Abstract
Objective
To use a Progressive Ratio (PR) computerized “work” paradigm to measure course and correlates of exercise motivation in inpatients with Anorexia Nervosa (AN).
Method
Sixteen inpatients with AN participated in a PR task assessing the relative reinforcing effect of 2 different increments of cash versus the opportunity to exercise for up to 30 minutes, twice; at low weight, and, for n=10 participants, after weight restoration.
Results
There was a trend toward a higher work for exercise with $2 versus $5 increments of cash as the alternative reinforcer. Exercise breakpoint did not differ between low and normal-weight states. Exercise breakpoint at each time point was correlated with pre-hospitalization exercise “commitment” (Commitment to Exercise Scale, r=0.613, p=0.012 at T1; r=0.634, p=0.049 at T2).
Discussion
Patients with AN will work at a PR task for access to even a small amount of exercise. Exercise motivation during hospitalization is correlated with pre-hospital exercise commitment, and does not appear to change consistently with weight restoration.
Background
Anorexia Nervosa (AN) is a serious illness featuring self-starvation with a high rate of relapse and chronicity (1). Exercise behavior occurs commonly, though not universally, in patients with AN, and has been linked in some studies to worsened clinical outcome (e.g., (2;3)). The avidity with which some individuals with AN pursue exercise suggests that it is highly reinforcing in at least a proportion of individuals with this disorder. Understanding the reinforcing effect of exercise, its course and its mediators in patients with AN, may ultimately lead to improved treatments for this challenging disorder.
The field of behavioral economics provides a set of tools to measure the reinforcing effects of a range of activities including eating, use of drugs of abuse, and other “motivated behaviors.” Laboratory paradigms quantify the reinforcing effect of a target behavior in terms of the amount of “work” an individual is willing to expend to access it (4). The progressive ratio (PR) task measures the reinforcing value of an object or behavior for a particular individual by requiring the individual to expend progressively increasing amounts of effort to earn access to it. Effort in laboratory animals is most often based on the number of bar presses while in humans effort or “work” is most often based on the number of key taps on a computer. Breakpoint for a stimulus is defined as the number of responses completed for a given reinforcer before he or she stops working for it. Total work and breakpoint are related, and the more reinforcing a stimulus is, the higher the breakpoint (4). Reinforcing effect is measured when one stimulus is offered and the relative reinforcing effect is measured when two or more alternative choices are provided concurrently. The latter paradigm provides the advantage of mimicking real-world situations in which engaging in one reinforcing behavior likely involves the choice of that behavior at the expense of alternative behaviors or reinforcers (e.g., an individual’s choice of drug versus food).
The PR and related tasks are well-established tools in drug abuse research, and recent investigations have extended them to examination of the reinforcing value of food under a variety of conditions (5) including under food deprivation (6), in varying populations (7);(8), and in association with genetic polymorphisms in the brain dopamine system (9). This procedure has also been applied to measuring the relative reinforcing value of different active and sedentary activities in children (10);(11) and in sedentary adults (12). Breakpoint is sensitive to the motivational state of an individual (e.g., food deprivation increases responding for food (4)), and may better predict actual consumption than subjective ratings of liking (5). The PR procedure can be used to guide treatment interventions by providing a means to assess the effects of pharmacological and other manipulations on the reinforcing value of a target behavior in a laboratory setting.
