Summary
Animal studies have implicated oxytocin and vasopressin in social bonding, physiological stress responses, and wound healing. In humans, endogenous oxytocin and vasopressin levels covary with perceptions of relationship quality, marital behaviors, and physiological stress responses. To investigate relationships among marital behavior, oxytocin, vasopressin, and wound healing, and to determine the characteristics of individuals with the highest neuropeptide levels, 37 couples were admitted for a 24-hour visit in a hospital research unit. After small blister wounds were created on their forearm, couples participated in a structured social support interaction task. Blister sites were monitored daily following discharge to assess wound repair speed. Blood samples were collected for oxytocin, vasopressin, and cytokine analyses. Higher oxytocin levels were associated with more positive communication behaviors during the structured interaction task. Furthermore, individuals in the upper oxytocin quartile healed blister wounds faster than participants in lower oxytocin quartiles. Higher vasopressin levels were related to fewer negative communication behaviors and greater tumor necrosis factor-α production. Moreover, women in the upper vasopressin quartile healed the experimental wounds faster than the remainder of the sample. These data confirm and extend prior evidence implicating oxytocin and vasopressin in couples' positive and negative communication behaviors, and also provide further evidence of their role in an important health outcome, wound healing.
Keywords: Oxytocin, Vasopressin, Marital Quality, Marriage, Inflammation, Cytokine
Introduction
Abundant evidence implicates the peptide hormones oxytocin and arginine vasopressin in the regulation of social behavior. Animal models have shown that both peptides are involved in parental behavior, sociability, and pair bonding (Pedersen, 1997; Cho, et al., 1999; Pitkow, et al., 2001). In humans, intranasal oxytocin administration increased trust and prosocial behaviors in experimental economic tasks (Kosfeld, et al., 2005; Zak, et al., 2007), and led to a higher ratio of positive to negative behaviors during the discussion of a marital disagreement (Ditzen, et al., 2009), compared to participants who received the placebo administration.
High peripheral oxytocin levels have been associated with better relationship quality. Individuals who reported greater perceived social support, spousal support, and a higher frequency of partner hugs and massages had higher oxytocin levels than those who reported less support and physical intimacy (Light et al., 2004; Grewen et al., 2005; Light et al., 2005a). Furthermore, a 4-week couple contact enhancement intervention increased salivary oxytocin levels, while no such change was observed in the control group (Holt-Lunstad, et al., 2008). Paradoxically, individuals reporting greater relationship stress have also sometimes had greater oxytocin levels (Turner, et al., 1999; Taylor, et al., 2006; Cyranowski, et al., 2008). The factors that modulate the association between relationship quality and plasma oxytocin levels remain unknown.
Although much less studied in humans, high vasopressin levels also appear to be associated with better relationship quality. Among men in a long-term relationship, a polymorphism of the vasopressin V1a receptor gene was related to lower self-reported partner bonding, more marital crises in the past year, and an increased likelihood of cohabiting rather than being married (Walum, et al., 2008). Moreover, orphanage-reared children who were living in stable homes at the time of testing had lower basal urinary vasopressin levels than family-reared children, suggesting that peripheral vasopressin levels may also be associated with a history of neglect (Fries, et al., 2005).
In addition to their role in social behavior, oxytocin and vasopressin may also impact circulating cytokine production. In cell cultures interleukin-6 (IL-6) production by stimulated THP-1 macrophage and endothelial cells was reduced following incubation with physiological levels of oxytocin (Szeto, et al., 2008). In rodents, injection of lipopolysaccaride (LPS) and intracerebral infusion of IL-1β increased the release of central and peripheral oxytocin and vasopressin (Landgraf, et al., 1995; Rivest and Laflamme, 1995; Palin, et al., 2009). Furthermore, intravenous oxytocin administration decreased IL-6 responses to LPS stimulation in humans (Clodi, et al., 2008). Thus, endogenous oxytocin and vasopressin levels may be capable of modulating inflammation.
Previous work showed that stress can slow the early stages of wound healing in humans and mice (Padgett, et al., 1998; Glaser, et al., 1999). Oxytocin and vasopressin may also influence wound healing. Restraint stress delayed wound healing in rodents (Detillion, et al., 2004), but when hamsters were exposed to the stressor with another animal, the presence of a companion buffered stress-induced delays in wound repair. When paired-housed hamsters received an oxytocin antagonist, they did not heal faster than individually-housed animals, suggesting that the increased oxytocin activity mediated the beneficial impact of social relationships on wound healing (Detillion et al., 2004). In rats who received peritoneal oxytocin administration healed a burn injury faster, compared to rats that received a placebo injection (Vitalo, et al., 2009), providing further evidence that the peptide may promote wound repair.
The goals of the present study were to evaluate the relationships between marital behavior and endogenous oxytocin and vasopressin, to dertermine the extent to which these peptides modulate proinflammatory cytokine production and wound healing, and to evaluate the characteristics of individuals with the highest neuropeptide levels. Married couples were invited for a 24-hour admission at a hospital research unit. During the visit, each couple underwent an experimental skin blistering procedure and participated in a structured social support interaction task. Participants provided blood samples during the visit for oxytocin, vasopressin, and proinflammatory cytokine analyses, and were monitored daily following discharge to assess wound healing.
