Abstract
Background
The impact of gender on major natural history endpoints in heart failure (HF) has not been examined in a propensity-matched study.
Methods
Of the 7788 chronic systolic and diastolic HF patients in the Digitalis Investigation Group trial 1926 were women. Propensity scores for female gender were used to assemble a cohort of 1669 pairs of men and women who were well-balanced on 32 measured baseline characteristics. Matched hazard ratios (HR) and 95% confidence intervals (CI) for outcomes associated with female gender were calculated using stratified Cox regression models.
Results
All-cause mortality occurred in 36% (rate, 1256/10,000 person-years) and 30% (rate, 1008/10,000 person-years) of matched men and women respectively during 5 years of follow up (HR when women were compared with men, 0.82, 95% CI, 0.72–0.94, P=0.004). Female gender was also associated with reduced cardiovascular mortality (matched HR, 0.85; 95% CI, 0.73–0.99, P=0.037) and a trend toward reduced non-cardiovascular mortality (matched HR, 0.73; 95% CI, 0.53–1.00; P=0.053). All-cause hospitalization occurred in 67% (rate, 4003/10,000 person-years) and 65% (rate, 3762/10,000 person-years) matched male and female patients respectively (HR for women, 1.03, 95% CI, 0.93–1.15, P=0.538). Female gender was not associated with cardiovascular or HF hospitalization but was associated with hospitalization due to unstable angina pectoris (matched HR, 1.38; 95%CI, 1.11–1.72; P=0.003) and stroke (matched HR, 0.65; 95%CI, 0.46–0.92; P=0.014).
Conclusions
In patients with chronic HF, female gender has a significant independent association with improved survival but has no association with all-cause, cardiovascular, or HF hospitalizations.
Keywords: Heart failure, gender, unstable angina pectoris, mortality, hospitalization
1. Introduction
Among patients with chronic heart failure (HF), female gender has generally been shown to be associated with more favorable outcomes [1–5]. However, to what extent these associations are independent of potential confounding due to imbalances in baseline characteristics is not well known. In the current study we examined the impact of gender on a wide variety of major natural history endpoints in a propensity matched population of ambulatory chronic HF patients in which men and women were well balanced on all measured baseline covariates.
2. Materials and methods
2.1. Source of data and study patients
We used the public-use copy of the Digitalis Investigation Group (DIG) trial dataset obtained from the National Heart, Lung and Blood Institute. The design and results from the DIG trial have been previously reported in detail [6]. Briefly, 7788 chronic HF patients were randomized to receive digoxin or placebo from 302 clinical centers across the United States (186 centers) and Canada (116 centers) between January 1991 and August 1993. Of these, 6800 patients had left ventricular ejection fraction <45% and most were receiving an angiotensin-converting enzyme inhibitor and diuretics. Of the 7788 DIG participants, 1926 (25%) were women.
2.2. Assembly of a balanced study cohort
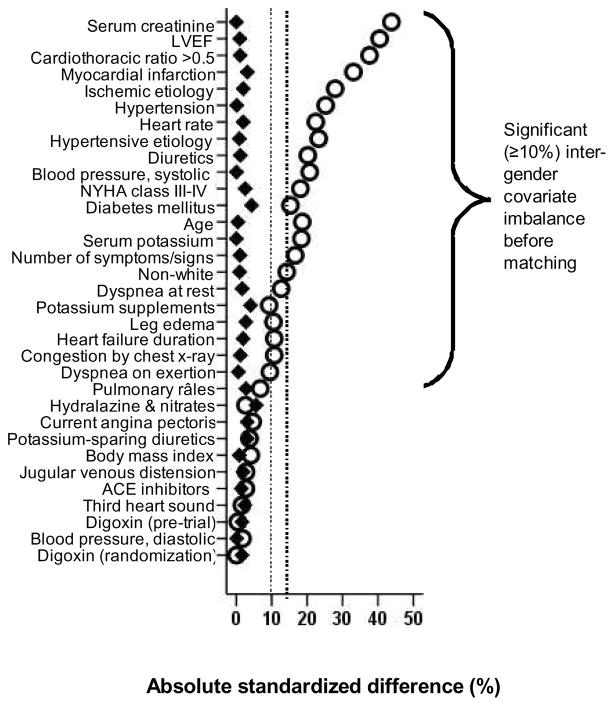
Because of the significant differences in baseline characteristics between gender groups (Table 1 and Figure 1), we used propensity scores to assemble a matched cohort in which male and female patients were well balanced on all measured baseline characteristics [7, 8]. Using a non-parsimonious multivariable logistic regression model, we estimated propensity scores for female gender for each of the 7788 DIG participants. In the model female gender was used as the dependent variable, and all clinically relevant baseline characteristics (n=32) displayed in Figure 1 were included as covariates. We then assembled a matched cohort of male and female patients who had similar propensity scores [9–19]. Overall, we were able to match 1669 pairs of men and women, which included 87% of all women. We assessed post-match balance for all measured covariates by estimating their between-gender absolute standardized differences and presented those findings as a Love plot [12]. An absolute standardized difference of 0% would suggest no residual bias, and that <10% is considered of inconsequential bias [12].
