Abstract
Despite recent advances in the management of breast and lung cancer, novel treatment strategies are still needed to further improve patient outcome. The targeting of cell death pathways has therefore been proposed to enhance therapeutic ratio in cancer. In this study, we examined the in vitro and in vivo effects of Z-VAD, a broad spectrum caspase inhibitor, on breast and lung cancer in association with radiation.
Using clonogenic assays, we observed that Z-VAD markedly radiosensitized breast and lung cancer cells, with a radiation dose enhancement ratio of 1.31 (p<0.003). For both models, the enhanced tumor cytotoxicity was associated with induction of autophagy. Furthermore, we found that administration of Z-VAD with radiation in both breast and lung cancer xenograft produced a significant tumor growth delay as compared to radiation alone, and was well tolerated. Interestingly, Z-VAD also had dramatic anti-angiogenic effect when combined with radiation both in vitro and in vivo, and thus represents an attractive anticancer therapeutic strategy. In conclusion, this preclinical study supports the therapeutic potential of Z-VAD as a radiosensitizer in breast and lung cancer. This study also suggests caspase inhibition as a promising strategy to enhance the therapeutic ratio of radiation therapy in solid tumors. Therefore, clinical trials are needed to determine the potential of this combination therapy in cancer patients.
Keywords: Angiogenesis, autophagy, breast cancer, caspase, lung cancer
Introduction
Solid tumors present formidable challenge in the multi-disciplinary management of cancer patients. Among solid tumors, breast and lung cancer account for an estimated 399,470 new cases in the United States in 2008, and despite multi-modality therapy, remain the leaders in cancer-related mortality (1). Since tumor resistance limits effectiveness of current treatments, such as radiotherapy, the identification of novel therapeutic strategies is critical for improving outcome. Manipulation of radiation-induced cell death is one such strategy (2), and has been proposed to enhance therapeutic ratio in cancer. Of the different cell death processes involved in radiation therapy, apoptosis has been the most well-studied (3). Apoptosis molecularly represents a cascade of caspase proteases released from the mitochondria, and is characterized by condensation, chromatin margination, early nuclear condensation and nucleosomal ladder formation. In the apoptosis pathway, caspases are the mediators of cellular destruction, and can schematically be divided in two groups: “initiator” caspases (including caspases-2,-8,-9, and -10) and effectors (or executors) caspase-3,-6 and -7 (4). However, radiation-induced apoptosis accounts for a minor portion of cell death in irradiated solid tumors (5). Consistent with these observations, we have previously demonstrated that inhibition of apoptosis via knockdown of Bak and Bax results in an increase in breast and lung cancer radiosensitivity in vitro through upregulation of autophagy, an alternate type of programmed cell death (6). Autophagy is an evolutionarily conserved process and a survival mechanism whose primary function is to degrade long-lived proteins and recycle cellular components (7). Under excessive stress conditions, autophagy can also lead to cell death by complete self-digestion, which is characterized by the formation of cytoplasmic double-membrane vacuoles (called autophagosomes) that fusion with lysosomes (8). The induction of cancer cell death by several agents underlines the potential of autophagy as a novel cancer treatment target (6, 9). In this study, we examine the radiosensitizing effects of Z-VAD, a broad spectrum irreversible caspase inhibitor (10), on MDA-MB-231 breast and H460 lung cancer cells in vitro and in both breast and lung cancer xenograft models.
Materials and Methods
Cell culture and chemical
MDA-MB-231 cells were cultured in DMEM (Invitrogen) and H460 cells were cultured in RPMI 1640 (Invitrogen, Grand Island, NY), both supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C and humidified 5% CO2. Z-VAD-fmk was purchased from Axxora (San Diego, CA). HUVECs were obtained from Clonetics and were maintained in EBM-2 medium supplemented with EGM-2 MV single aliquots (BioWhittaker).
Clonogenic Assay
MDA-MB-231 breast and H460 lung cancer cells were treated with DMSO, Z-VAD (50µM, for 24hrs), siRNAs against caspase-3 and-7, or siRNA control. Cells were irradiated with 0 to 6 Gy as indicated at a dose rate of 1.8 Gy/min, using a 137Cs irradiator (J.L. Shepherd and Asssociates, Glendale, CA). After irradiation, cells were incubated at 37°C for 8–10 days. Cells were fixed for 15min with 3:1 methanol: acetic acid and stained for 15min with 0.5% crystal violet (Sigma) in methanol. After staining, colonies were counted using a cutoff of 50 viable cells. Surviving fraction was calculated as (mean colony counts)/ (cells inoculated) × (plating efficiency (PE)), where PE was defined as (mean colony counts)/ (cells inoculated for non-irradiated controls). The radiation dose enhancement ratio (DER) was calculated as the dose (Gy) for radiation alone divided by the dose (Gy) for radiation plus Z-VAD (normalized for Z-VAD toxicity) necessary for a surviving fraction of 0.25. Experiments were conducted in triplicate and mean, SD, and P values (using a Student’s test) were calculated. Error bars were calculated as ± standard error (SE) by pooling of the results of 3 independent experiments.