Methods
Participants
Thirty-seven heterosexual, married couples (74 participants) were recruited as part of a larger study on marital stress and wound healing through newspaper and radio ads, notices posted on campus and in the community, and referrals from other participants (Kiecolt-Glaser, et al., 2005). Exclusion criteria included 1) health problems or related medications that had an obvious immunological or endocrinological component or consequence for wound healing (e.g., cancer, recent surgeries, strokes, diabetes mellitus, peripheral vascular disease, conditions such as asthma or arthritis that required regular use of anti-inflammatory medications), 2) blood pressure medication, smoking, or using excessive alcohol or caffeine, and 3) pregnancy or anti-diuretic medication use. All women were scheduled during the follicular phase of their menstrual cycle. The Institutional Biomedical Research Review Committee approved the project; all subjects gave written informed consent prior to participation.
Sixty-eight participants were Caucasian (91.3%), 3 were African-American, 2 Hispanic and 1 Asian. About half (56.5%) of the sample had a college education. Participants' ages ranged from 22 to 73 years with a mean of 38.47 (SD = 11.99). Couples had been married from 2 to 39 years, with a mean of 11 years (SD = 9.63). Most participants (78%) had only been married once. Couples had two children on average (SD=1.63).
Protocol
Individuals participated in a 24-hour visit at the CRC, a hospital-based research unit. At 07:00h, couples were admitted to the CRC, fed a standard breakfast (after fasting since midnight before admission), and given questionnaires to complete. A heparin well was inserted in each participant's arm to facilitate blood draws throughout the day. At 0915h, nurses performed the experimental blistering procedure for the wound healing study. At roughly 1045h, couples were positioned in chairs facing each other in front of a curtain, completed several questionnaires, and sat quietly for 10 minutes before starting the structured social support interaction task (Pasch and Bradbury, 1998). At 1130h, couples started a second interaction task during which they were asked to discuss the history of their relationship for 30 minutes (Veroff, et al., 1993). The marital interaction tasks were videotaped and the research team remained out of sight during all discussions. To ensure consistent physical activity across dyads and admissions, couples remained together in the same room for the remainder of the day.
Throughout the admission, couples were encouraged to drink water regularly to facilitate blood draws and prevent vasopressin fluctuations due to changes in plasma osmolality. Blood samples for oxytocin and vasopressin were taken at 0745h and 1200h. Blood samples for IL-6 and TNF-α were drawn on admission to the CRC, at the end of the interaction task, 3h later, and the next morning at 07:00h, respectively at 0, 4, 7, and 24 hours after the CRC admission.
Behavioral Observational Coding System
The first interaction task was designed to assess partners' behaviors when soliciting and offering social support. In the structured social support task, each spouse identified an important personal characteristic, problem, or issue that he or she wished to change; they were explicitly instructed to avoid discussing topics that might lead to marital dissension. Each spouse was then asked to talk about what they would like to change about themselves, while their partner could be involved in the discussion in whatever way they wished. Roles were reversed after 10 minutes of discussion (Pasch and Bradbury, 1998).
The Rapid Marital Interaction Coding System (Heyman, 2004) was used to quantify marital interaction data during the social support task. Negative behaviors were computed by summing five RMICS codes: psychological abuse, distress-maintaining attributions, hostility, dysphoric affect, and withdrawal. A positive behavior index was created by aggregating the acceptance, relationship-enhancing attribution, self-disclosure, and humour codes. Two coders rated the couples' interactions. The kappa coefficient for inter-rater reliability was .80. Couples displayed on average 18.70 (SD=10.93) positive behaviors and 4.08 (SD= 4.95) negative behaviors. Positive and negative behaviors were not significantly related to each other, r= .02, p=.90.
Health Behaviors
Health behaviors such as alcohol, caffeine, or medication use in the past week were evaluated (Kiecolt-Glaser and Glaser, 1988). Two questions from Baecke et al. (1982) were used to quantify recent physical activity. Height and weight measurements were taken to calculate participant's body mass index (BMI). The Pittsburgh Sleep Quality Index assessed sleep quality and sleep disturbances in the past month (Buysse, et al., 1989).
Plasma Oxytocin, Vasopressin and Cytokine Assays
Plasma was kept chilled and immediately frozen at -80°C prior to thawing for assays. The plasma was diluted in assay buffer (1:4) to give results reliably within the linear portion of the standard curve. Plasma oxytocin and vasopressin were assayed by enzyme immunoassay (Assay Designs, Ann Arbor, MI), per kit instructions. The EIAs are highly sensitive (minimal detection levels = 15 pg/ml oxytocin and 4 pg/ml vasopressin) with very little antibody cross-reactivity for other neuropeptides. For the oxytocin and vasopressin EIA kits, the cross-reactivity between oxytocin and vasopressin was <0.04%. All samples were run at the same time and the inter- and intra-assay coefficients of variation were less than 8% for both assays. Validation of the oxytocin assay is described elsewhere (Carter, et al., 2007). Due to technical difficulties, peptide analyses were not performed for one participant.
Plasma IL-6 and tumor necrosis factor-α (TNF-α) levels were assayed using an electrochemilluminescence method with kits purchased from Meso Scale Discovery (Gaithersburg, MD). Plates were read using the Meso Scale Discovery Sector Imager. All samples were run undiluted in duplicate, and all samples for a couple were run at the same time. Sensitivity for both IL-6 and TNF-α is .3 pg/ml. For IL-6, the inter- and intraassays coefficient of variation were 8.7% and 4.1%. For TNF-α, the inter- and intraassays coefficient of variation were 2.7% and 10.4%. Due to technical difficulties, cytokine data were not available for three participants.
