Abstract
Studies of environmental and toxic effects of polychlorinated biphenyls (PCBs) are ideally performed with PCB mixtures reflecting the composition of environmental PCB profiles to mimic actual effects and to account for complex interactions among individual PCB congeners. Unfortunately, only a few laboratory studies employing synthetic PCB mixtures have been reported, in part because of the challenges associated with the preparation of complex PCB mixtures containing many individual PCB congeners. The objective of this study was to develop a PCB mixture that resembles the average PCB profile recorded from 1996 to 2002 at a satellite station of the Integrated Atmospheric Deposition Network located at the Illinois Institute of Technology (IIT) in Chicago, Illinois, using commercial PCB mixtures. Initial simulations, using published Aroclor profiles, showed that a mixture containing 65% Aroclor 1242 and 35% Aroclor 1254 was a good approximation of the target profile. A synthetic Chicago air mixture (CAM) was prepared by mixing the respective Aroclor's in this ratio, followed by GC/MS/MS analysis. Comparison of the PCB profile of the synthetic mixture with the target profile suggests that the synthetic PCB mixture is a good approximation of the average IIT Chicago air profiles (similarity coefficient cos θ = 0.82; average relative percent difference = 84%). The synthetic CAM was also a reasonable approximation of the average of 184 PCB profiles analyzed in 2007 at 37 sites throughout Chicago as part of the University of Iowa Superfund Basic Research Program (isbrp), with a cos θ of 0.70 and an average relative percent difference of 118%. While the CAM and the two Chicago air profiles contained primarily di- to pentachlorobiphenyls, higher chlorinated congeners, including congeners with seven or eight chlorine atoms, were underrepresented in the synthetic CAM. The calculated TCDD toxic equivalency quotients of the synthetic CAM (2.7 ng/mg PCB) and the IIT Chicago air profile (1.6 ng/mg PCB) were comparable, but lower by two orders of magnitude than the isbrp Chicago air profile (865 ng/mg PCB) due to surprisingly high PCB 126 levels in Chicago air. In contrast, the calculated neurotoxic equivalency quotients of the CAM (0.33 mg/mg PCB) and the two Chicago air profiles (0.44 and 0.30 mg/mg PCB, respectively) were similar. This study demonstrates the challenges and methods of creating and characterizing synthetic, environmental mixtures of PCBs.
Keywords: Airborne PCBs, Aroclor, PCB homologue, Toxic Equivalency Quotient, TEQ, Neurotoxic Equivalency Quotient, NEQ, PCB atropisomers
1. Introduction
Congener profiles of polychlorinated biphenyls (PCBs) found in environmental samples are complex and frequently do not reflect the composition of commercial PCB formulations. Depending on their three-dimensional structure and their substitution pattern, PCB congeners bind to different cellular target sites and cause adverse effects by a variety of different mechanisms. For example, PCB congeners with multiple ortho chlorine substituents may bind to or activate the constitutive androstane receptor (CAR) (Denomme et al., 1983) and/or the pregnane X receptor (PXR) (Schuetz et al., 1998). Other ortho substituted PCB congeners interact with both the aryl hydrocarbon (Ah-)receptor and CAR (Parkinson et al., 1983). PCB congeners with zero ortho-chlorine atoms avidly bind to the Ah-receptor (Bandiera et al., 1982) and elicit a toxic response mimicking the action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (Goldstein et al., 1978; Safe, 1994). In addition, PCB congeners with multiple ortho substituents sensitize Ryanodine receptors (RyR) (Pessah et al., 2006), an interaction that has been shown to be enantiospecific in the case of PCB 136 (Pessah et al., 2009). Aside from receptor-mediated responses, lower chlorinated PCB congeners may be metabolically activated in vitro and in vivo to reactive intermediates, such as PCB epoxides, quinones and hydroquinones (Robertson and Gupta, 2000), which in turn can react with cellular targets or result in the formation of reactive oxygen species (Arif et al., 2003; Lin et al., 2000; Robertson and Gupta, 2000; Zhao et al., 2004). Primary metabolites of lower chlorinated PCBs may have a high affinity for the estrogen receptor (ER) (Korach et al., 1988) and, thus, may cause estrogenic effects.
The complex composition of environmental PCB mixtures and the different modes of action represent a significant challenge for both environmental and toxicity studies, partly because the congeners present in PCB mixtures can interact in complex ways to produce effects that are not apparent from single congener studies. In order to assess health risks in human populations, it is of particular importance to employ PCB mixtures resembling actual human exposures for toxicity studies. However, only few toxicity studies with environmentally relevant PCB mixtures have been reported and most have focused on the ingestion route of exposure and higher molecular weight congeners. For example, animals have been exposed to PCB-contaminated soils (Fouchecourt et al., 1998; Hansen et al., 1981b), extracts of soils (Kania-Korwel et al., 2005; Li and Hansen, 1996b) or pooled extracts of multiple high volume air samples (Li and Hansen, 1996a). Feeding Great Lakes fish to test animals was used to closely mimic this important exposure source (Restum et al., 1998; Shipp et al., 1998). Two studies reported by Larry Hansen and colleagues used fat from Aroclor-exposed animals to treat another species with the resulting PCB mixture (Hansen et al., 1981a; Hansen et al., 1983). Several other studies used analyses of exposure sources to formulate synthetic mixtures from individual PCB congeners. The advantage of this approach is a well-defined PCB mixture, but environmental mixtures prepared from individual congeners are inevitably incomplete (Altmann et al., 2001; Gyorkos et al., 1985; Hany et al., 1999; Lilienthal et al., 2000; Parkinson et al., 1980) and contain only major PCB congeners. A recent study reported the preparation of a PCB mixture resembling the PCB profile found in Fox river fish using commercial PCB mixtures (Kostyniak et al., 2005). This approach allows the preparation of large quantities of a well characterized synthetic mixture containing minor as well as major PCB congeners.