Immunoblotting
Following treatment with 50µM Z-VAD or not, MDA-MB-231 breast and H460 lung cancer cells (0.5×106) were treated with various doses of radiation and collected at various time points. The cells were harvested and then were washed with ice cold PBS twice before the addition of lysis buffer (20mM Tris-HCl, pH 7.4, 150mM NaCl, 20mM EDTA, 1% NP40, 50mM NaF, 1mM Na3Vo4, 1mM NaMO4 and cocktail inhibitor (sigma, 5µl/ml). Protein concentration was quantified by the Bio-Rad method. Equal amounts of protein were loaded into each well and separated by 10% SDS-PAGE gel, followed by transfer onto PVDF-membranes (BIO-RAD). Membranes were blocked with 5% nonfat dry milk in PBS-T for 1 h at room temperature. The blots were then incubated overnight at 4°C with the ATG5-ATG12 (a gift from Dr. Mizushima), Beclin-1 (Santa Cruz Biotechnologies), or Actin (Sigma) antibodies. After washing with PBS, membranes were incubated with goat anti-rabbit IgG secondary (1:1000, Santa Cruz Biotechnologies) antibody for 45 mins at room temperature. Immunoblots were developed by using the enhanced chemiluminescence (ECL) detection system (Amersham) and autoradiography.
Goat anti-rabbit IgG secondary (1:1000, Santa Cruse Biotechnologies) was incubated for 45mins at room temperature. Immunoblots were developed using the enhanced chemiluminescence (ECL) detection system (Amersham) and autoradiography.
Autophagy Assay
MDA-MB-231 and H460 cancer cells were transfected with GFP-LC3 plasmid (gift from Dr. Norboru Mizushima) (11) using a mixture of lipofectamine reagent (Invitrogen life technologies). After 24h, cells were treated with or without 50 µM Z-VAD and sham or 5 Gy irradiation. After 24h and 48h, GFP-LC3 fluorescence was observed using confocal fluorescence microscope. Characteristic punctate GFP-LC3 signal was considered a cell undergoing autophagy. The percentage of punctate GFP-LC3 cells per total GFP transfected cells was calculated and experiments were conducted three times.
siRNA Transfection
siRNAs against mouse caspase-3 and-7, Beclin, and control siRNA were purchased from Santa Cruz Biotechnologies. siRNA ATG5 (mouse) was synthesized by Dharmacon Research. The sense and antisense strands of ATG5 were begun at nucleotide, 5’-AACUUGCUUUACUCUCUCAUCAUU-3’ (Sense) and 3’-UUUUGAACGAAAUGAGAGAUAGU-5’ (Antisense). Cells were transfected with 25nM of siRNAs using LipofectAMINE 2000. The transfected cells were used for experiments 24h later.
Endothelial Cell Morphorgenesis assay: Tubule Formation
Human umbilical vein endothelial cells (HUVECs) were used to examine tubules formation for angiogenic function in vitro. HUVECs grown to ~70% confluency were treated with 50 µM Z-VAD, 5Gy, or combination therapy. Cells were then trypsinized and counted. They were seeded at 48000 per well on 24-well plates coated with 300 µl of Matrigel (BD Biosciences). These cells undergo differentiation into capillary-like tube structures and were periodically observed by microscope. One day later, cells were stained with haematoxylin and eosin (H&E) and photographs were taken via microscope. The average number of tubules was calculated from examination of three separate microscopic fields (100x) and representative photographs were taken.
Tumor volume assessment
Human MDA-MB-231 and H460 cells were used to generate a breast and lung xenograft model, respectively, in female athymic nude mice (nu/nu, 5 to 6 weeks old [Harlan Sprague Dawley Inc., Indianapolis, IN]). A suspension of 2 × 106 cells in 50 µL volume was injected subcutaneously into the left posterior flank of mice using a 1-cc syringe with 27½-gauge needle. Tumors were grown for 6 to 8 days until average tumor volume reached 0.28 cm3. Treatment groups consisted of vehicle control (in DMSO), Z-VAD, vehicle plus radiation, and Z-VAD plus radiation. Each treatment group contained 5 mice. Z-VAD was given daily by intraperitoneal (i.p.) injection at doses of 2 mg/kg for 7 consecutive days. DMSO was given by daily i.p. injection as a vehicle control. In the case of combination treatment, drug or vehicle was given for 2 days prior to the first dose of irradiation. Mice in radiation groups were irradiated 1 hour after drug or vehicle treatment with daily 2 Gy fractions given over 5 consecutive days. Tumors on the flanks of the mice were irradiated using an X-ray irradiator (Therapax, Agfa NDT, Inc., Lewis Town, PA). The non-tumor bearing parts of the mice were shielded by lead blocks. Tumors were measured 2–3 times weekly in 3 perpendicular dimensions using a Vernier caliper and volume was calculated using the modified ellipse volume formula (Volume = (Height × Width × Depth)/ 2). Growth delay was calculated for treatment groups relative to control tumors.