Suction Blister Studies
The suction blister protocol followed the methods described previously and used the same suction blister device (Neuro Probe, Cabin John, MD) (Glaser et al., 1999). A plastic template was taped to the volar surface of the nondominant forearm; a 350–mm Hg vacuum was applied through a pump attached to a regulator until blisters formed (1-1.5 hours). This gentle suction produced 8 small 8-mm blisters. Measurement of the rate of transepidermal water loss (TEWL) through human skin provided a noninvasive method to monitor changes in the stratum corneum barrier function of the skin, providing an excellent objective method for evaluation of wound healing. A computerized evaporimetry instrument, the DermaLab (CyberDERM, Media, PA), measured vapor pressure gradient in the air layers close to the skin surface, following established procedural guidelines. The 8 blister sites were assessed daily for 8 days and then again on day 12. Raw TEWL values were adjusted for daily variations in temperature and humidity. Daily control values from adjacent nonwounded skin were collected to provide information on normal fluctuations in TEWL. Daily control values were subtracted from the temperature-adjusted TEWL value. Healing was defined as the standard criterion of reaching 90% of the day 1 pre-blistering TEWL temperature- and control-adjusted baseline value. Due to technical and scheduling difficulties, wound healing data were not available for three participants.
Statistical analyses
For the marital behavior data, hierarchical linear models (HLM), used to account for the dependency between the husband and the wife data, were fitted with couples' positive and negative behaviors as dependent variables and oxytocin and vasopressin as individual-level predictors. Couple-level variables were created by summing the husbands and wives' positive and negative behaviors (Ewart, et al., 1991; Lindahl, et al., 1997). Positive and negative behaviors were entered simultaneously in the statistical model predicting each peptide variable. HLM models were also fitted to evaluate the extent to which circulating oxytocin and vasopressin levels impacted changes in plasma levels of IL-6 and TNF-α.
For the survival analysis of wound healing, the “event” was defined as reaching 90% of the day 1 TEWL measure. Participants with a ratio less than 90% at their last observed point, either by day 12 or earlier, were censored at that point. Missing data occurred at varying points because of technical difficulties and missed appointments; where necessary, time to healing was calculated using the last observation carried forward. Analyses were conducted using the Cox proportional hazards model with clustering on couple, to control for the non-independence within dyads.
Previous human studies have failed to find a relationship between laboratory social interaction or stressors and changes in oxytocin (Altemus, et al., 2001; Grewen et al., 2005; Taylor et al., 2006). In the present study, oxytocin, F(1,73) = 0.02, p = .90, and vasopressin, F(1,73) = 0.01, p = .93, levels did not significantly change from baseline to the end of the discussion between husbands and wives. This lack of change over time was observed for both men and women, ps > .23. Because there was no significant change in peptides over time, oxytocin and vasopressin measurements for each individual were averaged to obtain a better estimate of the individual's basal level of each peptide.
A series of multiple regressions were used to test a mediation model in which increased oxytocin production mediated the relationship between positive behaviors and wound healing. Bootstrapping resampling techniques were used to test the indirect effect (Shrout and Bolger, 2002; Preacher and Hayes, 2008).
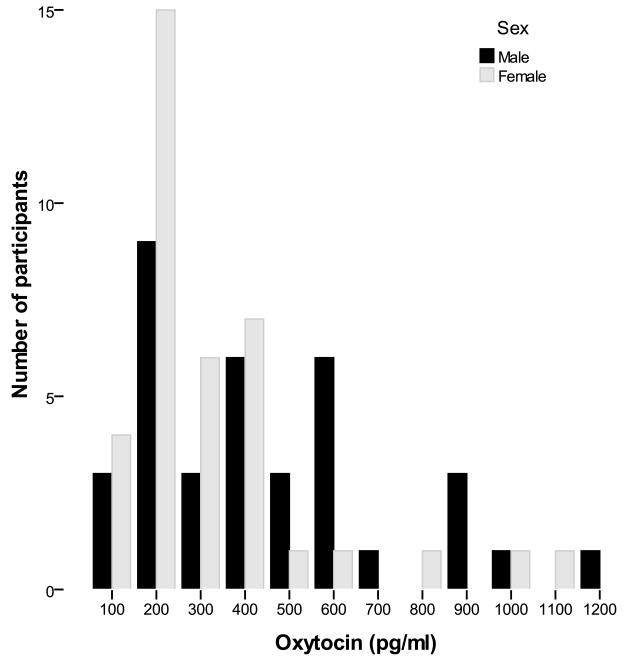
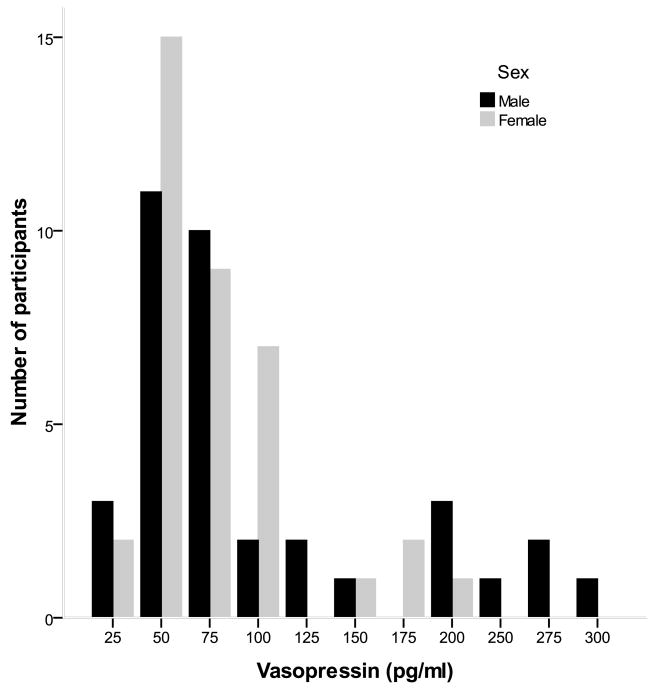
The distributions of oxytocin and vasopressin were severely, positively skewed (Figures 1 and 2). A base 10 logarithmic transformation successfully normalized their distribution. However, we were also interested in evaluating the characteristics of individuals with the highest oxytocin and vasopressin levels since they appear to present distinct characteristics (Light, et al., 2005b). Dichotomous variables were therefore created by comparing the upper quartile of each peptide variable to the remainder of the sample. All analyses were conducted using both the continuous and dichotomous oxytocin and vasopressin variables in separate models. The RMICS negative behaviors variables had an L-shaped distribution with more than 20% of the couples displaying no negative marital interaction. Given our inability to normalize its distribution with data transformations, a dichotomous variable was created using a median split of the negative behaviors data.
