Abstract
Objective
To assess ovarian follicle function in women with 46,XX spontaneous primary ovarian insufficiency
Design
Case-control with nested prospective cohort
Setting
Clinical Research Center, National Institutes of Health
Patients
Women with primary ovarian insufficiency without estrogen replacement for two weeks (N=97) and regularly menstruating control women (N=42)
Interventions
Single injection of 300 IU hrFSH
Main outcome measures
Change in serum estradiol at 24 hours
Results
Antral follicles ≥ 3 mm were detected in 73% (69/95) of patients; both serum estradiol and progesterone levels correlated significantly with maximum follicle diameter in these women. Patients with a maximum follicle diameter ≥ 8 mm had significantly higher serum estradiol and progesterone levels and significantly lower FSH and LH levels as compared to patients without such follicles. In controls estradiol levels increased significantly after FSH administration but in patients this was not the case despite the presence of an antral follicle ≥ 8 mm.
Conclusion
Most women with 46,XX spontaneous primary ovarian insufficiency have antral follicles detectable by ultrasound, suggesting that down-regulation of FSH receptors is not the predominant mechanism of follicle dysfunction. Evidence of progesterone secretion by antral follicles ≥ 8 mm in these patients is consistent with prior histologic evidence that follicle luteinization is the predominant mechanism of follicle dysfunction in this condition. Prospective controlled investigation designed to improve ovulatory function and fertility in these women is indicated.
Keywords: Primary ovarian insufficiency, hypergonadotropic hypogonadism, premature ovarian failure, premature menopause, lutienized Graafian follicle
Introduction
Menopause, defined as the permanent cessation of menses and irreversible termination of fertility, develops due to a depletion of potentially functional primordial follicles (1). Normal ovarian function depends on the presence of functional primordial follicles. The ovary is unique in the endocrine system in that it develops an entirely new secretory structure each month. This secretory structure, the Graafian follicle, arises from a primordial follicle. The maximum number of germ cells in the ovary peaks at about 7 million during week 20 of gestation. At birth the follicle count is approximately 2 million and at puberty approximately 400,000 (2).
In 1942 Fuller Albright et al. first reported a syndrome in young women that was characterized by amenorrhea and estrogen deficiency in association with menopausal FSH levels (3). He termed the condition “primary ovarian insufficiency” to make clear that it was ovarian function that was the primary defect rather than failure of FSH secretion by the pituitary (secondary ovarian insufficiency). Some women with this condition conceive years after the diagnosis, and for this reason the term “primary ovarian insufficiency” is more accurate, and may be less stigmatizing than the terms “premature menopause” and “premature ovarian failure” (4–6).
In 1982 Coulam reported that primordial follicles were found in ovarian biopsies in only 5 of 81 patients (6%) with 46,XX primary ovarian insufficiency (7). However, many women with primary ovarian insufficiency are known to experience intermittent and unpredictable ovarian function (8–12). In fact, over a 4 month period of serial sampling 50% of women with 46,XX spontaneous primary ovarian insufficiency had evidence of ovarian follicle function as defined by a serum estradiol level greater than 50 pg/ml (10). However, only four percent of women ovulated per month. We have previously demonstrated by ovarian biopsy and histologic examination that women with 46,XX spontaneous primary ovarian insufficiency frequently develop lutienized graafian follicles (10). Insight into the mechanisms that prevent most of these follicles from becoming ovulatory might lead to development of treatments to restore fertility.
The a priori aim of this study was to characterize the Graafian follicle function in response to FSH stimulation in women with 46,XX spontaneous primary ovarian insufficiency. Normal human follicles of 8mm in diameter or greater express aromatase and should secrete estradiol in response to FSH stimulation (13,14). We tested the hypothesis that women with primary ovarian insufficiency who have a follicle equal to or greater than 8 mm in diameter will respond to FSH stimulation by significantly increasing serum estradiol levels.
Material and Methods
The design of the study was case-control with a nested prospective cohort. From August 2000 to December 2005, we recruited women with 46, XX spontaneous primary ovarian insufficiency and control women. Patients were recruited by letters to physicians, notices in medical journals, and through the Internet. Controls were recruited by local advertisement. The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD) approved the study. Participants were compensated according to NIH guidelines. Guidelines for remuneration of research subjects in the NIH Intramural Research Program and registration in the clinical research volunteer program database are available online (15).
Women with primary ovarian insufficiency
To be eligible for the study, patients had to meet the following inclusion criteria: 1) diagnosis of spontaneous 46,XX primary ovarian insufficiency before the age of 40 years (i.e., at least 4 months of oligomenorrhea or amenorrhea and two FSH levels in the menopausal range, as defined by the local laboratory, confirmed on two separate occasions, at least 1 month apart); 2) current age between 18 and 42; 3) no iatrogenic cause of primary ovarian insufficiency or known chromosomal abnormality.
