Abstract
Previous work has demonstrated less accurate alignment of cortical structures for patients with schizophrenia than for matched control subjects when using affine registration techniques. Such a mismatch presents a potential confound for functional neuroimaging studies conducting between-group comparisons. Critically, the same issues may be present for subcortical structures. However, to date no study has explicitly investigated alignment precision for major subcortical structures in patients with schizophrenia. Thus, to address this question we used methods previously validated for assessment of cortical alignment precision to examine alignment precision of subcortical structures. In contrasts to our results with cortex, we found that major subcortical structures (i.e. amygdala, caudate, hippocampus, pallidum, putamen and thalamus) showed similar alignment precision for schizophrenia (N=48) and control subjects (N=45) regardless of the template used (other individuals with schizophrenia or healthy controls). Taken together, the present results show that, unlike cortex, alignment for six major subcortical structures is not compromised in patients with schizophrenia and as such is unlikely to confound between-group functional neuroimaging investigations.
Keywords: Schizophrenia, subcortex, registration, anatomical alignment
Introduction
A major challenge for most fMRI studies investigating functional differences between clinical and healthy populations is optimal alignment of anatomical and functional data across individuals, especially if there are reasons to believe that within-group alignment may be compromised in the clinical sample. Currently, most fMRI studies in schizophrenia use volumetric representations for data analysis (Acton and Friston, 1998; Friston et al., 1995; Worsley and Friston, 1995) and apply volume-based registration (VBR) to compensate for inter-individual variability in brain anatomy (Woods et al., 1998a; Woods et al., 1998b). Previously, we demonstrated that affine VBR may not be the optimal strategy for cortical alignment, possibly due to a mismatch between volume representations and cortical geometry, which is essentially a 2D folded sheet of tissue (Anticevic et al., 2008). Importantly, we also showed less accurate cortical alignment in patients with schizophrenia vs. healthy controls when using affine VBR (Anticevic et al., 2008), possibly owing to subtle abnormalities in cortical morphology in this illness, which may give rise to greater anatomical variability across schizophrenia subjects (Csernansky et al., 2008; Shenton et al., 2001).
The same problems may exist in patients with schizophrenia when considering subcortical alignment. To date, numerous studies have demonstrated subcortical size and shape abnormalities in this patient population for structures such as the hippocampal complex, amygdala and thalamus (Csernansky et al., 2008; Csernansky et al., 1998; Csernansky et al., 2004; Harms et al., 2007; Konick and Friedman, 2001; Lawrie et al., 2003; Mamah et al., 2007; Namiki et al., 2007; Shenton et al., 2001; Shenton et al., 2002; Velakoulis et al., 1999; Velakoulis et al., 2006; Wang et al., 2008; Wood et al., 2001). While these differences in brain morphology may be a consequence of the disease process and clinically significant in their own right, they further complicate functional investigations of these same regions. In principle, more spatial heterogeneity and smaller average size of subcortical structures could reduce alignment precision in the same way as demonstrated for cortical structures (Anticevic et al., 2008; Van Essen, 2005). In turn, as shown for cortical activations, lack of precise anatomical alignment may result in loss of statistical power when conducing fMRI analyses (Anticevic et al., 2008; Argall et al., 2006; Desai et al., 2005; Fischl et al., 1999a; Fischl et al., 1999b). Moreover, an exaggerated reduction in alignment precision in individuals with schizophrenia could further reduce power to detect significant task-related activation when compared to controls, which introduces a confound in the comparison of patients to healthy controls. Thus, some apparent differences in functional activation could arise from power discrepancies resulting from anatomical mismatches, and not true functional abnormalities (Anticevic et al., 2008). This presents a serious concern for fMRI studies conducting between-group comparisons and consequently may impact the interpretation of fMRI results with clinical populations. However, at present it remains unclear whether subcortical alignment precision is compromised in patients with schizophrenia.
