Abstract
Caulobacter phage phi 6, previously reported to adsorb specifically to bacterial flagella, was shown here to attach to pili more frequently than to flagella. Phage phi 6 was shown to contain double-stranded DNA by circular dichroism spectroscopy and thermal denaturation accompanied by a hyperchromic shift at 260 nm. Morphologically, phage phi 6 fits group B2 (H.-W. Ackermann, in A. I. Laskin and H. A. Lechevalier, ed., Handbook of Microbiology, vol. 1, p. 638-643, 1973) with a long, noncontractile tail and an elongate head. Pilus-less mutants of the host Caulobacter vibrioides CV6 are phage phi 6 resistant, whereas flagellum-less mutants, which produce pili, are phage susceptible. Treatments of susceptible cells which remove or immobilize pili and flagella, e.g., blending or cyanide, inhibited phage phi 6 infection. Our evidence suggests that phage of phi 6 initiates infection in a manner similar to the pilus-specific phages for Pseudomonas described previously (D. E. Bradley, Virology 51:489-492, 1973; D. E. Bradley and T. L. Pitt, J. Gen. Virol. 24:1-15, 1974).
Full text
PDF




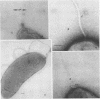
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Bacterial differentiation and phage infection. Virology. 1971 Apr;44(1):46–53. doi: 10.1016/0042-6822(71)90151-6. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. A pilus-dependent Pseudomonas aeruginosa bacteriophage with a long noncontractile tail. Virology. 1973 Feb;51(2):489–492. doi: 10.1016/0042-6822(73)90448-0. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Shortening of Pseudomonas aeruginosa pili after RNA-phage adsorption. J Gen Microbiol. 1972 Sep;72(2):303–319. doi: 10.1099/00221287-72-2-303. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iba H., Okada Y. A flagellotropic bacteriophage and flagella formation in Caulobacter. Virology. 1976 Jun;71(2):583–592. doi: 10.1016/0042-6822(76)90383-4. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollick J. D. Differential phage sensitivity of cell types in Caulobacter. J Gen Virol. 1972 Sep;16(3):405–407. doi: 10.1099/0022-1317-16-3-405. [DOI] [PubMed] [Google Scholar]
- Jollick J. D., Wright B. L. A flagella specific bacteriophage for caulobacter. J Gen Virol. 1974 Feb;22(2):197–205. doi: 10.1099/0022-1317-22-2-197. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Farmer S., Agabian N. Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology. 1977 Mar;77(1):401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
- Novotny C. P., Fives-Taylor P. Retraction of F pili. J Bacteriol. 1974 Mar;117(3):1306–1311. doi: 10.1128/jb.117.3.1306-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT J. M., STANIER R. Y. ISOLATION AND CHARACTERIZATION OF BACTERIOPHAGES ACTIVE AGAINST STALKED BACTERIA. J Gen Microbiol. 1965 Apr;39:95–107. doi: 10.1099/00221287-39-1-95. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Agabian-Keshishian N., Bendis I. Bacterial differentiation. Science. 1971 Sep 3;173(4000):884–892. doi: 10.1126/science.173.4000.884. [DOI] [PubMed] [Google Scholar]
- Wagner T. E., Vandegrift V., Moore D. S. Circular dichroism analysis of nucleoprotein complexes. Methods Enzymol. 1975;40:209–238. doi: 10.1016/s0076-6879(75)40019-2. [DOI] [PubMed] [Google Scholar]