Abstract
A polymorphism of the human Brain Derived Neurotrophic Factor (BDNF) gene that produces a valine-to-methionine substitution at codon 66 (Val66Met), is linked to adult anxiety and mood disorders, possibly through effects on brain circuitry function. Associations between BDNF gene variants and brain activity have not been explored in anxious and depressed adolescents. The current study investigated the association between BDNF genotype and amygdala-hippocampal responses to emotional stimuli in adolescents with anxiety disorders and/or major depressive disorder (MDD) and in healthy adolescents. Twenty-seven unmedicated patients with acutely-impairing current anxiety disorders and/or MDD and 31 healthy adolescents, matched on age, gender and IQ, rated their fear of fearful, angry, neutral and happy facial expressions during collection of fMRI data on the amygdala and hippocampus. Left and right amygdala and hippocampal responses were analyzed using Repeated-measures Analyses of Variance models, with Diagnosis (patients, healthy) and Genotype (Met-carriers, Val/Val homozygotes) as between-group factors and facial expression (fearful, angry, neutral, happy) as a within-subject factor. Significant effects of Diagnosis and Diagnosis-by-Genotype interactions (F’s>4, p’s<.05) characterized activations in amygdala and anterior hippocampal regions. Greater activations in patients than healthy adolescents were found. Critically, these hyperactivations were modulated by BDNF genotype: Met-carriers showed greater neural responses of emotional faces than Val/Val homozygotes in patients only. These data are first to demonstrate the contribution of BDNF gene variants to the neural correlates of adolescent anxiety and depression. Early “gene-brain” linkages may lay the foundation for longer-term patterns of neural dysfunction in affective disorders.
Keywords: Brain Derived Neurotrophic Factor Gene, adolescence, anxiety, depression, medial temporal lobe, fMRI, development
Anxiety and mood disorders are remarkably common (Kessler et al., 2007), posing huge costs to society (Bebbington, 2001; Rice and Miller, 1998). Anxiety disorders comprise a collection of syndromes characterized by exaggerated fear responses to perceived threats. Such threats extend to a wide range of situations in Generalized Anxiety Disorder (GAD), and to specific ones, such as social evaluation in Social Phobia (SoP) or separation from caregivers in Separation Anxiety Disorder (SAD). Major Depressive Disorder (MDD) is defined by episodes of low mood and/or anhedonia, and cognitive and vegetative symptoms. Despite differences in symptom profiles, anxiety and depression are highly comorbid (Mineka et al., 1998; Pine et al., 1998), share a common genetic basis (Eley and Stevenson, 1999; Kendler et al., 1992; Lesch et al., 1996) and similar pathophysiologic profile (Beesdo et al., 2009; Davidson, 2002; Davidson et al., 2002).
Interestingly most adult anxiety and mood disorders first emerge in adolescence (Gregory et al., 2007; Pine et al., 1998) highlighting the role of early risk mechanisms in precipitating later pathological outcomes. Early genetic factors have been found to influence continuity of adolescent anxiety and depression symptoms into young adulthood (Lau and Eley, 2006; Lau et al., 2007). These effects may be mediated through early neural dysfunction (Gross and Hen, 2004). To address this, the present study investigated emerging relationships between specific gene variants in the Brain Derived Neurotrophic Factor (BDNF) gene and brain responses in anxious and depressed adolescents and healthy comparison subjects. These early ‘gene-brain’ linkages in patients may lay the foundation for continuity in affective disorders.
Adolescent anxiety and depressive traits have been linked to several candidate genes, including the serotonin transporter (5HT-T) gene (Arbelle et al., 2003; Battaglia et al., 2005; Ebstein et al., 1998; Jorm et al., 2000) and, more recently, to allelic markers within the BDNF gene (Aguilera et al., 2009; Kaufman et al., 2006; Wichers et al., 2008). Excitement over such BDNF genotypes has been spurred by newer theories of depression, which focus on impairments in neuroplasticity and cellular resilience induced through stress (Duman and Monteggia, 2006; Manji et al., 2000). These alterations, which are particularly prominent in regions that regulate mood, may be mediated through a reduced expression of survival and growth promoting factors such as BDNF. Consistent with this, depressed patients manifest lower levels of BDNF than healthy volunteers, effects that reverse with antidepressant medication (Brunoni et al 2008; Sen et al 2008). While the role of inherited liability to these cellular events is unclear, it is plausible that polymorphisms altering BDNF expression may predict depression but also anxiety, given their shared genetic basis.
Indeed a functional variant of the BDNF gene, in humans, consisting of a single nucleotide polymorphism (SNP, rs6265) in the 5’ prodomain region, which produces a valine-to-methionine substitution at codon 66 (Val66Met) (Bath and Lee, 2006), has been explored with promising results in relation to both anxiety and depression behaviors. In rodents, this Val66Met polymorphism is associated with weaker activity-dependent secretion in both neuronal cultures and ex-vivo Met/Met homozygous knock-in mice (Chen et al., 2006). Therefore, the Met allele, which mitigates neurotrophic capacity, may confer vulnerability to affective dysregulation. Consistent with these predictions, transgenic BDNFMet/Met mice manifest anxious behaviors (Chen et al., 2006). Emerging data also show that carrying at least one copy of the Met allele predicts depressive behaviors in human adults (Sarchiapone et al., 2008; Schumacher et al., 2005; Verhagen et al., 2008) and adolescents (Aguilera et al., 2009; Kaufman et al., 2006; Wichers et al., 2008). In adults, the data occasionally show interactions with sex, where effects associated with the Met-allele manifest in males only (Verhagen et al., 2008). In adolescents, findings suggest BDNF-linked risks in the presence of traumatic events (Aguilera et al., 2009; Kaufman et al., 2006; Wichers et al., 2008).