To illustrate the point above, consider studies examining amygdala activation in patients with schizophrenia. To date, there is evidence suggesting that patients with schizophrenia under-recruit the amygdala in response to affective material (Aleman and Kahn, 2005; Li et al., 2009). As noted, some of these findings could – in theory – reflect reduced amygdala alignment fidelity for patients with schizophrenia when compared to healthy controls. As shown for cortical activations, reductions in amygdala alignment in patients could lead to reduced estimates of amygdala activation. Therefore, reduced alignment could, in principle, produce a positive finding of amygdala under-recruitment in patients when compared to controls, which would actually arise from an artifact of reduced alignment fidelity in the clinical sample. The same concerns are not limited to the amygdala, and logically extend to other subcortical structures. However, to our knowledge, no study to date has explicitly investigated subcortical alignment profiles in patients with schizophrenia and verified whether this confound can be ruled out when examining activation differences in major subcortical areas. To address these questions, we examined subcortical alignment precision in six major subcortical structures for a large sample of individuals with schizophrenia and matched healthy controls.
Materials and Methods
Subjects
Subjects were recruited through the clinical core of the Conte Center for Neuroscience of Mental Disorders (CCNMD) at Washington University in St. Louis. Clinical assessments were conducted by a research associate trained to administer the SCID-IV, who also regularly participated in the diagnostic rating sessions (First et al., 2002). An additional assessment session was conduced by an expert clinician using a semi-structured interview for DSM-IV as well as all available patient records. A consensus on each diagnosis was reached between the interviewer and the expert clinician. The complete sample included 48 subjects meeting DSM-IV diagnostic criteria for schizophrenia and 45 demographically matched healthy control subjects (Table 1). Control subjects were recruited using local advertisements in the same community from which schizophrenia subjects were recruited. Control subjects were not included if they had any lifetime history of Axis I psychiatric disorder or a first-degree relative with a psychotic disorder. Both control and schizophrenia subjects were also excluded if they: 1) met criteria for DSM-IV substance abuse or dependence within the past 6 months; 2) they had any severe medical complications that would compromise psychiatric assessment and diagnosis or render the subject unstable or at risk to participate; 3) they suffered head injury (past or present) with manifestation of neurological symptoms or loss of consciousness; or 4) met DSM-IV diagnostic criteria of mental retardation. All schizophrenia subjects were taking antipsychotic medication at the time of the scan and had to be stable for a period of at least 2 weeks. All subjects completed and signed an informed consent approved by the Washington University IRB and were assessed for handedness using the Edinburgh Handedness Inventory (Oldfield, 1971). Measurements of IQ (Wechsler, 1997), medication dose (converted to chlorpromazine equivalents) and symptom severity using the Schedules for the Assessment of Positive and Negative Symptoms (Andreasen, 1983a, b) were also obtained for each subject and their possible relationship with alignment quality was explored.
Table 1.
Demographics and clinical data
| Characteristic | Controls | Schizophrenia | Significance | |||
|---|---|---|---|---|---|---|
| M | S.D. | M | S.D. | T Value/Chi-Square | P Value | |
| Age (in years) | 23.81 | 9.62 | 25.67 | 8.10 | 1.02 | 0.31 |
| Gender (% male) | 0.58 | 0.77 | 3.75 | 0.053 | ||
| Parent's education (in years) | 13.94 | 2.50 | 13.51 | 3.79 | 0.73 | 0.46 |
| Participant's education (in years) | 13.45 | 2.29 | 11.76 | 2.36 | 1.94 | 0.06 |
| Handedness (% right) | 88.37 | 91.67 | 0.28 | 0.60 | ||
| Mean SAPS Global Item Score | 0.03 | 0.08 | 1.36 | 0.84 | ||
| Mean SANS Global Item Score | 0.27 | 0.31 | 1.96 | 0.80 | ||
| Disorganization | 0.88 | 1.21 | 3.90 | 2.50 | ||
| Poverty | 0.53 | 0.81 | 7.77 | 3.34 | ||
| Reality Disotortion | 0.02 | 0.16 | 3.56 | 2.72 | ||
Scanning
All subjects were scanned at the Washington University Medical School. The structural images from the participants were obtained from either a 1.5T Siemens VISION system (29 patients and 27 controls) or a 3T Tim TRIO system (19 patients and 18 controls). Although there could be concerns about combining data from different scanner platforms, the number of subjects in schizophrenia vs. control group scanned at either 1.5T and 3T data were similar (meaning that group was not confounded with scanner platform) and there was no interaction between the group factor and scanner platform (see Results). The structural images acquired on both scanners used a coronal MP-RAGE 3D T1-weighted sequence (for the 1.5T, TR=9.7 ms, TE=4 ms, flip=10°; voxel size=1× 1× 1.2 mm; for 3T, TR=2400 ms, TE=3.16 ms, flip=14.5°; voxel size=1× 1× 1 mm).