The present study describes the development of a PCB mixture that resembles the average PCB profile found in Chicago air using two commercial Aroclor mixtures. This approach was selected because it is very cumbersome to collect adequate quantities of airborne PCB mixtures, even for short term pilot studies (Li and Hansen, 1996a), and it is simply not feasible to collect sufficient PCB quantities from air to obtain an environmental mixture for definite studies. The synthetic mixture was analyzed by GC/MS/MS and compared to two average PCB air profiles from Chicago. Finally, the PCB homologue composition, the contributions of congeners to the TCDD and neuro-toxic equivalency quotients TEQ and NEQ and the distribution of chiral PCB congeners were determined from the congener profile to guide the toxicity assessment of this synthetic mixture.
2. Materials and methods
2.1. Determination of the average PCB profile observed in Chicago air
A representative, average PCB congener profile in Chicago air was first determined using an extensive set of airborne PCB congener profiles recorded at the satellite station of the Integrated Atmospheric Deposition Network (IADN) located at the Illinois Institute of Technology (IIT) in Chicago, Illinois (Sun et al., 2006). To determine an average congener profile, all the congener concentrations were normalized as a fraction of the total PCBs. That is, for each sample the congener concentration (pg/m3) was divided by the sum of the congener concentrations (Equation 1). ΣPCB is defined as the sum of the concentrations of 93 congeners or coeluting congener groups.
| (Equation 1) |
This was repeated for each of the 188 samples reported by IADN for the dates January 12, 1996 through Dec 9, 2002. The average congener fractions for these 188 samples were considered to be the target profile. To allow a comparison with our analytical data (see below), this IIT Chicago air profile was collapsed to a profile with 88 congeners or coeluting congeners groups representing 123 PCB congeners.
Recently, a second large data set with airborne PCB congener profiles became available. As part of the University of Iowa Superfund Basic Research Program (isbrp), a total of 184 PCB congener profiles were collected using an innovative sampling strategy of vehicle-mounted high-volume air samplers at more than 37 sites throughout Chicago, Illinois, in 2007 (Hu et al., 2008). In the following, the average profile obtained from these individual profiles will be referred to as the isbrp Chicago air profile.
2.2. Simulation of the synthetic Chicago Air Mixture (CAM)
Initial simulations based on detailed Aroclor compositions reported by George Frame and colleagues (Cochran and Frame, 1999; Frame, 2001; Frame et al., 1996a; Frame et al., 1996b; Hansen, 1999) and, subsequently, our own GC/MS/MS analyses (Figures 2 and 3) were performed to determine which combination of Aroclor 1242 and different Aroclor 1254 would give the best approximation of the average PCB profile observed in Chicago air. The contributions from each Aroclor were determined by the method of least squares. That is, the sum of the squares of the residuals (SSR) between the Chicago profile is minimized to determine the fraction of the mixture made up from Aroclor 1242 (x) and the fraction made up from Aroclor 1254 (y)
Figure 2.
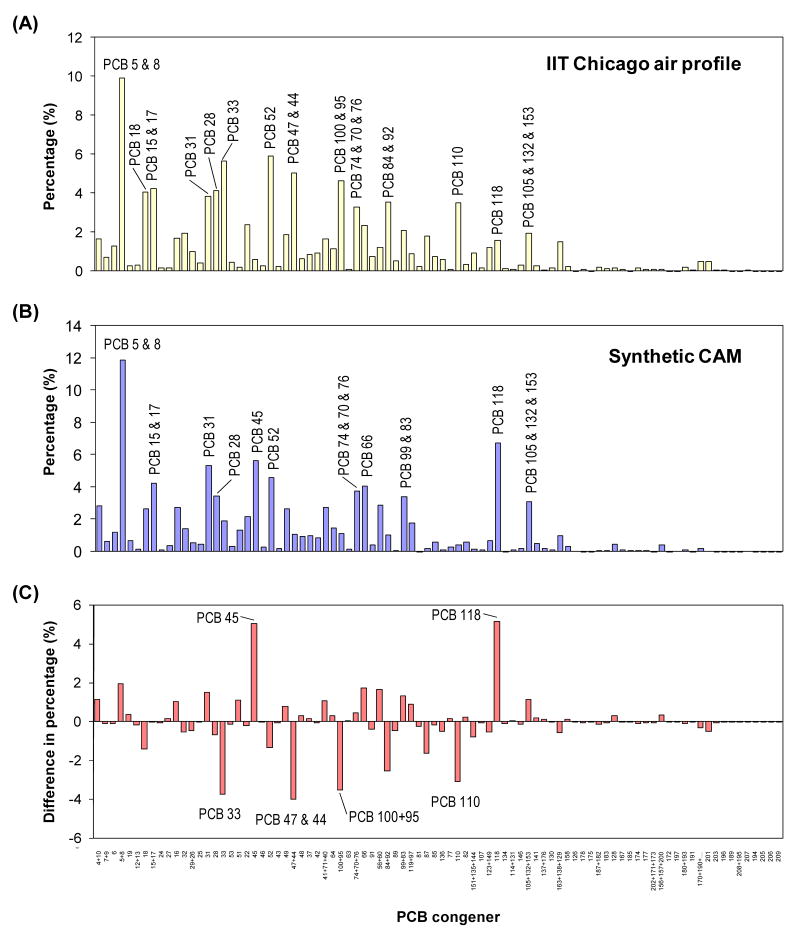
Comparison to the synthetic CAM with the average PCB profile in Chicago air. (A) Average PCB IIT Chicago air profile; (B) PCB profile of the synthetic CAM consisting of 65% Aroclor 1242 and 35% Aroclor 1254; (C) difference in percentage of the PCB congener profiles (synthetic CAM minus the IIT Chicago air profile).
Figure 3.
