Abstract
The hippocampal formation is a highly plastic brain region that is sensitive to stress. It receives extensive noradrenergic projections, and noradrenaline is released in the hippocampus in response to stressor exposure. The hippocampus expresses particularly high levels of the α1D adrenergic receptor (ADR) and we have previously demonstrated that α1d ADR mRNA expression in the rat hippocampus is modulated by corticosterone. One of the defining features of a stress response is activation of the hypothalamic pituitary adrenal (HPA) axis, resulting in the release of corticosterone from the adrenal glands. However, the effect of stress on hippocampal expression of α1d ADR mRNA has not been determined. In this study, male rats were exposed to inescapable tail shock, loud noise or restraint, and the effect on α1d ADR mRNA expression in the hippocampus was determined by semi-quantitative in situ hybridization. All three stressors resulted in a rapid upregulation of α1d ADR mRNA in the dentate gyrus, with expression peaking at approximately 90 minutes after the start of the stressor. Physical activity has previously been reported to counteract some of the effects of stress that occur within the dentate gyrus. However, 6 weeks of voluntary wheel running in rats did not prevent the restraint stress-induced increase in α1d ADR mRNA expression in the dentate gyrus. Although the function of the α1D ADR in the dentate gyrus is not known, these data provide further evidence for a close interaction between stress and the noradrenergic system in the hippocampus.
Keywords: noradrenergic, dentate gyrus, hippocampus, alpha 1d adrenergic receptor, stress, exercise
Introduction
The hippocampal formation is a highly plastic brain region that shows substantial stress sensitivity (McEwen, 2001). It is also the target of extensive noradrenergic innervation in both rats and humans (Loy et al., 1980; Powers et al., 1988). Considerable evidence supports a close interaction between stress and the noradrenergic system in the hippocampus, with a variety of stressors, including restraint, loud noise and intermittent tail shock, leading to an increase in noradrenaline release in the hippocampus (Abercrombie et al., 1988; Britton et al., 1992). Furthermore, the increase in noradrenaline release in the hippocampus induced by tail pinch stress has been correlated with the behavioral reactivity of rats to the stress of a novel environment (Rosario and Abercrombie, 1999). One of the defining features of a stress response is an increase in circulating glucocorticoids (corticosterone in rats; cortisol in humans), and interactions between glucocorticoids and the noradrenergic system in the hippocampus have also been reported. For example, noradrenergic input to the hippocampus has been reported to have a facilitatory effect on the hypothalamic pituitary adrenal (HPA) axis response to stress (Maccari et al., 1990a), while the response of hippocampal neurons to noradrenaline depends on the concentration of glucocorticoids and the relative binding of mineralocorticoid (MR) and glucocorticoid (GR) receptors (Joels et al., 1995).
To date, nine adrenergic receptors have been cloned, of which 3 are in the α1 ADR family. While levels of α1a and α1b ADR mRNA expression are relatively low in the hippocampus, α1d ADR mRNA is highly expressed in all subfields of the rat hippocampus (McCune et al., 1993; Pieribone et al., 1994; Day et al., 1997), and in the human hippocampus (Szot et al., 2005). There is extensive colocalization of α1d ADR mRNA with both MR and GR receptors within the rat hippocampal formation (Williams et al., 1997), and thus α1D ADRs are in an excellent position to both modulate glucocorticoid sensitive hippocampal neurons, and in turn be modulated by glucocorticoids. In vitro experiments have shown that glucocorticoids can upregulate α1d ADR mRNA expression (Rouppe van der Voort et al., 1999). We have previously demonstrated that levels of α1d ADR mRNA were profoundly decreased throughout the hippocampus and dentate gyrus as early as 1 day after adrenalectomy, and restored by corticosterone replacement (Day et al., 2008), providing additional evidence for regulation of this receptor by glucocorticoids.
Although a close relationship between glucocorticoids and the noradrenergic system in the hippocampus has been clearly demonstrated, to our knowledge, the impact of a stressor on levels of α1d ADR mRNA has not been reported. In the following series of experiments, adult male rats were exposed to inescapable tail shock, restraint or loud noise (100 dB sound pressure level, A scale; 100 dBA) stress, and the time course of α1d ADR mRNA expression in the hippocampus determined by semi-quantitative in situ hybridization. All stressors resulted in a rapid upregulation of α1d ADR mRNA in the dentate gyrus, which peaked around 90 min. after the start of stressor exposure. It has previously been reported that physical activity also affects the dentate gyrus, for example increasing brain-derived neurotrophic factor (BDNF) expression (Neeper et al., 1996; Russo-Neustadt et al., 2000) and neurogenesis (van Praag et al., 1999). Additionally, physical activity can counteract some of the effects of stress, including stress-induced decreases in BDNF expression in the dentate gyrus (Greenwood et al., 2007). In the current study, six weeks of prior wheel running resulted in a small increase in α1d ADR mRNA expression in the dentate gyrus, and did not prevent the restraint stress-induced increase in α1d ADR mRNA expression in this area. In contrast, and consistent with the literature, there was a clear interaction between exercise and stress on BDNF mRNA levels in the dentate gyrus of the same animals. The functional relevance of the α1D ADR in normal hippocampal functions, in particular in relation to stressor exposure, has yet to be determined. Nonetheless, these data provide further evidence for a close interaction between stress and the noradrenergic system in the hippocampus, and particularly expression of α1d ADR mRNA in the rat dentate gyrus.
Results
Experiment 1: Inescapable tail shock time course
One way ANOVA with “time” as a factor, revealed a significant increase in α1d ADR mRNA in the medial blade of the dentate gyrus (Figures 1 and 2; F(5,41) = 4.105; p = 0.004) after inescapable tail shock. A significant increase in α1d ADR mRNA was observed immediately (0 hour) and 1 hour after stressor termination (approximately 1.5 to 2.5 hours after the start of stress), compared to controls (p = 0.005 for both the 0 and 1 hour time points). There were no significant effects of time on α1d ADR mRNA levels in the CA1 (F(5,41) = 1.304; p = 0.281) or CA3 (F(5,41) = 0.381; p = 0.859) regions of the hippocampus, the lateral nucleus of the amygdala (F(5,39) = 0.677; p = 0.643) or the reticular nucleus of the thalamus (F(5,41) = 0.455; p = 0.807; Table 1). Although there was a trend for a decrease in α1d ADR mRNA expression in the lateral amygdala, 1 to 72 hours after the end of stress (Table 1), an additional one-way ANOVA with “stress” as a factor also was not significant (F(1,43) = 2.945; p = 0.093).
