Abstract
Diffusion tensor imaging (DTI) is a valuable tool for assessing presumptive white matter alterations in human disease and animal models. The current study used DTI to examine the effects of selective neurotoxic lesions of the hippocampus on major white matter tracts and anatomically related brain regions in macaque monkeys. Two years post-lesion, structural MRI and DTI sequences were acquired for each subject. Volumetric assessment revealed a substantial reduction in the size of the hippocampus in experimental subjects, averaging 72% relative to controls, without apparent damage to adjacent regions. DTI images were processed to yield measures of fractional anisotropy (FA), apparent diffusion coefficient (ADC), parallel diffusivity (lADC), and perpendicular diffusivity (tADC), as well as directional color maps. To evaluate potential changes in major projection systems, a region of interest (ROI) analysis was conducted including the corpus callosum, fornix, temporal stem, cingulum bundle, ventromedial prefrontal white matter and optic radiations. Lesion-related abnormalities in the integrity of the fiber tracts examined were limited to known hippocampal circuitry, including the fornix and ventromedial prefrontal white matter. These findings are consistent with the notion that hippocampal damage results in altered interactions with multiple memory-related brain regions, including portions of the prefrontal cortex.
The development of the magnetic resonance imaging (MRI) technique diffusion tensor imaging (DTI) permits the in vivo evaluation of the structural integrity of brain tissue. DTI reflects displacement of water molecules, which is affected by cellular transport and non-permeable membranes in the brain (for review see Beaulieu, 2002). Fractional anisotropy (FA) and apparent diffusion coefficient (ADC) are two measures commonly analyzed in DTI. FA measures the strength of orientation of water motion ranging from a value of 0, when movement is locally unrestricted and isotropic, as in the ventricles, to 1, reflecting highly directional, anisotropic movement, as in dense myelinated fiber tracts (Beaulieu, 2002). ADC measures diffusion of water molecules independent of the direction of motion. Alterations in anisotropy and diffusivity have been reported during normal brain development as well as following demyelination, axonal loss, and axonal reorganization (Beaulieu, 2002; Dijkhuizen, 2006; Sundgren et al., 2004; Voss et al., 2006). The precise microstructural basis of measures derived from DTI, however, remains to be confirmed (e.g Beaulieu, 2002; Schwartz and Hackney, 2003; Paus, 2009). The current study used DTI techniques to evaluate the long-term effects of hippocampal lesions on the integrity of major white matter projections in macaque monkeys.
Twelve young adult male rhesus monkeys (7–8 yrs; Macaca mulatta) served as subjects. Two years prior to DTI (at age 5–6 yrs), six of the monkeys received bilateral injections of the excitotoxin N-methyl-D-aspartic acid (NMDA) into the hippocampus via a longitudinal approach using methods described elsewhere (Hampton et al., 2004). The other six remained as unoperated controls. In the current study, structural MRI magnetization prepared rapid gradient echo (MPRAGE) and DTI sequences were acquired for each subject using a Siemens Allegra 3 T MRI scanner (Siemens Medical Solutions USA, Malvern, PA). First, a conventional 3D T1-weighted MPRAGE sequence was acquired with the following parameters: 208 slices, TR = 2,140 ms, TE = 4.38 ms, TI = 1100 ms, matrix = 96 × 96, field of view (FOV) = 16 × 16 cm, flip angle = 8°, with a scan time of 9:09 min. Second, fifteen 3.0 mm slices (skip 0.36 mm) were acquired for DTI analysis with the following scanning parameters: TR = 4,500 ms, TE = 78 ms, 12 diffusion directions, 13 averages, b = 1000 s/mm2, in plane resolution 1.3 × 1.0 mm, FOV = 128 × 128 mm, with a scan time of 12:45 min. Experimental procedures were approved by the NIMH and Mount Sinai School of Medicine Institutional Animal Care and Use Committees and conformed to the United States Public Health Service policy on Humane Care and Use of Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
To evaluate the extent of the hippocampal lesions, structural MRI images were processed into 3D isotropic voxels and aligned perpendicular to the long axis of the hippocampus. The hippocampus was traced as described previously (Shamy et al., 2006), and included the dentate gyrus, CA1–3 fields, presubiculum, subiculum and parasubiculum. Qualitative observation of the structural MRI scans revealed substantial atrophy of the hippocampus in all monkeys that sustained excitotoxic lesions, without apparent damage to neighboring regions. The quantitative volumetric data were analyzed by a repeated measures ANOVA with group (hippocampal lesion, control) as a between-subjects factor and hemisphere (left, right) as a within-subjects factor. As expected, this analysis revealed a significant main effect of group, confirming that the volume of the hippocampus was significantly smaller in monkeys that received NMDA injections than among controls (F(1,10) = 234.62, P < 0.001). Hippocampal volume averaged 379.02 mm3 for controls and 106.92 mm3 for monkeys with lesions, a 72% reduction (Supplementary Figure. 1). There was no significant main effect of hemisphere (F(1,10) < 0.01, P = 0.976) and no interaction between hemisphere and group (F(1,10) = 0.311, P = 0.590). This degree of atrophy is highly consistent with a post-mortem histological evaluation in hippocampectomized monkeys in which it was shown that 68–79% volume reduction corresponds to 96–99% neuronal damage (Malkova et al., 2001).
