Abstract
Studies of cardiovascular disease risk have explored the idea that exaggerated physiological responses to stress may signal increased risk of cardiovascular disease. We describe a neurophysiological model of brain structures and peripheral structures that may contribute to exaggerated reactivity. Level I in this model includes the limbic system and its interactions with the prefrontal cortex that determine stress appraisals and coping responses. Level II addresses the hypothalamus and brainstem that contribute outputs to the body and which also includes brainstem nuclei that feed back to Level I to modulate its functioning. Level III includes the peripheral tissues themselves. We then suggest that stress reactivity ranging from very low to very high has a normative midrange of intensity and present evidence that negative health outcomes may be associated with both exaggerated and diminished stress reactivity since both tendencies imply a loss of homeostatic regulation. In particular, dysregulation at Levels I and II in our heuristic model signify altered motivational function and an attendant alteration in outflow to the periphery and poor behavioral homeostasis. In consequence, poor affective and behavioral regulation would be expected to contribute to poor health behaviors therefore additionally impairing health. In conclusion, diminished as well as exaggerated physiological reactivity should be seen as nonoptimal functioning that can contribute to poor health outcomes. This conceptualization places physical health into the context of behavioral and physiological processes that contribute to homeostasis.
Overview
The idea that biased emotional reactions and physiological responses are an indication of poor health is as old as Hippocrates. Under the Greek worldview, the balance of four vital humors controlled a person’s temperament. States of imbalance would render the person prone to disease. The present paper will discuss altered states of health in light of emotions, brain mechanisms, temperament, and biases in physiological regulation. Our perspective is that reactions to stress should be seen as having a normal, or normative, magnitude, and that significant deviations in response, whether above or below normal, are indicative of biases in homeostasis. In consequence, both exaggerated and diminished reactivity to stress may signal vulnerabilities to psychosomatic diseases.
Historical Perspective
Greek thinkers held that the body consisted of four humors that had an optimal balance that defined a state of health (Hart, 2001). Each humor could influence the individual’s psychological and physiological disposition, and either an excess or a deficit would render the individual subject to particular behavioral traits and health outcomes dispositions. For example, yellow bile embodied the element of fire. It came from the gall bladder, and too much yellow bile made a person choleric (bad tempered and easily angered) and subject to inflammation and fevers. Therefore humoral imbalances were used to account for both personality characteristics and associated disease vulnerabilities. Seemingly outmoded, this model has a modern flavor as well. The humors are similar to the hormones and neurotransmitters that we now study in relation to our behaviors, moods, and health. For example, dopamine is viewed, a bit simplistically, as the brain’s reward substance. Individual differences in central dopamine function are associated with variations in moods, food consumption tendencies, and also with addictions (Koob & Le Moal, 1997). In this sense, our modern psychosomatic theories still rely on imbalances in physiological substances as affecting how we respond to the world, and we see these altered responses as signaling health and disease.
The noted physiologist, Walter Cannon, talked frequently about emotional reactions originating in the brain areas later termed the limbic system. In particular, he commented on how strong emotional reactions could affect hormonal and nervous system outputs to the body, with the potential to cause significant physical symptoms including death (Cannon, 1928, 1957). Cannon also recognized that a tendency to be emotionally reactive could be a persistent tendency underlying what we think of as individual differences in temperament. This approach to emotional reactions and medical consequences reached its high point as an all-encompassing system of thinking in Franz Alexander’s textbook of psychosomatic medicine (Alexander, 1950). In both Greek and more recent thinking, there is an interplay between unseen essential properties (the character of fire, for instance, or the emotional reactions to an event) and physiological counterparts or consequences. The historical parallels between the Greek system of medicine and the more recent history of psychosomatic thinking are therefore not so different in essential quality as they are in the details.
One outgrowth of this tradition is the reactivity hypothesis that traces its history to the early 1930’s. Hines and Brown, then at the Mayo Clinic, used the cold pressor test as a provocative challenge to investigate individual differences in risk for hypertension (Hines & Brown, 1932, 1933). These workers framed the hypothesis that immersing a hand or foot in ice water could cause a reflex rise in blood pressure that would be larger in persons who were at risk of future hypertension. This work provided the tester with a specific stressor and a simple measure of responsivity that proved to be reasonably reliable and predictive of disease risk. Larger blood pressure responses were found in children from families with hypertension (Hines, 1937; Matthews, et al., 1988), and they predicted risk of future hypertension (Matthews, et al., 2004). Such evidence provided an orientation for other studies of cardiovascular disease risk. In this tradition, studies of reactivity and disease all share the postulate that larger-than-normal responses are markers of subclinical disease or that they contribute to increased risk (Everson, Kaplan, Goldberg, & Salonen, 1996; Kaplan, Manuck, Clarkson, & Prichard, 1985; Manuck, Kaplan, & Clarkson, 1983). This paper will not review specific evidence for or against the predictive value of reactivity data as this has been done extensively elsewhere (Treiber, et al., 2003). Instead we will discuss possible sources of large or small stress responses and present evidence that deviations from the norm may have prognostic value.