Controls
Control women were healthy, had a BMI ranging from 17 to 27, had a previous pregnancy, were not pregnant at present, and were regularly menstruating (cycles between 21 and 35 days). They reportedly did not smoke more than two cigarettes per day nor abuse alcohol (<two drinks a day). They were taking no chronic medications, were not using hormonal contraception, and were not exercising more than 10 hours a week. The control women had a serum progesterone level exceeding 6 ng/ml in the menstrual cycle just before the study cycle.
Study Protocol
All participants denied any history of hypersensitivity to recombinant FSH. All signed a written informed consent. Baseline serum levels were obtained for FSH, LH, estradiol, and progesterone. We performed gray-scale and color Doppler transvaginal ultrasound by using a multifrequency transducer (5–8 MHz) on a Sequoia scanner (Acuson, Mountain View, CA) as reported previously (16). Scans were performed by two examiners (DJ and EM). We defined antral follicles as anechoic structures at least 3 mm in diameter that demonstrated no color flow. The baseline measurements were obtained on the third day of the menstrual cycle in the control group and after 2 weeks off hormone replacement therapy in women with primary ovarian insufficiency.
After baseline sampling we administered a single intramuscular dose of 300 IU of recombinant human FSH (Organon, West Orange, NJ). This dose was chosen since it has been used in prior studies to evaluate ovarian function (16,17). Repeat serum sampling was performed 24 hours later.
The measurements of estradiol, FSH and LH levels were performed at the NIH Clinical Center. Serum FSH and LH were analyzed by microparticle enzyme immunoassay (MEIA; Abbott Laboratories Abbott Park, IL). FSH intra-assay CV was 4.9% and the inter-assay CV was 6.5%; for LH these were 5.8% and 6.4%, respectively. Estradiol and progesterone were measured by competitive chemiluminescence immunoassay (Immulite 2000 analyzer, Diagnostic Products Corporation, Los Angeles, CA); intra-assay and inter-assay CV were <11.0% and <10% respectively.
Statistical Analysis
We used the signed rank test and rank sum test for paired and unpaired comparisons of populations. Correlation was assessed using the Spearman rank correlation. To compare proportions in two populations we use Fisher’s exact test. To account for the differences in age, age of onset of menstrual irregularity, age at diagnosis, and time since diagnosis of primary ovarian insufficiency on the comparison of hormone levels between the subgroups of patients defined by maximal follicle size, we used multivariate linear regression. First we estimated an optimal Box-Cox transformation for each hormone and then fit a multivariate regression to the transformed hormone values (18). This allowed us to test the adjusted differences by using the Wald p-value for the term in the multivariate regression indicating whether or not the largest follicle was ≥8mm. All analyses used SAS version 9 (SAS, Cary, NC). Statistical significance was set at the two-tailed level of P < 0.05.
Results
We recruited a total of 97 women with 46,XX spontaneous primary ovarian insufficiency and 42 healthy control women with regular menstrual cycles. Table 1 summarizes the baseline characteristics of the participants. Women with primary ovarian insufficiency were significantly younger than controls. There were no differences in BMI, age of menarche, or racial/ethnic distribution. Only 3.1% (3/97) of women with spontaneous primary ovarian insufficiency had primary amenorrhea.
Table 1.
Baseline clinical characteristics of control women (N=42) and women with primary ovarian insufficiency (POI) (N=97)
| Characteristic | Control Median (IQR) or N(%) | POI Median (IQR) or N(%) | P value |
|---|---|---|---|
| Age | 37.0 (31.0, 42.0) | 34.0 (28.0, 36.0) | 0.003 |
| BMI | 22.7 (21.0, 24.7) | 23.5 (20.9, 26.0) | 0.158 |
| Age at Menarche | 13.0 (12.0, 14.0) | 13.0 (12.0, 14.0) | 0.335 |
| Race % | 0.119 | ||
| Asian | 6 (14) | 6 (6) | |
| African American | 7 (17) | 11 (11) | |
| Hispanic | 3 (7) | 3 (3) | |
| Caucasian | 26 (62) | 77 (79) | |
| FSH (IU/L) | 8.0 (7.0, 11.0) | 81.0 (51.0, 111.0) | <0.001 |
| LH (IU/L) | ND | 49.0 (35.0, 68.0) | -------- |
| Estradiol (pg/mL) | 36.3 (28.0, 57.0) | 27.0 (20.0, 40.4) | 0.019 |
| Progesterone (ng/mL) | ND | 0.5 (0.3, 0.7) | -------- |
| Endometrial stripe (mm) | 4.0 (3.0, 6.0) | 2.0 (1.0, 3.0) | <0.001 |
| Right ovarian volume (cm3) | 6.9 (5.8, 8.9) | 1.4 (0.7, 2.6) | <0.001 |
| Left ovarian volume (cm3) | 6.6 (4.4, 8.7) | 1.4 (0.5, 2.4) | <0.001 |
| Largest follicle (mm) | 9.0 (8.0, 11.0) | 6.6 (2.3, 12.8) | 0.003 |
| Age at onset of menstrual irregularity | N/A | 28.4 (18.2, 32.9) | -------- |
| Age at POI diagnosis | N/A | 31.9 (25.3, 34.2) | -------- |
| Time since POI diagnosis (months) | N/A | 20.3 (5.8, 50.6) | -------- |
IQR = Interquartile range, ND = Not done, NA = Not applicable
All control women and 95 women (out of 97) with spontaneous primary ovarian insufficiency had a sonographic evaluation (2 patients did not have the ultrasound). As shown in Table 1, as expected, at baseline evaluation women with primary ovarian insufficiency had a higher serum FSH and LH and significantly higher serum estradiol levels as compared to controls. Women with spontaneous primary ovarian insufficiency had a significantly thinner endometrial stripe, a smaller total ovarian volume, and a significantly smaller largest follicle diameter.