To date, few studies have explored the degree to which BDNF allelic variants modulate the ‘neural signature’ of anxiety and depression. Establishing these associations may inform genetic pathways to clinical outcomes (Hariri and Weinberger, 2003). Encouraging results have been reported in healthy adults, where greater amygdala and hippocampal responses characterize Met carriers relative to Val/Val in response to emotional tasks (Montag et al., 2008; Schofield et al., 2008). These links have yet to be extended to adolescents. The aim of the present study was to examine the contributions of these BDNF gene variants to the neural underpinnings of anxiety and depression in affected and healthy youth. Our strategy took advantage of an fMRI paradigm that produces consistent hyperactivation of these structures in adolescent anxiety and mood patients relative to healthy peers (Beesdo et al., 2009; McClure et al., 2007). This task involves evaluation of internal fear to a range of emotional faces and elicits similar patterns of amygdala activation in anxious and depressed patients relative to one another (Beesdo et al., 2009). Based on adult data (Montag et al., 2008; Schofield et al., 2008), enhanced activation of the amygdala and hippocampus to these affective cues is expected among adolescents carrying the Met-allele. Yet extrapolating adult gene-brain relationships to younger samples may be complicated by developmental changes in the expression of BDNF from adolescence to young adulthood (Webster et al., 2002). Data of 5HT-T gene variants on brain function in youth also tentatively suggests clinical differences in gene effects in healthy versus ill adolescents (Lau et al., 2008). Finally, given sex-specific genetic effects on behavior (Verhagen et al., 2008) modulation of these effects by sex may also be expected.
Material and Methods
Subjects
Participants were 27 unmedicated adolescents with either a current anxiety disorder and/or MDD (age range: 9–18 years) and 31 psychiatrically healthy adolescents (age range: 9–17 years). Patients were recruited through local health centers via contacts with health practictioners while comparison subjects responded to various advertisements. Data from these subjects have been reported as part of larger samples in other publications on the neural correlates of disease (Beesdo et al., 2009; McClure et al., 2007; Roberson-Nay et al., 2006) as well as our recent report on the relationship between 5HT-T gene variants and amygdala responses in 32 patients and 33 healthy adolescents (Lau et al., 2008). These subjects were selected from the larger samples through the availability of genotype information. Due to missing BDNF genotype data, seven subjects included in the 5HT-T analyses were not reported here. Patients with either anxiety or MDD were combined into one group based on evidence, in adolescents, of strong co-morbidity (Pine et al., 1998), shared genetic risks (Eley and Stevenson, 1999; Lesch et al., 1996), and shared pathophysiology (Beesdo et al., 2009; Pine, 2007).
Patients and healthy subjects did not differ on age (t(56)=0.43, p=0.67), sex (χ2=2.31, p=0.16) or IQ (t(56)= 0.81, p=0.42), but patients reported lower socioeconomic status (t(47)=2.25, p<0.05) (Table 1). Subsequent analyses did covary for this group difference with no major changes to the results. To minimize spurious results associated with population stratification, we compared the ethnic origins of patients and controls using 186 ancestry informative markers that differentiate continental and certain subcontinental populations (Ducci et al., 2009; Hodgkinson et al., 2008). For each subject, ethnic ancestry factor scores for each of seven factors can be calculated based on these markers (Table 1). These factors were initially suggested through factor analysis of markers obtained from 1051 individuals compiled from 51 reference populations representing worldwide diversity (Centre d’Etude Polymorphisme Humain). As such, ancestry factor scores collectively reflect a quantitative profile of an individual’s genetic ancestry, capturing subtle gradations of ethnic differences between individuals, rather than binary measures supplied through self-reported ethnicity measures. The use of these continuous measures of genetic ancestry across several ethnic dimensions blurs boundaries between individuals, making the stratification of participants into sub-groups less clear. No significant differences in ancestry factor scores emerged between groups (t’s<1.37, p’s=n.s.) or between genotypes within groups (t’s<1.92, p’s=n.s.) (Table 1).
Table 1.
Demographic, diagnostic and genotypic characteristics of healthy subjects and patients
| Healthy (n=31) |
Patient (n=27) |
|
|---|---|---|
| Demographics, Mean(SD) | ||
| Age | 13.71 (2.61) | 13.44 (2.29) |
| Males, No.(%) | 14 (45) | 12 (45) |
| IQ | 110.23 (14.37) | 113.48 (16.24) |
| Socioeconomic Status | 48.74 (25.92) | 33.77 (19.24) |
| Ethnic Ancestry Factor Scores | ||
| Europe | 0.62 (0.39) | 0.62 (0.36) |
| Middle East | 0.10 (0.18) | 0.10 (0.17) |
| Africa | 0.09 (0.21) | 0.11 (0.24) |
| Central Asia | 0.08 (0.15) | 0.07 (0.18) |
| America | 0.07 (0.17) | 0.02 (0.04) |
| Far East Asia | 0.03 (0.09) | 0.07 (0.20) |
| Oceania | 0.01 (0.01) | 0.01 (0.01) |
| Current DSM-IV diagnoses, No.(%) | ||
| Anxiety disorder | 21 (78) | |
| Generalized Anxiety Disorder | 12 (44) | |
| Social phobia | 12 (44) | |
| Separation anxiety disorder | 5 (19) | |
| Generalized Anxiety Disorder only | 4 (15) | |
| Social Phobia only | 4 (15) | |
| Separation Anxiety Disorder only | 1 (4) | |
| Major depressive disorder | 13 (48) | |
| Major depressive disorder only | 5 (19) | |
| Genotype, No.(Mean age, % males) | ||
| Met Met | 0 (0, 0%) | 1 (11.33, 0%) |
| Val Met | 8 (13.06, 38%) | 8 (13.06, 25%) |
| Val Val | 23 (13.94, 48%) | 18 (13.72, 56%) |
All participants were interviewed using by trained clinicians using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS), which is a semi-structured interview (Kaufman et al., 2000) (Table 1). For patients, inclusion criteria comprised either current GAD, SoP, SAD or MDD for which patients sought treatment, and persistent symptoms during three weeks of supportive therapy. Given documented differences in their pathophysiology, patients with panic disorder, or patients who met criteria for specific phobia (excluding separation anxiety disorder) alone were not included in the current study. For controls, inclusion criteria comprised no current or lifetime psychiatric diagnoses. Exclusion criteria for all subjects were current Tourette’s syndrome, obsessive compulsive disorder, or conduct disorder; recent exposure to trauma; current use of psychoactive substance; suicidal ideation; lifetime history of mania, psychosis, or pervasive developmental disorder; and IQ<70. The study was approved by the NIMH Institutional Review Board. All participants/parents provided written informed assent/consent.