Sources of Individual Differences in Reactivity
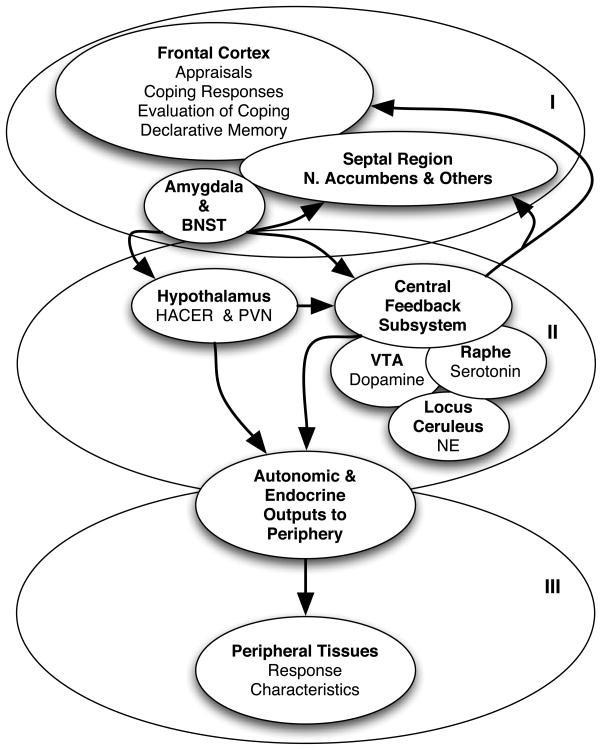
Studies of stress reactivity and disease risk have tended to avoid considerations of underlying mechanism and instead have focused on the chosen peripheral indicator of reactivity, such as blood pressure response, and then examined its association with future disease. Although this is a valid approach to the question of association, it diminishes the potential for understanding how the altered response tendency might interact with disease pathophysiology. We have described in recent publications how altered functioning of brain systems and peripheral tissues could underlie individual differences in reactivity to psychological and physical stressors (Lovallo, 2005a, 2005b; Lovallo & Gerin, 2003). Figure 1 shows the system divided for heuristic purposes into three levels of organization, with each level being a possible source of altered response tendencies.
Figure 1.
Three levels of systems organization that may contribute to individual differences in stress reactivity. BNST = bed nuclei of the stria terminalis; HACER = hypothalamic area controlling emotional reactions, the lateral-hypothalamic-perifornical region; PVN = paraventricular nucleus of the hypothalamus, a nucleus that regulates both stress endocrine outputs to the pituitary and sympathetic outflow via the brainstem; VTA = ventral tegmental area, the source of ascending dopaminergic fibers to the limbic system and prefrontal cortex; NE = norepinephrine.
Level I in the system incorporates the brain’s emotional apparatus and associated appraisal-based response system. Emotions are complex events that have four components: Emotions have cognitive inputs, and they can be evoked, heightened, and lessened by our thoughts (Schachter & Singer, 1962). Cognitive processes, including working memory and decision-making, underlie Lazarus’s system of primary appraisals of the threat value of an event and secondary appraisals of coping options and resources that influence emotional and physiological responses (Folkman & Lazarus, 1988; Lazarus & Folkman, 1984). These cognitive reactions are further refined by limbic system inputs, arising at the amygdala and forwarded via the bed nuclei of the stria terminalis to the orbitofrontal cortex and anterior cingulate gyrus (Lovallo, 2005b; Rolls, 2000). Emotions also have skeletal motor components; we convey our emotions in facial expressions and emotions are action dispositions that prepare us for behaviors to avoid danger and obtain things needed for survival (Ekman, 1993). These action dispositions are accompanied by visceral changes that prepare us to sustain the behavioral efforts necessary to accomplish the survival goals that are called for (Sinha, Lovallo, & Parsons, 1992). Finally, thoughts, visceral states, and muscle feedback together result in subjective sensations that we experience as the feeling of happiness, sadness, anxiety, etc. These Level I interactions between the prefrontal cortex and the limbic system also establish the outflow to Level II, which includes the hypothalamus and brainstem.
Level II structures have two important functions. The hypothalamus and brainstem form the final common pathways for outputs to the body. They can be influence reactivity because of variations in homeostatic set points and output gain factors. For example studies in hypertension risk indicate that persons that have equivalent responses at Level I may differ in output to the cardiovascular and endocrine systems because of characteristics of the hypothalamic paraventricular nucleus (al’Absi & Lovallo, 1993; Goncharuk, Van Heerikhuize, Swaab, & Buijs, 2002). In addition, Level II structures represent the brainstem’s “central feedback subsystem,” a term we have applied to the aminergic nuclei of the pons. Descending inputs from the hypothalamus and limbic system act on the brainstem’s noradrenergic, serotonergic, and dopaminergic nuclei, and these in turn acutely and chronically alter the responsivity of Level I structures and also influence peripheral outflow (Lovallo, 2005c).
Finally, at Level III are the peripheral tissues that can determine response magnitudes. Individual differences in response may reflect differences in autonomic outputs or intrinsic differences in tissue structure.
According to this model, individual differences at any of the three levels of systems organization could account for individual differences in response to stress and interact in different ways with disease mechanisms. In addition, appropriate study designs and use of emotion self-reports can help to identify which levels in the system are contributing to obtained reactivity differences (Lovallo, 2005a, 2005b; Lovallo & Gerin, 2003).
Large and Small Responses to Stress and Possible Disease Associations
The typical view of stress reactivity and disease assumes that larger responses are worse and smaller responses are better. This unstated assumption seems to be self-evident and in no need of examination, however we have recently questioned this assumption and advanced the idea that biases toward both very large and very small stress reactions are both indicators of poor homeostasis and are signals of possible disease risk (Carroll, Lovallo, & Phillips, 2009; Carroll, Phillips, & Lovallo, In press). If we assume that larger-than-normal responses of the cardiovascular and endocrine systems can signal systemic dysfunction (Manuck, Kaplan, Adams, & Clarkson, 1989), then it may be equally likely that smaller-than-normal responses can also signal systems dysfunction and contribute to pathophysiological processes.