Previously we presented the use of a Progressive Ratio (PR) paradigm to quantify the reinforcing effect of exercise relative to a monetary voucher in a sample of 16 inpatients with AN (13). In this investigation we found that patients performed an average of 1134 (±751) button presses for the opportunity to exercise on a treadmill for a maximum of either 15 or 30 minutes. Furthermore, exercise breakpoint was significantly correlated with self-reported depression per the Beck Depression Inventory (BDI), and a trend was found toward a correlation between exercise breakpoint and the Commitment to Exercise Scale (CES; (14)). Main limitations of the initial study were assessment at a single time point and use of an alternative reinforcer, an Amazon.com voucher with maximum value of $20, that appeared to be too low to effectively compete with exercise in this population. The current study addresses these limitations by assessing exercise reinforcement both at low weight and following weight restoration, and by modifying the reinforcers to be either 3 min per trial of exercise for a maximum earning of 30 min exercise upon completion of the maximum 10 trials, versus $2 or $5 in cash per trial, for a maximum possible earning of $20 or $50 per session (counterbalanced). The aims of the study were to determine (1) whether the relative reinforcing effect of exercise is modified in the setting of a variable (and potentially more valuable) alternative reinforcer; (2) whether the relative reinforcing effect of exercise changes with weight restoration; and (3) which clinical variables are correlated with the relative reinforcing effect of exercise among inpatients with AN.
Methods
Participants
Study participants were 16 patients hospitalized on the General Clinical Research Unit of the New York State Psychiatric Institute (NYSPI)/Columbia University Medical Center and receiving treatment for AN. All participants were women between the ages of 18 and 45 years, and all met DSM-IV criteria for AN (with the allowable exception of the requirement for amenorrhea (15)). Patients were required to be medically stable and weigh a minimum of 75% Ideal Body Weight (IBW) to participate. Potential participants were excluded if they had significant medical illness or pregnancy; substance dependence in the past 6 months; psychosis, or were at suicide risk. All patients were free of psychotropic medication for a minimum of 2 weeks prior to the first set of study sessions. One participant started sertraline between the two study time points; remaining participants took no psychotropic medication throughout the study. All participants signed informed consent prior to participation in this study, and all study procedures were approved of the NYSPI Institutional Review Board.
The GCRU features a behavioral protocol aimed at restoration of weight to 90% IBW. During hospitalization, physical activity is initially limited to activities of daily living within the confines of the inpatient unit. Failure to gain weight at an expected rate results in reduction of activity privileges (“bed rest”). As weight gain progresses, patients are permitted to have supervised off-unit time that was limited to select areas within the facility, and, once stable at or above 90% IBW (weight maintenance phase), are permitted to leave the inpatient unit for up to 24 hours on a pass. Formal exercise, including the use of exercise equipment, is not permitted during the weight restoration phase of treatment, and is highly discouraged during the weight maintenance phase. Throughout hospitalization, surreptitious exercise (e.g., in patients’ bedrooms) is feasible, but discouraged.
Ten of the sixteen patients enrolled in low-weight testing successfully completed weight restoration under this protocol and participated in the second testing sessions scheduled upon achieving 90% IBW. One additional patient completed the inpatient treatment but developed cardiac instability and was excluded from weight-restored study participation.
Progressive Ratio Task
At each time point, low-weight and 90% IBW, patients participated in two 40-min PR task sessions, scheduled at least 48 hours but no greater than two weeks (mean=4.9, SD=3.2 days) apart. The timing of the low-weight sessions depended on when patients achieved the minimum weight for testing and were medically stable. The second set of sessions was conducted as soon as possible after participants reached 90% IBW (mean of 34 ±10.4) days after first set of sessions). All PR sessions were conducted on a weekday morning (starting between 9:30 and 10:30 am) after patients ate a supervised breakfast at 8:00 am. Neither caloric content nor food content of the meal was standardized across patients.
During each session, an individual worked for all or part of a maximum amount of time (30 min) for which she would be permitted to exercise, or for all or part of a maximum amount of cash (either $20 or $50, randomized and counterbalanced across study days) she could earn. Work consisted of finger presses on a computer keyboard. Each PR session consisted of 10 trials, with 1/10th the maximum amount of exercise time or cash earned at each trial.