SNP Genotyping
Genomic DNA was extracted from EBV-transformed lymphoblastoid cell lines or untransformed white blood cells using Gentra Versagene Cell kits according to the manufacturer’s protocol. Genotyping was perfomed using the Illumina GoldenGate chemistry on the 1536 SNP NIAAA Addictions Array , with a >95% completion rate. Genotyping error was determined by replicating genotyping in 10% of the sample. These analyses showed 100% concordance across genotyped samples.
Subjects belonged to one of three genotype groups, either homozygous for Val or Met, or heterozygous (Table 1). Consistent with the low prevalence of homozygote Met carriers (approximately 2–3% in Caucasian populations), only one individual carried two copies of the Met allele. As such data from this individual was combined with Val-Met heterozygotes to comprise a group of Met-carriers as has been reported in other studies also investigating functional effects of the Met-allele (Montag et al., 2008). No differences emerged in genotypic distribution across patients and controls (χ2=0.40, p=0.57). Genotype frequencies did not deviate significantly from the expected values at the Hardy – Weinberg equilibrium test for either patients or controls (p > 0.05).
Emotion Paradigm
Procedures and stimuli have been described previously (Beesdo et al., 2009; Guyer et al., 2008; Lau et al., 2008; McClure et al., 2007; Monk et al., 2003; Pine et al., 2005; Rich et al., 2006; Roberson-Nay et al., 2006). Four epochs of 40 trials were presented: 32 trials showed different actors displaying different face-emotions (8 fearful, 8 angry, 8 happy, 8 neutral), and 8 trials contained a fixation point. For a given participant, each actor portrayed one single expression across the whole task. For example, while a given actor might be randomly selected to portray “fear” for one participant, the same actor might be randomly chosen to portray “happiness” for another subject, or “anger” for yet another participant. This design allowed us to control for variability in non-emotional features of the actors (e.g., ethnicity, hair color). These 40 trials were further divided into four blocks of 10 trials, in which 8 of the 32 faces and 2 fixation trials were presented in random order. In each block, subjects completed one of four tasks that varied in attentional focus: rating subjective fear levels to each face (“how afraid are you of this face?”); rating the nose width on each face (“how wide is the nose on this face?”); rating the perceived level of threat of each face (“how hostile is this face?”); or passively viewing the face. The present work focused on the fear rating blocks only. This was driven by three sets of prior findings. First, the face-rating task is most consistent at eliciting clinical differences between anxious and depressed patients and healthy adolescents in ‘affective’ neural circuitry (McClure et al., 2007; Beesdo et al., 2009). Second, relative to one another, anxious and depressed patients do not differ significantly in patterns of neural activation during this condition (Beesdo et al., 2009). Third, risks associated with the short allele of the 5HT-T gene emerged during this condition in a previous report (Lau et al., 2008). Ratings were made on a 5-point scale. Each block began with instructions (3000ms) followed by 10 trials (4000ms/trial). Order of blocks was fully randomized. Gray-scale face stimuli were derived from three sources (Ekman, 1976; Gur et al., 2001; Tottenham and Tanaka J., in press). Stimuli were displayed on the Avotec Silent Vision Glasses (Stuart, FL). Ratings were recorded with a five-key button box (Waukesha, WI) and reaction times (RT) noted.
MRI Data acquisition and processing
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired on a General Electric (Waukesha, WI) Signa 3-tesla scanner. Following sagittal localization and manual shimming, functional T2*-weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a matrix size of 64×64mm, repetition time (TR) of 2000ms, echo time (TE) of 40ms, field of view (FOV) of 240mm, and voxels of 3.75×3.75×5.0mm. Images were acquired in 23 contiguous axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure line. All functional data were gathered in a single 14-minute run per subject. After echo-planar imaging (EPI) acquisition, a high-resolution T1-weighted anatomical image was acquired to aid spatial normalization. A standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, FOV=256, number of excitations=1, TR=11.4ms, TE=4.4ms, matrix=256×256, time to inversion (TI)=300ms, bandwidth=130 Hz/pixel, 33 kHz/256 pixels) was used.
Reconstructed fMRI images were examined for excessive motion (>3mm in any plane) using MedX (Medical Numerics, Sterling, Virginia). Subsequent analyses were conducted with SPM99 (University College London) and Matlab6 (Mathworks, Massachusetts). Functional data were corrected for slice timing and motion, coregistered to anatomical data, resliced to isotropic 2mm voxels, and spatially normalized to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99. Images were inspected following normalization. We estimated event-related response amplitudes at the individual subject level for every face-emotion in each attention task using the General Linear Model. Dividing each contrast image by the subject-specific voxel time series means yielded percent fMRI signal change(Zarahn et al., 1997).
Statistical analyses
As mentioned above, the current analyses focused primarily on data collected during the assessment of internal fear, the so-called “afraid” rating state. This was driven primarily by prior findings that this attention-state yields the most reliable group differences in neural responses to face emotions between adolescent patients relative to healthy controls and between genetically at-risk and low-risk individuals (Beesdo et al., 2009; Lau et al., 2008; McClure et al., 2007). Moreover, similar patterns of amygdala activation are found to characterize both anxiety and depression patient groups in this condition compared to the passive viewing condition where attention is unconstrained (Beesdo et al., 2009). Finally, current sample sizes possessed limited statistical power to test for four-way Diagnosis-by-Genotype-by-Attention-by-Face-Emotion or five-way Diagnosis-by-Genotype-by-Attention-by-Face-Emotion-by-Sex interactions. We therefore adopted a strategy strictly constrained by a priori hypotheses derived from previous work.
Ratings and RTs to different face emotions during the “afraid” rating state were examined using repeated-measures analyses of variance (ANOVAs) with one within-subjects factor (Face-emotion: fearful, angry, happy, neutral) and three between-subjects factors (Diagnosis: patients, controls; Genotype: Val/Val homozygotes, Met-carriers; Sex: male, female). Given violations of sphericity on some analyses, adjustments were made by applying the Greenhouse-Geisser (G-G) procedure.