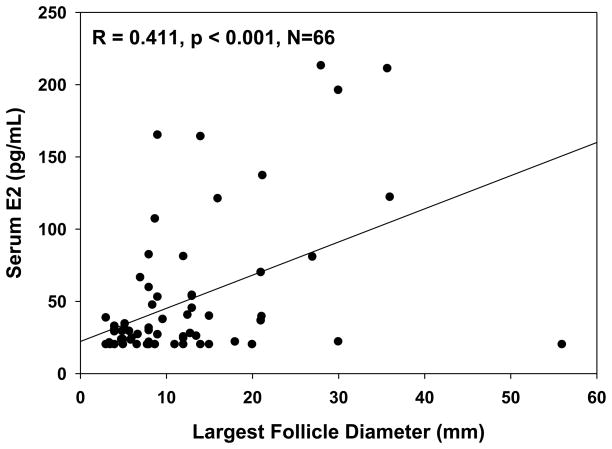
An antral follicle ≥ 3 mm was detected in 73% (69 out of 95) of women with 46,XX spontaneous primary ovarian insufficiency. In these women there was a significant correlation between follicle diameter and serum estradiol level (Figure 1) (N=66, as serum estradiol levels were missing in 3 women). An antral follicle ≥ 8 mm was detected in 45% (43 out of 95). Basal serum estradiol levels were significantly greater in patients who had an antral follicle ≥ 8 mm as compared to women who did not have such follicles; the same was true for serum progesterone levels (Table 2). Also, in those patients who had an antral follicle ≥ 3mm serum progesterone levels correlated significantly with maximum follicle diameter (r=0.26, p=0.035).
Figure 1.
Correlation between serum estradiol level and size of largest antral follicle detected by transvaginal ultrasound in women with primary ovarian insufficiency who had a follicle of at least 3 mm in diameter.
Table 2.
Baseline comparison of women with primary ovarian insufficiency segregated by the absence (N=52) or presence (N=43) of a follicle of 8mm in diameter or greater and after statistical adjustment of hormone values for age, age of onset of menstrual irregularity, age at diagnosis, and time since diagnosis.