Volume based registration (VBR)
The entire 3D structural volume (T1) was registered to a stereotaxic atlas space (Talairach and Tournoux, 1988) using 12-parameter affine transform and resampled to 1mm cubic representation (Buckner et al., 2004; Ojemann et al., 1997). The VBR method was identical to the approach taken when examining cortical alignment and corresponds to widely used methods in the schizophrenia literature (Anticevic et al., 2008).
Subcortical structure isolation
As noted, we sought to compare alignment precision in six different bilateral subcortical structures including the amygdala, caudate, hippocampus, pallidum, putamen, and thalamus. To this end, we isolated each subject’s bilateral structure using an automated subcortical segmentation algorithm implemented in the FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/), which was shown to have high reliability when compared to manual subcortical identification (Fischl et al., 2002; Fischl et al., 2004). Also, other authors have found comparable results between FreeSurfer and manual anatomical ratings in a clinical population (Tae et al., 2008). Subcortical binary masks for each structure were isolated using in-house algorithms and used for subsequent analyses. Results were visualized using the AFNI software package (Cox, 1996). Of note, no between-group volume differences across structures were found (all ps > 0.3). In addition, the two groups were compared in terms of equality of variances for each structure and each hemisphere. The only significant difference that emerged was in the left thalamus, which however did not show alignment differences between the groups (see Results).
Volume alignment precision
To quantitatively evaluate subcortical alignment a rigorously validated metric for volume alignment precision (VAP) (Anticevic et al., 2008) was used. The VAP metric is similar to the volume alignment consistency (VAC) and surface alignment consistency (SAC) measures introduced by Van Essen (2005), but has the advantage of allowing parametric statistical comparisons, as it computes a quantitative measure of overlap for each subject. Briefly, to obtain the VAP index, identified subcortical structures for each subject were summed to generate probabilistic maps that reflected, for each voxel, the number of subjects for whom that voxel was in the subcortical structure of interest (i.e. an overlap histogram). We then selected those voxels for a given structure that were labeled as belonging to that subcortical region for at least 50% of subjects. Next, the intersection between each individual subject’s mask for that structure and the group 50% overlap region for a particular structure was expressed as a fraction of the individual subject’s total structure volume and then averaged across all subjects, yielding the VAP for that structure:
where x(s) is the number of voxels in a given structure (s) for subject i that intersect the 50% overlap region; x(total) is the total number of voxels that belong to structure s for subject i; and n is the total number of subjects contributing to the VAP calculation. As shown previously, the 50% overlap criterion was fairly stringent and was likely to detect true between-group alignment differences (Anticevic et al., 2008).
Results
First, in order to qualitatively visualize the results of within-group alignment we generated the probabilistic overlap maps shown in figure 1. The red voxels mark locations of high overlap in a given structure, whereas the blue voxels mark areas of low overlap. Upon visual inspection, there are no discernible alignment precision differences between schizophrenia (left panel) and control subjects (right panel) for any of the examined structures (Figure 1A–L). Next, we verified these results quantitatively using the VAP index.
Figure 1. Probabilistic alignment maps for patients with schizophrenia and healthy controls.
An overlap histogram is shown for the amygdala, caudate, hippocampus, pallidum, putamen and thalamus for patients with schizophrenia on the left (A–F) and control participants on the right (G–L). Regions showing a high degree of across-subject overlap are shown in red, whereas regions showing minimal overlap across subjects are shown in blue.
