Abstract
Purpose
Androgen deprivation therapy (ADT) for prostate cancer increased substantially through the 1990s, but more recent national trends regarding incident and prevalent use have been incompletely characterized.
Methods
Linked Surveillance, Epidemiology and End Results-Medicare data were used to study patterns of ADT utilization. Prevalence of ADT in the male Medicare population was estimated by examining a cohort of prostate cancer patients and a 5% non-cancer control population, from 1991 to 2005. ADT use across different indications was examined for men with incident cancers from 2000 to 2002. Nested logit models were used to examine determinants of ADT use in men with lower risk prostate cancer not treated definitively by surgery or radiation.
Results
Prevalent ADT use increased through the 1990s, peaked in 2000 at 3.17% of all male Medicare beneficiaries, subsequently stabilized, then dropped in 2005 to 2.92%. Between 2000 and 2002, use in incident prostate cancer was stable, with 44.8% use in all cases, 15% of cases as an adjuvant with radiation, and 14% as a primary therapy. In the nested logit model, predictors of ADT use in a lower risk setting were older age, higher stage and grade, and elevated prostate-specific antigen levels.
Conclusions
Following a period of rapid expansion during the 1990s, incident and prevalent use of ADT has leveled, and may be starting to decline. Further research is needed to monitor how reductions in reimbursement for GnRH agonists will affect appropriate use of ADT as well as use in settings where its benefits may be marginal.
Keywords: prostate cancer, androgen deprivation therapy, practice patterns
Introduction
Although the potential benefits of androgen deprivation therapy (ADT) have been recognized for more than 50 years,1 solid evidence regarding its efficacy in prostate cancer has been limited to relatively few clinical indications, including in metastatic settings and as an adjunct to local therapy in high-risk disease.2 3 4 5 The use of ADT, however, increased markedly during the 1990s across all stages and grades. Between 1991 and 1999, for example, the proportion of men with prostate cancer treated with ADT within 6 months of diagnosis nearly doubled from 22.9% to 42.7%. During the same period, primary androgen deprivation increased dramatically from 3.7% to 30.9% in older men with localized cancers. 6
Identifying ongoing trends in use of ADT, particularly in low risk settings where its benefits are uncertain, is important for a number of reasons. The costs associated with ADT are substantial; Medicare expenditures for gonadotropin-releasing hormone (GnRH) agonists totaled over a billion dollars in the year 2003 alone.7 More recently, unintended adverse consequences related to ADT have become more apparent.8 9 10
Although recent evidence suggests that primary androgen deprivation therapy among men presenting with low risk disease has begun to decline,11 changes in practice trends have not yet been confirmed at a national level. Further, relatively little is known regarding the prevalence of androgen deprivation exposure,12 which may continue to increase from long-term use in settings of localized disease and biochemical recurrence. Accordingly, we undertook this study using linked Surveillance, Epidemiology, and End Results (SEER)-Medicare data to describe national trends in incident and prevalent use of ADT in the period beyond the 1990s, and to examine use in low-risk settings where evidence is lacking and the benefit of therapy may be marginal.
Methods
Data Source
Linked SEER-Medicare data including incident cancer cases through 2002 with health service claims through 2005 were used for analysis.13 14 The SEER program is overseen by the National Cancer Institute (NCI), consists of regional and state-based tumor registries located throughout the country, and represents approximately 25% of the US population.15 For the purposes of this analysis, 11 SEER sites were used (San Jose-Monterey, San Francisco-Oakland, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle-Puget Sound, Utah, Atlanta and Los Angeles). Medicare is a federal health services program covering 97% of US residents 65 years of age and older that provides information on healthcare services received by program beneficiaries.16 Both data sources have been linked to provide reliable information regarding incident cancer cases and related health services. The linked database also includes a 5% random sample control group of beneficiaries residing in SEER areas who do not have a registered diagnosis of cancer.
Study Subjects
Prevalence of androgen deprivation exposure in the male Medicare population was estimated using a cohort of men 65 years of age and older registered in SEER-Medicare with any cancer diagnosis in addition to men in the 5% random sample from 1991 through 2005 (prevalent ADT cohort). To reduce chances of underestimation, no attempt was made to limit this analysis to men with a diagnosis of prostate cancer either in SEER or in Medicare claims.17 To examine more recent trends, androgen deprivation use among incident cases diagnosed from 2000 through 2002 with follow-up through 2005 was examined (incident ADT cohort). In analyses focusing on time trends in the use of androgen deprivation in incident cases of lower-risk disease, ADT use among men with clinical stage T1 or T2, well to moderately differentiated (Gleason 2–7) prostate cancer was examined from 1992 to 2002 (lower-risk ADT cohort). The application of commonly used clinical risk categories was not possible due to the absence of prostate-specific antigen (PSA) values and predefined SEER grade classifications. To ensure complete claims information, men without both Medicare Part A and B coverage and those enrolled in a HMO at any time were excluded from analyses.