| Factor | <8mm follicle Median (IQR) or N (%) | ≥8 mm follicle Median (IQR) or N (%) | P value | Adjusted P value |
|---|---|---|---|---|
| Age | 31.5 (26.0,36.0) | 34.0 (32.0,36.0) | 0.063 | |
| BMI | 24.4 (21.5,27.9) | 22.6 (20.6, 25.4) | 0.107 | |
| Age at menarche | 13.0 (12.0, 13.0) | 13.0 (12.0, 14.0) | 0.976 | |
| Age at onset of menstrual irregularity | 26.0 (16.0, 31.0) | 31.5 (25.2, 33.3) | 0.009 | |
| Age at POI diagnosis | 29.0 (21.5, 33.4) | 33.5 (30.8, 34.7) | 0.002 | |
| Time since POI diagnosis (months) | 27.4 (6.4, 74.9) | 15.2 (4.8, 30.1) | 0.029 | |
| Serum estradiol (pg/mL) | 23.0 (20.0, 32.5) | 39.6 (21.9, 80.9) | <0.001 | 0.002 |
| Serum progesterone (ng/mL) | 0.5 (0.2, 0.6) | 0.6 (0.5, 0.8) | 0.002 | 0.004 |
| FSH (IU/L) | 93.0 (70.0, 115.0) | 57.0 (33.0, 106.0) | 0.001 | 0.002 |
| LH (IU/L) | 56.0 (42.0, 68.0) | 37.5 (23.0, 68.0) | 0.019 | 0.013 |
| History of smoking | 3 (6) | 5 (12) | 0.461 | |
| History of primary ovarian insufficiency in family | 9 (17) | 12 (28) | 0.227 | |
| Adrenal autoantibodies | 0 (0) | 3 (7) | 0.089 | |
| History of thyroid disease | 7 (14) | 7 (16) | 0.776 | |
| Race | 0.424 | |||
| Asian | 3 (6) | 3 (7) | ||
| African American | 7 (13) | 4 (9) | ||
| Hispanic | 3(6) | 0 (0) | ||
| Caucasian | 39 (75) | 36 (84) |
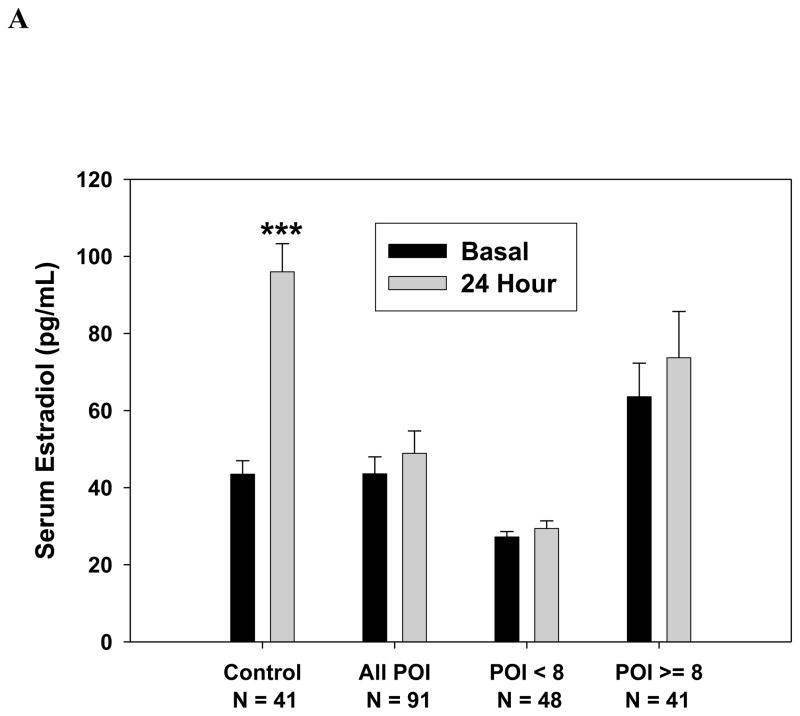
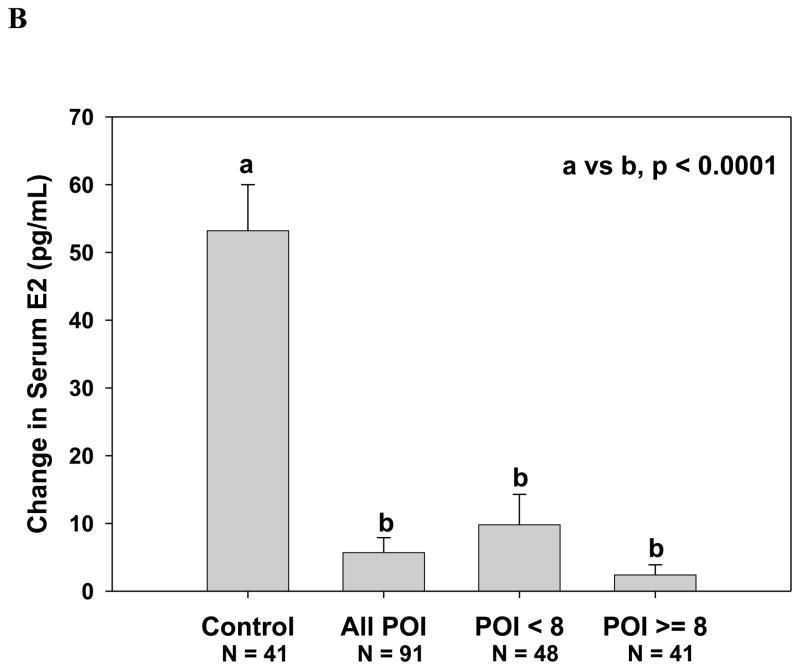
As shown in Figure 2A, among 41 women with primary ovarian insufficiency who had a follicle ≥ 8 mm on sonogram and had serum estradiol measurement results available, the serum estradiol did not increase significantly in response to FSH stimulation [median (interquartile range, IQR) IU/L, 39.6 ( 21.9, 80.9) to 39.8 (20.0, 88.8)) pg/ml, P=0.190]. In contrast, serum estradiol levels of control women significantly increased in response to FSH stimulation from 36.3 (28.0, 57.0) to 94.1 (67.0, 118.0) pg/ml (p < 0.001). Change in serum estradiol levels by group are shown in Figure 2B. Controls had a significantly greater change in serum estradiol in response to FSH stimulation when compared to patients. The estradiol response did not differ significantly between those patients who had an antral follicle greater than 8 mm and those 48 who did not (p=0.95). When evaluating all women with spontaneous primary ovarian insufficiency, the estradiol level increased in response to FSH marginally from median (IQR) 27.0 (20.0, 40.4) to 28.4 (20.0, 43.8) pg/ml, a rise of borderline significance (P=0.052). Administration of FSH did not significantly change serum progesterone levels [median (IQR) ng/ml 0.5 (0.3, 0.7) before vs. 0.5 (0.3, 0.7) after, p=0.111].