Histological sections, vWF, ki67, TUNEL, P62, and HMGB1 staining
Mice were implanted with MDA-MB-231 or H460 cells and treated as described above in the tumor volume studies. After 7 days of daily treatments, tumors from each mice group were resected and paraffin fixed. Slides from each treatment group were then stained for von Willebrand Factor (vWF) using anti-vWF polyclonal antibody (Chemicon). Blood vessels were quantified by randomly selecting three separate 400X fields and counting the number of blood vessels per field. This was done in triplicate and the average of the three counts was calculated. Ki67 (cell proliferation), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL), P62 (autophagy assay), and necrosis assay high mobility group box-1 (HMGB1) staining were performed in the Vanderbilt University Pathology Core laboratory using standard protocols. The TUNEL-Fluorescence Method detects and quantifies apoptosis at the cellular level by labeling free 3’-OH terminals that result from cleavage of genomic DNA during apoptosis (12). P62, a scaffold protein binding to LC3 is a preferred target for autophagy which controls its intracellular level, and a marker for autophagy (13). HMGB1 is a small acidic chromatin binding protein that is passively released from necrotic cells and a specific marker for necrosis (14). Number of positive cells per field were scored and graphed by averaging three repeated assessments. For each type of staining, representative photographs were taken.
Statistical analysis
Analysis of study results focused on testing the differences of the mean tumor volume among treatment groups and different time points. The data analysis was completed using the restricted/residual maximum likelihood-based mixed-effect model to adjust the intracorrelation effect for the mice that had multiple measurements. The model reported in the paper was selected on the basis of the Schwarz’s Bayesian criterion. All tests of significance were 2-sided, and differences were considered statistically significant when p was less than 0.05. A statistical package, SAS v8.2, was used for all analyses.
Results
Z-VAD radiosensitizes both MDA-MB-231 and H640 cancer cells
To investigate whether inhibition of apoptosis would result in radiosensitization of MDA-MB-231 breast or H460 lung cancer cells, we used a clonogenic assay to examine the effects of Z-VAD, a pan-caspase inhibitor. In addition, we also used siRNAs against caspase-3 and -7 to study more specifically the effect of Z-VAD on radiation sensitivity. Both MDA-MB-231 and H460 cells were treated with 50 µM Z-VAD, DMSO control, siRNAs against caspase-3/7, or siRNA control, and were then irradiated with 0–6 Gy. We used a Z-VAD concentration of 50 µM because this concentration has been used previously in many studies investigating apoptosis. Surviving colonies were counted 8 days later and graphed as survival curves (Figure 1). After Z-VAD treatment, enhanced radio-sensitivity was demonstrated in both breast (Figure 1A) and lung (Figure 1B) cancer cells, with a DER of 1.31 (p<0.003). As is commonly seen with DMSO treatment, which is known to possess some toxicity, there was a small reduction only in surviving fraction of DMSO treated cells at high doses of radiation. Figure 1 also shows that caspase-3/7 siRNAs sensitize MDA-MB-231 (DER=1.23, p<0.004) and H460 (DER=1.31, p<0.003) cancer cells to ionizing radiation. These cells are unable to undergo apoptosis since caspase-3/7, which execute the apoptotic process, are blocked. These results suggest that inhibition of caspases by their siRNAs or Z-VAD can significantly sensitize both MDA-MB-231 breast and H460 lung cancer cells to ionizing radiation.
Figure 1. Radiation sensitization of MDA-MB-2331 and H460 cancer cells following treatment with Z-VAD.
Clonogenic assay revealing radiosenstization of MDA-MB-2331 (Figure 1A) or H460 (Figure 1B) cells treated with pan-caspase inhibitor Z-VAD. Cells were treated with 50 µM Z-VAD or DMSO control, and were irradiated with the indicated doses of radiation. After 8 days, colonies were stained and scored for colony formation and the surviving fractions were plotted in a semi-log format. Shown in the figure are the mean ± the standard deviation (S.D.) of three separate repeated experiments.
Z-VAD induces autophagy in irradiated MDA-MB-231 and H460 cancer cells
Our previous published data suggested that by inhibiting apoptosis, cells can be radiosensitized through alternative cell death pathways, such as autophagy (6, 15). To further investigate this phenomenon in breast and lung cancer, we examined the effects of blocking caspase-dependent apoptosis with Z-VAD on autophagosome formation. Microtubule associated protein-1 LC3 is an important constituent of mammalian autophagosomes, and green fluorescent protein-tagged light-chain 3 (GFP-LC3) has been demonstrated to be an effective marker of their presence (11, 16). To determine whether autophagic cells were increased in cancer cells treated with Z-VAD and RT, GFP-LC3 plasmid was transfected into cancer cells prior treatment with Z-VAD and RT (5Gy). After MDA-MB-231 or H460 cells were exposed to the combined treatment, cells with characteristic punctate GFP-LC3 pattern were observed, suggesting the presence of autophagic cells (11). Quantitative analysis of this effect revealed only a slight increase in the punctate fluorescence pattern following radiation alone (~10%) or Z-VAD alone (~13–17%) at 24 hours (Figure 2A). However, when radiation and Z-VAD treatments were combined, there was a greater than additive effect with a major increase in the number of cells with the punctate GFP-LC3 pattern (p<0.0001) for both models. These results suggest a significant increase in autophagosome formation after combined Z-VAD/radiation treatment in MDA-MB-231 and H460 cancer cells. These results were also confirmed by assessing the level of Beclin-1, a component of a class III PI3 kinase complex, and ATG-5/ATG-12 complex, which are essential autophagy proteins (17, 18). As shown in Figure 2B, treatment with irradiation or Z-VAD alone only resulted in a moderate increase in expression of autophagic proteins. The greatest induction, however, was seen following combination treatment, particularly at the 24 h time point. These results suggest that cancer cells treatment with radiation/Z-VAD is associated with up-regulation of essential autophagic proteins.