Definitions
Androgen deprivation was defined as receipt of either GnRH agonist or orchiectomy.6 Use of antiandrogens could not be identified. Treatment with radical prostatectomy, external radiation therapy, interstitial radiation therapy or ADT was identified through SEER treatment codes or Medicare claims as previously described.6 Demographic information, including age, geographic region, race/ethnicity, and socioeconomic status was abstracted. Race and ethnicity were defined as White, Black, Hispanic and Other according to reporting used in SEER. Socioeconomic status was assigned based on the 2000 US census median income and education level corresponding to zip code of residence. Cancer characteristics were derived from SEER data. Cancer grade was categorized as well differentiated (Gleason grade 2–4), moderately differentiated (Gleason grade 5–7), and poorly or undifferentiated (Gleason grade 8–10) according to predefined SEER grading classifications. Clinical stage (T1–T4) was assigned according to the SEER Extent of Disease classification system. Information regarding prostate-specific antigen (PSA) was available for a subset of the cohort, although not as discrete values. Accordingly, PSA information was classified as within reference, above reference or missing in accordance with abstraction procedures used to collect SEER data. Comorbidity was assessed using a standard index by identifying pre-existing medical conditions present 12-month prior to prostate cancer diagnosis.18
Outcomes
The primary outcome was patient level ADT use. “Any ADT” was defined as use of at least one dose of a GnRH agonist or orchiectomy within one year of diagnosis for incident cases of prostate cancer, or within a given calendar year for prevalent cases. “Primary ADT” was defined as receipt of at least six doses of a GnRH agonist or orchiectomy in the absence of surgery or radiation within a year of diagnosis. “Adjuvant ADT” was defined as administration of at least six doses of a GnRH agonist or orchiectomy with radiation therapy or one dose of a GnRH agonist or orchiectomy with surgery within a year of diagnosis. No attempt was made to distinguish between adjuvant or neoadjuvant therapy given limitations in precisely identifying timing of treatment in Medicare claims and SEER data. In addition, it is possible that some of the cases classified as “Adjuvant ADT” in this way would actually represent early salvage use of ADT for progression within the first year. Remaining cases of ADT use were classified as “Other ADT.”
Statistical analysis
The estimated proportion of men with prevalent androgen deprivation exposure was calculated for each calendar year and plotted. Exposure to androgen deprivation in a given year was counted if claims were identified for: 1) one dose of a GnRH agonist in that year or 2) orchiectomy in that year or any previous year within the available study period. A limitation to this approach is that men managed with orchiectomy prior to 1991 were not identified as ADT cases during subsequent years of the study, potentially resulting in underestimation of androgen deprivation exposure. To provide national estimates, both numerator and denominator counts obtained from the 5% control group were multiplied by 20.
Androgen deprivation use for incident cases was defined as receipt of ADT in the first year following diagnosis. Incident use was calculated and stratified by cancer and patient characteristics. Differences in proportions of men treated with ADT were then analyzed using chi square statistics corrected for multiple comparisons.
A two-stage nested logit model was used to examine treatment of lower risk prostate cancer within the first year following diagnosis with a specific interest in factors predictive of ADT use. This modeling approach addresses scenarios with more than two alternative choices (radical prostatectomy, radiation, ADT or observation), but unlike the more commonly used multinomial logit model does not require the assumption of “independence of irrelevant alternatives”,19 which may be violated when some treatment alternatives are more similar than others. In this case, choice between definitive (surgery and radiation therapy) and non-definitive (ADT and observation) treatment alternatives are interrelated, and therefore not independent. The first stage (“upper level” model) addresses the initial choice between definitive therapy (defined here as radiation or surgery, regardless of whether adjuvant ADT is given) or not, and the second stage (“lower level” model) addresses the choice between ADT (defined here as orchiectomy or ≥1 dose of GnRH agonist) or not, given an initial choice of non-definitive therapy. Parameters of the nested logit model were estimated from the full log-likelihood function, which was obtained by adding the conditional log-likelihood function at each level. Odd ratios with associated 95% CI were calculated for independent variable categories. Standard statistical software (SAS v9.1, Cary, NC) was used for analyses, tests were 2-sided, and a p<0.05 was used to determine significance.