Figure 2.
Serum estradiol response to stimulation with 300 IU FSH. (A) Control women, women with primary ovarian insufficiency as a group, and women with primary ovarian insufficiency segregated by the absence or presence of an antral follicle 8 mm in diameter or greater (***, p < 0.0001 vs. baseline); B. Change in serum estradiol levels at 24 hours.
As shown in Table 2, when patients were segregated by the presence or absence of a follicle 8 mm in diameter or greater, significant differences were evident between the two groups. Patients who had a follicle ≥ 8 mm were significantly older at the onset of menstrual abnormality, older at the time of diagnosis, and had a shorter time interval since diagnosis. Serum estradiol and progesterone levels were significantly greater and serum FSH and LH were significantly lower in women with larger follicles. After these hormone levels were adjusted for age, age of onset of menstrual irregularity, age at diagnosis, and time since diagnosis of primary ovarian insufficiency, the estimated differences remained statistically significant and in the same direction.
Discussion
As the pool of preantral follicles grow in response to endogenous FSH stimulation and then reach an antral follicle size of approximately 8 mm in diameter or more, FSH dependent P450-aromatase mRNA expression is significantly increased along with follicular production of estradiol (13,19–22). In this prospective study of ovarian follicle function in women with 46, XX spontaneous primary ovarian insufficiency, we found an antral follicle of 8 mm in diameter or greater in nearly one-half of patients. The data lead us to conclude that these are indeed follicles rather than non-functional cystic structures. Serum estradiol and progesterone levels are significantly higher and serum FSH and LH levels are significantly lower in those patients with antral follicles of this size. Furthermore, we found that serum estradiol levels are significantly positively correlated with follicle diameter, which also supports a conclusion that these structures are endocrinologically active. However, we show that these follicles respond poorly to FSH stimulation as assessed by increase in measured serum estradiol level in response to exogenous FSH.
The data lead us to conclude that some pathologic process is interfering with normal follicle function in these patients. Clearly spontaneous 46,XX primary ovarian insufficiency in most cases is not simply a situation of complete follicle depletion. As evidenced here, in many cases this is a state of follicle dysfunction. It has been hypothesized that elevated FSH levels cause down-regulation of FSH receptors in the ovaries of these patients (4,23,24). Down-regulation of plasma membrane receptors may result from ligand-induced receptor internalization, sequestration, and regulation of receptor mRNA levels (25). However, to our knowledge there is no direct evidence demonstrating reduced FSH receptor expression in the follicles of young women with 46,XX spontaneous primary ovarian insufficiency.
FSH action is unequivocally required to support follicular growth beyond the preantral antral stage. Our findings lead us to conclude that FSH has been acting on the FSH receptor by two lines of functional evidence: 1) follicles have grown beyond the preantral stage (which requires FSH action), and 2) the follicles are associated with higher estradiol levels (which requires FSH action to induce aromatase activity in granulosa cells). It seems reasonable to conclude that FSH has been acting on its receptor. The fact that we detect antral follicles in 73% of these patients and that we found a positive correlation between follicle diameter and serum estradiol level are strong evidence that follicles are responding to FSH action by growing and secreting steroids. Our data call into question the validity of the hypothesis that down-regulation of FSH receptors is a common mechanism of follicle dysfunction in women with 46,XX spontaneous primary ovarian insufficiency.
The requirement of FSH to stimulate follicle growth in humans was first demonstrated elegantly by Rabin et al., wherein their investigation of a 22 year old woman with isolated FSH deficiency showed no antral follicle development as assessed by laparoscopy and ovarian biopsy (26). Subsequent clinical studies have confirmed the need for FSH to support follicle development and estradiol production (27). This has also been confirmed in an animal model; the FSH knockout mouse fails to develop follicles beyond the preantral stage (28). Furthermore, ovarian biopsy in patients with a mutation in the FSH receptor has demonstrated the presence of primordial follicles in 9 of 9 patients, but only one of these patients had an antral follicle detected histologically (29). Based on the present data we conclude that preantral follicles in most women with 46,XX spontaneous primary ovarian insufficiency are indeed capable of responding to the abnormally high endogenous FSH stimulation with follicle growth and estradiol production.