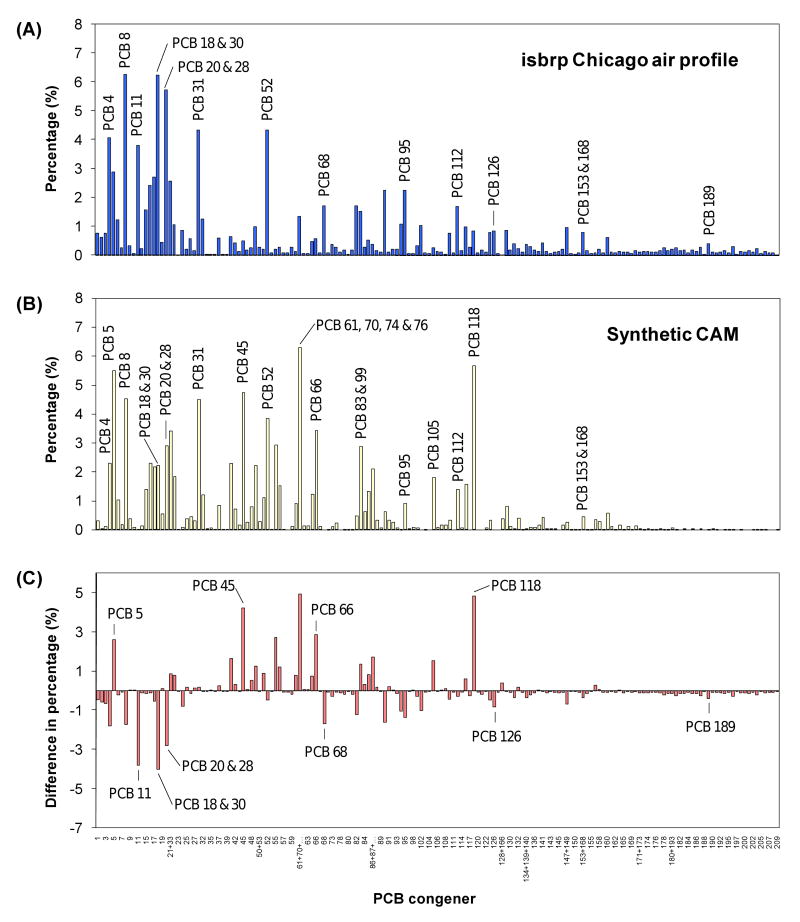
Comparison of the average 2007 PCB profile in Chicago air with the synthetic CAM. (A) Average PCB isbrp Chicago air profile; (B) PCB profile of the synthetic CAM consisting of 65% Aroclor 1242 and 35% Aroclor 1254; (C) difference in percentage of the PCB congener profiles of both mixtures (synthetic CAM minus the isbrp Chicago air profile). The labels on the x-axis show only every other PCB congeners.
where i refers to one of the 92 congeners considered. The terms fi,1242, fi,1254 and fi, IIT refer to the mass fraction of the total PCBs for each congener i in Aroclor 1242, Aroclor 1254 and the IIT congener profile, respectively. Since this is a binary mixture,
Microsoft Excel's Solver function was used to perform the iterative calculation and determine the value of x that would give the minimum SSR value. Using this approach, the best match of the Chicago air PCB profile was found to be a mixture of 65% Aroclor 1242 and 35% of a late-production Aroclor 1254 (Johnson et al., 2008). A slightly better approximation of the target profile could be achieved using a more typical, early production Aroclor 1254 lot. Unfortunately, large quantities of such an Aroclor 1254 lot were not available for this study. The average molecular weight of this mixture is 284 g/mol, as calculated from published molecular weights of Aroclor 1242 (261 g/mol) and Aroclor 1254 (327 g/mol) (Silberhorn et al., 1990).
2.3. Preparation of the synthetic CAM
Aroclor 1242 (Electrical Grade, Monsanto Lot KB-05-415) and Aroclor 1254 (Electrical Grade, Monsanto Lot KB-05-612) were generous gifts of Dr. Larry G. Hansen (University of Illinois, Urbana, Illinois, US). The Aroclor 1254 lot is a well characterized batch and contains a relatively high percentage of dioxin-like PCB congeners (Johnson et al., 2008). Both Aroclor lots, in addition to Aroclor's 1248 and 1260, have previously been employed to prepare a PCB mixture approximating a Fox River PCB profile (Kostyniak et al., 2005). Appropriate amounts of each Aroclor mixture were weighed into a cleaned and tared brown glass bottle to give 20 grams of a 65:35 mixture. The mixture was shaken continuously for 30 days and small aliquots were removed after 8, 14 and 30 days for congener specific PCB analysis. The PCB profiles for all three time points were in excellent agreement with each other.
2.4. GC/MS/MS analysis of the CAM and technical Aroclor mixtures
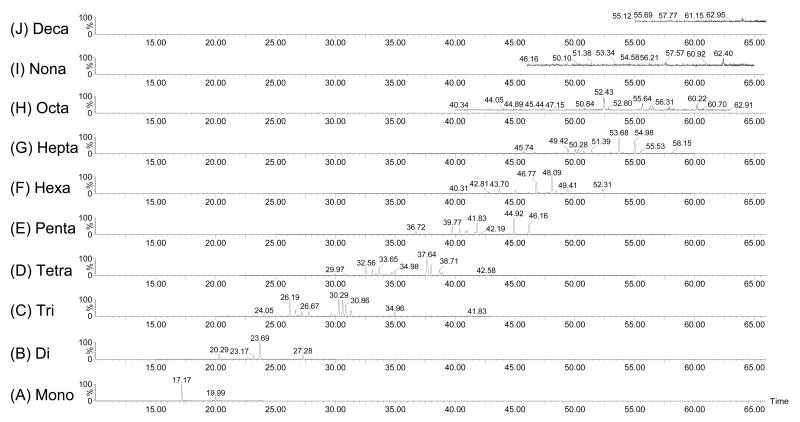
The quantification of PCB homologues was performed by GC/MS/MS with an Agilent 6890N gas chromatograph coupled to a Waters Micromass Quattro micro GC mass spectrometer (Milford, MA, USA) operating under electron impact (EI) positive mode at 70 eV and multiple reaction monitoring, and the trap current was 200 μA as described previously (Hu et al., 2008). The GC was equipped with an Agilent 7683 series autosampler. Two microlitres of the sample were injected in splitless mode in a Supelco SPB™-Octyl fused silica capillary column (30 m × 250 μm i.d. × 0.25 μm film thickness) with helium as the carrier gas at a constant flow rate of 0.8 ml min−1. The collision gas was ultra pure carrier grade Argon. The initial oven temperature was 75 °C which was held for 5 min. The gradient program ran to 150 °C at 15 °C/min and was held for 1 min. The second step of the gradient ran to 280 °C at 2.5 °C/min and was held for 3 min. A representative GC/MS/MS chromatogram of the mixture obtained under these conditions is shown in Figure 1. A chromatogram of the PCB standard is included in the Supporting Material (Figure S1). A total of 182 peaks corresponding to all 209 congeners were analyzed and the relative amount of each congener was normalized as a fraction of the total PCB mass in the sample as described above. The congener profile of Aroclor 1254 (i.e., lot KB-05-612) obtained with this method is in good agreement with the published PCB profile of this lot obtained using the same GC column (similarity coefficient cos θ = 0.92; average relative percent difference = 75) (Johnson et al., 2008).