Results
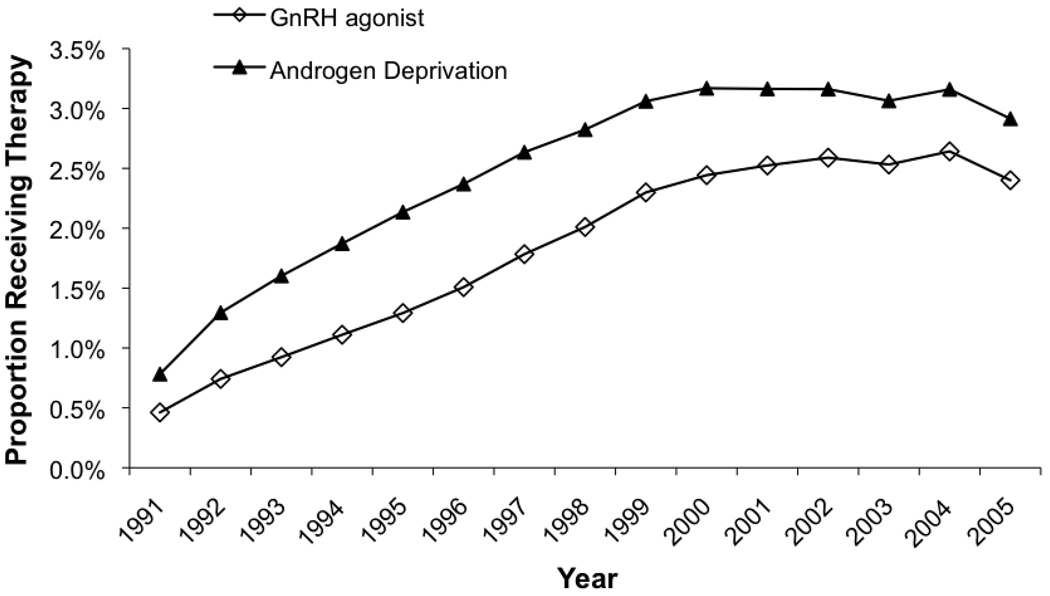
As shown in Figure 1, the estimated prevalence of androgen deprivation therapy among male Medicare beneficiaries increased steadily through the 1990s, peaking at 3.17% in 2000 from a low of 0.78% in 1991. Between 2000 and 2004, prevalence leveled and then decreased slightly to 2.92% in 2005. Prevalent use of GnRH agonists was primarily responsible for overall ADT use, and increased steadily throughout most of the period.
Figure 1.
Prevalence of androgen deprivation therapy among male Medicare beneficiaries between 1991 and 2005
Incident use is shown in Table 1. Between 2000 and 2002, nearly half (44.8%) of men with incident prostate cancer were exposed to ADT during the first year after diagnosis. Adjuvant ADT with radiation was the most common use of ADT comprising just over 15% of cases, followed by primary ADT use in 14% of cases. Other ADT use comprised a substantial proportion of cases (12.8%) and consisted mainly of short-term use (median of 4 months) either alone or together with radiation or surgery.
Table 1.
