-722), and from Alpha Diagnostic International (San Antonio, TX), including anti-GST-α (Catalog # GSTA11-S) and anti-GST-μ (Catalog # GSTM11-S). An antibody against NQO1 (Catalog # 3187) was purchased from Cell Signaling Technology (Beverly, MA), and an antibody against GAPDH was from Millipore (Billarica, MA).
Statistical analysis
Statistical significance of the in vivo data on enzyme activities was tested by ANOVA, followed by the Student-Newman-Keuls multiple comparisons test using Instat software (GraphPad, San Diego, CA).
Acknowledgments
This work was supported by a grant (R01CA120533) from the National Cancer Institute, USA.
Footnotes
Abbreviations: ADT, 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GCL, glutamate cysteine ligase; GST, glutathione S-transferase; NQO1, NAD(P)H:quinone oxidoreductase 1.
NMR signals are interchangeable.
References
- 1.Zhang Y, Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther. 2008;7:3470–9. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Gordon GB. A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther. 2004;3:885–93. [PubMed] [Google Scholar]
- 3.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–91. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 4.Munday R, Zhang Y, Munday CM, Li J. Structure-activity relationships in the induction of Phase II enzymes by derivatives of 3H-1,2-dithiole-3-thione in rats. Chem-Biol Interact. 2006;160:115–22. doi: 10.1016/j.cbi.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Munday R, Munday CM. Induction of phase II enzymes by 3H-1,2-dithiole-3-thione: dose-response study in rats. Carcinogenesis. 2004;25:1721–5. doi: 10.1093/carcin/bgh162. [DOI] [PubMed] [Google Scholar]
- 6.Paonessa JD, Munday CM, Mhawech-Fauceglia P, Munday R, Zhang Y. 5,6-Dihydrocyclopenta[c][1,2]-dithiole-3(4H)-thione is a promising cancer chemopreventive agent in the urinary bladder. Chem-Biol Interact. 2009;180:119–26. doi: 10.1016/j.cbi.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang L, Zhang Y. Isothiocyanates in the chemoprevention of bladder cancer. Curr Drug Metab. 2004;5:193–201. doi: 10.2174/1389200043489027. [DOI] [PubMed] [Google Scholar]
- 8.Bona M, Boudeville P, Zekri O, Christen MO, Burgot JL. Water/n-octanol partition coefficients of 1,2-dithiole-3-thiones. J Pharm Sci. 1995;84:1107–12. doi: 10.1002/jps.2600840914. [DOI] [PubMed] [Google Scholar]
- 9.Munday R, Zhang Y, Fahey JW, Jobson HE, Munday CM, Li J, Stephenson KK. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr Cancer. 2006;54:223–231. doi: 10.1207/s15327914nc5402_9. [DOI] [PubMed] [Google Scholar]
- 10.Komiya Y, Tsukino H, Nakao H, Kuroda Y, Imai H, Katoh T. Human glutathione S-transferase A1 polymorphism and susceptibility to urothelial cancer in the Japanese population. Cancer Lett. 2005;221:55–9. doi: 10.1016/j.canlet.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Salagovic J, Kalina I, Habalova V, Hrivnak M, Valansky L, Biros E. The role of human glutathione S-transferases M1 and T1 in individual susceptibility to bladder cancer. Physiol Res. 1999;48:465–71. [PubMed] [Google Scholar]
- 12.Schulz WA, Krummeck A, Rosinger I, Eickelmann P, Neuhaus C, Ebert T, Schmitz-Drager BJ, Sies H. Increased frequency of a null-allele for NAD(P)H: quinone oxidoreductase in patients with urological malignancies. Pharmacogenetics. 1997;7:235–9. doi: 10.1097/00008571-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Park SJ, Zhao H, Spitz MR, Grossman HB, Wu X. An association between NQO1 genetic polymorphism and risk of bladder cancer. Mutat Res. 2003;536:131–7. doi: 10.1016/s1383-5718(03)00041-x. [DOI] [PubMed] [Google Scholar]
- 14.Chollet M, Burgot JL. pKa values of 3-thioxo-1,2-dithiole-4- and -5-carboxylic acids and of 3-oxo-1,2-dithiole-4- and -5-carboxylic acids in aqueous solution at 298 K. J Chem Soc, Perkin Trans. 1998;2:387–391. [Google Scholar]
- 15.Bieder A, Decouvelaere B, Gaillard C, Depaire H, Heusse D, Ledoux C, Lemar M, Le Roy JP, Raynaud L, Snozzi C, Gregoire J. Comparison of the metabolism of oltipraz in the mouse, rat and monkey and in man. Distribution of the metabolites in each species. Arzneimittelforschung. 1983;33:1289–97. [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 17.Negri E, La Vecchia C. Epidemiology and prevention of bladder cancer. Eur J Cancer Prev. 2001;10:7–14. doi: 10.1097/00008469-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Meinetsberger E, Schöffer A, Behringer H. Eine einfache und ergiebige Herstellung von 3-Thioxo-1,2-dithiol (1,2-Trithion) Synthesis. 1977:802–803. [Google Scholar]
- 19.Mayer R, Faust J. Synthese des “Dithions” (1.2-dithia-cyclopentenon-(3)) Chem Ber. 1963;96:2702–2703. [Google Scholar]
- 20.Legrand L, Mollier Y, Lozac’h N. Sulfuration des composés organiques (IV). Dithiole-1-2 thiones-3 comportant des substituants hydrocarbonés ou des noyeaux condensés. Bull Soc Chim Fr. 1953:327–331. [Google Scholar]
- 21.Brown JP. 5-Thiation of 1,2-dithiole-3-thiones. J Chem Soc (C) 1968:1077–1082. [Google Scholar]
- 22.Trebaul C, Teste J. Composés soufrés hétérocycliques. IV. Action du pentasulfure de phosphore sur des esters β-cétoniques substitués en α par un -Cl, un -CN ou un - COOC2H5. Bull Soc Chim Fr. 1969:2456–2462. [Google Scholar]
- 23.Klingsberg E. 1,2-Dithiolium salts: a new pseudoaromatic cationoid system. Chemy Ind. 1960:1568–1569. [Google Scholar]
- 24.Böttcher B, Bauer F. Über Trithione, V. Mitteil.: Notiz über einige neue Trithione. Chem Ber. 1951;84:458–463. [Google Scholar]
- 25.Stachel HD, Zoukas T. Über die Farbreaktion nach Ogston. Arch Pharm (Weinheim) 1991;324:131–132. [Google Scholar]
- 26.Gewald K. Heterocyclen aus CH-aciden Nitrilen. V. Gemeinsame Einwirkung von Schwefel und Schwefelkohlenstoff auf methylenaktiv Nitrile. J Prakt Chem. 1966;31:214–220. [Google Scholar]
- 27.Mayer R, Wittig P, Fabian J, Heitmüller R. Trithione aus Enaminen, Schwefelkohlenstoff und Schwefel. Chem Ber. 1964;97:654–660. [Google Scholar]
- 28.Boberg F, Knoop J. 1.2-Dithia-cyclopentenone-(3) aus Trithionen. Liebigs Ann Chem. 1967;708:148–154. [Google Scholar]
- 29.Thuillier A, Vialle J. Composes organiques sufurés. VII. Condensation du sulfure de carbone et des cyclanones. Bull Soc Chim Fr. 1962:2194–2198. [Google Scholar]
- 30.Ernster E. DT diaphorase. Meth Enzymol. 1967;10:309–317. [Google Scholar]
- 31.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]