The following example illustrates this point. A healthy young man in a seated position should have a heart rate of about 60 – 65 beats per minute and a blood pressure of perhaps 115/65 mmHg. If this person rises to a standing position, the blood would tend to pool in the legs, but this would be opposed by the baroreceptor system, which initiates a vigorous sympathetic nervous system response to maintain adequate return of blood to the heart by contracting peripheral blood vessels and stimulating the heart to increase cardiac output (Cacioppo, et al., 1994; Guyton, 2000). Accordingly we might expect heart rate to go up to about 85 beats per minute, systolic blood pressure to rise slightly, and diastolic pressure to change very little. This response appears to be normative for the challenge of orthostasis.
Variations up or down from this normal response pattern are undesirable. If the heart rate were to go to 130 beats per minute or blood pressure to rise substantially, we might suspect a failure of the baroreceptors to have regulated the response appropriately (a failure at Level II). Similarly, a prehypertensive person with altered blood vessel wall thickness might have an abnormally heightened blood pressure response that would signal persistently elevated peripheral resistance, a Level III response alteration common in prehypertensive states (Folkow, 1990). We might therefore assume that there is an existing pathophysiology and that the persistence of such dysregulated responses might have damaging consequences.
Consider the alternative scenario. Our young man rises to a standing position and promptly faints. Such an outcome could indicate that the baroreceptors failed to trigger the necessary sympathetic output (a failure in Level II of the system), or the sympathetic outflow failed to evoke the necessary peripheral responses (a failure at Level III). This not-uncommon failure to regulate blood pressure and flow under the simple demand of orthostasis is typically a signal of an autonomic neuropathy, among other possibilities.
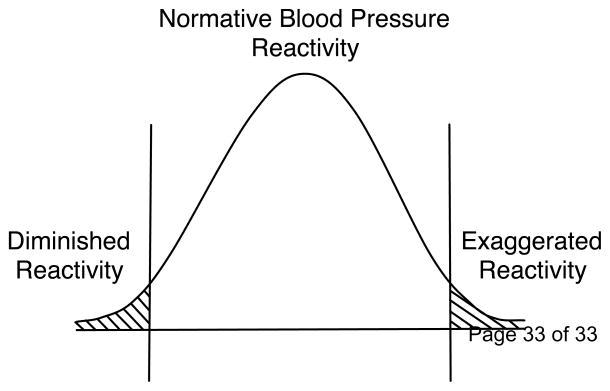
These contrasting examples illustrate that response deviations from normal in either direction may signal a loss of homeostatic regulation and indicate disease risk. If the system is organized homeostatically, or we may say normatively, then the system’s response and return to normal will be within normal limits and time parameters given the current demand. By definition responses that depart significantly from that norm in either direction could signal a potential systems dysregulation, pointing toward a reduced state of health. To illustrate this point, Figure 2 presents a normal curve as representing the response of a regulated physiological variable, such as heart rate, blood pressure, or cortisol, in a large population of otherwise healthy persons. The center of the distribution may be seen as representing a presumed normative range of reactions that captures the reactivity tendencies of the majority of persons being tested. At the tails of the distribution are persons who, based on statistical principles, do not represent the normative range, but are in the extremes. This is an argument in principle that the concept of reactivity its association with disease risk should include exaggerated responses and ones that are smaller than normal. The remainder of this discussion will focus on individual differences in emotional reactivity and coping and how these may affect stress reactivity and risk of disease.
Figure 2.
A hypothetical cardiovascular response distribution with areas under the curve identifying highly reactive individuals along the right tail and highly unreactive individuals under the left tail.
Level I and Level II Processes Can Determine Exaggerated or Diminished Outputs From the Brain to the Periphery
As noted above, emotional responses formed at Level I in the system determine outputs to Level II and ultimately how the body responds to stress. Alterations in these Level I relationships will affect a person’s emotional response characteristics, and it is likely that some alterations in emotional reactivity will result in diminished reactions to stress rather than larger ones. These diminished responses would then affect Levels II and III in the system with implications for physiological reactivity and health. In addition, altered emotional reactivity would affect motivational processes, and these would influence behavioral choices and habits that may have implications for health.
Damasio and colleagues have written persuasively about the impact of altered prefrontal-limbic integrations on cognition, behavior, emotional responses, and autonomic outflow (Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Damasio, 1994; Damasio, et al., 2000). The amygdala and its interactions with the striatum, orbitofrontal cortex, and anterior cingulate gyrus stand at the core of the brain’s emotional response system (Rolls, 1972, 1999). The amygdala contains inborn neuronal pattern recognition systems that carry survival benefit to the developing animal. Primates’ well-known innate fear of snakes is one such example, and this fear is known to depend on an intact amygdala. Young monkeys fear snakes on the first encounter, but amygdaloid ablation completely eliminates this fear response (Amaral, Price, Pitkanen, & Carmichael, 1992; Prather, et al., 2001). This example presents a case in which a small or absent response is clearly not adaptive and health promoting; young monkeys that are not afraid of snakes are at high risk of being bitten while those with large reactions are likely to avoid such a fate.