Table 1.
Baseline patient characteristics, by gender, before and after propensity score matching
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
| Male (n = 5862) | Female (n = 1926) | P Value | Male (n = 1669) | Female (n = 1669) | P Value | |
| Age (years) | 63 ± 10 | 65 ± 12 | <0.0001 | 65 ± 10 | 65 ± 12 | 0.889 |
| Non-white | 774 (13%) | 354 (18%) | <0.0001 | 303 (18%) | 297 (18%) | 0.820 |
| Body mass index (kg/m2) | 27 ± 5 | 27 ± 7 | 0.078 | 27 ± 5 | 27 ± 6 | 0.769 |
| Duration of HF (months) | 31 ± 37 | 27 ± 34 | <0.0001 | 27 ± 33 | 28 ± 33 | 0.581 |
| Primary cause of HF | ||||||
| Ischemic | 4225 (72%) | 1135 (59%) | 1022 (61%) | 1038 (62%) | ||
| Hypertensive | 496 (9%) | 309 (16%) | <0.0001 | 241 (14%) | 236 (14%) | 0.886 |
| Idiopathic | 762 (13%) | 349 (18%) | 298 (18%) | 292 (18%) | ||
| Others | 379 (7%) | 133 (7%) | 108 (7%) | 103 (6%) | ||
| Prior myocardial infarction | 3927 (67%) | 981 (51%) | <0.0001 | 886 (53%) | 912 (55%) | 0.362 |
| Current angina pectoris | 1562 (27%) | 553 (29%) | 0.077 | 455 (27%) | 478 (29%) | 0.399 |
| Hypertension | 2584 (44%) | 1090 (57%) | <0.0001 | 906 (54%) | 907 (54%) | 1.000 |
| Diabetes mellitus | 1568 (27%) | 650 (34%) | <0.0001 | 582 (35%) | 548 (33%) | 0.229 |
| Medications | ||||||
| Pre-trial digoxin use | 2530 (43%) | 835 (43%) | 0.881 | 730 (44%) | 743 (45%) | 0.676 |
| Trial use of digoxin | 2927 (50%) | 962 (50%) | 0.990 | 833 (50%) | 820 (49%) | 0.681 |
| ACE inhibitors | 5485 (94%) | 1789 (93%) | 0.296 | 1547 (93%) | 1553 (93%) | 0.741 |
| Diuretics | 4457 (76%) | 1619 (84%) | <0.0001 | 1395 (84%) | 1388 (83%) | 0.778 |
| PS diuretics | 434 (7%) | 162 (8%) | 0.149 | 126 (8%) | 140 (8%) | 0.407 |
| Potassium supplement | 1594 (27%) | 605 (31%) | <0.0001 | 532 (32%) | 501 (30%) | 0.264 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 1206 (21%) | 499 (26%) | <0.0001 | 433 (26%) | 421 (25%) | 0.665 |
| Dyspnea on exertion | 4354 (74%) | 1508 (78%) | <0.0001 | 1297 (78%) | 1293 (78%) | 0.901 |
| Jugular venous distension | 754 (13%) | 266 (14%) | 0.284 | 241 (14%) | 230 (14%) | 0.624 |
| Third heart sound | 1399 (24%) | 447 (23%) | 0.556 | 417 (25%) | 400 (24%) | 0.524 |
| Pulmonary râles | 942 (16%) | 359 (19%) | 0.009 | 328 (20%) | 310 (19%) | 0.464 |
| Lower extremity edema | 1166 (20%) | 467 (24%) | <0.0001 | 407 (24%) | 388 (23%) | 0.469 |
| Number of symptom/signs | 5.4 ± 2.1 | 5.7 ± 1.9 | <0.0001 | 5.7 ± 2.0 | 5.7 ± 1.9 | 0.764 |
| NYHA functional class | ||||||
| Class I | 913 (16%) | 190 (10%) | 174 (10%) | 177 (11%) | ||
| Class II | 3235 (55%) | 1009 (52%) | <0.0001 | 878 (53%) | 895 (54%) | 0.995 |
| Class III | 1606 (27%) | 681 (35%) | 579 (35%) | 560 (34%) | ||
| Class IV | 108 (2%) | 46 (2%) | 38 (2%) | 37 (2%) | ||
| Heart rate (beats/minute), | 78 ± 13 | 81 ± 13 | <0.0001 | 80 ± 12 | 80 ± 13 | 0.561 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 126 ± 20 | 131 ± 22 | <0.0001 | 129 ± 20 | 129 ± 21 | 0.993 |
| Diastolic | 75 ± 11 | 75 ± 12 | 0.495 | 75 ± 11 | 75 ± 12 | 0.966 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 779 (13%) | 330 (17%) | <0.0001 | 288 (17%) | 281 (17%) | 0.783 |
| Cardiothoracic ratio >0.5 | 3274 (56%) | 1416 (74%) | <0.0001 | 1201 (72%) | 1193 (72%) | 0.779 |
| Serum creatinine (mg/dL) | 1.32 ± 0.36 | 1.16 ± 0.37 | <0.0001 | 1.19 ± 0.28 | 1.19 ± 0.38 | 0.447 |
| Serum potassium (mEq/L) | 4.36 ± 0.45 | 4.28 ± 0.42 | <0.0001 | 4.3 ± 0.5 | 4.3 ± 0.4 | 0.939 |
| Ejection fraction (%) | 31 ± 12 | 36 ± 14 | <0.0001 | 34 ± 13 | 34 ± 13 | 0.758 |
| Ejection fraction >45%* | 581 (10%) | 407 (21%) | <0.0001 | 307 (18%) | 282 (17%) | 0.254 |
ACE = angiotensin-converting enzyme; NYHA = New York Heart Association; PS = potassium sparing
Variable not included in logistic regression model for propensity score
Figure 1.