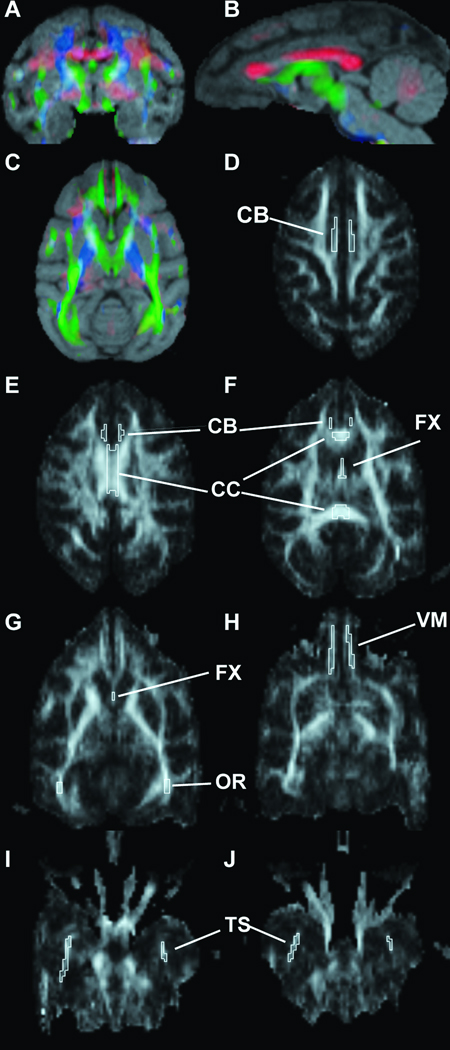
Potential alterations in the integrity of major white matter tracts were assessed using images acquired from the DTI sequences. Scans were analyzed using the Analyze 8.1 DTI add-on module to produce FA, ADC and directional color maps (Supplementary Figure. 2). Further, parallel diffusion (lADC, the diffusion along the primary direction of movement), and transverse diffusion (tADC, the diffusion perpendicular to the primary direction of motion), have been suggested to provide additional information about the integrity of axonal projections and myelin sheaths, respectively (Concha et al., 2006). Therefore, in-house software was used to calculate maps of lADC and tADC. Images were coregistered with structural MRI scans from each animal to provide an individualized anatomical reference (Fig 1a–c). A region of interest (ROI) analysis was conducted centering on the corpus callosum, fornix, temporal stem, cingulum bundle, ventromedial prefrontal white matter and optic radiations (Fig. 1d–j) and were traced on the colormaps using the co-registered structural image for reference. One lesion subject was excluded from the ventromedial prefrontal white matter analysis due to image distortion in the frontal lobe on that scan. For regions where the left and right sides were enclosed within individual borders, DTI measures were averaged across hemispheres for analysis. Intra-rater reliability was substantial, ranging from 0.95 to 0.97 as measured by tracing all regions for 3 subjects in each group twice.
Figure 1.
To provide a qualitative anatomical reference, the directional DTI color maps were coregistered with structural MRI scans for each animal (A–C). Colors indicate the orientation of the white matter tracts (blue = D/V; green = A/P; red = R/L). To evaluate potential changes in major projection systems, a region of interest (ROI) analysis was conducted on several areas including the cingulum bundle (CB; D–F), corpus callosum (CC; E,F), fornix (FX; F,G), optic radiations (OR; G), white matter adjacent to the ventromedial prefrontal cortex (VM; H) and the temporal stem (TS; I,J).