In addition to the amygdala’s innate response repertoire, the amygdala is essential to the formation of Pavlovian conditioned associations; the amygdala receives highly processed sensory inputs from cortical association areas, and also receives inputs from the viscera via the anterior insula (Davis, 2000). This pairing permits bodily states to be associated with external events and permits development of normally motivated responses to those events. Destruction of the amygdala abolishes the ability to form Pavlovian conditioning (Campeau & Davis, 1995). Loss of the ability to form Pavlovian associations, or to properly express innate response tendencies, leaves the person unable to develop appropriately motivated behaviors in response to situational demands. In humans bilateral amygdala damage disrupts emotional responsivity and this disruption is most severe in persons sustaining damage early in life because of a failure to develop a normal experiential background of motivated responses (Anderson, et al., 1999; Siebert, Markowitsch, & Bartel, 2003; Tranel, Gullickson, Koch, & Adolphs, 2006).
At least one study has shown that high levels of emotional stability and intelligence predict longer lifespan, suggesting that integrity of the central nervous system at Levels I and II can contribute to good health (Weiss, Gale, Batty, & Deary, 2009). Damage to the amygdala or its connections to the prefrontal cortex impairs emotional responsivity and diminishes the person’s ability to produce adequate behavioral coping strategies to challenges presented by external events. Recent neuroimaging work examining brain activity in relation to cardiovascular stress reactivity has provided substantial real-time evidence that amygdala connectivity is an important determinant of individual differences in cardiovascular response tendencies (Gianaros, Jennings, Sheu, Derbyshire, & Matthews, 2007; Gianaros, May, Siegle, & Jennings, 2005; Gianaros, et al., 2008).
Given the central role of these amygdala-prefrontal connections, it is perhaps not difficult to view more subtle Level I deficits as causing diminished emotional reactivity and accordingly smaller physiological responses to stressor challenge. There are several lines of evidence that this is so. Psychopaths typically display a lack of emotional response to social cues, and this is accompanied by deficient activity of the amygdala during tasks designed to evoke such responses (Blair, Colledge, Murray, & Mitchell, 2001; Kiehl, et al., 2001; Muller, et al., 2003; Rilling, et al., 2007). Such reactivity differences are mirrored in persons with varying numbers of alleles for a low-activity version of the catechol-O-methyltransferase (COMT) gene. Each person carries one copy from each parent and therefore can have 0, 1, or 2 copies of the low-activity variant or its high-activity counterpart allele. Persons with two copies of the low-activity allele are highly reactive to unpleasant stimuli in relevant frontal and limbic system areas (Smolka, et al., 2005). Persons with two copies of the high-activity allele are emotionally unreactive and have antisocial and disinhibitory behavioral characteristics (Goldman, Oroszi, & Ducci, 2005). Not surprisingly, persons with disinhibitory behavioral patterns also have smaller cardiovascular and cortisol reactions to threatening social situations, such as a public speaking task (Sorocco, Lovallo, Vincent, & Collins, 2006). The common factor tying behavioral disinhibition together with lack of emotional and physiological reactivity is that deficient inputs from the amygdala or inadequate connections to the prefrontal cortex would affect the persons ability to choose adaptive courses of action and would similarly influence Level II activity causing diminished physiological responsivity.
In addition to affecting Level II outputs to the periphery, altered amygdala-prefrontal communication have an impact on the actions of the brain’s central feedback subsystem consisting of the serotonergic raphe nuclei, the dopaminergic ventral tegmental nuclei, and the noradrenergic locus ceruleus all located in the pons (Iversen, Kupfermann, & Kandel, 2000; Swanson, 2000). These brainstem nuclei depend on inputs from higher centers to react to the external environment. Once they do react, they set the state of the central nervous system in response to such inputs, they also develop characteristic patterns of reaction that contribute to individual differences in relation to experience (Koob, 1992; Koob & Le Moal, 1997; Swanson, 2000). The serotonergic raphe nuclei have ascending fibers that affect the state of limbic system and prefrontal cortex communication. Alterations in their signaling would have an impact on long-term regulation of affect (Manuck, et al., 1999; Manuck, Flory, Ferrell, Mann, & Muldoon, 2000; Manuck, Kaplan, Rymeski, Fairbanks, & Wilson, 2003). In similar fashion, the activity and response level of the locus ceruleus to external events depends on normal activation of the brain’s corticotropin releasing factor neurons (Aston–Jones, Ennis, Pieribone, Nickell, & Shipley, 1986; Petrusz & Merchenthaler, 1992). The locus ceruleus is responsible for the global activational state of the central nervous system (Aston–Jones, et al., 1986). Finally, the dopaminergic fibers arising from the ventral tegmental area of the pons, and arriving at critical striatal areas such as the nucleus accumbens, are necessary for maintaining normal attention to cues signaling reward and motivating approach behaviors and cognition more generally (Arnsten, 1997; Dellu-Hagedorn, 2006; Hakyemez, Dagher, Smith, & Zald, 2008; Murphy, Arnsten, Goldman-Rakic, & Roth, 1996). Alterations in dopaminergic signaling to these areas are considered by many to be a source of altered approach-avoidance tendencies and differential response to reward signals. Accordingly, persons with deficient or excessive dopaminergic function may have altered behavioral tendencies and altered autonomic responses to environmental cues. Consequently, altered frontal-limbic interactions would alter the ability of the pontine nuclei to perform their functions and, in turn, altered pontine function would affect the background state of the central nervous system, both processes acting as neurophysiological underpinnings of individual differences in reactivity.