Love plot for absolute standardized differences for all measured covariates by gender, before and after propensity score matching
2.3. Study outcomes
The primary outcomes for the current analysis were mortality and hospitalizations due to all causes, cardiovascular causes and HF during 38 months of median follow-up. Secondary outcomes included a multitude of other cause-specific mortality and hospitalizations. Data on mortality and hospitalization were collected by study investigators and was 99% complete [20].
2.4. Statistical analysis
Baseline characteristics were compared using Pearson Chi square and Wilcoxon rank-sum tests for the pre-match data, and McNemar’s test and paired sample t-test for post-match comparisons, as appropriate. Kaplan Meier plots and matched Cox regression models were used to estimate associations of female gender with various outcomes. A formal sensitivity analysis was conducted to examine the strength of association an unmeasured confounder with a near-perfect association with outcome (e.g. mortality), would need to have with the exposure (viz., female gender) to change the conclusions of our study [21]. Subgroup analyses were conducted to examine heterogeneity of the association between gender and all-cause mortality. All statistical tests were two sided with p-values <0.05 considered significant. SPSS for Windows (Version 15) was used for all data analyses [22].
3. Results
3.1 Patient characteristics
Matched patients (n=3338) had a mean age (±SD) of 65 ± 11 years (range, 22 to 94 years), and 18% were non-white. Before matching, women in general were older and more likely to be non-white. They also had a shorter mean duration of HF, a higher mean LVEF, a lower prevalence of ischemic heart disease, but a higher prevalence of diabetes mellitus and a higher symptom burden such as angina pectoris and dyspnea both at rest and on exertion. These and other imbalances in baseline characteristics were well balanced after matching (Table 1 and Figure 1). Post match absolute standardized differences for all measured covariates were <5% suggesting substantial covariate balance across the groups (Figure 1).
3.2. Association between gender and mortality
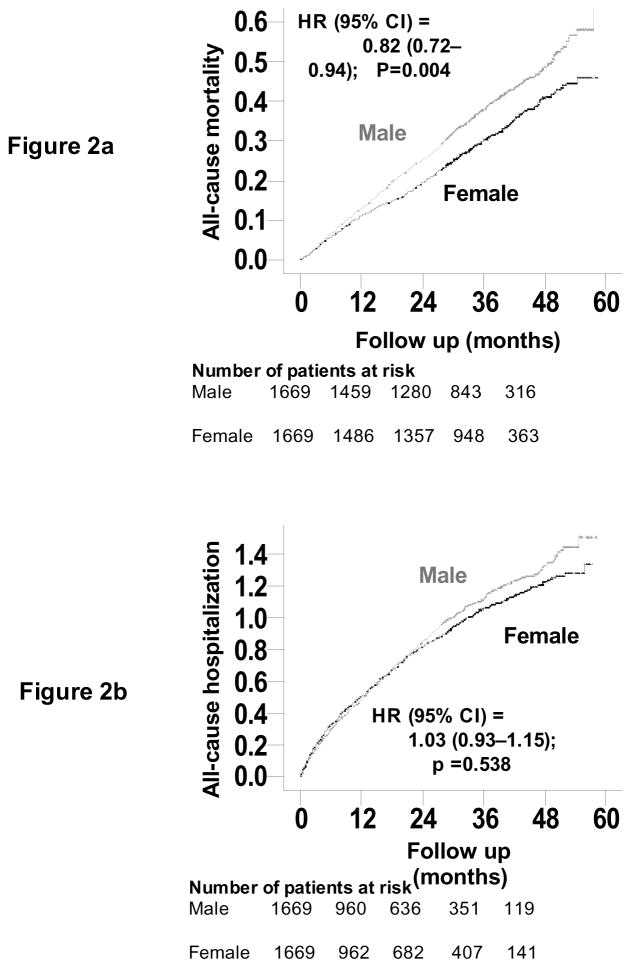
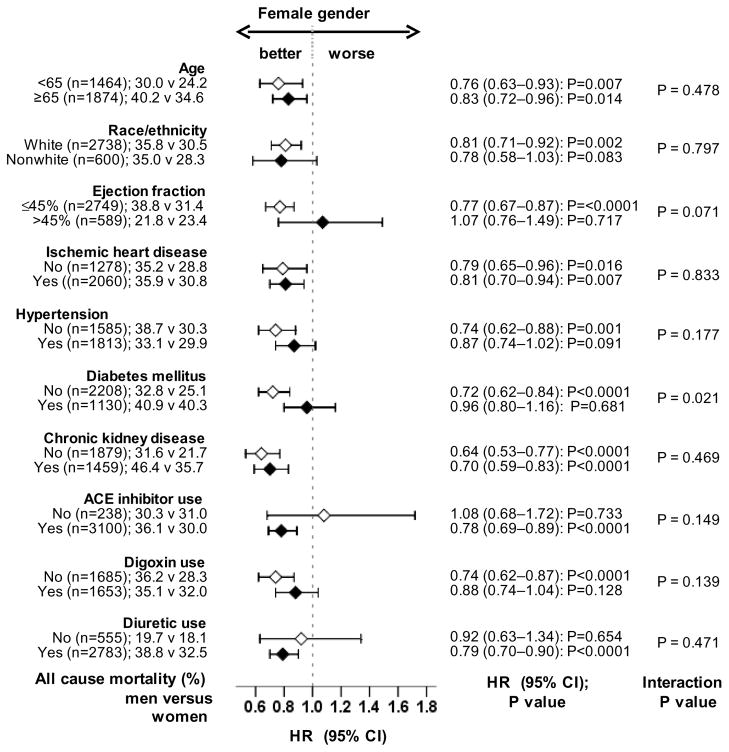
Overall, 1097 (33%) patients died from all causes. All-cause mortality occurred in 36% (rate, 1256/10,000 person-years) and 30% (rate, 1008/10,000 person-years) men and women respectively (matched hazard ratio {HR} when women were compared to men, 0.82; 95% CI, 0.72–0.94; P=0.004; Table 2 and Figure 2a). In the absence of hidden bias, a sign-score test for matched data with censoring provides strong evidence (P=0.005) that women clearly outlived men. An unmeasured binary covariate that is a near-perfect predictor