Figure 1.
Plasma oxytocin distribution as a function of sex. Both oxytocin and vasopressin measurements were averaged using the baseline and post-interaction task samples
Figure 2.
Plasma vasopressin distribution as a function of sex. Both oxytocin and vasopressin measurements were averaged using the baseline and post-interaction task samples
The covariates included in the behavioral data analyses were age and sex. Age was included in the model because of its significant relationship with plasma oxytocin levels, r =-.24, p= .04, Body mass index was also included as a covariate in the cytokine models. The covariates for the survival analysis were age and sex. Given the known sexually dimorphic effect of oxytocin and vasopressin, sex differences were tested in each model (Carter, Pournajafi-Nazarloo, Kramer, Ziegler, White-Traut, Bello and Schwertz, 2007). The α significance level was set at .05. Statistical analyses were performed using SAS version 9.1 and STATA version 9.0.
Results
Oxytocin, Vasopressin, and Marital Behavior
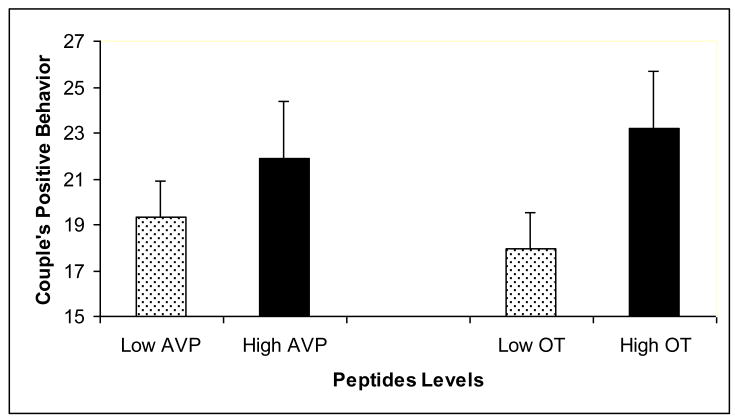
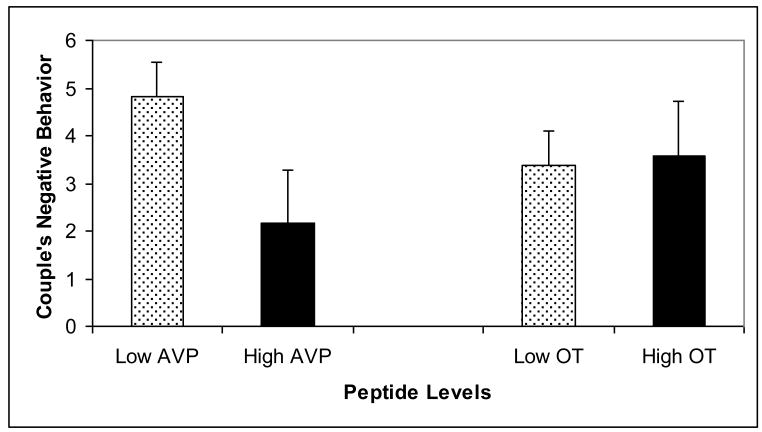
Hierarchical linear models showed that oxytocin and vasopressin were associated with marital behaviors, but the pattern of associations differed between positive and negative behaviors. Individuals who displayed more positive behaviors during the structured social support task had greater oxytocin levels, t(67)= 2.20, p = .03, than individuals who engaged in fewer positive behaviors (Figure 3). Positive communication behaviors were not related to vasopressin levels, t(67)= .25, p = .80. The inverse pattern was found for negative behaviors. Participants who engaged in more negative behaviors during the interaction task had lower vasopressin levels, t(67)= 2.39, p = .02 than individuals displaying fewer negative behaviors (Figure 4). However, negative behaviors were unrelated to oxytocin levels, t(67)= .48, p = .68. None of the oxytocin and vasopressin by sex interaction terms were significant predictors of marital behaviors, all ps >. 18.