Figure 1.
Representative GC/MS/MS chromatograms for the synthetic Chicago air mixture (CAM) consisting of 65% Aroclor 1242 and 35% Aroclor 1254 showing the homologue composition. The analysis was done in the monitored reaction mode. A detailed description of the gas chromatographic separation is provided under Materials and Methods.
3. Results and discussion
3.1. Comparison of the synthetic PCB congener profile with the IIT Chicago air profile
The concentrations and PCB congener profiles in Chicago air are well understood for the satellite stations of the IADN located at IIT in Chicago. This station has been operating since 1996, collecting samples every 12 days. The average PCB congener distribution signal for this IADN satellite station was selected as a target profile and is shown in Figure 2A. The objective of this study was to prepare a synthetic PCB mixture that resembles this Chicago air profile for further environmental and toxicity studies. Towards this goal, a PCB mixture was simulated using both detailed PCB congener profiles of Aroclor 1242 and 1254. A mixture containing 65% Aroclor 1242 and 35% Aroclor 1254 gave the best approximation, as determined by the method of least squares. This synthetic Chicago air mixture (CAM) was prepared by mixing the respective Aroclor's in a 65:35 ratio followed by thorough mixing for up to 30 days and analyzed by GC/MS/MS. A congener profile of this synthetic mixture with 88 PCB congeners or coeluting congener groups representing 123 congeners is shown in Figure 2B.
The differences in the percentage of each congener between the IIT Chicago air profile (Figure 2A) and the synthetic CAM (Figure 2B) are shown in Figure 2C. Although the differences in percentages were comparatively small, some congeners (e.g., PCB 45 and 118) were clearly overrepresented in the CAM, whereas other congeners were underrepresented (e.g., PCBs 33, 47&44, 100&95 and 110). This suggests that the IIT Chicago air profile is not a simple mixture of Aroclor 1242 and Aroclor 1254. This is not a surprising observation because PCBs undergo congener selective weathering in the environment (Hornbuckle et al., 2006).
One drawback of the IIT Chicago air profile (Figure 2A) is the fact that this profile consists only of a comparatively small number of PCB congeners. In particular, levels of monochlorinated PCB congeners are not included in the IIT Chicago air profile. However, these lower chlorinated PCB congeners are of particular interest because they can be metabolically activated to reactive and potentially toxic PCB metabolites, such as PCB epoxides, hydroquinones, semiquinone free radicals and quinones (Amaro et al., 1996; McLean et al., 1996; Song et al., 2008).
The overall similarity of the IIT Chicago air profile (Figure 2A) and the synthetic CAM (Figure 2B) was further assessed using the similarity coefficient cos θ (Magar et al., 2005) and the average relative percent difference (RPD) (Kostyniak et al., 2005). The similarity coefficient cos θ, determined using the PCB profiles shown in Figures 2A and 2B, was 0.82 (cos θ = 1 represents a perfect match). The average RPD, calculated for the RPD of all 88 congeners and coeluting congener groups was 84%. The RPD has been used previously to determine the similarity of a synthetic PCB mixture and PCB profile of fish from the Fox River (Kostyniak et al., 2005). In this study, the average RPD between the two congener profiles was 71% (64 peaks representing 92 PCB congeners). Considering that our study analyzed a larger number of peaks (88 peaks representing 123 congeners), the RPD of 84% in our study still suggests that the synthetic CAM is a good approximation of the IIT Chicago air profile.
3.2. Comparison of the synthetic PCB congener profile with the isbrp Chicago air profile
A second dataset with 184 PCB congener profiles collected in 2007 at over 37 locations in Chicago, Illinois, became recently available (Hu et al., 2008). The PCB profiles of this dataset were obtained using the same GC/MS/MS method and laboratory used to analyze the synthetic CAM, which allows a better comparison of the PCB profiles. In addition, this new dataset includes highly volatile, monochlorinated PCB congeners, which are frequently not analyzed in air samples due to analytical challenges. However, these monochlorinated PCB congeners are readily metabolized to reactive metabolites (Amaro et al., 1996; McLean et al., 1996) that have been implicated in carcinogenesis (Ludewig et al., 2008).
The average PCB profile showing 169 peaks representing all 209 PCB congeners is shown in Figure 3A. The analogous PCB profile of the CAM and the difference in the percentage between both profiles are shown in Figures 3B and 3C, respectively. Several PCB congeners were over- (e.g., PCBs 5, 45, 66 and 118) or underrepresented (e.g., PCBs 18&30, 20&28 and 68) in the CAM in comparison to the isbrp Chicago air profile in Figure 3A. PCB congeners underrepresented also include PCB 11, which is typically not found in technical Aroclor mixtures (Hu et al., 2008), and PCB 126, a highly toxic PCB congener (see below).
The CAM was also a reasonable approximation of the isbrp profile, with a similarity coefficient cos θ of 0.70 and an average RPD of 118%. As shown in Figure 3, the CAM was missing in particular higher chlorinated PCB congeners, which contributes to the comparatively small cos θ and the large average RPD. The smaller extent of similarity is in part due to much larger number of PCB congeners used in the comparison of the PCB profiles.