The evidence cited above indicates that significant motivational consequences occur when there are functional alterations of either prefrontal-limbic communication (providing inputs to Level II processes) or altered feedback from the Level II aminergic nuclei to Level I structures. These alterations in Level-I and -II interactions would therefore have an impact on outflow to the periphery via the brainstem and hypothalamus. Because these interactions affect coping processes and decision-making, they may also have significant consequences for health behaviors, including a tendency toward poor eating habits, risk-taking, smoking, and alcohol intake, among others.
There is no inherent reason why these alterations in peripheral outflow would only result in exaggerated outputs; they may equally well diminish normal physiological reactivity.
Health Implications of Reduced Levels of Physiological Reactivity
We recently summarized research indicating that low stress reactivity may accompany biases in food intake and fuel homeostasis and also abnormal motivational states involving risk of alcoholism (Carroll, et al., In press). Although a full accounting of existing evidence is beyond the scope of this paper, a few indications of the health context of reduced stress reactivity is in order. Briefly stated, we noted that persons with altered stress responses are shown to have changes in immune system response (Cacioppo, et al., 1998; Sheridan, Stark, Avitsur, & Padgett, 2000). Persons with robust cortisol stress responses also have more vigorous antibody responses to antigen challenge while blunted cortisol responses signal poorer antibody response (Phillips, Carroll, Burns, & Drayson, 2005). In this latter study, blunted stress cortisol responses were also accompanied by high levels of neuroticism, suggesting a poor regulation of affect in these persons, potentially implicating Levels I and II in our model. Other research shows that deficient stress cortisol responses may fail to keep immune activity in check, increasing the risk of autoimmune diseases. Women characteristically have diminished cortisol responsivity (but the same basal levels) relative to men, and they are about four times more likely to suffer from autoimmune disorders such as arthritis (Morell, 1995). The Lewis rat model of arthritis provides a mechanistically elaborated example of reduced stress reactivity and its implications for arthritis. The Lewis rat is genetically deficient in corticotropin releasing factor activity at the hypothalamic paraventricular nucleus. This leads to reduced cortisol activation to an immune system challenge such as injection of streptococcus bacteria cell wall preparations. Following such injections Lewis rats exhibit join inflammation and deformation analogous to human arthritis (Sternberg, et al., 1989; Sternberg, Wilder, Chrousos, & Gold, 1991).
Obesity research also suggests health implications of reduced stress reactivity in relation to Level I and II brain structures. One study examined central serotonergic reactivity to fenfluramine challenge and its relationship to the metabolic syndrome, a constellation of body mass index, abdominal obesity, hypertension, poor lipid profile, and insulin resistance, and found that low serotonergic responsivity was associated with greater prevalence of these metabolic risk factors (Muldoon, et al., 2004). More direct evidence comes from a Scottish longitudinal study that found low levels of cardiovascular response to stress predicted higher levels of obesity at entry and greater five-year progression of obesity (Carroll, Phillips, & Der, 2008). In a U.S. community-based study, perceived stress predicted a flattening of the diurnal cortisol curve, an indicator of reduced integrity of hypothalamic-pituitary-adrenocortical axis function (Farag, et al., 2008). However, when obesity was taken into account, this relationship disappeared and obesity alone accounted for the flattening of the cortisol curve. One way to interpret this evidence is that a flattened diurnal cortisol pattern is either contributes to obesity or is a reflection of obesity (Dallman, et al., 2003). An additional health consequence is that a flattening of the diurnal cortisol curve reduces the ability of peripheral cortisol to reset circadian cellular clocks in various tissues. A loss of this time signal could well be an indicator of poor systems integrity and poorer systems function (Buijs, van Eden, Goncharuk, & Kalsbeek, 2003).
How might we interpret the connection of reduced cortisol responsivity to obesity as a behavioral trait? There is increasing evidence in the field of addiction research that persons prone to smoking, alcoholism, gambling and other forms of substance abuse are relatively antisocial and behaviorally disinhibited (perhaps evidence of Levels I and II having altered function) and that these same people also have reduced stress cortisol reactivity (Acton, 2003; Anker, Perry, Gliddon, & Carroll, 2009; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000). Adolescent and young adult offspring of alcoholic or substance-abusing parents are themselves at increased lifetime risk of substance abuse, with a significant genetic contribution to this risk (Cloninger, Bohman, & Sigvardsson, 1981). In addition, a tendency toward substance use disorders is significantly associated with antisocial tendencies accompanied by poor mood regulation (Cadoret, O’Gorman, Troughton, & Heywood, 1985; Lovallo, Yechiam, Sorocco, Vincent, & Collins, 2006; Shedler & Block, 1990; Vanyukov, et al., 1993). In turn, antisocial and disinhibitory behavioral tendencies predict reduced cortisol stress reactivity which itself predicts experimentation with smoking in at-risk adolescents (Moss, Vanyukov, Yao, & Kirillova, 1999; Moss, Vanyukov, & Martin, 1995). A blunted stress cortisol response to stress is also seen in young adults with a family history of alcoholism (Sorocco, et al., 2006) and in alcoholic and polysubstance abusing patients (Lovallo, Dickensheets, Myers, Thomas, & Nixon, 2000). The blunted stress cortisol responses in these latter studies are accompanied by a diminished cardiovascular response to stress (Lovallo, et al., 2000; Panknin, Dickensheets, Nixon, & Lovallo, 2002).