of mortality could potentially explain away the association between female gender and mortality if that unmeasured covariate would increase the odds of its association with female gender by 6.15%. Except for patients with diabetes mellitus amongst whom the survival benefit of female gender appeared to be lost, the association between gender and mortality was homogeneous across various subgroups of matched patients (Figure 3). Unadjusted, multivariable-adjusted and propensity-adjusted HR’s (95% CI) for all-cause mortality associated with female gender were 0.83 (0.76–0.91; P<0.0001), 0.83 (0.75–0.91; P<0.0001) and 0.82 (0.74–0.91; P<0.0001) respectively. Associations between gender and other cause-specific mortalities among matched patients are displayed in Table 2.
Table 2.
Mortality by gender in propensity score matched heart failure patients
| Cause-specific mortality | Rate, per 10000 person-years (events/total follow up years) | Absolute rate difference* (per 10000 person-years) | Hazard ratio† (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Male (N=1669) | Female (N=1669) | ||||
| All-cause | 1256 (595/4736) | 1008 (502/4982) | −249 | 0.82 (0.72–0.94) | 0.004 |
| Cardiovascular | 961 (455/4736) | 783 (390/4982) | −178 | 0.85 (0.73–0.99) | 0.037 |
| Heart failure | 389 (184/4736) | 387 (193/4982) | −1 | 1.09 (0.87–1.37) | 0.452 |
| Other cardiovascular | 572 (271/4736) | 395 (197/4982) | −177 | 0.69 (0.26–0.85) | 0.001 |
| Non-cardiovascular | 230 (109/4736) | 171 (85/4982) | −60 | 0.73 (0.53–1.00) | 0.053 |
| Unknown | 65 (31/4736) | 54 (27/4982) | −11 | 0.73 (0.38–1.39) | 0.332 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the male group from the event rates in the female group (before values were rounded).
Hazard ratios and confidence intervals (CI) (when women were compared to men) were estimated from matched Cox proportional hazards models
Figure 2.
Kaplan-Meier plots for (a) mortality due to unstable all causes and (b) hospitalization due to all-causes by gender
Figure 3.
Association of gender with all-cause mortality in subgroups of propensity-matched patients (ACE=angiotensin-converting enzyme; CI= confidence interval; HR=hazard ratio)
3.3. Association between gender and hospitalization
Overall, 2207 (66%) patients were hospitalized due to all causes. All-cause hospitalization occurred in 67% (rate, 4003/10,000 person-years) and 65% (rate, 3762/10,000 person-years) of men and women respectively (matched HR when women were compared to men, 1.03; 95% CI, 0.93–1.15; P=0.538; Table 3 and Figure 2b). Unadjusted, multivariable-adjusted and propensity-adjusted HR’s (95% CI) for all-cause hospitalization associated with female gender were 0.99 (0.93–1.06; P=0.847), 0.93 (0.87–1.00; P=0.035) and 0.93 (0.87–1.00; P=0.055) respectively.
Table 3.