3.3. Comparison of the homologue composition
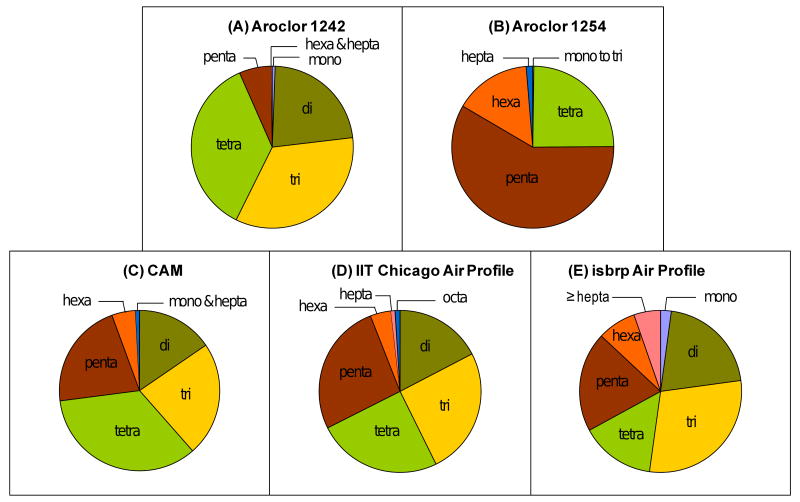
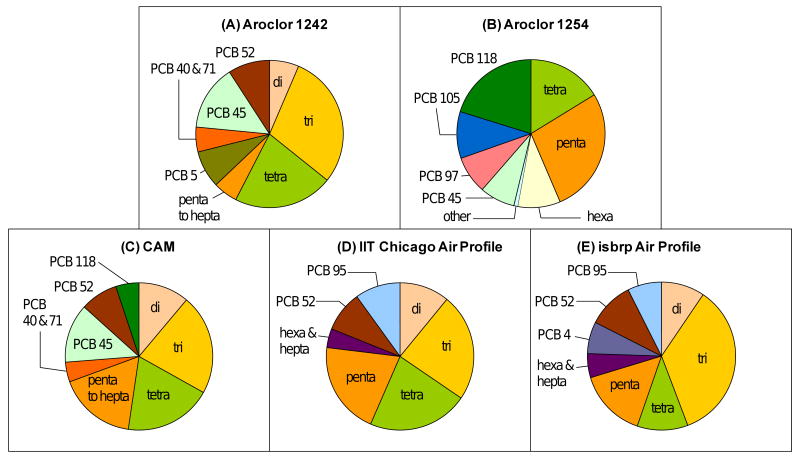
Figures 3A-3C show a comparison of the homologue composition of the CAM compared to the two Aroclor mixtures used in its preparation. The homologue composition of the average Chicago air profile at the IIT site (Figure 4D) and the average PCB profile recorded at different locations in Chicago as part of the isbrp (Figure 4E) are shown for comparison. While Aroclor 1254 was a medium chlorinated PCB mixture containing over 50% pentachlorobiphenyls, Aroclor 1242 contained only a small percentage of pentachlorobiphenyls (6 %). Instead, Aroclor 1242 was composed of comparable amounts of di-, tri- and tetrachlorobiphenyls (22-36%). This homologue composition is in agreement with the average molecular composition reported for these Aroclors (Silberhorn et al., 1990). Because the CAM is a 65:35 mixture of two Aroclor mixtures, it contained a much broader range of homologue groups, with di- to pentachlorobiphenyls making up significant percentages of this synthetic mixture (15-34%).
Figure 4.
Homologue composition of (A) Aroclor 1242, (B) Aroclor 1254, (C) the synthetic CAM, (D) the average IIT Chicago air profile and (E) the average isbrp Chicago air profile.
Similar to the CAM, the IIT and isbrp Chicago air profiles were composed of high percentages of di- to pentachlorobiphenyls (Figures 4D and 4E). However, the IIT Chicago air profile contained twice as many hepta chlorinated PCB congeners compared to the synthetic CAM (1.8 versus 0.8 %), i.e. these more persistent, higher chlorinated congeners were underrepresented in the synthetic mixture. Higher chlorinated PCB congeners with seven or eight chlorine atoms represented an even more significant percentage of the isbrp Chicago air profiles (13%). This observation is surprising considering the low vapor pressure of higher chlorinated PCB congeners (Hornbuckle et al., 2006).
3.4. Comparison of the TCDD Toxic Equivalence Quotient (TEQ)
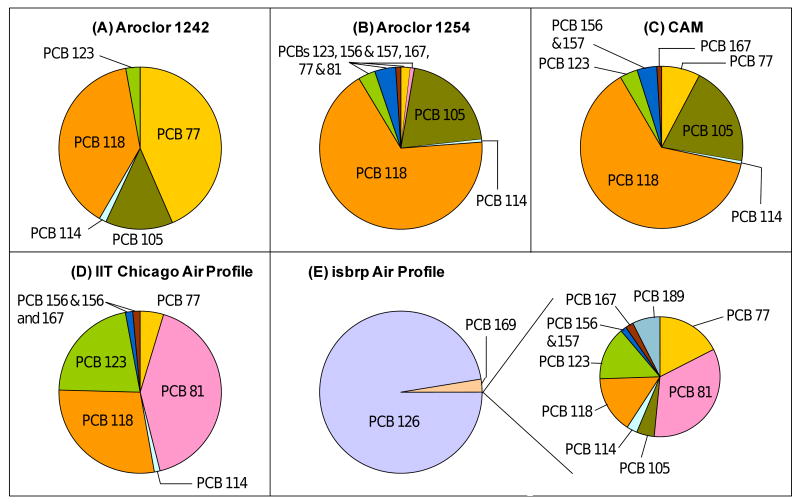
The number of ortho chlorine substituents determines the preferred conformation adopted by the two phenyl rings of a PCB congener (Lehmler et al., 2002; Shaikh et al., 2008) and, thus, determines its cellular targets. Several congeners with zero or one ortho chlorine atom can adopt a conformation similar to TCDD and interact with the aryl hydrocarbon (Ah)-receptor (Bandiera et al., 1982). These PCB congeners display dioxin-like toxicity and have been assigned a TCDD toxic equivalent factor (TEF) (Van den Berg et al., 2006). Nine dioxin-like PCB congeners, including PCBs 77, 81, 105, 114, 118, 123, 156, 157 and 167, were detected in the three synthetic mixtures, whereas PCBs 126, 167 and 169 were below the detection limit (Figure 5A-5C). The observation that PCB 126 was not detected is surprising because the Aroclor 1254 lot used in this study has been reported to resemble the 1254 A lot (Kostyniak et al., 2005) described by Frame et al. (Frame, 2001). Specifically, the Aroclor 1254 A lot contains 0.02% of PCB 126 and, thus, has a high TEQ activity compared to other Aroclor 1254 lots.