Clinical and demographic characteristics stratified by type of androgen deprivation use (any, primary, adjuvant and other)
| Characteristics | Category | N | Any ADT (%) |
Primary ADT (%) |
Adjuvant ADT with XRT (%) |
Adjuvant ADT with IRT (%) |
Adjuvant ADT with RP (%) |
Other ADT (%) |
|---|---|---|---|---|---|---|---|---|
| Overall cohort | 28,053 | 44.8 | 14.0 | 15.3 | 3.9 | 2.8 | 12.8 | |
| Age | 65–69 | 7,779 | 31.1 | 4.1 | 11.7 | 3.9 | 5.1 | 10.2 |
| 70–74 | 8,378 | 42.6 | 7.2 | 18.3 | 5.3 | 3.2 | 14.0 | |
| 75–79 | 6,816 | 51.8 | 15.3 | 19.6 | 4.5 | 1.3 | 15.6 | |
| 80+ | 5,080 | 60.1 | 38.5 | 9.9 | 1.1 | 0.4 | 11.4 | |
| Race/ethnicity | White | 21,800 | 44.2 | 13.2 | 15.5 | 4.0 | 2.7 | 12.8 |
| Black | 2,578 | 39.8 | 12.6 | 12.3 | 2.5 | 2.1 | 12.9 | |
| Hispanic | 1,397 | 46.8 | 14.2 | 13.3 | 3.8 | 6.2 | 13.1 | |
| Other | 2,278 | 55.1 | 22.8 | 17.5 | 5.1 | 2.2 | 12.6 | |
| SEER Region | San Jose | 1,192 | 55.6 | 18.4 | 18.5 | 7.9 | 4.9 | 13.9 |
| San Francisco | 1,820 | 44.5 | 12.4 | 13.9 | 3.5 | 2.1 | 16.2 | |
| Connecticut | 3,472 | 53.6 | 14.0 | 26.3 | 6.7 | 1.6 | 11.7 | |
| Detroit | 5,088 | 43.1 | 11.7 | 16.5 | 4.0 | 2.2 | 12.7 | |
| Hawaii | 781 | 57.5 | 18.1 | 25.7 | 8.5 | 1.5 | 12.2 | |
| Iowa | 3,646 | 50.1 | 19.9 | 15.7 | 2.7 | 2.5 | 12.0 | |
| New Mexico | 1,424 | 39.5 | 11.3 | 11.1 | 1.5 | 3.7 | 13.4 | |
| Seattle | 3,163 | 37.8 | 11.2 | 11.8 | 3.6 | 3.4 | 11.4 | |
| Utah | 2,006 | 40.3 | 13.2 | 7.3 | 2.8 | 2.2 | 17.6 | |
| Atlanta | 1,558 | 29.5 | 7.5 | 7.3 | 3.2 | 1.0 | 13.8 | |
| Los Angeles | 3,903 | 44.7 | 16.2 | 12.5 | 2.7 | 4.8 | 11.2 | |
| Marital status | Not married | 8,360 | 47.2 | 20.1 | 13.7 | 3.1 | 2.0 | 11.4 |
| Married | 19,693 | 43.8 | 11.4 | 15.9 | 4.3 | 3.1 | 13.4 | |
| Education level | <8.4% | 6,686 | 40.6 | 11.0 | 13.5 | 3.8 | 2.3 | 13.8 |
| 8.4–13.3% | 6,796 | 44.4 | 12.7 | 16.1 | 4.2 | 3.0 | 12.6 | |
| 13.4–20.3% | 6,750 | 47.2 | 16.3 | 16.4 | 4.1 | 2.3 | 12.3 | |
| ≥20.4% | 6,739 | 47.1 | 16.0 | 15.2 | 3.7 | 3.3 | 12.6 | |
| Income level | < $37,500 | 6,717 | 47.6 | 17.6 | 14.1 | 2.9 | 3.0 | 13.0 |
| $37,500 to $47,499 | 6,565 | 46.1 | 14.6 | 16.0 | 4.1 | 3.1 | 12.3 | |
| $47,500 to $61,999 | 6,959 | 43.4 | 13.7 | 14.9 | 3.9 | 2.3 | 12.5 | |
| ≥ $62,000 | 6,730 | 42.4 | 10.2 | 16.2 | 5.0 | 2.5 | 13.5 | |
| Comorbidity | 0 | 18,680 | 44.1 | 13.3 | 15.2 | 4.1 | 2.7 | 12.9 |
| 1 | 4,574 | 50.0 | 17.5 | 16.3 | 3.5 | 2.7 | 13.5 | |