Hospitalization by gender in propensity score matched heart failure patients
| Cause-specific hospitalization | Rate, per 10000 person-years (events/total follow up years) | Absolute rate difference* (per 10000 person-years) | Hazard ratio† (95% confidence interval) | P value | |
|---|---|---|---|---|---|
| Male (N=1669) | Female (N=1669) | ||||
| All cause | 4003 (1114/2783) | 3762 (1093/2905) | − 240 | 1.03 (0.93–1.15) | 0.538 |
| Cardiovascular | 2491 (834/3348) | 2570 (877/3412) | + 79 | 1.10 (0.98–1.23) | 0.121 |
| Heart failure | 1212 (489/4034) | 1215 (510/4198) | + 3 | 1.04 (0.90–1.20) | 0.610 |
| Myocardial infarction | 194 (90/4638) | 208 (101/4865) | +14 | 1.20 (0.86–1.66) | 0.281 |
| Unstable angina | 528 (177/3350) | 738 (252/3415) | +210 | 1.38 (1.11–1.72) | 0.003 |
| Stroke | 202 (93/4598) | 139 (68/4891) | −63 | 0.65 (0.46–0.92) | 0.014 |
| Other cardiovascular | 416 (183/4403) | 375 (175/4668) | −41 | 0.89 (0.70–1.12) | 0.309 |
| Non cardiovascular | 1560 (589/3776) | 1362 (550/4038) | −198 | 0.89 (0.78–1.02) | 0.100 |
Absolute differences in rates of events per 10,000 person-year of follow up were calculated by subtracting the event rates in the male group from the event rates in the female group (before values were rounded)
Hazard ratios and confidence intervals (CI) (when women were compared to men) were estimated from matched Cox proportional hazards models
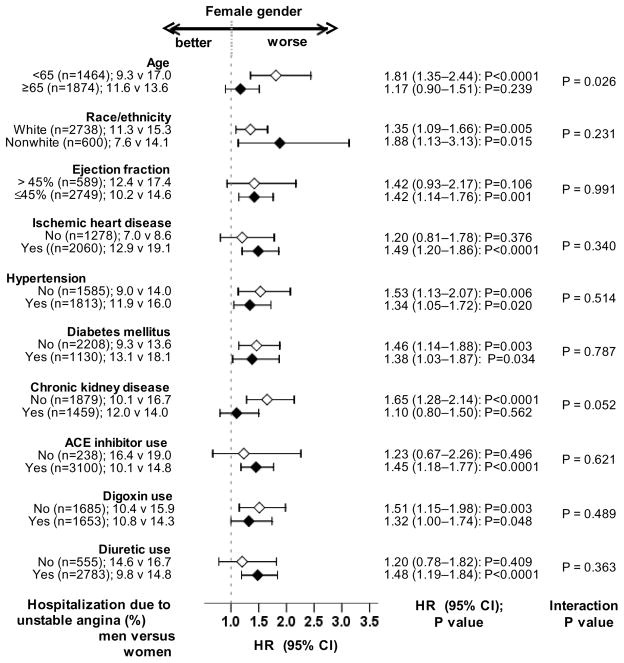
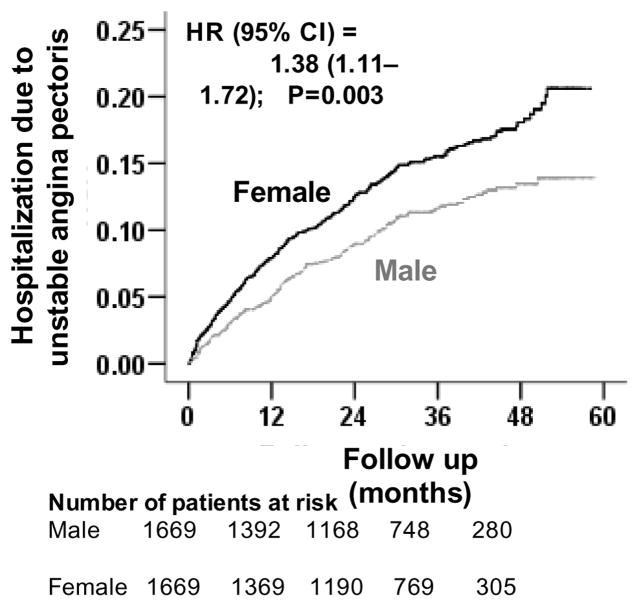
Hospitalization due to incident unstable angina pectoris occurred in 11% (528/10,000 person-years of follow up) and 15% (738/10,000 person-years of follow up) of matched men and women (matched HR when women were compared to men, 1.38; 95% CI, 1.11–1.72; P=0.003; Table 3 and Figure 3). In the absence of hidden bias, a sign-score test for matched data with censoring provides strong evidence (P=0.003) that women clearly had more hospitalizations due to unstable angina pectoris and that the odds of an unmeasured covariate to be associated with female gender would need to be increased by 11.39% to explain away this association. The association between gender and unstable angina pectoris was homogenous across various subgroups except for age (Figure 5). Unadjusted, multivariable-adjusted and propensity-adjusted HR’s (95% CI) for all-cause hospitalization associated with female gender were 1.33 (95% CI, 1.16–1.53; P<0.0001), 1.37 (95% CI, 1.18–1.58; P<0.0001) and 1.35 (95% CI, 1.16–1.59; P<0.0001) respectively. Gender had no association with hospitalization due to acute myocardial infarction (AMI) (Table 3). Association between gender and other cause-specific hospitalizations among matched patients are displayed in Table 3.