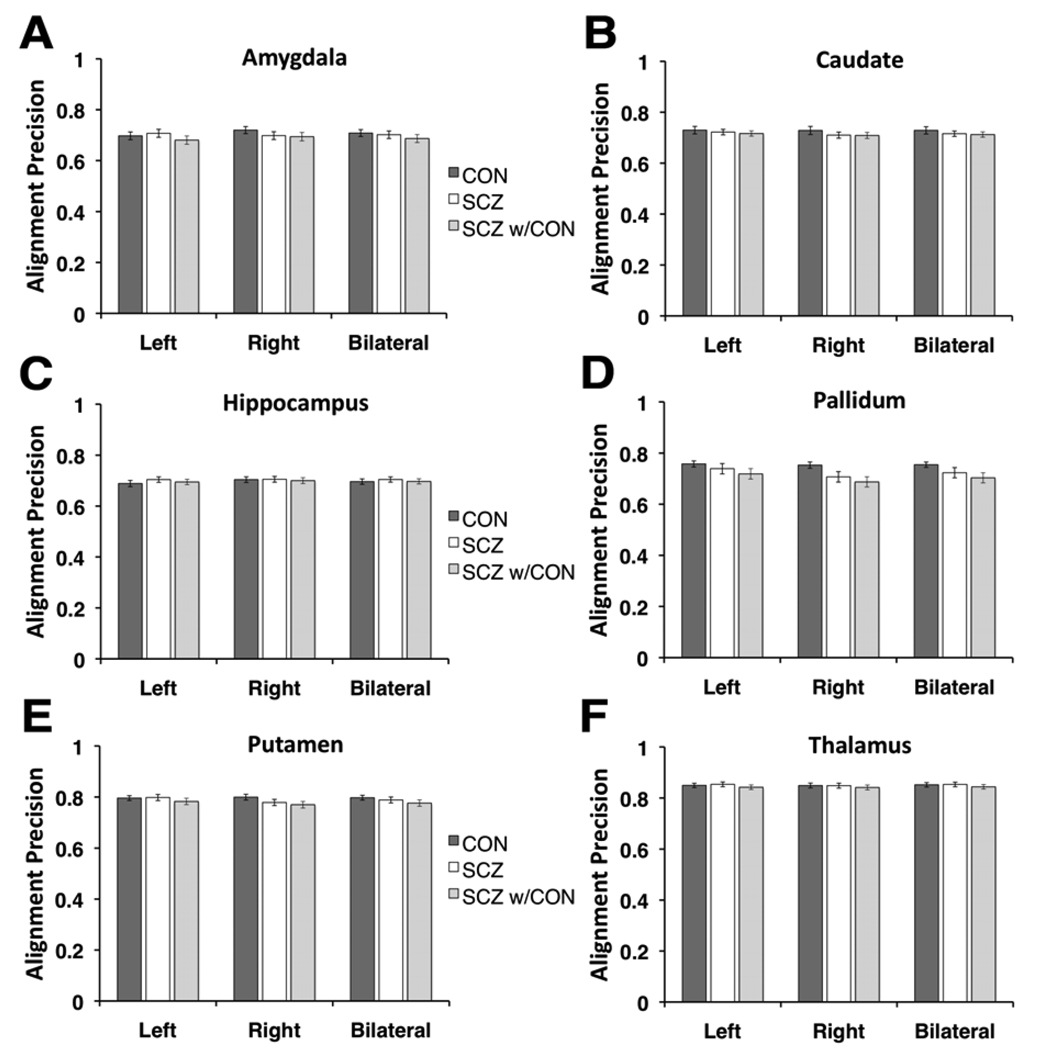
Figure 2 shows the average VAP indices for both hemispheres separately as well as bilaterally for all examined structures (Figure 2A–F). In line with the probabilistic maps shown in figure 1, the VAP measures showed numerically small differences between schizophrenia and control subjects. To confirm this statistically, we computed a 3-way ANOVA with one between-group factor (Group, 2 levels – schizophrenia & control subjects) and 2 within-group factors (Hemisphere, 2 levels – left & right; Region, 6 levels). The ANOVA results indicated no main effect of Group [F(1,91)=0.37, p=0.541]. Additionally, the interactions of Group × Region [F(5,455)=1.35, p=0.24] and Group × Hemisphere × Region [F(5,455)=1.01, p=0.40] failed to reach significance. The only interaction including the Group term that reached significance was the Group × Hemisphere interaction [F(1,91)=5.49,p<0.025], indicating that the VAP differed between schizophrenia and control subjects across the two hemispheres. The main source of the Group × Hemisphere interaction was somewhat lower VAP for the right relative to the left hemisphere in patients (schizophrenia right hemisphere VAP = 0.741; schizophrenia left hemisphere VAP = 0.754), which was especially prominent in the pallidum and putamen. In contrast, control subjects showed better VAP for the right compared to left hemisphere (control right hemisphere VAP = 0.760; schizophrenia left hemisphere VAP = 0.753). Although the numerical VAP differences across hemispheres were very small in both groups, post-hoc contrasts revealed that the hemispheric alignment difference was significant in the schizophrenia [t(47)=2.7, p<0.001], but not the control group [t(44)=0.90, p=0.37]. Of note, when including the scanner platform as a factor in the ANOVA (3T vs 1.5T) there was no significant interaction between the scanner platform and group [F(1,89)=0.08, p=0.77], indicating comparable group alignment differences irrespective of scanner.