| 2 | 1,374 | 54.4 | 22.6 | 16.4 | 3.6 | 1.3 | 14.2 | |
| ≥3 | 648 | 52.2 | 22.5 | 16.4 | 3.6 | 0.8 | 12.5 | |
| Year of diagnosis | 2000 | 8,944 | 45.5 | 14.8 | 14.1 | 3.4 | 3.1 | 13.5 |
| 2001 | 9,489 | 44.6 | 13.7 | 16.0 | 4.2 | 2.5 | 12.6 | |
| 2002 | 9,620 | 44.3 | 13.6 | 15.6 | 4.1 | 2.8 | 12.4 | |
| Clinical stage | T1 | 9,068 | 36.0 | 8.7 | 12.5 | 3.4 | 2.7 | 12.1 |
| T2 | 16,621 | 45.7 | 13.4 | 16.0 | 4.4 | 3.0 | 13.3 | |
| T3 | 561 | 75.6 | 17.1 | 37.1 | 9.5 | 2.0 | 19.4 | |
| T4 | 1,154 | 81.5 | 50.6 | 19.8 | 0.7 | 0.5 | 10.6 | |
| Gleason score | 2–4 | 849 | 26.4 | 9.4 | 6.7 | 2.2 | 0.8 | 9.4 |
| 5–7 | 19,544 | 38.8 | 10.1 | 13.6 | 4.0 | 2.3 | 12.8 | |
| 8–10 | 6,412 | 66.0 | 24.8 | 22.3 | 4.4 | 4.8 | 13.9 | |
| PSA | Above reference | 309 | 58.9 | 23.6 | 16.2 | 4.2 | 3.7 | 15.5 |
| Within reference | 251 | 47.8 | 8.4 | 19.9 | 7.6 | 2.4 | 17.1 | |
| Missing | 27,493 | 44.6 | 13.9 | 15.2 | 3.9 | 2.8 | 12.8 | |
| Lower risk cohort | 7,557 | 45.9 | 17.9 | 13.4 | 3.1 | 0.8 | 13.8 |
Primary ADT was associated with higher risk characteristics, with the highest rates of use occurring in late stage and high-grade cancers, as well as in those patients with elevated PSA levels. Nevertheless, even in men aged 75 and older with T1 or T2, low to moderate grade tumors, the rate of primary ADT use was 17.9% (with 0.7% as orchiectomy and 17.2% as GnRH agonists; proportion of ADT as orchiectomy, 3.9%). This contrasts with primary ADT use in men aged 75 and older who had higher risk features (T1 or T2 with high grade histology, or T3/T4 tumors) in which the rate of use was 48.7% (with 7.3% as orchiectomy and 41.4% as GnRH agonists; proportion of ADT as orchiectomy, 15.0%). Use of primary ADT was also higher with increasing age and comorbidity, in unmarried men, and in those living in areas with lower income and education.
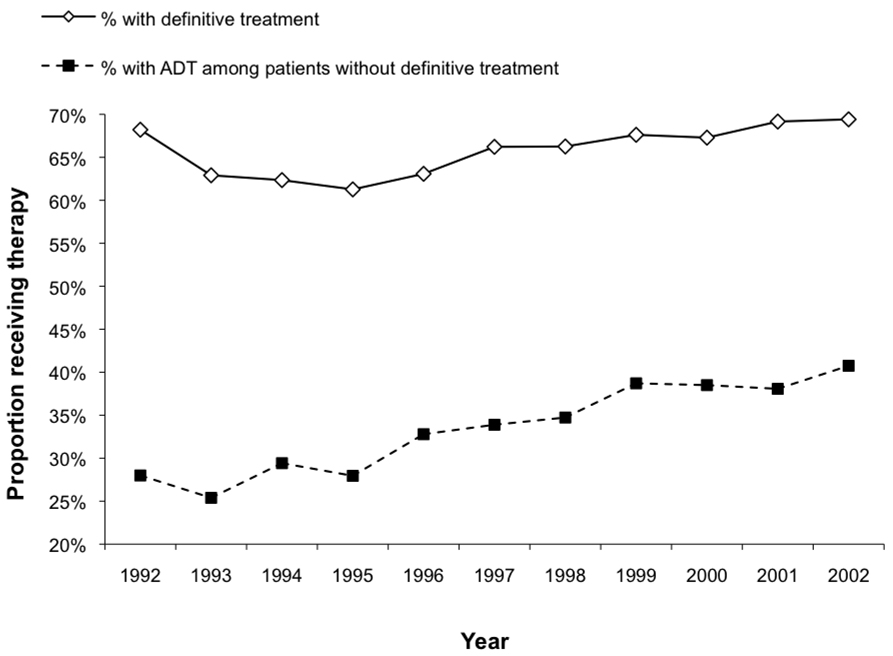
Figure 2 shows plots over time of the proportion of patients with lower risk prostate cancer (stage T1 or T2, low to moderate grade tumors) receiving definitive therapy (radiation or radical prostatectomy) and the proportion receiving any ADT (orchiectomy or ≥1 dose of GnRH agonist) among those not treated definitively in the first year following diagnosis. While rates of definitive therapy remained relatively stable, the use of ADT increased steadily from 25.4% in 1993 to 40.8% in 2002 among men managed without definitive therapy.
Figure 2.