Figure 2. Induction of autophagosome formation and essential autophagic proteins by treatment with Z-VAD/radiation.
GFP-LC3 transfected cells were treated with 5 Gy, 50 µM Z-VAD, or both and then examined by fluorescence microscopy after 24 hours. (A) Quantitative measurement of positive MDA-MB-231 and H460 cells, showing increased autophagosome formation in response to Z-VAD/radiation. The percentage of cells with punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. Error bars are shown as mean ± S.D. (B) MDA-MB-231 and H460 cells were treated with 5 Gy, 50 µM Z-VAD, or a combination of both. Cells were collected at noted time points and protein extracts were made for Western Immunoblotting. Shown are immunoblots of ATG5-ATG12 complex and Beclin-1 using lysates from the MDA-MB-231 and H460 cancer cells. Actin immunoblot was used for normalization.
Combination treatment of Z-VAD and radiation results in extended tumor growth delay and is well-tolerated in solid tumor xenograft models
Having established the in vitro effect of Z-VAD on solid tumor radiosensitivity, mouse heterotopic xenograft models were produced to explore the radiation response by Z-VAD in vivo. The treatment groups consisted of a vehicle control, Z-VAD (2 mg/kg i.p.), vehicle plus radiation, and combination Z-VAD plus radiation. MDA-MB-231 and H460 tumor xenografts in mice were treated as described in the Material and Methods section. Growth delay was calculated as the number of days required to reach a tumor volume of 2 cm3 for treatment groups relative to control tumors (Figure 3, A and B). In the breast xenograft model, a significant tumor growth delay was seen with combination therapy of Z-VAD and radiation compared to irradiation alone (22 vs 16 days, p<0.005), and Z-VAD alone did also significantly affect the tumor growth compared to control (~3 days delay, p=0.003). Similarly, combination therapy of Z-VAD/radiation resulted in a significant tumor growth delay in the lung xenograft model (9 vs 6 days, p=0.008) compared to irradiation alone. These results suggest that Z-VAD can increase solid tumors response in combination with radiotherapy. In addition, mouse body weights were also monitored to assess whether treatment with Z-VAD, radiation, or combination treatment yielded systemic toxicity (Supplemental Figure 1, A and B). As expected, the combination treatment group, which had the most prolonged tumor growth delay, had the smallest increase in body weight. Only minimal weight loss was seen at ten days following the combined Z-VAD and irradiation treatment, suggesting that the combined treatment was very well tolerated.
Figure 3. Combination therapy slows tumor xenograft growth and is well tolerated.
Human MDA-MB-231 or H460 cells were injected into athymic female nude mice and grown for 6–8 days to an average tumor volume of 0.28 cm3. Treatment groups consisted of vehicle control (DMSO), Z-VAD (2 mg/kg i.p.), vehicle plus radiation, and Z-VAD plus radiation. Drug treatment was given daily as intraperitoneal injections for 7 consecutive days while irradiation was given daily at 2 Gy per fraction for 5 consecutive days (detailed in Materials and Methods). Breast (A) and lung (B) tumors were measured regularly and volume was plotted vs time. Growth delay was calculated as the number of days required to reach a tumor volume of 2 cm3 for treatment groups relative to control tumors.