To begin a PR task session, the participant selected a computer screen icon that represented the reinforcer of her choice, opportunity to exercise or cash. Once this was selected, the participant was required to continue to work for that reinforcer until that trial was completed (or until she stopped working for the session). After completion of a trial, the participant had eight seconds to decide which reinforcer she would work for at the next trial. She could change her selection at the beginning of a trial but not within a trial (i.e., after she had pressed the keypad multiple times). The work required in the first trial for each reinforcer was 50 presses; however, the work requirement increased each subsequent trial that reinforcer was selected. At the first trial a given reinforcer was chosen, 50 presses were required. The second time it was selected, 250 presses were required, and then 450, 650, 850, 1050, 1250, 1450, 1650, and 1850 presses were required to complete the trial. If the participant selected the alternative reinforcer, it would require 50 button presses, then 250, 450, etc. Thus, the work requirement for each reinforcer increased independently during the session. To earn the maximum amount of exercise or cash, the participant had to perform 9,500 keyboard finger presses within the 40-min period. Fewer presses were required if choices were distributed between the two reinforcers. Patients were informed prior to beginning the experiment about these procedures. At the end of each trial, an icon representing the earned reinforcer was displayed on the right side of the computer screen. At the end of the 40-min session, total earnings were displayed.
Choice reinforcers were time to exercise and cash. For exercise earnings, individuals were allowed access to a treadmill, which was shown to participants prior to experimental sessions. Exercise earnings were up to 30 min brisk walking with a maximum speed of 4.0 mph. Exercise was monitored by a study physician using an earlobe pulse monitor with target heart rate in the 40–50% maximal heart rate range, consistent with light to moderate exercise (16). Potential monetary earnings (cash) was also shown to participants prior to experimental sessions and provided immediately after the PR session. Cash earnings were up to $20 or $50, counterbalanced by study session (day). The reason for measuring the reinforcing effect of exercise relative to two different increments of cash was to determine whether the breakpoint for exercise would differ as a function of alternative. In an optimal paradigm, a plateau of responding would be achieved over multiple “doses” of an alternative reinforcer, such that a participant would respond less for the reinforcer in question when a large enough or valuable enough alternative reinforcer was available (17).
Progressive Ratio Breakpoint
The largest completed number of button presses (i.e., trial) for a given reinforcer, termed the PR breakpoint, is thought to provide a measure of the reinforcing efficacy of that object or activity. A larger PR breakpoint indicates a more potent reinforcer. We hypothesized that the PR breakpoint would be relatively larger for exercise when the alternative cash reinforcer was $2 per trial as compared with $5 per trial. We also hypothesized that the breakpoint for exercise would be higher at low weight (75% IBW; first study time point or T1) than at 90% IBW (second study time point or T2). Furthermore we hypothesized that individual differences would be observed among patients in exercise breakpoint, with predicted clinical correlates of the breakpoint for exercise including the CES and the BDI (per our previous findings (13)).
Instruments
Initial self-report ratings were completed by participants within 2 weeks of hospital admission. These included the Commitment to Exercise Scale (CES(14)), an 8-item visual analogue scale assessing pathological and obligatory aspects of exercise behavior; total score on CES is expressed as a percentage (with 100 the maximum obtainable score); the Beck Depression Inventory (BDI, (18)), the Beck Anxiety Inventory (BAI, (19)), and the Eating Disorder Inventory (EDI, (20)). The CES was also completed by 8 of 10 participants upon weight restoration (within 2 weeks of the T2 PR task). The Pearson correlation coefficient was calculated to determine whether an association was present between results of the PR task and these clinical measures, as well as age and body mass index (BMI) upon hospitalization, and duration of illness in years.
Comparison with Previous Study
We also compared data obtained from the first study time point to those presented in our first paper (13), which was conducted using an essentially identical paradigm with an independent cohort of participants. As described in the Background section of the current paper, major differences between the 2 studies were the reinforcers: in the first study, the amount of exercise varied between 1.5 and 3 min per trial vs a constant dose of monetary voucher ($2); in the current study, the amount of cash varied between $2 and $5 per trial with a constant dose of exercise (3 min). Independent sample t-test was conducted to determine whether a significant difference was present between exercise breakpoint for 3 min exercise under the $2 cash alternative reinforcer condition (Study 2) versus $2 voucher condition (Study 1) condition (n=16 participants in each group), and under the $5 cash (Study 2) versus $2 voucher (Study 1) condition (n=16 in each group). Independent t-test was performed for clinical variables including age, admission BMI, duration of illness and BDI between the two cohorts.