Proportions of definitive treatment (radiation or surgery) and use of ADT in patients not managed with active treatment among men with lower-risk prostate cancer over time
Results of the nested logit model are shown in Table 2. The likelihood of receiving definitive therapy was significantly higher with younger age and lower comorbidity level, and significantly lower among black men, unmarried men, and those residing in areas of lower income or education. The likelihood of receiving ADT in those not undergoing definitive therapy was strongly influenced by age; men age 80 years or older were more than twice as likely to receive ADT than men less than 70 years old. Significant variation within relatively confined cancer characteristics was also apparent. Men with clinical stage T2 disease were greater than twice as likely to receive ADT than those with T1 cancers, and men with moderately differentiated tumors were nearly 2.5 times more likely to be treated with ADT than those with well differentiated cancers. Trends over time in use of ADT were confirmed in the model, with the likelihood of use in 2002 being greater than 40% higher than at the start of the study in 1992 (OR 1.42, 95% CI 1.26–1.59). The likelihood of ADT use, however, remained relatively stable from 1997 onwards. Significant geographic variation in the use of definitive therapy and ADT was evident.
Table 2.
Likelihood of management with definitive treatment (radiation or surgery) or androgen deprivation therapy (GnRH agonist) based on nested logit models among men with lower-risk prostate cancer (clinical T1/T2 stage, Gleason ≤ 7 grade)
| Nested Logit Regression Model | |||
|---|---|---|---|
|
Characteristics |
Category |
Upper Level Model: Use of Definitive Treatment (yes/no) OR (95% CI) |
Lower Level Model: Use of ADT, conditional on initial choice of non- definitive treatment (yes/no) OR (95% CI) |
| Age | 65–69 | 1.00 | 1.00 |
| 70–74 | 0.64 (0.60–0.67) | 1.18 (1.08–1.29) | |
| 75–79 | 0.25 (0.23–0.26) | 1.50 (1.37–1.64) | |
| ≥80 | 0.06 (0.05–0.06) | 2.04 (1.86–2.24) | |
| Race/ethnicity | White | 1.00 | 1.00 |
| Black | 0.73 (0.68–0.79) | 0.98 (0.88–1.08) | |
| Hispanic | 0.87 (0.79–0.96) | 1.19 (1.05–1.35) | |
| Other | 0.54 (0.49–0.59) | 1.21 (1.08–1.35) | |
| SEER Region | San Jose | 1.00 | 1.00 |
| San Francisco | 1.37 (1.22–1.54) | 0.55 (0.47–0.64) | |
| Connecticut | 1.39 (1.24–1.55) | 0.95 (0.83–1.10) | |
| Detroit | 1.75 (1.57–1.95) | 0.77 (0.67–0.88) | |
| Hawaii | 2.98 (2.51–3.54) | 0.83 (0.67–1.03) | |
| Iowa | 1.18 (1.05–1.34) | 1.15 (0.99–1.34) | |
| New Mexico | 1.18 (1.03–1.34) | 0.49 (0.41–0.59) | |
| Seattle | 1.43 (1.27–1.61) | 0.54 (0.46–0.63) | |
| Utah | 1.09 (0.96–1.24) | 0.75 (0.64–0.89) | |
| Atlanta | 1.95 (1.71–2.22) | 0.60 (0.50–0.71) | |
| Los Angeles | 1.38 (1.24–1.54) | 0.93 (0.82–1.07) | |
| Marital status | Not married | 1.00 | 1.00 |
| Married | 1.86 (1.79–1.94) | 1.00 (0.95–1.06) | |
| Education level | <8.4% | 1.00 | 1.00 |
| 8.4–13.3% | 0.96 (0.90–1.02) | 1.11 (1.03–1.21) | |
| 13.4–20.3% | 0.86 (0.81–0.92) | 1.10 (1.01–1.21) | |
| ≥20.4% | 0.87 (0.80–0.95) | 1.15 (1.03–1.29) | |
| Median income | < $37,500 | 1.00 | 1.00 |
| $37,500 to $47,499 | 1.19 (1.12–1.27) | 1.01 (0.94–1.09) | |
| $47,500 to $61,999 | 1.20 (1.12–1.29) | 1.07 (0.98–1.18) | |
| ≥ $62,000 | 1.19 (1.09–1.31) | 0.99 (0.88–1.11) | |
| Comorbidity | 0 | 1.00 | 1.00 |
| 1 | 0.80 (0.76–0.84) | 1.02 (0.96–1.09) | |
| 2 | 0.60 (0.55–0.66) | 1.00 (0.90–1.11) | |
| ≥3 | 0.34 (0.30–0.39) | 0.91 (0.79–1.05) | |
| Year of diagnosis | 1992 | 1.00 | 1.00 |
| 1993 | 0.71 (0.65–0.77) | 0.92 (0.82–1.03) | |
| 1994 | 0.64 (0.59–0.70) | 1.16 (1.04–1.30) | |
| 1995 | 0.63 (0.58–0.69) | 1.14 (1.01–1.27) | |
| 1996 | 0.71 (0.65–0.77) | 1.37 (1.22–1.53) | |
| 1997 | 0.82 (0.75–0.90) | 1.45 (1.30–1.63) | |
| 1998 | 0.85 (0.78–0.93) | 1.36 (1.21–1.53) | |
| 1999 | 0.88 (0.81–0.96) | 1.55 (1.38–1.73) | |
| 2000 | 0.87 (0.79–0.95) | 1.39 (1.24–1.56) | |
| 2001 | 0.94 (0.86–1.02) | 1.40 (1.25–1.59) | |
| 2002 | 0.94 (0.86–1.02) | 1.42 (1.26–1.59) | |
| Clinical stage | T1 | 1.00 | 1.00 |
| T2 | 1.71 (1.65–1.78) | 2.34 (2.20–2.48) | |
| Gleason score | 2–4 | 1.00 | 1.00 |
| 5–7 | 2.74 (2.60–2.89) | 2.48 (2.28–2.70) | |
| PSA | Within reference | 1.00 | 1.00 |
| Above reference | 0.86 (0.59–1.27) | 1.82 (1.07–3.09) | |
Discussion