These diminished endocrine and autonomic responses to stress are associated with poor regulation of affect and behavior in these family-history positive persons. We have observed persons with a positive family history of alcoholism to have antisocial tendencies, to be high in neuroticism, and to have higher depression scores than persons with no such history (Sorocco, et al., 2006). In addition, these same individuals have poorer working memory performance, and the males are biased toward attention to winnings in a gambling task (Lovallo, et al., 2006). These persons also make more impulsive errors on a Go-NoGo reaction time task (Saunders, et al., 2008). Neuroimaging work shows that otherwise healthy nonalcoholic young adults with a family history of alcoholism have reduced amygdala activation to emotional faces and that this blunted amygdala response is greater in persons with more antisocial tendencies (Glahn, Lovallo, & Fox, 2007). During work on the Iowa Gambling Task, these same persons with a positive family history have greater activation of anterior cingulate gyrus and the dorsal striatum (caudate nucleus) (Acheson, Robinson, Glahn, Lovallo, & Fox, 2009). We interpreted the striatal activation in the scanner as being associated with the greater attention to gains that we saw in the laboratory, an indication that the persons with a positive family history were playing the game more as a risky gamble, while the persons with a negative family history approached the game more as a cognitive challenge.
This evidence points to a connection between altered Level I and II systems functioning, reduced endocrine and autonomic stress reactivity, and behavioral dysregulation with consequences for health. The evidence for behavioral dysregulation includes standard tests of behavioral control in the lab, but it also seems to extend to dysregulated consumatory behaviors seen in obesity and addiction-proneness in daily life. A question worth asking is how does poor mood regulation accompanied by behavioral disinhibition relate to deficient cortisol and autonomic reactivity? We have discussed elsewhere that poor amygdala response to environmental challenge, and hence altered amygdala-prefrontal signaling, may result in risk taking behavior and overly active approach tendencies and deficient avoidance tendencies (Lovallo, 2007). The amygdala plays in a key role in signaling the system that danger is present, and helping to generate a normal stress response, including cortisol and sympathetic activation, to support fight-or-flight behaviors. Persons lacking a normal amygdala response are more likely to be attracted to situations that others perceive as dangerous and to be focused on hedonic experience at the expense of longer term planning. This model therefore is consistent with overconsumption of alcohol, recreational drugs, and high-calorie foods. As noted in this connection, Mary Dallman has proposed a related example of food overconsumption resulting from high levels of cortisol secretion. In this model, high levels of cortisol signal the system that a homeostatic threat is present, food intake dampens this ongoing cortisol activity, and the return to homeostasis registers as a reward signal supporting future intake behaviors (Dallman, 1993; Dallman, et al., 2003). It may seem contradictory that both high levels of cortisol and reduced cortisol reactivity would be associated with increased consumatory behavior, but this may equally well exemplify our basic thesis that elevated and reduced stress reactivity both signal systems dysregulation and potential health risks.
The present paper has avoided a specific focus on cardiovascular disease in relation to subnormal response dispositions because examples from other health problems are now more prevalent and examples from cardiovascular disease are few. Future studies may well focus on cardiovascular disease risk as a further test of the hypothesis advanced here.
Final Considerations and Implications for Research
The thrust of this discussion is that the study of stress reactivity and its implications for health should be broadened to incorporate both exaggerated and diminished physiological reactivity as candidates for predicting poorer health outcomes. Although there is good evidence that elevated reactivity may indicate greater cardiovascular disease risk, there is now a small but growing number of studies suggesting that reduced stress reactivity signals altered frontal-limbic integrations of behavior and physiological functioning. These latter alterations may be risk factors for altered eating behaviors and substance use disorders, among others.
In reference to Figure 2, we might consider several points about the ideas expressed above. First, a frequently used strategy for studying reactivity is a median split of the data, with the goal of comparing the 50% above the median with the 50% below. Examination of Figure 2 indicates that this could tend to wash out true effects operating at both ends of the distribution. Persons who are highly reactive are lumped in with many others near the median who represent the normative homeostatic range. Similarly, grouping those at the very low end with persons near the median obscures the consequences of low reactivity. A more fruitful research strategy would be to compare each end of the distribution against the middle.
Second, there are a several questions that come to mind when thinking about the people who inhabit these distribution extremes:
Who are they? An initial approach might be to do a multivariate analysis to identify the set of demographic or psychological characteristics that best define those low or high in reactivity without preconceptions about who they are.
What are the psychological or physiological characteristics of the low and high reactor groups?
Given the psychophysiological characteristics of the extreme groups, how do they differ from the normative middle group?
If we know the psychophysiological profile of persons at either extreme, we can then ask what disorders each extreme group might they be predisposed to, or protected against? Is it possible that persons with very small responses are disposed to risk-taking and consumatory disorders while high reactors are at risk of an entirely different set of disorders, such as hypertension. In this phase of the analysis, consideration of mechanisms operating at Levels I, II, vs. III of the model in Figure 1 may be of help in sorting out the potential risks.
As an initial suggestion for research to test our hypothesis, researchers with ready access to large prospective cardiovascular disease databases may be positioned to test this hypothesis without the collection of new data. For example, any study of blood pressure reactivity at entry and with long-term follow-up of health outcomes could be reexamined with an emphasis on persons at both ends of the response distribution, as illustrated in Figure 2. Relevant questions are whether the putative outcomes for the groups at the two extremes are both within the cardiovascular domain or not. If not, then what sorts of disorders cluster at the lower end of the distribution?