Another possible mechanism to explain the follicle dysfunction in this disorder deserves consideration. The normal menopausal elevation of serum LH is known to result from inadequate negative feedback due to a reduced number of follicles (30). A properly timed LH surge luteinizes a mature follicle, reduces granulosa cell mitogenic activity,(31) and induces changes in steady state level of messenger ribonucleic acids for enzymes involved in progesterone synthesis (32). Inappropriate early luteinization of a growing follicle would, thus, be expected to impair follicle growth and reduce estradiol production in response to FSH.
We previously demonstrated histologically that inappropriate luteinization is a major mechanism of follicle dysfunction in women with 46,XX spontaneous primary ovarian insufficiency (10). This action on granulosa cells to cause lutienization is evidence that LH receptors also have not been down-regulated by the high levels of serum LH in these patients. Furthermore, the data presented here are not only consistent with the prior histologic evidence of follicle luteinization in these patients but also supportive of this pathophysiologic mechanism of follicle dysfunction. We found that serum progesterone level is positively correlated with maximal follicle diameter in these women, which is convincing evidence that the progesterone is indeed arising from the follicles. Also, patients with 8 mm or greater follicles have serum progesterone levels significantly greater than those without such follicles. The progesterone levels are understandably modest because these are luteinized follicles not corpora lutea. Luteinization is a terminally differentiated state so that premature luteinization expectedly stops granulosa cell proliferation and reduces the amount of cell mass that can produce progesterone. Hence, the differences, while modest, are nevertheless statistically different and fit with the clinical picture regarding pathogenesis. We have no reason to expect that the patient groups differ with regard to progesterone secretion from the adrenal, so we don’t expect this to account for the difference. We are thus left with the conclusion that the difference in progesterone levels is most likely related to the presence of luteinized follicles. Our findings are consistent with a process in which activated primordial follicles in these patients grow in response to the high FSH levels acting on the FSH receptor. However, as a result of the elevated LH levels (likely due to inadequate negative feedback related to inadequate follicle cohort size), the tonic high LH levels induce inappropriately early luteinization and thus impair normal follicle function.
Taken together these data have important implications with regard to potential therapies to improve ovarian follicle function in those women with 46,XX spontaneous primary ovarian insufficiency who have follicles remaining in the ovary. At present there are no established therapies to improve ovulatory function in women with this condition (12,33–37). In women with normal ovarian function the large cohort of atresia-destined follicles may play a more important role than has been recognized (38). By providing negative feedback this cohort of follicles maintains a proper gonadotropin environment, and thus prevents premature luteinization (10,39,40).
Patients with follicles ≥ 8 mm at the time or our examination did not differ significantly with regard to age at menarche from those who did not meet this criterion. However, patients with follicles ≥ 8 mm were significantly older at the time of onset of menstrual irregularity, significantly older at the time of diagnosis, and participated in our study significantly sooner after diagnosis compared to patients without follicles ≥ 8 mm. However, regression analysis supports a conclusion that the differences in hormone levels we demonstrated are real despite these other differences. Possibly patients who had follicles ≥ 8 mm may represent a less severe form of the disease.
Our study has limitations. It was not population based, and thus there may be inherent acquisition bias that may limit application of these findings to all women with 46,XX spontaneous primary ovarian insufficiency. The study was not blinded with regard to patient or control, so there is possibility of bias accruing with regard to ovarian ultrasound examinations. This would not be expected to have an effect on hormonal levels. Serial examination of follicles after FSH stimulation, measures of AMH or inhibin B, and Doppler data on ovarian blood flow may have provided additional information. It is possible that with a larger sample of patients we could have demonstrated a statistically significant increase in serum estradiol levels in response to FSH stimulation. We, therefore, cannot conclude that all of the follicles in these patients fail to respond, or respond minimally, to FSH stimulation. Clearly, however, the response we observed is less than expected in these patients when characterized as a group.