Figure 5.
Association of gender with unstable angina pectoris in subgroups of propensity-matched patients (ACE=angiotensin-converting enzyme; CI= confidence interval; HR=hazard ratio)
4. Discussion
The findings of the current study demonstrate that despite a better risk profile (a shorter mean duration of HF, a higher mean LVEF, and a lower prevalence of ischemic heart disease) women with chronic HF in general had a higher symptom burden (higher prevalence of NYHA class III–IV). However, in keeping with the low baseline risk profile, women with chronic HF in our study had significantly reduced mortality. On the other hand, despite a higher symptom burden, female gender was associated with similar hospitalizations to males. These associations persisted when all measured baseline characteristics including risk profile and symptom burden were well-balanced after propensity score matching. These findings suggest that the intrinsic mortality benefit of women with HF is not translated into an intrinsic lower hospitalization. Additionally, female gender was associated with increased hospitalization due to unstable angina pectoris. Understanding reasons for a higher symptom burden among women with HF may help reduce hospitalization in HF, which is a leading cause of hospitalization among older adults. This is also important as with the aging of the population, the proportion of women with HF is projected to increase.
Bivariate associations between gender and outcomes are likely in part confounded by imbalances in baseline characteristics between men and women. However, these bivariate associations are true representations of the impact of gender on major natural history end points in chronic HF because many of these characteristics such as higher mean LVEF and a lower prevalence of ischemic heart disease are inseparably associated with female gender. Bivariate associations fail to inform us however to what extent these associations may have been due to an intrinsic impact of gender. Findings from our propensity-matched cohort suggest that women with chronic HF may have a significant intrinsic survival benefit that may not be explained by imbalances in baseline characteristics between men and women. Matched men and women in our study were well-balanced on 32 measured baseline characteristics that included many potential confounders such as HF duration and etiology, important comorbidities and New York Heart Association functional class.
The association of gender with outcomes in chronic HF may also be attributed to an unmeasured covariate. However, findings from our sensitivity analyses suggest that two of the key associations observed in our study, improved survival and increased risk of unstable angina pectoris hospitalization in women were relatively insensitive to an unmeasured confounder. Further, for an unmeasured characteristic to become a confounder, it must be associated with both gender and the outcome, and not be strongly associated with any of the 32 measured characteristics. Therefore, it is unlikely that the observed associations observed in our study can be due to the effect of an unmeasured confounder.
Although the exact underlying mechanism of the survival advantage of female HF patients is not clearly understood, an intrinsic impact of gender on outcome is mechanistically plausible. Findings from a genetic model of hypertension and HF in rats suggest a preserved adaptive hypertrophic reserve was observed in females, which may contribute to the lower morbidity and mortality of females with chronic HF [23]. Similarly, in a mouse model of AMI, male gender has been associated with maladaptive ventricular remodeling and delayed healing [24–26]. In humans with severe aortic stenosis, male gender has been associated with a more maladaptive ventricular remodeling than that seen in women [27]. There is also evidence that specific sex hormone receptors exist within the myocardium and that sex hormones may affect both mechanical and biochemical properties of the adult heart [28, 29]. Sex hormones may also contribute to sex-related differences in the activation of the renin-angiotensin-aldosterone system [29, 30]. While most women in our study were post-menopausal, there is also evidence suggesting an association between estrogen and improved survival in women with HF [31–33]. Therefore, while the improved survival of women with HF is incompletely understood, accumulating evidence suggests that this association is biologically plausible.
The dissociation in the impact of female gender on mortality and hospitalization in HF is intriguing. Despite reduced mortality, women had similar all-cause, cardiovascular and HF hospitalizations as in men. One potential explanation might be that men were more likely to experience sudden cardiac deaths, which may have precluded hospitalizations. However, it is also possible that women may have had developed more severe symptoms during follow-up, may have perceived their symptoms to be more severe, may have lacked social support necessitating hospitalization, or may have been undertreated for their symptoms [34]. However, regardless of the cause, it is essential to understand reasons for this mortality-hospitalization dissociation in women with HF so that interventions can be developed to reduce hospitalizations these patients. This is important as HF is the leading cause of hospitalization among older adults in developed nations and with the aging of the population, the prevalence of women with HF is projected to increase.