Figure 3.
Couple's Positive Behavior As a Function of Oxytocin (OT) and Vasopressin (AVP) Levels. Both oxytocin and vasopressin measurements were averaged using the baseline and post-interaction task samples. For illustration purposes, each peptide variable was dichotomized using a cut-off at the 75th percentile. Errors bars represent the standard error.
Figure 4.
Couple's Negative Behavior As a Function of Oxytocin (OT) and Vasopressin (AVP) Levels. Both oxytocin and vasopressin measurements were averaged using the baseline and post-interaction task samples. For illustration purposes, each peptide variable was dichotomized using a cut-off at the 75th percentile. Errors bars represent the standard error.
Oxytocin, Vasopressin, and Circulating Proinflammatory Cytokines
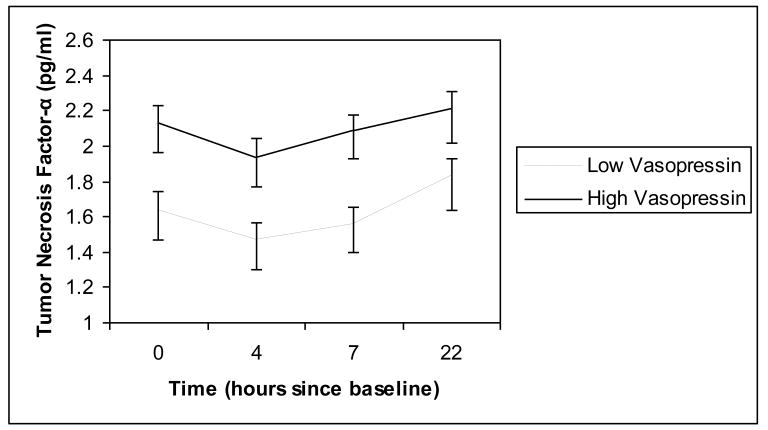
Unconditional hierarchical linear models revealed that cytokine production exhibited a curvilinear pattern of change over time, with IL-6 increasing until 7h and decreasing at 22h and TNF-α being stable during the first 7 hours and then increasing at 22h. Oxytocin levels were not significant predictors of changes over time for either IL-6 levels, F (1,75) = .23, p = .63, or in TNF-α levels, F (1,75) = .03, p = .90. Plasma vasopressin levels were positively associated with TNF-α production across the visit, F(1, 75) = 4.12, p = .05 (Figure 5). However, vasopressin levels did not predict changes over time in IL-6, F (1,75) = .51, p = .48. There was no significant sex by vasopressin or oxytocin interaction in predicting changes in cytokines over time, all ps > .17.
Figure 5.
Plasma tumor necrosis factor-α across the CRC visit as a function of plasma vasopressin levels. Vasopressin measurements were averaged using the baseline and post-interaction task samples. Plasma sample were collected at baseline, 4, 7, and 22 hours later. For illustration purposes, the vasopressin variable was dichotomized using a cut-off at the 75th percentile. Errors bars represent the standard error.
Oxytocin, Vasopressin, and Wound Healing
Cox survival analyses models determined that basal oxytocin and vasopressin levels were not related to the speed of repair of the experimental wound. The hazard ratio (HR) was 1.08 for oxytocin, p = .85 and .86 for vasopressin, p =.69. The sex by oxytocin and sex by vasopressin interactions were not significant predictors of wound healing, p > .34.
Characteristics of Individuals in the Upper Oxytocin and Vasopressin Quartiles
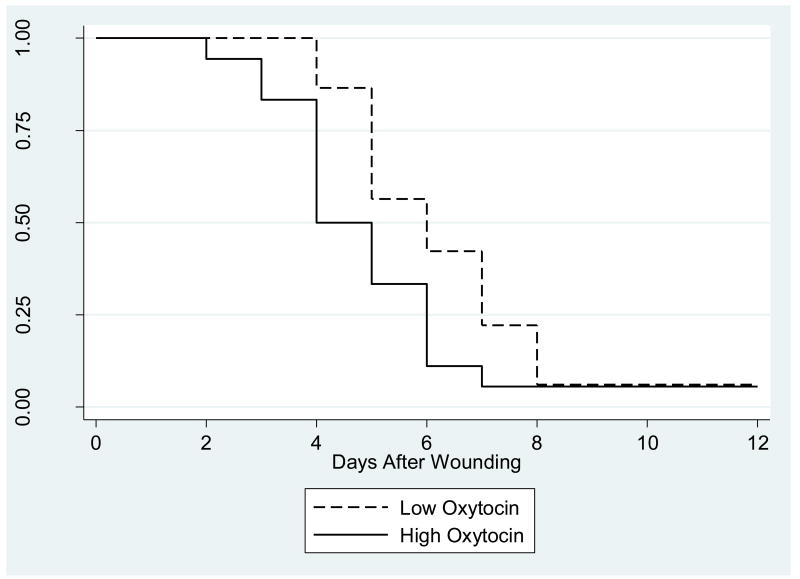
Individuals with the highest oxytocin levels display distinct characteristics, compared to individuals with lower oxytocin levels (Light, et al., 2005b). To investigate their differential behavioral and physiological characteristics, we compared individuals from the upper quartile to participants in the bottom three quartiles of each peptide variable. Individuals in the upper oxytocin quartile displayed a greater number of positive behaviors during the structured social support task than individuals in the lower oxytocin quartiles, t(67)= 2.15, p = .04. No association was found between the upper oxytocin quartile and negative communication behaviors, t(67)= 98, p = .33. Cox survival analyses revealed that individuals in the upper oxytocin quartile healed blister wounds faster than individuals in the lower quartiles, HR= 1.79, p = .04 (Figure 6). Furthermore, individuals who displayed more positive communication behaviors healed faster, compared to their less positive counterparts, t(64)= 2.03, p = .05. Bootstrapping resampling techniques tested whether the effect of positive behaviors on wound healing was mediated by increased oxytocin production. The indirect effect was not significant in the mediation model, z= 1.28, p = .20. This suggests that increased oxytocin production did not mediate the association between positive behaviors and wound healing, but that positive behaviors and oxytocin production were independent predictors of wound healing. There was no sex difference in the effects of oxytocin on marital behaviors, p>.65 and wound healing, p =.33.