For group-level fMRI analyses, a random effects model permitted population-level inferences (Holmes, 1998). Analyses were restricted to the amygdala and the anterior hippocampus using a region-of-interest (ROI) approach (Beesdo et al., 2009; Guyer et al., 2008; Lau et al., 2008; McClure et al., 2007; Monk et al., 2003; Perez-Edgar et al., 2007). The boundaries of these regions were defined in a mask using standard anatomical criteria on the single subject MNI template image (Szeszko et al., 1999). This mask was then applied to all normalized brains at the group level. BOLD signal changes for each event-type (fearful, angry, happy, neutral faces) during “afraid” ratings relative to fixations were averaged across all voxels in the left and right amygdala and the left and right anterior hippocampus for each subject. This yielded one value for each region for each event type, reducing problems related to multiple testing in many other fMRI studies. Data for each region was analyzed separately using four sets of repeated-measure ANOVAs in SPSS-14, examining main effects and interactions of one within-subjects factor (Face-emotion: fearful, angry, happy, neutral) and three between-subjects factors (Diagnosis: patients, controls; Genotype: Val/Val homozygotes, Met-carriers; Sex: male, female). As before, violations of sphericity were corrected through the G-G procedure. Of the demographic variables (age, IQ, SES, and ancestry), only ancestry scores for African, Middle Eastern, Asian and Oceanian origins correlated significantly with BOLD signal changes in these regions. However importantly, there were no significant differences in the ethnic profile derived from ancestry factor scores across groups or across BDNF genotype. Regardless, we added these covariates in subsequent analyses, ensuring that effects of genetic ancestry did not confound group or genotype differences on BOLD responses. Finally, voxel-wise SPM analyses using small volume Gaussian random field correction procedures for multiple comparisons, confirmed significant Genotype-by-Diagnosis interactions in the amygdala and anterior hippocampus during afraid ratings.
Results
Behavioral data
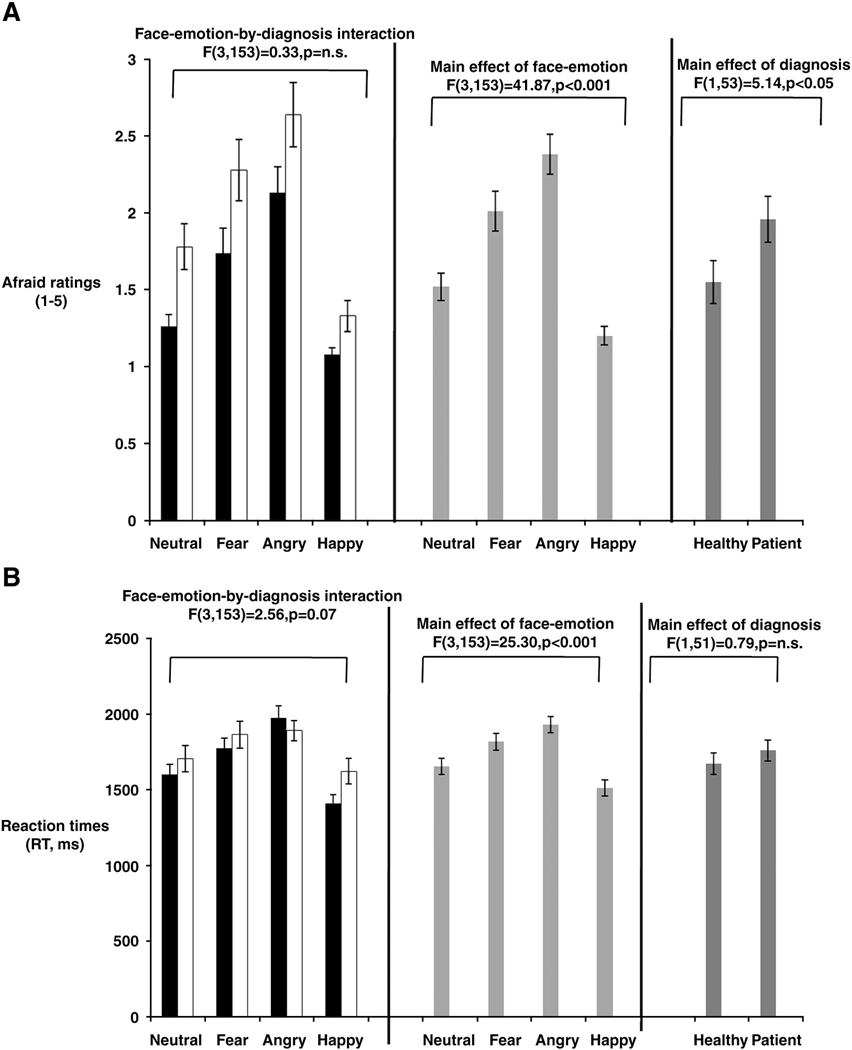
Behavioral responses for 3 healthy participants were not recorded due to technical problems encountered during scanning. Analysis of the remaining 55 data sets revealed significant effects of Face-emotions (F(3,153)=41.87, p<0.001) and Diagnosis (F(1,53)=5.14, p<0.05) on self-report of “afraid” ratings, but with no support for main or interaction effects associated with Genotype (Figure 1a). As illustrated, the mean “afraid” self-report scores across the whole sample were highest for angry faces, followed by fearful, neutral and happy faces. Moreover, patients gave higher “afraid” ratings to all faces than did healthy comparison subjects. Reaction time (RT) analysis likewise revealed significant differences across face-emotions (F(3,153)=25.30, p<0.001), but no main or interaction effects with subject group (Figure 1b).
Figure 1.
Figure 1a: Face-emotion and Diagnosis effects on “afraid” ratings across faces
Figure 1b: Face-emotion effects on reaction times (ms) to “afraid” ratings across faces
fMRI data
Repeated-measures ANOVAs of Diagnosis by Genotype by Emotion yielded similar results across the four analyzed regions (Table 2). For each region, while the 3-way interaction was not significant, the 2-way Diagnosis by Genotype interaction was statistically significant. The interactions of Emotion with Diagnosis or Genotype were not significant. These findings implied that the degree to which neural activation was modulated by Genotype varied between patients and comparison subjects, but not as a function of displayed emotion.
Table 2.