Despite leveling in more recent years, the results of our study indicate that through the 1990s, primary androgen deprivation was used increasingly in place of observation among men with lower risk prostate cancer. Use of androgen deprivation as primary therapy or as an adjuvant with radiation in low risk prostate cancer continues to be controversial. Notably, clinical guidelines do not support ADT use in low risk settings,20 21 and there is little evidence supporting its use for biochemical recurrence, which likely comprises a significant portion of the prevalent ADT use note in this study. Despite these controversies, findings from this and other studies have demonstrated dramatic growth of ADT in settings of uncertain benefit.6 22 23 24 In fact, the oldest men in our study were more likely to receive ADT, despite their low risk of death from prostate cancer.25 A large proportion of this use may have been driven by the favorable reimbursement for GnRH agonists and financially driven practices.26 27 Recent changes brought about by the Medicare Modernization Act, which reduced reimbursement for GnRH agonists by an estimated 50%28 may explain some of the decrease in ADT use noted in 2005. Because these changes took effect in 2005, however, additional years of data will be necessary to determine the extent of and time to lower ADT utilization.
Our findings are consistent with other recent work in this area. A study of national Medicare claims showed that reimbursement for androgen deprivation decreased from a peak of $1.2 billion in 2003 to $450 million in 2005 which was coupled with a more modest 10% decrease in androgen deprivation use.29 However, because the authors relied on summary counts of services as the primary data source, insight into important information regarding indications, clinical setting, incident use and prevalence of ADT was not possible. Observations from several community and academic practices suggest a decreased use of both primary and adjuvant ADT in low risk cancers between 2004–2006, but not in settings of high risk cancers, where supporting evidence is clearer.11 Our results support these finding, although in a broader and more representative range of practices across the United States.
Despite a decline in use in 2005, exposure to androgen deprivation remains high. Currently, ADT is used in approximately 3% of the male Medicare population, corresponding to over 400,000 men. Although, ADT will continue to be an important component of care given evidence of benefit in high risk and metastatic prostate cancer,2 3 4 the role of ADT in lower-risk settings and as primary therapy remains relatively unclear.30 31 While the apparent reduction in discretionary use of ADT is a positive development, a recent report noting decreased survival in men with cardiovascular comorbidity managed with ADT as an adjuvant to radiation raises important questions about androgen deprivation even in settings where the benefits were thought to be clear.32 33 Such concerns are particularly relevant in light of our results demonstrating considerable ADT use among men with multiple medical comorbidities (Table 1). Research directed toward identifying patients who are at risk for harm from ADT, as well as developing interventions that may mitigate the adverse effects of ADT, should therefore be a priority.
Several study limitations should be considered. The study cohort was limited to Medicare beneficiaries, so ADT use in men younger than 65 years of age or those insured through HMOs could not be assessed. Further, prevalence of androgen deprivation exposure was estimated from SEER regions, which may not be completely nationally representative. However, our results are very similar to that of previous work using a national 5% Medicare sample.11 Additional data limitations included predefined grade categories used in SEER grouping Gleason 5, 6 and 7 disease and absence of PSA values preventing application of commonly used clinical risk classifications. Information regarding use of antiantrogens was also not available, and overall use of ADT may have been underestimated due to misidentification of a small number of men treated with antiandrogen monotherapy. Because detailed clinical information such as changes in PSA values was not available, more subtle aspects of the decision to treat with ADT could not be investigated. Still, the use of ADT for a rising or high PSA level alone is not supported by clear evidence.