All statistical analyses were performed using SPSS version 16.0 (Chicago, IL).
RESULTS
Participant Characteristics
Sixteen women participated. Six met criteria for AN-Restricting subtype, while 10 met criteria for AN Binge/Purge subtype. Participants were a mean age of 27 (SD=7.8), with average reported duration of illness of 11.4 (±8.4) years, and body weight of 68.9 (±7.7) percent Ideal Body Weight (IBW) upon hospital admission. Participants were an average of 78.3 (±2.3) %IBW at the first study sessions and 91.2 (±2.8) %IBW at the second sessions (n=10). Patients were hospitalized an average of 23.1 (±16.7) days before the first study sessions and 56.2 (±20.9; n=10) days before the second sessions.
Progressive Ratio Task
When participants were allowed to work for exercise compared with the $2 cash alternative reinforcer, 15 patients completed all 10 trials and one completed 9 trials. The mean breakpoints for exercise are presented in Table 1. When participants were allowed to work for exercise compared with the $5 cash alternative reinforcer, 13 completed 10 trials at both sessions, two completed 9 trials, and one completed 8 trials. For three participants completing less than 10 trials, the session in which less than 10 trials were completed was the first study session. For the remaining participant who completed 9 trials, a computer error occurred preventing her completion of the task.
Table 1.
Exercise Breakpoints and Psychological Ratings at Timepoints 1 and 2
| Study Timepoint | Exercise Breakpoint, $2 cash condition Mean (SD) | Exercise Breakpoint, $5 cash condition Mean (SD) | CES Mean (SD) | BAI Mean (SD) | BDI Mean (SD) | EDI Mean (SD) |
|---|---|---|---|---|---|---|
| T1 (Approx. 75% IBW; n=16) | 793.7 (786.7) | 465.6 (607.4) | 61.7 (30.8) | 19.8a (12.4) | 26.9b (11.8) | 73.1 (29.5) |
| T2 (Approx. 90% IBW; n=10) | 530 (652) | 525 (762.8) | 45.3 (32.1; n=8) | 7.2 (6.6) | 14.4 (12.4) | 62.2 (36.9) |
CES = Commitment to Exercise Scale, BAI = Beck Anxiety Inventory, BDI = Beck Depression Inventory, EDI = Eating Disorder Inventory, total of all subscales. BAI, BDI and EDI data are from n=16 patients at low weight and n=8 patients at 90%IBW.
Significantly lower at T2 compared with T1, p=0.024.
Significantly lower at T2 compared with T1, p=0.004.
When comparison was made between the $2 alternative reinforcer condition and the $5 condition, at T1, a trend was observed for greater work for exercise when $2 cash was the alternative compared to when $5 cash was the alternative (p=0.096). Breakpoint for exercise did not differ significantly under these conditions (p=0.114). Nine participants had larger exercise breakpoints under the $2 condition, three had larger exercise breakpoints under the $5 condition, and four showed no difference between the two conditions. At T2, four participants had larger exercise breakpoint under the $2 condition, one had larger exercise breakpoint under the $5 condition, and four showed no difference between the two conditions.
When exercise breakpoint was compared within participants across study time points, four participants showed higher breakpoint for exercise at the T2 compared with T1, four showed lower breakpoint, and three showed equal breakpoint. Of the three who had equal breakpoint, all worked exclusively for cash at each study session. No significant difference was found in the group as a whole between breakpoints between T1 and T2.
Psychological Ratings
Scores on the BAI, BDI, and EDI at each time point, and score on the CES at hospital admission are also presented in Table 1. Scores on the BAI and BDI decreased significantly between T1 and T2 (p values <0.05). Scores on the CES were available for 8 of 10 completers at T2 and did not change significantly between assessment time points (p=0.163).