Analyses assessing main and interaction effects of Face emotion, Diagnosis, Sex and Genotype on regions-of-interest
| Main and interaction effects | Region of interest | |||
|---|---|---|---|---|
| Left amygdala | Right amygdala | Left anterior hippocampus |
Right anterior hippocampus |
|
| Face-emotion | p = n.s. | F(3,141) = 4.06* | p = n.s. | F(3,141) = 4.71* |
| Diagnosis | F(1,48) = 6.27* | F(1,47) = 8.06** | F(1,45) = 6.02* | F(1,47) = 11.18** |
| Sex | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Genotype | F(1,48) = 6.08* | p = n.s. | F(1,45) = 3.19† | p = n.s. |
| Face-emotion-by- Diagnosis | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by-Sex | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Diagnosis -by-Sex | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Diagnosis -by-Genotype | F(1,48) = 13.25*** | F(1,47) = 4.64* | F(1,45) = 4.16* | F(1,47) = 4.57* |
| Sex-by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by- Diagnosis -by-Sex | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by- Diagnosis -by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by-Sex-by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Diagnosis -by-Sex-by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
| Face-emotion-by- Diagnosis -by-Sex-by-Genotype | p = n.s. | p = n.s. | p = n.s. | p = n.s. |
p < 0.05
p < 0.01
p < 0.001
p < 0.10
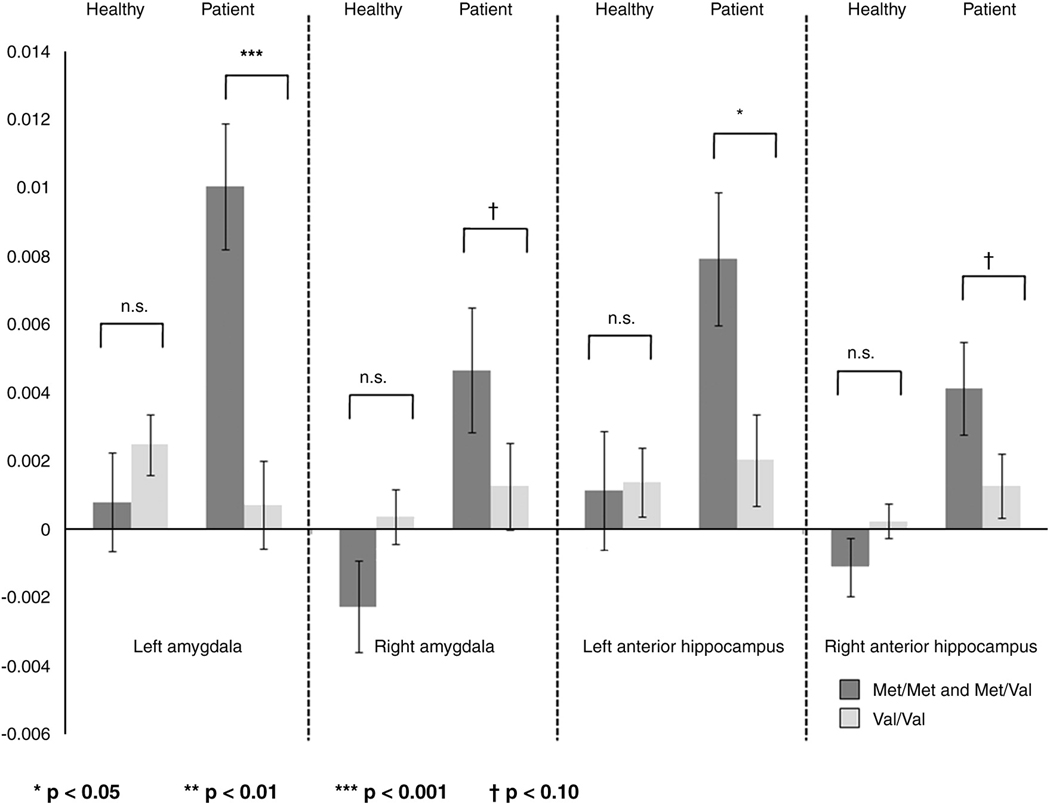
To decompose these Diagnosis-by-Genotype interactions, the effects of Genotype were examined in patients and healthy comparisons separately. Given a lack of significant main and interaction effects associated with sex, this factor was not included in these analyses. Genotypic differences on neural responses in each diagnostic group are illustrated for each region in Figure 2. The significant two-way interactions were driven by genotypic differences in patients: Met-carriers showed greater neural responses than Val-homozygotes. Effect sizes indexed by Cohen’s d for these group differences were 1.61 and 0.71 for the left and right amygdala respectively, and 1.14 and 0.80 for the left and right anterior hippocampus respectively. Such genotypic differences were not observed in the healthy comparison group.
Figure 2.
Significant Diagnosis-by-Genotype Interactions emerged in amygdala and anterior hippocampal regions
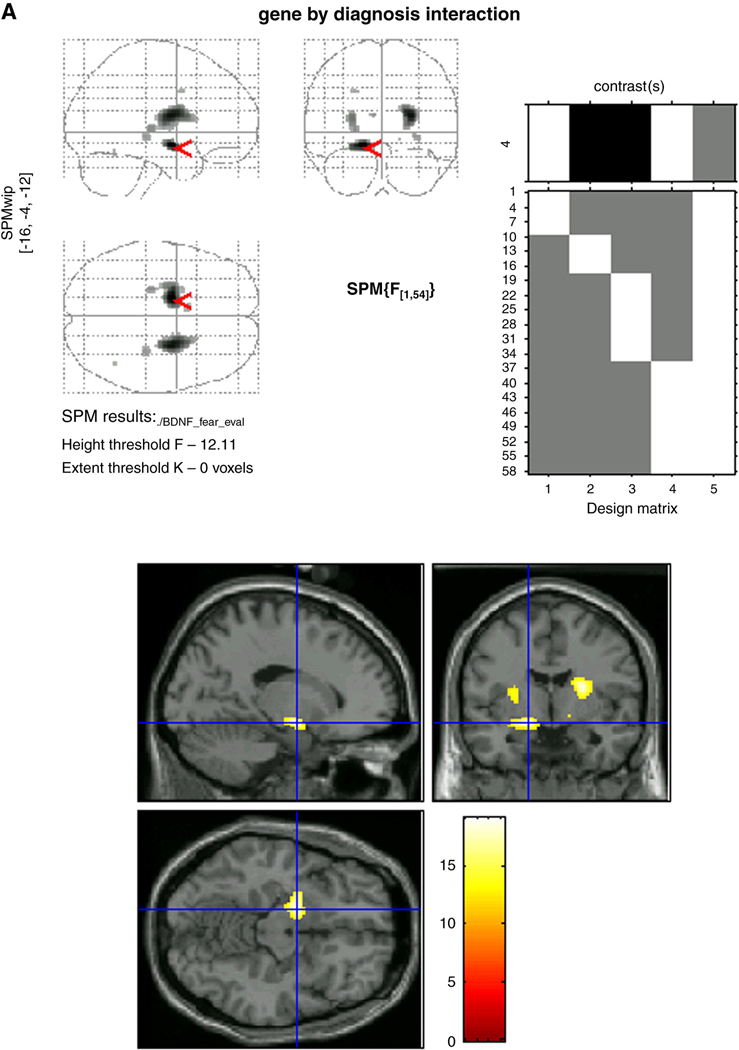
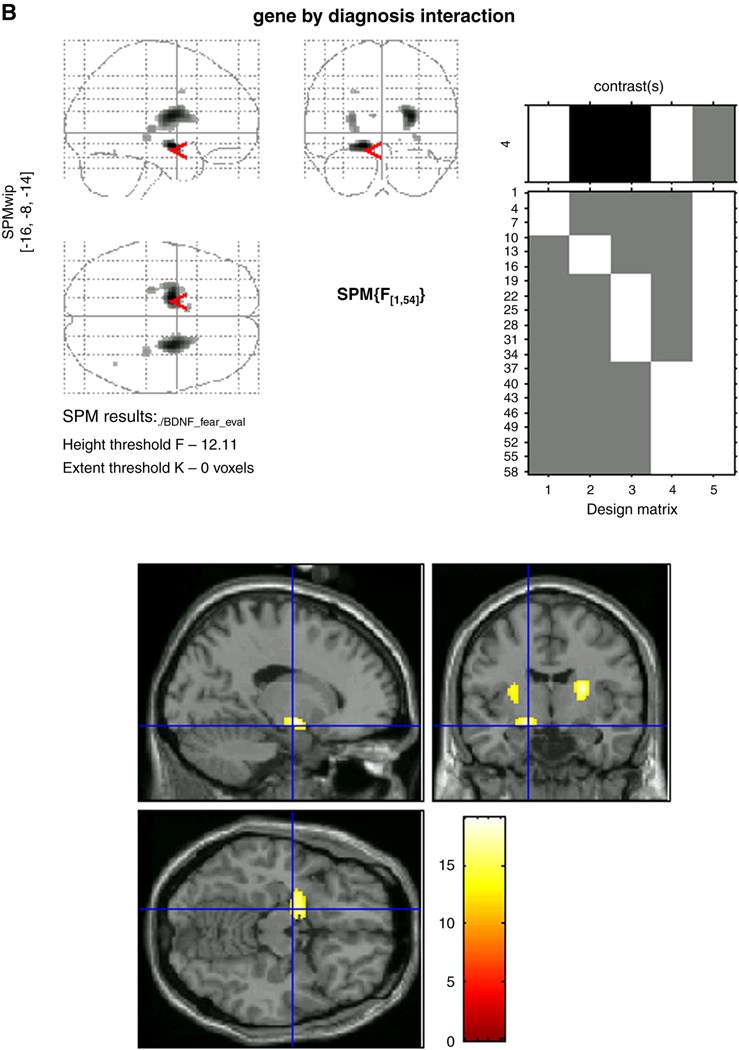