We conclude that in most cases 46,XX spontaneous primary ovarian insufficiency is not simply a situation of complete ovarian follicle depletion. Nearly three-fourths of women with this condition have antral follicles detectable by ultrasound. Furthermore, in nearly one-half of these patients primordial follicles are capable of responding to endogenous FSH stimulation as evidenced by growth to ≥ 8 mm in diameter and estradiol secretion. This, along with prior evidence demonstrating the development of lutienized Graafian follicles in many of these patients,(10) as well as the new evidence herein suggesting that follicles in these patients secrete progesterone, support a conclusion that down regulation of FSH receptors is not the predominant mechanism of follicle dysfunction in these patients. The present findings support and extend prior evidence that inappropriate luteinization of follicles is the more likely predominant mechanism. This would explain the resulting follicle dysfunction that 1) prevented us from observing the expected increase in serum estradiol following exogenous FSH administration, and 2) clinically impairs follicle growth to maturity and ovulation in these patients. If inappropriate luteinization is the major underlying mechanism, then suppression of the LH levels should improve ovulation and conception rates. There is a need for additional prospective controlled studies designed to improve ovulatory function and fertility in women with 46,XX spontaneous primary ovarian insufficiency.
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health. Vien H. Vanderhoof and Lawrence M. Nelson are Commissioned Officers in the United States Public Health Service.
The authors are grateful to the women who participated in this study as patients and as controls.
Footnotes
Where the work was done: National Institutes of Health, Bethesda, Maryland, USA,
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mattison DR, Thomford PJ, Jelovsek FR. Disposition of oocytes and age at menopause. N Engl J Med. 1988;318:644. doi: 10.1056/NEJM198803103181019. [DOI] [PubMed] [Google Scholar]
- 2.Baker TG. A quantitative and cytological study of germ cells in the human ovaries. Proc Roy Soc Lond B Biol Sci. 1963;158:417–433. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 3.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. American Journal of the Medical Sciences. 1942;204:625–648. [Google Scholar]
- 4.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–492. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 5.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 6.Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulam CB. Premature gonadal failure. Fertil Steril. 1982;38:645–655. doi: 10.1016/s0015-0282(16)46688-4. [DOI] [PubMed] [Google Scholar]
- 8.Rebar RW, Erickson GF, Yen SSC. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril. 1982;37:35–41. [PubMed] [Google Scholar]
- 9.Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53:804–810. [PubMed] [Google Scholar]
- 10.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized Graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–1475. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 11.Conway GS, Kaltsas G, Patel A, Davies MC, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertil Steril. 1996;65:337–341. doi: 10.1016/s0015-0282(16)58095-9. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AE, Adams JM, Mulder JE, Martin KA, Sluss PM, Crowley WFJ. A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J Clin Endocrinol Metab. 1996;81:3615–3621. doi: 10.1210/jcem.81.10.8855811. [DOI] [PubMed] [Google Scholar]
- 13.McNatty KP, Makris A, Reinhold VN, De GC, Osathanondh R, Ryan KJ. Metabolism of androstenedione by human ovarian tissues in vitro with particular reference to reductase and aromatase activity. Steroids. 1979;34:429–443. doi: 10.1016/0039-128x(79)90104-1. [DOI] [PubMed] [Google Scholar]
- 14.Sasano H, Okamoto M, Mason JI, Simpson ER, Mendelson CR, Sasano N, et al. Immunolocalization of aromatase, 17 alpha-hydroxylase and side-chain-cleavage cytochromes P-450 in the human ovary. J Reprod Fertil. 1989;85:163–169. doi: 10.1530/jrf.0.0850163. [DOI] [PubMed] [Google Scholar]
- 15.Guidelines for remuneration of research subjects in the Intramural Research Program. Office of Human Subjects Research, National Institutes of Health. 2-9-2009. 7-16-2009. Ref Type: Electronic Citation
- 16.Pastor CL, Vanderhoof VH, Lim LC, Calis KA, Premkumar A, Guerrero NT, et al. Pilot study investigating the age-related decline in ovarian function of regularly menstruating normal women. Fertil Steril. 2005;84:1462–1469. doi: 10.1016/j.fertnstert.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Fanchin R, de Ziegler D, Olivennes F, Taieb J, Dzik A, Frydman R. Exogenous follicle stimulating hormone ovarian reserve test (EFORT): a simple and reliable screening test for detecting ‘poor responders’ in in-vitro fertilization. Hum Reprod. 1994;9:1607–1611. doi: 10.1093/oxfordjournals.humrep.a138760. [DOI] [PubMed] [Google Scholar]