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Drug Alcohol Depend. 2009;100(1–2):17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton GS. Measurement of impulsivity in a hierarchical model of personality traits: implications for substance use. Subst Use Misuse. 2003;38(1):67–83. doi: 10.1081/ja-120016566. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Lovallo WR. Cortisol concentrations in serum of borderline hypertensive men exposed to a novel experimental setting. Psychoneuroendocrinology. 1993;18(5–6):355–363. doi: 10.1016/0306-4530(93)90011-9. [DOI] [PubMed] [Google Scholar]
- Alexander F. Psychosomatic Medicine, its principles and applications. New York: Norton; 1950. [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley-Liss, Inc; 1992. pp. 1–66. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2(11):1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacology, Biochemistry and Behavior. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Aston–Jones G, Ennis M, Pieribone RA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177(1):17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31(6):586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, O’Gorman TW, Troughton E, Heywood E. Alcoholism and antisocial personality. Interrelationships, genetic and environmental factors. Arch Gen Psychiatry. 1985;42(2):161–167. doi: 10.1001/archpsyc.1985.01790250055007. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci. 1995;15(3 Pt 2):2301–2311. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The mechanism of emotional disturbance of bodily functions. New England Journal of Medicine. 1928;198:165–172. [Google Scholar]
- Cannon WB. “Voodoo” death. Psychosomatic Medicine. 1957;19:182–190. doi: 10.1097/00006842-195705000-00003. [DOI] [PubMed] [Google Scholar]
- Carroll D, Lovallo WR, Phillips AC. Are large physiological reactions to acute psychological stress always bad for health? Social and Personality Psychology Compass. 2009;3:725–743. [Google Scholar]
- Carroll D, Phillips AC, Der G. Body mass index, abdominal adiposity, obesity, and cardiovascular reactions to psychological stress in a large community sample. Psychosom Med. 2008;70(6):653–660. doi: 10.1097/PSY.0b013e31817b9382. [DOI] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Lovallo WR. The behavioural and health corollaries of blunted physiological reactions to acute psychological stress: Revising the reactivity hypothesis. In: Wright R, Gendolla GHE, editors. Motivation perspectives on cardiovascular response. Washington, DC: American Psychological Association; In press. [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross fostering analysis of adopted men. Arch Gen Psychiat. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Adaptation of the hypothalamic–pituitary–adrenal axis to chronic stress. Trends in Endocrinology and Metabolism. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: Emotion, reason, and the human brain. New York: G.P. Putnam’s Sons; 1994. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford, England: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2:10. doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. Facial expression and emotion. Am Psychol. 1993;48(4):384–392. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure response to exercise predicts future high blood pressure in middle-aged men. Hypertension. 1996;27(5):1059–1064. doi: 10.1161/01.hyp.27.5.1059. [DOI] [PubMed] [Google Scholar]
- Farag NH, Moore WE, Lovallo WR, Mills PJ, Khandrika S, Eichner JE. Hypothalamic-pituitary-adrenal axis function: relative contributions of perceived stress and obesity in women. J Womens Health (Larchmt) 2008;17(10):1647–1655. doi: 10.1089/jwh.2008.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. Coping as a mediator of emotion. J Pers Soc Psychol. 1988;54(3):466–475. [PubMed] [Google Scholar]
- Folkow B. “Structural factor” in primary and secondary hypertension. Hypertension. 1990;16(1):89–101. doi: 10.1161/01.hyp.16.1.89. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49(1):134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosom Med. 2005;67(1):31–39. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61(11):1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Goncharuk VD, Van Heerikhuize J, Swaab DF, Buijs RM. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol. 2002;443(4):321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. Textbook of Medical Physiology. 10. New York: W. B. Saunders & Co; 2000. [Google Scholar]
- Hakyemez HS, Dagher A, Smith SD, Zald DH. Striatal dopamine transmission in healthy humans during a passive monetary reward task. Neuroimage. 2008;39(4):2058–2065. doi: 10.1016/j.neuroimage.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Hart GD. Descriptions of blood and blood disorders before the advent of laboratory studies. British Journal of Haematology. 2001;115(4):719–728. doi: 10.1046/j.1365-2141.2001.03130.x. [DOI] [PubMed] [Google Scholar]
- Hines EA., Jr Reaction of the blood pressure of 400 school children to a standard stimulus. Journal of the American Medical Association. 1937;108:1249–1250. [Google Scholar]
- Hines EA, Jr, Brown GE. Standard stimulus for measuring vasomotor reactions. Its application in the study of hypertension. Proceedings of the Staff Meeting of the Mayo Clinic. 1932;7:332–335. [Google Scholar]
- Hines EA, Jr, Brown GE. A standard test for measuring the variability of blood pressure: Its significance as an index of the prehypertensive state. Annal of Internal Medicine. 1933;7:209–217. [Google Scholar]
- Iversen S, Kupfermann I, Kandel ER. Emotional states and feelings. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. New York: McGraw-Hill; 2000. pp. 982–997. [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Prichard RW. Animal models of behavioral influences on atherogenesis. In: Manuck ESKSB, editor. Advances in Behavioral Medicine. Greenwich, CT: JAI Press; 1985. pp. 115–164. [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological mechansims of cocaine and opiate dependence. In: Faffe CPOBaJH., editor. Addictive States. New York: Raven press; 1992. [Google Scholar]
- Koob GF, Le Moal M. Drug Abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- Lovallo WR. Cardiovascular reactivity: Mechanisms and pathways to cardiovascular disease. International Journal of Psychophysiology. 2005a;58(2–3):119–132. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Stress & Health: Biological and Psychological Interactions. 2. Thousand Oaks, CA: Sage Publications; 2005b. [Google Scholar]