Voxel-wise analyses using small volume Gaussian random field correction procedures for multiple comparisons were conducted to confirm ROI results relative to whole brain response. These showed that while clear Diagnosis-by-Genotype interactions characterized the left amygdala (F=19.10, p<0.001) and left anterior hippocampus (F=19.10, p<0.001) (Figure 2), activations in each structure overlapped closely with one another, and emerged as one single region of activation with a peak in the left amygdala. When separated into ROIs, the topography of the peak activations within each structure (left amygdala: x=−16, y=−4, z=−12 and left anterior hippocampus: x=−16, y=−8, z=−14) are presented in Figure 3a and Figure 3b. For completeness, results of whole-brain voxel-wise analyses that revealed significant (p<.001) Diagnosis-by-Genotype interactions in other regions are also presented in Table 3. Of note, activations in the right amygdala and right anterior hippocampus were weaker than on the left, manifest only in the amygdala and at a comparatively more liberal threshold of p<0.005.
Figure 3.
Figure 3a: Topography of peak activations in the left amygdala
Figure 3b: Topography of peak activations in the left anterior hippocampus
Table 3.
Regions showing significant Diagnosis-by-Genotype interactions during “afraid” ratings
| Brodman Area |
Region | Volume, mm |
X | y | z | F | P Value |
|---|---|---|---|---|---|---|---|
| Medialdorsal amygdala | 218 | −16 | −4 | −12 | 19.1 | <0.001 | |
| Putamen | 128 | −26 | −6 | 12 | 14.64 | <0.001 | |
| Thalamus-pulvinar | 23 | −24 | −22 | 4 | 13.84 | <0.001 | |
| Caudate | 8 | −8 | 10 | 16 | 13.3 | <0.001 | |
| center posterior hippocampus | 26 | 30 | −28 | −4 | 13.29 | <0.001 | |
| 9 | Dorsal frontal gyrus | 9 | −24 | 4 | 36 | 12.83 | <0.001 |
| Lateral cerebellum | 4 | 42 | −56 | −18 | 12.45 | <0.001 | |
| Pallidum | 1 | 16 | −4 | −6 | 12.16 | <0.001 |
Discussion
Exciting new data implicates the Val66Met BDNF polymorphism in the pathophysiology of anxiety and mood disorders in adults (Sarchiapone et al., 2008; Schumacher et al., 2005; Verhagen et al., 2008) and in adolescents (Aguilera et al., 2009; Kaufman et al., 2006; Wichers et al., 2008). Recent “imaging genetics” studies suggest that risks associated with the Met allele manifest through neural correlates of emotional responding (Montag et al., 2008). So far, these hypotheses have been explored in healthy adults only, with studies reporting greater amygdala activation to emotional stimuli among Met-carriers. The present study extended these findings in two ways, by assessing these associations in affected and healthy adolescents. As many adult disorders have their roots in adolescence (Gregory et al., 2007; Pine et al., 1998), studying these associations in youth may critically inform pathways responsible for life-long anxiety and mood pathology. Moreover, given considerable work in basic science implicating developmental changes in BDNF gene expression (Webster et al., 2002), studying these relationships in healthy adolescents may also inform typical brain development (Huang and Reichardt, 2001).