Correlates of Exercise Breakpoint
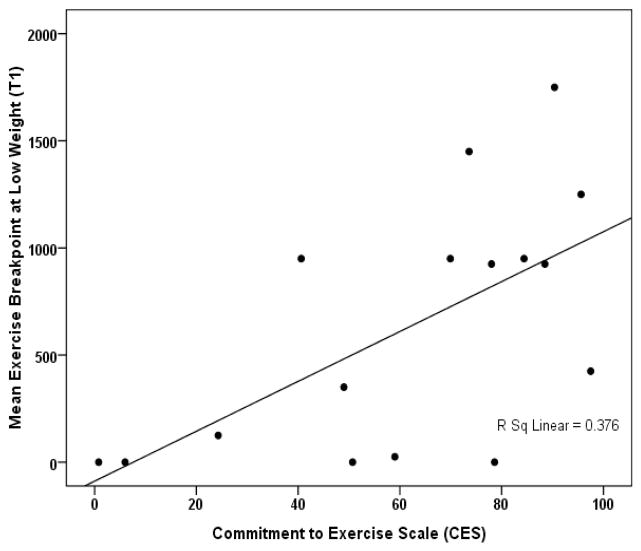
Breakpoint averaged over the first two sessions was correlated with CES score obtained on hospital admission (averaged over $2 and $5 alternative reinforcer conditions, r=0.613, p=0.012, Figure 1). When the two sessions were considered separately, CES better predicted exercise breakpoint obtained using the $2 cash alternative reinforcer (r=0.666, p=0.005) than that obtained using the $5 cash alternative reinforcer (r=0.317, p=0.231). For the 10 participants who completed the 90% IBW testing, mean exercise breakpoint at T2 was correlated with mean exercise breakpoint at T1 (r=0.863, p=0.001) and also with admission CES (r=0.634, p=0.049), though not with T2 CES (r=0.579, p=0.133). Breakpoint was not correlated with other clinical factors including age, duration of illness, or scores on psychological assessments, or with duration of time to the first study session or time in between sessions.
Figure 1.
Relationship between pre-admission CES and exercise breakpoint (averaged over two sessions) at 75% IBW in 16 inpatient women with AN.
Comparison of Subtypes and Study Completers versus Non-completers
No differences were found between the AN-R and AN-B/P subtype with regard to exercise breakpoint or total work for exercise at either time point. Clinical measures including CES did not differ between subtypes. Of AN-R participants, 3 of 6 completed the study; of AN-B/P participants, 7 of 10 completed the study.
Comparison of study completers with non-completers on clinical and behavioral measures showed a near-significant trend toward larger exercise breakpoint among non-completers compared with completers (1983 ±696 vs 825 ±1203, p=0.051). No difference was observed in admission percent IBW, CES, BDI, BAI or EDI, or age or duration of illness between the two groups of patients.
Comparison to Previous Study
When exercise breakpoint data were compared with data presented in our previous paper, patients had higher breakpoints for exercise under the $2 voucher condition (1128 ± 763) compared with the $5 cash session (466 ±607), but not compared with the $2 cash session (794 ±787; p values 0.011 and 0.232, respectively). Clinical measures compared did not differ between the two cohorts of patients. The %IBW at time of the first session was higher in Study 1 than in Study 2 (81.15 ±4.37 vs 78.28 ±2.32, respectively; t[df=30]=2.321, p=0.027).
Discussion
Previously we reported the feasibility and potential utility of employing a PR task, with demonstrated utility in other settings, to measure the reinforcing effect of exercise among inpatient women with AN. This study represents further development of this paradigm to assess a phenomenon that can be highly problematic in people with this disorder. While this study has several methodological limitations, discussed below, several aspects warrant further discussion.
First, this study replicates our previous finding that women with AN are willing to “work” at a PR task for the opportunity to exercise. In contrast, non-eating disordered people of normal and obese body weights have been found to prefer to work for sedentary activities compared with physical activity (21;22). The finding that 75% of our patient participants worked for at least some portion of exercise suggests that it is a commodity of some value to them. These data together provide further support the feasibility and potential utility of this paradigm in AN patients.