Figure 1.
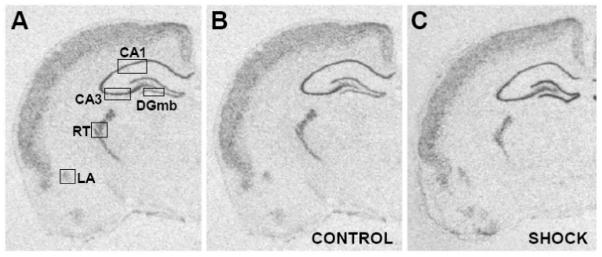
A) Representative section from a control animal showing the placement, size and shape of the templates used to analyze α1d ADR mRNA expression in the reticular thalamic nucleus (RT), lateral amygdala (LA), CA1 and CA3 regions of the hippocampus, and the medial blade (DGmb) of the dentate gyrus. B) Representative section to show α1d ADR mRNA expression in a home cage control rat (CONTROL) from Experiment 1. C) Representative section to show α1d ADR mRNA expression immediately after 100 inescapable tail shocks (SHOCK) representing the 0 hour time point in Experiment 1.
Figure 2.
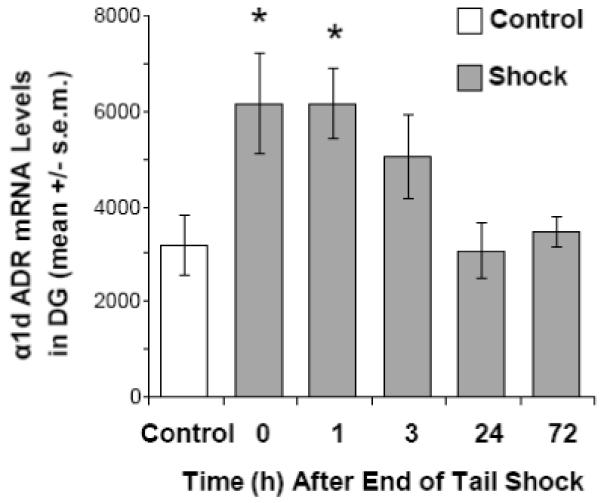
Experiment 1: the effect of inescapable tail shock (100, 1.5 mA, 5 sec. shocks, average inter-trial interval of 1 min.) on levels of α1d ADR mRNA levels in the dentate gyrus (DG). * p < 0.01 compared with control group.
Table 1.
Relative levels of α1d ADR mRNA expression in the CA1 and CA3 hippocampal regions, the reticular thalamic nucleus (RT) and the lateral amygdala (LA) after inescapable tail shock, restraint or loud noise (100 dBA) stress. Note that because α1d ADR mRNA expression in response to the three stressors was analyzed in three separate experiments, levels between stressors cannot be compared directly. Times represent the time after the start of stressor exposure, with the exception of inescapable tail shock, where values reflect the time after the end of the stressor. Inescapable tail shock exposure lasted approximately 100 min. There were no significant changes in α1d ADR mRNA expression in any of these brain regions after any of the stressors
| Time (h) | CA1 | CA3 | RT | LA |
|---|---|---|---|---|
| Experiment 1: Inescapable Tail Shock | ||||
| Control | 6997 ± 811 | 8210 ± 708 | 5399 ± 663 | 1203 ± 287 |
| 0 | 7361 ± 1091 | 7784 ± 954 | 5090 ± 1160 | 945 ± 283 |
| 1 | 7967 ± 1039 | 7961 ± 767 | 4542 ± 740 | 728 ± 170 |
| 3 | 9071 ± 946 | 9035 ± 1111 | 4873 ± 681 | 752 ± 169 |
| 24 | 5931 ± 870 | 7520 ± 762 | 3963 ± 528 | 873 ± 203 |
| 72 | 6888 ± 305 | 7880 ± 356 | 4897 ± 594 | 746 ± 169 |
| Experiment 2: Restraint Stress | ||||
| 0 | 4128 ± 1022 | 5422 ± 1287 | 2185 ± 665 | 707 ± 303 |
| 1.5 | 4327 ± 858 | 5073 ± 1279 | 2263 ± 953 | 552 ± 358 |
| 3 | 3455 ± 925 | 4258 ± 1048 | 1456 ± 347 | 283 ± 85 |
| 6 | 3578 ± 823 | 3960 ± 725 | 1420 ± 566 | 395 ± 214 |
| 24 | 3987 ± 1475 | 4700 ± 1220 | 2148 ± 1143 | 704 ± 454 |
| Experiment 3: Noise Stress (100 dBA) | ||||
| 0 | 4229 ± 428 | 6065 ± 549 | 2606 ± 504 | 839 ± 192 |
| 0.5 | 3430 ± 359 | 5294 ± 400 | 2430 ± 395 | 854 ± 171 |
| 1.5 | 4219 ± 291 | 6304 ± 656 | 2945 ± 620 | 542 ± 106 |
| 3 | 4703 ± 414 | 6315 ± 489 | 2817 ± 457 | 603 ± 125 |
Experiment 2: Restraint stress time course
One-way ANOVA with “time” as a factor initially suggested that there were no significant differences in α1d ADR mRNA in the medial blade of the dentate gyrus (F(3,23) = 2.929; p = 0.055). However, a visual inspection of the data (shown in Figure 3) suggested a relatively rapid time course, similar to that observed in Experiment 1. Further polynomial contrast analysis revealed a significant quadratic component for stressed animals for α1d ADR mRNA expression in the dentate gyrus (p = 0.019). Post-hoc analysis revealed a significant increase in α1d ADR mRNA in the dentate gyrus 90 min after the start of restraint, relative to the control group (p = 0.036). Levels of α1d ADR mRNA in the dentate gyrus returned to control values by 3 hours. One-way ANOVA with “time” as a factor did not reveal any significant effects on α1d ADR mRNA expression in the CA1 (F(3,23) = 0.356; p = 0.918) or CA3 (F(3,23) = 0.219; p = 0.958) regions of the hippocampus, the lateral nucleus of the amygdala (F(3,23) = 0.319; p = 0.922) or the reticular nucleus of the thalamus (F(3,23) = 0.323; p = 0.929; Table 1). Similarly, polynomial contrast did not reveal any significant effects on α1d ADR mRNA expression in any of these brain regions.