Figure 5.
Relative contribution of dioxin-like PCB congeners to the TCCD Toxic Equivalency Quotient (TEQ) of A) Aroclor 1242, (B) Aroclor 1254, (C) the synthetic CAM, (D) the average IIT Chicago air profile and (E) the average isbrp Chicago air profile. TEQ values were calculated from the PCB congener profiles using the 2005 WHO TCDD toxic equivalent factors (TEF) (Van den Berg et al., 2006). PCB 105 was not included in the TEQ calculation for the IIT Chicago air profile because it co-eluted with PCBs 153 and 132.
A theoretical TEQ based on the 2005 WHO TEF (Van den Berg et al., 2006) was calculated for the two Aroclors and the CAM by multiplying the weight percentage with the TEF of each congener. The TEQs – the sum of these products – are summarized in Table 1 and decreased in the order TEQAroclor 1254 > TEQCAM > TEQAroclor 1242. PCB 118, which was a major constituent of the three synthetic PCB mixtures, made the most significant overall contribution to the TEQ, followed by PCBs 105 and 77. The TEQ of the Chicago air profile determined at the IIT site appeared to be comparable to the synthetic PCB mixtures (Figure 5D). However, in contrast to the three synthetic PCB mixtures, PCBs 81 followed by PCBs 118 and 123 made major contributions to the TEQ of this Chicago air profile (PCB 105 was not included in the calculation of the TEQ because it co-eluted with PCBs 153 and 132).
Table 1.
Comparison of the TCCD Toxic Equivalency Quotient (TEQ) and Neurotoxic Equivalency Quotient (NEQ) of the PCB profiles.
| PCB mixture | TEQ [ng/mg PCB] | NEQ [mg/mg PCB] |
|---|---|---|
| Aroclor 1242 | 0.5 | 0.36 |
| Aroclor 1254 | 7.9 | 0.26 |
| CAM | 2.7 | 0.33 |
| IIT Chicago air profile | 1.6 | 0.44 |
| isbrp Chicago air profile | 865a | 0.30 |
PCBs 126 and 169 were detected only at 7% and 10% of the sampling sites.
The TEQ of the isbrp Chicago air profile was 865 ng/mg PCB as compared to 2.69 ng/mg PCB for the synthetic CAM and 1.59 ng/mg PCB for the IIT Chicago air profile (Table 1). Although all eleven PCB congeners with a TEF were reported for this profile, PCB 126 and, to a lesser extent, PCB 169 made the almost exclusive contributions to the TEQ of this profile (Figure 5E). Both PCB 126 and 169 have a high affinity for the Ah-receptor and, therefore, large TEF values (Van den Berg et al., 2006). However, the apparently high TEQ of the isbrp Chicago air profile needs to be interpreted with caution because PCBs 126 and 169 were detected only at 7% and 10% of the sampling sites.
3.5. Comparison of the Neurotoxic Equivalence Quotient (NEQ)
In contrast to dioxin-like PCB congeners, congeners with two or more ortho chlorine substituents adopt conformations with larger dihedral angles than dioxin like PCB congeners and, therefore, do not interact with the Ah-receptor (Bandiera et al., 1982). As discussed above, these non-dioxin like PCB congeners cause adverse effects, such as (developmental) neurotoxicity, by other modes of action. Simon et al. recently proposed a neurotoxic equivalence scheme of relative potency of PCB congeners (Simon et al., 2007). The NEQs calculated for all five PCB profiles using the neurotoxic equivalent values (NEV) estimated by Simon et al. are very similar (Table 1). In contrast to the TEQ, a large number of PCB congeners (52-64 out of 74 congeners with a NEV), mostly congeners with two to five chlorine substituents, made minor contributions (i.e., < 5%) to the NEQ (Figure 6). PCB 45 was a major neurotoxic PCB congener in the three synthetic PCB mixtures (7.7 to 14.3 % NEQ; Figure 6A-6C), but made only a minor contribution to the NEQ of the IIT and the isbrp Chicago air profiles (< 2 % NEQ; Figure 6D and 6E). PCB 52 and 95 made significant contributions to the NEQ of the IIT and the isbrp Chicago air profiles (> 15 % NEQ). While the contribution of PCB 52 to the NEQ in the CAM was comparable to its role in the two Chicago air profiles, PCB 95 was underrepresented in the CAM based on its contribution to the NEQ.
Figure 6.
Relative contribution of PCB congeners with a Neurotoxic Equivalency Factor (NEF) to the Neurotoxic Equivalency Quotient (NEQ) of A) Aroclor 1242, (B) Aroclor 1254, (C) the synthetic CAM, (D) the average IIT Chicago air profile and (E) the average isbrp Chicago air profile. The NEQ for each mixture was calculated from the PCB congener profiles using the neurotoxic equivalence factors proposed by Simon et al. (Simon et al., 2007).