The association between female gender and hospitalization due to unstable angina pectoris is unlikely to be explained by an imbalance in baseline ischemic heart disease as the prevalence of ischemic heart disease was lower among women before matching and was balanced after matching. A potential explanation may be a higher prevalence of left ventricular hypertrophy and diastolic HF in women. Although we had no data on the prevalence of left ventricular hypertrophy, women in our study had a higher prevalence of hypertension, cardiomegaly, and a higher mean LVEF before matching. Unstable angina pectoris may be more common in patients with left ventricular hypertrophy and diastolic HF, often due to subendocardial ischemia in viable myocardium [35–37]. Data from animal studies suggest that the intrinsic resistance against myocardial ischemia in females may be attenuated in hypertrophied myocardium as protein kinase B (Akt) and extracellular signal-regulated kinases (ERK 1/2) responsible for the protection may be deactivated in hypertrophied female hearts [38, 39]. Of note, the prevalence of ischemic heart disease in women was high (~60%) and similar to that in men. It is also possible that treatment of ischemic heart disease may have been suboptimal in women that may have resulted in the higher incidence of unstable angina. These findings suggest that women with HF and angina pectoris should be properly evaluated and treated for myocardial ischemia, if present.
Findings from our subgroup analyses deserve further discussion. We observed that female gender was associated with reduced mortality in patients with and without ischemic heart disease (Figure 3). In patients with more advanced systolic HF, the survival advantage of female gender has been variably reported to be present only among those with [4] and without [3, 40] ischemic heart disease. Our observation that the survival benefit of female gender was lost among HF patients with diabetes mellitus is consistent with our prior report of a significant gender-diabetes interaction in HF [41]. The lack of a statistically significant association between female gender and unstable angina pectoris hospitalization among elderly HF patients (Figure 5) may in part be due to the higher prevalence of silent myocardial ischemia in older adults [42, 43].
A potential limitation of our study is that these findings are based on trial-eligible, relatively young HF patients in normal sinus rhythm at baseline, from the pre-beta-blocker era of HF therapy, which may limit generalizability. Therefore, these findings may need to be replicated in more contemporary cohorts of HF patients.
In conclusion, in ambulatory chronic HF patients, women have a better risk profile, a higher symptom burden, a lower risk of death and a similar risk of hospitalization as in men. As HF is the leading cause of hospitalization among older adults and prevalence of women with HF is projected to increase in the developed nations, an understanding of underlying mechanisms responsible for this mortality-hospitalization dissociation in women may help in the development of interventions to reduce hospitalizations in women with HF.
Figure 4.
Kaplan-Meier plots for hospitalization due to unstable angina pectoris by gender
Acknowledgments
Funding support: Dr. Ahmed is supported by the National Institutes of Health through grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama.
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [44].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 2.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Jr, Sueta CA, Gheorghiade M, et al. Gender differences in survival in advanced heart failure. Insights from the FIRST study. Circulation. 1999;99:1816–21. doi: 10.1161/01.cir.99.14.1816. [DOI] [PubMed] [Google Scholar]
- 4.Simon T, Mary-Krause M, Funck-Brentano C, Jaillon P. Sex differences in the prognosis of congestive heart failure: results from the Cardiac Insufficiency Bisoprolol Study (CIBIS II) Circulation. 2001;103:375–80. doi: 10.1161/01.cir.103.3.375. [DOI] [PubMed] [Google Scholar]
- 5.Alla F, Al-Hindi AY, Lee CR, Schwartz TA, Patterson JH, Adams KF., Jr Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. Am Heart J. 2007;153:1074–80. doi: 10.1016/j.ahj.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 8.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 9.Adamopoulos C, Pitt B, Sui X, Love TE, Zannad F, Ahmed A. Low serum magnesium and cardiovascular mortality in chronic heart failure: A propensity-matched study. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Pitt B, Rahimtoola SH, et al. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: a propensity-matched study of the DIG trial. Int J Cardiol. 2008;123:138–46. doi: 10.1016/j.ijcard.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–53. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed MI, Ekundayo OJ, Mujib M, et al. Mild hyperkalemia and outcomes in chronic heart failure: A propensity matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alper AB, Campbell RC, Anker SD, et al. A propensity-matched study of low serum potassium and mortality in older adults with chronic heart failure. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekundayo OJ, Adamopoulos C, Ahmed MI, et al. Oral potassium supplement use and outcomes in chronic heart failure: A propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2008.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekundayo OJ, Dell’italia LJ, Sanders PW, et al. Association between hyperuricemia and incident heart failure among older adults: A propensity-matched study. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamouzis G, Agha SA, Ekundayo OJ, et al. Incident coronary revascularization and subsequent mortality in chronic heart failure: A propensity-matched study. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pitt B, Zannad F, Gheorghiade M, et al. Transatlantic similarities and differences in major natural history endpoints of heart failure after acute myocardial infarction: A propensity-matched study of the EPHESUS trial. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: A propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sui X, Gheorghiade M, Zannad F, Young JB, Ahmed A. A propensity matched study of the association of education and outcomes in chronic heart failure. Int J Cardiol. 2008;129:93–9. doi: 10.1016/j.ijcard.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–30. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum P. Sensitivity to Hidden Bias. In: Rosenbaum P, editor. Observational Studies. Vol. 1. New York, NY: Springer-Verlag; 2002. pp. 105–170. [Google Scholar]
- 22.SPSS for Windows, Rel. 15 program. SPSS Inc; Chicago, IL: 2008. [Google Scholar]
- 23.Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33:676–80. doi: 10.1161/01.hyp.33.2.676. [DOI] [PubMed] [Google Scholar]
- 24.Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. Gender differences in postinfarction left ventricular remodeling. Cardiology. 1999;91:173–83. doi: 10.1159/000006906. [DOI] [PubMed] [Google Scholar]
- 25.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75:2181–92. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Wu JC, Nasseri BA, Bloch KD, Picard MH, Scherrer-Crosbie M. Influence of sex on ventricular remodeling after myocardial infarction in mice. J Am Soc Echocardiogr. 2003;16:1158–62. doi: 10.1067/S0894-7317(03)00648-5. [DOI] [PubMed] [Google Scholar]
- 27.Carroll JD, Carroll EP, Feldman T, et al. Sex-associated differences in left ventricular function in aortic stenosis of the elderly. Circulation. 1992;86:1099–107. doi: 10.1161/01.cir.86.4.1099. [DOI] [PubMed] [Google Scholar]
- 28.Schaible TF, Malhotra A, Ciambrone G, Scheuer J. The effects of gonadectomy on left ventricular function and cardiac contractile proteins in male and female rats. Circ Res. 1984;54:38–49. doi: 10.1161/01.res.54.1.38. [DOI] [PubMed] [Google Scholar]
- 29.McGill HC, Jr, Sheridan PJ. Nuclear uptake of sex steroid hormones in the cardiovascular system of the baboon. Circ Res. 1981;48:238–44. doi: 10.1161/01.res.48.2.238. [DOI] [PubMed] [Google Scholar]
- 30.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Pina IL. A better survival for women with heart failure? It’s not so simple. J Am Coll Cardiol. 2003;42:2135–8. doi: 10.1016/j.jacc.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Reis SE, Holubkov R, Young JB, White BG, Cohn JN, Feldman AM. Estrogen is associated with improved survival in aging women with congestive heart failure: analysis of the vesnarinone studies. J Am Coll Cardiol. 2000;36:529–33. doi: 10.1016/s0735-1097(00)00738-5. [DOI] [PubMed] [Google Scholar]
- 33.Lindenfeld J, Ghali JK, Krause-Steinrauf HJ, et al. Hormone replacement therapy is associated with improved survival in women with advanced heart failure. J Am Coll Cardiol. 2003;42:1238–45. doi: 10.1016/s0735-1097(03)00938-0. [DOI] [PubMed] [Google Scholar]
- 34.Harjai KJ, Nunez E, Stewart Humphrey J, Turgut T, Shah M, Newman J. Does gender bias exist in the medical management of heart failure? Int J Cardiol. 2000;75:65–9. doi: 10.1016/s0167-5273(00)00298-9. [DOI] [PubMed] [Google Scholar]
- 35.Brush JE, Jr, Cannon RO, 3rd, Schenke WH, et al. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319:1302–7. doi: 10.1056/NEJM198811173192002. [DOI] [PubMed] [Google Scholar]
- 36.Iriarte M, Caso R, Murga N, et al. Microvascular angina pectoris in hypertensive patients with left ventricular hypertrophy and diagnostic value of exercise thallium-201 scintigraphy. Am J Cardiol. 1995;75:335–9. doi: 10.1016/s0002-9149(99)80549-9. [DOI] [PubMed] [Google Scholar]
- 37.Nijland F, Kamp O, Verhorst PM, de Voogt WG, Visser CA. In-hospital and long-term prognostic value of viable myocardium detected by dobutamine echocardiography early after acute myocardial infarction and its relation to indicators of left ventricular systolic dysfunction. Am J Cardiol. 2001;88:949–55. doi: 10.1016/s0002-9149(01)01968-3. [DOI] [PubMed] [Google Scholar]
- 38.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther. 2005;315:1125–35. doi: 10.1124/jpet.105.090803. [DOI] [PubMed] [Google Scholar]
- 39.Bell JR, Porrello ER, Huggins CE, Harrap SB, Delbridge LM. The intrinsic resistance of female hearts to an ischemic insult is abrogated in primary cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2008;294:H1514–22. doi: 10.1152/ajpheart.01283.2007. [DOI] [PubMed] [Google Scholar]
- 40.Ghali JK, Krause-Steinrauf HJ, Adams KF, et al. Gender differences in advanced heart failure: insights from the BEST study. J Am Coll Cardiol. 2003;42:2128–34. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed A, Aban IB, Vaccarino V, et al. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–90. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rich MW. Epidemiology, clinical features, and prognosis of acute myocardial infarction in the elderly. Am J Geriatr Cardiol. 2006;15:7–11. doi: 10.1111/j.1076-7460.2006.05273.x. quiz 12. [DOI] [PubMed] [Google Scholar]
- 43.de Bruyne MC, Mosterd A, Hoes AW, et al. Prevalence, determinants, and misclassification of myocardial infarction in the elderly. Epidemiology. 1997;8:495–500. doi: 10.1097/00001648-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–50. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]