Figure 3.
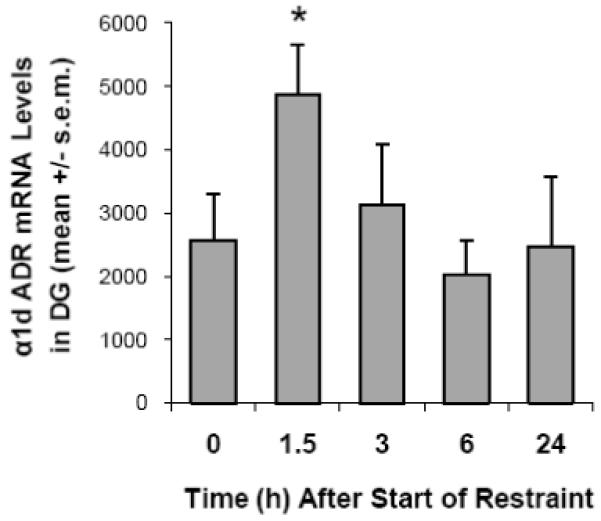
Experiment 2: the effect of 90 min. restraint on levels of α1d ADR mRNA levels in the dentate gyrus (DG). * p < 0.05 compared with control (0 min.) group.
Experiment 3: Noise stress time course
Data from nal̈ve rats and those experiencing background noise conditions were not significantly different from each other for corticosterone or α1d ADR mRNA expression in any brain region, and were pooled. The pooled data are referred to as “0 h time point”. As expected, exposure to 100 dBA noise resulted in a significant increase in corticosterone release over time (One-way ANOVA: F(3,21) = 14.791; p < 0.001). The plasma level of corticosterone peaked 30 min. after the start of stress (p < 0.001 compared with 0 h time point), and by 1.5 h was not significantly different from baseline (p = 0.562). Corticosterone values were: 0 h: 1.8 ± 0.8 μg/dl; 0.5 h: 17.8 ± 4.5 μg/dl; 1.5 h: 3.3 ± 0.8 μg/dl; 3 h: 1.8 ± 0.8 μg/dl. One way ANOVA with “time” as a factor, revealed a significant increase in α1d ADR mRNA in the dentate gyrus (Figure 4; F(3,21) = 5.579; p = 0.006) after exposure to 100 dBA noise for 30 minutes. Expression peaked 1.5 hours after the start of the stress, with levels of α1d ADR mRNA significantly higher at this time point compared to all others studied (p = 0.002 with respect to 0 h time point; p = 0.001 with respect to 0.5 h time point; p = 0.018 with respect to 3 h time point). There were no significant effects of time on α1d ADR mRNA levels in the CA1 (F(3,21) = 1.216; p = 0.328) or CA3 (F(3,21) = 0.559; p = 0.648) regions of the hippocampus, the lateral nucleus of the amygdala (F(3,20) = 1.232; p = 0.324) or the reticular nucleus of the thalamus (F(3,21) = 0.151; p = 0.928; Table 1).
Figure 4.
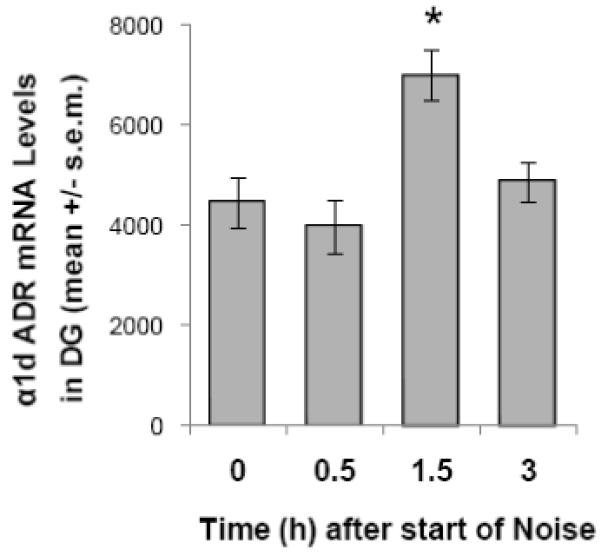
Experiment 3: the effect of 30 min. noise (100 dBA) on levels of α1d ADR mRNA levels in the dentate gyrus (DG). * p < 0.01 compared with control (0 min.) group.
Experiment 4: Effect of voluntary wheel running on restraint stress induced increase in α1d ADR mRNA expression in the hippocampus
Repeated measures ANOVA revealed a significant effect of time (F(6,192) = 465.3; p < 0.001), and a time by group (SED or RUN) interaction (F(6,192) = 13.352; p = 0.001), within subjects, on body weight. There was a significant difference in body weight between SED and RUN rats over time (F(1,32) = 11.674; p = 0.002). Initially, body weight did not differ between the 2 groups (p = 0.782). By the end of the first week of running wheel access, RUN rats weighed significantly less than SED rats (p < 0.001), and this difference persisted through the 6 weeks of the experiment. Body weights for SED and RUN rats are shown in Table 2. There was also a significant decrease in the thymus to body weight ratio in RUN compared to SED rats (F(1,32) = 16.38; p < 0.001; SED: 1.09 ± 0.03 g/kg; RUN: 0.89 ± 0.04 g/kg). There was a trend towards an increase in adrenal to body weight ratio in RUN compared to SED rats, but this was not significant (F(1,32) = 3.21; p = 0.08; SED: 150.1 ± 6.4 mg/kg; RUN: 165.3 ± 5.5 mg/kg). There was a significant effect of time on the average distance run per rat per 24 hour period (F(5,75) = 26.948; p < 0.001), with the distance run increasing over the course of the experiment, peaking during weeks 5 to 6 (Table 2).
Table 2.