Conclusion
The incident and prevalent use of androgen deprivation therapy has leveled in recent years, although a significant proportion of men with prostate cancer are managed with androgen deprivation during the course of their disease. In lower-risk settings where indications and benefits are unclear, androgen deprivation therapy is more commonly used in more recent years and among older men, suggesting that continued monitoring of ADT use will be necessary.
Acknowledgments
VBS is supported in part through a grant from the National Cancer Institute (CA116758)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huggins C, Hodges R. Studies on prostate cancer: 1. The effects of castration, or estrogen and androgen injection on serum phosphates in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 2.Medical Research Council Prostate Working Party Investigators Group. Immediate versus deferred treatment for advanced prostate cancer: initial results of the Medical Research Council trial. Br J Urol. 1997;79:235–246. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 3.Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85-31. J Clin Oncol. 1997;15:1013–1021. doi: 10.1200/JCO.1997.15.3.1013. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Gonzalez D, Warde P, et al. Omproved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 5.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 6.Shahinian VB, Yong-fang K, Freeman JL, et al. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 7.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:802–803. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;353:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 9.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 10.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Broering JM, Kantoff PW, et al. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–S19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry MJ, Delorenzo MA, Walker-Corkery ES, et al. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: a population-based cohort study. BJU Intl. 2006;98:973–978. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40 Suppl IV:3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 15. [accessed June 20, 2008];Overview of the SEER Program. Available from URL: http://seer.cancer.gov/about/
- 16. [accessed June 20, 2008];Medicare Program – General Information: Overview. Available from URL: http://www.cms.hhs.gov/MedicareGenInfo/
- 17.Kuo YF, Goodwin JS, Shahinian VB. Gonadotropin-releasing hormone agonist use in men without a cancer registry diagnosis of prostate cancer. BMC Health Serv Res. 2008;8:146. doi: 10.1186/1472-6963-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epid. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.Weiler W. A application of the nested multinomal logit model to enrollment choice behavior. Research in Higher Education. 1987;27:273–282. [Google Scholar]
- 20. [accessed June 20, 2008];AUA Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. doi: 10.1016/j.juro.2007.03.003. Available from URL: http://www.auanet.org/guidelines/main_reports/proscan07/content.pdf. [DOI] [PubMed]
- 21. [accessed June 20, 2008];NCCN Practice Guidelines in Oncology: Prostate Cancer V.1.2008. Available at URL: http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf.
- 22.Cooperberg MR, Grossfeld GD, Lubeck SP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Meng MV, Elkin EP, et al. Androgen deprivation use with external beam radiation for prostate cancer: results from CaPSURE. J Urol. 2005;174:1802–1807. doi: 10.1097/01.ju.0000177089.93728.20. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami J, Cowan JE, Elkin EP, et al. Androgen-deprivation therapy as primary treatment for localized prostate cancer. Cancer. 2006;106:1708–1714. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 25.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 26.Medicare: payments for covered drugs exceed providers costs. Washington, DC: General Accounting Office; 2001. General Accounting Office report. GAO-01-1118. [Google Scholar]
- 27.Shahinian VB, Kuo YF, Freeman JL, et al. Characteristics of urologists predict the use of androgen deprivation therapy for prostate cancer. J Clin Oncol. 2007;25:5359–5365. doi: 10.1200/JCO.2006.09.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PE. Changes in reimbursement rates and rules associated with Medicare Prescription Drug Improvement and Modernization Act. Am J Health-Syst Pharm. 2006;63 Suppl 7:S2–S6. doi: 10.2146/ajhp060460. [DOI] [PubMed] [Google Scholar]
- 29.Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the US Medicare population. Cancer. 2008;112:2195–2201. doi: 10.1002/cncr.23421. [DOI] [PubMed] [Google Scholar]
- 30.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong YN, Freedland SJ, Egleston B, et al. The role of primary androgen deprivation therapy in localized prostate cancer. Eur Urol. 2009;56:609–616. doi: 10.1016/j.eururo.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Amico AV, Chen MH, Renshaw AA, et al. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289–295. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 33.Nanda A, Chen MH, Braccioforte MH, et al. Hormonal therapy use for prostate cancer and mortality in men with coronoary artery disease induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]