Second, these findings suggest that the relative reinforcing effect of exercise is influenced at least to some extent by the “dose” of the alternative reinforcer. Although only a modest, non-significant difference was present in the current study between the $2 per trial and $5 per trial alternative reinforcer conditions, we did find a difference between the $5 cash condition and the $2 voucher condition used in our previous study, such that patients worked harder for exercise (had higher breakpoints) when the alternative was relatively less valuable. While limited conclusions can be drawn from comparison between two different groups of patients, these findings taken together do suggest that the relative reinforcing effect of exercise might be modified among AN inpatients by adjusting the “cost” of exercise relative to an alternative reinforcer. Potential implications of this are elaborated below. Alternative explanations for the difference in exercise breakpoint between the two studies include the possibility that the maximum total earnings from the second study influenced participants (i.e., opportunity to earn a total of up to $140 may have persuaded some participants to work exclusively for cash); that different patient cohorts influenced their attitudes toward study participation; and that patients participating in the first study, who were more likely to be in the middle phase of treatment, had greater motivation for exercise than patients in our second study, who were either low weight or weight restored. These possibilities would have to be addressed via studies assessing exercise breakpoint at several timepoints, and compared with additional doses and types of alternative reinforcers within a single group of participants.
Third, breakpoint for exercise did not decrease significantly between the low and restored-weight testing point, as we had predicted. On the contrary, individual patients demonstrated variable changes in the exercise breakpoint, which likely corresponded with individual psychological factors. For example, some patients upon debriefing who chose exercise cited “having a bad body-image day” as the reason for doing so, in attempt to modify their body habitus. Others however reported knowledge that they could have access to other physical activity off the inpatient unit that would be preferable to the treadmill exercise, and that cash was more useful to them at that point when they were in the process of discharge planning. Prohibition of other off-unit activities on days of and surrounding this task would have produced a more controlled “value” of the exercise offered at the weight-restored time point (though would have limited the acceptability of this study to patients and the feasibility of conducting it). It is of interest to note that despite having access to off-unit activities, 60% of patients at 90% IBW still chose to work for exercise during at least one study session (compared with 75% of patients at low weight), supporting the conclusion that exercise, even in this artificial laboratory situation, retained some motivating value for patients. Factors influencing choice behavior in this paradigm are likely complex and subject to further investigation.
Psychological correlates of exercise breakpoint in this study included the admission CES score, which is a measure of pre-hospitalization exercise motivation. Exercise breakpoints at the two different study time points were also highly correlated. These associations provide further validation of both CES and breakpoint assessments, and suggest that motivation to exercise is not simply a “state” measure in individuals: patients who were motivated to exercise before hospital admission were still relatively more motivated to exercise in the beginning and at the end of treatment. It would be of interest to examine measures of exercise reinforcement over yet a longer period of time in people with AN. Previous studies using variable measures of exercise avidity (e.g., assessments of “compulsive exercise,” “exercise dependence” and “hyperactivity” suggest that some 40–60% of patients with AN on cross-sectional assessment exhibit symptoms consistent with problematic exercise (e.g., (23–26)). Typically these measures are conducted by assessing exercise behavior among outpatients, who do not have restrictions on their activity. A laboratory paradigm that can objectively measure exercise motivation among inpatients with AN, who do not otherwise have access to exercise, permits tracking of this phenomenon across treatment, and also permits measurement of interventions aimed at modifying it, as discussed below.
The lack of an association between breakpoint and CES obtained at T2 is not surprising: the CES, much like other self-report assessments of exercise motivation, assesses participants’ recent exercise attitudes and behavior; questions include, for example, whether participants have a “set routine” for exercise or “feel ‘guilty’” when an exercise session is missed. On the inpatient unit, exercise behavior was not permitted prior to this study, thus scores obtained on the T2 assessment are more difficult to interpret compared with admission CES, which reflects pre-admission exercise attitudes and behavior. That the CES at the second time point was nonzero suggests that either some aspect of exercise ideation remains among weight-restored patients (which would also not be surprising) and/or that patients’ answers are influenced by their pre-admission exercise behavior. Either way, even though behavior in the PR task may similarly be multi-determined, we propose it to be a more valid cross-sectional assessment of the reinforcing effect of exercise in a setting in which patients have not recently had access to this behavior.