- Lovallo WR. Stress & Health: Biological and Psychological Interactions. 2. Thousand Oaks, CA: Sage Publications; 2005c. [Google Scholar]
- Lovallo WR. Individual differences in response to stress and risk for addiction. In: al’Absi M, editor. Stress and addiction: Biological and psychological mechanisms. 1. Burlington, MA: Academic Press; 2007. pp. 227–248. [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24(5):651–658. [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosom Med. 2003;65(1):36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2006;30(5):763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Dent KM, Mann JJ, Muldoon MF. Aggression and anger-related traits associated with a polymorphism of the tryptophan hydroxylase gene. Biol Psychiatry. 1999;45(5):603–614. doi: 10.1016/s0006-3223(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95(1):9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Adams MR, Clarkson TB. Behaviorally elicited heart rate reactivty and atherosclerosis in female cynomolgus monkeys (Macaca fascicularis) Psychosomatic Medicine. 1989;51:306–318. doi: 10.1097/00006842-198905000-00005. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Clarkson TB. Behaviorally induced heart rate reactivity and atherosclerosis in cynomolgus monkeys. Psychosom Med. 1983;45(2):95–108. doi: 10.1097/00006842-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Rymeski BA, Fairbanks LA, Wilson ME. Approach to a social stranger is associated with low central nervous system serotonergic responsivity in female cynomolgus monkeys (Macaca fascicularis) Am J Primatol. 2003;61(4):187–194. doi: 10.1002/ajp.10118. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110(1):74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Manuck SB, Stoney CM, Rakaczky CJ, McCann BS, Saab PG, et al. Familial aggregation of blood pressure and heart rate responses during behavioral stress. Psychosom Med. 1988;50(4):341–352. doi: 10.1097/00006842-198807000-00003. [DOI] [PubMed] [Google Scholar]
- Morell V. Zeroing in on how hormones affect the immune system. Science. 1995;269(5225):773–775. doi: 10.1126/science.7638587. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao JK, Kirillova GP. Salivary cortisol responses in prepubertal boys: the effects of parental substance abuse and association with drug use behavior during adolescence. Biol Psychiatry. 1999;45(10):1293–1299. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry. 1995;38(8):547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89(1):266–271. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biol Psychiatry. 2003;54(2):152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93(3):1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panknin TL, Dickensheets SL, Nixon SJ, Lovallo WR. Attenuated heart rate responses to public speaking in individuals with alcohol dependence. Alcoholism-Clinical and Experimental Research. 2002;26(6):841–847. [PubMed] [Google Scholar]
- Petrusz P, Merchenthaler I. The corticotropin-releasing factor system. In: Nemeroff CB, editor. Neuroendocrinology. 1. Boca Raton, FL: CRC Press; 1992. pp. 129–183. [Google Scholar]
- Phillips AC, Carroll D, Burns VE, Drayson M. Neuroticism, cortisol reactivity, and antibody response to vaccination. Psychophysiology. 2005;42(2):232–238. doi: 10.1111/j.1469-8986.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106(4):653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57(2):119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128–119. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Activation of amygdaloid neurones in reward, eating and drinking elicited by electrical stimulation of the brain. Brain Res. 1972;45(2):365–381. doi: 10.1016/0006-8993(72)90468-4. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The Brain and Emotion. New York: Oxford Univ. Press; 1999. [Google Scholar]
- Rolls ET. Precis of The brain and emotion. Behav Brain Sci. 2000;23(2):177–191. doi: 10.1017/s0140525x00002429. discussion 192–233. [DOI] [PubMed] [Google Scholar]
- Saunders B, Farag N, Vincent AS, Collins FL, Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32(5):888–894. doi: 10.1111/j.1530-0277.2008.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter S, Singer JE. Cognitive, social, and physiological determinants of emotional state. Psychol Rev. 1962;69:379–399. doi: 10.1037/h0046234. [DOI] [PubMed] [Google Scholar]
- Shedler J, Block J. Adolescent drug use and psychological health. A longitudinal inquiry. The American Psychologist. 1990;45(5):612–630. doi: 10.1037//0003-066x.45.5.612. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126(Pt 12):2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosomatic Medicine. 1992;54(4):422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, et al. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25(4):836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. International Journal of Psychophysiology. 2006;59(3):210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM, Hill JM, Chrousos GP, Kamilaris T, Listwak SJ, Gold PW, et al. Inflammatory mediator–induced hypothalamic–pituitary–adrenal axis activation is defective in streptococcal cell wall arthritis susceptible Lewis rats. Proceedings of the National Academy of Sciences USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg EM, Wilder RL, Chrousos GP, Gold PW. Stress responses and the pathogenesis of arthritis. In: McCubbin PGKJA, Nemeroff CB, editors. Stress, neuropeptides, and systemic disease. New York, NY: Academic Stress; 1991. pp. 287–300. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. [Review] Brain Research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cogn Neuropsychiatry. 2006;11(3):219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease States. Psychosom Med. 2003;65(1):46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: associations with cortisol. Psychiatry Res. 1993;46(1):9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Weiss A, Gale CR, Batty GD, Deary IJ. Emotionally stable, intelligent men live longer: the Vietnam experience study cohort. Psychosomatic Medicine. 2009;71(4):385–394. doi: 10.1097/PSY.0b013e318198de78. [DOI] [PubMed] [Google Scholar]