Consistent support was found for Diagnosis-by-Genotype interactions on brain activity within a region that encompassed both the amygdala and the hippocampus. These interactions were driven by genetic differences among patients, with greater responses in Met-carriers than Val-homozygotes. Such genotype differences were absent in healthy adolescents. Both prior (Beesdo et al., 2009; Forbes et al., 2006; McClure et al., 2007; Roberson-Nay et al., 2006; Thomas et al., 2001) and current data have documented excessive activation of the amygdala and hippocampus to emotional stimuli in anxious and depressed adolescents relative to healthy individuals. As such, the profile of enhanced responses forms a ‘neural signature’ of adolescent anxiety and depression. Our findings crucially suggest that genetic factors can influence this neural expression in affected individuals. None of these effects were modulated by sex.
BDNF genetic variants and adolescent emotional disorders
This study is the first to use “imaging genetics” to reveal BDNF contributions to the neural correlates of adolescent anxiety and depression. As such, these findings support previous work establishing links between the Val66Met Variant and behavioral outcomes of anxiety and depression in animals (Chen et al., 2006) and humans (Sarchiapone et al., 2008; Schumacher et al., 2005; Verhagen et al., 2008). Presumably, these links arise through complex relationships between gene expression associated with the Met-substitution and circulating levels of BDNF (Chen et al., 2006). Here, we extend this research by showing that altered BDNF expression also influences neural dysfunction in brain regions that process emotional material. Notably these effects were stronger in left subcortical regions than right, consistent with recent findings suggesting that the left amygdala is more often activated (Baas et al., 2004; Wager et al., 2003). Although prior data from adults have already shown that this BDNF polymorphism does indeed modulate variations in brain function among healthy individuals (Montag et al., 2008; Schofield et al., 2008), our findings in affected individuals place these gene-brain linkages within trajectories of pathology. Moreover, as this gene-neural substrate link characterized affected youth, our data suggest a potential role for BDNF in early manifestations of life-long disorders. This may be particularly crucial for long-term maintenance of symptoms, given the role of BDNF in regulating synaptic plasticity and connectivity in the brain (Huang and Reichardt, 2001). Whether the reduction of BDNF levels associated with the Met-substitution simply reflect symptom expression during adolescence, or also plays a role in long-term effects on brain plasticity is a central question that has implications for disease etiology.
Whereas the Met allele clearly enhanced amygdala and hippocampal activation in patients, comparable predictions of neural activity did not characterize healthy adolescents. While further research needs to verify these findings, the absence of a gene-brain linkage in ‘normal’ development may suggest that anxiety and depression, like many other complex traits, arise from multiple interacting risk factors (Plomin et al., 1994). Here, the effects of risks associated with the Met allele could be elicited because of the presence of other factors in patients, yet similarly, they may be buffered by the presence of other factors in healthy adolescents. These ‘moderating’ factors could include other gene polymorphisms or environmental factors that serve to enhance risk or resilience, leading to pathological versus healthy outcomes (Aguilera et al., 2009; Eley et al., 2004; Kaufman et al., 2006; Kaufman et al., 2004; Wichers et al., 2008). Consistent with this, BDNF expression is thought to mediate the effects of stress-induced neuronal atrophy, cell loss and inhibition of neurogenesis on mood dysregulation. As such adverse environmental factors may be necessary to unmask these gene-brain associations. Links between the Met-variant and brain activity in healthy adults could therefore reflect an accumulation of environmental risks across time. Nevertheless, the paucity of current research into associations between this specific gene variant and neural function prevents any further interpretations of this discrepancy until more research is conducted.
Limitations
Several caveats of these data need to be recognized. First, results are preliminary, and need replication in larger samples. This is especially critical as understanding of the relationship between alterations in BDNF levels and manifestation of emotional behaviours is still relatively limited, and sometimes characterized by inconsistencies and contradictions (Groves, 2007). Within this context, understanding the effects of the Val66Met polymorphism on human brain function is in its infancy (Groves, 2007). Our results are nevertheless consistent with emerging data on gene-behavior associations in animals and humans. Moreover, we also find large effect sizes (Cohen’s d >0.70) of these genetic differences on brain function among patients. Due to our relatively small sample size and the low frequency of individuals carrying two copies of the Met allele, we were also unable to explore the possibility of a dose-response relationship between number of Met-alleles and subcortical engagement during emotion among patients. Such a question awaits further analysis in larger samples. Another question that will also require large samples concerns the degree of interaction between different allelic variants (e.g. 5HTT and BDNF genes) on the pathophysiology of anxiety and depression. Indeed, recent work has documented moderation of the short allele of the serotonin transporter gene by BDNF polymorphic variation on depressive symptoms in samples of maltreated children, samples substantially larger than those available to neuroimaging studies (Aguilera et al., 2009; Kaufman et al., 2006; Wichers et al., 2008).
A second caveat of these results is that gene effects on behavioral performance (ratings and RTs) of the evaluation of internal fear were absent. This is a contentious issue in fMRI research. While some argue for the relevance of behavioral differences in interpreting group fMRI findings (Wilkinson and Halligan, 2004), others treat these as experimental confounds in task performance that could explain differences in neural activation (Callicott et al., 2003). Still, others argue that the lack of gene-behavior associations reinforces the need to search for endophenotypes (Gottesman and Gould, 2003), which lie ‘upstream’ to the products of gene expression but ‘downstream’ from behavior. As these markers (including brain function) fall intermediate between genes and behavior, they are expected to show stronger genetic effects than behavioral measures.
Finally, our patient group comprised individuals with GAD, SoP, SAD and MDD. Whereas BDNF theories have mainly applied to depression, more recently, variation in the genotype have been linked to anxiety-related traits in rodents (Chen et al., 2006) and in humans such as neuroticism (Sen et al., 2003). However findings are less consistent, with suggestions that the Val-allele rather than the Met polymorphism increased neuroticism scores (Sen et al., 2003). Nevertheless many studies show a shared genetic basis of various subtypes of adolescent anxiety and of depression (Eley and Stevenson, 1999) in addition to similar pathophysiologic profiles, notably, amygdala activations to fear processing (Beesdo et al., 2009; Pine, 2007). Thus there is a strong rationale for combining these disorders in the current study. Nevertheless, to confirm this ‘shared diathesis’, future studies employing larger sample sizes and enhanced statistical power should investigate differences associated with allelic variants across different diagnoses.