Figure 6.
Kaplan-Meier survival curves for time to healing of the standard wound by high- and low- oxytocin groups. Oxytocin measurements were averaged using the baseline and post-interaction task samples. High- and low- oxytocin groups are based on a 75/25 percentile split.
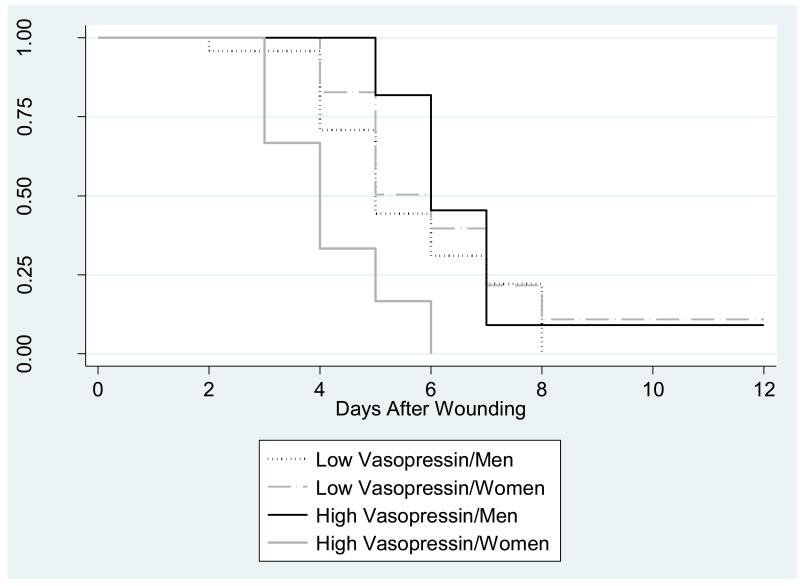
There was a marginally significant association between the upper vasopressin quartile and negative behaviors, t(67) = 1.73 p = .08. The upper vasopressin quartile was not related to positive behaviors, t(67)= .98, p = .33. There was no sex difference in the associations among the upper vasopressin quartile and negative or positive behaviors,.p > .44. Individuals in the upper vasopressin quartile had greater plasma TNF-α levels across the visit, compared to individuals in lower quartiles, F(1,75) = 7.24, p = .01. Furthermore, women in the upper vasopressin quartile healed significantly faster than men and women in the lower vasopressin quartiles, HR= 7.08, p = .002 (Figure 7).
Figure 7.
Kaplan-Meier survival curves for time to healing of the standard wound by high- and low- vasopressin groups and sex. Both oxytocin and vasopressin measurements were averaged using the baseline and post-interaction task samples High- and low-vasopressin groups are based on a 75/25 percentile split.
Oxytocin, Vasopressin, and Health Behaviors
Relationship stress can impact health behavior practices, which may in turn influence neuropeptides production. To evaluate this possibility, correlations among peptide levels and alcohol and caffeine use, exercise, body mass index, and sleep disturbances were computed. None of the correlations were significant at the .05 levels, all ps >.15. Furthermore, women using estrogen medication had similar oxytocin levels than women not using hormonal supplementation, F(1,37)= 72, p =.40. Health behaviors and estrogen medication variables were included in each model to evaluate the possibility that these variables may confound the relationships among peptides, behaviors, and wound healing. All the main effects of interest (i.e. the associations among peptides, marital behavior, and wound healing) remained significant even after adjusting for differences in health behaviors, all ps ≤ .05.
Discussion
Individuals with higher oxytocin levels displayed more positive communication behaviors during a structured social support task than participants with lower oxytocin levels. Individuals with higher vasopressin levels exhibited fewer negative behaviors during the marital interaction task, and had greater TNF-α levels throughout the CRC visit, compared to participants with lower vasopressin levels. Furthermore, individuals in the upper oxytocin quartile and women in the upper vasopressin quartile healed experimental wounds faster than the remainder of the sample. The current findings corroborate and extend rodent studies showing that oxytocin can facilitate social bonding and wound healing (Cho et al., 1999; Detillion et al., 2004).