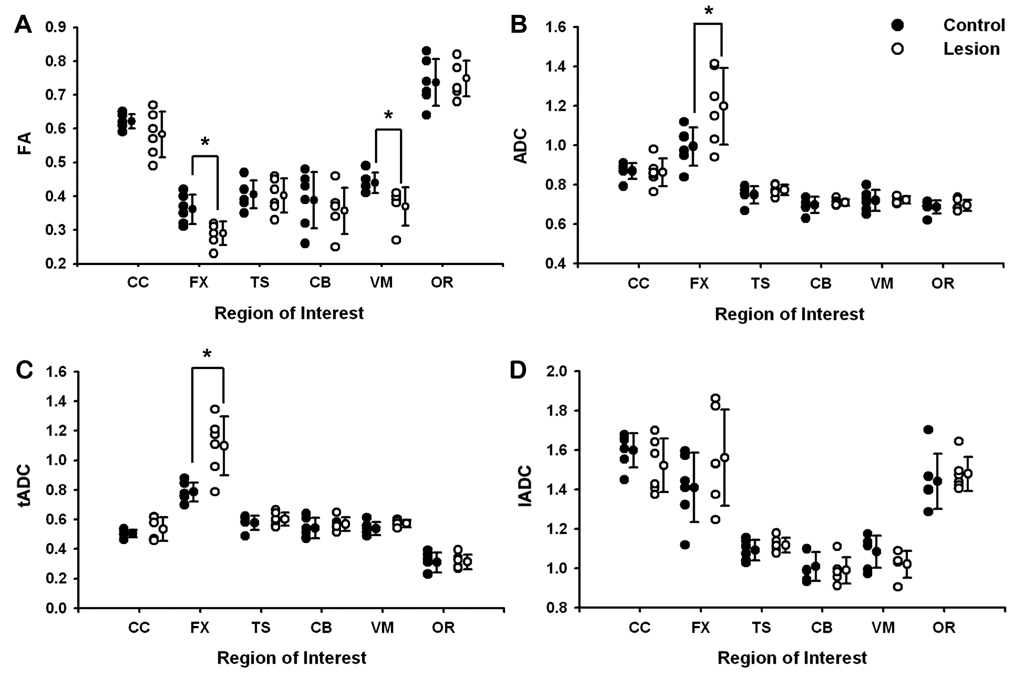
Means and standard errors for FA, ADC, tADC, and lADC for each ROI are reported in Supplementary Table 1. Lesion-related changes in the integrity of fiber tracts were observed in the fornix and white matter adjacent to the ventromedial prefrontal cortex. In the fornix, FA was decreased (t = 3.13, p = 0.011), whereas ADC (t = 2.30, p = 0.044) and tADC were greater (t = 3.66, p = 0.004) in lesion subjects relative to controls. Fornix lADC, in contrast, was statistically equivalent across groups. FA was also decreased in the ventromedial prefrontal white matter of experimental animals compared with controls (t = 2.61, p = 0.028), but without corresponding changes in measures of diffusivity. DTI measures were preserved in the corpus callosum, temporal stem, cingulum bundle, and optic radiations (p-values > 0.05; Fig. 2).
Table 1.
Means and SE for DTI measures by group
| N | FA | ADC | tADC | lADC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | |||
| CC | Control | 6 | 0.62 | 0.01 | 0.87 | 0.02 | 0.50 | 0.01 | 1.60 | 0.04 |
| Lesion | 6 | 0.58 | 0.03 | 0.86 | 0.03 | 0.53 | 0.03 | 1.52 | 0.06 | |
| FX | Control | 6 | 0.36 | 0.02 | 0.99 | 0.04 | 0.79 | 0.03 | 1.41 | 0.07 |
| Lesion | 6 | 0.29 | 0.01 | 1.20 | 0.08 | 1.10 | 0.08 | 1.56 | 0.10 | |
| TS | Control | 6 | 0.41 | 0.02 | 0.75 | 0.02 | 0.58 | 0.02 | 1.09 | 0.02 |
| Lesion | 6 | 0.40 | 0.02 | 0.77 | 0.01 | 0.60 | 0.02 | 1.12 | 0.02 | |
| CB | Control | 6 | 0.39 | 0.03 | 0.70 | 0.02 | 0.54 | 0.03 | 1.01 | 0.03 |
| Lesion | 6 | 0.36 | 0.03 | 0.71 | 0.01 | 0.57 | 0.02 | 0.99 | 0.03 | |
| VM | Control | 6 | 0.44 | 0.01 | 0.72 | 0.02 | 0.54 | 0.02 | 1.08 | 0.03 |
| Lesion | 5 | 0.37 | 0.03 | 0.72 | 0.01 | 0.57 | 0.01 | 1.02 | 0.03 | |
| OR | Control | 6 | 0.74 | 0.03 | 0.69 | 0.01 | 0.31 | 0.03 | 1.44 | 0.06 |
| Lesion | 6 | 0.75 | 0.02 | 0.70 | 0.01 | 0.31 | 0.02 | 1.48 | 0.04 |
FA in Arbitrary Units, ADC, tADC, lADC in (10−3 mm2/s)
Figure 2.