- 18.Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society, Series B. 1964;26:211–252. [Google Scholar]
- 19.Ryan KJ, Petro Z. Steroid biosynthesis by human ovarian granulosa and thecal cells. J Clin Endocrinol Metab. 1966;26:46–52. doi: 10.1210/jcem-26-1-46. [DOI] [PubMed] [Google Scholar]
- 20.Westergaard L, Christensen IJ, McNatty KP. Steroid levels in ovarian follicular fluid related to follicle size and health status during the normal menstrual cycle in women. Hum Reprod. 1986;1:227–232. doi: 10.1093/oxfordjournals.humrep.a136390. [DOI] [PubMed] [Google Scholar]
- 21.van Santbrink EJ, Hop WC, van Dessel TJ, de Jong FH, Fauser BC. Decremental follicle-stimulating hormone and dominant follicle development during the normal menstrual cycle. Fertil Steril. 1995;64:37–43. [PubMed] [Google Scholar]
- 22.van Dessel HJ, Schipper I, Pache TD, van Geldorp H, de Jong FH, Fauser BC. Normal human follicle development: an evaluation of correlations with oestradiol, androstenedione and progesterone levels in individual follicles. Clin Endocrinol. 1996;44:191–198. doi: 10.1046/j.1365-2265.1996.662483.x. [DOI] [PubMed] [Google Scholar]
- 23.Check JH, Nowroozi K, Chase JS, Nazari A, Shapse D, Vaze M. Ovulation induction and pregnancies in 100 consecutive women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53:811–816. doi: 10.1016/s0015-0282(16)53514-6. [DOI] [PubMed] [Google Scholar]
- 24.Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris) 2003;64:87–92. [PubMed] [Google Scholar]
- 25.LaPolt PS, Jia XC, Sincich C, Hsueh AJ. Ligand-induced down-regulation of testicular and ovarian luteinizing hormone (LH) receptors is preceded by tissue-specific inhibition of alternatively processed LH receptor transcripts. Mol Endocrinol. 1991;5:397–403. doi: 10.1210/mend-5-3-397. [DOI] [PubMed] [Google Scholar]
- 26.Rabin D, Spitz I, Bercovici B, Bell J, Laufer A, Benveniste R, et al. Isolated deficiency of follicle-stimulating hormone: clinical and laboratory features. N Engl J Med. 1972;287:1313–1317. doi: 10.1056/NEJM197212282872602. [DOI] [PubMed] [Google Scholar]
- 27.Layman LC, Lee EJ, Peak DB, Namnoum AB, Vu KV, van Lingen BL, et al. Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med. 1997;337:607–611. doi: 10.1056/NEJM199708283370905. [DOI] [PubMed] [Google Scholar]
- 28.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 29.Aittomaki K, Herva R, Stenman UH, Juntunen K, Ylostalo P, Hovatta O, et al. Clinical features of primary ovarian failure caused by a point mutation in the follicle-stimulating hormone receptor gene. J Clin Endocrinol Metab. 1996;81:3722–3726. doi: 10.1210/jcem.81.10.8855829. [DOI] [PubMed] [Google Scholar]
- 30.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55:699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yong EL, Baird DT, Yates R, Reichert LE, Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–849. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 32.Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:1041–1045. doi: 10.1210/jcem-70-4-1041. [DOI] [PubMed] [Google Scholar]
- 33.Surrey ES, Cedars MI. The effect of gonadotropin suppression on the induction of ovulation in premature ovarian failure patients. Fertil Steril. 1989;52:36–41. doi: 10.1016/s0015-0282(16)60785-9. [DOI] [PubMed] [Google Scholar]
- 34.Nelson LM, Kimzey LM, White BJ, Merriam GR. Gonadotropin suppression for the treatment of karyotypically normal spontaneous premature ovarian failure: a controlled trial. Fertil Steril. 1992;57:50–55. doi: 10.1016/s0015-0282(16)54775-x. [DOI] [PubMed] [Google Scholar]
- 35.Morris RS, Sauer MV. New advances in the treatment of infertility in women with ovarian failure. Curr Opin Obstet Gynecol. 1993;5:368–377. [PubMed] [Google Scholar]
- 36.Anasti JN, Kimzey LM, Defensor RA, White B, Nelson LM. A controlled study of danazol for the treatment of karyotypically normal spontaneous premature ovarian failure. Fertil Steril. 1994;62:726–730. doi: 10.1016/s0015-0282(16)56996-9. [DOI] [PubMed] [Google Scholar]
- 37.van Kasteren YM, Hoek A, Schoemaker J. Ovulation induction in premature ovarian failure: a placebo-controlled randomized trial combining pituitary suppression with gonadotropin stimulation. Fertil Steril. 1995;64:273–278. doi: 10.1016/s0015-0282(16)57722-x. [DOI] [PubMed] [Google Scholar]
- 38.Popat VB, Vanderhoof VH, Calis KA, Troendle JF, Nelson LM. Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2008;89:429–433. doi: 10.1016/j.fertnstert.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacker HM, Ethan A. How do the ovaries count? Mathematical Biosciences. 1988;90:305–332. [Google Scholar]
- 40.Duncan M, Cummings L, Chada K. Germ cell deficient (gcd) mouse as a model of premature ovarian failure. Biol Reprod. 1993;49:221–227. doi: 10.1095/biolreprod49.2.221. [DOI] [PubMed] [Google Scholar]