Body Weights and Distances Run for rats from Experiment 4: Effect of voluntary wheel running on restraint stress induced increase in α1d mRNA expression in the hippocampus. SED: rats housed under sedentary conditions for the duration of the experiment (n = 18). RUN: rats housed with constant access to a running wheel for 6 weeks (n = 16). Weights for week 0 were taken immediately before the RUN rats started access to the running wheel. Weeks 1-6 refer to the weeks of running wheel access. Distance run represents the mean distance run per rat per 24 hour period for each week. All values are expressed as mean ± 1 s.e.m.
| Week | SED: Body Weight (g) | RUN: Body Weight (g) | RUN: Distance run (km) |
|---|---|---|---|
| 0 | 231.9 ± 2.3 | 232.9 ± 1.9 | - |
| 1 | 268.3 ± 3.7 | 249.4 ± 2.8* | 1.2 ± 0.1 |
| 2 | 292.4 ± 5.3 | 269.3 ± 3.9* | 2.9 ± 0.4 |
| 3 | 317.3 ± 6.6 | 289.7 ± 4.3* | 4.3 ± 0.6 |
| 4 | 328.4 ± 7.3 | 296.0 ± 4.9* | 5.1 ± 0.7 |
| 5 | 341.1 ± 8.1 | 306.7 ± 5.6* | 5.3 ± 0.7 |
| 6 | 359.0 ± 8.7 | 322.3 ± 5.6* | 5.3 ± 0.8 |
p < 0.001 with respect to the respective SED group. There was also a significant effect of time on the average distance run per rat per 24 hour period (p < 0.001).
One-way ANOVA revealed a significant effect of time on ACTH (F(2,48) = 56.96; p < 0.001) and corticosterone (F(2,48) = 27.70; p < 0.001) release in response to 90 min. restraint stress (Table 3). Post-hoc comparisons by group (LSD) revealed that levels of both ACTH and corticosterone were significantly elevated at both the 30 and 90 min. time points, for both SED and RUN rats, compared to respective baseline groups (p < 0.001 for all comparisons except corticosterone for Run 90 group, p < 0.05). Although there was a trend for decreased ACTH and corticosterone responses to restraint in RUN compared to SED rats, there was no significant effect of exercise on either plasma ACTH (F(1,49) = 0.911; p = 0.344) or corticosterone (F(1,49) = 2.997; p = 0.09). There was no effect of exercise on baseline levels of either ACTH (p = 0.976) or corticosterone (p = 0.919).
Table 3.
Plasma levels of corticosterone and ACTH in response to 90 min. restraint stress. SED: rats housed under sedentary conditions for the duration of the experiment (n = 18). RUN: rats housed with constant access to a running wheel for 6 weeks (n = 16). Values represent the group mean ± 1 s.e.m.
| Exercise | Time (min.) | ACTH (pg/ml) | Corticosterone (μg/dl) |
|---|---|---|---|
| SED | 0 | 36 ± 2.3 | 1.5 ± 0.5 |
| SED | 30 | 323 ± 43.7† | 20.8 ± 1.9† |
| SED | 90 | 101 ± 20.6† | 13.1 ± 2.4† |
| RUN | 0 | 37 ± 2.3 | 1.8 ± 1.1 |
| RUN | 30 | 258 ± 34.9† | 13.4 ± 2.2† |
| RUN | 90 | 56 ± 11.0† | 7.6 ± 2.8* |
p < 0.05
P < 0.01 with respect to appropriate baseline (time = 0 min.) group.
One-way ANOVA revealed a significant effect of stress on α1d ADR mRNA expression in the dentate gyrus (F(1,30) = 25.056; p < 0.001; Figure 5A). There was also a small but significant effect of exercise on α1d ADR mRNA expression in the dentate gyrus, with slightly greater expression in RUN compared with SED rats (F(1,30) = 4.441, p = 0.044; Figure 5A). However, there was no exercise by stress interaction (F(1,30) = 0.110; p = 0.742). In order to determine if this exercise paradigm did have some effect on stress-induced changes in the dentate gyrus, as has previously been reported, expression of BDNF mRNA in this region was also analyzed. Several groups have reported that BDNF mRNA expression increases in the dentate gyrus following voluntary wheel running, and decreases after acute stressor exposure (Smith et al., 1995; Russo-Neustadt et al., 2000; Cotman et al., 2007), and an interaction between stress and exercise on BDNF mRNA expression in the dentate gyrus has previously been reported by Greenwood and colleagues (Greenwood et al., 2007). One-way ANOVA revealed a significant effect of restraint stress on BDNF mRNA expression in the dentate gyrus (F(1,30) = 15.710; p < 0.001), with expression diminished in stressed compared with non-stressed rats (Figure 5B). There was also a significant effect of exercise, with RUN rats expressing greater levels of BDNF mRNA in the dentate gyrus (F(1,30) = 30.115, p < 0.001). In addition, there was a significant interaction between exercise and stress (F(1,30) = 5.890; p = 0.021).
Figure 5.
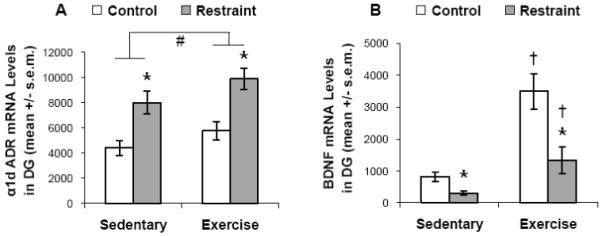
Experiment 4: the effect of 6 weeks of prior wheel running (exercise) on the restraint stress (90 min.) induced expression of α1d ADR or BDNF mRNA levels in the dentate gyrus (DG). # p < 0.05 (main effect of exercise); * p < 0.05 with respect to relevant control group; † p < 0.05 with respect to relevant sedentary group.
Discussion
The data reported here provide additional evidence for a close relationship between stress and the noradrenergic system in the hippocampus, specifically in the dentate gyrus. A rapid and transient increase in α1d ADR mRNA expression was observed in the rat dentate gyrus after stressor exposure (inescapable tail shock, restraint or loud noise stress), which was not influenced by exercise history (6 weeks of prior wheel running). The increase appeared to be limited to the dentate gyrus, with no significant changes observed in the CA1 or CA3 regions of the hippocampus, reticular nucleus of the thalamus or lateral nucleus of the amygdala.