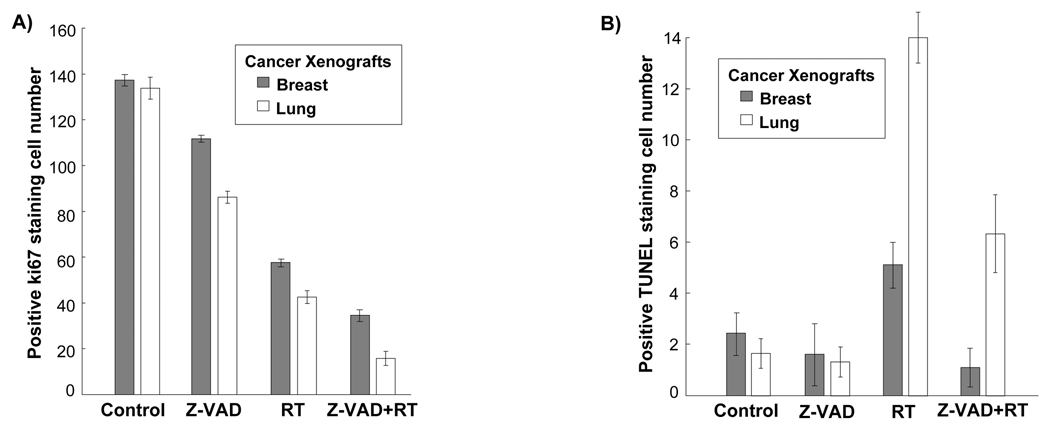
Z-VAD reduces tumor proliferation index despite marked decrease in apoptosis in irradiated MDA-MB-231 and H460 mouse xenografts
To further characterize the effect of Z-VAD shown in the tumor growth delay models, we examined fixed MDA-MB-231 and H460 tumor sections in all treatment groups for proliferation (Ki67 staining), and apoptosis (TUNEL staining). The four treatment groups were identical to those used for the tumor growth delay study. As shown in Figure 4A, Ki67 staining revealed lower breast (35 vs 59, p<0.0001) and lung (16 vs 44, p<0.0001) cellular proliferation in the combination treatment group compared to the irradiation alone group. Apoptosis levels in fixed MDA-MB-231 and H460 tumor sections were assessed using TUNEL staining. As shown in Figure 4B, combined Z-VAD and radiation treatment resulted in ~80% reduction in apoptosis, compared to radiation alone in both breast (p=0.002) and lung (p<0.001) tumor xenografts. These results suggest that Z-VAD can reduce radiation-induced apoptosis and further reduce tumor cell proliferation after irradiation.
Figure 4. Z-VAD reduces cell proliferation, and apoptosis in irradiated MDA-MB-231 and H460 cancer models.
Histologic sections were obtained from the tumors of the mice in each treatment group from the tumor volume study. Number of positive cells were scored and graphed by averaging three repeated assessments. (A) Average Ki67 proliferative index of each treatment group was determined by counting positive cells per microscopic field in breast and lung tumor sections. This was repeated thrice. Columns, mean; bars, SD. (B) TUNEL staining was also performed on breast and lung tumor sections, and apoptotic index was similarly calculated by counting positive TUNEL stained cells per microscopic field. Column, mean; bars, SD.
Z-VAD induces both autophagy and necrosis in irradiated MDA-MB-231 and H460 mouse xenografts
To explore the mechanisms of cell death resulting from Z-VAD in vivo, we examined fixed MDA-MB-231 and H460 tumor sections in all treatment groups for autophagy (P62 staining), and necrosis (HMGB1 staining). The four treatment groups were identical to those used for the tumor growth delay study. P62 interacts and binds to LC3 and is removed in lysosomes by autophagy, which controls its turnover. Representative histological photographs of P62 staining on breast and lung tumor sections are shown in Figure 5C and Supplemental Figure 2A, respectively. As shown in Figure 5A, P62 staining revealed lower breast (17.3 vs 55, p<0.001) and lung (19.7 vs 55.3, p<0.001) positive cells in the combination treatment group compared to the irradiation alone group. Necrosis levels in fixed MDA-MB-231 and H460 tumor sections were assessed using HMGB1 staining. HMGB1 is a nuclear protein that binds tightly to chromatin in apoptotic cells, whereas during necrosis, it is released into the extracellular environment. Representative histological photographs of HMGB1 staining on breast and lung tumor sections are shown in Figure 5D and Supplemental Figure 2B, respectively. Nuclear staining is present in the untreated tissue, whereas cytoplasmic and extranuclear staining of HMGB1 is found in the tumor tissue treated with Z-VAD, radiation or both. As shown in Figure 5B, combined Z-VAD and radiation treatment resulted in ~70% and ~73% increase in cytoplasmic HMGB1 positive cells, compared to radiation alone in breast (p=0.002) and lung (p=0.0005) tumor xenografts, respectively. Taken together, these results suggest that Z-VAD induces autophagy and necrosis after irradiation in vivo.
Figure 5. Z-VAD induces autophagy and necrosis in irradiated MDA-MB-231 and H460 cancer in vivo models.
Histologic sections were obtained from the tumors of the mice in each treatment group from the tumor volume study. Number of positive cells were scored and graphed by averaging three repeated assessments. (A) Average P62 index of each treatment group was determined by counting positive cells per microscopic field in breast and lung tumor sections. This was repeated thrice. Columns, mean; bars, SD. (B) HMGB1 staining was also performed on breast and lung tumor sections, and quantification was similarly performed by counting positive cytoplasmic HMGB1 stained cells per microscopic field. Column, mean; bars, SD. (C) Representative histological photographs of MDA-MB-231 tumor sections following P62 staining. (D) Representative histological photographs of MDA-MB-231 tumor sections following HMGB1 staining.