Quantitative results for individual subjects (symbols) and group means (± S.E.M.) for fractional anisotropy (A), apparent diffusion coefficient (B), transverse diffusivity (C), and parallel diffusivity (D). Lesion-related changes in the integrity of the fiber tracts examined were limited to the fornix and white matter adjacent to the ventromedial prefrontal cortex. DTI measures were unchanged in the corpus callosum, temporal stem, cingulum bundle and optic radiations. ADC (10−3 mm2/s); CC, corpus callosum; FX, fornix; TS, temporal stem; CB, cingulum bundle; VM, white matter adjacent to the ventromedial prefrontal cortex; and OR, optic radiations.
This study is among the first to demonstrate alterations of white matter integrity following selective lesions of the hippocampus using in vivo DTI methods. Our findings for the fornix are consistent with previous reports that chronic neuronal or axonal injury results in increased tADC (Concha et al., 2006; Schwartz and Hackney, 2003), potentially reflecting disruption of the myelin sheath (George and Griffin, 1994). The acute effects of such lesions, by comparison, lead to decreased lADC (Concha et al., 2006; Schwartz and Hackney, 2003) that may represent the disruption of axons (George and Griffin, 1994; Kerschensteiner et al., 2005).
As predicted, excitotoxic hippocampal lesions were associated with selective alterations in the integrity of the fornix and white matter in the region of the ventromedial prefrontal cortex. The observation that compromised integrity was regionally selective is consistent with the known anatomical organization of the pathways examined. The uncinate fascicle, for example, carries reciprocal projections between inferior temporal cortex and lateral and orbital prefrontal cortex (Ungerleider et al., 1989). Lacking a major hippocampal contribution, preservation following lesions of the hippocampus was expected in these projections. In contrast, many of the connections between the hippocampus and cortical and subcortical regions are conveyed via the fornix (Aggleton and Saunders, 1997; Rosene and Van Hoesen, 1977), including those to the orbital and medial regions of the prefrontal cortex (for review see Cavada et al., 2000). Our results are also consistent with findings reported by Croxson et al. (2005) using diffusion-weighted imaging tractography to examine connections of the prefrontal cortex in macaque monkeys and humans. That study found that the fornix in both species contains fibers terminating in the ventromedial portion of the prefrontal cortex (their PFCom) as well as in medial frontal cortical areas in the anterior cingulate gyrus.
Although the hippocampus receives the bulk of its inputs directly and indirectly from the entorhinal and perirhinal cortex - structures known to be essential for certain types of memory - it is of interest that impairments in recall are often associated with disrupted interaction across a network of anatomically connected brain regions including the hippocampus, mammillary bodies (which receive their hippocampal input entirely via the fornix), the anterior thalamic nuclei, and portions of the orbital and medial prefrontal cortex (Aggleton and Brown, 1999). Transection of the fornix in macaque monkeys, and partial or complete damage to the fornix in humans, produces memory impairment (humans: Aggleton et al., 2000; Gaffan et al., 1991; monkeys: Brasted et al., 2003; Brasted et al., 2005; Gaffan, 1994). Moreover, in one study with a large cohort of patients who underwent removal of colloid cysts, and therefore risked fornix damage, volumes of the mammillary bodies and fornix best predicted measures of recall (Tsivilis et al., 2008). This same study reported a relationship between the volume of the ventromedial prefrontal cortex (their OMPFC) and recall, albeit a much less consistent relationship than for the mammillary bodies and fornix (Tsivilis et al., 2008). The findings are noteworthy here because many of the hippocampal fibers terminating in the ventromedial prefrontal cortex travel via the fimbria-fornix (Cavada et al., 2000). Results of the present study extend this background, demonstrating that DTI is sufficiently sensitive to detect the effects of cell body-selective, neurotoxic lesions of the hippocampus on the organization of projections between memory-related brain regions in monkeys. Our observations are consistent with the proposal that memory deficits following damage restricted to the hippocampus arise from disrupted network interactions that include the prefrontal cortex.
Supplementary Material
Hippocampal ROIs as shown in an intact control (A) and lesioned (B) monkey. Qualitative observation revealed substantial atrophy in all lesion subjects without apparent additional damage to neighboring regions. Lesions were extensive throughout the rostro-caudal extent of the hippocampus (C) and resulted in a 72% reduction in volume bilaterally (D). H, Hippocampus; TLV, Temporal horn of the lateral ventricle; WM, white matter.