The time course of the stress-induced increase in α1d ADR mRNA in the dentate gyrus was surprisingly rapid, with expression peaking at around 90 minutes after the start of stress. This increase was transient, with expression declining by 3 hours after the start of stressor exposure. The mechanism for this regulation is not known at present. However, both MR (or type I corticosteroid receptor) and GR (or type II corticosteroid receptor) are highly expressed in the hippocampal formation (Reul and de Kloet, 1985; Herman et al., 1989), making this a region that is highly susceptible to changes in circulating glucocorticoids (McEwen, 2007). We have previously reported that expression of α1d ADR mRNA in the hippocampal formation is dependent on circulating levels of corticosterone, with adrenalectomy resulting in a relatively quick and profound decrease in α1d ADR mRNA expression in all hippocampal subfields and the dentate gyrus, that was restored with corticosterone replacement (Day et al., 2008). Whether the effect of corticosterone on α1d ADR mRNA is direct or indirect is not known. Despite the fact that the 5′-flanking region (1.6 kb) of the rat α1d ADR gene has been sequenced (Xin et al., 1999), to our knowledge, a functional glucocorticoid response element (GRE) sequence has not been demonstrated in this gene. Although plasma corticosterone was not analyzed in all experiments, we have previously demonstrated that inescapable tail shock, restraint and noise stress each elicit a robust corticosterone response (Fleshner et al., 1993; Campeau et al., 1997; Campeau and Watson, 1997; Campisi et al., 2003; Burow et al., 2005; Day et al., 2009), typically peaking around 30 minutes after the start of stressor exposure. This is consistent with the possibility that corticosterone could regulate expression of α1d ADR mRNA after stressor exposure. However, the stress-induced increase in α1d ADR mRNA was observed only in the dentate gyrus, whereas adrenalectomy resulted in a decrease in α1d ADR mRNA expression in the dentate gyrus and all hippocampal subfields, which was restored with corticosterone replacement (Day et al., 2008). A similar selectivity of effect has been observed following propagation of cortical spreading depression, with increased alpha 1 adrenergic receptor binding reported in the dentate gyrus, but not the CA1 or CA3 regions of the hippocampus (Haghir et al., 2009). One possibility is that the dentate gyrus may be more susceptible than other hippocampal regions to smaller or more transient fluctuations in corticosterone levels. Or, there may be counter-regulatory mechanisms in the other hippocampal regions to prevent the regulatory effects of corticosterone. Alternatively, other mechanisms, independent of corticosterone, could regulate α1d ADR mRNA expression in the dentate gyrus. Clearly additional experiments are required to begin to address these questions.
A history of voluntary exercise has been reported to counteract some effects of stress (Moraska and Fleshner, 2001; Fleshner, 2005; Dishman et al., 2006; Cotman et al., 2007; Greenwood and Fleshner, 2008). In particular, exercise increases neurogenesis in the dentate gyrus (van Praag et al., 1999) as well as expression of neurotrophic factors, such as nerve growth factor and BDNF in the dentate gyrus (Neeper et al., 1996; Russo-Neustadt et al., 2000). In the current study, access to a running wheel for 6 weeks led to a slight increase in α1d ADR mRNA in the dentate gyrus. Although voluntary wheel running is not considered to be a psychological stressor, it nonetheless results in increases in corticosterone release in the first few weeks of wheel running, although this effect appears to habituate (Fediuc et al., 2006). Although basal levels of corticosterone were not different between SED and RUN rats, the fact that there was significant thymus involution in RUN rats suggests that there was increased release of corticosterone in RUN rats over the time course of the experiment which may have contributed to the slight increase in basal levels of α1d ADR mRNA observed in the dentate gyrus of RUN rats. Despite the slight basal elevation of α1d ADR mRNA in the dentate gyrus, a similar increase in expression in response to restraint stress was observed in rats with prior access to the running wheel for 6 weeks, compared with rats housed under sedentary conditions, suggesting that a history of voluntary exercise may not buffer this particular effect of acute stress. Although there was no effect of exercise on the restraint stress induced increase in α1d ADR mRNA in the dentate gyrus, there was an interaction between exercise and stress on BDNF mRNA expression in the dentate gyrus, consistent with data reported previously by Greenwood and colleagues, using voluntary wheel running and inescapable tail shock stress (Greenwood et al., 2007). Also consistent with the literature, exercised rats had significantly increased expression of BDNF mRNA in the dentate gyrus, in contrast to the effect of stress to decrease expression of BDNF in this region (Smith et al., 1995; Russo-Neustadt et al., 2000; Cotman et al., 2007). In accordance with the literature, there were no significant differences between exercised and sedentary rats in the ACTH or corticosterone response to restraint stress (Droste et al., 2003; Droste et al., 2006). The fact that exercised and sedentary rats had similar plasma corticosterone and dentate gyrus α1d ADR mRNA levels in response to restraint stress is consistent with the idea that corticosterone could play a role in the regulation of α1d ADR mRNA.
It is not known at present if the stress-induced increase in α1d ADR mRNA expression in the dentate gyrus is translated into protein, and the functional relevance of any transient increase in α1D ADR in this area is not clear. However, although the increase in α1d ADR mRNA is transient after an acute stressor, the data from rats exposed to wheel running suggest that longer lasting changes are possible. It will be important to determine if chronic psychological stress may result in longer term changes in mRNA and protein expression, which may have a greater functional relevance. Of the three α1 ADRs that have been cloned, α1d ADR mRNA is the most highly expressed in the dentate gyrus (Day et al., 1997). Data specific for the α1D ADR is sparse, primarily because of the lack of specific ligands for the receptor. However, data from α1D ADR knockout mice suggest that this receptor is important in switching from basal locomotor behavior to an activated state, in response to either environmental modulation or amphetamine administration, although the specific role of hippocampal α1D ADR in these behaviors has not been demonstrated (Sadalge et al., 2003). Although the specific function of α1D ADR in the dentate gyrus is not known, the noradrenergic system has been implicated extensively in stress and anxiety type responses (Bremner et al., 1996; Morilak et al., 2005). In particular, stressor exposure results in a marked increase in noradrenaline in the hippocampus (Tanaka et al., 1983; Nisenbaum et al., 1991). The noradrenergic system has been implicated in glucocorticoid release with lesions of ascending noradrenergic pathways resulting in increased MR binding in the hippocampus and attenuated corticosterone responses to stress (Maccari et al., 1990b; Maccari et al., 1992). α1D ADR receptors are extensively colocalized with both MR and GR within the hippocampal formation (Williams et al., 1997) and activation of α1 ADR receptors leads to a down-regulation of both MRs and GRs (Kabbaj et al., 1995). Interestingly, it has been shown that chronic prazosin treatment, an α1 ADR receptor antagonist, differentially alters hippocampal MR and GR binding in “high responder” and “low responder” rats that have differential stress response profiles (Kabbaj et al., 2007). In addition, the hippocampus is recognized as an area important in memory formation, and both the noradrenergic system and stress have been shown to influence hippocampal dependent memory (Howland and Wang, 2008; de Quervain et al., 2009). Norepinephrine has been shown to enhance both calcium and potassium fluxes in the dentate gyrus during high-frequency stimulation of a type able to elicit long-term potentiation (LTP) (Stanton and Heinemann, 1986). More recently, α1 ADR activation has been associated with a novel form of long-term synaptic depression in rat hippocampus, which has been suggested to be involved in cognitive function (Scheiderer et al., 2004). In aged rats, an increase in α1 ADR binding in the hippocampus, including the dentate gyrus, has been associated with a decline in spatial memory function (Topic et al., 2007). Consistent with this idea, the density of noradrenergic innervation is reduced and abnormal noradrenergic axons are observed in humans with Alzheimer’s disease (Powers et al., 1988).