Figure 2. Volume alignment precision index across six subcortical regions for patients and healthy controls.
Average volume alignment precision results are shown for each of the examined structures (A–F) for left and right hemispheres separately as well as bilaterally. Dark gray bars and white bars indicate results for control and schizophrenia groups respectively, whereas the light gray bars indicate across-group alignment results (i.e. how well patients with schizophrenia align with the control group 50% overlap region). Error bars are +/− 1 standard error.
In addition to the statistical comparisons, we quantified the degree of between-group differences observed for each structure using effect size calculations (i.e. Cohen’s d) (Cohen, 1992). Table 2 shows effect sizes for differences between schizophrenia and control VAP indices for each structure and hemisphere. The average effect size across both hemispheres and all structures was 0.14, which by standard convention would be considered a negligible effect (Cohen, 1992). Furthermore, 10/18 individual comparisons had negligible effect sizes (i.e. Cohen’s d less than 0.15) and no effect size exceeded the value of 0.4, thus falling below the lower cutoff for what would conventionally be considered a medium effect. Taken together, these results suggest minimal VAP differences between schizophrenia and control subjects when using affine registration techniques.
Table 2. Statistical comparisons.
The t value, p value and an effect size measure (Cohen’s d) are shown for each examined comparison for both hemispheres as well as bilaterally. Results are shown comparing schizophrenia group alignment results with that of controls. Also, results are shown comparing original schizophrenia group alignment results to those obtained when using the control group 50% overlap region.
| Structure | Hemisphere | Schizophrenia vs Control Group VAP | Schizophrenia VAP vs. Results using control group 50% overlap template |
||||
|---|---|---|---|---|---|---|---|
| t-value | alpha | Effect Size (Cohen's d) | t-value | alpha | Effect Size (Cohen's d) | ||
| L | 0.45 | 0.65 | 0.09 | 1.18 | 0.24 | 0.25 | |
| Amygdala | R | 1.05 | 0.30 | 0.22 | 0.18 | 0.86 | 0.04 |
| Bilateral | 0.32 | 0.75 | 0.07 | 0.70 | 0.49 | 0.15 | |
| L | 0.42 | 0.67 | 0.09 | 0.37 | 0.71 | 0.08 | |
| Caudate | R | 0.94 | 0.35 | 0.20 | 0.07 | 0.94 | 0.01 |
| Bilateral | 0.74 | 0.46 | 0.16 | 0.23 | 0.82 | 0.05 | |
| L | 0.94 | 0.35 | 0.20 | 0.62 | 0.54 | 0.13 | |
| Hippocampus | R | 0.08 | 0.93 | 0.02 | 0.32 | 0.75 | 0.07 |
| Bilateral | 0.55 | 0.58 | 0.12 | 0.51 | 0.61 | 0.11 | |
| L | 0.79 | 0.43 | 0.17 | 0.70 | 0.49 | 0.15 | |
| Pallidum | R | 1.88 | 0.06 | 0.39 | 0.69 | 0.49 | 0.14 |
| Bilateral | 1.37 | 0.17 | 0.29 | 0.70 | 0.48 | 0.15 | |
| L | 0.15 | 0.88 | 0.03 | 0.89 | 0.37 | 0.19 | |
| Putamen | R | 1.26 | 0.21 | 0.26 | 0.50 | 0.62 | 0.10 |
| Bilateral | 0.59 | 0.56 | 0.12 | 0.72 | 0.47 | 0.15 | |
| L | 0.32 | 0.75 | 0.07 | 0.88 | 0.38 | 0.18 | |
| Thalamus | R | 0.04 | 0.97 | 0.01 | 0.53 | 0.60 | 0.11 |
| Bilateral | 0.11 | 0.91 | 0.02 | 0.72 | 0.47 | 0.15 | |
Although the above analysis demonstrates equivalent within-group alignment, it does not verify whether the across-group alignment is adequate. In other words, it is critical to show that schizophrenia subjects align at least approximately as well to the control subjects as they do to themselves. We examined this question by using the 50% overlap region from the control sample as the intersection volume when computing the VAP for the schizophrenia group (see Method Section). Figure 2 (light gray bars) indicate that the results remain largely unchanged when using schizophrenia subjects’ anatomies and the control 50% overlap template. To verify this statistically, we computed a 3-way ANOVA identical to that used for the within-group VAP scores. The ANOVA results indicated no main effect of Group [F(1,94)=0.55, p=0.46]. Additionally, the interactions of Group × Region [F(5,470)=0.483, p=0.78], Group × Hemisphere [F(1,94)=0.15, p=0.69] and Group × Hemisphere × Region [F(5,470)=0.31, p=0.90] failed to reach significance. As before, effect size calculations indicated negligible differences between original VAP values for schizophrenia group and VAP values when using the control 50% overlap region (Table 2). The average effect size was 0.12, which would again be considered negligible by standard convention. Moreover, no effect size calculations exceeded 0.25 and 10/18 fell in the negligible range (i.e. Cohen’s d smaller than 0.15), confirming that subcortical VAP for schizophrenia subjects remains similar even when using the 50% overlap region derived from the control group.