To evaluate the effects of neurotoxic hippocampal lesions on major white matter tracts, DTI scans were processed into fractional anisotropy (FA; A), directional color maps (B), 3D structural MRI stacks (C), apparent diffusion coefficient (ADC, D), parallel diffusion coefficient (lADC; E), and transverse apparent diffusion coefficient (tADC; F). D/V, dorsal-ventral; R/L, right-left; A/P, anterior-posterior; WM, white matter; GM, gray matter; EX, excluded areas outside of the brain.
Acknowledgments
We would like to thank Frank Macaluso, and Drs. Reginald Miller and Gregg Goldschlager for their support and expert assistance throughout the study and Drs. Paula Croxon and Mark Baxter for their thoughtful comments on the manuscript. Support contributed by NIH grants AG10606, MH62448, MH58911, and in part by the Intramural Research Programs of the NIA and NIMH.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22(3):425–444. discussion 444–89. [PubMed] [Google Scholar]
- Aggleton JP, McMackin D, Carpenter K, Hornak J, Kapur N, Halpin S, Wiles CM, Kamel H, Brennan P, Carton S, et al. Differential cognitive effects of colloid cysts in the third ventricle that spare or compromise the fornix. Brain. 2000;123(Pt 4):800–815. doi: 10.1093/brain/123.4.800. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Saunders RC. The relationships between temporal lobe and diencephalic structures implicated in anterograde amnesia. Memory. 1997;5(1–2):49–71. doi: 10.1080/741941143. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Role of the hippocampal system in associative learning beyond the spatial domain. Brain. 2003;126(Pt 5):1202–1223. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Bussey TJ, Murray EA, Wise SP. Conditional motor learning in the nonspatial domain: effects of errorless learning and the contribution of the fornix to one-trial learning. Behav Neurosci. 2005;119(3):662–676. doi: 10.1037/0735-7044.119.3.662. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci. 2004;24(8):2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. 2006:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25(39):8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM. Application of magnetic resonance imaging to study pathophysiology in brain disease models. Methods Mol Med. 2006;124:251–278. doi: 10.1385/1-59745-010-3:249. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transaction. J. Cog. Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Gaffan D, Hodges JR. Amnesia following damage to the left fornix and to other sites. A comparative study. Brain. 1991;114(Pt 3):1297–1313. doi: 10.1093/brain/114.3.1297. [DOI] [PubMed] [Google Scholar]
- George R, Griffin JW. The proximo-distal spread of axonal degeneration in the dorsal columns of the rat. J Neurocytol. 1994;23(11):657–667. doi: 10.1007/BF01181641. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Hampstead BM, Murray EA. Selective hippocampal damage in rhesus monkeys impairs spatial memory in an open-field test. Hippocampus. 2004;14(7):808–818. doi: 10.1002/hipo.10217. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11(5):572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Malkova L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11(4):361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition. 2009 doi: 10.1016/j.bandc.2009.06.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198(4314):315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, Hackney DB. Diffusion-weighted MRI and the evaluation of spinal cord axonal integrity following injury and treatment. Exp Neurol. 2003;184(2):570–589. doi: 10.1016/S0014-4886(03)00295-4. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27(10):1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46(5):339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11(7):834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Gaffan D, Pelak VS. Projections from inferior temporal cortex to prefrontal cortex via the uncinate fascicle in rhesus monkeys. Exp Brain Res. 1989;76(3):473–484. doi: 10.1007/BF00248903. [DOI] [PubMed] [Google Scholar]
- Voss HU, Uluc AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, Heier LA, Beattie BJ, Hamacher KA, Vallabhajosula S, et al. Possible axonal regrowth in late recovery from the minimally conscious state. J Clin Invest. 2006;116(7):2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hippocampal ROIs as shown in an intact control (A) and lesioned (B) monkey. Qualitative observation revealed substantial atrophy in all lesion subjects without apparent additional damage to neighboring regions. Lesions were extensive throughout the rostro-caudal extent of the hippocampus (C) and resulted in a 72% reduction in volume bilaterally (D). H, Hippocampus; TLV, Temporal horn of the lateral ventricle; WM, white matter.
To evaluate the effects of neurotoxic hippocampal lesions on major white matter tracts, DTI scans were processed into fractional anisotropy (FA; A), directional color maps (B), 3D structural MRI stacks (C), apparent diffusion coefficient (ADC, D), parallel diffusion coefficient (lADC; E), and transverse apparent diffusion coefficient (tADC; F). D/V, dorsal-ventral; R/L, right-left; A/P, anterior-posterior; WM, white matter; GM, gray matter; EX, excluded areas outside of the brain.