In summary, while the precise function of the α1D ADR is not known, these data provide further evidence for a close relationship between stress and the noradrenergic system in the hippocampal formation, with possible implications for cognitive and stress-related processes.
Experimental Procedure
Animals
All procedures described were approved by the University of Colorado, Boulder, Institutional Animal Care and Use Committee. Adult male Sprague Dawley rats (Harlan, Indianapolis, IN), 2 to 4 months old, were used. All animals were allowed to habituate to the housing conditions for at least 7 days prior to any experimental manipulation. Rats were group-housed, up to 5 per cage under conditions of constant temperature and humidity, on a 12 h light/dark cycle (lights on at 7:00 a.m.), with access to food and water ad libitum. At the end of each experiment, animals were killed by decapitation, and the brains removed and frozen in isopentane cooled to −20 to −30°C. Brains were stored at −80°C until processing for in situ hybridization for α1d ADR mRNA.
Experiment 1: Inescapable tail shock time course
Rats (N = 49) were randomly assigned either to be exposed to uncontrollable tail shock (n = 40) or to remain in their home cages (control; n = 9). Stressed rats were given 100 tail shocks (5 sec, 1.5 mA) on a 1 min variable-interval schedule while being restrained in Plexiglas tubes (23.4 cm long and 7.0 cm in diameter). Rats were stressed during their inactive (light) cycle, between 8 and 10 a.m. The tail shock protocol used in this experiment was based on a protocol known to produce learned helplessness (Greenwood et al., 2003). After stressor termination, rats were either killed immediately (Time = 0; n = 8) or returned to their home cages for 1, 3, 24 or 72 hours (n = 8 per group) before being killed as described above.
Experiment 2: Restraint stress time course
Rats (N = 34) were randomly assigned to restraint stress (n = 27) or home cage control (n = 7) groups. Animals in the restraint stress group were restrained for 90 min. in clear Plexiglas tubes (23.5 cm in length and 7 cm in diameter) with tails protruding. The size of the tube restricted movement in all directions, but did not interfere with breathing. Animals were either killed immediately after restraint (Time = 90 min.; n = 7) or were returned to their home cages, and killed either 3 h (n = 7), 6 h (n = 7) or 24 h (n = 6) after the start of restraint. All animals were killed on the same day, between 11:00 a.m. and 3:00 p.m. to limit changes due to diurnal variations.
Experiment 3: Noise stress time course
Rats (N = 25) were randomly assigned to noise stress (n = 15), background noise (n=5) or home cage control (n = 5) groups. Animals in the home cage control group remained group housed until they were killed. Rats in the noise stress and background noise groups were group housed initially, but then single-housed the night before noise exposure. They were transported to the behavioral suite within the same wing, and placed individually, within their home cages, in sound attenuation chambers overnight. A full description of the chambers and noise generation procedure has been published previously (Day et al., 2005). The following morning rats either remained under background noise conditions (approximately 60 dBA; n = 5) or were exposed to 100 dBA white noise for 30 min. Animals were killed 30 min., 90 min. or 3 h after the start of noise exposure (n = 5 per group). In this experiment, trunk blood was also collected into chilled vacutainers containing EDTA. Plasma aliquots were stored at −80°C before being analyzed for corticosterone. All animals were killed between 11:30 a.m. and 1:00 p.m. to reduce the possibility of changes due to diurnal variations.
Experiment 4: Effect of voluntary wheel running on restraint stress induced increase in a1d mRNA expression in the hippocampus
Rats (N = 34) were randomly assigned to sedentary (SED) or voluntary wheel run (RUN) groups. After 1 week of acclimation to the colony, and at 2 months of age, rats in the SED group (n = 18) were singly housed in regular polycarbonate cages (14 × 9.5 × 7.5”) for 6 weeks. Rats in the RUN group (n = 16) were housed in equivalent cages with access to a running wheel (Nalge Nunc Intl., Rochester, NY) for 6 weeks. Animals were weighed weekly and running distances tracked with Vital View Software (Mini-Mitter/Respironics, Bend, OR). Apart from the weekly weight check, and the changing of cages, animals were not handled. After 6 weeks, half of the rats in each of the SED (n = 9) and RUN (n = 8) groups were taken from their home cages (Control Group), killed, and trunk blood and brains collected as described in Experiment 3. The remaining SED (n = 9) and RUN (n = 8) rats were restrained for 90 min., as described in Experiment 2. A blood sample was taken from restrained rats 30 min. after the start of restraint, by puncturing the lateral tail vein with the corner of a clean razor blade. Blood (300 - 400 μl) was collected with a capillary tube and placed into tubes containing 20 μl EDTA, tetrasodium salt (20 mg/ml) on ice. The procedure lasted less than 3 minutes. Animals were killed 90 min. after the start of restraint and brains and trunk blood collected. In this experiment, brains were stored at −80°C before being processed for in situ hybridization for α1d ADR and BDNF mRNAs. Plasma was stored at −80°C before analysis of ACTH and corticosterone.
In situ Hybridization
Sections (10 μm) were cut on a cryostat through the hippocampus, and stored at −80°C until processing for in situ hybridization (Day et al., 1997). Briefly, cRNA probes to detect α1d mRNA, 531 mer, corresponding to nucleotides 262-793 in the coding region (cDNA courtesy of Dr. Dianne M. Perez, The Cleveland Clinic Foundation, Cleveland, OH), or pan-BDNF, 759 mer, spanning the common 3′ exon (IX) that encodes the mature BDNF protein (courtesy of Dr. James Herman, University of Cincinnati College of Medicine, Cincinnati, OH), were generated using standard transcription technology and labeled with 35S-UTP (GE Healthcare, Piscataway, NJ). Sections were fixed for 1 hour in 4% phosphate-buffered paraformaldehyde at room temperature. Following a series of washes in 2x standard saline citrate (SSC; 1x SSC = 150 mM sodium chloride and 15 mM sodium citrate), sections were incubated in 0.1 M triethanolamine with 0.25% acetic acid for 10 minutes. The tissue was rinsed in distilled water and dehydrated through a series of alcohols. Sections were incubated overnight at 55°C with diluted probe, in a 50% formamide hybridization buffer containing 10 mM dithiothreitol, to yield an approximate concentration of 2 × 106 c.p.m. per 70 μl. The following day sections were washed in 2x SSC and incubated in RNase A (200 μg/ml) at 37°C for 1 hour. Sections were then washed to a final stringency of 0.1x SSC at 65°C for 1 hour. Tissue was dehydrated through a series of alcohols and exposed to x-ray film (Kodak, Biomax-MR) for up to 1 week.