The finding that study non-completers showed a trend towards higher exercise breakpoints than completers raises the provocative suggestion that exercise motivation at low weight as measured by this PR task may predict some aspect of treatment response. It would be premature to draw such a conclusion from the current data, but further investigation with a larger study sample and longer duration of follow-up could clarify this question.
A final observation is that our patients, in general, expended substantial effort to earn their chosen reinforcer. Even though the current study found a higher proportion of sessions split between work for the two commodities (and thus overall less work) compared with our previous data, we still found that the rate of completed trials with exclusive work for one commodity (and thus maximal keyboard tapping) was 50%. Overall, 10 of 16 patients (62.5%) were willing to push on a computer keyboard 9500 times at least once in order to earn the commodity of their choice. Drug abusing participants will also respond a large number of times for their drug of choice (e.g., (27)), suggesting a parallel between the AN patients and drug abusers. It is also possible that the AN patients “liked” responding or even viewed responding per se as preferred activity compared with quiescence. This would be consistent with laboratory animal studies showing that the rewarding effects of a wide range of reinforcers are higher in the setting of food deprivation (28). Intriguingly, it is also consistent with the behavioral phenomenon of AN itself, which typically requires a great deal of commitment to a goal (i.e., weight loss), unhealthy as that may be.
This study had a number of limitations. First, the relatively small sample size and the attrition between low and restored body weights reduces the certainty with which we can conclude that weight gain does not affect exercise motivation. Second, as noted above, cash does not have a universal value for all individuals, and the variable value of this alternative reinforcer across participants could have further complicated our findings. Third, we were unable to completely prevent surreptitious exercise on the part of patients in the study, which may have dissuaded some participants from choosing this commodity. Fourth, although we informed patients that we would not discuss their choices with unit staff, who are generally discouraging of exercise, we could not prevent patients from discussing their experience of this task with each other, and expectations of participants of the task and/or the impact of their participation on treatment were not completely controlled. Finally, without a control group we are unable to comment on how the reinforcing effect of exercise in our population of AN patients would compare to that of a non-patient sample tested under the same conditions.
Clinical Implications
Limitations aside, several implications may be drawn from the above findings. First, this task is feasible and amenable to conduct on an inpatient treatment unit. It appears to provide an objective measure of exercise reinforcement during a time in which patients are not able to freely exercise, and thus paves the way for assessments of future treatments aimed at modifying exercise motivation among inpatients with AN. For example, several interventions informed by behavioral economics have been aimed at providing behavioral incentives to alter choice behavior; other interventions could include psychotherapy or even pharmacotherapy aimed directly at modifying exercise reinforcement in AN patients. This PR paradigm provides a compelling means by which the efficacy of such interventions could be tested. Given the variable value of cash to individuals of different socioeconomic backgrounds, as discussed above, additional alternative reinforcers such as pleasurable sedentary activities, social privileges, and non-monetary options should also be considered. Finally, longer-term assessments are needed including determination of the impact of in-hospital exercise reinforcement on post-discharge weight loss and relapse, which remain extremely problematic in AN patients. Given the findings by prior research that some measure of drive for activity predicts worsened outcome among AN patients (2;3)), an in-hospital tool to assess exercise drive and to measure the efficacy of potential treatment interventions could be of great value to both clinicians and researchers.
Acknowledgments
This study was supported by funding from the NIH (MH65024, PI Walsh, MH71285, PI Klein; MH083795, PI Klein) and from NARSAD (Young Investigator Award to Klein). We thank staff members of the GCRU and patient participants for their contribution to this project.