In summary, our data show greater activation of medial temporal lobe structures (amygdala, hippocampus) in response to affective cues among anxious and depressed patients carrying the Met variant. These data are novel in demonstrating BDNF modulation of the neural substrates of mood and anxiety disorders, and carry exciting implications for risk mechanisms in mood and anxiety pathology, particularly as they pertain to adolescents, whose brains are undergoing unique maturational changes (Ernst, 2009). These early links between BDNF allelic variants and brain responses may even lay the foundation for longer-term, sustained patterns of neural dysfunction in mood and anxiety disorders.
Acknowledgements
The authors would like to thank Michelle Goldwin, Nina Shiffrin, and Veronica Temple for data processing assistance; Longina Akhtar, Elena Gorodetsky, and Pei-Hong Shen for assistance with genotyping; Harvey Iwamoto for programming and computer support; and Jennifer Cameron, Ken Towbin, and Alan Zametkin for medical oversight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibanez MI, Ruiperez MA, Ortet G, Fananas L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009:1–8. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. Am J Psychiatry. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, Maffei C, Marino C. Influence of the serotonin transporter promoter gene and shyness on children's cerebral responses to facial expressions. Arch Gen Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Bebbington P. The World Health Report 2001. Soc Psychiatry Psychiatr Epidemiol. 2001;36:473–474. doi: 10.1007/s001270170010. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, McClure-Tone EB, Guyer AE, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and specific amygdala engagement perturbations in depressed versus anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, Goldman LR, Goldman D. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am J Psychiatry. 2009;166:1031–1040. doi: 10.1176/appi.ajp.2009.08071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Levine J, Geller V, Auerbach J, Gritsenko I, Belmaker RH. Dopamine D4 receptor and serotonin transporter promoter in the determination of neonatal temperament. Mol Psychiatry. 1998;3:238–246. doi: 10.1038/sj.mp.4000363. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychology Press; 1976. [Google Scholar]
- Eley TC, Stevenson J. Using genetic analyses to clarify the distinction between depressive and anxious symptoms in children. J Abnorm Child Psychol. 1999;27:105–114. doi: 10.1023/a:1021947113860. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge J. Adolescence: On the neural path to adulthood. Anatomy, connectivity and ontogeny of the nodes of the triadic model. In: Potenza M, Grant J, editors. Young Adult Mental Health. Oxford University Press; 2009. [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, Koenen K, Eley TC, Poulton R. Juvenile mental health histories of adults with anxiety disorders. Am J Psychiatry. 2007;164:301–308. doi: 10.1176/ajp.2007.164.2.301. [DOI] [PubMed] [Google Scholar]
- Gross C, Hen R. Genetic and environmental factors interact to influence anxiety. Neurotox Res. 2004;6:493–501. doi: 10.1007/BF03033286. [DOI] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43:505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Prior M, Sanson A, Smart D, Zhang Y, Easteal S. Association of a functional polymorphism of the serotonin transporter gene with anxiety-related temperament and behavior problems in children: a longitudinal study from infancy to the mid-teens. Mol Psychiatry. 2000;5:542–547. doi: 10.1038/sj.mp.4000782. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry. 1992;49:716–722. doi: 10.1001/archpsyc.1992.01820090044008. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, R DEG, Demyttenaere K, Gasquet I, G DEG, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustun TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Eley TC. Changes in genetic and environmental influences on depressive symptoms across adolescence and young adulthood. Br J Psychiatry. 2006;189:422–427. doi: 10.1192/bjp.bp.105.018721. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen PH, Pine DS, Ernst M. Amygdala Function and 5-HTT Gene Variants in Adolescent Anxiety and Major Depressive Disorder. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Gregory AM, Goldwin MA, Pine DS, Eley TC. Assessing gene-environment interactions on anxiety symptom subtypes across childhood and adolescence. Dev Psychopathol. 2007;19:1129–1146. doi: 10.1017/S0954579407000582. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Manji HK, Moore GJ, Rajkowska G, Chen G. Neuroplasticity and cellular resilience in mood disorders. Mol Psychiatry. 2000;5:578–593. doi: 10.1038/sj.mp.4000811. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: evidence from a genetic imaging study. Neuroimage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Klein RG, Mannuzza S, Moulton JL, 3rd, Lissek S, Guardino M, Woldehawariat G. Face-emotion processing in offspring at risk for panic disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:664–672. doi: 10.1097/01.chi.0000162580.92029.f4. [DOI] [PubMed] [Google Scholar]
- Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science. 1994;264:1733–1739. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- Rice DP, Miller LS. Health economics and cost implications of anxiety and other mental disorders in the United States. Br J Psychiatry Suppl. 1998:4–9. [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella MC, di Giannantonio M, Janiri L, de Gaetano M, Janal MN. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology. 2008;57:139–145. doi: 10.1159/000142361. [DOI] [PubMed] [Google Scholar]
- Schofield PR, Williams LM, Paul RH, Gatt JM, Brown K, Luty A, Cooper N, Grieve S, Dobson-Stone C, Morris C, Kuan SA, Gordon E. Disturbances in selective information processing associated with the BDNF Val66Met polymorphism: Evidence from cognition, the P300 and fronto-hippocampal systems. Biol Psychol. 2008 doi: 10.1016/j.biopsycho.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Jamra RA, Becker T, Ohlraun S, Klopp N, Binder EB, Schulze TG, Deschner M, Schmal C, Hofels S, Zobel A, Illig T, Propping P, Holsboer F, Rietschel M, Nothen MM, Cichon S. Evidence for a relationship between genetic variants at the 29 brain-derived neurotrophic factor (BDNF) locus and major depression. Biol Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, Wu H, Bogerts B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JLAC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: judgements from untrained research participants. Psychiatry Research. doi: 10.1016/j.psychres.2008.05.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.109. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Brain Res Dev Brain Res. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van Os J. The BDNF Val(66)Met × 5-HTTLPR x child adversity interaction and depressive symptoms: An attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:120–123. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M. A trial-based experimental design for fMRI. Neuroimage. 1997;6:122–138. doi: 10.1006/nimg.1997.0279. [DOI] [PubMed] [Google Scholar]