Semi quantitative mRNA Analysis
Levels of α1d ADR or BDNF mRNA were analyzed by computer assisted optical densitometry. Brain section images from in situ hybridization experiments were captured digitally (CCD camera, model XC-77, Sony, Tokyo, Japan), and analyzed using Scion Image version 4.0.3 for Windows (Scion Corporation). A macro was written (Dr. Serge Campeau) which enabled signal above background to be automatically determined as follows. For each section, a background sample was taken over an area of tissue expressing background levels of α1d ADR or BDNF mRNA, and the signal threshold calculated as mean gray value of background + 3.5 x standard deviation. The section was automatically density sliced at this value, so that only pixels with gray values exceeding these criteria were included in the analysis. The 3.5 standard deviations above the mean of background was chosen as a relatively stringent threshold criterion, which resulted in only a few pixels above threshold on the film background on areas of tissue previously shown to have undetectable α1d ADR mRNA expression, such as the habenula and parafascicular nucleus of the thalamus (Day et al., 1997). Despite the relatively stringent threshold conditions, the fact that a few pixels remained above threshold outside of the tissue section indicates that this method is suitable for detecting even relative low intensity mRNA expression. A template was used for each brain region, (CA1, CA3, dentate gyrus, reticular thalamic nucleus, lateral nucleus of the amygdala; Figure 1A), so that the same area was included in the analysis for each region. The number of pixels above background was multiplied by the signal above background, to give an integrated density value. This method has been shown to reflect both the number of cells expressing mRNA and the expression level per cell, as determined by cell and grain counts of emulsion dipped slides (Day et al., 2005). For each brain region, 5-6 sections were analyzed, left and right sides separately, and the mean of these values was calculated to give a single value for each animal.
ACTH Radioimmunoassay
Plasma (200 μl) was assayed for levels of ACTH using an Immunoradiometric Assay kit (Diasorin, Stillwater, MN). Briefly, the plasma was incubated overnight with a 125I-labeled monoclonal antibody specific for ACTH 1-17, a goat polyclonal antibody specific for ACTH 26-39, and a polystyrene bead coated with a mouse anti-goat antibody. Only ACTH 1-39 in the sample bound both antibodies to form an antibody complex. Beads were washed to remove unbound radioactivity, counted with a gamma counter, and the concentrations of ACTH determined by comparison to a standard curve generated concurrently. All samples from a particular experiment were run in the same assay.
Corticosterone ELISA
Levels of corticosterone in plasma were determined by a commercially available Enzyme Linked ImmunoSorbent Assay (ELISA) kit (Assay Designs, Ann Arbor, MI). Briefly, plasma or corticosterone standard was incubated in a 96 well plate coated with donkey anti-sheep antibody, together with a sheep polyclonal antibody specific for corticosterone, and corticosterone that was covalently attached to an alkaline phosphatase molecule. After a 2 hour incubation period, unbound reagents were washed from the plate and substrate added. After a 1 hour incubation period, the reaction was stopped and the intensity of the yellow color generated read with a microplate reader (Biotek EL808, Winooski, VT) at 405 nm. The concentration of corticosterone in the samples was calculated by comparison to a standard curve generated concurrently. Kit directions were followed except with an adaptation for using a smaller volume of plasma as follows. The steroid displacement reagent (provided with the kit) was added to the assay buffer at a concentration of 0.5 μl/ml. Plasma (typically 10 μl) was diluted 1:50 with the amended assay buffer. The diluted plasma sample (100 μl) was then processed as described in the kit directions. This method used equivalent final concentrations of steroid displacement reagent, and was determined to result in equivalent assayed corticosterone levels as the standard method, in which 2.5 μl steroid displacement reagent is added to 97.5 μl plasma, and the plasma diluted with standard assay buffer (data not shown). All samples from a particular experiment were run in the same assay.
Statistical Analysis
Data were analyzed by one-way (Experiments 1-3) or two-way (Experiment 4) analysis of variance (ANOVA) followed by Fisher’s LSD (Least Significant Difference) post-hoc multiple comparisons tests, as indicated in the text. Significance was set at p = 0.05.