Z-VAD reduces vascular density in irradiated solid tumor models and sensitizes HUVECs to radiation
To determine the effect of Z-VAD on tumor vasculature, vascular density study was performed using von Willebrand Factor (vWF) staining in each breast and lung cancer xenograft treatment groups. The number of vessels per microscopic field was then quantified for each treatment group. As shown in Figure 6A, combination therapy of Z-VAD and radiation in both xenograft models resulted in a dramatic 5-fold reduction (p<0.0001) in the average number of vessels per microscopic field in comparison to control and a ~2-fold reduction (p=0.006) relative to radiation therapy alone. To further investigate the effects of Z-VAD and radiation on blood vessel formation, an endothelial cell morphogenesis assay was performed to examine the ability of treated HUVECs to produce capillary-like tubular structures. The mean number of counted tubules in three separate (×100) fields and a representative image are shown in Figure 6B and 6C, respectively. Treatment with Z-VAD combined to radiation significantly decreased tubule formation compared to radiation alone (6.0 vs 23.33, p<0.0001). No treatment control had 47.66 tubules (SD=0.57) per microscopic field and Z-VAD alone had 18 tubules (SD=1.0), suggesting an anti-angiogenic effect in addition to the radiosensitization effect of Z-VAD.
Figure 6. Z-VAD reduces vascular density in irradiated solid tumors in vivo and sensitizes vascular endothelial cells to ionizing radiation in vitro.
Histologic sections were obtained from the tumors of the mice in each treatment group from the in vivo tumor volume study, and stained for blood vessels using an antibody for vWF. (A) Blood vessels were quantified by randomly selecting 400X fields and counting the number of blood vessels per field in breast and lung tumor sections. This was done in triplicate and the average of the three counts was calculated. Columns, average; bars, SD. (B) Human umbilical vein endothelial cells (HUVECs) were treated with 50 µM Z-VAD, 5 Gy, or combination therapy. Six hours later, cells were trypsinized and replated on 24-well plates coated with Matrigel. After 24 h, cells were fixed and stained with H&E. The slides were examined by microscopy (x100), and stained tubules were then counted in three separate, randomly selected fields. Columns, mean number of tubules counted per microscopic field; bars, SD. (C) Representative photographs of H&E stained HUVECs showing tubule formation.
Discussion
In the present report, we study the effects of Z-VAD, a pan-caspase inhibitor, which resulted in the effective radiosensitization of breast and lung cancer cells in vitro. The potential therapeutic effects of caspase inhibition by Z-VAD were then demonstrated in both breast and lung cancer xenograft models. This study also suggests that Z-VAD increase radiation effects on vasculature and thus may contribute to the extended tumor growth delay. Consistent with previous study, in vitro experiments also showed that Z-VAD induces autophagy, which may play a dual role, either protection or mediation of cell death. Importantly, this report suggests that this novel strategy has great potential for enhancing the treatment of solid tumors with radiotherapy.
It has been shown that Z-VAD is a competitive, irreversible and broad specificity inhibitor of all 10 caspases, except for caspase-2 which is only weakly inhibited (19). We showed that the administration of Z-VAD in combination with ionizing radiation decreased dramatically the survival of MDA-MB-231 breast and H460 lung cancer cells (Figure 1). Moreover, siRNAs against caspase-3/7 sensitized lung cancer cells to ionizing radiation, suggesting that Z-VAD acts by inhibition of caspase-3/7. Interestingly, siRNAs against caspase-3/7 also sensitized breast cancer cells to ionizing radiation, although to a lower extent as compared to Z-VAD, possibly because the latter can inhibit caspase-3 and-7 and also other main caspases that are involved in apoptosis. These results suggest that Z-VAD has the potential to achieve a high degree of radio-sensitization in breast and lung cancer cells in vitro.
In our breast and lung xenograft models, Z-VAD resulted in extended tumor growth delay when administered with radiation. Not surprisingly, Z-VAD decreased radiation-induced apoptosis as determined by the TUNEL staining in vivo (Figure 4B). Indeed, Z-VAD blocks caspase protease activity required for apoptosis. More interestingly, Z-VAD was also able to reduce tumor cell proliferation (Figure 4A). These results suggest that an alternative death pathway, other than apoptosis, might be involved in response to the combined treatment Z-VAD/radiation. Autophagy is such a death mechanism, as previously demonstrated in irradiated cancer cells (9). We have previously shown that inhibition of apoptosis with the pan-caspase inhibitor Z-VAD or siRNA knockout of Bax/Bak upregulates autophagy in radiosensitized breast and prostate cancer cell lines (15). Consistent with our previous studies targeting upstream apoptotic regulators (15), we observed that the combination of Z-VAD and irradiation was associated with an increased in radiation-induced autophagy in both breast and lung cancer cells (Figure 2). Indeed, ionizing radiation has previously been shown to induce autophagy in cancers of the breast (15), lung (6), prostate (15), and malignant glioma (20, 21). In addition, autophagy was shown to enhance radiation cytotoxicity in cancer cells (22), and thus represents an attractive target for cancer therapy. Consistent with these data, it has been reported that EB 1089, a vitamin D3 analogue, was able to enhance radiation sensitivity of breast cancer cells via induction of autophagy (23). Recent studies demonstrated the induction of autophagic cell death after exposure to Z-VAD or specific caspase-8 knockdown in L929 fibrosarcoma cells (24), suggesting that the absence of caspase activity can induce autophagic cell death. In light of these data, autophagy may explain the enhancement of radiation response in our xenograft models.