3.6. Comparison of the relative composition of chiral PCB congeners
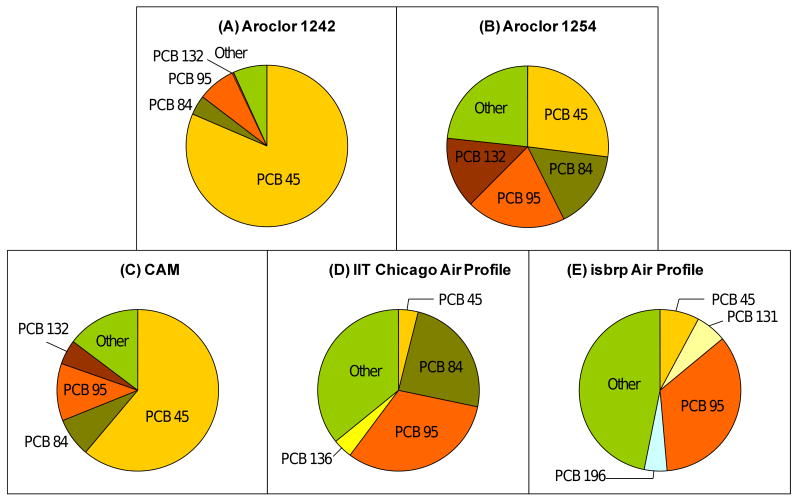
Some neurotoxic PCB congeners with three or four ortho chlorine substituents and unsymmetrical substitution patterns in both phenyl rings are chiral due to the hindered rotation around the biphenyl bond and, like other chiral organic compounds, can be subject to enantioselective disposition processes or interact with cellular targets in an enantio-selective or –specific manner (Lehmler and Robertson, 2001; Pessah et al., 2009). Figures 7A-C show that the congener distribution of chiral PCBs differs among the three synthetic mixtures. All three synthetic mixtures contain comparable total amounts of chiral PCBs ranging from 7.2 to 8.3 %, with PCBs 45, 84, 95 and 132 comprising about 6.3 to 6.7 % of the respective mixture. However, the overall distribution of chiral PCB congeners changes significantly with increasing degree of chlorination of the three synthetic mixtures. For example, the percentage of PCB 45 decreases from 5.9 % (80% of chiral PCBs) in Aroclor 1242 to 2.2 % (27 % of chiral PCBs) in Aroclor 1254. Furthermore, Aroclor 1254 contains traces of many higher chlorinated, chiral PCBs, such as PCBs 88, 91, 135, 136, 144, 149, 171, 174 and 183. Chiral PCBs 131, 175, 176, 196 and 197 were not detected in any of the PCB mixtures. In contrast, the two Chicago air profiles display a different composition with regards to chiral PCB congeners (Figures 7D and 7E). Specifically, the neurotoxic PCB 95 is a major chiral PCB congener in both Chicago air profiles, whereas PCB 45 represents only a comparatively small percentage of chiral PCB congeners.
Figure 7.
Weight percentage of chiral PCB congeners in (A) Aroclor 1242, (B) Aroclor 1254, (C) the synthetic CAM, (D) the average IIT Chicago air profile and (E) the average isbrp Chicago air profile. Only major chiral PCB congeners are shown.
Supplementary Material
Acknowledgments
This research was supported by grants ES05605, ES012475 and ES013661 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and Major Research Instrumentation grant BES-0420378 from the National Science Foundation. Contents of this manuscript are solely the reponsibility of the authors and do not necessarily represent the official views of NIEHS and NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann L, Mundy WR, Ward TR, Fastabend A, Lilienthal H. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on long-term potentiation and [3H]MK-801 binding in occipital cortex and hippocampus. Toxicol Sci. 2001;61:321–330. doi: 10.1093/toxsci/61.2.321. [DOI] [PubMed] [Google Scholar]
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: Reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- Arif JM, Lehmler HJ, Robertson LW, Gupta RC. Interaction of benzoquinone- and hydroquinone-derivatives of lower chlorinated biphenyls with DNA and nucleotides in vitro. Chem Biol Interact. 2003;142:307–316. doi: 10.1016/s0009-2797(02)00141-2. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone, 3-methylcholanthrene or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Cochran JW, Frame GM. Recent developments in the high-resolution gas chromatography of polychlorinated biphenyls. J Chromatogr A. 1999;843:323–368. doi: 10.1016/s0021-9673(99)00063-1. [DOI] [PubMed] [Google Scholar]
- Denomme MA, Bandiera S, Lambert I, Copp L, Safe L, Safe S. Polychlorinated biphenyls as phenobarbitone-type inducers of microsomal enzymes. Structure-activity relationships for a series of 2,4-dichloro-substituted congeners. Biochem Pharmacol. 1983;32:2955–2963. doi: 10.1016/0006-2952(83)90402-1. [DOI] [PubMed] [Google Scholar]
- Fouchecourt MO, Berny P, Riviere JL. Bioavailability of PCBs to male laboratory rats maintained on litters of contaminated soils: PCB burden and induction of alkoxyresorufin O-dealkylase activities in liver and lung. Arch Environ Contam Toxicol. 1998;35:680–687. doi: 10.1007/s002449900431. [DOI] [PubMed] [Google Scholar]
- Frame GM, Robertson LW, Hansen LG. The current state-of-the-art of comprehensive, quantitative, congener-specific PCB analysis, and what we now know about the distributions of individual congeners in commercial Aroclor mixtures. Lexington: University Press of Kentucky; 2001. pp. 3–9. [Google Scholar]
- Frame GM, Cochran JW, Bowadt SS. Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. J High Resolut Chromatogr. 1996a;19:657–668. [Google Scholar]
- Frame GM, Wagner RE, Carnahan JC, Brown JF, Jr, May RJ, Smullen LA, Bedard DL. Comprehensive, quantitative congener-specific analyses of eight Aroclors and complete PCB congener assignments on DB-1 capillary GC columns. Chemosphere. 1996b;33:603–623. [Google Scholar]
- Goldstein JA, Hass JR, Linko P, Harvan DJ. 2,3,7,8-Tetrachlorodibenzofuran in a commercially available 99% pure polychlorinated biphenyl isomer identified as the inducer of hepatic cytochrome P-448 and aryl hydrocarbon hydroxylase in the rat. Drug Metab Dispos. 1978;6:258–264. [PubMed] [Google Scholar]
- Gyorkos J, Denomme MA, Leece B, Homonko K, Valli VE, Safe S. Reconstituted halogenated hydrocarbon pesticide and pollutant mixtures found in human tissues: effects on the immature male Wistar rat after short-term exposure. Can J Physiol Pharmacol. 1985;63:36–43. doi: 10.1139/y85-006. [DOI] [PubMed] [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Kluwer Academic Publishers; Boston: 1999. [Google Scholar]
- Hansen LG, Strik JJ, TWA, Koeman JH, Kan CA. Biological activity of technical Aroclor 1254 compared to Aroclor 1254 residues: swine fat residues fed to broiler cockerels. Toxicology. 1981a;21:203–12. doi: 10.1016/0300-483x(81)90156-6. [DOI] [PubMed] [Google Scholar]
- Hansen LG, Tuinstra LG, Kan CA, Strik JJ, Koeman JH. Accumulation of chlorobiphenyls in chicken fat and liver after feeding Aroclor 1254 directly or fat from swine fed Aroclor 1254. J Agric Food Chem. 1983;31:254–60. doi: 10.1021/jf00116a017. [DOI] [PubMed] [Google Scholar]