Acknowledgements
These studies were supported by R01 MH-067988 (HD), a NARSAD Young Investigator Award (HD) and RO1 MH-068283 (MF). The authors would like to thank Dr. Robert Spencer (University of Colorado, Psychology and Neuroscience Department) for the use of his rat restrainers in Experiments 2 and 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Classification Terms:
40.016: Catecholamine receptors
50.001: Hypothalamic-pituitary-adrenal regulation
90.020: Stress
Section: Regulatory Systems
Literature Cited
- Abercrombie ED, Keller RW, Jr., Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: Pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Burow A, Day HE, Campeau S. A detailed characterization of loud noise stress: Intensity analysis of hypothalamo-pituitary-adrenocortical axis and brain activation. Brain Res. 2005;1062:63–73. doi: 10.1016/j.brainres.2005.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Akil H, Watson SJ. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mrna in the forebrain associated with audiogenic stress in rats. J. Neurosci. 1997;17:5979–5992. doi: 10.1523/JNEUROSCI.17-15-05979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Greenwood BN, Hansen MK, Moraska A, Higgins K, Smith TP, Fleshner M. Habitual physical activity facilitates stress-induced hsp72 induction in brain, peripheral, and immune tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R520–530. doi: 10.1152/ajpregu.00513.2002. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. Reversible inactivation of the auditory thalamus disrupts hpa axis habituation to repeated loud noise stress exposures. Brain Res. 2009;1276:123–130. doi: 10.1016/j.brainres.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr., Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mrna in the rat brain and spinal cord. J. Chem. Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur. J. Neurosci. 2005;21:441–454. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Kryskow EM, Watson SJ, Akil H, Campeau S. Regulation of hippocampal alpha1d adrenergic receptor mrna by corticosterone in adrenalectomized rats. Brain Res. 2008;1218:132–140. doi: 10.1016/j.brainres.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front. Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: Impact of concurrent treatment with the antidepressant drug tianeptine. J. Neuroendocrinol. 2006;18:915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on hpa axis responsiveness to restraint stress in sprague-dawley rats. J. Appl. Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Physical activity and stress resistance: Sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005;33:120–126. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Watkins LR, Lockwood LL, Grahn RE, Gerhardt G, Meaney MJ, Laudenslager ML, Maier SF. Blockade of the hypothalamic-pituitary-adrenal response to stress by intraventricular injection of dexamethasone: A method for studying the stress-induced peripheral effects of glucocorticoids. Psychoneuroendocrinology. 1993;18:251–263. doi: 10.1016/0306-4530(93)90022-d. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Med. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144:1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghir H, Kovac S, Speckmann EJ, Zilles K, Gorji A. Patterns of neurotransmitter receptor distributions following cortical spreading depression. Neuroscience. 2009;163:1340–1352. doi: 10.1016/j.neuroscience.2009.07.067. [DOI] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger rnas in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Howland JG, Wang YT. Synaptic plasticity in learning and memory: Stress effects in the hippocampus. Prog. Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- Joels M, Hesen W, de Kloet ER. Long-term control of neuronal excitability by corticosteroid hormones. J. Steroid Biochem. Mol Biol. 1995;53:315–323. doi: 10.1016/0960-0760(95)00069-c. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Morley-Fletcher S, Le Moal M, Maccari S. Individual differences in the effects of chronic prazosin hydrochloride treatment on hippocampal mineralocorticoid and glucocorticoid receptors. Eur J Neurosci. 2007;25:3312–3318. doi: 10.1111/j.1460-9568.2007.05585.x. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Piazza PV, Simon H, Le Moal M, Maccari S. Opposite effects on hippocampal corticosteroid receptors induced by stimulation of beta and alpha-1 noradrenergic receptors. Neuroscience. 1995;66:539–545. doi: 10.1016/0306-4522(94)00620-k. [DOI] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J. Comp. Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- Maccari S, Le Moal M, Angelucci L, Mormede P. Influence of 6-ohda lesion of central noradrenergic systems on corticosteroid receptors and neuroendocrine responses to stress. Brain Res. 1990a;533:60–65. doi: 10.1016/0006-8993(90)91795-i. [DOI] [PubMed] [Google Scholar]
- Maccari S, Le Moal M, Angelucci L, Mormede P. Influence of 6-ohda lesion of central noradrenergic systems on corticosteroid receptors and neuroendocrine responses to stress. Brain Res. 1990b;533:60–65. doi: 10.1016/0006-8993(90)91795-i. [DOI] [PubMed] [Google Scholar]
- Maccari S, Mormede P, Piazza PV, Simon H, Angelucci L, Le Moal M. Hippocampal type i and type ii corticosteroid receptors are modulated by central noradrenergic systems. Psychoneuroendocrinology. 1992;17:103–112. doi: 10.1016/0306-4530(92)90049-d. [DOI] [PubMed] [Google Scholar]
- McCune S, Voigt M, Hill J. Expression of multiple alpha adrenergic receptor subtype mrnas in the adult rat brain. Neuroscience. 1993;57:143–151. doi: 10.1016/0306-4522(93)90116-w. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-klh antibody suppression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R484–489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mrna for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone V, Nicholas A, Dagerlind A, Hokfelt T. Distribution of a-1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J. Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RE, Struble RG, Casanova MF, O’Connor DT, Kitt CA, Price DL. Innervation of human hippocampus by noradrenergic systems: Normal anatomy and structural abnormalities in aging and in alzheimer’s disease. Neuroscience. 1988;25:401–417. doi: 10.1016/0306-4522(88)90248-5. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: Correlation with stress-induced norepinephrine efflux in the hippocampus of sprague-dawley rats. Brain Res. Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Rouppe van der Voort C, Kavelaars A, van de Pol M, Heijnen CJ. Neuroendocrine mediators up-regulate alpha1b- and alpha1d-adrenergic receptor subtypes in human monocytes. J. Neuroimmunol. 1999;95:165–173. doi: 10.1016/s0165-5728(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sadalge A, Coughlin L, Fu H, Wang B, Valladares O, Valentino R, Blendy JA. Alpha 1d adrenoceptor signaling is required for stimulus induced locomotor activity. Mol Psychiatry. 2003;8:664–672. doi: 10.1038/sj.mp.4001351. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mrnas in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Heinemann U. Norepinephrine enhances stimulus-evoked calcium and potassium concentration changes in dentate granule cell layer. Neurosci. Lett. 1986;67:233–238. doi: 10.1016/0304-3940(86)90314-9. [DOI] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Alpha1-adrenoreceptor in human hippocampus: Binding and receptor subtype mrna expression. Mol. Brain Res. 2005;139:367–371. doi: 10.1016/j.molbrainres.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kohno Y, Nakagawa R, Ida Y, Takeda S, Nagasaki N, Noda Y. Regional characteristics of stress-induced increases in brain noradrenaline release in rats. Pharmacol Biochem Behav. 1983;19:543–547. doi: 10.1016/0091-3057(83)90132-6. [DOI] [PubMed] [Google Scholar]
- Topic B, Willuhn I, Palomero-Gallagher N, Zilles K, Huston JP, Hasenohrl RU. Impaired maze performance in aged rats is accompanied by increased density of nmda, 5-ht1a, and alpha-adrenoceptor binding in hippocampus. Hippocampus. 2007;17:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Williams A, Nguyen M, Morilak D. Co-localization of alpha-1d adrenergic receptor mrna with mineralocorticoid and glucocorticoid receptor mrna in hippocampus. J. Neuroendocrinol. 1997;9:113–119. doi: 10.1046/j.1365-2826.1997.00522.x. [DOI] [PubMed] [Google Scholar]
- Xin X, Yang N, Faber JE. Platelet-derived growth factor-bb inhibits rat alpha1d-adrenergic receptor gene expression in vascular smooth muscle cells by inducing ap-2-like protein binding to alpha1d proximal promoter region. Mol Pharmacol. 1999;56:1152–1161. doi: 10.1124/mol.56.6.1152. [DOI] [PubMed] [Google Scholar]