Both positive and negative relationships have been observed between marital quality and oxytocin levels. In the present study, individuals who displayed more positive behaviors in a marital interaction task had higher oxytocin levels than their less positive counterparts, and individuals who exhibited more negative behaviors had lower vasopressin levels than those who had fewer negative behaviors. These results are consistent with data showing that intranasal oxytocin administration increased the ratio of positive to negative behaviors during a marital conflict discussion (Ditzen et al., 2009). The impact of an intranasal oxytocin administration may vary as a function of the social context; the presence of a best friend acted synergistically with an intranasal oxytocin administration to dampen cortisol and anxiety responses to a laboratory stressor (Heinrichs, et al., 2003). This suggests that the presence of a romantic partner may also modulate the association between relationship quality and peripheral oxytocin levels. In accord with this hypothesis, among studies in which participants were engaged in an experimental task in the presence of a loved one, individuals who reported greater social support had higher oxytocin levels (Grewen et al., 2005a). Conversely, greater relationship stress was associated with higher oxytocin levels in studies in which individuals were required to be involved in an experimental task alone (Turner et al., 1999; Taylor et al., 2006; Cyranowski et al., 2008).
The evidence that high levels of oxytocin and vasopressin were associated with faster wound healing corroborates and extends animal work that showed oxytocin promoted wound repair (Detillion et al., 2004; Vitalo et al., 2009). The current results also parallel experimental studies in rodents in which an oxytocin antagonist attenuated the beneficial effect of pair-housing on wound healing (Detillion et al. 2004). Indeed, the associations of oxytocin and vasopressin with both marital behavior and wound healing reinforce the idea that these peptides may be physiological mediators of the beneficial impact of social relationships on health (Neumann, 2009). These data also complement a line of work suggesting that increases in oxytocin associated with social interaction can attenuate neuroendocrine and autonomic responses to stress, providing a potential mechanism by which these peptides may impact wound healing and health (Carter, 1998; Light et al., 2000; Grewen et al., 2005).
In accord with previous studies, individuals with the highest oxytocin levels exhibited distinctive physiological characteristics (Light, et al., 2005b). In the present study, significant wound healing effects were observed only among individuals in the upper oxytocin and vasopressin quartiles. Furthermore, individuals in the upper oxytocin quartile exhibited more positive behaviors during the interaction task, and there was a trend for participants in the upper vasopressin quartile to display fewer negative marital behaviors, compared to participants in the lower quartiles. It is possible that exposure to high levels of these peptides may be necessary to differentially influence either wound healing or behavior.
The association between plasma peptide levels, marital behavior, and wound healing can be explained by two broad perspectives. First, individual differences in oxytocin and vasopressin production may represent a third variable, such as genetics, that influences both marital bonding and wound healing. For example, polymorphisms of the AVP V1 receptors were associated with marital bonding among men in a long-term romantic relationship (Walum et al., 2008). Furthermore, polymorphisms of the oxytocin receptor gene were related to both a behavioral measure of empathy and heart rate reactivity to an acoustic startle stimulus (Rodriguez et al., 2009). Individual variation in the oxytocin and vasopressin genes may therefore have consequences for the integrity of these peptidergic systems and their implication in social and emotional processes (Heinrichs et al., 2009). However, the relationships between the polymorphisms of the oxytocin and vasopressin receptor genes and the circulating levels of these peptides are still unknown (Rodriguez et al., 2009).
Alternatively, it is possible that supportive marital interactions may promote greater oxytocin and vasopressin production, thereby modulating stress responses, and facilitating wound healing. For instance, the impact of spousal support on norepinephrine plasma levels was mediated by oxytocin production (Grewen et al., 2005). In the current study, the impact of positive marital behaviors on wound healing did not appear to be meditated by peripheral oxytocin production. However, these results must be interpreted with caution. In accord with previous marital interaction studies, the social support interaction task did not elicit change in circulating peptide levels (Grewen et al., 2005). The lack of changes in peptide production associated with the marital interaction task precludes inferences of a causal relationship between positive behaviors and oxytocin production. However, it is possible that the mere presence of a romantic partner may have influenced oxytocin and vasopressin production. Furthermore, the interaction task may have elicited changes in brain, but not plasma levels of the peptides. Indeed, some studies have found concordance between central and peripheral release of oxytocin (Moos and Richard, 1989), while other studies have observed small or non-significant associations between central and peripheral oxytocin and vasopressin in response to certain stimuli (Landgraf and Neumann, 2004).
We originally hypothesized that oxytocin and vasopressin would be associated with plasma levels of IL-6, and TNF-α. Contrary to our expectations, only vasopressin was associated with TNF-α. The interactive effects of peptides and inflammatory processes are not well understood and its biological significance unknown. Methodological differences between studies may have contributed to the discrepant findings. In previous studies, exogenous oxytocin administration impacted cytokine responses, while in the present study no associations were found between endogenous peptides and cytokine levels. It is possible that pharmacological doses of neuropeptides are necessary to observe stronger association between peptides and cytokine plasma levels.
Alternatively, acute versus chronic forms of stress may have different consequences for these systems. For example, in prairie voles, brief social isolation and other forms of stress result in the release of various hormones from the HPA axis, while chronic isolation is associated with elevations in oxytocin, without a measurable change in glucocorticoids or vasopressin (Grippo, et al., 2007). Although acute exogenous oxytocin administration typically leads to reductions in glucocorticoid secretion in rats or voles (Carter, 1998; Neumann, 2002; Grippo, et al., 2009), elevations in endogenous plasma oxytocin have been associated with relationships stress and higher basal salivary cortisol among older women (Taylor et al., 2006).