In addition, we examined whether medication status, IQ, or symptom severity impacted the quality of alignment precision in the patient group. The average correlation across all structures for the left and right hemisphere between medication dose and alignment precision was r=0.06 and r=−0.02 respectively. When examining each structure separately, only one correlation emerged at p<0.05 level in the left caudate (r=−0.31). However, given the number of comparisons this finding would not survive even a liberal multiple-comparison correction. The average correlation across all structures for the left and right hemisphere between IQ and alignment precision was r=0.26 and r=0.15 respectively. When examining each structure separately no correlations were significant at p<0.05. In addition, we calculated symptom severity based on SAPS and SANS ratings and correlated the obtained values with alignment precision across left and right hemispheres in three broad symptom domains: i) Disorganization symptoms [average correlations in left hemisphere: r=−0.18; right hemisphere: r=−0.12]; ii) Reality Distortion symptoms [left hemisphere: r=0.04; right hemisphere: r=−0.01]; and iii) Poverty symptoms [left hemisphere: r=−0.25; right hemisphere: r=−0.20]. No individual correlations reached significance.
Discussion
We demonstrate, using the same techniques previously applied to cortical regions, that subcortical alignment in patients with schizophrenia is comparable to that of control subjects when using 12-parameter affine VBR. Furthermore, we cross-validate these results across groups, demonstrating that, when using the control subjects’ derived overlap region, results change minimally as compared to schizophrenia within-group alignment.
Subcortical versus cortical alignment
The main finding in the current study is relatively similar alignment of subcortical structures in schizophrenia and control subjects, supported by negligible effect sizes when comparing group differences in alignment (Cohen, 1992). Moreover, the largest observed VAP differences (right palladium and right putamen) did not reach what, by standard convention, would be considered even a medium effect size. Admittedly, this pattern of results was surprising given our previous findings for cortical landmarks (Anticevic et al., 2008). We would have expected reduced VAP for at least some subcortical structures in schizophrenia subjects given numerous reports of anatomical and morphological abnormalities in some of the investigated regions (Csernansky et al., 2008; Csernansky et al., 1998; Csernansky et al., 2004; Harms et al., 2007; Konick and Friedman, 2001; Lawrie et al., 2003; Mamah et al., 2007; Namiki et al., 2007; Shenton et al., 2001; Shenton et al., 2002; Velakoulis et al., 1999; Velakoulis et al., 2006; Wang et al., 2008; Wood et al., 2001). One possible explanation for our findings may be the inherent geometry of subcortical relative to cortical anatomy. In other words, given that cortex is a 2D folded sheet of tissue, most cortical landmarks take the geometric representations of ‘ribbons’ or folded ‘sheets’ in 3D space with high surface-to-volume ratio. In turn, any type of movement (i.e. translation or rotation) or distortion (i.e. subtle anatomical shape abnormalities) will have a large impact on alignment of such a brain region relative to the group average. In contrast, most subcortical structures have a much smaller surface-to-volume ratio and more closely approximate spherical configurations (with the possible exception of the hippocampus). In turn, closer approximation of spherical shape may render across-subject subcortical alignment more robust to subtle shape abnormalities and/or shifts in space.