However, the role of autophagy is more complex (25). It has been implicated in cell survival by self-degradation of proteins in stress-induced conditions such as amino acid deprivation. In our study, the application of Z-VAD and irradiation resulted in a transient burst of autophagic protein expression at 24 hours (Figure 2B). The noted overexpression of autophagic proteins may possibly represent an acute survival mechanism adopted by breast tumors subjected to stress conditions (26). In the lung model, however, the overexpression of autophagic proteins is more sustained at both 24 and 48 hours (Figure 2B). Nevertheless, an increasing body of evidence suggests that the role of autophagy may dependent on cells and circumstances, such as the type and quantity of stress exposure. Thus, in an ionizing radiation model, excessive autophagy may function as an alternate death pathway for apoptosis-inhibited cancer cells (6, 21). Based on the interconnection between autophagy and apoptosis (27), these mechanisms may serve as “backup” for each other, becoming activated when one pathway or the other is suppressed. It should also be noted that irreversibly damaged cells upon Z-VAD have alternative route to death besides autophagy, such as necrosis (28). Recent studies in different models showed that autophagy may have a role in determining cell fate in response to stress in apoptosis-deficient cells (29) towards necrosis, and contributing to cellular destruction during necrosis in a yeast model (30). Here, we focused our study on the in vivo biological efficacy and effects of Z-VAD, mainly to take into account the complex microenvironment of tumors. Interestingly, we found that the combined treatment radiation plus Z-VAD induces not only autophagy but also necrosis in vivo as showed by the HMGB1 staining on breast and lung tumor sections (Figure 5B). Our findings are consistent with the previously published data showing that autophagy can promote cell death in apoptosis-deficient murine embryonic fibroblasts (MEF) cells (29). Of note, this is the first in vivo report showing that Z-VAD promotes both autophagy and necrosis in irradiated breast and lung cancer models.
Our results also support the potential effects of Z-VAD on the tumor microenvironment. As we know, radiotherapy induce the production of pro-angiogenic factors such as the vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) (31–34), which result in an increase in radioresistance and thus attenuates vasculature damage by radiation. This survival mechanism potentially limits the effectiveness of ionizing radiation for cancer therapy. It is also well known that excessive angiogenesis is a decisive step for tumorigenesis and cancer progression (35). In addition, microvessel density was shown to be a prognostic factor predictive of neoplastic transformation (36–39), and poor survival (40, 41). As illustrated by the vWF staining in Figure 4, the combination Z-VAD/radiation further reduced the vessels density compared to radiation alone (p=0.006). To verify the vascular effects of Z-VAD observed in vivo, we studied the ability of treated endothelial cells to produce capillary-like tubular structures in vitro (Figure 4C). Our results are consistent with a report suggesting that apoptosis has an important role in angiogenesis by allowing the formation and remodeling of a new functional blood vessel network (42). More recently, it has also been shown that glioblastoma cells exhibit a basal constitutive caspase activity promoting migratory and invasive activities (43), and that caspase-8 can regulate cell adhesion and motility (44), which are characteristics needed for angiogenesis. Therefore, we speculated that Z-VAD may possibly exert its radiosensitizing activity in solid tumors at least partially via antiangiogenic effects by either inhibiting the caspase function in invasiveness and motility, or disrupting the ability to form functional vascular network. Of note, the results observed in the vivo experiment may be limited by the relative short half-life of Z-VAD. It is thus possible that higher or more frequent drug dosing may result in bigger differences between treatment groups. Nevertheless, this study strongly demonstrates the enhancement of radiotherapy with the concurrent administration of Z-VAD, and suggests an even more interesting trend for caspase inhibition.
In conclusion, this preclinical study supports the therapeutic potential of Z-VAD as a radiosensitizer in breast and lung cancer. We also demonstrated the anti-vascular effects of Z-VAD (in vivo and in vitro), which thus represents an attractive anticancer therapeutic strategy in combination with radiation therapy. This report suggests pan-caspase inhibition as a novel concept for the enhancement of radiation therapy in breast and lung cancer. Further clinical investigation using this proof-of-principle is warranted in combination with radiotherapy for breast and lung cancer.
Supplementary Material
Body weights were measured in human MDA-MB-231 breast (A) and H460 lung (B) cancer xenografts every 5 days and body weight ratio was calculated relative to baseline measurement. Body weight change of >10% was considered excessive.
(A) Representative histological photographs of H460 tumor sections following P62 staining. (B) Representative histological photographs of H460 tumor sections following HMGB1 staining.