References
- 1.Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiol Behav. 2004;81:359–374. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychol Med. 2004;34:671–679. doi: 10.1017/S0033291703001168. [DOI] [PubMed] [Google Scholar]
- 3.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22:339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 5.Epstein LH, Leddy JJ. Food reinforcement. Appetite. 2006;46:22–25. doi: 10.1016/j.appet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Raynor HA, Epstein LH. The relative-reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 7.Temple J, Bulkley A, Badawy R, Krause N, McCann S, Epstein LH. Differential effects of daily snack food intake on the reinforcing value of food in obese and nonobese women. Am J Clin Nutr. 2009 May 20; doi: 10.3945/ajcn.2008.27283. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill C, Saxton J, Webber L, Blundell J, Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. Am J Clin Nutr. 2009 Jun 17; doi: 10.3945/ajcn.2009.27479. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, Kaufmann V, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–242. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 10.Barkley J, Epstein L, Roemmich J. Reinforcing value of interval and continuous physical activity in children. Physiol Behav. 2009;98:31–36. doi: 10.1016/j.physbeh.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein LH, Roemmich JN, Saad FG, Handley EA. The value of sedentary alternatives influences child physical activity choice. Int J Behav Med. 2004;11:236–242. doi: 10.1207/s15327558ijbm1104_7. [DOI] [PubMed] [Google Scholar]
- 12.Saelens BE, Epstein LH. The rate of sedentary activities determines the reinforcing value of physical activity. Health Psychol. 1999;18:655–659. doi: 10.1037//0278-6133.18.6.655. [DOI] [PubMed] [Google Scholar]
- 13.Schebendach JE, Klein DA, Foltin RW, Devlin MJ, Walsh BT. Relative reinforcing value of exercise in inpatients with anorexia nervosa: model development and pilot data. Int J Eat Dis. 2007;40:446–453. doi: 10.1002/eat.20392. [DOI] [PubMed] [Google Scholar]
- 14.Davis C, Brewer H, Ratusny D. Behavioral frequency and psychological commitment: necessary concepts in the study of excessive exercising. J Behav Med. 1993;16:611–628. doi: 10.1007/BF00844722. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JE, Cook-Myers T, Wonderlich SA. Diagnostic criteria for anorexia nervosa: Looking ahead to DSM-V. Int J Eat Disord. 2005;37:595–597. doi: 10.1002/eat.20125. [DOI] [PubMed] [Google Scholar]
- 16.Pollock ML, Gaesser GA, Butcher JD, et al. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 17.Comer SD, Collins ED, Wilson ST, Donovan MR, Foltin RW, Fischman MW. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 20.Garner DM, Olmsted MP. The eating disorder inventory manual. Odessa, FL: Psychological Assessment Resources; 1984. [Google Scholar]
- 21.Vara LS, Epstein LH. Laboratory assessment of choice between exercise or sedentary behaviors. Res Q Exerc Sport. 1993;64:356–360. doi: 10.1080/02701367.1993.10608822. [DOI] [PubMed] [Google Scholar]
- 22.Coleman KJ, Gonzalez EG, Cooley T. An objective measure of reinforcement and its implications for exercise promotion in sedentary Hispanic and Anglo women. Ann Behav Med. 2000;2:229–236. doi: 10.1007/BF02895118. [DOI] [PubMed] [Google Scholar]
- 23.Klein DA, Bennett AS, Schebendach J, Foltin RW, Devlin MJ, Walsh BT. Exercise “addiction” in anorexia nervosa: model development and pilot data. CNS Spectr. 2004;9:531–537. doi: 10.1017/s1092852900009627. [DOI] [PubMed] [Google Scholar]
- 24.Brewerton TD, Stellefson EJ, Hibbs N, Hodges EL, Cochrane CE. Comparison of eating disorder patients with and without compulsive exercising. Int J Eat Disord. 1995;17:413–416. doi: 10.1002/1098-108x(199505)17:4<413::aid-eat2260170414>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Davis C, Katzman DK, Kaptein S, et al. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr Psychiatry. 1997;38:321–326. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- 26.Shroff H, Reba L, Thornton LM, et al. Features associated with excessive exercise in women with eating disorders. Int J Eat Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- 27.Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- 28.Carr KD. Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]