Implications for fMRI studies and statistical power
It is important to note that our findings do no imply that subtle shape deformations in subcortex are not clinically important. However, our findings do suggest that alignment of subcortical structures is unlikely to confound subcortical fMRI investigations in patients with schizophrenia. There are two main pieces of supporting evidence for this assertion. First, the overall magnitude of effect size differences was negligible when directly comparing schizophrenia vs. control group alignment profiles for each structure. Second, we found that when testing across-group alignment (e.g., alignment of schizophrenia to control subjects) the results remain largely unchanged, suggesting that schizophrenia subjects’ subcortical structures align relatively well with respect to control group templates. If schizophrenia subjects’ anatomies were shifted or much more variable relative to the control group then the across-group alignment would have been poorer, but we failed to observe such an effect.
It should be noted that we found a Group × Hemisphere interaction with respect to alignment precision in the within-group alignment analyses, which indicated that schizophrenia subjects showed relatively worse alignment in the right when compared to left hemisphere structures, whereas no such finding was observed for controls. While a statistically reliable finding, the magnitude of the hemispheric alignment difference in the schizophrenia group was very small for almost all regions, and schizophrenia and control groups did not differ for either the right or the left hemisphere. Given that the only hemispheric difference approaching the medium effect size was in the right pallidum, functional neuroimaging studies examining this region may want to verify that alignment in their sample is not compromised or alternatively use a ROI-based analytic strategy that would eliminate the problem of an alignment mismatch. Of note, one possibility is that our findings reflect exaggerated hemispheric asymmetries previously reported in schizophrenia (Crow, 1999; Crow et al., 1989a; Crow et al., 1989b; Pearlson and Marsh, 1999; Qiu et al., 2009; Shenton et al., 2001), which may lead to greater alignment mismatches in certain left hemisphere subcortical structures. Finally, the present findings also suggest that subcortical alignment differences may have a negligible impact on other methods relying on similar registration techniques for aligning subcortical structures (e.g. voxel-based morphometry studies) (Modinos et al., 2009).
Possibility for further improvements
While we demonstrated that alignment precision shows negligible discrepancies between schizophrenia and control subjects following 12-parameter affine registration, there were still differences in specific structures that approached medium effect sizes. Such differences may be completely eliminated with current state-of-the-art subcortical segmentation techniques using structure-specific alignment. For instance, one approach – large deformation diffeomorphic metric mapping (LDDMM) of subcortical structures introduced by Khan and colleagues (Khan et al., 2008) – which is integrated with the FreeSurfer software package employs sample-specific template-based registration while producing a ‘smoother’ segmentation of subcortical structures. In turn, superior segmentation should further minimize across-subject variability for a given structure. In addition to improved segmentation, LDDMM affords non-linear structure-specific registration, which further minimizes alignment discrepancies and should, in principle, yield an even better power profile for functional neuroimaging investigations. Additionally, state-of-the-art whole-brain nonlinear deformation algorithms evaluated by Klein and colleagues (Klein et al., 2009) may further reduce alignment discrepancies observed in the current study.
Limitations
We demonstrated that VAP for major subcortical structures is largely equivalent in schizophrenia and control subjects. However, we did not explicitly examine the relationship between VAP and power profiles for fMRI signal within subcortical structures. The implication of the current findings is that the observed negligible effect sizes for between-group differences in subcortical alignment do not present a plausible confound for fMRI comparisons. However, it will be important for future work to explicitly test this hypothesis. Given that we focused on six major subcortical structures, it would be quite challenging to design a single experiment that reliably engages all of the examined structures in one study in the same participants. However, for future validations it would be feasible to investigate one or a few regions in focused studies (e.g. the amygdala in response to facial affect) to explicitly validate that even minimal subcortical alignment mismatches do not confound fMRI activation profiles. In addition, while we did not find alignment differences in the present study, we still cannot rule out the possibility that if we were to examine a more chronic and severe sample some differences may be more apparent.
Conclusions
In summary, we demonstrated that, in contrast to cortex, alignment of subcortical structures is largely similar when comparing patients with schizophrenia and healthy controls. This has important implications for studies examining functional differences in subcortical structures, particularly those using whole-brain approaches, as it suggests that group differences in alignment may not present a major confound for interpreting group differences in functional activation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alan Anticevic, Department of Psychology, Washington University in St. Louis.
Grega Repovs, Department of Psychology, University of Ljubljana.