Acknowledgments
Grant support: This work is supported in part by NCI 1R01 CA125842-01A1 and DOD BC030542
Abbreviations
- Z-VAD
N-benzyloxycarbonyl-Valyl-Alanyl-Aspartyl-fluoromethylketone (Z-VAD.fmk)
Footnotes
Competing Interests: Authors declare no competing interests.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 3.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301:133–142. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Lenardo MJ. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci. 2000;113(Pt 5):753–757. doi: 10.1242/jcs.113.5.753. [DOI] [PubMed] [Google Scholar]
- 5.Abend M. Reasons to reconsider the significance of apoptosis for cancer therapy. Int J Radiat Biol. 2003;79:927–941. doi: 10.1080/09553000310001632958. [DOI] [PubMed] [Google Scholar]
- 6.Kim KW, Mutter RW, Cao C, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281:36883–36890. doi: 10.1074/jbc.M607094200. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 8.Reggiori F, Klionsky DJ. Autophagosomes: biogenesis from scratch? Curr Opin Cell Biol. 2005;17:415–422. doi: 10.1016/j.ceb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Paglin S, Hollister T, Delohery T, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 10.Han Y, Giroux A, Colucci J, et al. Novel pyrazinone mono-amides as potent and reversible caspase-3 inhibitors. Bioorg Med Chem Lett. 2005;15:1173–1180. doi: 10.1016/j.bmcl.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Mizushima N, Yamamoto A, Hatano M, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komatsu M, Waguri S, Koike M, et al. Homeostatic Levels of p62 Control Cytoplasmic Inclusion Body Formation in Autophagy-Deficient Mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 15.Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 16.Contessa JN, Hampton J, Lammering G, et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002;21:4032–4041. doi: 10.1038/sj.onc.1205500. [DOI] [PubMed] [Google Scholar]
- 17.Liang XH, Yu J, Brown K, Levine B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 2001;61:3443–3449. [PubMed] [Google Scholar]
- 18.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- 20.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 21.Daido S, Yamamoto A, Fujiwara K, Sawaya R, Kondo S, Kondo Y. Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res. 2005;65:4368–4375. doi: 10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24:5414–5422. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]
- 23.Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther. 2006;5:2786–2797. doi: 10.1158/1535-7163.MCT-06-0316. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer-Hansen M, Jaattela M. Autophagy - an emerging target for cancer therapy. Autophagy. 2008:4. doi: 10.4161/auto.5921. [DOI] [PubMed] [Google Scholar]
- 26.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:963. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 27.Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 28.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 29.Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX. Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ. 2008;15:422–425. doi: 10.1038/sj.cdd.4402234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samara C, Syntichaki P, Tavernarakis N. Autophagy is required for necrotic cell death in Caenorhabditis elegans. Cell Death Differ. 2008;15:105–112. doi: 10.1038/sj.cdd.4402231. [DOI] [PubMed] [Google Scholar]
- 31.Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol. 1999;276:G249–G258. doi: 10.1152/ajpgi.1999.276.1.G249. [DOI] [PubMed] [Google Scholar]
- 32.Thornton SC, Walsh BJ, Bennett S, et al. Both in vitro and in vivo irradiation are associated with induction of macrophage-derived fibroblast growth factors. Clin Exp Immunol. 1996;103:67–73. doi: 10.1046/j.1365-2249.1996.898598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 34.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 35.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 36.Brem SS, Gullino PM, Medina D. Angiogenesis: a marker for neoplastic transformation of mammary papillary hyperplasia. Science. 1977;195:880–882. doi: 10.1126/science.402692. [DOI] [PubMed] [Google Scholar]
- 37.Guidi AJ, Fischer L, Harris JR, Schnitt SJ. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994;86:614–619. doi: 10.1093/jnci/86.8.614. [DOI] [PubMed] [Google Scholar]
- 38.Gullino PM. Natural history of breast cancer. Progression from hyperplasia to neoplasia as predicted by angiogenesis. Cancer. 1977;39:2697–2703. doi: 10.1002/1097-0142(197706)39:6<2697::aid-cncr2820390656>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Ohta Y, Endo Y, Tanaka M, et al. Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res. 1996;2:1411–1416. [PubMed] [Google Scholar]
- 40.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer. 2006;51:143–158. doi: 10.1016/j.lungcan.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 42.Segura I, Serrano A, De Buitrago GG, et al. Inhibition of programmed cell death impairs in vitro vascular-like structure formation and reduces in vivo angiogenesis. Faseb J. 2002;16:833–841. doi: 10.1096/fj.01-0819com. [DOI] [PubMed] [Google Scholar]
- 43.Gdynia G, Grund K, Eckert A, et al. Basal caspase activity promotes migration and invasiveness in glioblastoma cells. Mol Cancer Res. 2007;5:1232–1240. doi: 10.1158/1541-7786.MCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 44.Senft J, Helfer B, Frisch SM. Caspase-8 interacts with the p85 subunit of phosphatidylinositol 3-kinase to regulate cell adhesion and motility. Cancer Res. 2007;67:11505–11509. doi: 10.1158/0008-5472.CAN-07-5755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Body weights were measured in human MDA-MB-231 breast (A) and H460 lung (B) cancer xenografts every 5 days and body weight ratio was calculated relative to baseline measurement. Body weight change of >10% was considered excessive.
(A) Representative histological photographs of H460 tumor sections following P62 staining. (B) Representative histological photographs of H460 tumor sections following HMGB1 staining.