Research in animals suggests that the effects of central oxytocin and vasopressin on behavior are sexually dimorphic (Carter et al., 2007). However, in the present study no sex differences were found in the association between plasma oxytocin, vasopressin, and marital behaviors. This is consistent with other studies that have not found differences between men and women on the association between oxytocin and marital behaviors (Grewen et al., 2005; Ditzen et al., 2009). The only sex differences observed were with vasopressin and wound healing; women with high levels of these peptides had faster wound repair than women with lower vasopressin and men. Menopausal status and the use of estrogen medication are potential variables that may attenuate sex differences in the effects of oxytocin. However, even when these variables were included in the statistical models, they did not alter the findings.
Multiple oxytocin and vasopressin measurements immediately before and after the marital interaction task would have provided a better test of the impact of this experimental manipulation on peptide levels. These peptides are metabolized within minutes and might have returned to baseline levels by the time our second sample was taken. The marital interaction task resulted in a low base rate of negative behaviors, another limitation of the study. Furthermore, the study included a relatively small sample of couples given the number of statistical analyses being conducted. A replication of these results is therefore clearly warranted. Despite these limitations, the data are provocative and in line with the results of other animal and human studies.
The present study confirmed and extended the association between oxytocin, vasopressin, social bonding, and wound healing previously described in the animal literature. Oxytocin and vasopressin are peptides involved in both social and stress processes.
Acknowledgments
We thank Ms. Liisa Hantsoo who assisted with the preparation and proof-reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Redwine LS, Leong YM, Frye CA, Porges SW, Carter CS. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63(5):814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295(3):E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom Med. 2008;70(9):967–75. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology. 2004;29(8):1004–1011. doi: 10.1016/j.psyneuen.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ewart CK, Taylor CB, Kraemer HC, Agras WS. High blood pressure and marital discord: Not being nasty matters more than being nice. Health Psychol. 1991;10:155–163. doi: 10.1037//0278-6133.10.3.155. [DOI] [PubMed] [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102(47):17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Marucha PT, MacCallum RC, Laskowski BF, Malarkey WB. Stress-related changes in proinflammatory cytokine production in wounds. Arch Gen Psychiatry. 1999;56:450–456. doi: 10.1001/archpsyc.56.5.450. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67(4):531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32(8-10):966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54(12):1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heyman RE. Rapid marital interaction coding system (RMICS) Nahwah, New Jersey: Lawrence Erlbaum Associates; 2004. Trans ed Vol. [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70(9):976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Methodological issues in behavioral immunology research with humans. Brain Behav Immun. 1988;2:67–78. doi: 10.1016/0889-1591(88)90007-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Holsboer F, Pittman QJ. Interleukin-1 beta stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci. 1995;7(4):592–598. doi: 10.1111/j.1460-9568.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25(3-4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005a;69(1):5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29(8):1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Brownley KA, West SG, Hinderliter AL, Girdler SS. Oxytocinergic activity is linked to lower blood pressure and vascular resistance during stress in postmenopausal women on estrogen replacement. Horm Behav. 2005b;47(5):540–548. doi: 10.1016/j.yhbeh.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 2000;19(6):560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Lindahl KM, Clements M, Markman H. Predicting marital and parent functioning in dyads and triads: A longitudinal investigation of marital processes. J Fam Psychol. 1997;11:139–151. [Google Scholar]
- Moos F, Richard P. Paraventricular and supraoptic bursting oxytocin cells in rat are locally regulated by oxytocin and functionally related. J Physiol. 1989;408:1–18. doi: 10.1113/jphysiol.1989.sp017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Neumann ID. The advantage of social living: brain neuropeptides mediate the beneficial consequences of sex and motherhood. Front Neuroendocrinol. 2009;30(4):483–496. doi: 10.1016/j.yfrne.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Palin K, Moreau ML, Sauvant J, Orcel H, Nadjar A, Duvoid-Guillou A, Dudit J, Rabie A, Moos F. Interleukin-6 activates arginine vasopressin neurons in the supraoptic nucleus during immune challenge in rats. Am J Physiol Endocrinol Metab. 2009;296(6):E1289–E1299. doi: 10.1152/ajpendo.90489.2008. [DOI] [PubMed] [Google Scholar]
- Pasch LA, Bradbury TN. Social support, conflict, and the development of marital dysfunction. J Consult Clin Psychol. 1998;66:219–230. doi: 10.1037//0022-006x.66.2.219. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. Oxytocin control of maternal behavior. Regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21(18):7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol. 1995;7(7):501–525. doi: 10.1111/j.1365-2826.1995.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7(4):422–445. [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295(6):1495–1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68(2):238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Turner RA, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: Investigating emotion and interpersonal distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- Veroff J, Sutherland L, Chadiha L, Ortega RM. Newlyweds tell their stories: A narrative method for assessing marital experiences. J Soc Pers Relat. 1993;10:437–457. [Google Scholar]
- Vitalo A, Fricchione J, Casali M, Berdichevsky Y, Hoge EA, Rauch SL, Berthiaume F, Yarmush ML, Benson H, Fricchione GL, Levine JB. Nest making and oxytocin comparably promote wound healing in isolation reared rats. PLoS ONE. 2009;4(5):e5523. doi: 10.1371/journal.pone.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci U S A. 2008;105(37):14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]