- Hansen LG, Washko PW, Tuinstra LGMT, Dorn SB, Hinesly TD. Polychlorinated biphenyl, pesticide, and heavy metal residues in swine foraging on sewage sludge amended soils. J Agric Food Chem. 1981b;29:1012–1017. [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or Aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol. 1999;158:231–243. doi: 10.1006/taap.1999.8710. [DOI] [PubMed] [Google Scholar]
- Hornbuckle KC, Carlson DL, Swackhamer DL, Baker JE, Eisenreich SJ, Hites R. Polychlorinated Biphenyls in the Great Lakes. Vol. 5. Berlin, Heidelberg: Springer Verlag; 2006. pp. 13–70. [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Discovery of non-Aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GW, Hansen LG, Hamilton MC, Fowler B, Hermanson MH. PCB, PCDD and PCDF congener profiles in two types of Aroclor 1254. Environ Toxicol Pharmacol. 2008;25:156–163. doi: 10.1016/j.etap.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, Espandiari P, Gairola CG, Lehmler HJ. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ Sci Technol. 2005:3513–3520. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- Korach KS, Sarver P, Chae K, McLachlan JA, McKinney JD. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Mol Pharmacol. 1988;33:120–126. [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJK, Seegal RF, Pessah IN, Schantz SL. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Parkin S, Robertson LW. The three-dimensional structure of 3,3′,5′-trichloro-4-methoxybiphenyl, a “coplanar” polychlorinated biphenyl (PCB) derivative. Chemosphere. 2002;46:485–488. doi: 10.1016/s0045-6535(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Robertson LW, Hansen L. Atropisomers of PCBs. Lexington: University Press of Kentucky; 2001. pp. 62–65. [Google Scholar]
- Li MH, Hansen LG. Enzyme induction and acute endocrine effects in prepubertal female rats receiving environmental PCB/PCDF/PCDD mixtures. Environ Health Perspect. 1996a;104:712–22. doi: 10.1289/ehp.96104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Hansen LG. Responses of prepubertal female rats to environmental PCBs with high and low dioxin equivalencies. Fundam Appl Toxicol. 1996b;33:282–93. doi: 10.1006/faat.1996.0166. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Fastabend A, Hany J, Kaya H, Roth-Harer A, Dunemann L, Winneke G. Reduced levels of 1,25-dihydroxyvitamin D3 in rat dams and offspring after exposure to a reconstituted PCB mixture. Toxicol Sci. 2000;57:292–301. doi: 10.1093/toxsci/57.2.292. [DOI] [PubMed] [Google Scholar]
- Lin PH, Sangaiah R, Ranasinghe A, Upton PB, La DK, Gold A, Swenberg JA. Formation of quinonoid-derived protein adducts in the liver and brain of Sprague-Dawley rats treated with 2,2′,5, 5′-tetrachlorobiphenyl. Chem Res Toxicol. 2000;13:710–718. doi: 10.1021/tx000030f. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Lehmann L, Esch H, Robertson LW. Metabolic activation of PCBs to carcinogens in vivo. A review. Environ Toxicol Pharmacol. 2008;25:241–246. doi: 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar VS, Johnson GW, Brenner RC, Quensen JF, 3rd, Foote EA, Durell G, Ickes JA, Peven-McCarthy C. Long-term recovery of PCB-contaminated sediments at the Lake Hartwell superfund site: PCB dechlorination. 1. End-member characterization. Environ Sci Technol. 2005;39:3538–3547. doi: 10.1021/es048622y. [DOI] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Robertson LW, Safe S. Reconstituted human breast milk PCBs as potent inducers of aryl hydrocarbon hydroxylase. Biochem Biophys Res Commun. 1980;96:882–889. doi: 10.1016/0006-291x(80)91438-2. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphyenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (-)-2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22:201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restum JC, Bursian SJ, Giesy JP, Render JA, Helferich WG, Shipp EB, Verbrugge DA, Aulerich RJ. Multigenerational study of the effects of consumption of PCB-contaminated carp from Saginaw Bay, Lake Huron, on mink. 1. Effects on mink reproduction, kit growth and survival, and selected biological parameters. J Toxicol Environ Health A. 1998;54:343–375. doi: 10.1080/009841098158791. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Gupta RC. Metabolism of polychlorinated biphenyls (PCBs) generates electrophiles and reactive oxygen species that damage DNA. 2000:16–32. [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh NS, Parkin S, Luthe G, Lehmler HJ. The three-dimensional structure of 3,3′,4,4′-tetrachlorobiphenyl, a dioxin-like polychlorinated biphenyl (PCB) Chemosphere. 2008;70:1694–1698. doi: 10.1016/j.chemosphere.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp EB, Restum JC, Giesy JP, Bursian SJ, Aulerich RJ, Helferich WG. Multigenerational study of the effects of consumption of PCB-contaminated carp from Saginaw Bay, Lake Huron, on mink. 2. Liver PCB concentration and induction of hepatic cytochrome P-450 activity as a potential biomarker for PCB exposure. J Toxicol Environ Health A. 1998;54:377–401. doi: 10.1080/009841098158809. [DOI] [PubMed] [Google Scholar]
- Silberhorn E, Glauert HP, Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:439–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- Simon T, Britt JK, James RC. Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul Toxicol Pharmacol. 2007;48:148–170. doi: 10.1016/j.yrtph.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Song Y, Wagner BA, Lehmler HJ, Buettner GR. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies. Chem Res Toxicol. 2008;21:1359–1367. doi: 10.1021/tx8000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Basu I, Hites RA. Temporal trends of polychlorinated biphenyls in precipitation and air at Chicago. Environ Sci Technol. 2006;40:1178–1183. doi: 10.1021/es051725b. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Narang A, Ding X, Eadon G. Characterization and quantitative analysis of DNA adducts formed from lower chlorinated PCB-derived quinones. Chem Res Toxicol. 2004;17:502–511. doi: 10.1021/tx034245b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.