Jared X. Van Snellenberg, Department of Psychology, Columbia University
John G. Csernansky, Department of Psychiatry, Northwestern University
Deanna M. Barch, Department of Psychology, Washington University in St. Louis
References
- Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. European journal of nuclear medicine. 1998;25:663–667. [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983a. [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) Iowa City: University of Iowa; 1983b. [Google Scholar]
- Anticevic A, Dierker DL, Gillespie SK, Repovs G, Csernansky JG, Van Essen DC, Barch DM. Comparing surface-based and volume-based analyses of functional neuroimaging data in patients with schizophrenia. NeuroImage. 2008;41:835–848. doi: 10.1016/j.neuroimage.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argall BD, Saad ZS, Beauchamp MS. Simplified intersubject averaging on the cortical surface using SUMA. Human brain mapping. 2006;27:14–27. doi: 10.1002/hbm.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crow TJ. Twin studies of psychosis and the genetics of cerebral asymmetry. The British journal of psychiatry : the journal of mental science. 1999;175:399–401. doi: 10.1192/bjp.175.5.399. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Ball J, Bloom SR, Brown R, Bruton CJ, Colter N, Frith CD, Johnstone EC, Owens DG, Roberts GW. Schizophrenia as an anomaly of development of cerebral asymmetry. A postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989a;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- Crow TJ, Colter N, Frith CD, Johnstone EC, Owens DG. Developmental arrest of cerebral asymmetries in early onset schizophrenia. Psychiatry research. 1989b;29:247–253. doi: 10.1016/0165-1781(89)90053-x. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Gillespie SK, Dierker DL, Anticevic A, Wang L, Barch DM, Van Essen DC. Symmetric abnormalities in sulcal patterning in schizophrenia. NeuroImage. 2008;43:440–446. doi: 10.1016/j.neuroimage.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proceedings of the National Academy of Sciences. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. The American journal of psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- Desai R, Liebenthal E, Possing ET, Waldron E, Binder JR. Volumetric vs. surface-based alignment for localization of auditory cortex activation. NeuroImage. 2005;26:1019–1029. doi: 10.1016/j.neuroimage.2005.03.024. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M. Whole Brain Segmentation Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23 Suppl 1:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. NeuroImage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. NeuroImage. 2008;41:735–746. doi: 10.1016/j.neuroimage.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Job DE, Johnstone EC. Structural and functional abnormalities of the amygdala in schizophrenia. Annals of the New York Academy of Sciences. 2003;985:445–460. doi: 10.1111/j.1749-6632.2003.tb07099.x. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RC, McAlonan GM, Gong QY. Facial Emotion Processing in Schizophrenia: A Meta-analysis of Functional Neuroimaging Data. Schizophrenia bulletin Epub. 2009 doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophr Res. 2007;89:59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural covariance in the hallucinating brain: a voxel-based morphometry study. J Psychiatry Neurosci. 2009;34:465–469. [PMC free article] [PubMed] [Google Scholar]
- Namiki C, Hirao K, Yamada M, Hanakawa T, Fukuyama H, Hayashi T, Murai T. Impaired facial emotion recognition and reduced amygdalar volume in schizophrenia. Psychiatry research. 2007;156:23–32. doi: 10.1016/j.pscychresns.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Marsh L. Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry. 1999;46:627–649. doi: 10.1016/s0006-3223(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Qiu A, Wang L, Younes L, Harms MP, Ratnanather JT, Miller MI, Csernansky JG. Neuroanatomical asymmetry patterns in individuals with schizophrenia and their non-psychotic siblings. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Gerig G, McCarley RW, Székely G, Kikinis R. Amygdala-hippocampal shape differences in schizophrenia: the application of 3D shape models to volumetric MR data. Psychiatry research. 2002;115:15–35. doi: 10.1016/s0925-4927(02)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam E-C, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–581. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. NeuroImage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Thompson PA, Barch DM, Miller MI, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biol Psychiatry. 2008;64:1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wood SJ, Velakoulis D, Smith DJ, Bond D, Stuart GW, McGorry PD, Brewer WJ, Bridle N, Eritaia J, Desmond P, Singh B, Copolov D, Pantelis C. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2001;52:37–46. doi: 10.1016/s0920-9964(01)00175-x. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. general methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998a;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC. Automated image registration: II. intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998b;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited - again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]